Abstract
Apicomplexan parasites have challenged researchers for nearly a century. A major challenge to developing efficient treatments and vaccines is the parasite’s ability to change its cellular and molecular makeup to develop intracellular and extracellular niches in its hosts. Ca2+ signaling is an important messenger for the egress of the malaria parasite from the infected erythrocyte, gametogenesis, ookinete motility in the mosquito, and sporozoite invasion of mammalian hepatocytes. Calcium-dependent protein kinases (CDPKs) have crucial functions in calcium signaling at various stages of the parasite’s life cycle; this therefore makes them attractive drug targets against malaria. Here, we summarize the functions of the various CDPK isoforms in relation to the malaria life cycle by emphasizing the molecular mechanism of developmental progression within host tissues. We also discuss the current development of anti-malarial drugs, such as how specific bumped kinase inhibitors (BKIs) for parasite CDPKs have been shown to reduce infection in Toxoplasma gondii, Cryptosporidium parvum, and Plasmodium falciparum. Our suggested combinations of BKIs, artemisinin derivatives with peroxide bridge, and inhibitors on the Ca(2+)-ATPase PfATP6 as a potential target should be inspected further as a treatment against malaria.
Keywords: CDPK, anti-malarial drug, oocyst, merozoite, sporozoite
Introduction
Apicomplexan parasites are a distinct group of protozoan parasites that cause several human diseases. For example, Toxoplasma gondii infects about one-third of the global human population and causes severe disease in immunocompromised patients and pregnant women via brain inflammation and lung infection1,2. Plasmodium falciparum, the causative agent of malaria, is another apicomplexan parasite which causes over 1 million deaths per year worldwide1,3. Unique to these protozoan parasites are a conserved set of specialized apical organelles, called apicoplasts, and a shared mechanism for motility that is important for active penetration into their host cells4,5. The complexity of apicomplexan parasite biology has been a major obstacle to the development of a fully protective vaccine for diseases like malaria. A major obstacle to vaccine development is that the parasite has the ability to change its own cellular and molecular contents, for development in both mammalian hosts and the mosquito vector6.
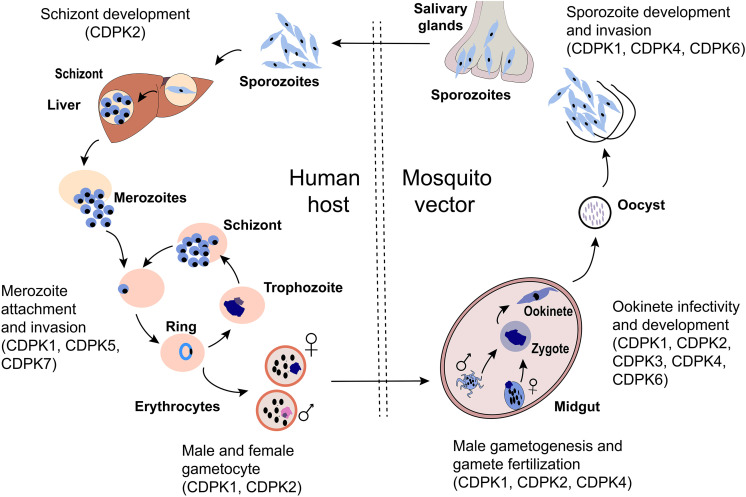
Secondary messengers like calcium (Ca2+) control a number of significant pathways in apicomplexan parasites and are important intermediaries in the stages of the apicomplexan life cycle7. In apicomplexans, a major intermediary of Ca2+ signaling is a family of calcium-dependent protein kinase (CDPK), a serine/threonine kinase that is also present in plants, green algae, and ciliates, but not mammals7–9. Although CDPK variants have a similar structure and mechanism of action, they have different roles throughout the parasite’s life cycle due to their expression timing and pattern, post-translation regulation and modification, and substrate sensitivity and specificity toward calcium10. Fig. 1 shows the life cycle-dependent expression of different CDPKs. This figure exhibits the essential role for the regulation of CDPKs for development at each stage of the Plasmodium sp. life cycle. Because CDPKs play an important role in calcium signaling at various stages of the parasite’s life cycle8,10, they are attractive drug targets for malarial treatment and/or prevention11.
Figure 1.
The malaria parasite life cycle depicted in the mosquito and human host. During a blood meal, a mosquito inoculates sporozoites into a human host. Sporozoites human infect liver cells and mature into schizonts, which rupture and release merozoites. Liberated merozoites invade erythrocytes and undergo successive rounds of intracellular replication, egress, and reinvasion as ring-stage trophozoites, schizonts, and merozoites, respectively. During this time, some parasites differentiate into male microgametyocytes and female macrogametocytes and are ingested by a mosquito during a blood meal. Inside the mosquito, the microgametes penetrate the macrogametes to form zygotes. The zygotes develop into ookinetes which invade the mid-gut wall of the mosquito and develop into oocysts. The oocysts grow, rupture, and release sporozoites, which travel to the mosquito’s salivary glands where they are re-transmitted into a new host. CDPKs have been involved in many steps involving motility and development as shown in the diagram and discussed further in the text.
Characteristics of Calcium-dependent Protein Kinases in Malaria Parasite
Toxoplasma possesses more than 20 different CDPKs, while Plasmodium sp. and Cryptosporidium sp. have less than 10. Most of these CDPKs have been linked to protein secretion, invasion, and differentiation12,13. P. falciparum CDPK (PfCDPK) is important for transduction pathways that lead to increased calcium concentrations associated with important physiological processes11. CDPK structure is typically composed of an N-terminal kinase domain separated from the C-terminal calmodulin-like domain by a small junction domain11. The calmodulin-like domain has four EF-hand motifs which can be activated by calcium ions8,11. A number of CDPK members contain additional structural features which include N-terminal extensions, a varied number of EF hands, and additional domains such as pleckstrin homology (PH)14.
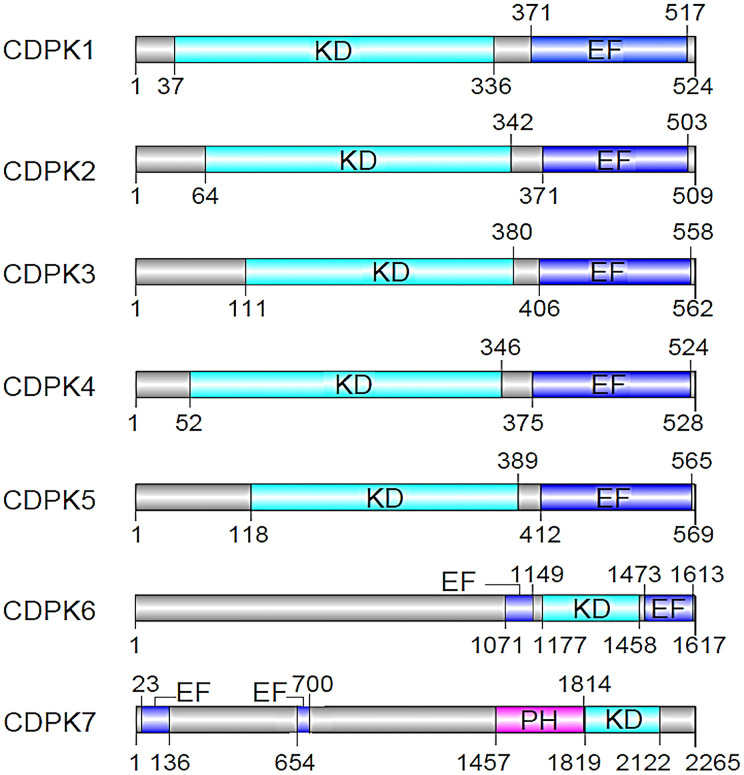
The calcium-binding domain of the four EF hands of CDPK is highly homologous to calmodulin-dependent kinases (CaMKs)4. Although CaMKs are auto-inhibited by a C-terminal helix4, the CDPK counterparts are regulated by their calcium-binding domain. Specifically, CDPKs undergo conformational changes that activate their ability to regulate other proteins by means of phosphorylation in response to rapid and transient increases in the concentration of cytoplasmic calcium. Apicomplexans have five main classes of CDPKs based on protein domain structures7,8,11; however, the CDPK family in P. falciparum has seven members (CDPK1-7) that are categorized into only four of the five classes11. The structures of these seven CDPK proteins can be found in Fig. 2. It is these subtle differences in CDPK proteins that allows for differential function within the different stages of the Plasmodium sp. life cycle. In this summary, we recapitulate the unique characteristics of P. falciparum CDPKs and the various functions they are linked to in certain stages of the Plasmodium life cycle.
