Abstract
Background:
Chimeric antigen receptor T cells (CAR-Ts) constitute a novel therapeutic strategy for relapsed/refractory B-cell malignancies. CAR-T therapy has been extensively applied in the clinical setting; however, few systematic studies have evaluated the cost of CAR-T treatment. This study was conducted to evaluate the total cost and cost structure of CAR-T therapy and identify potential risk factors leading to increased costs.
Methods:
We identified the associated risk factors in 89 patients in a phase 1/2 study. The cohort included patients with acute lymphoblastic leukemia (ALL, n = 55) and non-Hodgkin’s lymphoma (NHL, n = 34).
Results:
Overall, the treatment of the ALL cohort was costlier than that of the NHL cohort (P < 0.001). Furthermore, in the ALL cohort, it was costlier to treat patients with a high tumor burden (P < 0.001), high cytokine release syndrome (CRS) grade (P < 0.001), and complications of infection after CAR-T cell infusion (CTI) in the whole cohort (P = 0.013) than patients with a low tumor burden, with low CRS grade, and without infection, respectively. CRS grade and length of stay (P ≤ 0.005) were independent risk factors associated with the total cost in both the ALL and NHL cohorts during CAR-T therapy. A high tumor burden, duration of fever, and treatment with tocilizumab were independent risk factors associated with the total cost in the ALL cohort (P < 0.05).
Conclusions:
CAR-T treatment should be extended to patients with a low tumor burden or patients in a state of complete remission, and a corticosteroid approach, as opposed to tocilizumab, may reduce costs.
Keywords: chimeric antigen receptor T-cell therapy, B-cell malignancies, total cost, risk
Introduction
Patients with relapsed/refractory (R/R) B-cell malignancies have a very poor prognosis, with low complete remission (CR) rates and a short overall survival (OS) time after salvage chemotherapy. For B-cell lymphoma, the CR rate is only 7% with a median OS of 6.3 months1. For B-cell acute lymphoblastic leukemia (ALL), the CR rate is 43%, with a median OS of 6.1 months2. Therefore, effective therapeutic strategies are urgently needed for these patients.
Recently, chimeric antigen receptor T cells (CAR-Ts) have been successfully employed to improve treatment outcomes for B-cell malignancies; CR rates of 70%–95% and 50%–70% for CAR-T treatment of R/R ALL2–8 and B-cell lymphoma9–13 have been reported in independent clinical trials, respectively. Because of the breakthrough efficacy of CAR-T therapy, on August 30, 2017, the FDA approved tisagenlecleucel (Kymriah®, Novartis, Basel, Switzerland) in the United States for treating patients up to 25 years old with R/R ALL14. Subsequently, axicabtagene ciloleucel (Yescarta®, Gilead, Foster City, CA, USA) was approved for treating adult R/R large B-cell lymphoma and tisagenlecleucel for R/R diffuse large B-cell lymphoma, for patients ineligible for or relapsing after autologous stem cell transplantation15. Many patients have benefited from CAR-T therapy, and the frequency of this treatment has steadily increased.
Despite the incredible efficacy of CAR-T treatment, the cost is high. Kymriah has a listed price of US$475,000 and Yescarta has a listed price of US$373,00016. In the United States, all costs, including a lengthy hospital stay, outpatient follow-up, and supportive care for the prevention and treatment of complications, are expected to amount to more than US$547,000, possibly resulting in total expenses exceeding US$1 million per patient16. Costs may further increase with complications, including severe cytokine release syndrome (CRS), CAR-T cell-related encephalopathy syndrome, pancytopenia, and infection17–23. Currently, the economic burden associated with CAR-T treatment is poorly defined and not well studied. Therefore, data regarding the cost of CAR-T therapy episodes are of special interest.
In China, CAR-T therapy has not been approved for clinical application and is currently in clinical trials; the CAR-T product is free. Detailed real-world cost estimates associated with CAR-T treatment episodes, excluding the CAR-T production fee, are limited. Given this lack of evidence, we assessed the costs, symptoms, and toxicities associated with CAR-T treatment in 89 Chinese patients with R/R B-cell malignancies and identified the associated factors. Depending on the medical insurance policy, 50%–100% of the total cost of the 89 patients is borne by the government, and the remainder is borne by the patients themselves, which is different from the third-party payer systems in other countries.
