Abstract
Ovarian cancer (OC) has a high mortality rate among women worldwide. However, even with the advances in detection and therapeutics, the number of cases is increasing worldwide. Increasingly, microRNAs (miRNAs), including miR-497-5p, have been implicated in the progression of many cancers, but the role of miR-497-5p in OC remains unknown. The purpose of this study was to investigate the underlying molecular mechanism of miR-497-5p in OC. Herein, we find that miR-497-5p is down-regulated in OC tissues, and overexpression of miR-497-5p enhances apoptosis in OC cells. The increased apoptosis was correlated with enhanced expression of apoptosis-related proteins. MiR-497-5p directly bound the 3’-untranslated region of metadherin (MTDH), leading to the reduction of MTDH in mRNA and protein levels. Moreover, MTDH knockout promoted the apoptosis of OC cells. Taken together, we conclude that miR-497-5p contributes to cell apoptosis in OC by regulating MTDH.
Keywords: apoptosis, ovarian cancer, miR-497-5p, MTDH
Introduction
Ovarian cancer (OC) is the gynecologic malignancy with the highest death rate1. However, even with the advances in detection and therapeutics, the number of cases is increasing worldwide. Therefore, clearly there is a compelling need for improvement in existing therapy.
miRNA, a small noncoding RNA targeting multiple mRNAs and regulation of gene expression by triggering translation repression and/or RNA degradation, has been identified as the crux of gene regulation involved in cancer2. Accumulated studies have demonstrated that the loss or gain of function of specific miRNAs may be key events in the OC process3,4. Previous studies demonstrated that miR-136 is associated with the primary cisplatin resistance of human epithelial OC5. miR-200c and miR-141 have also been validated as potential diagnostic and prognostic biomarkers for OC6. However, the biological function and molecular mechanism of miR-497-5p in OC still remain unclear.
Metadherin (MTDH) was initially cloned in 20027. MTDH is also known as astrocyte elevated gene-1 (AEG-1) and lysine-rich CEACAM1 coisolated (LYRIC), and plays pivotal roles in the tumorigenesis and progression of different types of cancers8,9. Thus, MTDH fulfills many characteristics to serve as an important molecule regulating multiple events in carcinogenesis. Numerous studies have shown that MTDH plays important roles in OC10. However, the role of MTDH in the miR-497-5p network is still unclear in OC.
In this study, we explored the expression of miR-497-5p in OC tissues and its biological function in OC. Moreover, the potential targets of miR-497-5p and gene functional analysis were investigated to decipher possible mechanisms in apoptosis in OC.
Materials and Methods
Cell Culture
The human OC cell lines SKOV-3 and TOV-112D were commercially available from ATCC (Manassas, VA, USA). Cells were cultured in DMEM (Gibco, Gaithersburg, MD, USA) with 10% fetal bovine serum (Gibco, Gaithersburg, MD, USA) at 37°C in 95% air and 5% CO2.
Tissue Collection
Human OC tissues were obtained from 30 patients diagnosed at the oncology division in the department of Gynecology and obstetrics of China-Japan Friendship Hospital (Beijing, China), between April 2018 and December 2018. All the specimens were reviewed in the pathology division and the relevant clinical data were collected by retrospective review of the patients’ files. This study was approved by the Ethics Committee of China-Japan Friendship Hospital and informed consent was obtained from each patient. Patients with nonepithelial-type neoplasia and treated before or without operation were excluded. Borderline tumors of the ovary were also excluded from this study. Biopsies were frozen in liquid nitrogen and stored at –80°C until subsequent analysis.
RNA Isolation and Quantitative Real-Time PCR
Total RNA from tissue samples and cells was extracted by using mirVanaTM miRNA isolation kit (Thermo Fisher Scientific, Shanghai, China) according to the manufacturer’s instructions. Then, RNAs were reverse-transcribed into cDNA using the high-capacity RNA-to-cDNA kit (Thermo Fisher Scientific, Shanghai, China). Taqman qRT-PCR was performed to determine MTDH gene expression on a QuantStudio 6 Flex system (Life Technologies, Gaithersburg, MD, USA). β-actin was used as an internal control to normalize the expression of the target gene(s).
