Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal disease of motor neuron degeneration in the brain and spinal cord. Progressive paralysis of the diaphragm and other respiratory muscles leading to respiratory dysfunction and failure is the most common cause of death in ALS patients. Respiratory impairment has also been shown in animal models of ALS. Vascular pathology is another recently recognized hallmark of ALS pathogenesis. Central nervous system (CNS) capillary damage is a shared disease element in ALS rodent models and ALS patients. Microvascular impairment outside of the CNS, such as in the lungs, may occur in ALS, triggering lung damage and affecting breathing function. Stem cell therapy is a promising treatment for ALS. However, this therapeutic strategy has primarily targeted rescue of degenerated motor neurons. We showed functional benefits from intravenous delivery of human bone marrow (hBM) stem cells on restoration of capillary integrity in the CNS of an superoxide dismutase 1 (SOD1) mouse model of ALS. Due to the widespread distribution of transplanted cells via this route, administered cells may enter the lungs and effectively restore microvasculature in this respiratory organ. Here, we provided preliminary evidence of the potential role of microvasculature dysfunction in prompting lung damage and treatment approaches for repair of respiratory function in ALS. Our initial studies showed proof-of-principle that microvascular damage in ALS mice results in lung petechiae at the late stage of disease and that systemic transplantation of mainly hBM-derived endothelial progenitor cells shows potential to promote lung restoration via re-established vascular integrity. Our new understanding of previously underexplored lung competence in this disease may facilitate therapy targeting restoration of respiratory function in ALS.
Keywords: ALS, respiratory dysfunction, lungs, microvasculature, repair
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by motor neuron degeneration in the brain and spinal cord leading to muscle atrophy and paralysis1–3. Regardless of disease type, sporadic or familial, ALS patients usually die within 3–5 years of diagnosis due to respiratory failure4–6. Respiratory complications (e.g., dyspnea or orthopnea), ensuing about 3 years from disease onset, are the most common causes of death for ALS patients7–9. Patients with bulbar onset typically display early respiratory problems associated with more rapid disease progression. Riluzole and edaravone (Radicava®) are the only Food and Drug Administration-approved treatment drugs10–12. Although these drugs have a modest survival benefit, they offer no improvement in clinical symptoms13. Progressive respiratory muscle weakness leads to impaired diaphragm function14,15, resulting in ineffective breathing and pneumonia in ALS patients16,17. Moreover, a recent study18 showed that respiratory dysfunction in ALS patients is associated with weakness of cervical spinal cord muscles. Due to these complications, palliative approaches such as tracheostomy or breathing tube to support ventilation and airway clearance are demanded7,19,20, which may prolong survival and improve quality of life in ALS patients for at least two additional years. Since about 95% of ALS patients in the United States decline implantation of mechanical ventilation devices6, management of respiratory failure represents a significant unmet clinical need in addition to development of effective therapeutic strategies.
Cell-based regenerative medicine has emerged as a potent therapy in a variety of neurological disorders, including ALS21–25 . Stem cell therapy, however, has primarily targeted rescue of motor neurons from degeneration via a neuroprotective action rather than cell replacement as discussed26–29. For instance, intrathecal administration of bone marrow mesenchymal stem cells into ALS patients (phase I/IIa clinical trial) showed safety and efficacy in treatment by providing potential neurotrophic support to motor neurons30. Moreover, combining autologous adult neural stem cell treatment with T-cell vaccination might be a feasible therapeutic approach for ALS patients31. In addition, autologous hematopoietic stem cell transplantation into the frontal motor cortex of ALS patients was shown to be safe and well tolerated by patients32. Important considerations for using stem cell treatment for ALS, specifically in early stage clinical trials, are comprehensively discussed24.
Despite intensive investigations into potential ALS treatments, strategies to restore and/or preserve respiratory function remain limited. In an effort to address this issue, Nichols et al.33 showed that transplantation of human neural progenitor cells into the cervical spinal cord of pre-symptomatic G93A SOD1 mutant rats preserved breathing capacity and improved phrenic motor neuron survival. Also, administration of glial-restricted precursors into the cervical spinal cord of a rat model of ALS revealed increased animal survival in addition to moderating the decline in respiratory function in cell-treated rats34. Moreover, to our knowledge, only one phase I clinical trial examined the effect of intramedullary infusion of autologous bone marrow mononuclear cells at the thoracic level on the breathing patterns of ALS patients35. Results of this study demonstrated safety of the cell injection; however, no significant differences were observed in oxygen saturation or inspiratory flow after 1 year. Although preclinical and clinical studies are important for targeting respiratory function by preserving breathing capability, the invasiveness of cell injections into the spinal cord may preclude this treatment for a large population of ALS patients.
Accumulated evidence identified ALS as a neurovascular disease36,37, which led to investigations into additional effectors in motor neuron degeneration. Our38–41 and other42–47 studies demonstrated impairment of microvessel endothelial cells (ECs) in the brain and spinal cord of rodent models of disease and ALS patients, resulting in compromised blood–central nervous system (CNS) barrier (B-CNS-B) integrity. Since ECs play a key role in lung vascular development and function48,49, it is possible that microvascular EC dysfunction in lungs may occur and lead to and/or even initiate respiratory complications in ALS.