Materials and Methods
Patients and Clinical Protocols
This study (ChiCTR-ORN-16008948, ChiCTR-OIC-17011310, ChiCTR1800015575) was performed according to the Declaration of Helsinki. Patients with R/R ALL or non-Hodgkin’s lymphoma (NHL) who were administered lymphodepletion chemotherapy and CAR-T cell infusion (CTI) from July 2015 to May 2019 in a phase 1/2 open-label single-institution clinical trial were included after approval by the ethics committee of the First Affiliated Hospital of Zhejiang University. The inclusion criteria were as follows: (1) age less than 75 years; (2) R/R CD19+ ALL, R/R CD19+ diffuse large B-cell lymphoma or follicular lymphoma; (3) relapse after hematopoietic stem cell transplantation (HSCT) without evidence of graft-versus-host disease and not requiring immunosuppression therapy; (4) measurable disease and adequate performance status and organ function; and (5) patients with adequate renal and hepatic function with an Eastern Cooperative Oncology Group performance status of 0–2. Patients with central nervous system leukemia were ineligible. The study protocol was approved by the institutional review board of the First Affiliated Hospital, School of Medicine, Zhejiang University, and all patients provided written, informed consent. Patients with R/R ALL underwent disease assessment with bone marrow aspiration before CTI, and patients with NHL underwent positron emission tomography/computed tomography (PET/CT) examination. All patients were administered a single cycle of lymphodepletion chemotherapy, followed by CTI at doses of 1.0 × 106–10.0 × 106 cells/kg. All CAR-T cells were from the same costimulatory domains, as described in our clinical trials3. Granulocyte colony-stimulating factor (5 μg/kg) was administered daily when the absolute neutrophil count (ANC) was <500 cells/mm3, and patients were admitted in a laminar flow ward. The serum immunoglobulin G levels were evaluated prior to and approximately weekly after CTI, and immunoglobulin (400 mg/kg, intravenous injection IV) was recommended if the serum immunoglobulin G levels were less than 600 mg/dL. Patients with a body temperature of ≥38°C and neutropenia were immediately treated with 1 g of meropenem/imipenem every 8 h; blood cultures were carried out for patients with body temperatures of ≥38°C and repeated if necessary. Tocilizumab and corticosteroid were administered to terminate CRS, which was dependent on the patient’s condition.
Cost Calculation and Structure
When a patient was enrolled in this clinical trial, all treatment-related costs began to be calculated until the patients were discharged or died after CTI. Hospitalization costs were extracted from the hospital information system, which provided inpatient personal information, diagnosis, treatment process, nursing records, and hospital bills. The costs were classified using 10 components as follows: drugs, laboratory tests, radiology examinations, transfusions, nursing, physicians, treatment, oxygen supply, room charges, and others. In this study, drugs mainly included chemotherapy drugs, immunoglobulin, antibiotics, and tocilizumab. The treatment cost was mainly related to the costs of leukapheresis, injection, infusion, and monitoring. Several patients were administered donor’s CAR-T, and thus the donor’s inpatient costs were incorporated in the patient’s cost. Several patients died from infection or primary disease just after CTI and were not included in the analysis, as they did not complete the entire treatment process. In this study, all the costs were inpatient costs and did not include indirect costs. In our calculations, we intentionally excluded costs associated with stem cell transplantation, as the objective of our study was to estimate costs strictly related to the administration and adverse effects of CAR-T therapy, rather than all costs associated with the potential downstream pathways of CAR-T recipients.
Statistical Analysis
Summary statistics were determined for categorical variables, along with median and ranges for continuous variables. To compare two or three sets of data, we used nonparametric tests. To evaluate the risk factors for total cost, we performed linear regression analysis. Because the cost data are not orthodox, before regression analysis we log-transformed the data and confirmed that the data were normally distributed. Neutrophil recovery was defined as the first of 3 consecutive days with an ANC ≥ 500 cells/mm3. Patients with body temperatures of ≥38°C within 72 h before CTI were considered to have a fever before CTI. The body temperatures of every patient were recorded from the first day of inpatient treatment to death or discharge. The duration of fever was defined as a body temperature of >38°C from the first day up to the third consecutive day with a body temperature of <37.3°C.
Results
Clinical and Treatment Characteristics
During the study period, 89 patients with R/R ALL or NHL were administered CTI. Patients and treatment characteristics are listed in Table 1. The median age was 35 years (range 7–71 years), and 41 patients (46.1%) were women. The study patients were pretreated with a median of 6 prior lines of chemotherapy (range 2–24) before CTI, and 21 patients (23.6%) underwent a prior autologous and/or allogeneic HSCT. Before CTI, 17 patients (19.1%) had an ANC < 500 cells/mm3. Thirty-one patients (34.8%) had fever. Sixty patients (67.4%), with blast cells in the bone marrow >20% or stage III–IV according to PET/CT scan before CTI, were considered to be in a state of high tumor burden. Twenty-nine patients (32.6%), with blast cells in the bone marrow ≤20% or stage I–II according to PET/CT scan before CTI, were considered to be in a state of low tumor burden. In the total cohort, the median length of stay was 27 days (range 11–104 days), whereas the ALL cohort had a longer stay than the NHL cohort at 33 days (range 14–103 days) versus 18 days (range 11–104 days), respectively. Additionally, the median duration of neutropenia in the ALL cohort was longer than that in the NHL cohort at 6 days (range 0–78 days) versus 1.5 days (range 1–36 days), respectively. Furthermore, we divided the patients into remission and nonremission groups according to the evaluation after treatment. There were 63 patients in the remission group and 26 patients in the nonremission group. Forty-seven (85.4%) patients with ALL and 16 (47.1%) patients with NHL were in the remission group.