For the quantification of the mature miRNAs, total RNA was reverse-transcribed using the Taqman advanced miRNA cDNA synthesis kit following the manufacturer’s instructions (Applied Biosystems, Waltham, MA, USA). The miR-497-5p levels were normalized using the U6 small nuclear RNA level. U6 was reverse-transcribed using the TaqmanTM microRNA reverse transcription kit according to the manufacturer’s protocol (Applied Biosystems, Waltham, MA, USA).
qRT-PCR primers for miR-497-5p and MTDH expression were available from Applied Biosystems (Beverly, MA, USA). The relative expression levels of specific gene and miRNA were normalized to their relative endogenous controls and was expressed as 2^(ΔΔCt)11. All reactions were performed independently in triplicate.
Western Blotting
Total protein concentration was measured by bicinchoninic acid assay kit (Sigma Aldrich, Shanghai, China). The same quality of protein (15 μg) was fractionated on 4–15% polyacrylamide gels and transferred onto nitrocellulose (Bio-Rad, Philadelphia, PA, USA). The levels of MTDH were analyzed by western blot using an anti-MTDH at 1:700 dilution (Thermo Fisher Scientific, Shanghai, China). Additionally, antibodies detecting cleaved PARP and caspase-3 (1:1000; Cell Signaling, Beverly, MA) were used to assess apoptosis activity. Membranes were washed three times with TBST and incubated with the goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:2000; Bio-Rad, Philadelphia, PA, USA). Normalization was performed by blotting the same samples with an antibody against β-actin (Abcam, Burlingame, CA, USA).
miR-497-5p Overexpression
The lentiviral system was used to stably overexpress miR-497-5p in the SKOV-3 and TOV-112D cells. A nonrelevant sequence insert lentivirus acted as a negative control (miR-NC). We constructed the lentiviral miR-497-5p and miR-NC expression vector as previously described12. The lentiviruses were generated in HEK 293 T cells using Lenti-X HTX Packaging Mix (Clontech, Mountain View, CA, USA), titered, and stored at –80°C until use.
The lentivirus was infected to cells with 5 μg/mL polybrene (Sigma Aldrich, St. Louis, MO, USA). Cells were incubated in complete growth medium with 10 μg/mL puromycin (Sigma Aldrich, St. Louis, MO, USA) for the stable clone selection.
MTDH Knockout
The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) double nickase system can cleave genomic DNA in a site-specific manner with minimal off-target effects13–15. The MTDH knockout (MTDH-KO) was generated by using CRISPR double nickase system (Santa Cruz Biotechnology, Dallas, TX, USA). A control double nickase plasmid was transfected as a vector control (Ctrl-KO). The double nickase plasmid transfection followed the manufacturer’s protocol.
Dual-Luciferase Reporter Assay
SKOV-3 or TOV-112D cells were cultured in 6-well plates (0.25 M cells/well) with complete media. After 24 h, the cells were co-transfected with 20 nM of miR-497-5p or miR-NC and 1 μg of MTDH 3’-UTR luciferase reporter construct using Lipofectamine 2000 with Opti-MEM (Gibco, Gaithersburg, MD, USA). Luciferase activities were assayed using a luciferase assay kit (Promega, Madison, WI, USA) 48 h after transfection.
The 3’-UTR binding site of MTDH (–70–3056 bp) for miR-497-5p was purchased from GeneCopoeia (Rockville, MD, USA).
Flow Cytometric Analysis (FACS)
Cells (0.5 M cells/well) with miR-497-5p overexpression, MTDH knockout or their respective controls were cultured in 6-well plates for 48 h. Cells were harvested and apoptotic cells were detected by staining with Annexin V-FITC and propidium iodide according to the manufacturer’s protocol (BD Biosciences, Bedford, MA, USA). The stained cells were immediately analyzed by a flow cytometer.