Figure 2.
P. falciparum calcium-dependent protein kinases (CDPKs). EF: EF-hand motifs, KD: kinase domain, PH: pleckstrin homology. CDPKs: an N-terminal protein kinase domain, a C-terminal calmodulin-like domain with calcium-binding EF-hand motifs. CDPK6 and CDPK7 are unique from the rest of the CDPKs.
CDPK belongs to the category of serine/threonine-protein kinases that phosphorylates the hydroxyl group of serine or threonine in the presence of the cellular Ca2+ /calmodulin messenger14–16. The shared sequence of all family member identities ranges from nearly 53% (CDPK1 and CDPK2) to 31% (CDPK6 and CDPK7), as indicated in Table 1. The differences in length and sequence indicate that members of the CDPK family can act upon a range of substrates to fulfill a variety of functions (Tables 1 and 2). CDPKs have two main domains: a Ser/Thr kinase domain and an EF-hand-type calcium-binding domain1. Thus, the model structure of CDPK consists of four parts: a variable N-terminus, a catalytic kinase domain (KD), a junction domain (JD) and the calmodulin-like domain (CLD) responsible for calcium binding. The N-terminal region is less conserved among CDPK proteins and plays a significant role in the CDPK–membrane association during myristoylation15. The catalytic KD is the conserved region to which the ATP binds and phosphorylates the hydroxyl residues of serine and threonine in the substrates. The CLD is composed of four EF-hand motifs (helix–loop–helix type) and is implicated in binding intracellular calcium. The JD is the autoregulatory domain that connects the KD and the CLD, and plays a key role in the regulation of CDPKs. The CDPK is normally activated by Ca2+ via binding of the CLD to the junction. The junction and calmodulin-like regions form the CDPK activation domain (CAD), which is responsible for activating the enzyme in the presence of calcium and maintaining the enzyme in an inactive state in the absence of calcium15–17.
Table 1.
The Protein Sequence Identities (%) between the seven CDPKs of P. falciparium.
| CDPK1 | CDPK2 | CDPK3 | CDPK4 | CDPK5 | CDPK6 | |
|---|---|---|---|---|---|---|
| CDPK1 | ||||||
| CDPK2 | 39 | |||||
| CDPK3 | 43 | 45 | ||||
| CDPK4 | 53 | 41 | 39 | |||
| CDPK5 | 40 | 45 | 47 | 41 | ||
| CDPK6 | 35 | 35 | 32 | 33 | 33 | |
| CDPK7 | 38 | 39 | 39 | 42 | 38 | 31 |
Table 2.
The Physical Properties of P. falciparium CDPK Proteins.
| Gene | Location | Transcript (bp) | Protein (aa) | Protein MW | Isoelectric point | Formula |
|---|---|---|---|---|---|---|
| CDPK1 | Chr 2: 720,437-722,661 | 1575 | 524 | 60799.53 | 7.48 | C2623H4143N687O796S16 |
| CDPK2 | Chr 6: 449,291-451,461 | 1530 | 509 | 58464.75 | 6.66 | C2620H4138N686O794S16 |
| CDPK3 | Chr 3: 422,379-424,680 | 1689 | 562 | 65288.09 | 9.36 | C2945H4680N772O837S31 |
| CDPK4 | Chr 7: 755,763-757,696 | 1587 | 528 | 60779.74 | 6.50 | C2717H4330N712O823S20 |
| CDPK5 | Chr 13: 1,528,502 -1,530,208 | 1707 | 568 | 66248.07 | 6.12 | C2967H4684N770O890S27 |
| CDPK6 | Chr 11: 872,031-877,678 | 4854 | 1617 | 191814.14 | 5.32 | C8454H13035N2289O2719S49 |
| CDPK7 | Chr 11: 909,366-916,324 | 6798 | 2265 | 265136.39 | 5.73 | C11479H18210N3238O3834S69 |
As mentioned earlier, P. falciparum CDPKs are classified into four categories1,14. The first category contains proteins with canonical CDPK structures containing four C-terminal EF-hand motifs. The second category contains proteins with three C-terminal EF hands. The other two groups of CDPKs have one or more N-terminal EF hands followed by a Ser/Thr KD and three or four C-terminal EF-hand motifs1. In Fig. 2, we summarize the structures of all the 7 CDPKs in P. falciparum, indicating the positions of the various domains.
The physical properties of the CDPK proteins are summarized in Table 2. Briefly, the molecular weights of the CDPK proteins range from 58.4 to 265.1 kDa, while their isoelectric points (pI) fall between 5.32 and 9.36. Also, the number of amino acids and formula of each CDPK is provided (Table 2). Schwartz et al. in 2001 indicated that whole proteome pI values and subcellular localization are correlated18. Knowing the pI can be a powerful tool for predicting protein and protein–membrane interactions, or to determine the family member isoforms19. The pI values of different isoforms are significant to pharmaceutical applications, sensing, and nonspecific adsorption. These pharmaceutical numbers are affected by the amino acid composition, chemical side chain modifications, and the three-dimensional molecular conformation20,21.
Of all the CDPKs, CDPK1 exhibits unique structural features which have strong potential for therapeutic purposes. An extended ATP-binding pocket was observed in the crystal structure of CDPK1 showing the presence of the smallest amino acid, present in a “gate-keeper” position. The smallest amino acid, glycine, is close to the adenine recognition site22. Therefore, inhibitors of CDPKs can achieve analogous selectivity for their targets via interacting with this hydrophobic pocket at the back of the ATP binding site, access to which is blocked in most eukaryotic protein kinases by a big “gate-keeper” residue.
CDPKs in the Development of the Plasmodium sp
Gametogenesis in the mid-gut of the mosquito is activated by the ingestion of gametocytes during a blood meal (Fig. 1)6. The emergence of the parasite relies on the secretion of osmiophilic bodies from the cytosol of the gametocyte and these may contain enzymatic intercessors of host cell lysis23. CDPK1 is expressed throughout the parasite’s life cycle, which suggests it has multiple roles in different stages of the parasite (Fig. 1)7. In gametogenesis and ookinete formation, CDPK1 is critical for the transcription of a subset of translationally restrained mRNAs which encode the major ookinete surface proteins, p28 and p25. Several groups have shown that a knockdown of P. berghei CDPK1 (PbCDPK1) expression prevents ookinete development7,23,24. Additionally, Bansal et al. (2018) showed that CDPK1 knock-out parasites exhibit slow-asexual proliferation, indicating a critical defect in both male and female gamete formation. Correspondingly, in female macrogametocytes, the CDPK1 initiates the release of the translational repression of mRNAs and is treated as a potential target for malaria infection23. This, together with the important role of CDPK1 in mosquito infection and parasite invasion, reaffirms the role of CDPK1 in gametocyte motility.