Table 1.
Clinical and Treatment Characteristics Before and After Chimeric Antigen Receptor T-Cell Therapy.
| ALL, n = 55 | NHL, n = 34 | P | Total, n = 89 | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, median (range), years | 27 (7–67) | 48 (23–71) | <0.001 | 35 (7–71) |
| Female | 28 (50.9%) | 13 (38.2%) | 0.246 | 41 (46.1%) |
| Prior antitumor treatment regimens | 5 (2–24) | 8 (5–24) | <0.001 | 6 (2–24) |
| IgG, median (range), mg/dL | 745 (302–1,856) | 700 (195–1,321) | 0.430 | 744 (195–1,856) |
| ANC < 500 cells/mm3 before CTI | 15 (27.3%) | 2 (5.9%) | 0.013 | 17 (19.1%) |
| Fever before CTI | 24 (43.6%) | 7 (20.6%) | 0.027 | 31 (34.8%) |
| Prior autologous and/or allogeneic HSCT | 17 (30.9%) | 4 (11.8%) | 0.040 | 21 (23.6%) |
| Infection in prior treatment | 15 (27.3%) | 5 (14.7%) | 0.170 | 20 (22.5%) |
| Disease status | ||||
| ≤20% blastsa/I–IIb | 20 (36.4%) | 9 (26.5%) | 0.362 | 29 (32.6%) |
| >20% blastsa/III–IVb | 35 (63.6%) | 25 (73.5%) | 60 (67.4%) | |
| Post CAR-T cell characteristics | ||||
| Duration of neutropenia | 6 (0–78) | 1.5 (1–36) | <0.001 | 4 (0–78) |
| Duration of fever | 7 (0–45) | 6 (0–63) | 0.091 | 6 (0–63) |
| CRS gradec | ||||
| 0 | 7 (12.7%) | 5 (14.7%) | 0.001 | 12 (13.5%) |
| 1–2 | 29 (52.7%) | 23 (67.6%) | 52 (58.4%) | |
| 3–5 | 19 (34.7%) | 6 (17.6%) | 25 (28.1%) | |
| Corticosteroid | 6 (10.9%) | 5 (14.7%) | 0.599 | 11 (12.4%)d |
| Tocilizumab | 18 (32.7%) | 5 (14.7%) | 0.061 | 23 (25.8%)d |
| Length of stay, median (range), days | 33 (14–103) | 18 (11–104) | <0.001 | 27 (11–104) |
ALL: acute lymphoblastic leukemia; ANC: absolute neutrophil count; CAR-T: chimeric antigen receptor T cell; CICAE: common terminology criteria for adverse events; CRS: cytokine release syndrome; CTI: CAR-T cell infusion; HSCT: hematopoietic stem cell transplantation; IgG: immunoglobulin G; NHL: non-Hodgkin’s lymphoma.
aBlasts referred to the blast cells in the bone marrow.
b Patients with NHL were examined via positron emission tomographic/computed tomographic scanning before CTI and staged in accordance with the results.
c The severity of CRS was graded according to 4.0.
d Fourteen patients received only tocilizumab, two patients received only corticosteroids, and nine patients received both.
Total Cost of CAR-T Treatment
The median total hospitalization cost was US$8,514 (range US$1,861–120,029) for patients in the total cohort versus US$13,354 (range US$2,605–54,268) for patients in the ALL cohort and US$5,707 (range US$1,861–120,029) in the NHL cohort. The total cost of the ALL cohort was significantly higher than in the NHL cohort (P < 0.001).
Structure of Cost during CAR-T Therapy
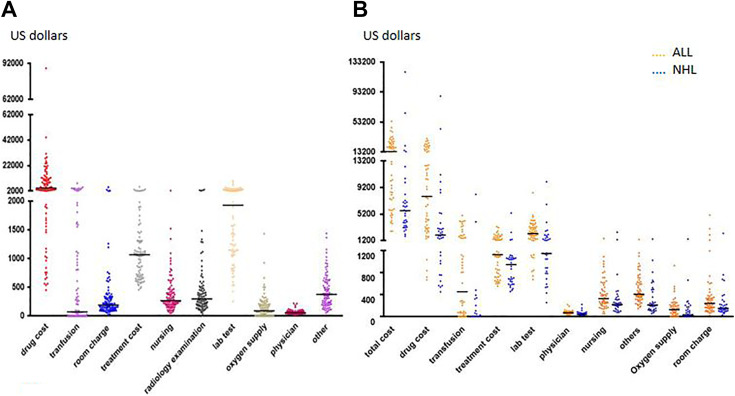
The descriptive findings following CAR-T treatment during hospitalization are presented in Fig. 1A. The drug cost was the main expenditure, ranging from 18% to 80%, with a median ratio of 51%. The median drug cost was US$4,269 (range US$451–87,883). Lab tests were the second highest cost, with the median lab tests cost at US$1,930 (range US$255–10,066). Treatment costs ranked third, with a median of US$1,068 (range US$457–5,361). “Other” costs (mainly materials) ranked fourth, and the median was US$375 (range US$54–1,438). Next were radiology examinations, nursing, and room charges, with median costs of US$299 (range US$0–3,182), US$267 (range US$51–2,457), and US$192 (range US$26–5,052), respectively. The lowest cost was oxygen supply, transfusions, and physicians, with median costs of US$91 (range US$0–1,432), US$72 (range US$0–8,167), and US$57 (range US$23–221), respectively.
