Abstract
During the process of human islet isolation a cascade of stressful events are triggered and negatively influence islet yield, viability, and function, including the production of proinflammatory cytokines and activation of apoptosis. Carbon monoxide-releasing molecule 2 (CORM-2) is a donor of carbon monoxide (CO) and can release CO spontaneously. Accumulating studies suggest that CORM-2 exerts cytoprotective and anti-inflammatory properties. However, the effect of CORM-2 on islet isolation is still unclear. In this study, we found that CORM-2 pretreatment significantly decreased the expression of critical inflammatory genes, including tissue factor, intercellular adhesion molecule-1, chemokine (C-C motif) ligand 2, C-X-C motif chemokine 10, Toll-like receptor 4, interleukin-1β, interleukin-6, and tumor necrosis factor-α (TNF-α). The isolated islets of the CORM-2 pretreatment group showed reduced apoptotic rate, improved viability, and higher glucose-stimulated insulin secretion, and functional gene expression in comparison to control group. Importantly, CORM-2 pretreatment prevented the impairment caused by TNF-α, evidenced by the improved glucose-stimulated index and transplantation outcomes. The present study demonstrated the anti-inflammatory property of CORM-2 during human islet isolation, and we suggest that CORM-2 pretreatment is an appealing treatment to mitigate inflammation-mediated islet dysfunction during isolation and culture ex vivo and to preserve long-term islet survival and function.
Keywords: islet transplantation, inflammation, CORM-2, islet function, TNF-α
Introduction
Islet transplantation is a promising treatment for type 1 diabetes mellitus, which can significantly reduce the incidence of severe hypoglycemia events1. Both the islet quantity and quality are critical factors influencing the efficacy of clinical islet transplantation. The donor islets are isolated using enzyme perfusion method. However, the enzymatic and mechanical digestion process during islet isolation has been reported as a compromised function of islets after transplantation due to the related inflammation events2–4. Reducing the inflammation during islet isolation might benefit the maintenance of the islet function and consequently improve the transplantation outcomes.
Carbon monoxide (CO) is synthesized by heme oxygenase during heme degradation. Recent evidence has proved that low-dose exogenous CO can attenuate the impairment of oxidative stress and local and systemic inflammation5–7. Carbon monoxide-releasing molecule 2 (CORM-2) is often used as a CO donor. Lin et al. found that CORM-2 decreased tumor necrosis factor-α (TNF-α)-induced lung inflammation8. Megias et al. reported that CORM-2 suppressed cytokine-induced inflammation in a human colonic epithelial cell line9. Katada et al. found that CORM-2 inhibited ischemia/reperfusion-induced inflammation in the small intestine10. These observations highlighted the anti-inflammatory properties of CORM-2. Yet, whether CORM-2 has an anti-inflammation effect and mitigates the consequent islet dysfunction during the islet isolation is unknown.
In the present study, we showed that CORM-2 pretreatment inhibited the expression of inflammatory cytokines, improved islet viability and function, and reduced the apoptotic rate of isolated islet cells. TNF-α is a potential mediator of β-cell death after transplantation11. Here we also demonstrated that CORM-2 pretreatment attenuated the islet dysfunction induced by TNF-α and improved transplant outcomes. Taken together, we demonstrated the beneficial effect of CORM-2 on islet isolation, which is associated with suppression of inflammation.
Materials and Methods
Mice
Male ICR and BALB/c mice, 8 to 10 weeks, were purchased from Beijing Huafukang Biosciences (Beijing, China). The islets used in the in vitro experiments were isolated from male ICR mice because of their high yield of islets. Both the islet donor and recipient mice were male BALB/c in the in vivo islet transplantation experiment. All mice were fed normal chow and maintained on a 12-h light–dark cycle (lights on at 7:00 AM). The Nankai University Institutional Animal Care and Utilization Committee approved all experiments.
Islet Isolation and Treatment
Islets were isolated from ICR or BALB/c mice using a previously described method12. Briefly, the pancreata were perfused with collagenase P (0.5 mg/ml, Roche, Basel, Switzerland) containing either 0.1% DMSO (control; Solarbio, Beijing, China) or 100 μM CORM-2 (Sigma-Aldrich, St. Louis, MO, USA) and incubated on ice for 30 min, followed by digestion at 37 °C for 11 min and purification by density gradient (Histopaque 1077, Sigma-Aldrich, St Louis, MO, USA). Isolated islets were either used immediately or cultured in RPMI 1640 supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS) at 37 °C and 5% CO2.
The islets were incubated with 50 ng/ml mouse TNF-α (Peprotech, Rocky Hill, NJ, USA) for 72 h.