In addition to CDPK1, CDPK2 has been shown to be critical in male gametocyte development. Bansal et al. (2017) showed PfCDPK2 as a significant factor in the development of the male gametes inside red blood cells (RBCs)11. Furthermore, PfCDPK2 may function in the fertilization of the female gametes by male gametes, as well as the development of ookinetes11. However, the exact role PfCDPK2 plays in the female gamete fertilization remains to be identified.
Cytoplasmic Plasmodium CDPK3 is produced in the ookinete stage of the malaria parasite, and regulates ookinete motility for the invasion of the mosquito mid-gut6,24–26. Thus, the signaling pathway implicating intracellular calcium and CDPK3 may activate ookinete motility for crossing through this phase, promoting the ookinete invasion in the mid-gut8. The activation of ookinete motility is critical for the survival and proliferation of the parasite. Therefore, linking CDPK3 to this role is not farfetched (Table 3).
Table 3.
Summary of CDPK Proteins Involvement in Malaria Parasite Development.
| Protein | PubMed ID | Function | Species | Reference |
|---|---|---|---|---|
| CDPK1 | XP_001349680.1 | Ookinete development; release of translational repression of mRNAs; exflagellation | P. falciparum | Sebastian et al., 2012 |
| P. berghei | ||||
| CDPK2 | XP_966095.1 | Ookinete development; fertilization of the female gametes by male gametes | P. falciparum | Bansal et al., 2017 |
| CDPK3 | XP_001351174.1 | Ookinete motility; mosquito mid-gut invasion | P. berghei | Ishino et al., 2006; |
| Siden-Kiamos et al., 2006 | ||||
| CDPK4 | XP_001349078.1 | Microgamete egress and invasion; DNA synthesis; transmission to the mosquito | P. falciparum | Billker et al., 2004; |
| P. berghei | Ojo et al., 2014 | |||
| CDPK5 | XP_001350105.1 | Egress of merozoites | P. falciparum | Dvorin et al., 2010 |
| CDPK6 | XP_001347910.1 | Oocyst development | P. falciparum | Wang et al., 2015 |
| CDPK7 | XP_001347913.1 | Parasite development in the RBCs | P. falciparum | Wang et al., 2015 |
Note: CDPK, calcium-dependent protein kinase; RBCs, red blood cells.
CDPK4 has an essential function in male gametogenesis induced by mosquito xanthurenic acid following intracellular calcium mobilization6,8,27. It is possible that CDPK4 is involved in different stages of genome replication during the male gametocytes exflagellation11,28. In microgametocyte activation, CDPK4 is required to enter S-phase in the rodent parasite P. berghei, as shown through studies using selective inhibitors of CDKP4 blocking exflagellation27,28. Furthermore, CDPK4 activity is required to initiate axoneme motility and eventually to complete cytokinesis28. Consequently, CDPK4 is considered a potential target for producing the vaccine responsible for blocking transmission29. When the CDPK4 is blocked, the parasite cannot enter the S-phase, leading to an abrupt halt of the cell cycle.
The presence of ookinetes in the hemocoel of mosquitoes can sequester to the mid-gut wall and transform into oocysts that subsequently lead to formation of sporozoites6. The survival of the malaria parasite in the mosquito and its transmission to the host relies on the ookinetes that cross the mid-gut epithelial barrier for onward development of oocysts. When there are defects in gametogenesis, fertilization, ookinete formation and motility, cell traversal, and oocyst development, there will be a reduction in the number of mature oocysts6. A recent report suggested that Toxoplasma gondii CDPK3, the ortholog of PfCDPK1, and CDPK6 may be involved in oocyst development30. Deletion of PbCDPK3 leads to a defect in ookinete transmission to the mid-gut of the mosquito, terminating oocysts production1,8,26. Likewise, Plasmodium CDPK4 has been reported to be important for oocyst formation27,28 (Table 3). That is, the disruption of the Plasmodium CDPK4 gene causes severe defects in both sexual reproduction and mosquito transmission27.
CDPKs in Infection and Maturation of Sporozoites
According to Aly et al., the Plasmodium genome possesses a large number of predicted kinases including a family of CDPKs6. CDPKs are responsible for the triggering of calcium-mediated signaling pathways within the sporozoite, and are thought to be essential to induce exocytosis of molecules required for sporozoite invasion6. In malaria parasites, intracellular Ca2+ signals are translated into stage-specific cellular responses by CDPKs, which combine its N-terminal KD with a C-terminal, calmodulin-like regulatory domain in a single polypeptide14. Interestingly, this domain architecture is limited to alveolates and plants, which promotes a unique activation mechanism distinct from that of related mammal calmodulin-dependent protein kinases4,31. As a result, the CDPKs are the best target for therapeutic purposes.
The significant role of CDPK4 in malaria transmission is explained by its multiple functions28. CDPK4 has been reported to be important for sporozoite motility27,28. Remarkably, in relation to the apicomplexan parasite T. gondii, the functional ortholog of CDPK4 (TgCDPK1) is essential in the egress and invasion of the parasite into the host cell28,32. Furthermore, the transcription level of a newly described member of the Plasmodium CDPK6 was high in P. falciparum sporozoites6,30, indicating a potential role in formation of sporozoites.
In a recent study, PfCDPK7 was found to be an effector of phosphatidylinositol phosphate (PIP) signaling. Thus, PfCDPK7 forms a complex with PI(4,5)P2 and leads to parasite development in the erythrocyte33.The transcription profiles get to the pinnacle at ring and late trophozoite stages, respectively34. Therefore, these kinases are both pleiotropic regulators of the cell cycle during gametogenesis, as well as a wide range of controllers of multiple other biological processes28 (Table 3). A recent study by Kumar and colleagues on PfCDPK7 knock-out parasites indicated that the ring development was greatly affected, which was suggested by stunted parasite morphology. That is, PfCDPK7 disruption caused a major reduction in the growth of the erythrocyte stage parasites, as maturation of ring-form trophozoites (rings) to mature trophozoites was significantly stalled33. Also, the number of merozoites in PfCDPK7 knock-out schizont saw lower expression than in the wild-type parasites. Thus, it is indeed possible that defects in the early life cycle developmental stages, including inefficient nutrient uptake, affect parasite division and that PfCDPK7 may be involved in cell division33. Gene disruption of the PfCDPK7 ortholog in T. gondii suggests its essential role for parasite growth via cell division. Also, TgCDPK7-KO parasites showed defective parasite division and weakened centrosome duplication33,35. Since Plasmodium and Toxoplasma divide through rather different processes of schizogony and endodyogeny, the CDPK7 may regulate the division in these parasites through different mechanisms33.