Statistical Analysis
All the experiments were performed in triplicate. Data were expressed as means ± standard errors of three independent experiments. The two-tailed, unpaired student’s t-test was used to evaluate the differences between two treatments. Multiple group comparisons were analyzed using ANOVA with a post hoc test. The p (probability) < 0.05 was considered as a statistically significant result. The SPSS software package (version 20.0, SPSS Inc) was used for statistical analyses.
Results
miR-497-5p is Down-Regulated in Ovarian Cancer Tissues
Based on our preliminary miRNA microarray data, miR-497-5p is found to be down-regulated in OC tissues. In the present study, we first determined the miR-497-5p expression levels in 12 OC tissues and the paired non-tumorous tissues with Taqman qRT-PCR. Consistent with our preliminary data, miR-497-5p expression levels were significant down-regulated compared with the paired non-tumorous tissues (p < 0.05, Fig. 1).
Figure 1.
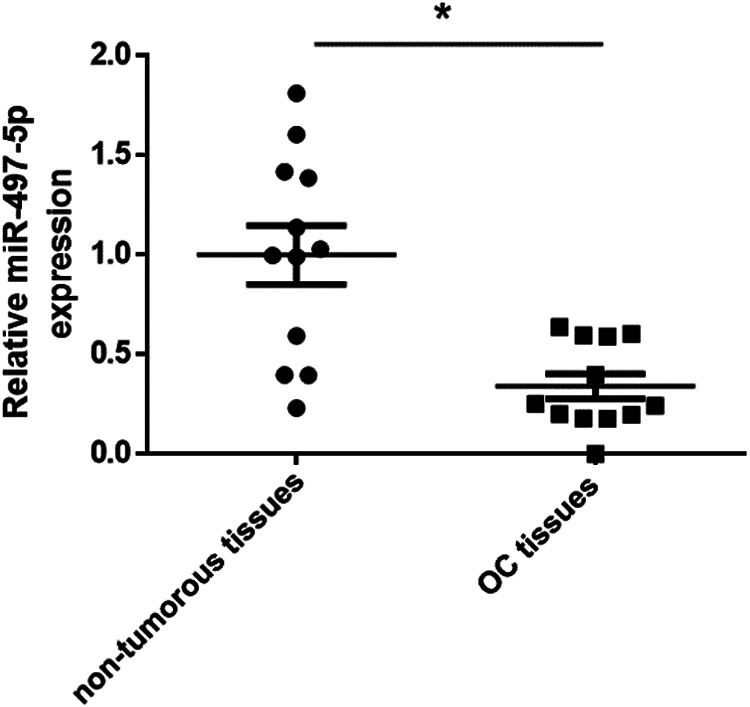
miR-497-5p expression was down-regulated in OC tissues. miR-497-5p expression was detected by qRT-PCR in 12 paired OC tissues and paired non-tumorous tissues. *p < 0.05 vs. non-tumorous tissues.
miR-497-5p Overexpression Enhances Ovarian Cancer Cell Apoptosis
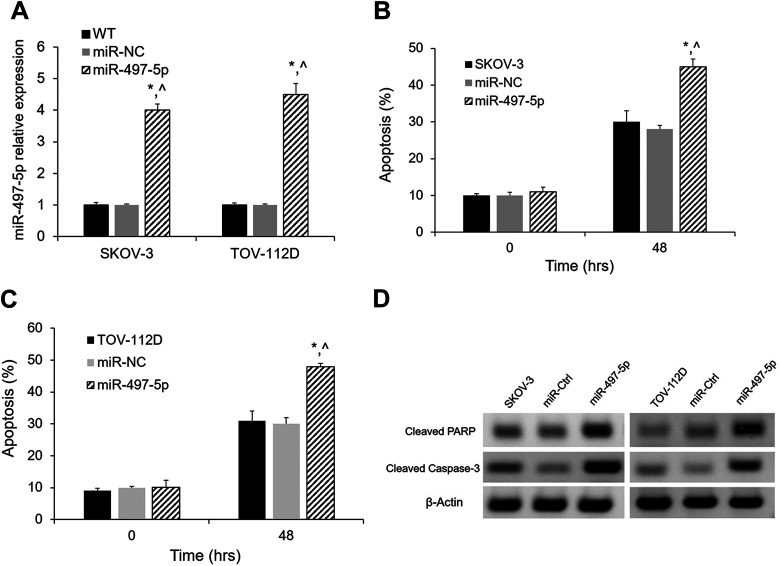
In order to elucidate the biological function of miR-497-5p in the malignancy of OC, we stably overexpressed miR-497-5p in SKOV-3 and TOV-112D cells by lentiviral system. The overexpression was validated by qRT-PCR (Fig. 2A). MiR-497-5p overexpression effects on the apoptosis of SKOV-3 and TOV-112D cells were quantitated by FACS after 48 h. As shown in Fig. 2B and 2C, the percentage of apoptosis was significantly increased in the cells overexpressing miR-497-5p. Furthermore, cleaved PARP and caspase-3 apoptosis markers were increased in cells with overexpression of miR-497-5p (Fig. 2D). These results demonstrated that miR-497-5p overexpression led to increased apoptosis.
