Abstract
Neurotrophins are a family of proteins that play an important role in the regulation of the growth, survival, and differentiation of neurons in the central and peripheral nervous system. Neurotrophins were earlier characterized by their role in early development, growth, maintenance, and the plasticity of the nervous system during development, but recent findings also indicate their complex role during normal physiology in both neuronal and non-neuronal tissues. Therefore, it is important to recognize a deficiency in the expression of neurotrophins, a major factor driving the debilitating features of several neurologic and psychiatric diseases/disorders. On the other hand, overexpression of neurotrophins is well known to play a critical role in pathogenesis of chronic pain and afferent sensitization, underlying conditions such as lower urinary tract symptoms (LUTS)/disorders and osteoarthritis. The existence of a redundant receptor system of high-and low-affinity receptors accounts for the diverse, often antagonistic, effects of neurotrophins in neurons and non-neuronal tissues in a spatial and temporal manner. In addition, studies looking at bladder dysfunction because of conditions such as spinal cord injury and diabetes mellitus have found alterations in the levels of these neurotrophins in the bladder, as well as in sensory afferent neurons, which further opens a new avenue for therapeutic targets. In this review, we will discuss the characteristics and roles of key neurotrophins and their involvement in the central and periphery nervous system in both normal and diseased conditions.
Keywords: Neurotrophins, NGF (nerve growth factor), BDNF (brain-derived neurotrophic factor), Bladder and development
Introduction
The physiological role of neurotrophins (NTs) has been an exciting area of study, allowing for a better understanding of the fate of neurons in both the developing and adult nervous system. Of the neurotrophin family, the nerve growth factor (NGF) was the first to be identified and phenotyped by Levi-Montalcini [1]. NGF was then used to derive the properties of the neurotrophin family. It has been shown that a neurotrophic factors can support the maintenance, survival, differentiation, and growth of neurons and promote nerve regeneration in the setting of spinal cord injury [2]. They arrive in this neuronal environment by secretion from a target tissue, which can be either neuronal or non-neuronal, where they act on the neuronal supply in tissue to support differentiation and survival [3, 4].
Since the discovery of NGF, dozens of other neutrophic factors have also been recognized, each with unique characteristics and roles in biological activity, with distinct expression that confers a functional specificity to target tissue. In addition, factors previously discovered and known for their effects in other systems, including insulin-like growth factors or tumor-derived factors, have also been classified as neurotrophic factors. However, these factors have instead been grouped into different families according to the homology of the nucleotide sequence and, hence, evolutionary relatedness [5].
Indeed, of these related families, the most well-understood and widely expressed factors in the brain seem to be neurotrophins (NTs) [6, 7]. While NGF is the model for the neurotrophin family (NF), it now also includes other factors, namely, brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), and neurotrophin 4 (NT-4) [4]. As their names suggest, all trophins have demonstrated some level of survival activity on nervous tissue. Interestingly, neurotrophins have also been found to influence growth and development by means other than recovering neurons from cell death. For example, they could alter phenotypes of cholinergic neurons innervating bladder and, therefore, manipulate the function of cholinergic neurons in the bladder [8]. Some neurotrophins support neuron survival up to the point of naturally occurring cell death. After this, they become ineffective as a result of an apparent switch in the neurotrophin receptor expressed by the neuron [9–11]. In discussing their influence on neuronal proliferation, some neurotrophins such as NT-3 have been shown to exert profound survival/neurite outgrowth effects on neural crest (NC) progenitor cells and were also found mitogenic to somite cells in the mixed NC/somite cultures. NT-3 also has potential to increase the survival of the dividing sympathetic neuroblast population; however, it does not directly stimulate mitosis [12–15]. In addition, neurotrophins have been found to influence synaptic activity as increase neurotrophin expression confers a role in the plasticity of the nervous system. Thus, the availability of mice to study with specific neurotrophin disruption is important in deciphering their roles in the central and peripheral nervous system. Studies from these mutant mice reflect that the lacking neurotrophin may not cause cell death, but indeed, it may have compromised their differentiated potential and, hence, altered functional activity [16–19].
The classical “neurotrophin theory” [12] explains the loss of sets of peripheral sympathetic neurons [20], sensory neurons [21], proprioceptive neurons [22], and subsets of sensory neurons in NGF, BDNF, NT-3, and NT-4 knockout mice, respectively [23]. The time course of NT expression is intriguing as it points to the important function played by these NTs in both developing and adult nervous system [7, 24, 25]. Further, the expression of these factors has been shown to be high during early development, a time highlighted by substantial growth, differentiation, and modeling of the nervous system. Expression of NTs subsides in the adult as the number of neurons innervating a target tissue exceeds that usually found in the mature innervated target. The final number of neurons is further reduced through apoptosis, which also reflects the availability of neurotrophins [26]. Although, some data are available about their role in some diseases (Table 1), but indeed, there is still much to be learned regarding the roles of the neurotrophic factors in the brain and during the earlier development. In this review, we will discuss diverse roles of NTs in the central and peripheral nervous system as well as recent advancement of NT-mediated therapies in different disease conditions (Fig. 1).
Table 1.
NTs involved in different diseases and their targets
| Disease | Neurotrophic factor(s) | Targets |
|---|---|---|
| Alzheimer’s disease | NGF, BDNF, and GDNF | Cholinergic neurons |
| Parkinson’s disease | GDNF/neurturin | Striatal neurons |
| Huntington’s disease | BDNF | Striatal neurons |
| Spinal cord injury | BDNF and NT-3 | Site of injury |
| Supranuclear palsy | GDNF | Several in CNS |
| Amyotrophic lateral sclerosis (ALS) | NGF and BDNF | Motor neurons |
| Down syndrome | NGF | Cholinergic neurons |
| Sensory neuropathy | NGF | Sensory and sympathetic neurons |
| Overactive bladder (OAB) | NGF and BDNF | Bladder |
| Bladder pain | NGF | Bladder |
Fig. 1.
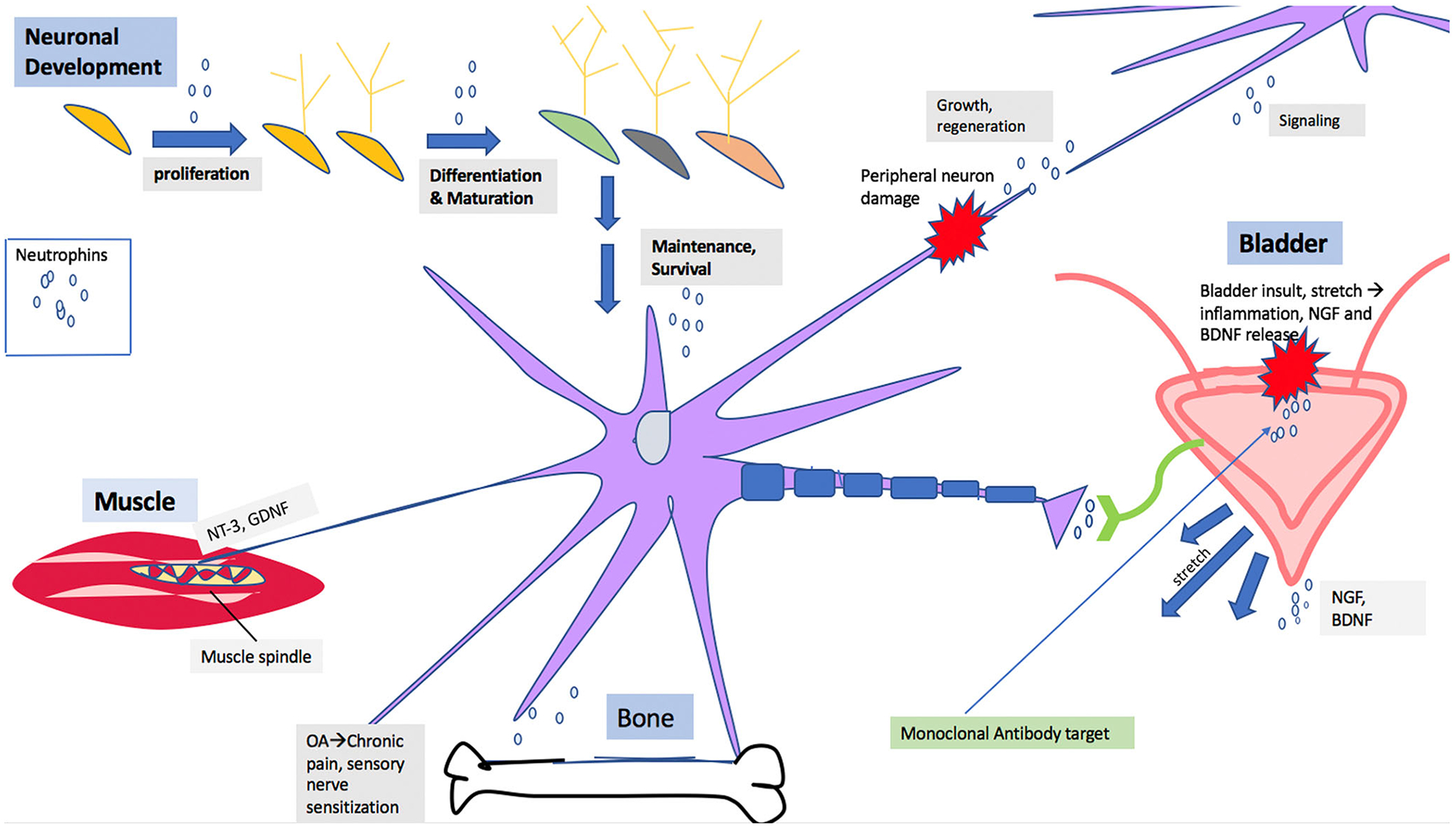
Schematic diagram showing role of neurotrophins in neuronal development and in disease pathology
Structural Information of Neurotrophins
Neurotrophins are synthesized with ~ 250-amino acid residue precursors and secreted in the form of non-covalently linked dimers, consisting of approximately 120-amino acid residues [27]. Mature forms of specific neurotrophins show extremely high sequence homology and remain conserved during evolution among vertebrates. Even different neurotrophins such as BDNF [28], NT-3, and NT-4 [29] have around 50% sequence homology to NGF. The crystal structure of NGF also exhibits a high similarity to other neurotrophins such as BDNF, NT-3, and NT-4, in which homologous regions in the hydrophobic core lie between three pairs of antiparallel β strands. This hydrophobic core is further stacked by intramolecular cysteine disul-fide bonds between these three strands [30]. This core is further attached with a flat surface, allowing two subunits to give a 2-fold axis symmetry. Further, it is in the regions of the three β hairpin loops (amino acids 29–35, 43–48, and 92–98; numbers refer to NGF), the reverse turns (amino acids 59–66), and the carboxyl and amino terminus that amino acid variations between the neurotrophins can be deciphered. As chimeric molecules contain portions of various neurotrophins, it is the basic residues within the variable regions that are critical for binding the appropriate tyrosine receptor kinase (Trks) [29, 31, 32]. Alternatively, it appears that a different, yet overlapping, set of basic residues is important for binding the P75 low-affinity neurotrophin receptor (p75NGFR) [33].
Receptors Binding Neurotrophins
Tyrosine receptor kinases (Trks), found in multiple forms ranging from a single active protein to large heteromeric complexes, appear to be better conserved than their neurotrophin ligands [34, 35]. Trks have a receptor family of glycosylated proteins of approximately 825-amino acid residues [29, 31, 32]. The name Trk derived from their identification as troponin/receptor kinase gene fusion identified in colon carcinoma; their normal (proto-oncogene) forms have now also been identified. Each Trk receptor consists of an extracellular ligand-binding domain, a single transmembrane domain to transduce signals, and an intracellular tyrosine kinase domain which leads intracellular signaling [34, 35]. These domains may be a part of a single protein or may be distributed among several interacting proteins. Interestingly, it has been shown that each receptor, when transfected into a cell line, can transduce the appropriate NT signal independently of other receptor proteins [36].