To address therapeutic strategies for repair of the damaged endothelium in ALS, our research team focused on the effects of intravenously (iv) administered human bone marrow-derived CD34+ cells (hBM34+) or endothelial progenitor cells (hBM-EPCs) into symptomatic G93A SOD1 mutant mice. Results showed structural and functional benefits of transplanted cells on restoration of barrier competence in the CNS, leading to increased motor neuron survival50–53. The B-CNS-B repair was due to the widespread transplanted cell engraftment into capillary lumen in the brain and spinal cord, mainly in hBM-EPC-treated ALS mice. Taking into account that the majority of iv-transplanted cells are initially trapped in the lungs54, this pulmonary first-pass effect could be favorable for therapeutic options in lungs. This possibility should be investigated.
In this study, we briefly explored the potential role of the microvasculature in triggering lung damage in ALS. A specific focus was to determine the effects of intravenous stem cell transplants on promoting microvasculature EC repair in the lungs. Our understanding of the underexplored lung complications in ALS may foster development of targeted therapies for restoration of respiratory function in ALS.
Respiratory Dysfunction and Lung Damage in Rodent Models of ALS
Based on notable respiratory complications in ALS patients, studies on respiratory function in animal models of disease were initiated. Respiratory dysfunction resulting in altered breathing patterns and metabolism (i.e., O2 consumption and CO2 production) has been shown in SOD1 mutant G93A mouse55 and rat33,56 models of ALS until the late disease stage, mainly by motor impairment of phrenic nerve. Also, rats with fused in sarcoma RNA-binding protein (FUS) gene transfer showed progressive motor deficiencies in association with breathing abnormalities and arterial blood acidosis57. Importantly, Tankersley et al.55 demonstrated three phases of respiratory phenotype in G93A SOD1 mutant mice according to their ages: stable breathing characteristics (10–14 wk of age), breathing pattern and respiratory apparatus less efficient (16–18 wk of age), and rapid decline in ventilator function (after 18 wk of age). However, these studies or others did not evaluate the lung as a respiratory organ in an animal model of ALS. The importance of the interaction between the lung parenchyma and the airways has been comprehensively discussed58. The authors emphasized that the interdependence between respiratory system components such as the airways, lung parenchyma, and vasculature is imperative for the physiological respiratory function and that the weakening of any these components contributes to “airway dysfunction and gas exchange impairment”58.
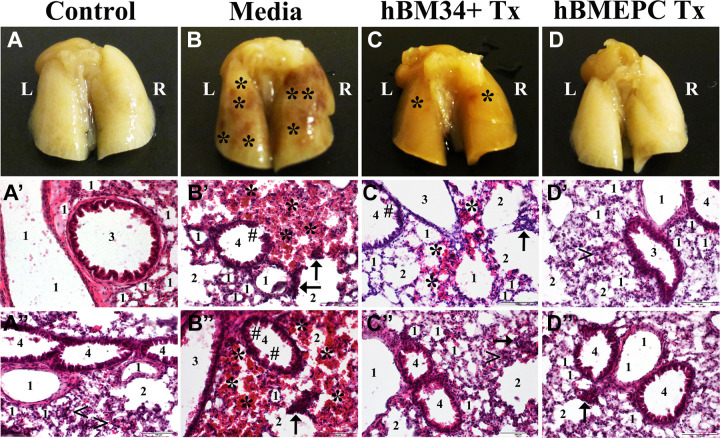
We initiated studies to evaluate the lung status in G93A SOD1 mutant mice and effect(s) of systemically administered hBM-derived stem cells. The lungs were obtained from randomly selected ALS mice used in our previous studies50–52. These mice received iv hBM34+ cells or hBM-EPC transplants at equal cell doses of 1 × 106 cells/mouse at early symptomatic stage. Also, media-injected mice and mice not carrying the mutant SOD1 gene were used as controls. Cell-transplanted and media-injected animals received cyclosporine A (50 mg/mL ampules, NDC 0574-0866-10, Perrigo, Minneapolis, MN, USA) (10 mg/kg, ip) for the entire post-transplant period. All mice were sacrificed at 17 wk of age (4 wk post-cell or media administration) under pressure-controlled fluid delivery to avoid capillary rupture as described in our published papers mentioned above. The left lung lobe was removed and processed for histological analysis using a routine hematoxylin & eosin (H&E) staining. Results showed extensive lung damage in media-treated ALS mice versus controls (Fig. 1). Numerous microhemorrhages (mh) were found in both lung lobes in these mice (Fig. 1B). Substantial reduction of mh was noted after hBM34+ cell transplantation; only a few mh were detected (Fig. 1C). In mice receiving hBM-EPCs at the same cell concentration, no mh were observed and the lung tissues were grossly similar to those in control mice (Fig. 1D).
Figure 1.