Flow Cytometry
A PE Annexin V Apoptosis Detection Kit (BD Bioscience, San Jose, CA, USA) was used to detect cell apoptosis. Islets were harvested after 72-h culture ex vivo and dispersed into single islet cells. Briefly, islets were incubated with 0.25% trypsin and put into a 37°C shaking incubator with 100 rpm/min. After 10 min, the dissociation process was stopped and the cells were filtered through a 40-µm cell strainer to a single-cell suspension. Cells were washed twice with 1 × PBS and incubated with Annexin-V-PE and 7-amino-actinomycin D (7-AAD) at room temperature for 15 min in the dark. Then, the samples were analyzed by a FACScan (BD Bioscience) flow cytometry. Signals Annexin-V–/7-AAD–, Annexin-V+/7-AAD–, and Annexin-V+/7-AAD+ indicated living, early, and late apoptotic cells, respectively.
Real-Time Polymerase Chain Reaction (RT-PCR) Assay
Total RNA was extracted from islet samples using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Total RNA was reverse transcribed to cDNA using a reverse transcriptase reaction kit (Takara, Kohoku-cho, Kusatsu, Japan). RT-PCR was performed using FS Essential DNA Green Master (Roche). The primers used to assess the expression of mRNA are shown in Table 1.
Table 1.
Primers for Real-Time Polymerase Chain Reaction.
| Genes | Forward | Reverse |
|---|---|---|
| TF | GGAGGAGCCGCCATTTACAAA | AAACTGCTGAATTACTGGCTGT |
| ICAM-1 | GTGATGCTCAGGTATCCATCCA | CACAGTTCTCAAAGCACAGCG |
|
CCL2
CXCL10 |
TAAAAACCTGGATCGGAACCAAA CCAAGTGCTGCCGTCATTTTC |
GCATTAGCTTCAGATTTACGGGT GGCTCGCAGGGATGATTTCAA |
| TLR4 | GCCTTTCAGGGAATTAAGCTCC | GATCAACCGATGGACGTGTAAA |
| IL-1β | TTCAGGCAGGCAGTATCACTC | GAAGGTCCACGGGAAAGACAC |
| IL-6 | AGCCAGAGTCCTTCAGA | GGTCCTTAGCCACTCCT |
| TNF-α | CCCTCACACTCAGATCATCTTCT | TGCTACGACGTGGGCTACAG |
| INS | CAATCATAGACCATCAGCAAGC | AGAAACCACGTTCCCCAC |
| PDX1 | CCCGAGCTTCTGAAAACTTTG | CTTTTCATTGTCCTCAGTTGGG |
| MAFA | AGGAGGAGGTCATCCGACTG | CTTCTCGCTCTCCAGAATGTG |
| NKX6.1 | CGCTTGGCCTATTCTCTGGG | CTGCGTGCTTCTTTCTCCA |
| GLUT2 | TGTGCTGCTGGATAAATTCGCCTG | AACCATGAACCAAGGGATTGGACC |
| GAPDH | CATGGCCTTCCGTGTTCCTA | GCGGCACGTCAGATCCA |
TF: tissue factor; ICAM-1: intercellular adhesion molecule; CCL2: chemokine (C-C motif) ligand 2; CXCL10: C-X-C motif chemokine 10; TLR4: Toll-like receptor 4; IL-1β: interleukin-1β; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α.
Islet Viability Assay
To determine the viability, the isolated islets were incubated with fluorescein diacetate (FDA; Sigma-Aldrich) for 2 min followed by propidium iodide (PI; Sigma-Aldrich) for 30 s. The living cells were stained green and dead cells were stained red. The positive area stained by each dye was measured separately using Image J software. The viability (%) of each islet = [1 – PI positive area/(FDA positive area + PI positive area)] × 100%.
Insulin Secretion Assay
Ten islets were pretreated in 1 ml 1.67 mM low-glucose Krebs–Ringer bicarbonate buffer (KRB; supplemented with 0.5% BSA, pH 7.4) for 1 h in 12-well plate, then the medium was removed, followed by sequential treatment with 1 ml low-glucose KRB solution (1.67 mM) for 1 h and high-glucose KRB solution (16.7 mM) for 1 h. The media at low and high glucose levels were collected separately. Insulin concentration was measured by enzyme-linked immunosorbent assay (ELISA; Mouse Insulin ELISA Kit; Mercodia, Uppsala, Sweden). Insulin secretion of islets was expressed as the glucose-stimulated index (GSI; insulin content in the 16.7 mM glucose media/insulin content in the 1.67 mM glucose media) to assess glucose responsiveness.
Immunohistochemistry
The pancreatic isolated islets were fixed and paraffin embedded. Tissue sections were incubated with primary insulin antibody (Abcam, Cambridge, MA, USA), along with secondary AffiniPure Goat Anti-Mouse IgG H&L antibody (Jackson Immunoresearch Laboratories and Molecular Probes, West Grove, PA, USA). Counterstaining was performed with DAPI (Vector, Burlingame, CA, USA). Pannoramic MIDI and Pannoramic Viewer (3DHistech, Budapest, Hungary) were used to scan stained slides and capture images. All the pictures were quantified and analyzed using Image-Pro Plus software.