Initially, neurotrophins are synthesized in a pro-form, from which they then undergo proteolytic cleavage to become mature neurotrophins. Proteolytic cleavage can occur either inside the cell by plasmin and furin or outside the cell by matrix metalloproteinase. From here, NTs exhibit a strong affinity to bind with Trk receptors and a lesser affinity to the p75 neurotrophin receptor (p75NTR) [37]. Upon binding with neurotrophins, these receptors first dimerize at the cell surface, where the receptor tyrosine residues are next autophosphorylated. While the phosphorylation of tyrosine residues represents a small proportion of proteins phosphorylated in the cell, it is a critical part of neurotrophin-mediated signal transduction and downstream function. The phosphorylated tyrosine residues then recruit intracellular proteins with their ability to transduce signals. At physiologic NT concentrations, a specificity among the Trk receptors exists with binding constants for the high-affinity ligands in the range of 10–11 nM. In this way, neurotrophin receptors can be further classified. NGF binds with TrkA; BDNF and NT-4 bind with TrkB; and NT-3 binds with TrkC. However, there is some overlap due to some redundancy in the structure of these neurotrophin receptors; for example, NT-3 can also bind to TrkA and TrkB, although with lower affinity than to TrkC. In addition, truncated isoforms of TrkB [38] and TrkC [39] also exist. However, because these truncated forms lack cytoplasmic tyrosine kinase catalytic regions, they are unable to elicit the same response to neurotrophin binding. Like the intact receptors, these truncated isoforms are also expressed throughout the developing and adult central and peripheral nervous systems. As the full-length TrkB form is preferentially expressed in neurons, the kinase-deficient TrkB isoform appears to be preferentially expressed in non-neuronal cells [40]. Further, it is still not clear whether the non-catalytic forms of the receptors act as agonists or antagonists, as this may depend on the context in which they are found. They could serve as agonists by concentrating a neurotrophin after neuron injury [41], or, perhaps, an antagonist action also exists, as their presence might decrease the availability of a specific neurotrophin available to competing neurons during innervation. Further, all four neurotrophins can also bind to the low-affinity nerve growth factor receptor, p75NTR. The exact role of the p75NTR receptor has yet to be ascertained. One theory is that it may serve a role in increasing the specificity of the Trk receptor to the neurotrophic ligand. Another idea centers on its potential influences in the retrograde transport of bound neurotrophins [37]. Interestingly, in the study of mutated NT-4, NT binding to p75NTR was hindered, translating to a much less potent TrkB inducer of phosphorylation [32]. These findings then suggest that p75NTR may also a play crucial role in NT-4 signaling.
While Trks are the primary receptors for neurotrophins, the characteristics and various roles of p75NTR are also relevant. This receptor belongs to the tumor necrosis factor receptor superfamily (TNFR), and it contains extracellular cysteine-rich domains (CRDs) and an intracellular death domain. It is the action of intracellular adaptor proteins on the intracellular domain that promotes the enzymatic activity of p75 receptors. p75NTR has a unique ability to interface with both pro- and mature neurotrophin dimers. It is p75NTR signaling with pro-neurotrophins that has been thought to play diverse roles in mediating the fate of a neuron. Interestingly, one structural model was constructed showing an ability of p75NTR to fit into a tertiary complex with TrkA/NGF, although this has yet to be proven [42]. Thus, this receptor has been linked to a wide array of functions in the developing and injured neuron, including apoptosis, remodeling, and synaptic plasticity [43]. On the one hand, p75NTR has been shown to promote Trk-NT neuron survival signaling; however, other studies have found that this receptor has the ability to interact with sortilin (SORCS2) to trigger pro-neurotrophin cell death signals. Further, it has also been reported that this receptor could also interact with Nogo to promote myelin growth inhibitory effects [42, 43]. While sortilin is unable to contribute to the binding of p75NTR with NTs, it has been shown to regulate the rate of p75NTR cleavage, a critical step in the pathway of pro-neurotrophin cell death [43]. Indeed, the sortilin/p75NTR/ pro-neurotrophin complex has been implicated in the pathway of the pro-neurotrophin induction of cell death [44]. Further study would be valuable to reveal different binding changes induced by pro- and mature neurotrophins, as well as how they are relevant in influencing the fate of the life and death of neurons.
Neurotrophic Factor-Mediated Intracellular Signaling Pathways
Ras/Erk (Mapk) Cascade
The Ras/ERK pathway is regulated by the activity of proteins, namely, Ras. The small, membrane-associated protein Ras transduces signals from tyrosine kinase to the extracellular signal-regulated kinase (ERK) proteins, among other activities [45]. Ras is a G protein, as its activity depends on the type of guanine nucleotide that is bound to it. However, it is distinct from the heterotrimeric G proteins coupled to several neurotransmitter receptors. Ras becomes active when it binds to guanosine triphosphate (GTP), while the guanosine diphosphate (GDP) binding form remains inactive. Upon the activation of Trks, an adapter protein in the Shc family binds to these receptors. The Shc protein then becomes tyrosine phosphorylated and binds a Grb protein, one example being Grb2. While the Shc protein contains one PTB domain and an SH-2 domain, Grb2 contains two SH-2 domains and one SH-3 domain. Together, these proteins form a complex that activates a GDP–GTP exchange factor, namely, SOS, which subsequently activates Ras through GTP binding. Once activated, Ras recruits a serine kinase (Raf family) to the membrane, where it is then activated. A cascade is then initiated in which Raf activates MEK (from MAPK or ERK kinase). MEK then phosphorylates ERKs [46]. ERKs, also known as mitogen-activated protein kinases (MAPKs), are multifunctional kinases with various activities within the cell. Previously, ERKs have been shown to phosphorylate a diverse array of proteins such as tyrosine hydroxylase (TH), different transcription factors, protein translation regulators, proteins involved in microtubule formation, and many others [16, 17]. ERKs have also been shown to mediate neuron survival, elongation of neuritic processes [18], and levels of specific neuronal enzymes and ion channels [19]. Furthermore, ERKs have been identified as important in the hippocampal long-term potentiation (LTP) in brain slices [47]. While some effects of ERK activation are very rapid because of phosphorylation of membrane receptors, leading to increased activity, others are delayed and persistent because of the genomic changes. The ERK activation is also involved in sensitization of the transient receptor potential cation channel subfamily V member 1 (TRPV1) receptor of sensory neurons by NGF in the spinal cord, which underlies chronic pain [48, 49].
Phospholipase C, Gamma 1 (PLC-γ) Cascade
A second NT-signaling pathway involves phospholipase C, gamma 1 (PLC-γ) activation [50, 51]. As seen in the well-understood PLC-β, PLC-γ also cleaves the phospholipid 4, 5-bisphosphatidylinositol phosphates (PIP2) into diacylglycerol (DAG), and inositol 1,4,5-trisphosphate (IP3). DAG can then activate protein kinase C (PKC), whereas IP3 propagates the release of intracellular stores of calcium. Intracellular calcium can influence many pathways, including the activation of Ca2+/calmodulin-dependent protein kinases or the production of cyclic adenosine monophosphate (cAMP, cyclic AMP, or 3′,5′-cyclic adenosine monophosphate) through some adenylyl cyclases. Consequently, these mechanisms are known to have powerful effects on neurons. Unlike PLC-β, which is under the regulation of heterotrimeric G protein-coupled receptors, PLC-γ is regulated by tyrosine phosphorylation. PLC-γ contains two domains, namely, the SH-2 and SH-3 domains. Once bound to tyrosine phosphorylated receptors, it is recruited to the membrane, where it is phosphorylated, and this phosphorylation then activates PLC activity [52]. However, there is virtually nothing known about the role of PLC-γ in the intact brain; however, it has been proposed to likely exert important effects on neuronal function.
Interaction of Neurotrophin Signaling Cascades
The interactions of downstream signaling pathways of the NT receptors have been found to be somewhat complex. For instance, PI-3-K has been shown to contribute to ERK activation by both Ras-dependent and Ras-independent pathways [53–55]. Further, Ras has been shown to contribute to AKT activation, and both SHC and IRS-1 can bind to the same phosphorylated tyrosine site, but with differing affinities [56]. PLC-γ, however, can activate ERK in neurons and in theory can also terminate PI-3-K signaling by cleaving phosphotidylinositol-3′-phosphate [57]. In addition, many of these proteins exist in multiple different isoforms because of different genes or differential splicing of the same gene. While these isoforms are differentially expressed during development and in different brain regions, still there is a great deal of overlap. Further, the complexity of protein expression and cross talk in these signaling pathways and expression pose a tremendous opportunity for potential sites of drug discovery in brain and the periphery.
Role of Neurotrophins in the Development of Sensory and Motor Neurons in the Periphery
The neurotrophin hypothesis describes the event of neurotrophins located in the target tissue of a neuron binding to the cell membrane, being internalized and traveling in a retrograde fashion to the cell body where they can then affect metabolic function. Developmentally, there is evidence that neurotrophin levels are upregulated as fibers innervate the target peripheral tissue. Later, levels of neurotrophins have been found to decrease in the periphery as a means of controlling neuronal cell number and innervation density [58–60]. Studies on developing sensory ganglion cells have confirmed the influence of neurotrophins on the development of various populations of sensory neurons. Many investigators have found that small, peptide-containing neurons require neurotrophin NGF during development. Furthermore, the exposure of the adult animal or the fetus (via placental circulation) to anti-NGF has been found to prevent the survival of cells expressing TrkA [61]. NGF has been implicated in the growth and differentiation of sympathetic nerve development, and its withdrawal has been linked to pro-apoptotic pathways [62].
NT-3 has been shown to play a pivotal role in the development of excitatory synapses. It is through the binding of TrkC that a TrkC–presynaptic protein tyrosine phosphatase δ (PTPδ) complex can be formed, which in turn promotes synapse activity. Interestingly, NT-3 action has been found to be independent of receptor concentration when mediating presynaptic differentiation, yet postsynaptic differentiation is mediated by NT-3 in a manner dependent on concentration [63]. This role of NT-3 in enhancing PTPδ–TrkC binding was shown to promote the presynapse-inducing activity of TrkC in rat hippocampal neurons [64]. Further, the significance of this neurotrophin–TrkC interaction has been highlighted by studies involving genetically modified animals demonstrating the relationship between neurotrophins and the sensory fibers’ development. Mice lacking TrkC isoforms have been found to die early in postnatal development while expressing a phenotype suggestive of proprioceptive nerve involvement. Moreover, a loss of type Ia sensory fibers and abnormally myelinated axons within sensory tracts of the spinal column have been found in mice with tyrosine kinase lacking TrkC receptors [65]. Interestingly, when transgenic mice were studied with overexpression of the catalytic TrkC isoform in neuronal cells, an anxious behavioral phenotype was noticed, and increased excitatory postsynaptic potentials were recorded in the hippocampal region. The distribution of TrkA and TrkC receptors on sensory neurons supports the neurotrophic action of NGF and NT-3, respectively. Therefore, these findings suggest a fine regulation of the neurotrophin-Trk on neuronal development, with evidence of both low and high activity translating to aberrant neuronal development [65].
Neutralization of NGF by Antibody and Antisense
Neurotrophins have also been implicated as a potential target of pain therapy, as NTs such as NGF are now recognized as key mediators of pain signaling and tissue injury [66]. In this section, we are discussing multiple studies that have recognized neurotrophin expression and potential treatments applied in the setting of bladder disorders. Therefore, drugs such as tanezumab, a monoclonal antibody to NGF, have been studied and found to confer a reduction in pain in chronic conditions such as osteoarthritis, interstitial cystitis, and chronic lower back pain (CLBP) [67]. In a study by Belanger et al., the effect of tanezumab on the sympathetic nervous system (SNS) of primates given supratherapeutic doses was observed. Of note, they found that administration of this drug for a time period of up to 6 months did not confer any adverse morphologic or functional alteration in the SNS; moreover, they also found that it was not associated with neuronal cell death in adult primates [68].