Figure 2.
Apoptosis effects of miR-497-5p overexpression in SKOV-3 and TOV-112D cells. (A) Relative levels of miR-497-5p in SKOV-3 and TOV-112D cells were determined by qRT-PCR after miR-497-5p overexpression. (B, C) miR-497-5p overexpression significantly increased apoptosis in SKOV-3 and TOV-112D cells. (D, E) Western blot showing increased apoptotic proteins (cleaved PARP and caspase-3) in both in SKOV-3 and TOV-112D cells overexpressing miR-497-5p. *p < 0.05 vs. untransfected cells; ^p < 0.05 vs. miR-NC; n = 3.
MTDH is Up-Regulated in Ovarian Cancer Tissues
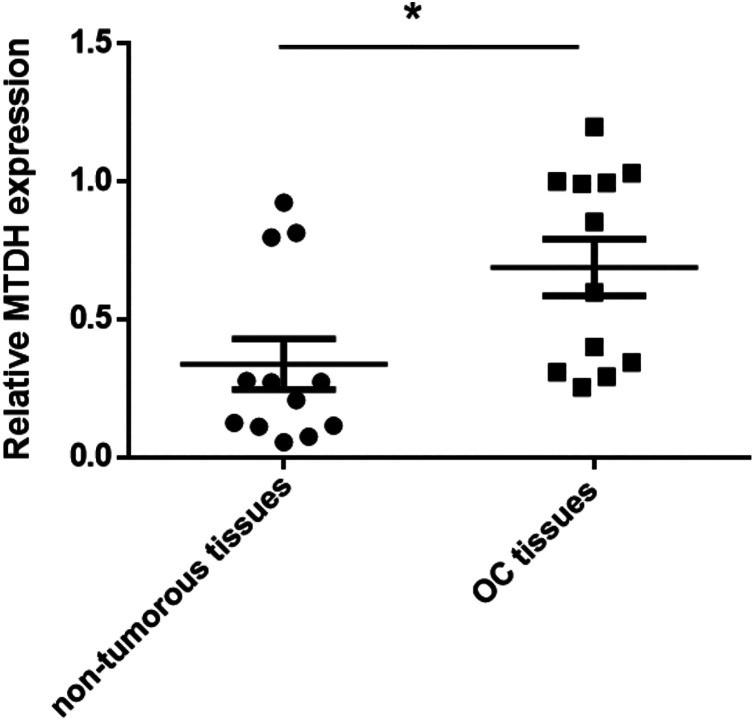
In previous studies, MTDH has been verified as the direct target of mR-497 in hepatocellular carcinoma (HCC)16. Overexpression of MTDH is observed in a variety of cancers across all biological systems. In our study, we determined the mRNA in expression levels of MTDH in 12 OC tissues and paired non-tumorous tissues using Taqman qRT-PCR. MTDH mRNA levels were significant up-regulated compared with the normal tissues (p < 0.05, Fig. 3).
Figure 3.
MTDH expression was up-regulated in OC tissues. MTDH mRNA levels were detected by qRT-PCR in 12 paired OC tissues and paired non-tumorous tissues. *p < 0.05 vs. non-tumorous tissues.