Characteristic of the lungs in G93A SOD1 mice at the late disease stage. Gross view of the lungs with dorsal side up showed abundant microhemorrhages (mh) in media-treated mice (B) versus controls (A). At 4 wk post-treatment, a substantial decrease of mh was noted after hBM34+ cell treatment (C) whereas no mh were found in ALS mice receiving hBM-EPCs (D). The H&E staining in left lung lobe revealed typical appearance of all lung compartments, including cellular components in control mice (A′, A″). In contrast, diffuse eosinophilic infiltration around bronchioles and bronchiolar epithelium damage were seen in media-treated mice (B′, B″). Also, ruptured capillaries near alveoli or alveolar sacs were noted. Mice treated with hBM34+ cells demonstrated some areas of eosinophilic permeation, mild damage of bronchiolar epithelium, and a few burst capillaries (C′, C″). Near normal presence of airway components was observed in ALS mice receiving hBM-EPCs (D′, D″). Only scarce ruptured capillaries around alveolar sacs were found (D″). Scale bar in A′–D″ is 100 µm. L: left lobe; R: right lobe; 1: alveolus; 2: alveolar sac; 3: bronchus; 4: bronchiole; <: typical capillary; ←: ruptured capillary; *: microhemorrhage; #: damaged bronchiolar epithelium; Tx: cell transplant; ALS: amyotrophic lateral sclerosis; hBM34+: human bone marrow-derived CD34+ cells; hBM-EPCs: human bone marrow endothelial progenitor cells; H&E: hematoxylin & eosin.
The H&E staining confirmed the gross lung observations. Control mice showed typical morphologies of all lung compartments and cellular components (Fig. 1A′, A″). However, diffuse eosinophilic infiltration around bronchioles and bronchiolar cuboidal epithelium damage were seen in many lung patches of media-injected mice (Fig. 1B′, B″). Also, ruptured capillaries, as indicated by nucleated cell extravasation near alveoli or alveolar sacs, were noted. Mice with hBM34+ cell treatment demonstrated some areas of eosinophilic permeation, mild damage of bronchiolar epithelium, and a few burst capillaries (Fig. 1C′, C″). Near normal appearance of airway components and ciliated cuboidal epithelial cells were observed in ALS mice receiving hBM-EPCs; only scarce ruptured capillaries around alveolar sacs were found (Fig. 1D′, D″).
Altogether, our study results demonstrated significant lung damage in ALS mice at late symptomatic stage with notable mh by ruptured capillaries near the alveolar wall resulting in lung petechiae. Typically, numerous capillaries surrounding the alveolar walls allow rapid diffusion of O2 into the bloodstream and CO2 waste from blood into alveoli. This gas exchange occurs through a thin respiratory membrane (i.e., blood–gas barrier) located at the outer capillary membrane59. Any damage to microvessels in the lung may lead to airflow obstruction or restriction. Our novel finding on lung petechiae via microvessel damage in G93A SOD1 mutant mice near the end stage of disease may explain previously reported declines in breathing function in this animal model of ALS at the same age55. Importantly, intravenous transplantation of hBM-derived stem cells into symptomatic ALS mice attenuated lung hemorrhagic damage. More efficacious repair was determined by the administration of hBM-EPCs versus hBM34+ cells. Similar results on the reduction of mh in the spinal cords of symptomatic ALS mice treated with hBM stem cells were reported in our previous study53.
Capillary Permeability in the Lungs of G93A SOD1 Mutant Mice
Our previous studies demonstrated substantial capillary leakage in the spinal cords of late-stage G93A SOD1 mutant mice and that iv transplantation of hBM34+ cells51 or hBM-EPCs52 significantly decreased vascular permeability as determined by the analysis of Evans blue (EB) dye extravasation into the spinal cord parenchyma. These results, along with other effects of cell transplantation, indicate functional capillary repair in the spinal cords of ALS mice.
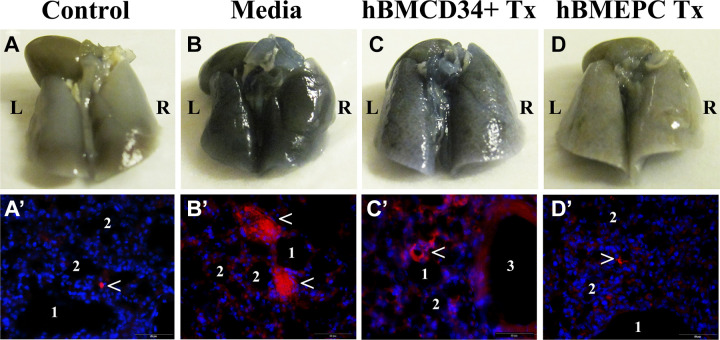
In our current initial study, lungs were obtained from randomly selected cell-treated or media-treated G93A SOD1 mice and controls, which received EB injection as described in our above-mentioned studies. The left lung lobe was removed and processed for the examination of EB extravasation into tissue parenchyma. Gross observation of the lungs from mice at 17 wk of age showed intensive blue color in both lung lobes from media-treated ALS mice (Fig. 2B) compared to controls (Fig. 2A). Reduced color intensity was detected in the lungs of ALS mice receiving hBM34+ cell transplant (Fig. 2C). However, some blue patches were seen in the lungs of these mice. The lung tissues observed in mice after transplantation of hBM-EPCs were grossly similar to controls (Fig. 2D). Detailed examination of the lung tissues in control mice revealed only a few spots of EB extravasation close to alveolar sacs (Fig. 2A′). In contrast, large areas of permeated EB dye near alveoli or alveolar sacs were detected in media-treated mice (Fig. 2B′). In the lungs from ALS mice treated with hBM34+ cells, EB leakage from several capillaries was found (Fig. 2C′). Analogous to control mice, minor EB extravasation from some lung capillaries was observed in ALS mice receiving hBM-EPCs (Fig. 2D′).