Successful outcomes in pain reduction of animal models and in patients with chronic pain in osteoarthritis following sequestration of NGF with monoclonal antibody (tanezumab) further support the critical role of NGF in the maintenance of sensory neurons and their sensitization. Autonomic dysreflexia (AD) following spinal cord injury is characterized by NGF overexpression in bladder wall and the activation of C-fiber afferent nerves by bladder distension. NGF overexpression in bladder of patients suffering from AD secondary to spinal cord injury is presumed to sensitize the C-fiber bladder afferent pathways via upregulation of TRPV1 and TRPA1 expression, and sensitized bladder afferents are known to ex-aggerate the arterial blood pressure responses to bladder distention in AD. Yoshizawa et al. reported that NGF overexpression accompanied an increase in TRP channels’ (capsaicin receptors, TRPV1 and TRPA1) mRNA levels in the bladder afferent neurons when measured against rats with intact spinal cords. Increased expression of NGF and TRP channel [69] was linked to significantly increased blood pressure in AD via bladder-vascular reflex. Kadewaka et al. found that intravesical application of NGF antisense complexed with cationic liposomes is a promising treatment for AD [70]. Further, delivery and complexation methods for NGF antisense have been investigated. Kashyap et al. found that NGF antisense oligonucleotides complexed with cationic peptide protamine instead of liposomes reduced the constitutive expression of NGF, as well as its receptor p75 (NTR) in the bladder [71]. It is known that acute exposure of bladder to noxious stimuli induces NGF overexpression, and Majima et al. looked at the effectiveness of liposomal suppression of NGF in the setting of bladder cystitis. They too used an NGF antisense oligonucleotide (OND) complexed to cationic liposomes and evaluated pain behavior in a rat cystitis model. In cystitis rats, intravesical application of liposome-OND improved bladder hypersensitivity [72].
Kashyap et al. also supported these findings by reporting the suppression of NGF overexpression evoked by acute acetic acid exposure. Sequence-specific downregulation of the NGF expression in bladder reduced the inflammation and reduced the frequency of reflex voiding in rodents [66]. Acute exposure to acetic acid is known to cause NGF overexpression, which is linked to overexpression of soluble inter-cellular adhesion molecule-1 (sICAM-1), sE-selectin, chemokine (C–X–C motif) ligand 10 (CXCL-10) and CXCL-1, leptin, monocyte chemoattractant protein-1 (MCP-1), and vascular endothelial growth factor (VEGF) in rat bladder. NGF-antisense treatment downregulated the expression of NGF and other molecules in rat bladder after acute exposure to acetic acid [66]. Furthermore, elevated NGF expression has been associated with differential expression of miRNA, specifically the upregulation of miR-132 and downregulation of miR-221 in the bladder of rats with acetic acid-induced overactivity [73]. MicroRNAs (miRs) represent a new level of epigenetic posttranscriptional control of gene expression and are now considered molecular determinants of clinical outcomes. A clinical study found a differential expression of bladder miR-221 in patients with overactive bladder (OAB) following intradetrusor injection of onabotulinum toxin A [74]. In patients who responded positively to this therapy with low postresidual volumes (PVR), the expression of miR-221 and miR-125b was found to be upregulated by 11- and 2-fold, respectively. Moreover, in patients with high PVR following treatment, a reduced miR-221 expression was implicated in regulating the differentiating action of NGF based on biochemical studies [74].
Urine levels of NGF were found to be elevated in patients with voiding symptoms, implying that elevation in urine is caused by overexpression in the bladder. Analysis of urine specimens of 140 overactive bladder patients (OAB), ranging from 25 to 90 years old, justified the proposed age-related association with a low-grade chronic inflammatory processes in OAB [75]. While urinary NGF is increased in interstitial cystitis, it is also elevated in patients with OAB and detrusor overactivity DO. A recent study proposed use of urinary NGF levels for monitoring the therapeutic response and disease progression [76].
Brain-Derived Neurotrophic Factor
BDNF and its corresponding receptor, TrkB, have been found to be widely expressed in the developing and adult nervous system. Interestingly, BDNF–TrkB signaling has been linked to an increase in BDNF mRNA expression, suggesting the ability of this BDNF to upregulate pro-BDNF release [37]. Mice lacking TrkB receptors [77] have sensory deficits that depict impaired function in large-diameter cutaneous afferents. Mice lacking neurotrophin BDNF were found to be ataxic, and this reflects a loss of dorsal root ganglions (DRGs), as well as myelinated axons [78]. Although muscle spindles were normal in these knockout animals, the major deficit was in the mechanoreceptors that are in skin of these animals. Furthermore, DRGs show similar losses in cell number in both knockout mice; however, loss in trigeminal ganglia was greater in TrkB knockout than the BDNF one. Therefore, it can be concluded that the TrkB receptor could also bind with ligands other than BDNF, supported by the significant depletion of motorneurons in TrkB knockouts compared to an unchanged number of these motor neurons in BDNF knockouts.
In the mice with lost Bdnf gene, 80% have a reduction in vestibular ganglion neurons innervating the inner ear organs and responsible for balance and movement. Furthermore, a 25–44% reduction in neurons of the trigeminal that connects the hindbrain to whisker pads and a 55–65% reduction have been identified in neurons of the nodose and petrosal ganglia (relaying information from the heart, major vessels, lung, and gut to the brain stem). Moreover, a significant reduction in mechanoreceptor neurons of the dorsal root ganglion was also noticed in these mice [78–80]. Mice with disrupted TrkB gene have disruption in sensory neurons [77]. These findings are in accordance with the understanding of BDNF’s ability to help cell growth of trigeminal neurons derived from embryos in early stages of innervation [81]. Moreover, BDNF is also important for the differentiation process and supports the differentiation of cortical and hippocampal GABAergic interneurons. Although the number of GABAergic neurons and neural gamma-aminobutyric acid (GABA) was normal in BDNF-lacking mice, the neuronal markers of GABAergic cells, namely, parvalbumin, calbindin, and neuropeptide Y, were decreased. In this way, it is reasonable to consider the compromised synaptic inhibitory activity of these neurons because of downregulated levels of calcium-binding proteins such as parvalbumin and calbindin. Moreover, reduced expression of calbindin was also observed in the medium-sized striatal spiny neurons and the striatal projection to the substantia nigra. Interestingly, reduced expression of neuropeptide Y was noticed only in mutant mice with the specific age of 15–20 days, prompting the question of whether the lack of BDNF prevented altogether, rather than delayed, normal differentiation of a subset of cortical and hippocampal GABAergic neurons. In contrast, BDNF infusion increases the expression of neuropeptide Y and other neuropeptides in hippocampal and cortical neurons. Therefore, reduced neuropeptide Y expression in BDNF knockout mice is corroborated by the increased neuropeptide Yexpression in the setting of BDNF infused into the hippocampus, cortex, and striatum of normal adult rats [82, 83]. Similarly, rats overexpressing BDNF gene in the bladder wall have shown enhanced calcium signaling due to BDNF-mediated enhanced expression and activation of the L-type calcium channel [40].
The role of BDNF in vivo as it influences the development of motor neurons is less clear. BDNF has been shown to support motor neuron survival in lesioned animals [84–86], as well as prevent naturally occurring death and also increase levels of neuronal markers in cultured embryonic motor neurons [87]. In a study of TrkB knockout mice, a role for BDNF in motor neuron development has also been proposed. In the TrkB knockout mice, a reduced number of lumbar spinal and facial nuclear motor neurons has been recorded [77]. Yet, interesting findings were discovered in the motor neurons of BDNF knockout mice as well as mice missing both BDNF and NT-4. Both of which are high-affinity ligands for the TrkB receptor [78]. Surprisingly, in these mice, motor neuron development was found normal. However, since NT-3 can also activate TrkB, it is possible for some redundancy to exist that allows for sufficient motor neuron development. Thus, while BDNF may participate in motor neuron development, it seems that an absence of this NT can be compensated by other neurotrophins.
Importantly, the differentiation of cholinergic neurons, which are important in cognitive functions and involved in Alzheimer’s disease, are influenced by exogenous BDNF. BDNF administration has been shown to protect cholinergic neurons of the basal forebrain against lesion-induced death, influence the neurotransmitter production, and increase the expression of cholinergic markers in vivo and in vitro [87, 88]. It is surprising that deficiencies in cholinergic neurons have yet to be reported for the BDNF knockout, BDNF/NT-4 double knockout, and TrkB knockout mice; however, BDNF overexpression has been shown to induce the expression of choline acetyltransferase at cholinergic nerve terminals innervating the bladder [8, 88]. Furthermore, the enhanced levels of choline acetyltransferase was functionally active as proven by higher contraction amplitude in bladder strips overexpressing BDNF relative to bladder strip overexpression luciferase at a frequency of 8 Hz and above (known to stimulate parasympathetic nerves) in electric field stimulation (EFS) studies using organ bath [88]. BDNF also affects differentiation of dopaminergic neurons. In addition, there have also been no gross deficits in the dopaminergic neurons of the midbrain reported in these mice. For these reasons, a more detailed analysis of these mutant mice is needed to clarify the role of BDNF in the differentiation of these sets of neurons.
BDNF has been associated with the survival of in vitro cultured neuronal cells, namely, rat retinal ganglion cells, cerebellar granule neurons, and neurons of the locus coeruleus when derived from embryonic and adult animals at the appropriate developmental stage. BDNF also interacts with other neurotrophins [89–91], such as trigeminal sensory neurons [92] and cerebellar neurons [91], which appear to switch their dependence from BDNF to NGF and NT-3, respectively. Interestingly, the adult rat hippocampal and cerebral cortical neurons have been shown to express TrkB and TrkC, the receptor for NT-3, indicating that these neurons can respond to both BDNF and NT-3 [94]. BDNF could also act in an autocrine manner. BDNF and TrkB receptors are co-expressed in many hippocampal and cortical neurons [91–94]. Antisense oligonucleotides to BDNF can inhibit survival of cultured neurons from the dorsal root ganglion [95, 96], while overexpression of BDNF in rat bladder was associated with increased retrograde transport of BDNF and NGF in L6 and S1 dorsal root ganglion and hence induced afferent sensitization as shown by increased voiding frequency in rats overexpressing the BDNF gene in their bladder [88]. Further, BDNF expression can be modulated by activity of the GABAergic and cholinergic systems [88, 96, 97], and BDNF in turn can influence neuronal synaptic activity. Thus, the reciprocal influence shared between BDNF and neuronal activity, in addition to the stabilizing effect of BDNF on interneuronal synaptic connections, suggests a complex inter-play that may influence both synaptic plasticity and anatomical reorganization in the mature nervous system [98].
Neurotrophin-3
NT-3 and its receptor, TrkC [99], are also widely present in the developing and adult nervous system [21]. Mice with NT-3 disruption [21, 100, 101] have severe loss of proprioceptive neurons, which is an important finding, as these nerves are responsible for relaying information regarding limb position. Proprioceptive 1a afferents are an integral part of the muscle-motor neuron circuit that mediates the stretch reflex, also known as the muscle spindle. They are key in relaying information from the peripheral muscle spindles to motor neurons in the spinal cord and ascending projections of spinocerebellar neurons. Therefore, the greatly decreased or missing population of 1a afferents and muscle spindles is observed in mice lacking NT-3 and in the NT-3 receptor (TrkC) [99]. Of the mice that were heterozygous for disrupted NT-3, muscle spindles were present at 50% the normal number, which is indicative of a direct relationship between NT-3 availability with 1a afferents’ survival. The observed loss of proprioceptive neurons in the NT-3-disrupted mice occurs in parallel with the previous findings that NT-3 is expressed in the target tissues, muscles, and motor neurons. Further, it has been found that proprioceptive neurons expressing TrkC and NT-3 support the viability of in vitro cultured proprioceptive neurons derived from the trigeminal mesencephalic nucleus [102]. In addition, proprioceptive Golgi tendon organs were also found to be missing, suggesting that 1b afferents synapsing with these structures would also be absent. Furthermore, the motor neurons of the ventral roots were reduced by approximately 30%, in contrast with motor neurons of the spinal cord, which were normal.