Fig. 1.
Distribution of costs and comparison of costs between groups. (A) Distribution of costs in total cohort. (B) Comparison of costs between the ALL and NHL cohorts (total cost, P < 0.001; drug cost, P < 0.001; transfusion, P < 0.001; treatment cost, P = 0.001; lab test, P < 0.001; physician, P < 0.001; nursing, P = 0.012; others, P < 0.001; oxygen supply, P = 0.015; room charge, P = 0.023). ALL: acute lymphoblastic leukemia; NHL: non-Hodgkin’s lymphoma.
We compared 10 cost components between the ALL and the NHL cohorts. For the NHL cohort, the cost was significantly lower than in the ALL cohort: drugs (median US$7,854 vs 2,037, P < 0.001), transfusion costs (median US$451 vs 0, P < 0.001), treatment costs (median US$1,131 vs 948, P = 0.001), lab test costs (median US$2,261 vs 1,150, P < 0.001), physicians costs (median US$68 vs 39, P < 0.001), others (median US$407 vs 211, P < 0.001), nursing (median US$327 vs 218, P = 0.012), oxygen supply (median US$137 vs 30, P = 0.015), and room charges (median US$239 vs 149, P = 0.023; Fig. 1B). Additionally, the NHL cohort had a shorter length of stay (median 33 days vs 18.5 days, P < 0.001).
During CAR-T treatment, we divided the 89 patients into two cohorts according to their CRS grade: CRS grade 0–2 and CRS grade 3–5; patients with CRS grade 3 or higher cost more, with the total cost and the above-mentioned 10 cost components being higher: total cost (median US$20,123 vs 6,400, P < 0.001), drug costs (median US$12,612 vs 2,813, P < 0.001), transfusion costs (median US$806 vs 0, P = 0.001), treatment costs (median US$1,275 vs 1,051, P = 0.002), lab test costs (median US$3,142 vs 1,447, P = 0.001), radiology examination costs (median US$429 vs 237, P < 0.001), nursing costs (median US$402 vs 242, P = 0.002), oxygen supply costs (median US$203 vs 44, P < 0.001), room charges (median US$327 vs 166, P = 0.009), physician costs (median US$79 vs 52, P = 0.005), and others (median US$526 vs 306, P = 0.001; Fig. 2A).
Fig. 2.
Cost comparison between cohorts according to CRS grade or infection. (A) Comparison of costs between patients with CRS grade 0–2 and CRS grade 3–5 (total cost, P < 0.001; drug cost, P < 0.001; transfusion, P = 0.001; room charge, P = 0.009; treatment cost, P = 0.002; nursing, P = 0.002; radiology examination, P < 0.001; lab test, P = 0.001; oxygen supply, P < 0.001; physician, P = 0.005; other, P = 0.001). (B) Comparison of costs between infected and noninfected patients (total cost, P = 0.013; drug cost, P = 0.020; transfusion, P = 0.012; room charge, P = 0.017; nursing, P = 0.048; lab test, P = 0.007; oxygen supply, P = 0.007; physician, P = 0.029). CRS: cytokine release syndrome.
During CAR-T treatment, 13 patients were considered as infected according to microbiological tests; we divided the 89 patients into two cohorts, the noninfected and the infected cohorts, and determined the total cost. Seven cost components were increased: total cost (median US$7,451 vs 19,449, P = 0.013), drug costs (median US$3,171 vs 11,132, P = 0.020), transfusion costs (median US$54 vs 1,806, P = 0.012), lab tests (median US$1,568 vs 2,893, P = 0.007), nursing (median US$252 vs 578, P = 0.048), oxygen supply (median US$69 vs 265, P = 0.007), room charges (median US$171 vs 371, P = 0.017), and physician costs (median US$52 vs 72, P = 0.029; Fig. 2B).