MTDH is the Direct Target of miR-497-5p
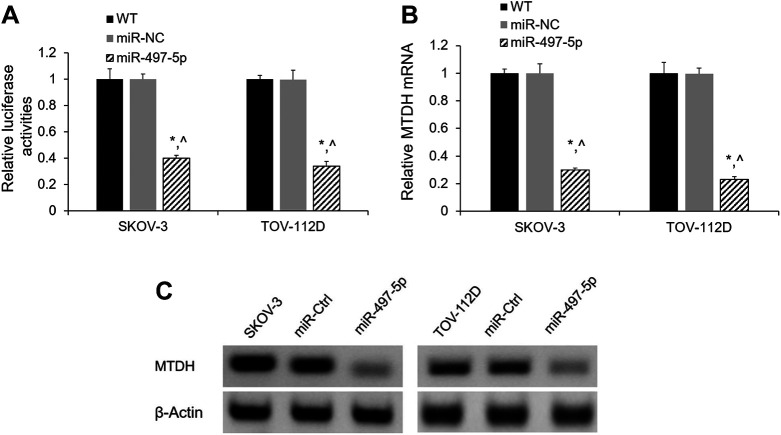
To decipher the molecular mechanism underlying how miR-497-5p inhibits apoptosis of SKOV-3 and TOV-112D cells, we next determined the biological function target of miR-497-5p. To verify whether MTDH was a potential target of miR-497-5p in OC cells, SKOV-3 and TOV-112D cells were co-transfected with the MTDH 3’-UTR and miR-497-5p or miR-Ctrl. The dual-luciferase assay revealed that miR-497-5p significantly inhibited the activity of luciferase reporter with the 3’-UTR of MTDH compared with miR-NC and the untransfected cells (Fig. 4A). In addition, SKOV-3 and TOV-112D cells that overexpressed miR-497-5p showed significantly decreased MTDH in both mRNA and protein levels compared with the untransfected cells and cells overexpressed with miR-NC (Fig. 4B and 4C).
Figure 4.
MTDH is a target of miR-497-5p. (A) 3’-UTR luciferase reporter assays. SKOV-3 and TOV-112D cells were co-transfected with miR-497-5p or miR-NC and MTDH 3’-UTR luciferase reporter construct. Firefly luciferase activity was normalized to Renilla luciferase activity. (B) Relative level of MTDH mRNA in SKOV-3 and TOV-112D cells was determined by qRT-PCR after miR-497-5p overexpression. (C) MTDH protein expression in SKOV-3 and TOV-112D cells was determined by western blotting after miR-497-5p overexpression. *p < 0.05 vs. untransfected cells; ^p < 0.05 vs. miR-NC; n = 3.
MTDH Knockout Mimics the Effect of miR-497-5p Overexpression on Apoptosis
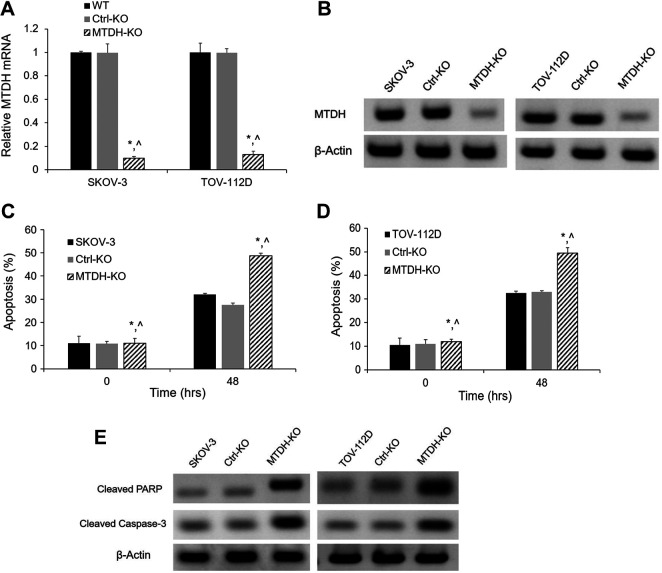
To elucidate the role of MTDH in the miR-497-5p network of OC, MTDH was knocked out using the CRISPR double nickase system. The knockout system generated efficient genome modification, which was observed by the significantly decreased MTDH mRNA and protein levels in SKOV-3 and TOV-112D cells (Fig. 5A and 5B). Knockout of MTDH mimics the effect of miR-497-5p overexpression on apoptosis measured by FACS. Inhibition of MTDH led to an increased apoptosis compared with the Ctrl-KO and untransfected cells (Fig. 5C and 5D). As shown in Fig. 5E, cleaved PARP and caspase-3 apoptosis markers were increased in SKOV-3 and TOV-112D cells with MTDH knockout.