NT-3-lacking mice have a decreased population of sympathetic neurons present in the superior cervical ganglion [21] without any change in peripheral sympathetic sensory innervation, which was observed in the NGF and TrkA knockout mice [103, 104]. Sympathetic neurons depend on NGF for survival during the innervation of targets, so mice lacking NT-3 could reflect all of the NT-3-dependent events that occur prior to target innervation. Furthermore, NT-3 also promotes in vitro survival of sympathetic neuroblasts, which later become sympathetic precursors [13]. Thus, a reduced population of sympathetic neurons in mice lacking the NT-3 gene reflects that NT-3 has earlier effects on sympathetic phenotype. The effect of NT-3 on the neuronal population of the nodose ganglia is much more clear as the reduction of neurons was partial (47%) in the NT-3-lacking mice, and the NT-3 antibody also reduced neurons’ population in the nodose ganglion in the developing quail embryo [21]. This decrease in nodose neuronal population indicates decreased neuronal interconnection to the nodose ganglion rather than protection against naturally occurring death. This was theorized as the timing of NT-3 neutralization is very important and preceded the stage of neuronal death [105]. An involvement of NT-3 in early development is also suggested as it was highly expressed in other areas of the developing embryo before natural cell death. NT-3 can induce the differentiation of cultured enteric multipotent neural crest cells into neurons or glia [14], and work as a mitogen for neural crest cells cultured in the presence of somites and also those cultured with oligodendrocyte precursor cells [106]. Further, NT-3 could help in the differentiation of neural tube progenitors to motor neurons expressing the BEN/SC1 epitope [15] and could carry signals from the neural tube for conversion of the epithelial dermatome progenitors to mesenchyme cells of the dermis [107]. In-depth studies of the NT-3 knockout mice could clear its role in early embryogenesis.
NT-3 enhances the survival of in vitro cultured embryonic noradrenergic neurons of the locus coeruleus, as well as in vivo survival of noradrenergic neurons of the locus coeruleus after the exposure of 6-hydroxydopamine. Reports are also there showing that NT-3 mediates survival and differentiation of cultured dopaminergic and cholinergic neurons derived from the developing substantia nigra [108], induces the cholinergic phenotype in cultured rat motor neurons and protection of Purkinje cells [109], and stimulates neurite outgrowth in cultured hippocampal pyramidal neurons [110]. NT-3 enhances GABA release in cultured developing hypothalamic neurons of rat during the developmental period when GABA is depolarizing and calcium elevating, but not later when GABA is inhibitory, suggesting that one mechanism through which NT-3 may influence neuronal development is via presynaptic potentiation of GABA excitation [111].
Neurotrophin-4
NT-4 (also called NT-4/5 or NT-5) mRNA is present in major regions of the brain, as well as several other tissues. Unlike all other neurotrophins or their receptor knockout mice, the mice lacking NT-4 are found to thrive and exhibit no overt phenotypic abnormalities, and they are able to reproduce [22]. However, mice lacking NT-4 have around 57% loss in the sensory population of nodose–petrosal and a 50% loss in the geniculate ganglion’s population. As mentioned previously, BDNF and NT-4 share the same TrkB receptor. Therefore, mice lacking BDNF have around 61% reduction of nodose- petrosal neurons and a 48% reduction in geniculate population [22, 23]. However, double-knockout mice lacking BDNF and NT-4 genes reflect that this reduction of nodose–petrosal neurons was in different subsets of the neuronal population, as double knockout mice have around 94% reduction in number of neurons of nodose–petrosal ganglion [112]. On the other hand, trigeminal sensory ganglia, which showed neuronal loss in the BDNF knockout mouse, remained unaffected in the mouse lacking NT-4. The difference in the lost population in the NT-4/BDNF double knockout (79–94%) to the 47% loss of nodose neurons in the single NT-3 or BDNF knockout mouse [21] showed that some populations of nodose neurons have a dichotomous nature, which is affected by NT-3 and either NTF-4 or BDNF. It was previously discussed in the BDNF section that disruption of NT-4 alone or NT-4 and BDNF together did not affect the same number of motor neurons, the absence of this phenomenon was surprising, as the survival and differentiation promoting the activity of NT-4 on cultured motor neurons and axotomized motor neurons was predicted to be a result of decreased motor neuron population [59, 86].
As both NT-4 and BDNF share a similar TrkB receptor, it is not surprising that, in most cases, the addition of exogenous NT-4 to culture neurons or in vivo administration of NT-4 has similar effects as administration of BDNF. NT-4 induces differentiation of cultured spinal [113] and basal forebrain cholinergic neurons, hippocampal neurons, and cerebellar granule cells [114, 115], in addition to survival of in vitro embryonic culture of dopaminergic neurons from mesencephalon and noradrenergic neurons from the locus coeruleus [116]. Further, the differentiation of dopaminergic, GABAergic, and serotonergic neurons in and near the substantia nigra can also be activated by administration of NT-4 in vivo [117]. NT-4 also appears to transiently support the survival of trigeminal and jugular neurons during embryonic development by exerting these effects in a parallel manner as seen in BDNF.
Neurotrophins in the CNS
Of the neurotrophin family members, NGF has the most restricted specificity in CNS and targets specifically cholinergic neurons in the brain region. However, in periphery and other regions of the nervous system, it acts on sympathetic and sensory nerves. Both BDNF and NT-3 are expressed in cortical and neocortical structures, while NT-4 is widely expressed in adult mammals compared to other NTs [118, 119], but its levels remain low compared to other NTs. Interestingly, and perhaps not surprisingly, studies have clearly depicted that after development, these factors are involved in the ongoing remodeling of neuronal functionality underlying the plasticity of neurons [2, 33]. These findings lead to the discussion of a major limitation in the treatment of CNS diseases, which is the loss of regenerative capacity of the brain at an early age. Improper development of nerve circuits is devastating in adults, which makes restorative therapy more difficult. This was previously seen in a broad range of neurologic disorders, such as Parkinson’s or Alzheimer’s disease, stroke, and other inflammatory diseases, including multiple sclerosis [120]. In addition, disorders such as myoclonus or epilepsy reflect in-appropriate neural connections or the lack of the proper feed-back loop of neuronal functionality. Moreover, in some psychiatric diseases, similar deficits or the lack of proper functionality is seen. Chemical and anatomic abnormalities in the nervous system have been documented in affective diseases, such as anxiety disorders and schizophrenia [121]. Therefore, methods that stimulate the regeneration potential of the brain, as in during development, are likely to show great potential in reversing the anomalous functions to normal functions in these neurologic disorders or psychiatric diseases [122].
In some cases, specific neurotrophins and other factors have shown their role in these anomalous changes, including that of hippocampal plasticity and long-term potentiation [123–125] to the acquisition of new songs by songbirds [126–128]. Nowadays, neuronal models are explained by the alteration of neurotrophic factor signaling to prove synaptic alterations or strengthening. In contrast to the original models, which describe retrograde neurotrophin signaling from a target organ, these signaling events also work in an anterograde or autocrine manner. Moreover, studies have shown a change in the rapid expression of these neurotrophic factors in adults, which proves their active involvement with other environment factors [129, 130].
A new finding regards the ability of DRG neurons to express mRNA for neurotrophins and corresponding Trk receptors [131]. Therefore, there is potential for an autocrine or paracrine mechanism. In this way, the release of a neurotrophin from the soma or dendrites is then able to activate receptors in the same or nearby cells. This idea significantly differs from the neurotrophic hypothesis, which is discussed above. Furthermore, the idea of axonal release also suggests other targets for neurotrophins, such as Schwann cells, other spinal cord neurons (with the ability to propagate axo–axonal synapses on sensory terminals), and peripheral tissues innervated by peripheral axons [132, 133]. Thus, it is reasonable to indicate the roles of neurotrophins to extend well beyond the mechanism implied by the neurotrophic hypothesis. As noted previously, motor neurons are also highly selective in their expression of neurotrophins and their corresponding receptors. It has been found that, in the developmental period of the nerve, BDNF is transported to a much greater extent than NT-3 or NGF in both neonates and in adults [77, 82]. However, there has been the observation of some NT-3 found in the ventral horn after muscle injection, although this was not in association with large motor neurons. One possibility is for NT-3 to be transported by spindle afferents, which have TrkC receptors, to their terminals in the ventral horn. As presumed from the transport result, motor neurons express the receptor for BDNF (TrkB) [29]. Motor neurons also express the mRNA for NT-3 [134], and it is not unreasonable to suggest a paracrine action of NT-3 on spindle afferents, as they project monosynaptically to motor neurons and are known to express Trk C receptors. The functional importance of BDNF to motor neurons during development has been demonstrated by its ability to protect axotomized motor neurons from cell death when applied to these specific axons in neonates [135]. This result may parallel the observed effects of NGF in rescuing sympathetic neurons from the effects of axotomy [136]. Still, it is not known whether this protection of axotomized motor neurons further extends to reversing desynapsis [137], as was found in the case of NGF and axotomized sympathetic neurons. However, the role of BDNF in motor development is not an exclusive one as it has already been shown in BDNF-deficient mice [138]. This suggests the possibility of TrkB receptors’ interaction and activation on motor neurons by a different neurotrophin [139], and in fact, it was proven recently that NT-4/5 could also interact with TrkB receptors [139, 140]. In accordance with the presumed role of TrkB in motor neuron development, mice lacking the TrkB gene suffer from significant loss of motor neurons. Therefore, a continued area of study could further characterize the role of an activated TrkB receptor in modulating motor neuron survival into adulthood.
Multiple Roles of Neurotrophins
Studies involving NGF indicate a much clearer role of this NT and its ability to persist after the early developmental processes, as well as to alter the nociceptive properties of nerves. BDNF overexpression in rat bladder by transfection of the BDNF transgene enhanced the micturition frequency in bladder and enhanced the retrograde transport of BDNF to DRG. These and other studies indicate that BDNF also has effects on sensory afferents to implicate BDNF’s role in afferent sensitization [141–144]. NGF is well known to affect afferent sensitization [145, 146]. It is also important to understand that nociceptive afferents have completely different physiological and pharmacological properties, both peripherally and centrally (i.e., sensitization), than low-threshold afferents used as targets of the other neurotrophins. Another function that has been demonstrated by neurotrophins in adults is the ability to restore function to damage neurons [31, 89] and also increase the function of neighboring normal neurons [147]. In support of these ideas, it is reported that peripheral nerve damage leads to a rapid increase in the expression of NGF mRNA Schwann cells, as well as in damaged tissue followed by inflammatory cell invasion [66, 140, 148]. In another study, a larger, yet slower, increase in BDNF mRNA limited to Schwann cells of the distal stump has also been reported [149, 150]. Because of what we know thus far, maximum efforts have been made to investigate whether neurotrophins can stimulate the growth or prevent the degeneration at CNS those that are damaged in neurodegenerative disorders such as Alzheimer’s and Parkinson’s [151]. Now, people are looking at other roles of neurotrophins, at peripheral and other tissue systems. The NGF’s role gives a new idea of relevance to NT molecules originally viewed as factors, as important in development. These molecules show potential use as agents to reverse neurodegenerative processes, as well as broader influences on neural function. Thus, the neurotrophins occupy a wide influence over both the central and peripheral nervous system, with activity throughout the span of a life cycle.