Islet Transplantation
STZ-induced diabetic (single intraperitoneal injection of 165 mg/kg STZ; Sigma-Aldrich) BALB/c mice were used as recipients. The mice were considered diabetic when nonfasting blood glucose was larger than 300 mg/dl for two consecutive days. One hundred and fifty islets isolated from BALB/c mice were transplanted into kidney subcapsular space of diabetic recipients. Blood glucose values were measured every day for 33 days. Before sacrificing the mice, the islet graft-bearing kidneys were removed on day 30.
Intraperitoneal Glucose Tolerance Test
Intraperitoneal glucose tolerance test (IPGTT) was performed after an overnight fasting with free access to water. Blood glucose was measured at 0, 15, 30, 60, and 120 min after each mouse was given an intraperitoneal injection of glucose (2 g/kg body weight) using a Roche glucometer. Area under the curve between 0 and 120 min was calculated for each mouse.
Statistical Analysis
All data were analyzed by two-tailed Student’s t test or one- or two-way ANOVA with post hoc Tukey’s test using GraphPad Prism Version 7.0d. P values of <0.05 were considered statistically significant.
Results
CORM-2 Pretreatment Attenuates the Inflammation in Isolated Islets
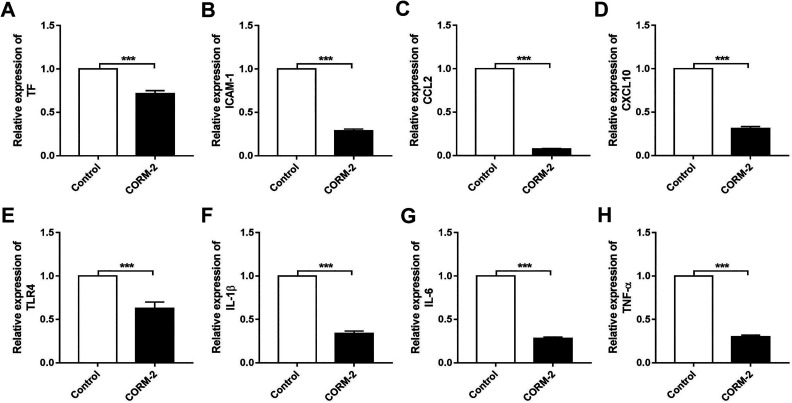
To examine the inflammatory level of islets between CORM-2 pretreatment and control groups, we analyzed the mRNA expressions of proinflammatory cytokines of islets immediately after isolation which are well known to impact graft function and transplant outcomes. As shown in Fig. 1, we observed markedly decreased levels of tissue factor (TF), intercellular adhesion molecule (ICAM-1), chemokine (C-C motif) ligand 2 (CCL2), C-X-C motif chemokine 10 (CXCL10), Toll-like receptor 4 (TLR4), interleukin-1β (IL-1β), interleukin-6 (IL-6), TNF-α in CORM-2 pretreatment group. These findings suggest that CORM-2 has anti-inflammatory properties in islet isolation.
Fig. 1.
Carbon monoxide-releasing molecule 2 (CORM-2) pretreatment attenuates islet inflammation after isolation. Relative mRNA expressions of (A) TF, (B) ICAM-1, (C) CCL2, (D) CXCL10, (E) TLR4, (F) IL-1β, (G) IL-6, and (H) TNF-α in islets pretreated with or without CORM-2. Data are shown as mean ± SD. ***P < 0.001. CORM-2: carbon monoxide-releasing molecule 2; TF: tissue factor; ICAM-1: intercellular adhesion molecule; CCL2: chemokine (C-C motif) ligand 2; CXCL10: C-X-C motif chemokine 10; TLR4: Toll-like receptor 4; IL-1β: interleukin-1β; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α.
CORM-2 Pretreatment Inhibits the Apoptosis of Isolated Islets
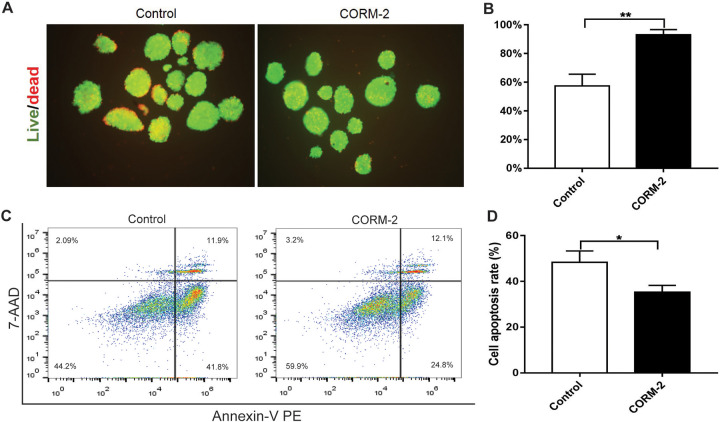
To test the capacity of CORM-2 to protect islet viability, FDA/PI staining followed by fluorescence microscopy was carried out. As shown in Fig. 2A, B, after 72-h culture ex vivo, CORM-2 pretreatment group showed significantly higher viability compared to the control group. Moreover, we found the apoptotic rate of islet cells in the CORM-2 group was lower than that in the control group (Fig. 2C, D). These data suggest that CORM-2 contributes to maintaining the viability of isolated islets cultured ex vivo.
