Abstract
We report the single-strand Recombinase Polymerase Amplification (ssRPA) method, which merges the fast, isothermal amplification of RPA with subsequent rapid conversion of the double-strand DNA amplicon to single strands, and hence enables facile hybridization-based, high-specificity readout. We demonstrate the utility of ssRPA for sensitive and rapid (4 copies per 50 μL reaction within 10 min, or 8 copies within 8 min) visual detection of SARS-CoV-2 RNA spiked samples, as well as clinical saliva and nasopharyngeal swabs in VTM or water, on lateral flow devices. The ssRPA method promises rapid, sensitive, and accessible RNA detection to facilitate mass testing in the COVID-19 pandemic.
Introduction.
Effective and accessible mass testing can help to limit the spread of the current SARS-CoV-2 pandemic. While serology testing reveals recent and past exposure, RNA testing allows early detection of active infection. Standard RT-qPCR1 and relatives2–10 achieve high analytical sensitivity (1–100 copies of viral RNA per input μl)11, but take hours and require relatively complex equipment. New sequencing approaches promise high multiplexing but slow response further12–15. In contrast, isothermal methods such as Recombinase Polymerase Amplification (RPA)16–22, Recombinase-Aided Amplification (RAA)23, Nicking Enzyme Amplification Reaction (NEAR)24, Loop-mediated isothermal Amplification (LAMP)25–39, and Rapid Amplification (RAMP, an RPA + LAMP combination)25 typically detect 10–1000 copies of RNA per reaction with minimal or no instrumentation (see review40). The RPA reaction can generate double-stranded DNA (dsDNA) amplicons particularly quickly, but its recombinase-driven priming process is prone to multi-base mismatching that necessitates an additional specificity check39,41. Augmentation of RPA with conditionally extensible primers or cleavable inter-primer probes enhances specificity16,42,43, but tends to reduce reaction speed. Alternatively, Cas1234 or Cas1319,44 nucleases applied to amplification products generate signal in a sequence specific manner, but incur substantial increases in workflow complexity and reaction time. Here, we describe “single-strand RPA” (ssRPA) method, which applies (1) rapid amplification of dsDNA, (2) conversion to ssDNA, and (3) sequence-specific, hybridization-based readout, arranged to maintain both optimal speed and accuracy (Fig. 1a). For amplification, we apply basic RT-RPA16 in the absence of any specificity-enhancing components that may inhibit speed. For ssDNA conversion, we use an exonuclease to digest strands except those chemically protected. Finally, hybridization-based readout is shown with LFDs.
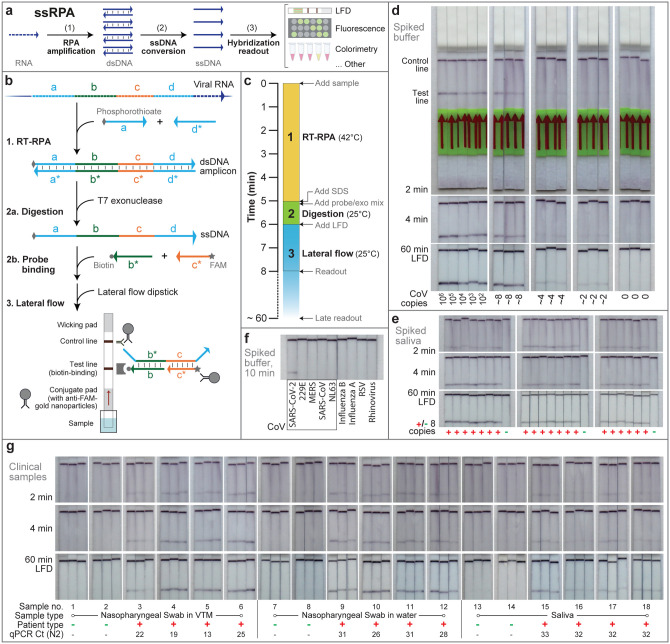
Figure 1: ssRPA assay design, workflow, and characterization.