On the other hand, with neurotrophins such as NGF showing high expression relating to pain in inflammatory conditions, drugs that can block these NTs are currently a focus in studies today. Alternatively, the regenerative and antiapoptotic roles of NTs and neuronal dysfunction in the bladder have been studied in the setting of other chronic diseases, such as diabetes mellitus. In one study looking at streptozotocin (STZ)-induced diabetic rats, a progressive increase in the bladder weight, urine volume produced, residual urine, and intercontraction intervals was found [152]. Consequently, a hyposensitive, underactive bladder was induced by DM in these rats, which further resulted in signs of inflammation with increased urine NGF levels, prostaglandin receptor EP1 and EP3, yet a decreased bladder urothelial NGF and prostaglandin E2 (PGE2) expression. Therefore, with this inverse relationship between NGF in the urine and the bladder, it has been proposed that there might be some destruction of NGF receptors, resulting in increased NGF loss in the urine [152]. Therefore, in this situation, a possible blunting of the regenerative and antiapoptotic characteristics of this NT by loss in the urine could be one promising area of study in diabetes. Perhaps, because low levels have been recorded in diabetes, there is some role for gene therapy to restore NGF levels in these patients suffering from diminished bladder function [152]. There is much evidence to date indicating the powerful biological effects of NGF in the setting of neuronal disease, and so, there is a strong incentive to analyze further their functional actions and to develop techniques with localized delivery to modulate their action.
Other Classes of Neurotrophic Factors and Their Signaling Systems
Neurotrophic factors form other families of protein such as the glial cell line-derived neurotrophic factor (GDNF), which have been found to have profound effects on both dopaminergic and motor neurons, as well as enteric neurons [153–155]. For example, in the setting of disc degeneration, NGF and GDNF have been shown to instigate symptoms such as pain, bowel urgency, and paraesthesia. However, artemin, a member of the GDNF family, has been shown to transduce signals through the GFR-α receptor in sensory afferent nerves, proposing a potential use for it in the repair of sensory afferent nerves. In a recent study, artemin injection was linked to the re-establishment of spinal connections and nerve regeneration. However, although proof of pain reduction in humans who received artemin in phase II trials is still to be established, its safe and tolerable nature further promotes it as a potential analgesic therapy [67, 156]. In addition to artemin, the GDNF family also includes persephin and neurturin. This family is closer to the transforming growth factor-β (TGF-β) family, and it signals through a heteromeric signaling complex [157]. Further, a common component of GDNF receptor complexes is the Ret protein. This protein spans the membrane and expresses an intracellular tyrosine kinase (TK) domain. While this domain is less understood compared to the Trk receptor, it has been shown to activate some of the same intracellular signaling pathways as Trk receptors, including the Ras/ERK pathway and PI-3-K [158]. Ret interacts with several receptor-binding proteins, GFR-α subunits 1–4, which do not span the membrane, but have interactions with the extracellular surface through a glycosylphosphatidylinositol moiety. The α subunit of GFR is responsible for ligand specificity and participates in the activation of the Ret. Furthermore, these subunits can also prompt the activation of src-like tyrosine kinases, which is independent of Ret. Because of the known neuroprotective activities of the GDNF family of proteins, an interest has developed in understanding their role in the pathogenesis and potential mechanisms of treatment in diseases such as addiction, Parkinson’s disease, and amyotrophic lateral sclerosis [154, 155]. Another large class of receptors that couple to intracellular tyrosine kinases comprises the Janus kinases (JAKs) [159, 160]. The JAKs consist of extracellular binding components, one of which can span the membrane, yet is unable to exert intrinsic activity. Instead, these components employ an effect by activating other specific JAK family members. The JAKs could then activate several other intracellular effector molecules such as IRS proteins, SHC, and others. Moreover, they are well known to interact with another group of proteins called STATs, which work as signal transducers and activators of transcription proteins. STAT proteins bind to JAK, and upon phosphorylation of tyrosine residues, they are then released and translocate to the nucleus. Here, they exert a direct effect as DNA-binding transcriptional activators. Importantly, many other neurotrophic factors could also activate the JAK/STAT pathway, including ciliary neurotrophic factor (CNTF), growth hormone, leptin, and many cytokines [161]. Still, while it is currently an area of intense study, there is much to be learned regarding the role of STAT-mediated transcription, especially in the brain.
Conclusion
The action of neurotrophins on the Trk family of receptors plays an important role in regulating the survival and growth of neurons in both the central and peripheral nervous system (Fig. 2). Drugs acting on these receptors are currently being tested in the clinic to halt neurodegenerative diseases. Overexpression of NGF in peripheral organs is being targeted by monoclonal antibodies to reduce pain and afferent sensitization. Future research is warranted to advance the understanding of neurotrophins in physiology and pharmacology.
Fig. 2.
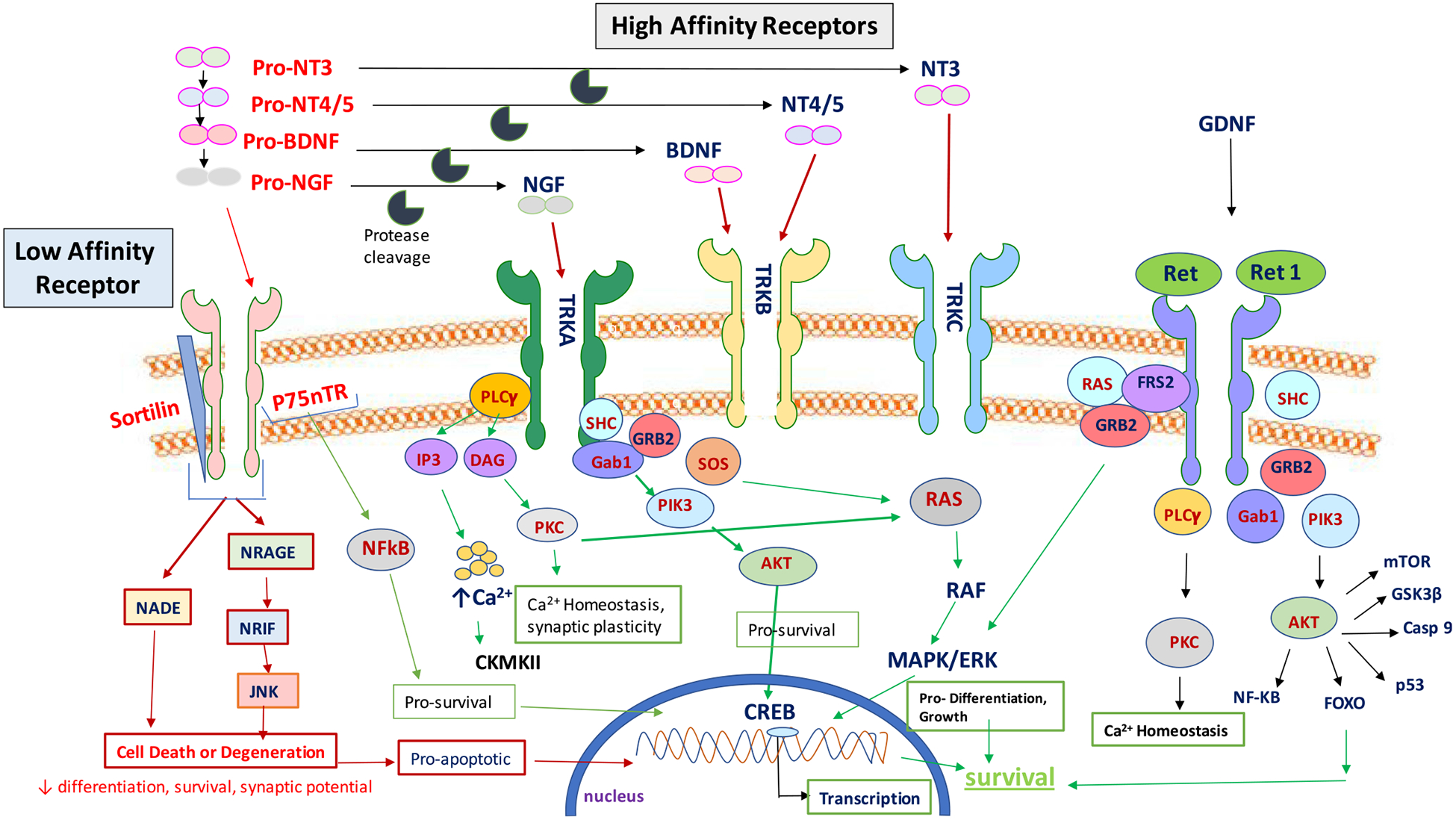
Schematic illustration of neurotrophin signaling. (NT = neurotrophins, BDNF = brain-derived neurotrophic factor, TRK = tyrosine receptor kinase, GDNF = glial-derived neurotrophic factor, PLC = phospholipase C, RAS = Ras proteins, GRB = growth factor receptor-bound protein 2, PIK3 = phosphatidylinositol-4,5-bisphosphate 3-kinase, AKT = AKT serine/threonine–protein kinases, FOXO = The forkhead box O transcription factor, PKC = protein kinase C, CREB = the cAMP-responsive element-binding protein)
Acknowledgments
This work was supported by National Institutes of Health grants DK088836, and authors also acknowledge the Research Scholar Award from Allergan and the Urology Care Foundation to Mahendra Pratap Kashyap.
Footnotes
Compliance with Ethical Standards
Conflicts of Interest The authors declare that they have no conflicts of interest.