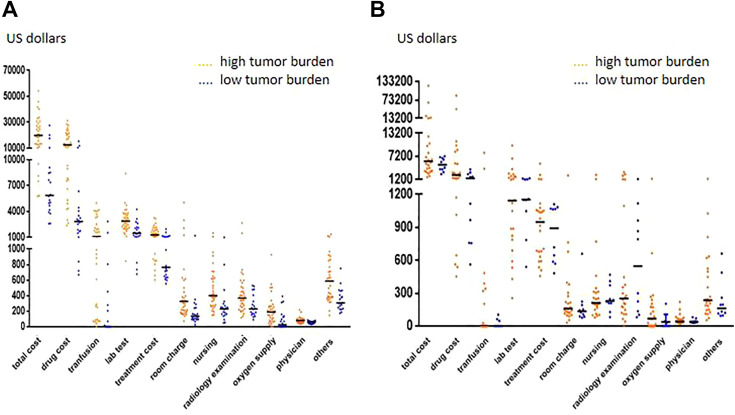
In the ALL cohort, patients with a high tumor burden had higher costs, and the total cost and 10 cost components were increased: total cost (median US$5,872 vs 19,854, P < 0.001), drug costs (median US$2,851 vs 12,499, P < 0.001), transfusion costs (median US$0 vs 1,095, P < 0.001), treatment cost (median US$764 vs 1,309, P < 0.001), lab tests (median US$1,504 vs 2,983, P < 0.001), radiology examinations (median US$229 vs 368, P = 0.007), nursing (median US$230 vs 402, P < 0.001), oxygen supply (median US$27 vs 189, P = 0.001), room charges (median US$136 vs 325, P < 0.001), physician costs (median US$58 vs 79, P = 0.003), and others (median US$306 vs 588, P < 0.001; Fig. 3A). In the NHL cohort, there was no significant difference (P > 0.05) between patients with high or low tumor burdens (Fig. 3B).
Fig. 3.
Comparison of costs between high and low tumor burden groups. (A) Comparison of costs between high and low tumor burdens in the ALL cohort (total cost, P < 0.001; drug cost, P < 0.001; transfusion, P < 0.001; lab test, P < 0.001; treatment cost, P < 0.001; room charge, P < 0.001; nursing, P < 0.001; radiology examination, P = 0.007; oxygen supply, P = 0.001; physician, P = 0.003; others, P < 0.001). (B) Comparison of costs between high and low tumor burdens in the NHL cohort (total cost, P > 0.05; drug cost, P > 0.05; transfusion, P > 0.05; lab test, P > 0.05; treatment cost, P > 0.05; room charge, P > 0.05; nursing, P > 0.05; radiology examination, P > 0.05; oxygen supply, P > 0.05; physician, P > 0.05; others, P > 0.05). ALL: acute lymphoblastic leukemia; NHL: non-Hodgkin’s lymphoma.
Risk Factors Associated with Cost during CAR-T Therapy
We then analyzed various pre- and posttreatment clinical factors to identify risk factors associated with total costs. Risk factors associated with costs via univariate and multivariate analysis are shown in Table 2. According to our previous description, there were significant differences in cost between the ALL and the NHL cohorts. Age, sex, prior chemotherapy, and prior autologous and/or allogeneic HSCT were not correlated with increased total costs (Table 2). CRS grade (B: 0.163, 95% CI: 0.053–0.274, P = 0.005; B: 0.303, 95% CI: 0.132–0.474, P = 0.001, respectively) and length of stay (B: 0.023, 95% CI: 0.014–0.031, P < 0.001; B: 0.036, 95% CI: 0.017–0.054, P < 0.001, respectively) were independent risk factors associated with total cost during CAR-T therapy for both the ALL and the NHL cohorts. For the ALL cohort, the tumor burden before CTI (B: 0.007, 95% CI: 0.002–0.011, P = 0.002), duration of fever (B: 0.017, 95% CI: 0.033–0.040, P = 0.001), and treatment with tocilizumab (B: 0.377, 95% CI: 0.001–0.033, P = 0.040) were independent risk factors associated with the total cost during CAR-T therapy.
Table 2.
Risk Factors Affecting the Total Cost, Determined by Linear Proportional Hazards Regression Analysis.