(a) Key to ssRPA design is the rapid generation of many ssDNA copies from a single RNA target. ssDNA output offers straightforward specific readout by fluorescence or colorimetric/visual methods such as lateral flow devices. (b) Step 1: Target viral RNA region (of domains a-b-c-d) is reverse transcribed into cDNA via extension of the reverse primer (d*) by the Reverse Transcriptase in the reaction mixture48. Subsequently, the cDNA is amplified via isothermal RPA at 42 °C by templated extension of the forward (a) and reverse primers (d*). The forward primer has a 6-nucleotide long poly-T segment with phosphorothioate bonds. Step 2a: Products of RPA are amended with SDS and transferred to the exo/LFD buffer that contains T7 exonuclease (a dsDNA-specific 5′ to 3′ exonuclease) and detection probes. The resulting mixture is incubated for 1 min at ambient temperature and reverse strand of the dsDNA amplicon products get preferentially digested yielding ssDNA amplicon (a-b-c-d) homologous to the target RNA sequence. Step 2b: The 3′ biotin (b*) and 5′ FAM (c*) modified detection probes, already added, hybridize to target sequences. These probes make the assay directly compatible with commercially available test strips that feature a biotin-binding test line and gold nanoparticles conjugated to rabbit anti-FAM IgG on the conjugate pad. Step 3: The test strip is vertically inserted into the resulting 100 μl mixture. The right ssDNA amplicon acts as a bridge that binds both the biotin-probe and the FAM-probe independently resulting in immobilization of the complex at the test line, where formation of a colored line indicates a positive result. The control line formed of rabbit secondary antibodies captures the remaining gold nanoparticle conjugates by binding to rabbit anti-FAM IgG. (c) Timeline of the assay shows the incubation conditions and duration of the 3 main steps in ssRPA: (1) RT-RPA, (2) exonuclease digestion and (3) lateral flow. The test line and control line can be visualized as early as 1–2 min or as late as 60+ min without false positives. (d) Sensitivity of ssRPA-LFD demonstrated by serial dilution of synthetic SARS-CoV-2 full genome standard (Twist) from ~ 1,000,000 copies down to ~ 2 copies per reaction. A 5 μl volume of genomic viral RNA in DNase/RNase-free water was used as input for a 50 μl reaction volume. After RT-RPA for 5 minutes at 42 °C, 8 μl product was mixed with 12 μl of 10% SDS and then transferred into 80 μl of exo/LFD buffer. Following 1 min T7 exonuclease digestion at room temperature, samples were applied to commercial HybriDetect strips for ≥ 1 min. A time series for the same strip is shown in each column. Note the zero test line signal in negative controls, even at 60 min. (e) Using the same procedure as in (d), but spiking 8 copies cultured, heat inactivated SARS-CoV-2 virus (BEI) into 5 μl of SARS-CoV-2-negative human saliva in 20 repeats. No-template negative controls are also shown. (f) Specificity was shown by testing 8 other respiratory virus genomic samples, including genomically similar coronaviruses 229E, MERS, (2003) SARS-CoV, and NL63, and alternative diagnoses of influenza B, influenza A, respiratory syncytial virus (RSV), and rhinovirus 17, each at >105 copies per assay and spiked in DNase/RNase-free water. A sample with virus-derived SARS-CoV-2 RNA at low copy is shown as a positive control. Strips show the readout at 10 min of lateral flow. (g) Clinical samples were taken as NP swabs in VTM, NP swabs in water, or saliva, from SARS-CoV-2 positive and negative patients alike, and heat inactivated at vendor for safety. Sample order was randomized prior to ssRPA, and technician was blinded as to patient type or qPCR status to avoid experimental bias. During assay, samples further underwent a 2 min extraction protocol with Lucigen DNA extraction buffer at 50% dilution and 95 °C, and were tested at 10% v/v into ssRPA for 5′ spike targets (see Methods). Non-extracted samples yielded little or no signal (Fig. S6). Presumptive diagnosis from supplier testing is noted. Concomitant qPCR quantification in our lab, using CDC N2 primers from buffer-exchanged samples, is shown with each result (See also Fig. S7).
Results.
We selected the 5′ end of the SARS-CoV-2 spike protein sequence as our main detection target. Per the detailed protocol of Fig. 1b (and Supplementary Protocol), we diluted the sample in a basic RT-RPA reaction mixture, modifying the forward primer with a 5′ tail of 6 phosphorothioate-linked bases to confer exonuclease protection45,46. The reaction was run for 5 min at 42°C on a heating block. A sample of the product was then treated with sodium dodecyl sulfate (SDS) and diluted into an exonuclease and lateral flow (exo/LFD) buffer, where the unprotected strand in the dsDNA was rapidly (≤ 1 min) digested by a T7 exonuclease to yield the protected ssDNA target45,46. A pair of 3′-biotin and 5′-FAM modified probes in the digestion buffer were available to target sequences in between the amplification priming domains, providing specificity not achievable with RPA priming alone. The correct target ssDNA therefore acted as a bridge that co-localized both detection probes within an LFD, ultimately binding gold nanoparticles to the biotin-ligand test line to produce visual readout as early as 1–2 minutes. Full amplification and detection timelines are described in Fig. 1c.