Figure 2.
Characteristic of capillary permeability in the lungs of G93A SOD1 mice at the late disease stage. Gross view of the lungs with dorsal side up showed intensive blue-colored lung tissues in media-treated ALS mice (B) compared to controls (A). At 4 wk post-treatment, reduced color intensity was detected in the lungs of ALS mice receiving hBM34+ cell transplant (C). Almost similar to controls, the lung tissues were grossly observed in mice after transplantation of hBM-EPCs (D). Examination of the lung tissues in control mice demonstrated only a few spots of EB extravasation close to alveolar sacs (red, arrowhead, A′). The large areas of EB extravasation near alveoli or alveolar sacs were detected in media-treated mice (red, arrowheads, B′). In the lungs from ALS mice treated with hBM34+ cells, EB leakage from several capillaries was found (red, arrowhead, C′). Minor EB permeation from some lung capillaries was observed in ALS mice receiving hBM-EPCs (red, arrowhead, D′) similar to control mice. DAPI (blue) was used as counterstaining for cell nuclei. Scale bar in A′–D′ is 50 µm. L: left lobe; R: right lobe; 1: alveolus; 2: alveolar sac; 3: bronchiole; EB: Evans blue; Tx: cell transplant; DAPI: 4′,6-diamidino-2-phenylindole; ALS: amyotrophic lateral sclerosis; hBM34+: human bone marrow-derived CD34+ cells; hBM-EPCs: human bone marrow endothelial progenitor cells.
Taken together, these study results demonstrating capillary permeability in the lungs of G93A SOD1 mutant mice at the late stage of disease supported our histological analysis described above. Since capillaries in the lungs are fenestrated, a slight amount of EB permeation into the lung parenchyma was detected in control mice. However, taking into account that the molecular size of EB is 961 Da, the observed extensive dye extravasation in the lung tissues of media-treated ALS mice may confirm significant rupture of capillaries. Also, the reduction of lung hemorrhagic damage after iv cell transplantation, mainly observed after hBM-EPC transplants, potentially resulted in functional microvessel repair as determined by decreased EB extravasation.
Cellular Immunophenotypic Analysis of Migrated Cells Into the Lungs of G93A SOD1 Mutant Mice After Intravenous Cell Transplantation
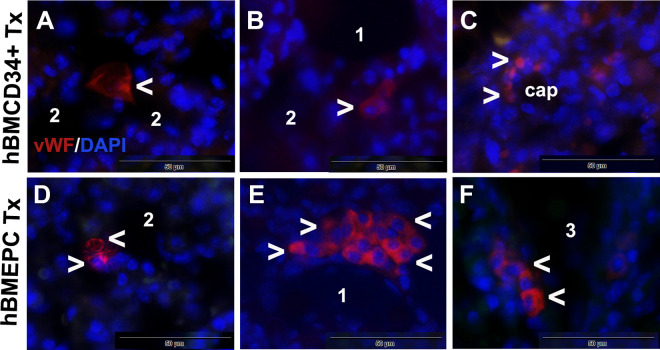
To determine that structural and functional lung improvements were related to a cell treatment effect, immunohistochemical staining of residual parts of the lung tissues from ALS mice which received hBM34+ cells and hBM-EPC transplants in our histological study was performed via detection of human anti-von Willibrand factor (hvWF) antigen as we previously described50,52. At 4 wk post-transplant, there were a few hvWF-positive cells in the lungs of mice treated with hBM34+ cells that displayed epithelial-like morphology and were located near the alveolar sac (Fig. 3A, B). Several rounded cells were found in the capillary wall (Fig. 3C). In the lungs of ALS mice that received hBM-EPCs, numerous cells immunoexpressing hvWF exhibited EC-like (Fig. 3D) or epithelial-like (Fig. 3D, F) cell phenotype. Interestingly, cells with epithelial morphology were detected in the bronchiolar wall (Fig. 3D).
Figure 3.
Cellular immunoexpression of human vWF in the lungs of G93A SOD1 mice 4 wk after cell transplantation. A few hBM34+ transplanted cells positive for vWF marker (red, arrowheads) showed epithelial-like morphology (A, B) or presented as rounded cells in capillary wall (C). A number of cells immunoexpressing vWF (red, arrowheads) with EC-like (D) or epithelial-like (E, F) morphology were detected in the lungs after hBM-EPC transplant. DAPI (blue) was used as counterstaining for cell nuclei. Scale bar in A–F is 50 µm. 1: alveolus; 2: alveolar sac; 3: bronchiole; cap: capillary; vWF: von Willibrand factor; hBM34+: human bone marrow-derived CD34+ cells; hBM-EPC: human bone marrow endothelial progenitor cell; DAPI: 4′,6-diamidino-2-phenylindole; EC: endothelial cells.