CDPK1 is potentially significant in asexual blood stages since it has resisted disruption in P. falciparum and P. berghei 25,36. Transcription of CDPK1 is similar to that of genes responsible for invasion of erythrocytes by merozoites via molecular motors23. PfCDPK1 is linked to the egress of merozoites from mature schizonts36,37, phosphorylation of motor complex proteins38, and the invasion of merozoites into RBCs11,39,40. However, PbCDPK1’s function appears to be redundant during invasion and egress within asexual stages and sporozoites. P. berghei has six CDPK homologs and multiple CDPKs are expressed in the same parasitic stage, so it is likely that their functions overlap7. For example, TgCDPK3 as the T. gondii ortholog of CDPK1 is dispensable for tachyzoite invasion and motility, and recent reports indicate from the destruction of the immune system that CDPK1 may not be essential in erythrocyte stages of P. falciparum 23,41. In 2012, Sebastian et al. showed that when a promoter-swap technique is employed to express P. berghei CDPK1 (PbCDPK1) at almost undetectable levels in asexual blood stages, no effect was detected on parasite development during these stages23. Again, a study that knocked out the Pbcdpk1 gene conclusively demonstrated that PbCDPK1 is entirely expendable in blood stages, with no effect on any stage of asexual parasite development7,41. Overall, this suggests that CDPK1 in P. falciparum may also be redundant in the erythrocytic stages of its life cycle.
Recent research indicates that PfCDPK2 is one of the CDPKs whose transcription levels peak at both ring and trophozoite stages, and that failure to disrupt PfCDPK2 via knock-out suggests an indispensable role of the kinase in the asexual stages of the malaria parasite42. Phosphopeptides for CDPK2 have also been identified in mature schizont-stage parasites, indicating that the protein becomes phosphorylated in vivo 11,43. Lauciello et al. recently identified potential phosphorylated substrates of pfCDPK2 via sequence alignment with the CDPK artificial substrate myelin basic protein (MBP) including, but not limited to, mitochondria phosphate carrier protein (MCP), histone H2B, and PF3D7_0704100, a conserved membrane protein. Since there is a 91% probability of mitochondrial signal peptide in PfCDPK2, there is good reason to believe that CDPK2 does phosphorylate MCP as calcium signals for other metabolic events during the ring and trophozoite stages44,45. Quite recent post-translational modification analysis of the schizont stage has identified PfCDPK2 phosphopeptides, indicating an in vivo phosphorylation on three sites, namely Ser-18, Thr-227, and Thr-22833,43.
In addition, PfCDPK5 has been demonstrated to play important roles in the egress of merozoites from schizonts46 (Fig. 1, Table 3). Here, PfCDPK5 lacking parasites stalled as complete schizonts, despite normal maturation of egress proteases and ligands for invasion. Also, merozoites released from arrested schizonts were capable of invading new erythrocytes, showing separate pathways of egress and invasion. The arrest of the role of cyclic guanosine monophosphate-dependent protein kinase and independent processing of protease was downstream. Thus, PfCDPK5 plays an essential role during the blood stage of malaria replication46.
To migrate from skin to hepatocytes, sporozoites must be able to evade immune recognition. While migration within RBCs equips the sporozoites with a unique capacity to avoid detection by the host’s immune system, thereby avoiding destruction by phagocytic cells47, it also facilitates the contact of sporozoites with several other host factors that influence sporozoite infectivity to hepatocytes. CDPK6 regulates the sporozoite’s switch from migratory to invasive upon sensing high levels of hepatocyte-specific sulfate proteoglycans (HSPGs) once the RBCs reach the liver48. Therefore, inhibiting CDPK6 would likely halt the life cycle progression of the malaria parasite by preventing progression to schizonts in the liver, and is a potential therapeutic target.
Essential CDPKs as Potential Drug Targets against the Malaria Parasite
An urgent need for the development of new therapeutic drugs with a novel mechanism of action against infection caused by malaria has promoted massive efforts on developing new therapeutic treatments. However, a major difficulty for therapeutic drug targeting is achieving selectivity by blocking multiple essential parasite enzymes to prevent the rapid emergence of drug resistance, while being non-toxic to the host25. To improve upon current treatment and prevention methodologies with the next generation of anti-malarial drugs, new research models must be developed and targeted at various critical stages of the malaria life cycle. Ideally, these new targets would be found only within the parasite without any homologs in the host. Since the CDPKs are present in the parasite only, there is hope for the design of selective inhibitors against the P. falciparum parasite associated with calcium signaling pathway-related factors such as ryanodine receptors, potential IP3 receptors, sarco/endoplasmic reticulum Ca2-ATPase PfATP6, and dihydroartemisinin15,49–51.
The functional and structural properties of various CDPKs have been studied comprehensively. Based on the findings, bumped kinase inhibitors (BKIs) have been developed. This group of inhibitors is composed of bulky C3 aryl substituents that are able to enter the hydrophobic pocket in the ATP binding site and act as ATP competitive inhibitors. Studies have shown that the BKIs inhibit T. gondii CDPK1 at low nano-molecular levels and interrupt the infection of cells at early stages12,52. Currently, a library of BKIs has been created and successfully examined against T. gondii and C. parvum 12,53,54. Although BKIs selectively inhibit CDPK1 from apicomplexans within a respectable structure–activity relationship22,55, they do not block mammalian kinases because mammalian kinases have larger amino acid residues adjacent to the hydrophobic pocket, which block the entry of the bulky C3 aryl group. In Plasmodium sp., BKIs do not affect intra- and extraerythrocytic stages in humans. Rather, they inhibit microgametocyte exflagellation, oocyst formation, and sporozoite production, which is necessary for transmission to mammals and in mosquitoes in the sexual stages12,29.
As we previously described, CDPK1 is essential for microneme secretion, host cell invasion, and egress of T. gondii 12, properties which constitute a potential target in T. gondii and related apicomplexans such as Cryptosporidium 12,56. Some BKI derivatives, especially BKI-1294, are efficacious against Neospora caninum CDPK1 in functional assays12,31. Moreover, BKI-1294 was also confirmed with high efficacy against acute neosporosis and toxoplasmosis31,57 in vivo, and offers prominent protection against vertical transmission of N. caninum in the pregnant mouse model12,58. Furthermore, in a C. parvum bovine model, BKI-1294 targeting CDPK1 reduces the oocyst shedding with no prominent side effects59. Apart from BK-1294, two other more effective BKIs, BKI-1517 and BKI-1553, have been shown to significantly reduce infection60.
Although the BKIs show excellent potency in vitro and in vivo as anti-malarial drugs, they also exhibited significant inhibition on human Ether-Related Gene (hERG) with potential cardiotoxicity57,61. This side effect promoted further extensive research and finally led to the discovery of the current TgCDPK1 inhibitor that combines high activity and distinct selectivity with promising pharmacokinetic properties along with low hERG activity61,62. Furthermore, the use of iterative design led to a change in direction away from hERG inhibitory activity and higher toxicity levels to humans to produce BKI-1517 and BKI-155360. According to Schaefer et al., further studies will be needed to examine whether BKI-1517 and BKI-1553 will be efficacious at a dosage acceptable for humans60.
Often, kinases are targeted in therapies to inhibit signaling pathways; however, a kinase-based drug for malaria has not yet been discovered. Some attempts for targeting the P. falciparum kinases by utilizing cyclin-dependent kinases, glycogen synthase kinase 3 (GSK3), protein kinase 5 (PK5), along with CDPKs are currently being explored with their structurally diverse inhibitors in search of a potent anti-malarial drug15,63–67. There is potential in prioritizing multiple essential targets within parasite kinases, including CDPKs, which possess a malaria-only domain architecture that is not found in mammals.
In a recent study by Miller et al., the ability of PfCDPK1 to catalyze the transfer of the γ-phosphate group from (γ-33P) ATP to the biotinylated substrate peptide in the presence of potential PfCDPK1 inhibitors was identified68. The capabilities of about 50 compounds to inhibit the kinase activity, were identified36,68, including a compound named purfalcamine, reported to bind to PfCDPK1 and blocks the development of late schizonts68. In another study, 70 PfCDPK1 inhibiting compounds with sub-micromolar activities were identified68,69. Pyrazolopyrimidines, azabenzimididazoles, and imidazopyridazines are other inhibitors of PfCDPK1 recently identified69–71 (Table 4).