We first tested ssRPA on buffer-spiked samples. Figure 1d (and Supplementary Fig. S1) shows LFD detection of synthetic SARS-CoV-2 full genome standard serially diluted in DNase/RNase-free water, photographed at multiple intervals on the same strips. Concentrations of input RNA were quantified by RT-qPCR and direct comparison to spectrophotometrically-quantified synthetic standards (Fig. S2). Results show detection of 8 copies per 50 μL reaction by naked eye within 8 min (2 min LFD), or 4 copies within 10 min (4 min LFD), and a dynamic range of at least 5 orders of magnitude. No test line signal formed for the no-template negative controls over >60 min of LFD incubation. To test for assay reliability, we spiked 5 μL human saliva with 8 copies of cultured, heat-inactivated virus and showed 20 of 20 positive tests within 8 min (Figs. 1e and S3). To test for specificity, we performed ssRPA on water spiked with viral RNA from 8 other respiratory viruses, including genomically similar coronaviruses and alternative diagnoses, each at >105 copies per assay. None exhibited false positive signals (Figs. 1f and S4). Finally, we performed a blinded assay on clinical samples, with a technician unaware of patient type or qPCR result correctly identifying the type (positive or negative) of all 18 samples at 2 min LFD time. Nasopharyngeal (NP) swabs stored in viral transport media (VTM), NP swabs stored in water, and saliva samples were processed by single-tube RNA extraction (1:1 mixture with extraction buffer, 95 °C × 2 min)47 and used at 10% v/v in RT-RPA (Figs. 1g and S5). All clinical samples were also semi-quantified by RT-qPCR, using column extraction/concentration and CDC N2 primers (Fig. 1g, S7). Results of this limited study demonstrate 100% sensitivity and 100% specificity across all sample types, including NP swabs in water and saliva samples with lower titers (high Ct).
Discussion.
The ssRPA method combines the speed of established RT-RPA16 with the sequence specificity of ssDNA hybridization by serially applying RPA and exonuclease steps. It enables best-in-class performance on the principal axes of reaction time and sensitivity, enabling the detection of 8 copies in 8 min or 4 copies in 10 min. The conceptual framework could be generalized to other isothermal readout methods with dsDNA output for achieving optimal sensitivity and speed. The present method may also be further developed to achieve single-nucleotide specificity, for example by using toehold probe readout19, and spatial multiplexing readout on LFDs with multiple test positions. We also expect future development, including simplified workflow and use of lyophilized reagents for ambient distribution and storage, will further facilitate mass testing.
Supplementary Material
Acknowledgements.
We acknowledge support from NIH 1DP1GM133052-01 and the Wyss Institute Molecular Robotics Initiative. We thank Girija Goyal and Don Ingber for inactivated SARS-CoV-2 samples, and Robert Rasmussen for help with safety and regulatory compliance. Noted reagents were obtained through BEI Resources, NIAID, NIH.
Footnotes
Competing interests. The authors have applied for patents based on the work. P.Y. is a co-founder and director of Ultivue, Inc., NuProbe Global, and Torus Biosystems, Inc.
References.
- 1.CDC. 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel Instructions for Use.pdf. [DOI] [PMC free article] [PubMed]
- 2.Wee S. K., Sivalingam S. P. & Yap E. P. H. Rapid direct nucleic acid amplification test without RNA extraction for SARS-CoV-2 using a portable PCR thermocycler. http://biorxiv.org/lookup/doi/10.1101/2020.04.17.042366 (2020) doi: 10.1101/2020.04.17.042366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladha A., Joung J., Abudayyeh O. O., Gootenberg J. S. & Zhang F. A 5-min RNA preparation method for COVID-19 detection with RT-qPCR. 6. [Google Scholar]
- 4.Bruce E. A. et al. RT-qPCR Detection of SARS-CoV-2 RNA from patient nasopharyngeal swab using Qiagen RNeasy kits or directly via omission of an RNA extraction step. bioRxiv 2020.03.20.001008 (2020) doi: 10.1101/2020.03.20.001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arumugam A. & Wong S. The Potential Use of Unprocessed Sample for RT-qPCR Detection of COVID-19 without an RNA Extraction Step. bioRxiv 2020.04.06.028811 (2020) doi: 10.1101/2020.04.06.028811. [DOI] [Google Scholar]
- 6.Marzinotto S. et al. A streamlined approach to rapidly detect SARS-CoV-2 infection, avoiding RNA extraction. medRxiv 2020.04.06.20054114 (2020) doi: 10.1101/2020.04.06.20054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Won J. et al. Development of a Laboratory-safe and Low-cost Detection Protocol for SARS-CoV-2 of the Coronavirus Disease 2019 (COVID-19). Exp. Neurobiol. 29, 107–119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Z. et al. A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. http://biorxiv.org/lookup/doi/10.1101/2020.02.22.961268 (2020) doi: 10.1101/2020.02.22.961268. [DOI] [Google Scholar]
- 9.Brown J. R. et al. Comparison of SARS-CoV2 N gene real-time RT-PCR targets and commercially available mastermixes. http://biorxiv.org/lookup/doi/10.1101/2020.04.17.047118 (2020) doi: 10.1101/2020.04.17.047118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xpert Xpress SARS-CoV-2 EUA Instructions for Use, rev D.pdf.