| Variables | ALL | NHL | ||||||
|---|---|---|---|---|---|---|---|---|
| Ba (95% CI) | P | Adjusted Ba (95% CI) | P c | Ba (95% CI) | P | Adjusted Ba (95% CI) | P c | |
| Age | 0.001 (−0.013 to 0.015) | 0.892 | 0.004 (−0.018 to 0.026) | 0.721 | ||||
| Sex | −0.328 (−0.741 to 0.085) | 0.118 | 0.365 (−0.262 to 0.992) | 0.244 | ||||
| Infection in prior treatment | −0.205 (−0.676 to 0.266) | 0.387 | 0.743 (−0.094 to 1.580) | 0.080 | 0.054 (−0.371 to 0.479) | 0.796 | ||
| Prior treatments | 0.015 (−0.028 to 0.059) | 0.484 | −0.015 (−0.089 to 0.060) | 0.687 | ||||
| Prior autologous and/or allogenic HSCT | −0.466 (−0.905 to −0.027) | 0.038 | −0.185 (−0.407 to 0.038) | 0.101 | 0.027 (0.939–0.993) | 0.955 | ||
| Tumor burdenb | 0.016 (0.010–0.022) | <0.001 | 0.007 (0.002–0.011) | 0.002 | 0.213 (−0.129 to 0.554) | 0.214 | ||
| Neutropenia before CTI | 0.555 (0.105–1.004) | 0.017 | 0.246 (−0.020 to 0.512) | 0.069 | −0.176 (−1.497 to 1.146) | 0.788 | ||
| Fever before CTI | 0.483 (0.078–0.888) | 0.020 | 0.047 (−0.169 to 0.263) | 0.666 | 0.510 (−0.238 to 1.257) | 0.175 | ||
| CRS | 0.311 (0.136–0.485) | 0.001 | 0.163 (0.053–0.274) | 0.005 | 0.515 (0.243–0.787) | 0.001 | 0.303 (0.132–0.474) | 0.001 |
| Corticosteroid | 0.490 (−0.174 to 1.155) | 0.145 | 1.701 (1.071–2.332) | <0.001 | 0.185 (−0.562 to 0.933) | 0.615 | ||
| Tocilizumab | 0.807 (0.415–1.198) | <0.001 | 0.377 (−0.038 to 0.625) | 0.006 | 1.113 (0.331–1.895) | 0.007 | −0.059 (−0.520 to 0.638) | 0.837 |
| Duration of fever | 0.059 (0.035–0.084) | <0.001 | 0.017 (0.001–0.033) | 0.040 | 0.062 (0.048–0.076) | <0.001 | −0.004 (−0.035 to 0.026) | 0.779 |
| Duration of neutropenia | 0.032 (0.019–0.045) | <0.001 | −0.001 (−0.011 to 0.008) | 0.767 | −0.007 (−0.058 to 0.044) | 0.792 | ||
| Length of stay | 0.030 (0.020–0.039) | <0.001 | 0.023 (0.014–0.031) | <0.001 | 0.040 (0.032–0.049) | <0.001 | 0.036 (0.017–0.054) | <0.001 |
ALL: acute lymphoblastic leukemia; CRS: cytokine release syndrome; CTI: chimeric antigen receptor T-cell infusion; HSCT: hematopoietic stem cell transplantation; NHL: non-Hodgkin’s lymphoma.
a B stands for beta, the direction of the effect and the unit increment per variable.
b Tumor burden in ALL cohort refers to blast cells in bone marrow, and in NHL cohort staging according to PETCT before CTI, with stage III–IV considered as high tumor burden and stage I–II considered as low tumor burden.
c Correlation coefficient of ALL cohort was 0.920, adjusted correlation coefficient was 0.815; correlation coefficient of NHL cohort was 0.931, adjusted correlation coefficient was 0.838.
Discussion
This study describes the total cost and cost structure in patients with R/R ALL and NHL during CAR-T treatment. We compared the ALL cohort and the NHL cohort; the ALL cohort showed higher costs, particularly the cohort with a high tumor burden. We found that the CRS grade and length of stay were independent risk factors associated with the total cost in both cohorts, and the CRS grade, length of stay, tumor burden, duration of fever, and treatment with tocilizumab were independent risk factors in the ALL cohort.
In recent decades, salvage chemotherapy and salvage allogeneic HSCT were the main treatment strategies for R/R hematological malignancies24–27, although there are some limitations. Patients treated with salvage chemotherapy have a low incidence of remission and poor OS19. Although allo-HSCT is a potentially curative treatment option for hematological malignancies, pretransplantation minimal residual disease negativity plays an important role in long-term free survival28,29; the outcome of salvage allo-HSCT is poor. Moreover, old age is typically associated with poor prognosis both in salvage chemotherapy and in allo-HSCT. Terwilliger found that age is a factor affecting poor prognosis24, as the elderly tend to have disease with more comorbidities, which increase the costs. In our study, we found that age was not correlated with increased total costs, and high CR rates of B-cell malignancies have been reported in our center and other independent clinical trials3–13. Thus, our study showed that CAR-T treatment may be a better choice for R/R B-cell malignancies compared to salvage chemotherapy and salvage allo-HSCT.
In our study, the NHL cohort showed lower costs than the ALL cohort. This may be related to the following factors: (1) the ALL cohort had a longer length of stay (P < 0.001); a long length of stay is correlated to the use of a room and higher nursing and physician costs, as well as a high risk of nosocomial infection, which can further increase the costs. Our study showed that the length of stay was an independent risk factor associated with the total cost of all 89 patients; (2) the ALL cohort had a long duration of neutropenia (P < 0.001), which is correlated with higher costs.