Fig. 2.
Improvement of islet viability after CORM-2 pretreatment. (A) Representative ex vivo viability in isolated islets cultured for 72 h. Green, living cells; red, dead cells. Original magnification ×100. (B) Quantitative evaluation of islet viability. Hundred islets in three independent experiments were examined, and the average viability in each experiment was calculated. (C) The distribution of apoptotic cells on the diagram of flow cytometry. (D) Quantitative evaluation of apoptotic cells. Data are shown as mean ± SD. *P < 0.05, **P < 0.01. CORM-2: carbon monoxide-releasing molecule 2.
CORM-2 Pretreatment Improves the Function of Isolated Islets
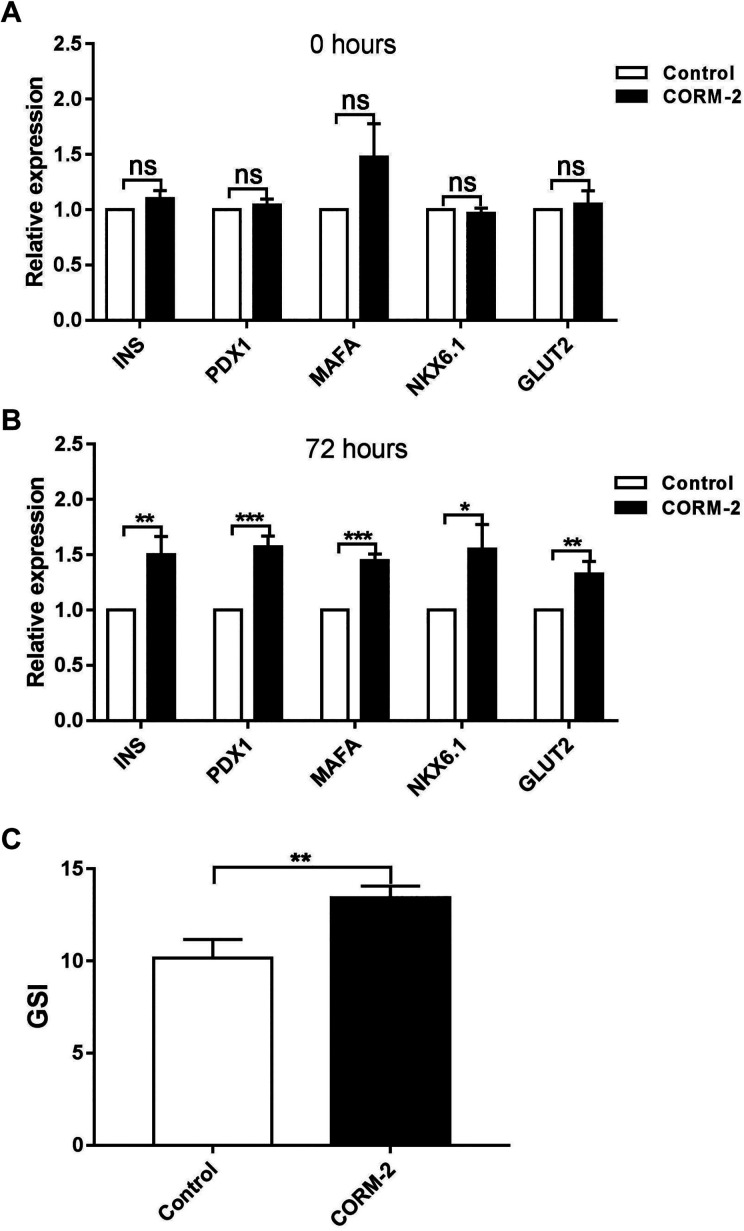
To determine the protective effect of CORM-2 on islet function, islets isolated with or without CORM-2 supplementation were cultured ex vivo; then the expression of functional genes were examined. As shown in Fig. 3A, no significant changes were detected immediately after isolation. However, the improvement was observed after 72 h of culturing ex vivo (Fig. 3B), with key islet functional genes INS, PDX1, MAFA, NKX6.1, and GLUT2 dramatically upregulated after CORM-2 pretreatment. To further validate the CORM-2 effect, we assessed GSI of islets with CORM-2 pretreatment. As shown in Fig. 3C, the GSI of islets was significantly increased after CORM-2 pretreatment.
Fig. 3.