Figure 5.
Apoptosis effects of MTDH knockout in SKOV-3 and TOV-112D cells. (A) Relative levels of MTDH mRNA in SKOV-3 and TOV-112D cells were determined by qRT-PCR after MTDH knockout. (B) MTDH protein expression in SKOV-3 and TOV-112D cells was determined by western blotting after MTDH knockout. (C) (D) MTDH knockout overexpression significantly increased apoptosis in SKOV-3 and TOV-112D cells. (E) Western blot showing increased apoptotic proteins (cleaved PARP and caspase-3) in both in SKOV-3 and TOV-112D cells after knockout. *p < 0.05 vs. untransfected cells; ^p < 0.05 vs. miR-NC; n = 3.
Discussion
It is widely known that miRNAs play crucial roles in the regulation of various processes during tumorigenesis and progression, including apoptosis, cell proliferation and metastasis17–19. MiR-497-5p belongs to the miR-15/16/195/424/497 family. There have been some reports showing the important roles that miR-497-5p plays in the progression of angiosarcoma, colorectal cancer, and so on20,21. However, the expressions of miRNAs show high tissue and cell-type specificity. MiR-497 was reported to suppress HCC metastasis and invasion in vitro by targeting MTDH15. MiR-497-5p has also been shown to inhibit tumor cell growth and invasion by targeting SOX5 in non-small-cell lung cancer22. In addition, miR-497-5p has been identified to suppress tumor cell growth of osteosarcoma by targeting ADP ribosylation factor-like protein 223. Recently, miR-497-5p has been reported to inhibit proliferation and invasion of non-small-cell lung cancer by regulating FGF224. However, elucidating the features of expression and roles of miR-497-5p in OC remains an ongoing process. In this study, we found miR-497-5p was down-regulated in OC tissues compared with paired non-tumorous tissues. MiR-497-5p overexpression enhanced cell apoptosis in SKOV-3 and TOV-112D cells. These results suggest that miR-497-5p was involved in the progression of OC.
As a recognized cancer gene, MTDH and its roles in cancer have been widely investigated in various cancers, such as malignant pleural mesothelioma, breast cancer and pancreatic cancer25–27. It has been reported that efficient and tumor-specific knockdown of the MTDH gene attenuates paclitaxel resistance of breast cancer cells both in vivo and in vitro 9. Down-regulation of MTDH expression can inhibit breast cancer progression and cell proliferation, and induce cell apoptosis in breast cancer28. Silencing the expression of MTDH increases the radiation sensitivity of SKOV3 OC cells and reduces their proliferation and metastasis10. Despite all these studies, the biological function of MTDH in the miR-497-5p network in OC is still unknown. In this study, we verified that MTDH is the direct target of miR-497-5p in SKOV-3 and TOV-112D cells. Moreover, the knockout of MTDH mimics the effect of miR-497-5p overexpression on the apoptosis of SKOV-3 and TOV-112D cells. However, it is worth verifying this model in vivo, and considering additional rescue experiments to demonstrate that miR-497-5p functions via MTDH in future studies.
In summary, our study indicates that miR-497-5p inhibits cell apoptosis in OC cells by directly targeting MTDH. Our results highlight the therapeutic potential of the miR-497-5p/MTDH pathway in OC.