High tumor burden was another risk factor associated with the increased total cost in the ALL cohort. The following factors may have been related: (1) The high tumor burden cohort showed a longer duration of neutropenia after CTI; even before CTI, these patients were already in a state of neutropenia; the patients should be treated with granulocyte colony-stimulating factor and antibiotics for infection prevention, and admitted to a laminar flow ward, which results in higher costs. (2) The high tumor burden cohort was also accompanied by low platelets and low hemoglobin; this cohort required frequent transfusion as support treatment; in our study, transfusion costs were significantly increased. A higher disease burden is associated with the development of severe CRS and severe neurotoxicity, and higher burden malignancy involvement in the bone marrow has been established as a risk factor for toxicity in patients with B-cell malignancies4,19,21,30. Park found that a low tumor burden before CAR-T treatment was associated with favorable outcomes in patients with R/R ALL4. Hay et al. identified a high marrow tumor burden as an independent predictor of CRS and delayed hematopoietic recovery in patients with grade > 4 CRS, which can result in higher costs19. Taken together, CAR-T treatment can be performed in patients with a low tumor burden R/R ALL to improve long-term free survival, decrease treatment-associated complications, and reduce treatment costs. Treating patients with low malignancy burdens may be optimal, in terms of both efficacy and cost savings, but may not always be possible.
Here, CRS grade was an independent risk factor associated with the total cost of all 89 patients, and the duration of fever was an independent risk factor associated with the total cost in the ALL cohort. It has been reported that the first presenting symptom of CRS is typically fever. Park et al. compared serum cytokine panels in patients with CRS who had infections to those of uninfected patients, but found no differences31. Currently, CRS and infection cannot be distinguished in patients administered CAR-T treatment and presenting with fever. Furthermore, the incidence of infection in CAR-T treatment was comparable to observations from salvage or primary therapy for patients with B-cell malignancies31,32. For patients with fever after CTI, antibiotics are required, and patients should be treated until the temperature returns to normal. In our study, the main expenditure was drug costs, and antibiotics are the main expenditure among these costs. Shortening the duration of fever would reduce costs. Corticosteroid treatment clearly has a role in toxicity management, and it has been reported that timely and effective cytokine-directed treatment with corticosteroids is important for avoiding CRS-associated death33. Tocilizumab enables CAR-T cells to be administered safely to many patients without significantly compromising efficacy34; however, the price is high. In our study, treatment with tocilizumab was an independent factor associated with the total cost because of its high price. Corticosteroids can reduce costs but also potentially reduce the efficacy of CAR-T cell therapy, specifically when used as a replacement for tocilizumab. To reduce costs, corticosteroids are a better choice.
Increasing numbers of patients will be treated with CAR-T therapy because of its ability to induce a high remission rate. At present, CAR-T treatment in China remains in the clinical trial stage. Currently, there are few reports of the cost of CAR-T treatment35. Our study provides an overview and analysis of the data in patients who underwent CAR-T treatment, providing a reference for government and insurance companies.
This study had several limitations, including its retrospective nature and limited sample size which may have influenced the reliability of the statistical analysis. The choice of covariates for multivariable analysis may have been constrained by the small number of observed events, including costs of the donors’ hospitalization in cases of donor-derived CAR-T cells which may overestimate costs compared to patients administered autologous products, which is far more common and currently the only method of administering commercially available CAR-T cell products. Additionally, excluding patients who died shortly after treatment may have underestimated costs, as these patients likely required more supportive care for complications and therefore would have increased costs.
Conclusions
This study provides a broad and comprehensive description of the total costs and structure of costs in this novel cohort. The results suggest that CAR-T treatment should be extended to patients with a low tumor burden or patients in a state of CR. Further studies of larger cohorts in multiple centers are required to obtain more extensive data pertaining to the overall cost of CAR-T treatment.
Footnotes
Ethical Approval: Ethical approval to report this study was obtained from the Ethics Committee of The First Affiliated Hospital, School of Medicine, Zhejiang University (No. 2018-747-2).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the study protocol approved by the Ethics Committee of The First Affiliated Hospital, School of Medicine, Zhejiang University.
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the 973 Program (grant number 2015CB964900); Natural Science Foundation of China (grant numbers 81470341, 81770201, and 81730008); Key Project of Science and Technology, Department of Zhejiang Province (grant number 2018C03016-2); and Zhejiang Public Welfare Foundation (grant number GF18H180002).