References
- 1.Levi-Montalcini R (1987) The nerve growth factor 35 years later. Science 237(4819):1154–1162. 10.1126/science.3306916 [DOI] [PubMed] [Google Scholar]
- 2.Fang H, Liu C, Yang M, Li H, Zhang F, Zhang W, Zhang J (2017) Neurotrophic factor and Trk signaling mechanisms underlying the promotion of motor recovery after acute spinal cord injury in rats. Exp Ther Med 14(1):652–656. 10.3892/etm.2017.4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowianski P, Lietzau G, Czuba E, Waskow M, Steliga A, Morys J (2017) BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. 10.1007/s10571-017-0510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keefe KM, Sheikh IS, Smith GM (2017) Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci 18(3). 10.3390/ijms18030548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito S, Menard M, Atkinson T, Brown L, Whitfield J, Chakravarthy B (2016) Relative expression of the p75 neurotrophin receptor, tyrosine receptor kinase A, and insulin receptor in SH-SY5Y neuroblastoma cells and hippocampi from Alzheimer’s disease patients. Neurochem Int 101:22–29. 10.1016/j.neuint.2016.09.015 [DOI] [PubMed] [Google Scholar]
- 6.Thoenen H (1991) The changing scene of neurotrophic factors. Trends Neurosci 14(5):165–170. 10.1016/0166-2236(91)90097-E [DOI] [PubMed] [Google Scholar]
- 7.Barde YA (1990) The nerve growth factor family. Prog Growth Factor Res 2(4):237–248. 10.1016/0955-2235(90)90021-B [DOI] [PubMed] [Google Scholar]
- 8.Kashyap M, Pore S, de Groat WC, Chermansky C, Yoshimura N, Tyagi P (2015) BDNF overexpression alters the phenotype of cholinergic neurons in rat bladder. J Urol 193(4):e1108 10.1016/j.juro.2015.02.1819 [DOI] [Google Scholar]
- 9.Buchman VL, Davies AM (1993) Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development 118(3): 989–1001 [DOI] [PubMed] [Google Scholar]
- 10.Davies AM (1994) The role of neurotrophins during successive stages of sensory neuron development. Prog Growth Factor Res 5(3):263–289. 10.1016/0955-2235(94)90010-8 [DOI] [PubMed] [Google Scholar]
- 11.Davies AM (1994) The role of neurotrophins in the developing nervous system. J Neurobiol 25(11):1334–1348. 10.1002/neu.480251103 [DOI] [PubMed] [Google Scholar]
- 12.Kalcheim C, Carmeli C, Rosenthal A (1992) Neurotrophin 3 is a mitogen for cultured neural crest cells. Proc Natl Acad Sci U S A 89(5):1661–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiCicco-Bloom E, Friedman WJ, Black IB (1993) NT-3 stimulates sympathetic neuroblast proliferation by promoting precursor survival. Neuron 11(6):1101–1111. 10.1016/0896-6273(93)90223-E [DOI] [PubMed] [Google Scholar]
- 14.Chalazonitis A, Rothman TP, Chen J, Lamballe F, Barbacid M, Gershon MD (1994) Neurotrophin-3 induces neural crest-derived cells from fetal rat gut to develop in vitro as neurons or glia. J Neurosci 14(11 Pt 1):6571–6584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Averbuch-Heller L, Pruginin M, Kahane N, Tsoulfas P, Parada L, Rosenthal A, Kalcheim C (1994) Neurotrophin 3 stimulates the differentiation of motoneurons from avian neural tube progenitor cells. Proc Natl Acad Sci U S A 91(8):3247–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis RJ (1993) The mitogen-activated protein kinase signal transduction pathway. J Biol Chem 268(20):14553–14556 [PubMed] [Google Scholar]
- 17.Cobb MH (1999) MAP kinase pathways. Prog Biophys Mol Biol 71(3–4):479–500. 10.1016/S0079-6107(98)00056-X [DOI] [PubMed] [Google Scholar]
- 18.Fukuda M, Gotoh Y, Tachibana T, Dell K, Hattori S, Yoneda Y, Nishida E (1995) Induction of neurite outgrowth by MAP kinase in PC12 cells. Oncogene 11(2):239–244 [PubMed] [Google Scholar]
- 19.Grewal SS, York RD, Stork PJ (1999) Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol 9(5):544–553. 10.1016/S0959-4388(99)00010-0 [DOI] [PubMed] [Google Scholar]
- 20.Ernfors P, Lee KF, Kucera J, Jaenisch R (1994) Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell 77(4):503–512. 10.1016/0092-8674(94)90213-5 [DOI] [PubMed] [Google Scholar]
- 21.Tessarollo L, Vogel KS, Palko ME, Reid SW, Parada LF (1994) Targeted mutation in the neurotrophin-3 gene results in loss of muscle sensory neurons. Proc Natl Acad Sci U S A 91(25): 11844–11848. 10.1073/pnas.91.25.11844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M et al. (1995) Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature 375(6528):235–238. 10.1038/375235a0 [DOI] [PubMed] [Google Scholar]
- 23.Segal RA, Takahashi H, McKay RD (1992) Changes in neurotrophin responsiveness during the development of cerebellar granule neurons. Neuron 9(6):1041–1052 [DOI] [PubMed] [Google Scholar]
- 24.Lu B, Figurov A (1997) Role of neurotrophins in synapse development and plasticity. Rev Neurosci 8(1):1–12. 10.1515/REVNEURO.1997.8.1.1 [DOI] [PubMed] [Google Scholar]
- 25.Chao MV (2000) Trophic factors: An evolutionary cul-de-sac or door into higher neuronal function? J Neurosci Res 59(3):353–355. [DOI] [PubMed] [Google Scholar]
- 26.Oppenheim RW (1991) Cell death during development of the nervous system. Annu Rev Neurosci 14(1):453–501. 10.1146/annurev.ne.14.030191.002321 [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Sanchez J, Arevalo JC (2017) A review on ubiquitination of neurotrophin receptors: facts and perspectives. Int J Mol Sci 18(3). 10.3390/ijms18030630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popova NK, Ilchibaeva TV, Naumenko VS (2017) Neurotrophic factors (BDNF and GDNF) and the serotonergic system of the brain. Biochemistry (Mosc) 82(3):308–317. 10.1134/S0006297917030099 [DOI] [PubMed] [Google Scholar]
- 29.Bothwell M (2014) NGF, BDNF, NT3, and NT4. Handb Exp Pharmacol 220:3–15. 10.1007/978-3-642-45106-5_1 [DOI] [PubMed] [Google Scholar]
- 30.Conover JC, Yancopoulos GD (1997) Neurotrophin regulation of the developing nervous system: analyses of knockout mice. Rev Neurosci 8(1):13–27 [DOI] [PubMed] [Google Scholar]
- 31.Gupta VK, You Y, Gupta VB, Klistorner A, Graham SL (2013) TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders. Int J Mol Sci 14(5):10122–10142. 10.3390/ijms140510122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibanez CF (1996) Neurotrophin-4: the odd one out in the neurotrophin family. Neurochem Res 21(7):787–793. 10.1007/BF02532301 [DOI] [PubMed] [Google Scholar]
- 33.Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE (2007) The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res 4(2):143–151. 10.2174/156720207780637216 [DOI] [PubMed] [Google Scholar]
- 34.Barbacid M (1994) The Trk family of neurotrophin receptors. J Neurobiol 25(11):1386–1403. 10.1002/neu.480251107 [DOI] [PubMed] [Google Scholar]
- 35.Ip NY, Yancopoulos GD (1994) Neurotrophic factors and their receptors. Ann Neurol 35(Suppl):S13–S16. 10.1002/ana.410350706 [DOI] [PubMed] [Google Scholar]
- 36.Ip NY, Stitt TN, Tapley P, Klein R, Glass DJ, Fandl J, Greene LA, Barbacid M et al. (1993) Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron 10(2):137–149. 10.1016/0896-6273(93)90306-C [DOI] [PubMed] [Google Scholar]
- 37.Mohamed R, El-Remessy AB (2015) Imbalance of the nerve growth factor and its precursor: implication in diabetic retinopathy. J Clin Exp Ophthalmol 6(5). 10.4172/2155-9570.1000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proenca CC, Song M, Lee FS (2016) Differential effects of BDNF and neurotrophin 4 (NT4) on endocytic sorting of TrkB receptors. J Neurochem 138(3):397–406. 10.1111/jnc.13676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherrard RM, Bower AJ (2002) Climbing fiber development: do neurotrophins have a part to play? Cerebellum 1(4):265–275. 10.1080/147342202320883579 [DOI] [PubMed] [Google Scholar]
- 40.Kashyap M, de Groat WC, Yoshimura N, Tyagi P (2016) BDNF enhances detrusor excitability through TRKB.T1 mediated activation of calcium channels. J Urol 195(4):e413–e414. 10.1016/j.juro.2016.02.1239 [DOI] [Google Scholar]
- 41.Kashyap M, de Groat WC, Yoshimura N, Tyagi P (2016) Pathogenic role of truncated TRKB receptor isoform (TRKB.T1) in BDNF induced detrusor overactivity (DO). The Journal of Urology 195(4):e795 [Google Scholar]
- 42.Anastasia A, Barker PA, Chao MV, Hempstead BL (2015) Detection of p75NTR trimers: implications for receptor stoichiometry and activation. J Neurosci 35(34):11911–11920. 10.1523/JNEUROSCI.0591-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skeldal S, Sykes AM, Glerup S, Matusica D, Palstra N, Autio H, Boskovic Z, Madsen P et al. (2012) Mapping of the interaction site between sortilin and the p75 neurotrophin receptor reveals a regulatory role for the sortilin intracellular domain in p75 neurotrophin receptor shedding and apoptosis. J Biol Chem 287(52):43798–43809. 10.1074/jbc.M112.374710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng D, Kim T, Ozkan E, Light M, Torkin R, Teng KK, Hempstead BL, Garcia KC (2010) Molecular and structural insight into proNGF engagement of p75NTR and sortilin. J Mol Biol 396(4):967–984. 10.1016/j.jmb.2009.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denhardt DT (1996) Signal-transducing protein phosphorylation cascades mediated by Ras/Rho proteins in the mammalian cell: the potential for multiplex signalling. Biochem J 318(Pt 3):729–747. 10.1042/bj3180729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn NG, Seger R, Krebs EG (1992) The mitogen-activated protein kinase activator. Curr Opin Cell Biol 4(6):992–999 [DOI] [PubMed] [Google Scholar]
- 47.English JD, Sweatt JD (1997) A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem 272(31):19103–19106. 10.1074/jbc.272.31.19103 [DOI] [PubMed] [Google Scholar]
- 48.Yu SJ, Xia CM, Kay JC, Qiao LY (2012) Activation of extracellular signal-regulated protein kinase 5 is essential for cystitis- and nerve growth factor-induced calcitonin gene-related peptide expression in sensory neurons. Mol Pain 8:48 10.1186/1744-8069-8-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhuang ZY, Xu H, Clapham DE, Ji RR (2004) Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci 24(38):8300–8309. 10.1523/JNEUROSCI.2893-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wahl MI, Jones GA, Nishibe S, Rhee SG, Carpenter G (1992) Growth factor stimulation of phospholipase C-gamma 1 activity. Comparative properties of control and activated enzymes. J Biol Chem 267(15):10447–10456 [PubMed] [Google Scholar]
- 51.Carpenter G, Ji Q (1999) Phospholipase C-gamma as a signal-transducing element. Exp Cell Res 253(1):15–24. 10.1006/excr.1999.4671 [DOI] [PubMed] [Google Scholar]
- 52.Nishibe S, Wahl MI, Hernandez-Sotomayor SM, Tonks NK, Rhee SG, Carpenter G (1990) Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science 250(4985):1253–1256. 10.1126/science.1700866 [DOI] [PubMed] [Google Scholar]
- 53.Wymann MP, Pirola L (1998) Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta 1436(1–2): 127–150. 10.1016/S0005-2760(98)00139-8 [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J (1994) Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370(6490):527–532. 10.1038/370527a0 [DOI] [PubMed] [Google Scholar]
- 55.Carpenter CL, Cantley LC (1996) Phosphoinositide kinases. Curr Opin Cell Biol 8(2):153–158. 10.1016/S0955-0674(96)80060-3 [DOI] [PubMed] [Google Scholar]
- 56.Wolf G, Trub T, Ottinger E, Groninga L, Lynch A, White MF, Miyazaki M, Lee J et al. (1995) PTB domains of IRS-1 and Shc have distinct but overlapping binding specificities. J Biol Chem 270(46):27407–27410. 10.1074/jbc.270.46.27407 [DOI] [PubMed] [Google Scholar]
- 57.Hashmi F, Liu M, Shen S, Qiao LY (2016) EXPRESS: Phospholipase C gamma mediates endogenous brain-derived neurotrophic factor-regulated calcitonin gene-related peptide expression in colitis-induced visceral pain. Mol Pain 12. doi: 10.1177/1744806916657088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korsching S, Thoenen H (1983) Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: correlation with density of sympathetic innervation. Proc Natl Acad Sci U S A 80(11):3513–3516. 10.1073/pnas.80.11.3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL (1993) Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron 10(3):359–367. 10.1016/0896-6273(93)90326-M [DOI] [PubMed] [Google Scholar]