Thus, iv-transplanted cells were found to migrate and engraft in the lung tissues of G93A SOD1 mice. More cells in the lungs were detected in ALS mice after transplantation of hBM-EPCs and may support our previous observations on advanced repair of damaged lungs via this cell type. We recently showed60 that hBM-EPCs release angiogenic factors (vascular endothelial growth factor [VEGF] and angiogenin) in vitro, so these cells might also maintain endogenous EC function and/or promote angiogenesis in the lungs. Additionally, hBM-EPCs may release nanosized vesicles, vesicles which potentially mimic cell transplant effects and primarily act as mediators of intercellular communications61,62. Reportedly, human EPC-derived microvesicles (MVs) have demonstrated in vivo benefits through promotion of angiogenesis63,64. According to the authors, these MVs internalized and delivered bioactive molecules into target ECs, fostering angiogenesis and/or promoting neoangiogenesis. Human EPC-derived MVs also promoted EC survival, proliferation, and development of capillary-like structures in vitro and stimulated angiogenesis in vivo using immunodeficient severe combined immunodeficiency disease (SCID) mice64. Further, inflammatory signs were lessened after the application of extracellular vesicles (EVs) obtained from hBM mesenchymal stromal cells (MSCs) in a mouse model of lipopolysaccharide-induced lung injury65 or lung ischemia-reperfusion injury66. In a recent review, Namba67 discussed the efficacy of conditioned media or exosomes derived from human MSCs in treating preterm infants with bronchopulmonary dysplasia. The author emphasized paracrine effects of MSCs via secretion of various bioactive substances (i.e., interleukin [IL]-6, IL-8, VEGF, extracellular matrix proteins, and exosomes), important for anti-inflammatory protection of pulmonary epithelial and microvascular ECs. Similarly to actions of MSC-derived factors, hBM-EPCs may exert their angiogenic, anti-inflammatory, and/or immunomodulatory paracrine effects for repair of damaged lungs in ALS. However, the specific paracrine humoral factors, such as cytokines, chemokines, and growth factors, mediating beneficial cell transplant effects on residential endothelial and/or epithelial cells in lungs should be determined. In our ongoing study, characteristics of EVs derived from hBM-EPCs, including proteomics, should be informative regarding vesicle contents for establishing lung homeostasis.
Conclusions and Perspectives
ALS is a multifactorial fatal disease with limited therapies. Respiratory dysfunction is the most common cause of death for ALS patients. Similarly, respiratory complications have also been identified in SOD1 mutant rodent models of ALS. Integrated function of all respiratory system components, including the lungs, is vital for proper physiological respiration. However, the lung status has not been previously explored in animal ALS models or ALS patients.
We initiated studies to evaluate the lung condition of G93A SOD1 mutant mice at the late stage of disease. Our preliminary study results demonstrated numerous mh resulting in lung petechiae in ALS mice at 17 wk of age. This lung damage occurred due to microvascular rupture, as confirmed by EB dye extravasation into the lung tissues. The lung microvasculature impairment is concurrent with our and others’ findings on structural and functional capillary deficiencies within the CNS in both ALS patients and animal models of ALS. We believe that microvascular lung damage caused by EC dysfunction initiates respiratory complications by obstruction of breathing in ALS. However, since the lung hemorrhagic damage was observed in ALS mice at the late disease stage, evaluation of this respiratory organ should be performed, at least, in early symptomatic mice to confirm our supposition. Also, it might be necessary to examine the lungs in ALS patients for the appearance of mh in order to provide proper treatment.
Although intensive investigations of effective treatment for ALS are ongoing, development of appropriate therapeutic strategies to restore and/or preserve respiratory function is an extreme need. A few preclinical and clinical studies showed the promise of stem cell transplant approaches for targeting respiratory function by preserving breathing capability; however, invasive route of cell delivery into the spinal cord may not to be feasible for a large cohort of ALS patients. Re-establishing lung microvasculature via intravenous cell administration may be a promising minimally invasive therapeutic strategy. We showed the benefits of iv-transplanted hBM-derived stem cells into symptomatic G93A SOD1 mice by repairing CNS endothelium. In the current study, migration and enrichment of these transplanted cells in the lungs of ALS mice at 4 wk post-transplant support our suggestion. While hBM34+ cell treatment attenuated lung hemorrhagic damage, hBM-EPC transplantation demonstrated more benefits on lung repair. Differences in outcomes between these hBM-derived stem cells suggest that a restricted cell lineage, such as EPCs, versus hematopoietic CD34+ stem cells provides enhanced restorative results on damaged microvessels in the lungs of ALS mice.
Thus, our initial study demonstrated a proof-of-principle that lung microvascular damage might be an essential effector leading to respiratory dysfunction in ALS. Repair of lung capillaries via intravenous hBM-EPC transplantation is promising and may form the basis for a therapeutic approach toward lung restoration. However, studies regarding post-transplant efficiency of blood gas exchanges for proper breathing capacity and capillary integrity are needed to prove our concept that hBM-EPC treatment targets the vasculature for repair of damaged lungs in ALS. These studies will be addressed in our future investigations.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors SGD, PRS, and CVB disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NIH, NINDS (grant number 1R01NS090962).