Improvement of islet function after CORM-2 pretreatment. (A) Relative mRNA expressions of functional genes in islets pretreated with or without CORM-2 immediately after isolation. (B) Relative mRNA expressions of functional genes in islets cultured ex vivo for 72 h. (C) GSI of islets ex vivo cultured for 72 h. Data are shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. CORM-2: carbon monoxide-releasing molecule 2; GSI: glucose-stimulated index.
CORM-2 Pretreatment Attenuates the TNF-α-Induced Functional Damage of Isolated Islets
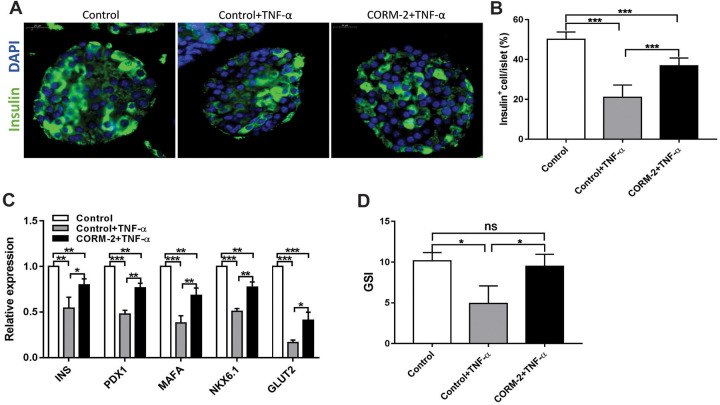
TNF-α is a potential inflammatory mediator of β-cell death after transplantation11. The percentages of insulin-positive cells (Fig. 4A, B), functional gene expressions (Fig. 4C), and glucose-stimulated insulin secretion (Fig. 4D) of islets treated with TNF-α were significantly decreased, suggesting that the presence of TNF-α impairs β-cell function. Thus we used TNF-α treatment as a representative inflammatory stimulus on islets, in order to investigate the potential protective role of CORM-2 pretreatment in resisting inflammatory destruction. We compared the TNF-α-induced impairment on islets with or without CORM-2 pretreatment. Results showed that the insulin-positive cell percentage per islet (Fig. 4A, B), β-cell functional gene expression (Fig. 4C), and the glucose-stimulated insulin secretion (Fig. 4D) in the islets with CORM-2 pretreatment (CORM-2 + TNF-α) were significantly higher than that detected in the islets without CORM-2 pretreatment in the presence of TNF-α (control + TNF-α). These results implied that CORM-2 has a protective effect on islets against TNF-α-induced damage.
Fig. 4.
CORM-2 pretreatment attenuates the TNF-α-induced function damage of islets in ex vivo culture. (A) Immunofluorescence with insulin (green) and DAPI (blue) of islets pretreated with or without CORM-2 followed by 50 ng/ml TNF-α treatment for 72 h (control + TNF-α group, CORM-2 + TNF-α group) or the control islets alone (control group). Scale bars = 20 μm. (B) Quantification of insulin+ cells per islet. (C, D) Relative mRNA expressions of functional genes (C) and GSI (D) in islets pretreated with or without CORM-2 following with or without 50 ng/ml TNF-α treatment for 72 h. Fig. 3(C) and 4(D) share the same control data. Data are shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. CORM-2: carbon monoxide-releasing molecule 2; TNF-α: tumor necrosis factor-α; GSI: glucose-stimulated index.
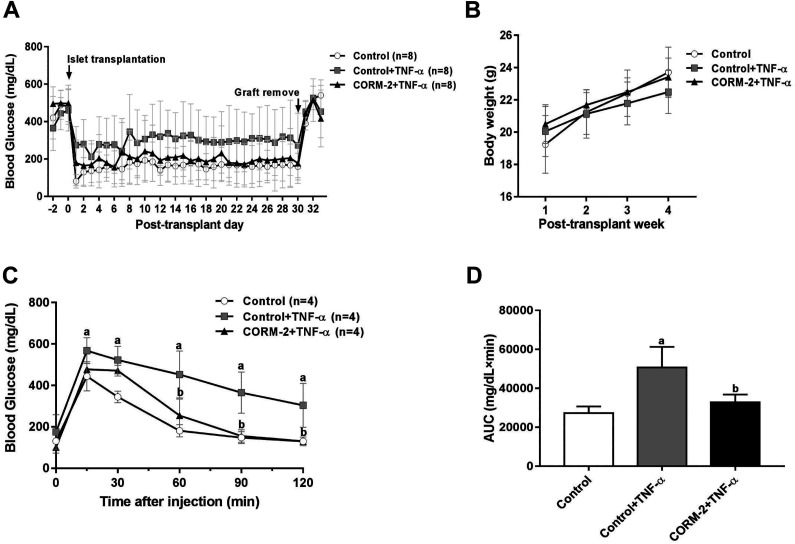
We further evaluated the function of islets in vivo using a syngeneic transplantation model. After TNF-α treatment for 48 h during ex vivo culture, islets with or without CORM-2 pretreatment were transplanted to STZ-induced diabetic mice (control + TNF-α group vs CORM-2 + TNF-α group). Meanwhile, an equal quantity of islets without any treatment were transplanted as a control for effective TNF-α treatment, as well as a positive control for successful transplantation surgery. The nonfasting blood glucose levels in CORM-2 + TNF-α group were lower than that in control + TNF-α group (Fig. 5A). IPGTT results showed that the glucose tolerance in CORM-2 + TNF-α group was superior than that in control + TNF-α group (Fig. 5C, D). These results demonstrate that CORM-2 treatment during islet isolation procedure is sufficient to significantly improve the transplant outcomes. These in vivo results confirmed the protective effect of CORM-2 pretreatment in resisting TNF-α-induced impairment.