Table 4.
Potential Drugs Targeting CDPKs.
| Drug name | Company | CDPK targets | Parasite stage targets | References |
|---|---|---|---|---|
| Purfalcamine | Sigma-Aldrich | CDPK1 | Merozoite | Miller et al., 2013 |
| Azabenzimididazoles | Sigma-Aldrich | CDPK1 | Schizogony | Lemercier et al., 2009; Ansell et al., 2014 |
| Imidazopyridazines | Merck | CDPK1 CDPK4 | Schizogony Trophozoite | Lemercier et al., 2009; Ansell et al., 2014 |
| Pyrazolopyrimidines | Sigma-Aldrich | CDPK1 CDPK4 | Gametocyte | Ansell et al., 2014; Chapman et al., 2013 |
In another recent study, using a functional model of Eimeria tenella, researchers performed a screen against EtCDPK4 and identified seven specific inhibitors. These include W-7, H-7, H-89, staurosporine, Ro-31-8220, myristoylated peptide, and D-sphingosine. The assessment of the seven specific inhibitors was based on the effect of Ca2+ concentrations on EtCDPK4 kinase activity. Out of the seven inhibitors, only D-sphingosine had no effect on EtCDPK4 kinase activity1.
Current efforts to inhibit CDPK1function could be enhanced by CDPK5 as an extra target since it was recently confirmed to be involved in P. falciparum schizont rupture25,26. Also, due to its early requirement for transmission, CDPK4 is treated as an important secondary target for a pan-CDPK inhibitor, as the other CDPK family members are either not essential in at least one Plasmodium species or absent from most malaria species infecting humans. Since the gate-keeper residues within CDPK1 and CDPK4 are perfectly conserved between P. falciparum and P. berghei, the ATP-binding pocket of CDPK1 and CDPK4 may be the best targets for the design of selective inhibitors for multiple apicomplexan species25.
Another compound, artemisinin, together with its semi-synthetic derivatives including dihydroartemisinin, artesunate, and artemether, has been used in the clinic for anti-malarial therapy for many years70. As a sesquiterpene lactone anti-malarial drug containing an infrequent peroxide bridge, which is considered as responsible targeting site for the drug acting mechanism, calcium homeostasis is very important along with CDPKs50,51,72–74. Pharmacological evidence demonstrated that the attendance of IP3 and ryanodine receptor channels signaling as calcium-induced calcium release are responsible for this calcium homeostasis, because these derivatives with infrequent peroxide bridge have several P-type Ca2+ ATPases involved in the ER-type reuptake mechanism at the proposed target sites70. Recently, research into extracellular Ca2+ linking to the trichocysts associated with fastest dense core-secretory vesicle exocytosis has elucidated that calcium homeostasis is critical in many physiological and pathological processes including protein secretion, motility, cell invasion, and differentiation51,75. With the involvement of putative mitochondrial Ca(2+)/H(+) exchanger, IP3/cADPR-mediated endoreticular calcium release via RyRs, and parasites-only CDPKs, our suggestion of a combination of different compounds from diverse calcium signaling pathways, such as a cocktail of BKI plus artemisinin, is worthy of more intensive studies.
A number of studies indicated an effective treatment of uncomplicated P. falciparum malaria by dihydroartemisinin–piperaquine and a combination of artemisinin semi-synthetic derivatives76–78. Further findings have suggested that using dihydroartemisinin–piperaquine for intermittent preventive treatment of malaria is associated with a 68% reduction in the risk of infection at delivery, an 84% reduction in the incidence of clinical malaria during pregnancy, and a 22% reduced risk of anemia at delivery compared with the current intermittent preventive treatment of sulfadoxine–pyrimethamine strategy77. A report has suggested a near complete prevention of malaria transmission in all patients given 0.125 mg/kg or higher dosage of primaquine when given in combination with dihydroartemisinin–piperaquine78. However, recent studies have reported a 15–60% rate of late clinical failures after the treatment of malaria with dihydroartemisinin–piperaquine in Cambodia79. As discussed above, BKIs as inhibitors of CDPKs have been developed with efficient properties12,28,53,54,60, and artemisinin with its semi-synthetic derivatives functions with an infrequent peroxide bridge80. Recently, Ca(2+)-ATPase PfATP6 was observed as strong potential drug target72,73. However, the declining effectiveness of artemisinin-based combination therapies (ACTs), especially of dihydroartemisinin–piperaquine, may destroy the gains made during the last decade in controlling malaria79. It is essential to develop broader combinations not only within the artemisinin derivatives, but also with other calcium-associated signaling pathways to design enhanced combination treatments. The development of new treatments often leads to a reduction in resistance, but drug discovery is a daunting task that takes years of effort and huge investment81. A marked decrease in deaths caused by malaria worldwide is linked to the use of effective ACTs against P. falciparum and recently P. vivax. However, resistance to these artemisinins is now causing the failure of P. falciparum ACTs in Africa and Southeast Asia82,83. The primary gene in P. falciparum ACT resistance is multidrug resistance 1 (Pfmdr1). Unveiling the basis of its role is essential for the design of improved anti-malarial therapeutics83. However, it is reported that parasites that were cleared slowly after artemisinin treatment have mutations of malarial kelch13 (K13) gene in the propeller domain81, which leads to a prolonged ring stage with an increased stress response84,85.
Conclusion
Since CDPKs have essential functions in apicomplexan parasites and are absent in mammalian hosts, the mechanism involved in CDPKs would provide promising targets for research on drugs against related apicomplexan parasites such as those that cause toxoplasmosis and malaria. Therefore, the CDPK family has received massive attention as a source of potential drug targets. Although multiple studies have identified CDPKs’ critical role in regulating several biological processes in apicomplexan parasites, the molecular mechanism remains unclear in these protozoan parasites. The critical roles of CDPKs in transduction pathways under physiological calcium levels associated with gametogenesis, zygote, ookinete formation, oocyst formation, and parasite development in host erythrocytes will be key components to developing effective anti-malarial drugs. The crucial functions of the CDPKs on the regulation of invasive motility in Plasmodium indicate the CDPKs as a potential therapeutic target for preventing parasite motility and blocking malarial infection.
Because CDPK1 has an enlarged ATP-binding pocket due to the presence of glycine at the “gate-keeper” position near to the adenine recognition site, some selective inhibitors against their kinase activity, such as BKI-1294, have been generated. Furthermore, CDPK5 has been confirmed to function in P. falciparum schizont rupture, whereas CDPK4 has the perfect and early requirement for transmission. Therefore, the current efforts to inhibit CDPK1 function enhanced by both CDPK4 and CDPK5 as extra targets have produced strong and potential anti-malarial drugs including W-7, H-7, H-89, staurosporine, Ro-31-8220, myristoylated peptide, and D-sphingosine. Furthermore, some suggested combinations of BKIs, artemisinin derivatives with peroxide bridge, and inhibitors of the Ca(2+)-ATPase PfATP6 as potential targets should be further examined as therapeutic treatments against malaria.
Acknowledgements
XHX and GGK conceived and designed the study. GGK, ZL, RY, DW, YS, YL, QY, XZ and LW comparative study and analyzed all the data. GGK, KG, OJ, LW, JB, WI, JM, JNB, and XHX prepared and all authors edited the manuscript. All authors read and approved the final version of the manuscript.