ORCID iD: Svitlana Garbuzova-Davis  https://orcid.org/0000-0001-5816-0937
https://orcid.org/0000-0001-5816-0937
References
- 1. Talbot K. Motor neuron disease: the bare essentials. Pract Neurol. 2009;9(5):303–309. [DOI] [PubMed] [Google Scholar]
- 2. Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–955. [DOI] [PubMed] [Google Scholar]
- 4. Braun AT, Caballero-Eraso C, Lechtzin N. Amyotrophic lateral sclerosis and the respiratory system. Clin Chest Med. 2018;39(2):391–400. [DOI] [PubMed] [Google Scholar]
- 5. Nichols NL, Van Dyke J, Nashold L, Satriotomo I, Suzuki M, Mitchell GS. Ventilatory control in ALS. Respir Physiol Neurobiol. 2013;189(2):429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niedermeyer S, Murn M, Choi PJ. Respiratory failure in amyotrophic lateral sclerosis. Chest. 2019;155(2):401–408. [DOI] [PubMed] [Google Scholar]
- 7. Lyall RA, Donaldson N, Polkey MI, Leigh PN, Moxham J. Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain. 2001;124(Pt 10):2000–2013. [DOI] [PubMed] [Google Scholar]
- 8. Ackrivo J, Hansen-Flaschen J, Wileyto EP, Schwab RJ, Elman L, Kawut SM. Development of a prognostic model of respiratory insufficiency or death in amyotrophic lateral sclerosis. Eur Respir J. 2019;53(4):1802237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhattacharya R, Harvey RA, Abraham K, Rosen J, Mehta P. Amyotrophic lateral sclerosis among patients with a Medicare Advantage prescription drug plan; prevalence, survival and patient characteristics. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(3–4):251–259. [DOI] [PubMed] [Google Scholar]
- 10. Hugon J. Riluzole and ALS therapy. Wien Med Wochenschr. 1996;146(9–10):185–187. [PubMed] [Google Scholar]
- 11. Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4(3):191–206. [PubMed] [Google Scholar]
- 12. Rothstein JD. Edaravone: a new drug approved for ALS. Cell. 2017;171(4):725. [DOI] [PubMed] [Google Scholar]
- 13. Inoue-Shibui A, Kato M, Suzuki N, Kobayashi J, Takai Y, Izumi R, Kawauchi Y, Kuroda H, Warita H, Aoki M. Interstitial pneumonia and other adverse events in riluzole-administered amyotrophic lateral sclerosis patients: a retrospective observational study. BMC Neurol. 2019;19(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Carvalho M, Matias T, Coelho F, Evangelista T, Pinto A, Luís ML. Motor neuron disease presenting with respiratory failure. J Neurol Sci. 1996;139(Suppl):117–122. [DOI] [PubMed] [Google Scholar]
- 15. Lechtzin N. Respiratory effects of amyotrophic lateral sclerosis: problems and solutions. Respir Care. 2006;51(8):871–881; discussion 881-884. [PubMed] [Google Scholar]
- 16. Corcia P, Pradat P-F, Salachas F, Bruneteau G, Forestier NL, Seilhean D, Hauw J-J, Meininger V. Causes of death in a post-mortem series of ALS patients. Amyotroph Lateral Scler. 2008;9(1):59–62. [DOI] [PubMed] [Google Scholar]
- 17. DiPALS Writing Committee, DiPALS Study Group Collaborators. Safety and efficacy of diaphragm pacing in patients with respiratory insufficiency due to amyotrophic lateral sclerosis (DiPALS): a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2015;14(9):883–892. [DOI] [PubMed] [Google Scholar]
- 18. Pinto S, Gromicho M, Swash M, deCarvalho M. Cervical muscle weakness is a marker of respiratory dysfunction in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2019;91(3):323–324. [DOI] [PubMed] [Google Scholar]
- 19. Bourke SC, Shaw PJ, Gibson GJ. Respiratory function vs sleep-disordered breathing as predictors of QOL in ALS. Neurology. 2001;57(11):2040–2044. [DOI] [PubMed] [Google Scholar]
- 20. Gautier G, Verschueren A, Monnier A, Attarian S, Salort-Campana E, Pouget J. ALS with respiratory onset: clinical features and effects of non-invasive ventilation on the prognosis. Amyotroph Lateral Scler. 2010;11(4):379–382. [DOI] [PubMed] [Google Scholar]
- 21. Mazzini L, Vescovi A, Cantello R, Gelati M, Vercelli A. Stem cells therapy for ALS. Expert Opin Biol Ther. 2016;16(2):187–199. [DOI] [PubMed] [Google Scholar]
- 22. Tang BL. The use of mesenchymal stem cells (MSCs) for amyotrophic lateral sclerosis (ALS) therapy - a perspective on cell biological mechanisms. Rev Neurosci. 2017;28(7):725–738. [DOI] [PubMed] [Google Scholar]
- 23. Sanberg PR, Eve DJ, Willing AE, Garbuzova-Davis S, Tan J, Sanberg CD, Allickson JG, Cruz LE, Borlongan CV. The treatment of neurodegenerative disorders using umbilical cord blood and menstrual blood-derived stem cells. Cell Transplant. 2011;20(1):85–94. [DOI] [PubMed] [Google Scholar]