Fig. 5.
Evaluation of islet function in vivo of TNF-α-treated islets that were isolated with or without CORM-2 pretreatment. (A, B) Results of blood glucose (A) and body weight (B) measurements (n = 8). (C) Blood glucose levels of mice in IPGTT on day 14. (D) Area under the curve of blood glucose levels of mice in IPGTT on day 14. Data are shown as mean ± SD. a P < 0.05: control group vs control + TNF-α group; b P < 0.05: CORM-2 + TNF-α treatment group vs control + TNF-α group (one- (D) or two-way (C) ANOVA with post hoc Tukey’s test). CORM-2: carbon monoxide-releasing molecule 2; IPGTT: intraperitoneal glucose tolerance test; TNF-α: tumor necrosis factor-α.
Discussion
In order to restore insulin independence after islet transplantation, more than one cadaveric pancreata are usually needed to achieve sufficient islets for one recipient13,14. One reason is that the inflammation stress during the islet isolation impairs islet viability and function15–18. The inflammatory signals produced during the isolation procedure can be further amplified through a positive feedback mechanism in the following ex vivo culture period before transplantation, leading to further damage to the islets and affecting transplantation outcomes. Inhibiting the inflammatory stress during islet isolation is a potential approach to improving islet quality and quantity15. In the present study, we identified that CORM-2 effectively reduced the expressions of inflammation factors in the isolated islets, including TF, ICAM-1, CCL2, CXCL10, TLR4, IL-1β, IL-6, and TNF-α (Fig. 1), suggesting an inhibition of inflammation responses. These results imply that CORM-2 is an appealing treatment which is beneficial for the resolution of inflammation during the initial digestion period of islets’ isolation.
A short-term ex vivo culture is usually inevitable for the freshly isolated islets for quality assessment and surgery preparation19. Additionally, accumulating studies suggested that the short-term culture of islets enhances the purity and ultrastructural integrity and decreases immunogenicity of islets20,21. However, due to the continuing inflammation stress in this period, the ex vivo culture also results in decreased viability and function of islets22–24. In this study, we demonstrated that the treatment of islets with CORM-2 during the isolation procedure can not only improve the islet viability (Fig. 2A, B), but also evidently reduce the number of apoptotic islet cells (Fig. 2D). Due to the dissociation procedure during the flow cytometry assay, islets in Fig. 2D showed attenuated viability compared with islets in Fig. 2B. Additionally, CORM-2 supplementation during islet isolation enhances the expressions of islet functional genes INS, PDX-1, MAFA, NKX6.1, and GLUT2 (Fig. 3B), which is possibly due to the inhibition of inflammation by CORM-2 and the consequent amplification of inflammatory signals.
Multiple studies demonstrated that the innate inflammatory events in the site of islet graft result in early graft loss after transplantation25–27. TNF-α is an important inflammatory cytokine which significantly impacts the clinical outcome of islet transplantation28. Early studies have verified that TNF-α induces islet destruction29. In this study, we also confirmed that TNF-α can significantly impair the islet function (Fig. 4). Importantly, we found that CORM-2 can prevent TNF-α-mediated islet function damage (Fig. 4A–C) and protect glucose responsiveness of islets (Fig. 4D). Ulteriorly, using an islet transplant mice model, we demonstrated that mice receiving CORM-2 pretreatment islets showed improved transplant outcomes as evidenced by a superior islet function (Fig. 5C, D). Taken together, these findings suggest that CORM-2 pretreatment may have a potential in protecting islet graft from inflammatory damage in the recipients.
In summary, our findings demonstrated that the presence of CORM-2 in the collagenase solution during islet isolation reduced the innate inflammation level in the islets and thus might protect islets from inflammatory damage from the beginning of islets’ isolation. Besides, CORM-2 pretreatment can further attenuate the inflammatory damage on cell viability and function of ex vivo cultured islets, suggesting that CORM-2 might also have a potential in enlarging the window of ex vivo culture before transplantation. In addition, CORM-2-pretreated islets also have better resistance to exogenous inflammatory cytokines, suggesting that the islets isolated with CORM-2 pretreatment might have higher survival ability in front of inflammatory cytokines attack after transplantation. We proposed that CORM-2 is a potential agent to prevent islet inflammation during the isolation procedure, which may improve the quantity and quality of human isolated islets and the further transplantation outcome.