Footnotes
Ethical Approval: This study was approved by the Administration Committee of Experiment Animal, Shaanxi Province, China
Statement of Human and Animal Rights: All of the experimental procedures involving animals were conducted in accordance with the Institutional Animal Care guidelines of Shaanxi Normal University, Shaanxi Province, China.
Statement of Informed Consent: There are no human subjects in this articles and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (#31571273/ 31771277/31371256), the Foreign Distinguished Scientist Program from the National Department of Education (#MS2014SXSF038), the National Department of Education Central Universities Research Fund (#GK20130100/201701005/GERP-17-45), US Maryland Stem Cell Research Fund (2009MSCRFE008300). The role of the funding support in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript are declared in this research.
ORCID iD: George Ghartey-Kwansah  https://orcid.org/0000-0002-1875-3137
https://orcid.org/0000-0002-1875-3137
Qinan Yin  https://orcid.org/0000-0001-8560-5751
https://orcid.org/0000-0001-8560-5751
Xuehong Xu  https://orcid.org/0000-0003-2172-6997
https://orcid.org/0000-0003-2172-6997
Availability of Data and Material: All data generated or analyzed during this study are included in this published article.
Reference
- 1. Wang Z, Huang B, Dong H, Zhao Q, Zhu S, Xia W, Xu S, Xie Y, Cui X, Tang M, Men Q, et al. Molecular characterization and functional analysis of a novel calcium-dependent protein kinase 4 from Eimeria tenella . PLoS One. 2016;11(12):e0168132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010;26(4):190–196. [DOI] [PubMed] [Google Scholar]
- 3. Sachs J, Malaney P. The economic and social burden of malaria. Nature 2002;415(6872):680–685. [DOI] [PubMed] [Google Scholar]
- 4. Wernimont AK, Artz JD, Finerty PJ, Lin YH, Amani M, Allali-Hassani A, Senisterra G, Vedadi M, Tempel W, Mackenzie F, Chau I, et al. Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium. Nat Struct Mol Biol. 2010;17(5):596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sibley LD. Intracellular parasite invasion strategies. Science. 2004;304(5668):248–253. [DOI] [PubMed] [Google Scholar]
- 6. Aly ASI, Vaughan AM, Kappe SHI. Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol. 2009;63:195–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jebiwott S, Govindaswamy K, Mbugua A, Bhanot P. Plasmodium berghei calcium dependent protein kinase 1 is not required for host cell invasion. PLoS One. 2013;8(11):e79171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the mid-gut epithelial cell. Mol Microbiol. 2006;59(4):1175–1184. [DOI] [PubMed] [Google Scholar]
- 9. Philip N, Waters AP. Conditional degradation of plasmodium calcineurin reveals functions in parasite colonization of both host and vector. Cell Host Microbe. 2015;18(1):122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kadian K, Gupta Y, Kempaiah P, Gupta N, Sharma A, Rawat M. Calcium dependent protein kinases (CDPKs): key to malaria eradication. Curr Top Med Chem. 2017;17(19):2215–2220. [DOI] [PubMed] [Google Scholar]
- 11. Bansal A, Molina-Cruz A, Brzostowski J, Mu J, Miller LH. Plasmodium falciparum calcium-dependent protein kinase 2 is critical for male gametocyte exflagellation but not essential for asexual proliferation. MBio. 2017;8(5):e01656–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Müller J, Hemphill A. Drug target identification in protozoan parasites. Expert Opin Drug Discov. 2016;11(8):815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii . Nature. 2008;451(7175):207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Billker O, Lourido S, Sibley LD. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe. 2009;5(6):612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aher RB, Roy K. Exploring structural requirements for the inhibition of Plasmodium falciparum calcium-dependent protein kinase-4 (PfCDPK-4) using multiple in silico approaches. RSC Adv. 2016;6:51957–51982. [Google Scholar]
- 16. Holder AA, Ridzuan MAM, Green JL. Calcium dependent protein kinase 1 and calcium fluxes in the malaria parasite. Microbes Infect. 2012;14(10):825–830. [DOI] [PubMed] [Google Scholar]
- 17. Wernimont AK, Amani M, Qiu W, Pizarro JC, Artz JD, Lin YH, Lew J, Hutchinson A, Hui R. Structures of parasitic CDPK domains point to a common mechanism of activation. Proteins. 2011;79(3):803–820. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz R, Ting CS, King J. Whole proteome pI values correlate with subcellular localizations of proteins for organisms within the three domains of life. Genome Res. 2001;11(5):703–709. [DOI] [PubMed] [Google Scholar]
- 19. Stekhoven FMAHS, Gorissen MHAG, Flik G. The isoelectric point, a key to understanding a variety of biochemical problems: a minireview. Fish Physiol Biochem. 2008;34(1):1–8. [DOI] [PubMed] [Google Scholar]
- 20. Hughes AJ, Tentori AM, Herr AE. Bistable isoelectric point photoswitching in green fluorescent proteins observed by dynamic immunoprobed isoelectric focusing. J Am Chem Soc. 2012;134(42):17582–17591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo S, Zhu X, Jańczewski D, Lee SSC, He T, Teo SLM, Vancso GJ. Measuring protein isoelectric points by AFM-based force spectroscopy using trace amounts of sample. Nat Nanotechnol. 2016;11(9):817–823. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Z, Ojo KK, Vidadala R, Huang W, Geiger JA, Scheele S, Choi R, Reid MC, Keyloun KR, Rivas K, Siddaramaiah LK, et al. Potent and selective inhibitors of CDPK1 from T. gondii and C. parvum based on a 5-Aminopyrazole-4-carboxamide scaffold. ACS Med Chem Lett. 2014;5(1):40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sebastian S, Brochet M, Collins MO, Schwach F, Jones ML, Goulding D, Rayner JC, Choudhary JS, Billker O. A plasmodium calcium-dependent protein kinase controls zygote development and transmission by translationally activating repressed mRNAs. Cell Host Microbe. 2012;12(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bennink S, Kiesow MJ, Pradel G. The development of malaria parasites in the mosquito mid-gut. Cell Microbiol. 2016;18(7):905–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tewari R, Straschil U, Bateman A, Bohme U, Cherevach I, Gong P, Pain A, Billker O. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe. 2010;8(4):377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siden-Kiamos I, Ecker A, Nybäck S, Louis C, Sinden RE, Billker O. Plasmodium berghei calcium-dependent protein kinase 3 is required for ookinete gliding motility and mosquito mid-gut invasion. Mol Microbiol. 2006;60(6):1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell Host Microbe. 2004;117(4):503–514. [DOI] [PubMed] [Google Scholar]