- 11.Wölfel R. et al. Virological assessment of hospitalized patients with COVID-2019. Nature (2020) doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 12.Chandler-Brown D., Bueno A. M., Atay O. & Tsao D. S. A Highly Scalable and Rapidly Deployable RNA Extraction-Free COVID-19 Assay by Quantitative Sanger Sequencing. http://biorxiv.org/lookup/doi/10.1101/2020.04.07.029199 (2020) doi: 10.1101/2020.04.07.029199. [DOI] [Google Scholar]
- 13.Octant SwabSeq SARS-CoV-2 testing. Notion https://www.notion.so. [Google Scholar]
- 14.Illumina CovidSeq Test Instructions for Use (# 1000000123387). 36 (2020). [Google Scholar]
- 15.Wang M. et al. Nanopore Targeted Sequencing for the Accurate and Comprehensive Detection of SARS-CoV-2 and Other Respiratory Viruses. Small 2002169 (2020) doi: 10.1002/smll.202002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piepenburg O., Williams C. H., Stemple D. L. & Armes N. A. DNA Detection Using Recombination Proteins. PLOS Biol. 4, e204 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding X., Yin K., Li Z. & Liu C. All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) Assay: A Case for Rapid, Ultrasensitive and Visual Detection of Novel Coronavirus SARS-CoV-2 and HIV virus. http://biorxiv.org/lookup/doi/10.1101/2020.03.19.998724 (2020) doi: 10.1101/2020.03.19.998724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucia C., Federico P.-B. & Alejandra G. C. An ultrasensitive, rapid, and portable coronavirus SARS-CoV-2 sequence detection method based on CRISPR-Cas12. http://biorxiv.org/lookup/doi/10.1101/2020.02.29.971127 (2020) doi: 10.1101/2020.02.29.971127. [DOI] [Google Scholar]
- 19.Zhang F., Abudayyeh O. O. & Gootenberg J. S. A protocol for detection of COVID-19 using CRISPR diagnostics. 8. [Google Scholar]
- 20.Metsky H. C., Freije C. A., Kosoko-Thoroddsen T.-S. F., Sabeti P. C. & Myhrvold C. CRISPR-based surveillance for COVID-19 using genomically-comprehensive machine learning design. http://biorxiv.org/lookup/doi/10.1101/2020.02.26.967026 (2020) doi: 10.1101/2020.02.26.967026. [DOI] [Google Scholar]
- 21.Hou T. et al. Development and Evaluation of A CRISPR-based Diagnostic For 2019-novel Coronavirus. http://medrxiv.org/lookup/doi/10.1101/2020.02.22.20025460 (2020) doi: 10.1101/2020.02.22.20025460. [DOI] [Google Scholar]
- 22.Qian J. et al. An enhanced isothermal amplification assay for viral detection. http://biorxiv.org/lookup/doi/10.1101/2020.05.28.118059 (2020) doi: 10.1101/2020.05.28.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo L. et al. SARS-CoV-2 detection with CRISPR diagnostics. Cell Discov. 6, 34 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbott ID-Now SARS-CoV-2 EUA Instructions for Use.