Footnotes
Authors’ Note: Xiang-Heng Cai and Guan-Qiao Wang contributed equally to this work.
Author Contributions: Conceptualization: ZS and SW; formal analysis: XC and GW; investigation: XC, GW, RL, WL, TL, and JZ; project administration: JZ, NL, and YL; supervision: ZS and SW; writing original draft: XC and GW; review and editing: ZS and SW.
Ethical Approval: Animal experiments were approved by the Medical Ethical Committee of Tianjin First Central Hospital.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Medical Ethical Committee of Tianjin First Central Hospital approved protocol.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Natural Science Foundation of China (81870535), National Key Research and Development Program (2016YFC1305104), key projects of Tianjin Natural Science Foundation (18JCZDJC33100), Tianjin Clinical Research Center for Organ Transplantation Project (15ZXLCSY00070), and Foundation of State Key Laboratory of Medicinal Chemical Biology (Nankai University) (2018016).
ORCID iD: Shu-Sen Wang  https://orcid.org/0000-0002-2323-6564
https://orcid.org/0000-0002-2323-6564
References
- 1. Jeong S, Lee S, Mee-Lee C, Shim IK, Kim SC. Role of high-mobility group box 1 (HMGB1) in transplantation of rat pancreatic islets. Ann Transplant. 2017;22:121–127. DOI: 10.12659/AOT.900731. [DOI] [PubMed] [Google Scholar]
- 2. Truong W, Shapiro AM. Progress in islet transplantation in patients with type 1 diabetes mellitus. Treat Endocrinol. 2006;5(3):147–158. [DOI] [PubMed] [Google Scholar]
- 3. Abdelli S, Ansite J, Roduit R, Borsello T, Matsumoto I, Sawada T, Allaman-Pillet N, Henry H, Beckmann JS, Hering BJ, Bonny C. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 2004;53(11):2815–2823. [DOI] [PubMed] [Google Scholar]
- 4. Itoh T, Iwahashi S, Shimoda M, Chujo D, Takita M, SoRelle JA, Naziruddin B, Levy MF, Matsumoto S. High-mobility group box 1 expressions in hypoxia-induced damaged mouse islets. Transplant Proc. 2011;43(9):3156–3160. [DOI] [PubMed] [Google Scholar]
- 5. Petrick L, Rosenblat M, Aviram M. In vitro effects of exogenous carbon monoxide on oxidative stress and lipid metabolism in macrophages. Toxicol Ind Health. 2016;32(7):1318–1323. [DOI] [PubMed] [Google Scholar]
- 6. Abe T, Yazawa K, Fujino M, Imamura R, Hatayama N, Kakuta Y, Tsutahara K, Okumi M, Ichimaru N, Kaimori JY, Isaka Y, et al. High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab Invest. 2017;97(4):468–477. [DOI] [PubMed] [Google Scholar]
- 7. Cheng Y, Rong J. Therapeutic potential of heme oxygenase-1/carbon monoxide system against ischemia-reperfusion injury. Curr Pharm Des. 2017;23(26):3884–3898. [DOI] [PubMed] [Google Scholar]
- 8. Lin CC, Chiang YC, Cho RL, Lin WN, Yang CC, Hsiao LD, Yang CM. Up-regulation of PYK2/PKCalpha-dependent haem oxygenase-1 by CO-releasing molecule-2 attenuates TNF-alpha-induced lung inflammation. Br J Pharmacol. 2018;175(3):456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Megias J, Busserolles J, Alcaraz MJ. The carbon monoxide-releasing molecule CORM-2 inhibits the inflammatory response induced by cytokines in Caco-2 cells. Br J Pharmacol. 2007;150(8):977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katada K, Bihari A, Mizuguchi S, Yoshida N, Yoshikawa T, Fraser DD, Potter RF, Cepinskas G. Carbon monoxide liberated from CO-releasing molecule (CORM-2) attenuates ischemia/reperfusion (I/R)-induced inflammation in the small intestine. Inflammation. 2010;33(2):92–100. [DOI] [PubMed] [Google Scholar]
- 11. Cetkovic-Cvrlje M, Eizirik DL. TNF-alpha and IFN-gamma potentiate the deleterious effects of IL-1 beta on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine. 1994;6(4):399–406. [DOI] [PubMed] [Google Scholar]