- 24. Goutman SA, Savelieff MG, Sakowski SA, Feldman EL. Stem cell treatments for amyotrophic lateral sclerosis: a critical overview of early phase trials. Expert Opin Investig Drugs. 2019;28(6):525–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mazzini L, Ferrari D, Andjus PR, Buzanska L, Cantello R, De Marchi F, Gelati M, Giniatullin R, Glover JC, Grilli M, Kozlova EN, et al. Advances in stem cell therapy for amyotrophic lateral sclerosis. Expert Opin Biol Ther. 2018;18(8):865–881. [DOI] [PubMed] [Google Scholar]
- 26. Abati E, Bresolin N, Comi G, Corti S. Advances, challenges, and perspectives in translational stem cell therapy for amyotrophic lateral sclerosis. Mol Neurobiol. 2019;56(10):6703–6715. [DOI] [PubMed] [Google Scholar]
- 27. Silani V, Calzarossa C, Cova L, Ticozzi N. Stem cells in amyotrophic lateral sclerosis: motor neuron protection or replacement? CNS Neurol Disord Drug Targets. 2010;9(3):314–324. [DOI] [PubMed] [Google Scholar]
- 28. Lunn JS, Sakowski SA, Feldman EL. Concise review: Stem cell therapies for amyotrophic lateral sclerosis: recent advances and prospects for the future. Stem Cells. 2014;32(5):1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomsen GM, Gowing G, Svendsen S, Svendsen CN. The past, present and future of stem cell clinical trials for ALS. Exp Neurol. 2014;262(Pt B):127–137. [DOI] [PubMed] [Google Scholar]
- 30. Syková E, Rychmach P, Drahorádová I, Konrádová Š, Růžičková K, Voříšek I, Forostyak S, Homola A, Bojar M. Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Transplant. 2017;26(4):647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moviglia GA, Moviglia-Brandolino MT, Varela GS, Albanese G, Piccone S, Echegaray G, Martinez G, Blasseti N, Farias J, Farina P, Perusso A, et al. Feasibility, safety, and preliminary proof of principles of autologous neural stem cell treatment combined with T-cell vaccination for ALS patients. Cell Transplant. 2012;21(Suppl 1):S57–63. [DOI] [PubMed] [Google Scholar]
- 32. Martínez HR, Molina-Lopez JF, González-Garza MT, Moreno-Cuevas JE, Caro-Osorio E, Gil-Valadez A, Gutierrez-Jimenez E, Zazueta-Fierro OE, Meza JA, Couret-Alcaraz P, Hernandez-Torre M. Stem cell transplantation in amyotrophic lateral sclerosis patients: methodological approach, safety, and feasibility. Cell Transplant. 2012;21(9):1899–1907. [DOI] [PubMed] [Google Scholar]
- 33. Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone PL, McHugh J, Svendsen CN, Mitchell GS. Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 2013;187(5):535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11(11):1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruiz-López FJ, Guardiola J, Izura V, Gómez-Espuch J, Iniesta F, Blanquer M, López-San Román J, Saez V, De Mingo P, Martínez S, Moraleda JM. Breathing pattern in a phase I clinical trial of intraspinal injection of autologous bone marrow mononuclear cells in patients with amyotrophic lateral sclerosis. Respir Physiol Neurobiol. 2016;221:54–58. [DOI] [PubMed] [Google Scholar]
- 36. Garbuzova-Davis S, Rodrigues MCO, Hernandez-Ontiveros DG, Louis MK, Willing AE, Borlongan CV, Sanberg PR. Amyotrophic lateral sclerosis: a neurovascular disease. Brain Res. 2011;1398:113–125. [DOI] [PubMed] [Google Scholar]
- 37. Rodrigues MCO, Hernandez-Ontiveros DG, Louis MK, Willing AE, Borlongan CV, Sanberg PR, Voltarelli JC, Garbuzova-Davis S. Neurovascular aspects of amyotrophic lateral sclerosis. Int Rev Neurobiol. 2012;102:91–106. [DOI] [PubMed] [Google Scholar]
- 38. Garbuzova-Davis S, Haller E, Saporta S, Kolomey I, Nicosia SV, Sanberg PR. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res. 2007;1157:126–137. [DOI] [PubMed] [Google Scholar]
- 39. Garbuzova-Davis S, Saporta S, Haller E, Kolomey I, Bennett SP, Potter H, Sanberg PR. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS ONE. 2007;2(11): e1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garbuzova-Davis S, Hernandez-Ontiveros DG, Rodrigues MCO, Haller E, Frisina-Deyo A, Mirtyl S, Sallot S, Saporta S, Borlongan CV, Sanberg PR. Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res. 2012;1469:114–128. [DOI] [PubMed] [Google Scholar]
- 41. Garbuzova-Davis S, Sanberg PR. Blood-CNS barrier impairment in ALS patients versus an animal model. Front Cell Neurosci. 2014;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nicaise C, Mitrecic D, Demetter P, De Decker R, Authelet M, Boom A, Pochet R. Impaired blood-brain and blood-spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res. 2009;1301:152–162. [DOI] [PubMed] [Google Scholar]
- 43. Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miyazaki K, Ohta Y, Nagai M, Morimoto N, Kurata T, Takehisa Y, Ikeda Y, Matsuura T, Abe K. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J Neurosci Res. 2011;89(5):718–728. [DOI] [PubMed] [Google Scholar]
- 45. Henkel JS, Beers DR, Wen S, Bowser R, Appel SH. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology. 2009;72(18):1614–1616. [DOI] [PubMed] [Google Scholar]