- 60.Richner M, Ulrichsen M, Elmegaard SL, Dieu R, Pallesen LT, Vaegter CB (2014) Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol Neurobiol 50(3):945–970. 10.1007/s12035-014-8706-9 [DOI] [PubMed] [Google Scholar]
- 61.Carroll SL, Silos-Santiago I, Frese SE, Ruit KG, Milbrandt J, Snider WD (1992) Dorsal root ganglion neurons expressing trk are selectively sensitive to NGF deprivation in utero. Neuron 9(4): 779–788. 10.1016/0896-6273(92)90040-K [DOI] [PubMed] [Google Scholar]
- 62.Kristiansen M, Ham J (2014) Programmed cell death during neuronal development: the sympathetic neuron model. Cell Death Differ 21(7):1025–1035. 10.1038/cdd.2014.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han KA, Woo D, Kim S, Choii G, Jeon S, Won SY, Kim HM, Heo WD et al. (2016) Neurotrophin-3 regulates synapse development by modulating TrkC-PTPsigma synaptic adhesion and intracellular signaling pathways. J Neurosci 36(17):4816–4831. 10.1523/JNEUROSCI.4024-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ammendrup-Johnsen I, Naito Y, Craig AM, Takahashi H (2015) Neurotrophin-3 enhances the synaptic organizing function of TrkC-protein tyrosine phosphatase sigma in rat hippocampal neurons. J Neurosci 35(36):12425–12431. 10.1523/JNEUROSCI.1330-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naito Y, Lee AK, Takahashi H (2017) Emerging roles of the neurotrophin receptor TrkC in synapse organization. Neurosci Res 116:10–17. 10.1016/j.neures.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 66.Kashyap M, Kawamorita N, Tyagi V, Sugino Y, Chancellor M, Yoshimura N, Tyagi P (2013) Down-regulation of nerve growth factor expression in the bladder by antisense oligonucleotides as new treatment for overactive bladder. J Urol 190(2):757–764. 10.1016/j.juro.2013.02.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhangare KP, Kaye AD, Knezevic NN, Candido KD, Urman RD (2017) An analysis of new approaches and drug formulations for treatment of chronic low back pain. Anesthesiol Clin 35(2):341–350. 10.1016/j.anclin.2017.01.023 [DOI] [PubMed] [Google Scholar]
- 68.Belanger P, Butler P, Butt M, Bhatt S, Foote S, Shelton D, Evans M, Arends R et al. (2017) Evaluation of the effects of tanezumab, a monoclonal antibody against nerve growth factor, on the sympathetic nervous system in adult cynomolgus monkeys (Macaca fascicularis): a stereologic, histomorphologic, and cardiofunctional assessment. Toxicol Sci 158(2):319–333. 10.1093/toxsci/kfx089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshizawa T, Kadekawa K, Tyagi P, Yoshikawa S, Takahashi R, Takahashi S, Yoshimura N (2014) Mechanisms inducing autonomic dysreflexia during urinary bladder distention in rats with spinal cord injury. Spinal Cord 53(3):190–194. 10.1038/sc.2014.233 [DOI] [PubMed] [Google Scholar]
- 70.Kadekawa K, Yoshizawa T, Wada N, Shimizu T, Majima T, Tyagi P, de Groat WC, Sugaya K et al. (2017) Effects of liposome-based local suppression of nerve growth factor in the bladder on autonomic dysreflexia during urinary bladder distention in rats with spinal cord injury. Exp Neurol 291:44–50. 10.1016/j.expneurol.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kashyap M, Pore S, Yoshimura N, Tyagi P (2016) Constitutive expression of NGF and P75(NTR) affected by bladder distension and NGF antisense treatment. Life Sci 148:93–98. 10.1016/j.lfs.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Majima T, Tyagi P, Dogishi K, Kashyap M, Funahashi Y, Gotoh M, Chancellor MB, Yoshimura N (2017) Effect of Intravesical liposome-based nerve growth factor antisense therapy on bladder overactivity and nociception in a rat model of cystitis induced by hydrogen peroxide. Hum Gene Ther 28(7):598–609. 10.1089/hum.2016.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kashyap M, Pore S, Chancellor M, Yoshimura N, Tyagi P (2016) Bladder overactivity involves overexpression of MicroRNA 132 and nerve growth factor. Life Sci 167:98–104. 10.1016/j.lfs.2016.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chermansky CJ, Kadow BT, Kashyap M, Tyagi P (2017) MicroRNAs as potential biomarkers to predict the risk of urinary retention following intradetrusor onabotulinumtoxin-A injection. Neurourol Urodyn. 10.1002/nau.23296 [DOI] [PubMed] [Google Scholar]
- 75.Tyagi P, Tyagi V, Qu X, Lin HT, Kuo HC, Chuang YC, Chancellor M (2014) Association of inflammaging (inflammation + aging) with higher prevalence of OAB in elderly population. Int Urol Nephrol 46(5):871–877. 10.1007/s11255-013-0621-x [DOI] [PubMed] [Google Scholar]
- 76.Kuo HC, Liu HT, Guan Z, Tyagi P, Chancellor MB (2011) Promise of urinary nerve growth factor for assessment of overactive bladder syndrome. Low Urin Tract Symptoms 3(1):2–9. 10.1111/j.1757-5672.2011.00087.x [DOI] [PubMed] [Google Scholar]
- 77.Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M (1993) Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 75(1):113–122. 10.1016/S0092-8674(05)80088-1 [DOI] [PubMed] [Google Scholar]
- 78.Jones KR, Farinas I, Backus C, Reichardt LF (1994) Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 76(6):989–999. 10.1016/0092-8674(94)90377-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ernfors P, Lee KF, Jaenisch R (1994) Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature 368(6467):147–150. 10.1038/368147a0 [DOI] [PubMed] [Google Scholar]
- 80.Snider WD (1994) Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77(5):627–638. 10.1016/0092-8674(94)90048-5 [DOI] [PubMed] [Google Scholar]
- 81.Paul G, Davies AM (1995) Trigeminal sensory neurons require extrinsic signals to switch neurotrophin dependence during the early stages of target field innervation. Dev Biol 171(2):590–605. 10.1006/dbio.1995.1307 [DOI] [PubMed] [Google Scholar]
- 82.Croll SD, Wiegand SJ, Anderson KD, Lindsay RM, Nawa H (1994) Regulation of neuropeptides in adult rat forebrain by the neurotrophins BDNF and NGF. Eur J Neurosci 6(8):1343–1353. 10.1111/j.1460-9568.1994.tb00325.x [DOI] [PubMed] [Google Scholar]
- 83.Mizuno K, Carnahan J, Nawa H (1994) Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol 165(1):243–256. 10.1006/dbio.1994.1250 [DOI] [PubMed] [Google Scholar]
- 84.Yan Q, Matheson C, Lopez OT, Miller JA (1994) The biological responses of axotomized adult motoneurons to brain-derived neurotrophic factor. J Neurosci 14(9):5281–5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clatterbuck RE, Price DL, Koliatsos VE (1994) Further characterization of the effects of brain-derived neurotrophic factor and ciliary neurotrophic factor on axotomized neonatal and adult mammalian motor neurons. J Comp Neurol 342(1):45–56. 10.1002/cne.903420106 [DOI] [PubMed] [Google Scholar]
- 86.Friedman B, Kleinfeld D, Ip NY, Verge VM, Moulton R, Boland P, Zlotchenko E, Lindsay RM et al. (1995) BDNF and NT-4/5 exert neurotrophic influences on injured adult spinal motor neurons. J Neurosci 15(2):1044–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde YA (1992) Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature 360(6406):757–759. 10.1038/360757a0 [DOI] [PubMed] [Google Scholar]
- 88.Kashyap M, Pore S, de Groat WC, Chermansky C, Yoshimura N, Tyagi P (2017) BDNF overexpression in bladder induces neuronal changes to mediate bladder overactivity. Am J Physiol Renal Physiol:ajprenal 00386:02017 10.1152/ajprenal.00386.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen AI, Zang K, Masliah E, Reichardt LF (2016) Glutamatergic axon-derived BDNF controls GABAergic synaptic differentiation in the cerebellum. Sci Rep 6(1):20201 10.1038/srep20201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wuhanqimuge AM (2013) Lysophosphatidylcholine potentiates BDNF-induced TrkB phosphorylation and downstream signals in cerebellar granule neurons. Biosci Biotechnol Biochem 77(12):2510–2513. 10.1271/bbb.130622 [DOI] [PubMed] [Google Scholar]
- 91.Vakili Zahir N, Abkhezr M, Khaje Piri Z, Ostad SN, Kebriaezade A, Ghahremani MH (2012) The time course of JNK and P38 activation in cerebellar granule neurons following glucose deprivation and BDNF treatment. Iran J Pharm Res 11(1):315–323 [PMC free article] [PubMed] [Google Scholar]
- 92.Takeda M, Takahashi M, Kitagawa J, Kanazawa T, Nasu M, Matsumoto S (2013) Brain-derived neurotrophic factor enhances the excitability of small-diameter trigeminal ganglion neurons projecting to the trigeminal nucleus interpolaris/caudalis transition zone following masseter muscle inflammation. Mol Pain 9:49 10.1186/1744-8069-9-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinon S, Garcia-Vences E, Toscano-Tejeida D, Flores-Romero A, Rodriguez-Barrera R, Ferrusquia M, Hernandez-Munoz RE, Ibarra A (2016) Long-term production of BDNF and NT-3 induced by A91-immunization after spinal cord injury. BMC Neurosci 17(1):42 10.1186/s12868-016-0267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pae CU, Marks DM, Han C, Patkar AA, Steffens D (2008) Does neurotropin-3 have a therapeutic implication in major depression? Int J Neurosci 118(11):1515–1522. 10.1080/00207450802174589 [DOI] [PubMed] [Google Scholar]
- 95.Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD et al. (1995) A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 374(6521):450–453. 10.1038/374450a0 [DOI] [PubMed] [Google Scholar]
- 96.Nijs J, Meeus M, Versijpt J, Moens M, Bos I, Knaepen K, Meeusen R (2015) Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: a new therapeutic target? Expert Opin Ther Targets 19(4):565–576. 10.1517/14728222.2014.994506 [DOI] [PubMed] [Google Scholar]
- 97.Thoenen H, Zafra F, Hengerer B, Lindholm D (1991) The synthesis of nerve growth factor and brain-derived neurotrophic factor in hippocampal and cortical neurons is regulated by specific transmitter systems. Ann N Y Acad Sci 640(1):86–90. 10.1111/j.1749-6632.1991.tb00196.x [DOI] [PubMed] [Google Scholar]
- 98.Kazim SF, Iqbal K (2016) Neurotrophic factor small-molecule mimetics mediated neuroregeneration and synaptic repair: emerging therapeutic modality for Alzheimer’s disease. Mol Neurodegener 11(1):50 10.1186/s13024-016-0119-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD et al. (1994) Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature 368(6468):249–251. 10.1038/368249a0 [DOI] [PubMed] [Google Scholar]
- 100.Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF (1994) Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature 369(6482):658–661. 10.1038/369658a0 [DOI] [PubMed] [Google Scholar]
- 101.Liu X, Ernfors P, Wu H, Jaenisch R (1995) Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature 375(6528):238–241. 10.1038/375238a0 [DOI] [PubMed] [Google Scholar]
- 102.Hory-Lee F, Russell M, Lindsay RM, Frank E (1993) Neurotrophin 3 supports the survival of developing muscle sensory neurons in culture. Proc Natl Acad Sci U S A 90(7):2613–2617. 10.1073/pnas.90.7.2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB et al. (1994) Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76(6):1001–1011. 10.1016/0092-8674(94)90378-6 [DOI] [PubMed] [Google Scholar]
- 104.Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M (1994) Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 368(6468):246–249. 10.1038/368246a0 [DOI] [PubMed] [Google Scholar]
- 105.Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD (1990) NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron 5(4):501–509. 10.1016/0896-6273(90)90089-X [DOI] [PubMed] [Google Scholar]
- 106.Barres BA, Raff MC, Gaese F, Bartke I, Dechant G, Barde YA (1994) A crucial role for neurotrophin-3 in oligodendrocyte development. Nature 367(6461):371–375. 10.1038/367371a0 [DOI] [PubMed] [Google Scholar]
- 107.Brill G, Kahane N, Carmeli C, von Schack D, Barde YA, Kalcheim C (1995) Epithelial-mesenchymal conversion of dermatome progenitors requires neural tube-derived signals: characterization of the role of Neurotrophin-3. Development 121(8):2583–2594 [DOI] [PubMed] [Google Scholar]
- 108.Hyman C, Juhasz M, Jackson C, Wright P, Ip NY, Lindsay RM (1994) Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci 14(1):335–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mount HT, Dreyfus CF, Black IB (1994) Neurotrophin-3 selectively increases cultured Purkinje cell survival. Neuroreport 5(18): 2497–2500. 10.1097/00001756-199412000-00023 [DOI] [PubMed] [Google Scholar]