- 25.El-Tholoth M., Bau H. H. & Song J. A Single and Two-Stage, Closed-Tube, Molecular Test for the 2019 Novel Coronavirus (COVID-19) at Home, Clinic, and Points of Entry. 21. [Google Scholar]
- 26.Notomi T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28, 63e–663 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Gonzalez E. et al. Scaling diagnostics in times of COVID-19: Colorimetric Loop-mediated Isothermal Amplification (LAMP) assisted by a 3D-printed incubator for cost-effective and scalable detection of SARS-CoV-2. medRxiv 2020.04.09.20058651 (2020) doi: 10.1101/2020.04.09.20058651. [DOI] [Google Scholar]
- 28.Zhang Y. et al. Rapid Molecular Detection of SARS-CoV-2 (COVID-19) Virus RNA Using Colorimetric LAMP. http://medrxiv.org/lookup/doi/10.1101/2020.02.26.20028373 (2020) doi: 10.1101/2020.02.26.20028373. [DOI] [Google Scholar]
- 29.Park G.-S. et al. Development of Reverse Transcription Loop-Mediated Isothermal Amplification Assays Targeting Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). J. Mol. Diagn. 22, 729–735 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan C. et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin. Microbiol. Infect. 26, 773–779 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W. et al. Rapid Detection of SARS-CoV-2 Using Reverse transcription RT-LAMP method. http://medrxiv.org/lookup/doi/10.1101/2020.03.02.20030130 (2020) doi: 10.1101/2020.03.02.20030130. [DOI] [Google Scholar]
- 32.Yu L. et al. Rapid colorimetric detection of COVID-19 coronavirus using a reverse tran-scriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic plat-form: iLACO. medRxiv 2020.02.20.20025874 (2020) doi: 10.1101/2020.02.20.20025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang M. et al. Development and Validation of a Rapid, Single-Step Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) System Potentially to Be Used for Reliable and High-Throughput Screening of COVID-19. Front. Cell. Infect. Microbiol. 10, 331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broughton J. P. et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. (2020) doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joung J. et al. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. http://medrxiv.org/lookup/doi/10.1101/2020.05.04.20091231 (2020) doi: 10.1101/2020.05.04.20091231. [DOI] [Google Scholar]
- 36.Sherlock SARS-CoV-2 EUA, Instructions for Use.
- 37.Wei S. et al. Field-deployable, rapid diagnostic testing of saliva samples for SARS-CoV-2. http://medrxiv.org/lookup/doi/10.1101/2020.06.13.20129841 (2020) doi: 10.1101/2020.06.13.20129841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabe B. A. & Cepko C. SARS-CoV-2 Detection Using an Isothermal Amplification Reaction and a Rapid, Inexpensive Protocol for Sample Inactivation and Purification. http://medrxiv.org/lookup/doi/10.1101/2020.04.23.20076877 (2020) doi: 10.1101/2020.04.23.20076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhadra S., Riedel T. E., Lakhotia S., Tran N. D. & Ellington A. D. High-surety isothermal amplification and detection of SARS-CoV-2, including with crude enzymes. http://biorxiv.org/lookup/doi/10.1101/2020.04.13.039941 (2020) doi: 10.1101/2020.04.13.039941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esbin M. N. et al. Overcoming the bottleneck to widespread testing: A rapid review of nucleic acid testing approaches for COVID-19 detection. RNA rna.076232.120 (2020) doi: 10.1261/rna.076232.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.TwistAmp DNA Amplification Kits: Assay Design Manual. [Google Scholar]
- 42.Powell M. L. et al. New Fpg probe chemistry for direct detection of recombinase polymerase amplification on lateral flow strips. Anal. Biochem. 543, 108–115 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Xia S. & Chen X. Ultrasensitive and Whole-Course Encapsulated Field Detection of 2019-nCoV Gene Applying Exponential Amplification from RNA Combined with Chemical Probes. (2020) doi: 10.26434/chemrxiv.12012789.v1. [DOI] [Google Scholar]
- 44.Kellner M. J., Koob J. G., Gootenberg J. S., Abudayyeh O. O. & Zhang F. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat. Protoc. 14, 2986–3012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han D. et al. Single-stranded DNA and RNA origami. Science 358, eaao2648 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sayers J. R., Schmidt W. & Eckstein F. 5′–3′ Exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 16, 791–802 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joung J. et al. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. medRxiv 2020.05.04.20091231 (2020) doi: 10.1101/2020.05.04.20091231. [DOI] [Google Scholar]
- 48.Wahed A. A. E. et al. Recombinase Polymerase Amplification Assay for Rapid Diagnostics of Dengue Infection. PLOS ONE 10, e0129682 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yurke B., Turberfield A. J., Mills A. P. Jr., Simmel F. C. & Neumann J. L. A DNA-fuelled molecular machine made of DNA. Nature 406, 605–608 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Zhang D. Y., Chen S. X. & Yin P. Optimizing the specificity of nucleic acid hybridization. Nat. Chem. 4, 208–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.