ORCID iD: Feng Zhu  https://orcid.org/0000-0003-3140-4289
https://orcid.org/0000-0003-3140-4289
References
- 1. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L, Boussetta S, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, Dombret H, Fielding AK, Heffner L, Larson RA, Neumann S, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. [DOI] [PubMed] [Google Scholar]
- 3. Orlowski RJ, Porter DL, Frey NV. The promise of chimeric antigen receptor T cells (CARTs) in leukaemia. Br J Haematol. 2017;177(1):13–26. [DOI] [PubMed] [Google Scholar]
- 4. Park JH, Riviere I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(1):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu Y, Wu Z, Luo Y, Shi J, Yu J, Pu C, Liang Z, Wei G, Cui Q, Sun J, Jiang J, et al. Potent anti-leukemia activities of chimeric antigen receptor-modified T cells against CD19 in Chinese patients with relapsed/refractory acute lymphocytic leukemia. Clin Cancer Res. 2017;23(3):3297–3306. [DOI] [PubMed] [Google Scholar]
- 6. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bade P, Verneris MR, Stefanski HE, Myers GD, Qayed M, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S, Kelly-Spratt KS, Hoglund V. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, Xue A, Goff SL, Yang JC, Sherry RM, Klebanoff CA. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35(16):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 13. Ayyappan S, Maddocks K. Novel and emerging therapies for B cell lymphoma. J Hematol Oncol. 2019;12(82):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bach PB, Giralt SA, Saltz LB. FDA approval of tisagenlecleucel: promise and complexities of a $475 000 cancer drug. JAMA. 2017;318(19):1861–1862. [DOI] [PubMed] [Google Scholar]
- 15. Kite Pharma, Inc. YESCARTA (axicabtagene ciloleucel) suspension for intravenous infusion: prescribing information [internet]. 2017. Available from https://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm581222.htm.
- 16. de Lima Lopes G, Nahas GR. Chimeric antigen receptor T cells, a savior with a high price. Chin Clin Oncol. 2018;7(2):21. [DOI] [PubMed] [Google Scholar]
- 17. Hu Y, Sun J, Wu Z, Yu J, Cui Q, Pu C, Liang B, Luo Y, Shi J, Jin A, Xiao L, et al. Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. J Hematol Oncol. 2016;9(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang LN, Song Y, Liu D. CD19 CAR-T cell therapy for relapsed/refractory acute lymphoblastic leukemia: factors affecting toxicities and long-term efficacies. J Hematol Oncol. 2018;11(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, López JA, Chen J, Chung D, Harju-Baker S, Cherian S, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor modified T-cell therapy. Blood. 2017;130(21):2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, Chen J. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, Halton E, Wang X, Senechal B, Purdon T, Cross JR. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jin A, Feng J, Wang Z, Jiang L, Hu Y, Zhao K, Huang H. Severe dyspnea caused by rapid enlargement of cervical lymph node in a relapsed/refractory B-cell lymphoma patient following chimeric antigen receptor T-cell therapy. Bone Marrow Transplant. 2019;54(7):969–972. [DOI] [PubMed] [Google Scholar]
- 24. Terwiliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gale RP. Standardizing haematopoietic cell transplants in China. J Hematol Oncol. 2018;11(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, Liu D, Liu Q, Liu T, Jiang M, Ren H, et al. The consensus on indications, conditioning regimen and donor selection of allogeneichematopoiteic cell transplantation for hematological disease in China- recommendations from the Chinese Society of Hematology. J Hematol Oncol. 2018;11(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fathi AT, DeAngelo DJ, Stevenson KE, Kolitz JE, Asch JD, Amrein PC, Attar EC, Steensma DP, Wadleigh M, Foster J, Connolly C, et al. Phase 2 study of intensified chemotherapy and allogeneic hematopoietic stem cell transplantation for older patients with acute lymphoblastic leukemia. Cancer. 2016;122(15):2379–2388. [DOI] [PubMed] [Google Scholar]
- 28. Pavlů J, Labopin M, Niittyvuopio R, Socié G, Yakoub-Agha I, Wu D, Remenyi P, Passweg J, Beelen DW, Aljurf M, Kröger N, et al. Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia: a retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT. Hematol Oncol. 2019;12(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sutton R, Shaw PJ, Venn NC, Law T, Dissanayake A, Kilo T, Haber M, Noris MD, Fraser C, Alvaro F, Revesz T, et al. Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol. 2015;168(3):395–404. [DOI] [PubMed] [Google Scholar]
- 30. Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, et al. CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, Seo SK. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis. 2018;67(4):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, Riddell SR, Maloney DG, Boeckh M, Turtle CJ. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu X J, Tang Y M. Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Cancer Lett. 2014;343(2):172–178. [DOI] [PubMed] [Google Scholar]
- 34. Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-)T cell therapy. Br J Haematol. 2018;183(3):364–374. [DOI] [PubMed] [Google Scholar]
- 35. Hernandez I, Prasad V, Gellad WF. Total Costs of Chimeric Antigen Receptor T-Cell Immunotherapy. JAMA Oncol. 2018;4(7):994–996. [DOI] [PMC free article] [PubMed] [Google Scholar]