Acknowledgments
The authors would like to thank the Pathology division of China-Japan Friendship Hospital in Beijing for the pathologic diagnosis.
Footnotes
Authors’ Contributions: SH conceived the study. SH and CL designed the experiments and wrote the manuscript. CL collected the samples and completed the experimental part of the study. AB and SH analyzed the data and revised the manuscript.
Ethical Approval: Ethical approval was obtained for all experimental procedures by the Ethics Committee of China-Japan Friendship Hospital, Beijing, China.
Statement of Human and Animal Rights: All procedures with human subjects in this study were conducted in accordance with the human ethics committee of China-Japan Friendship Hospital in Beijing, China. This article does not contain any studies with animals.
Statement of Informed Consent: Verbal informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Suhui Han  https://orcid.org/0000-0002-5839-3316
https://orcid.org/0000-0002-5839-3316
Reference
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Deb B, Uddin A, Chakraborty S. miRNAs and ovarian cancer: an overview. J Cell Physiol. 2018;233(5):3846–3854. [DOI] [PubMed] [Google Scholar]
- 3. Choi YE, Meghani K, Brault ME, Leclerc L, He YJ, Day TA, Elias KM, Drapkin R, Weinstock DM, Dao F, Shih KK, et al. Platinum and PARP inhibitor resistance due to overexpression of MicroRNA-622 in BRCA1-mutant ovarian cancer. Cell Rep. 2016;14(3):429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dwivedi SK, Mustafi SB, Mangala LS, Jiang D, Pradeep S, Rodriguez-Aguayo C, Ling H, Ivan C, Mukherjee P, Calin GA, Lopez-Berestein G, et al. Therapeutic evaluation of microRNA-15a and microRNA-16 in ovarian cancer. Oncotarget. 2016;7(12):15093–15104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao H, Liu S, Wang G, Wu X, Ding Y, Guo G, Jiang J, Cui S. Expression of miR-136 is associated with the primary cisplatin resistance of human epithelial ovarian cancer. Oncol Rep. 2015;33(2):591–598. [DOI] [PubMed] [Google Scholar]
- 6. Gao YC, Wu J. MicroRNA-200c and microRNA-141 as potential diagnostic and prognostic biomarkers for ovarian cancer. Tumour Biol. 2015;36(6):4843–4850. [DOI] [PubMed] [Google Scholar]
- 7. Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, Fisher PB. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21(22):3592–3602. [DOI] [PubMed] [Google Scholar]
- 8. Pan D, Jia Z, Li W, Dou Z. The targeting of MTDH by miR1455p or miR1453p is associated with prognosis and regulates the growth and metastasis of prostate cancer cells. Int J Oncol. 2019;54(6):1955–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang L, Tian Y, Leong WS, Song H, Yang W, Wang M, Wang X, Kong J, Shan B, Song Z. Efficient and tumor-specific knockdown of MTDH gene attenuates paclitaxel resistance of breast cancer cells both in vivo and in vitro . Breast Cancer Res. 2018;20(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen J, Jia Y, Jia ZH, Zhu Y, Jin YM. Silencing the expression of MTDH increases the radiation sensitivity of SKOV3 ovarian cancer cells and reduces their proliferation and metastasis. Int J Oncol. 2018;53(5):2180–2190. [DOI] [PubMed] [Google Scholar]
- 11. Yin Q, Fischer L, Neothling C, Schaefer WR. In vitro-assessment of putative antiprogestin activities of phytochemicals and synthetic UV absorbers in human endometrial Ishikawa cells. Gynecol Endocrinol. 2015;31(7):578–581. [DOI] [PubMed] [Google Scholar]