- 12. Montane J, de Pablo S, Castano C, Rodriguez-Comas J, Cadavez L, Obach M, Visa M, Alcarraz-Vizan G, Sanchez-Martinez M, Nonell-Canals A, Parrizas M, et al. Amyloid-induced beta-cell dysfunction and islet inflammation are ameliorated by 4-phenylbutyrate (PBA) treatment. FASEB J. 2017;31(12):5296–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuoka N, Itoh T, Watarai H, Sekine-Kondo E, Nagata N, Okamoto K, Mera T, Yamamoto H, Yamada S, Maruyama I, Taniguchi M, et al. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120(3):735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsumoto S, Takita M, Chaussabel D, Noguchi H, Shimoda M, Sugimoto K, Itoh T, Chujo D, SoRelle J, Onaca N, Naziruddin B, et al. Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1beta and TNF-alpha. Cell Transplant. 2011;20(10):1641–1647. [DOI] [PubMed] [Google Scholar]
- 15. Chang CA, Murphy K, Kane RR, Lawrence MC, Naziruddin B. Early TLR4 blockade attenuates sterile inflammation-mediated stress in islets during isolation and promotes successful transplant outcomes. Transplantation. 2018;102(9):1505–1513. [DOI] [PubMed] [Google Scholar]
- 16. Citro A, Cantarelli E, Pellegrini S, Dugnani E, Piemonti L. Anti-inflammatory strategies in intrahepatic islet transplantation: a comparative study in preclinical models. Transplantation. 2018;102(2):240–248. [DOI] [PubMed] [Google Scholar]
- 17. Bender C, Christen S, Scholich K, Bayer M, Pfeilschifter JM, Hintermann E, Christen U. Islet-expressed CXCL10 promotes autoimmune destruction of islet Isografts in mice with type 1 diabetes. Diabetes. 2017;66(1):113–126. [DOI] [PubMed] [Google Scholar]
- 18. Cowley MJ, Weinberg A, Zammit NW, Walters SN, Hawthorne WJ, Loudovaris T, Thomas H, Kay T, Gunton JE, Alexander SI, Kaplan W, et al. Human islets express a marked proinflammatory molecular signature prior to transplantation. Cell Transplant. 2012;21(9):2063–2078. [DOI] [PubMed] [Google Scholar]
- 19. Ihm SH, Matsumoto I, Zhang HJ, Ansite JD, Hering BJ. Effect of short-term culture on functional and stress-related parameters in isolated human islets. Transpl Int. 2009;22(2):207–216. [DOI] [PubMed] [Google Scholar]
- 20. Mythili DM, Patra SS, Gunasekaran S. Culture prior to transplantation preserves the ultrastructural integrity of monkey pancreatic islets. J Electron Microsc (Tokyo). 2003;52(4):399–405. [DOI] [PubMed] [Google Scholar]
- 21. Sabek OM, Marshall DR, Penmetsa R, Scarborough O, Gaber AO. Examination of gene expression profile of functional human pancreatic islets after 2-week culture. Transplant Proc. 2006;38(10):3678–3679. [DOI] [PubMed] [Google Scholar]
- 22. Murray HE, Paget MB, Downing R. Preservation of glucose responsiveness in human islets maintained in a rotational cell culture system. Mol Cell Endocrinol. 2005;238(1-2):39–49. [DOI] [PubMed] [Google Scholar]
- 23. Omori K, Komatsu H, Rawson J, Mullen Y. Pharmacological strategies for protection of extrahepatic islet transplantation. Minerva Endocrinol. 2015;40(2):85–103. [PubMed] [Google Scholar]
- 24. Noguchi H, Yamada Y, Okitsu T, Iwanaga Y, Nagata H, Kobayashi N, Hayashi S, Matsumoto S. Secretory unit of islet in transplantation (SUIT) and engrafted islet rate (EIR) indexes are useful for evaluating single islet transplantation. Cell Transplant. 2008;17(1–2):121–128. [DOI] [PubMed] [Google Scholar]
- 25. Citro A, Cantarelli E, Maffi P, Nano R, Melzi R, Mercalli A, Dugnani E, Sordi V, Magistretti P, Daffonchio L, Ruffini PA, et al. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest. 2012;122(10):3647–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melzi R, Mercalli A, Sordi V, Cantarelli E, Nano R, Maffi P, Sitia G, Guidotti LG, Secchi A, Bonifacio E, Piemonti L. Role of CCL2/MCP-1 in islet transplantation. Cell Transplant. 2010;19(8):1031–1046. [DOI] [PubMed] [Google Scholar]
- 27. Espes D, Pekna M, Nilsson B, Carlsson PO. Activation of complement C3 does not hamper the outcome of experimental intramuscular islet transplantation. Transplantation. 2016;100(3):e6–e7. [DOI] [PubMed] [Google Scholar]
- 28. Alejandro R, Barton FB, Hering BJ, Wease S. Collaborative islet transplant registry I. 2008 Update from the collaborative islet transplant registry. Transplantation 2008;86(12):1783–1788. [DOI] [PubMed] [Google Scholar]
- 29. Omori K, Mitsuhashi M, Ishiyama K, Nair I, Rawson J, Todorov I, Kandeel F, Mullen Y. mRNA of the pro-apoptotic gene BBC3 serves as a molecular marker for TNF-alpha-induced islet damage in humans. Diabetologia 2011;54(8):2056–2066. [DOI] [PubMed] [Google Scholar]