- 28. Fang H, Klages N, Baechler B, Hillner E, Yu L, Pardo M, Choudhary J, Brochet M. Multiple short windows of calcium-dependent protein kinase 4 activity coordinate distinct cell cycle events during Plasmodium gametogenesis. Elife. 2017;6:e26524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ojo KK, Pfander C, Mueller NR, Burstroem C, Larson ET, Bryan CM, Fox AM, Reid MC, Johnson SM, Murphy RC, Kennedy M, et al. Transmission of malaria to mosquitoes blocked by bumped kinase inhibitors. J Clin Invest. 2012;122(6):2301–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang J, Huang S, Zhang N, Chen J, Zhu X. Genome-wide expression patterns of calcium-dependent protein kinases in Toxoplasma gondii . Parasit Vectors. 2015;8:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ojo KK, Eastman RT, Vidadala R, Zhang Z, Rivas KL, Choi R, Lutz JD, Reid MC, Fox AM, Hulverson MA, Kennedy M, et al. A specific inhibitor of PfCDPK4 blocks malaria transmission: chemical-genetic validation. J Infect Dis. 2014;209(2):275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465(7296):359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar P, Tripathi A, Ranjan R, Halbert J, Gilberger T, Doerig C, Sharma P. Regulation of Plasmodium falciparum development by calcium-dependent protein kinase 7 (PfCDPK7). J Biol Chem. 2014;289(29):20386–20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brochet M, Billker O. Calcium signalling in malaria parasites. Mol Microbiol. 2016;100(3):397–408. [DOI] [PubMed] [Google Scholar]
- 35. Morlon-Guyot J, Berry L, Chen CT, Gubbels MJ, Lebrun M, Daher W. The Toxoplasma gondii calcium dependent protein kinase 7 is involved in early steps of parasite division and is crucial for parasite survival. Cell Microbiol. 2014;16(1):95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kato N, Sakata T, Breton G, Le Roch KG, Nagle A, Andersen C, Bursulaya B, Henson K, Johnson J, Kumar KA, Marr F, et al. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat Chem Biol. 2008;4(6):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Azevedo MF, Sanders PR, Krejany E, Nie CQ, Fu P, Bach LA, Wunderlich G, Crabb BS, Gilson PR. Inhibition of Plasmodium falciparum CDPK1 by conditional expression of its J-domain demonstrates a key role in schizont development. Biochem J. 2013;452(2):433–441. [DOI] [PubMed] [Google Scholar]
- 38. Green JL, Rees-Channer RR, Howell SA, Martin SR, Knuepfer E, Taylor HM, Grainger M, Holder AA. The motor complex of Plasmodium falciparum: phosphorylation by a calcium-dependent protein kinase. J Biol Chem. 2008;283(45):30980–30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bansal A, Singh S, More KR, Hans D, Nangalia K, Yogavel M, Sharma A, Chitnis CE. Characterization of Plasmodium falciparum calcium-dependent protein kinase 1 (PfCDPK1) and its role in microneme secretion during erythrocyte invasion. J Biol Chem. 2013;288(3):1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kumar S, Kumar M, Ekka R, Dvorin JD, Paul AS, Madugundu AK, Gilberger T, Gowda H, Duraisingh MT, Keshava Prasad TS, Sharma P. PfCDPK1mediated signaling in erythrocytic stages of Plasmodium falciparum . Nat Commun. 2017;8(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Green JL, Moon RW, Whalley D, Bowyer PW, Wallace C, Rochani A, Nageshan RK, Howell SA, Grainger M, Jones HM, Ansell KH, et al. Imidazopyridazine inhibitors of Plasmodium falciparum calcium-dependent protein kinase 1 also target cyclic GMP-dependent protein kinase and heat shock protein 90 to kill the parasite at different stages of intracellular development. Antimicrob Agents Chemother. 2016;60(3):1464–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Solyakov L, Halbert J, Alam MM, Semblat JP, Dorin-Semblat D, Reininger L, Bottrill AR, Mistry S, Abdi A, Fennell C, Holland Z, et al. Global kinomic and phospho-proteomic analyses of the human malaria parasite Plasmodium falciparum . Nat Commun 2011;2:565. [DOI] [PubMed] [Google Scholar]
- 43. Treeck M, Sanders JL, Elias JE, Boothroyd JC. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites’ boundaries. Cell Host Microbe. 2011;10(10):410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lauciello L, Kappes B, Scapozza L, Perozzo R. Expression, purification and biochemical characterization of recombinant Ca-dependent protein kinase 2 of the malaria parasite Plasmodium falciparum . Protein Expr Purif. 2013;90(2):170–177. [DOI] [PubMed] [Google Scholar]
- 45. Ginsburg H. Progress in in silico functional genomics: the malaria metabolic pathways database. Trends Parasitol. 2006;22(6):238–240. [DOI] [PubMed] [Google Scholar]
- 46. Dvorin JD, Martyn DC, Patel SD, Grimley JS, Collins CR, Hopp CS, Bright AT, Westenberger S, Winzeler E, Blackman MJ, Baker DA, et al. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science. 2010;328(5980):910–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, Dubremetz JF, Prévost MC, Ishino T, Yuda M, Ménard R. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe. 2008;3(2):88–96. [DOI] [PubMed] [Google Scholar]
- 48. Kumar KA, Mishra S. Plasmodium pre-erythrocytic stages: biology, whole parasite vaccines and transgenic models. Am J Immunol. 2012;8(3):88–100. [Google Scholar]
- 49. Doerig C, Billker O, Haystead T, Sharma P, Tobin AB, Waters NC. Protein kinases of malaria parasites: an update. Trends Parasitol. 2008;24(12):570–577. [DOI] [PubMed] [Google Scholar]
- 50. Nagamune CK, Moreno SN, Chini EN, Sibley LD. Calcium regulation and signaling in apicomplexan parasites. Subcell Biochem. 2008;47:70–81. [DOI] [PubMed] [Google Scholar]
- 51. Lourido SD, Moreno SN. The calcium signaling toolkit of the Apicomplexan parasites Toxoplasma gondii and Plasmodium spp. Cell Calcium. 2015;57(3):186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ojo KK, Larson ET, Keyloun KR, Castaneda LJ, Derocher AE, Inampudi KK, Kim JE, Arakaki TL, Murphy RC, Zhang L, Napuli AJ, et al. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat Struct Mol Biol. 2010;17(5):602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johnson SM, Murphy RC, Geiger JA, DeRocher AE, Zhang Z, Ojo KK, Larson ET, Perera BG, Dale EJ, He P, Reid MC, et al. Development of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitors with potent anti-toxoplasma activity. J Med Chem. 2012;55(5):2416–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Larson ET, Ojo KK, Murphy RC, Johnson SM, Zhang Z, Kim JE, Leibly DJ, Fox AM, Reid MC, Dale EJ, Perera BG, et al. Multiple determinants for selective inhibition of apicomplexan calcium-dependent protein kinase CDPK1. J Med Chem. 2012;55(6):2803–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keyloun KR, Reid MC, Choi R, Song Y. The gatekeeper residue and beyond: homologous calcium-dependent protein kinases as drug development targets for veterinarian Apicomplexa parasites. Parasitology. 2014;141(11):1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pedroni MJ, Vidadala RS, Choi R, Keyloun KR, Reid MC, Murphy RC, Barrett LK, Van Voorhis WC, Maly DJ, Ojo KK, Lau AO. Bumped kinase inhibitor prohibits egression in Babesia bovis . Vet Parasitol. 2016;215:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Doggett JS, Ojo KK, Fan E, Maly DJ, Van Voorhis WC. Bumped kinase inhibitor 1294 treats established Toxoplasma gondii infection. Antimicrob Agents Chemother. 2014;58(6):3547–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Winzer P, Müller J, Aguado-Martínez A, Rahman M, Balmer V, Manser V, Ortega-Mora LM, Ojo KK, Fan E, Maly DJ, Van Voorhis WC, et al. In vitro and in vivo effects of the bumped kinase inhibitor 1294 in the related cyst-forming apicomplexans Toxoplasma gondii and Neospora caninum . Antimicrob Agents Chemother. 