- 12. Zhao C, Huang C, Weng T, Xiao X, Ma H, Liu L. Computational prediction of MicroRNAs targeting GABA receptors and experimental verification of miR-181, miR-216 and miR-203 targets in GABA-A receptor. BMC Res Notes. 2012;5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154(6):1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yan JJ, Zhang YN, Liao JZ, Ke KP, Chang Y, Li PY, Wang M, Lin JS, He XX. MiR-497 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting VEGFA and AEG-1. Oncotarget. 2015;6(30):29527–29542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Croset M, Pantano F, Kan CWS, Bonnelye E, Descotes F, Alix-Panabieres C, Lecellier CH, Bachelier R, Allioli N, Hong SS, Bartkowiak K, et al. miRNA-30 family members inhibit breast cancer invasion, osteomimicry, and bone destruction by directly targeting multiple bone metastasis-associated genes. Cancer Res. 2018;78(18):5259–5273. [DOI] [PubMed] [Google Scholar]
- 18. Hou XW, Sun X, Yu Y, Zhao HM, Yang ZJ, Wang X, Cao XC. miR-361-5p suppresses lung cancer cell lines progression by targeting FOXM1. Neoplasma. 2017;64(4):526–534. [DOI] [PubMed] [Google Scholar]
- 19. Mansoori B, Mohammadi A, Ghasabi M, Shirjang S, Dehghan R, Montazeri V, Holmskov U, Kazemi T, Duijf P, Gjerstorff M, Baradaran B. miR-142-3p as tumor suppressor miRNA in the regulation of tumorigenicity, invasion and migration of human breast cancer by targeting Bach-1 expression. J Cell Physiol. 2019;234(6):9816–9825. [DOI] [PubMed] [Google Scholar]
- 20. Chen Y, Kuang D, Zhao X, Chen D, Wang X, Yang Q, Wan J, Zhu Y, Wang Y, Zhang S, Wang Y, et al. miR-497-5p inhibits cell proliferation and invasion by targeting KCa3.1 in angiosarcoma. Oncotarget. 2016;7(36):58148–58161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qiu Y, Yu H, Shi X, Xu K, Tang Q, Liang B, Hu S, Bao Y, Xu J, Cai J, Peng W, et al. microRNA-497 inhibits invasion and metastasis of colorectal cancer cells by targeting vascular endothelial growth factor-A. Cell Prolif. 2016;49(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li G, Wang K, Wang J, Qin S, Sun X, Ren H. miR-497-5p inhibits tumor cell growth and invasion by targeting SOX5 in non-small-cell lung cancer. J Cell Biochem. 2019;120(6):10587–10595. [DOI] [PubMed] [Google Scholar]
- 23. Sun Z, Li A, Yu Z, Li X, Guo X, Chen R. MicroRNA-497-5p suppresses tumor cell growth of osteosarcoma by targeting ADP ribosylation factor-like protein 2. Cancer Biother Radiopharm. 2017;32(10):371–378. [DOI] [PubMed] [Google Scholar]
- 24. Huang X, Wang L, Liu W, Li F. MicroRNA-497-5p inhibits proliferation and invasion of non-small cell lung cancer by regulating FGF2. Oncol Lett. 2019;17(3):3425–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen X, Li XY, Long M, Wang X, Gao ZW, Cui Y, Ren J, Zhang Z, Liu C, Dong K, Zhang H. The FBXW7 tumor suppressor inhibits breast cancer proliferation and promotes apoptosis by targeting MTDH for degradation. Neoplasma. 2018;65(2):201–209. [DOI] [PubMed] [Google Scholar]
- 26. Chen Z, Ma Y, Pan Y, Zhu H, Yu C, Sun C. MiR-1297 suppresses pancreatic cancer cell proliferation and metastasis by targeting MTDH. Mol Cell Probes. 2018;40:19–26. [DOI] [PubMed] [Google Scholar]
- 27. Zhang L, Singh A, Plaisier C, Pruett N, Ripley RT, Schrump DS, Hoang CD. Metadherin is a prognostic apoptosis modulator in mesothelioma induced via NF-kappaB-mediated signaling. Transl Oncol. 2019;12(6):859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tian W, Hao S, Gao B, Jiang Y, Zhang X, Zhang S, Guo L, Zhao J, Zhang G, Chen Y, Li Z, et al. Lobaplatin inhibits breast cancer progression, cell proliferation while it induces cell apoptosis by downregulating MTDH expression. Drug Des Devel Ther. 2018;12:3563–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]