- 46. Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125(1):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sasaki S. Alterations of the blood-spinal cord barrier in sporadic amyotrophic lateral sclerosis. Neuropathology. 2015;35(6):518–528. [DOI] [PubMed] [Google Scholar]
- 48. Hwangbo C, Lee H-W, Kang H, Ju H, Wiley DS, Papangeli I, Han J, Kim J-D, Dunworth WP, Hu X, Lee S, et al. Modulation of endothelial bone morphogenetic protein receptor type 2 activity by vascular endothelial growth factor receptor 3 in pulmonary arterial hypertension. Circulation. 2017;135(23):2288–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Madurga A, Golec A, Pozarska A, Ishii I, Mižíková I, Nardiello C, Vadász I, Herold S, Mayer K, Reichenberger F, Fehrenbach H, et al. The H2S-generating enzymes cystathionine β-synthase and cystathionine γ-lyase play a role in vascular development during normal lung alveolarization. Am J Physiol Lung Cell Mol Physiol. 2015;309(7):L710–724. [DOI] [PubMed] [Google Scholar]
- 50. Garbuzova-Davis S, Kurien C, Thomson A, Falco D, Ahmad S, Staffetti J, Steiner G, Abraham S, James G, Mahendrasah A, Sanberg PR, et al. Endothelial and astrocytic support by human bone marrow stem cell grafts into symptomatic ALS mice towards blood-spinal cord barrier repair. Sci Rep. 2017;7(1):884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garbuzova-Davis S, Haller E, Navarro S, Besong TE, Boccio KJ, Hailu S, Khatib M, Sanberg PR, Appel SH, Borlongan CV. Transplantation of human bone marrow stem cells into symptomatic ALS mice enhances structural and functional blood-spinal cord barrier repair. Exp Neurol. 2018;310:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Garbuzova-Davis S, Kurien C, Haller E, Eve DJ, Navarro S, Steiner G, Mahendrasah A, Hailu S, Khatib M, Boccio KJ, Borlongan CV, et al. Human bone marrow endothelial progenitor cell transplantation into symptomatic ALS mice delays disease progression and increases motor neuron survival by repairing blood-spinal cord barrier. Sci Rep. 2019;9(1):5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eve DJ, Steiner G, Mahendrasah A, Sanberg PR, Kurien C, Thomson A, Borlongan CV, Garbuzova-Davis S. Reduction of microhemorrhages in the spinal cord of symptomatic ALS mice after intravenous human bone marrow stem cell transplantation accompanies repair of the blood-spinal cord barrier. Oncotarget. 2018;9(12):10621–10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tankersley CG, Haenggeli C, Rothstein JD. Respiratory impairment in a mouse model of amyotrophic lateral sclerosis. J Appl Physiol. 2007;102(3):926–932. [DOI] [PubMed] [Google Scholar]
- 56. Nichols NL, Satriotomo I, Harrigan DJ, Mitchell GS. Acute intermittent hypoxia induced phrenic long-term facilitation despite increased SOD1 expression in a rat model of ALS. Exp Neurol. 2015;273:138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jackson KL, Dhaibar HA, Dayton RD, Cananzi SG, Mayhan WG, Glasscock E, Klein RL. Severe respiratory changes at end stage in a FUS-induced disease state in adult rats. BMC Neurosci. 2016;17(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paré PD, Mitzner W. Airway-parenchymal interdependence. Compr Physiol. 2012;2(3):1921–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Irvin CG, Bates JHT. Measuring the lung function in the mouse: the challenge of size. Respir Res. 2003;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garbuzova-Davis S, Ehrhart J, Mustafa H, Llauget A, Boccio KJ, Sanberg PR, Appel SH, Borlongan CV. Phenotypic characteristics of human bone marrow-derived endothelial progenitor cells in vitro support cell effectiveness for repair of the blood-spinal cord barrier in ALS. Brain Res. 2019;1724:146428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838–848. [DOI] [PubMed] [Google Scholar]
- 62. Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine. 2013;44(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, Galimi F, Romagnoli R, Salizzoni M, Tetta C, Segoloni GP, et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 2012;21(6):1305–1320. [DOI] [PubMed] [Google Scholar]
- 64. Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110(7):2440–2448. [DOI] [PubMed] [Google Scholar]
- 65. Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, Krasnodembskaya AD. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stone ML, Zhao Y, Robert Smith J, Weiss ML, Kron IL, Laubach VE, Sharma AK. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res. 2017;18(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Namba F. Mesenchymal stem cells for the prevention of bronchopulmonary dysplasia. Pediatr Int. 2019;61(10):945–950. [DOI] [PubMed] [Google Scholar]