- 110.Morfini G, DiTella MC, Feiguin F, Carri N, Caceres A (1994) Neurotrophin-3 enhances neurite outgrowth in cultured hippocampal pyramidal neurons. J Neurosci Res 39(2):219–232. 10.1002/jnr.490390212 [DOI] [PubMed] [Google Scholar]
- 111.Kim HG, Wang T, Olafsson P, Lu B (1994) Neurotrophin 3 potentiates neuronal activity and inhibits gamma-aminobutyratergic synaptic transmission in cortical neurons. Proc Natl Acad Sci U S A 91(25):12341–12345. 10.1073/pnas.91.25.12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murphy MC, Fox EA (2010) Mice deficient in brain-derived neurotrophic factor have altered development of gastric vagal sensory innervation. J Comp Neurol 518(15):2934–2951. 10.1002/cne.22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wong V, Arriaga R, Ip NY, Lindsay RM (1993) The neurotrophins BDNF, NT-3 and NT-4/5, but not NGF, up-regulate the cholinergic phenotype of developing motor neurons. Eur J Neurosci 5(5):466–474. 10.1111/j.1460-9568.1993.tb00513.x [DOI] [PubMed] [Google Scholar]
- 114.Ip NY, Li Y, Yancopoulos GD, Lindsay RM (1993) Cultured hippocampal neurons show responses to BDNF, NT-3, and NT-4, but not NGF. J Neurosci 13(8):3394–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gao WQ, Zheng JL, Karihaloo M (1995) Neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor (BDNF) act at later stages of cerebellar granule cell differentiation. J Neurosci 15(4):2656–2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Friedman WJ, Ibanez CF, Hallbook F, Persson H, Cain LD, Dreyfus CF, Black IB (1993) Differential actions of neurotrophins in the locus coeruleus and basal forebrain. Exp Neurol 119(1):72–78. 10.1006/exnr.1993.1007 [DOI] [PubMed] [Google Scholar]
- 117.Altar CA, Boylan CB, Fritsche M, Jackson C, Hyman C, Lindsay RM (1994) The neurotrophins NT-4/5 and BDNF augment serotonin, dopamine, and GABAergic systems during behaviorally effective infusions to the substantia nigra. Exp Neurol 130(1): 31–40. 10.1006/exnr.1994.1182 [DOI] [PubMed] [Google Scholar]
- 118.Kawakami T, Wakabayashi Y, Isono T, Aimi Y, Okada Y (2002) Expression of neurotrophin messenger RNAs during rat urinary bladder development. Neurosci Lett 329(1):77–80 [DOI] [PubMed] [Google Scholar]
- 119.Li Y, Xia B, Li R, Yin D, Wang Y, Liang W (2017) Expression of brain-derived neurotrophic factors, neurotrophin-3, and neurotrophin-4 in the nucleus accumbens during heroin dependency and withdrawal. Neuroreport 28(11):654–660. 10.1097/WNR.0000000000000810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Briken S, Rosenkranz SC, Keminer O, Patra S, Ketels G, Heesen C, Hellweg R, Pless O et al. (2016) Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J Neuroimmunol 299:53–58. 10.1016/j.jneuroim.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 121.Kudlek Mikulic S, Mihaljevic-Peles A, Sagud M, Bajs Janovic M, Ganoci L, Grubisin J, Kuzman Rojnic M, Vuksan Cusa B, Bradas Z, Bozina N (2017) Brain-derived neurotrophic factor serum and plasma levels in the treatment of acute schizophrenia with olanzapine or risperidone: 6-week prospective study. Nord J Psychiatry:1–8. doi: 10.1080/08039488.2017.1340518 [DOI] [PubMed] [Google Scholar]
- 122.Boyce VS, Mendell LM (2014) Neurotrophins and spinal circuit function. Front Neural Circuits 8:59 10.3389/fncir.2014.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang H, Schuman EM (1995) Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267(5204):1658–1662. 10.1126/science.7886457 [DOI] [PubMed] [Google Scholar]
- 124.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER (1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16(6):1137–1145. 10.1016/S0896-6273(00)80140-3 [DOI] [PubMed] [Google Scholar]
- 125.Messaoudi E, Bardsen K, Srebro B, Bramham CR (1998) Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol 79(1):496–499. 10.1152/jn.1998.79.1.496 [DOI] [PubMed] [Google Scholar]
- 126.Akutagawa E, Konishi M (1998) Transient expression and transport of brain-derived neurotrophic factor in the male zebra finch’s song system during vocal development. Proc Natl Acad Sci U S A 95(19):11429–11434. 10.1073/pnas.95.19.11429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rasika S, Alvarez-Buylla A, Nottebohm F (1999) BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron 22(1):53–62 [DOI] [PubMed] [Google Scholar]
- 128.Dittrich F, Feng Y, Metzdorf R, Gahr M (1999) Estrogen-inducible, sex-specific expression of brain-derived neurotrophic factor mRNA in a forebrain song control nucleus of the juvenile zebra finch. Proc Natl Acad Sci U S A 96(14):8241–8246. 10.1073/pnas.96.14.8241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ (1997) Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389(6653):856–860. 10.1038/39885 [DOI] [PubMed] [Google Scholar]
- 130.Vaegter CB, Jansen P, Fjorback AW, Glerup S, Skeldal S, Kjolby M, Richner M, Erdmann B et al. (2011) Sortilin associates with Trk receptors to enhance anterograde transport and neurotrophin signaling. Nat Neurosci 14(1):54–61. 10.1038/nn.2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mu X, Silos-Santiago I, Carroll SL, Snider WD (1993) Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J Neurosci 13(9):4029–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhong W, Yang M, Zhang W, Visocchi M, Chen X, Liao C (2017) Improved neural microcirculation and regeneration after peripheral nerve decompression in DPN rats. Neurol Res 39(4):285–291. 10.1080/01616412.2017.1297557 [DOI] [PubMed] [Google Scholar]
- 133.Qin J, Wang L, Sun Y, Sun X, Wen C, Shahmoradi M, Zhou Y (2016) Concentrated growth factor increases Schwann cell proliferation and neurotrophic factor secretion and promotes functional nerve recovery in vivo. Int J Mol Med 37(2):493–500. 10.3892/ijmm.2015.2438 [DOI] [PubMed] [Google Scholar]
- 134.Wen X, Li X, Liang H, Yang C, Zhong J, Wang H, Liu H (2017) One cell model establishment to inhibit CaMKIIgamma mRNA expression in the dorsal root ganglion neuron by RNA interfere. Artif Cells Nanomed Biotechnol 45(6):1–7. 10.1080/21691401.2016.1216860 [DOI] [PubMed] [Google Scholar]
- 135.Mariga A, Mitre M, Chao MV (2017) Consequences of brain-derived neurotrophic factor withdrawal in CNS neurons and implications in disease. Neurobiol Dis 97(Pt B):73–79. 10.1016/j.nbd.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT (1998) Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol 152(1):74–87. 10.1006/exnr.1998.6835 [DOI] [PubMed] [Google Scholar]
- 137.Mendell LM (1984) Modifiability of spinal synapses. Physiol Rev 64(1):260–324. 10.1152/physrev.1984.64.1.260 [DOI] [PubMed] [Google Scholar]
- 138.Baker SA, Stanford LE, Brown RE, Hagg T (2005) Maturation but not survival of dopaminergic nigrostriatal neurons is affected in developing and aging BDNF-deficient mice. Brain Res 1039(1– 2):177–188. 10.1016/j.brainres.2005.01.052 [DOI] [PubMed] [Google Scholar]
- 139.Nie S, Xu Y, Chen G, Ma K, Han C, Guo Z, Zhang Z, Ye K et al. (2015) Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents. Neuropharmacology 99:448–458. 10.1016/j.neuropharm.2015.08.016 [DOI] [PubMed] [Google Scholar]
- 140.Luberg K, Wong J, Weickert CS, Timmusk T (2010) Human TrkB gene: novel alternative transcripts, protein isoforms and expression pattern in the prefrontal cerebral cortex during postnatal development. J Neurochem 113(4):952–964. 10.1111/j.1471-4159.2010.06662.x [DOI] [PubMed] [Google Scholar]
- 141.Frias B, Santos J, Morgado M, Sousa MM, Gray SM, McCloskey KD, Allen S, Cruz F et al. (2015) The role of brain-derived neurotrophic factor (BDNF) in the development of neurogenic detrusor overactivity (NDO). J Neurosci 35(5):2146–2160. 10.1523/JNEUROSCI.0373-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pinto R, Frias B, Allen S, Dawbarn D, SB MM, Cruz F, Cruz CD (2010) Sequestration of brain derived nerve factor by intravenous delivery of TrkB-Ig2 reduces bladder overactivity and noxious input in animals with chronic cystitis. Neuroscience 166(3):907–916. 10.1016/j.neuroscience.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 143.Qiao LY, Yu SJ, Kay JC, Xia CM (2013) In vivo regulation of brain-derived neurotrophic factor in dorsal root ganglia is mediated by nerve growth factor-triggered Akt activation during cystitis. PLoS One 8(11):e81547 10.1371/journal.pone.0081547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xia C, Shen S, Hashmi F, Qiao LY (2016) Colitis-induced bladder afferent neuronal activation is regulated by BDNF through PLCgamma pathway. Experimental neurology 285(Pt B):126–135. 10.1016/j.expneurol.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Goins WF, Yoshimura N, Phelan MW, Yokoyama T, Fraser MO, Ozawa H, Bennett NJ, de Groat WC et al. (2001) Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J Urol 165(5):1748–1754. 10.1016/S0022-5347(05)66407-5 [DOI] [PubMed] [Google Scholar]
- 146.Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S (2006) Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 26(42):10847–10855. 10.1523/JNEUROSCI.3023-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Diamond J, Holmes M, Coughlin M (1992) Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J Neurosci 12(4):1454–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H (1987) Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci U S A 84(23):8735–8739. 10.1073/pnas.84.23.8735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bennett MR, Lagopoulos J (2014) Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol 112:80–99. 10.1016/j.pneurobio.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 150.Schecterson LC, Bothwell M (1992) Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron 9(3):449–463. 10.1016/0896-6273(92)90183-E [DOI] [PubMed] [Google Scholar]
- 151.Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS (1994) Neurotrophic factors: from molecule to man. Trends Neurosci 17(5):182–190. 10.1016/0166-2236(94)90099-X [DOI] [PubMed] [Google Scholar]
- 152.Nirmal J, Tyagi P, Chuang YC, Lee WC, Yoshimura N, Huang CC, Rajaganapathy B, Chancellor MB (2014) Functional and molecular characterization of hyposensitive underactive bladder tissue and urine in streptozotocin-induced diabetic rat. PLoS One 9(7):e102644 10.1371/journal.pone.0102644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260(5111):1130–1132. 10.1126/science.8493557 [DOI] [PubMed] [Google Scholar]
- 154.Shen L, Figurov A, Lu B (1997) Recent progress in studies of neurotrophic factors and their clinical implications. J Mol Med (Berl) 75(9):637–644. 10.1007/s001090050147 [DOI] [PubMed] [Google Scholar]
- 155.Gash DM, Zhang Z, Gerhardt G (1998) Neuroprotective and neurorestorative properties of GDNF. Ann Neurol 44(3 Suppl 1):S121–S125. 10.1002/ana.410440718 [DOI] [PubMed] [Google Scholar]
- 156.Knezevic NN, Mandalia S, Raasch J, Knezevic I, Candido KD (2017) Treatment of chronic low back pain—new approaches on the horizon. J Pain Res 10:1111–1123. 10.2147/JPR.S132769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP et al. (1996) Characterization of a multicomponent receptor for GDNF. Nature 382(6586):80–83. 10.1038/382080a0 [DOI] [PubMed] [Google Scholar]
- 158.van Weering DH, Bos JL (1998) Signal transduction by the receptor tyrosine kinase Ret. Recent Results Cancer Res 154:271–281. 10.1007/978-3-642-46870-4_18 [DOI] [PubMed] [Google Scholar]
- 159.Cattaneo E, Conti L, De-Fraja C (1999) Signalling through the JAK-STAT pathway in the developing brain. Trends Neurosci 22(8):365–369 [DOI] [PubMed] [Google Scholar]
- 160.Stahl N, Yancopoulos GD (1994) The tripartite CNTF receptor complex: activation and signaling involves components shared with other cytokines. J Neurobiol 25(11):1454–1466. 10.1002/neu.480251111 [DOI] [PubMed] [Google Scholar]
- 161.Lin G, Zhang H, Sun F, Lu Z, Reed-Maldonado A, Lee YC, Wang G, Banie L et al. (2016) Brain-derived neurotrophic factor promotes nerve regeneration by activating the JAK/STAT pathway in Schwann cells. Transl Androl Urol 5(2):167–175. 10.21037/tau.2016.02.03 [DOI] [PMC free article] [PubMed] [Google Scholar]