2015;59(10):6361–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lendner M, Böttcher D, Delling C, Ojo KK, Van Voorhis WC, Daugschies A. A novel CDPK1 inhibitor-a potential treatment for cryptosporidiosis in calves? Parasitol Res. 2015;114(1):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schaefer DA, Betzer DP, Smith KD, Millman ZG, Michalski HC, Menchaca SE, Zambriski JA, Ojo KK, Hulverson MA, Arnold SL, Rivas KL. Novel bumped kinase inhibitors are safe and effective therapeutics in the calf clinical model for cryptosporidiosis. J Infect Dis. 2016;214(12):1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cardew EM, Verlinde CLMJ, Pohl E. The calcium-dependent protein kinase1 from Toxoplasma gondii as target for structure-based drug design. Parasitology. 2018;145(2):210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vidadala RS, Rivas KL, Ojo KK, Hulverson MA, Zambriski JA, Bruzual I, Schultz TL, Huang W, Zhang Z, Scheele S, DeRocher AE, et al. Development of an orally available and central nervous system (CNS) penetrant Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitor with minimal human ether-a-go-go-related gene (hERG) activity for the treatment of toxoplasmosis. J Med Chem. 2016;59(13):6531–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aher RB, Roy K. First report on exploring classification and regression based QSAR modelling of Plasmodium falciparum glycogen synthase kinase (PfGSK-3) inhibitors. SAR QSAR Environ Res. 2015;26(11):959–976. [DOI] [PubMed] [Google Scholar]
- 64. Vidadala RSR, Ojo KK, Johnson SM, Zhang Z, Leonard SE, Mitra A, Choi R, Reid MC, Keyloun KR, Fox AM, Kennedy M, et al. Development of potent and selective Plasmodium falciparum calcium-dependent protein kinase 4 (PfCDPK4) inhibitors that block the transmission of malaria to mosquitoes. Eur J Med Chem. 2014;74:562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Keenan SM, Welsh WJ. Characteristics of the Plasmodium falciparum PK5 ATP-binding site: implications for the design of novel antimalarial agents. J Mol Graphics Modell. 2004;22(3):241–247. [DOI] [PubMed] [Google Scholar]
- 66. Geyer JA, Keenan SM, Woodard CL, Thompson PA, Gerena L, Nichols DA, Gutteridge CE, Waters NC. Selective inhibition of Pfmrk, a Plasmodium falciparum CDK, by antimalarial 1,3-diaryl-2-propenones. Bioorg Med Chem Lett. 2009;19(7):1982–1985. [DOI] [PubMed] [Google Scholar]
- 67. Cui H, Carrero-Lerida J, Silva APG, Whittingham JL, Brannigan JA, Ruiz-Perez LM, Read KD, Wilson KS, González-Pacanowska D, Gilbert IH. Synthesis and evaluation of α-thymidine analogues as novel antimalarials. J Med Chem. 2012;55(24):10948–10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Miller LH, Ackerman HC, Su X, Wellems TE. Malaria biology and disease pathogenesis: insights for new treatments. Nat Med. 2013;19(2):156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lemercier G, Fernandez-Montalvan A, Shaw JP, Kugelstadt D, Bomke J, Domostoj M, Schwarz MK, Scheer A, Kappes B, Leroy D. Identification and characterization of novel small molecules as potent inhibitors of the plasmodial calcium-dependent protein kinase 1. Biochemistry. 2009;48(27):6379–6389. [DOI] [PubMed] [Google Scholar]
- 70. Ansell KH, Jones HM, Whalley D, Hearn A, Taylor DL, Patin EC, Chapman TM, Osborne SA, Wallace C, Birchall K, Large J, et al. Biochemical and antiparasitic properties of inhibitors of the Plasmodium falciparum calcium-dependent protein kinase PfCDPK1. Antimicrob Agents Chemother. 2014;58(10):6032–6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chapman TM, Osborne SA, Bouloc N, Large JM, Wallace C, Birchall K, Ansell KH, Jones HM, Taylor D, Clough B, Green JL, et al. Substituted imidazopyridazines are potent and selective inhibitors of Plasmodium falciparum calcium-dependent protein kinase 1 (PfCDPK1). Bioorg Med Chem Lett. 2013;23(10):3064–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Arnou B, Montigny C, Morth JP, Nissen P, Jaxel C, Møller JV, Maire M. The Plasmodium falciparum Ca(2+)-ATPase PfATP6: insensitive to artemisinin, but a potential drug target. Biochem Soc Trans. 2011;39(3):823–831. [DOI] [PubMed] [Google Scholar]
- 73. Guimarães DS, Fonseca AL, Batista R, Comar Junior M, Oliveira AB, Taranto AG, Varotti Fde P. Structure-based drug design studies of the interactions of ent-kaurane diterpenes derived from Wedelia paludosa with the Plasmodium falciparum sarco/endoplasmic reticulum Ca2-ATPase PfATP6. Mem Inst Oswaldo Cruz. 2015;110(2):255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Plattner H, Sehring IM, Mohamed IK, Miranda K, De Souza W, Billington R, Genazzani A, Ladenburger EM. Calcium signaling in closely related protozoan groups (Alveolata): non-parasitic ciliates (Paramecium, Tetrahymena) vs. parasitic Apicomplexa (Plasmodium, Toxoplasma). Cell Calcium. 2012;51(5):351–382. [DOI] [PubMed] [Google Scholar]
- 75. Wang L, Xu MM, Li Z, Shi M, Zhou X, Jiang X, Bryant J, Balk S, Ma J, Isaacs WB, Xu Xu. Calcium and CaSR/IP3 R in prostate cancer development. Cell Biosci. 2018;8(1):16–24. [Google Scholar]
- 76. Keating GM. Dihydroartemisinin/piperaquine a review of its use in the treatment of uncomplicated Plasmodium falciparum malaria. Drugs. 2012;72(7):937–961. [DOI] [PubMed] [Google Scholar]
- 77. Desai M, Gutman J, Llanziva A, Otieno K, Juma E, Kariuki S, Ouma P, Were V, Laserson K, Katana A, Williamson J, et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin–piperaquine versus intermittent preventive treatment with sulfadoxine–pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three group, randomised controlled superiority trial. Lancet. 2015;386(10012):2507–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dicko A, Brown JM, Diawara H, Baber I, Mahamar A, Soumare HM, Sanogo K, Koita F, Keita S, Traore SF, Chen I. Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis. 2016;16(6):674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Duru V, Khim N, Leang R, Kim S, Domergue A, Kloeung N, Ke S, Chy S, Eam R, Khean C, Loch K, et al. Plasmodium falciparum dihydroartemisinin-piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: retrospective and prospective investigations. BMC Medicine. 2015;13:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen T, Li M, Zhang R, Wang H. Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J Cell Mol Med. 2009;13(7):1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang J, Xu C, Liao F L, Jiang T, Krishna S, Youyou Tu Y. A temporizing solution to“artemisinin resistance”. N Engl J Med. 2019;380(22):2087–2089. doi: 10.1056/NEJMp1901233. [DOI] [PubMed] [Google Scholar]
- 82. Haldar K, Bhattacharjee S, Safeukui I. Drug resistance in Plasmodium. Nat Rev Microbiol. 2018;16(3):156–170. doi: 10.1038/nrmicro.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gil JP, Krishna S. pfmdr1 (Plasmodium falciparum multidrug drug resistance gene 1): a pivotal factor in malaria resistance to artemisinin combination therapies. Expert Rev Anti Infect Ther. 2017;15(6):527–543. doi: 10.1080/14787210.2017.1313703. [DOI] [PubMed] [Google Scholar]
- 84. Wang J, Zhang CJ, Chia WN, Loh CC, Li Z, Lee YM, He Y, Yuan LX, Lim TK, Liu M, Liew CX, et al. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum . Nat Commun. 2015;6:10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, et al. Tracking Resistance to Artemisinin Collaboration (TRAC). Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371(5):411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]