Abstract
Deep vein thrombosis (DVT) is one of the most common circulating vascular diseases with an incidence of ~0.1% worldwide. Although anticoagulant medication remains to be the main therapeutic approach for patients with DVT, existing thrombus and pulmonary embolisms still pose as a threat to patient life. Therefore, effective targeted therapies need to be developed and studies are required to improve understanding of this condition. Endothelial progenitor cells (EPCs) originate from the bone marrow, are located in the peripheral blood and are involved in thrombus resolution. Long non-coding RNAs (lncRNAs) are non-coding RNAs that are >200 nucleotides in length. LncRNAs are associated with the development of numerous vascular diseases. Among these lncRNAs, metastasis associated lung adenocarcinoma transcript 1 (MALAT1) is downregulated in human atherosclerotic plaques. Furthermore, MALAT1 polymorphism resulted in vascular disease in Chinese populations. In the present study, the expression profile and potential functions of MALAT1 in DVT were investigated. The results revealed that MALAT1 was upregulated in DVT tissues. Furthermore, MALAT1 was able to regulate the biological behaviors of EPCs, including proliferation, migration, cell cycle arrest and apoptosis. In addition, the Wnt/β-catenin signaling pathway is a promising downstream target of MALAT1 in DVT. The changes in biological behaviors in EPCs caused by silenced MALAT1 were reversed by inhibition of the Wnt/β-catenin signaling pathway. In summary, the data indicated the roles of MALAT1 in the pathogenesis of DVT, and the MALAT1/Wnt/β-catenin axis could be a novel therapeutic target for the treatment of DVT.
Keywords: deep vein thrombosis, long non-coding RNA metastasis associated lung adenocarcinoma transcript 1, proliferation, migration
Introduction
DVT is a type of vascular disease, and its occurrence is ~0.1% globally (1). DVT can lead to swelling, ulceration and post-thrombotic syndromes in the legs, as well as pulmonary embolism, which results in ~15% of mortality in the first three months post-diagnosis (1). Although anticoagulants are widely used for the treatment of DVT, existing thrombus and pulmonary embolisms still affect life quality of DVT patients (2). Therefore, it is urgent to develop of effective targeted therapies against DVT, and studies are required to elucidate the mechanisms underlying the development of this disease.
EPCs are derived from bone marrow, are present in the circulating blood and have the potential to differentiate into mature endothelial cells during vascular injury repair (3). Additionally, they are considered to be potential biomarkers and promising regenerative medicine for cardiovascular diseases (3). Previous studies have indicated that peripheral EPCs serve essential roles in the resolution of thrombus (4-6). These data also provided novel insights on the therapeutic approaches of DVT treatment.
LncRNAs are novel non-protein-coding RNAs that are >200 nucleotides in length (7). Previous studies have suggested that lncRNAs are associated with the progression of a variety of vascular diseases, where impaired levels of lncRNAs were observed (8-13). Among these non-coding RNAs, lncRNA metastasis associated lung adenocarcinoma transcript 1 (MALAT1) has been indicated to be involved in the pathogenesis of vascular diseases, as it is downregulated in plaques and is associated with endothelial phenotypic switch (14). Furthermore, a previous study revealed that MALAT1 polymorphism could result in vascular disease in the Chinese population (15). However, the expression profiles and potential functions of MALAT1 in DVT have not been fully elucidated and require further investigation.
The Wnt pathway is an evolutionarily conserved signaling pathway and is widely involved in a variety of biological processes including cell proliferation and migration (16-19). This axis is activated by the Wnt ligand and when activated, β-catenin escapes serine and threonine phosphorylation by glycogen synthase kinase-3β (GSK3β) at the N-terminus, which dictates the stability of the β-catenin destruction complex (17). As a result, β-catenin accumulates in the cytoplasm and translocates into the nucleus, further modulating the expression of Wnt target genes (16). The Wnt pathway serves an essential role in the progression of vascular diseases (16-19). Furthermore, this pathway is also involved in the proliferation, differentiation and apoptosis of EPCs (20,21). In addition, MALAT1 could modulate the growth and migration of a variety of cell types through Wnt signaling (22,23). Taken together, the MALAT1/Wnt/β-catenin axis may also serve a role in the proliferation and migration of EPCs, consequently contributing to the progression of DVT.
In the present study, the effects of MALAT1-regulated signaling on the growth and migration of EPCs were investigated. The results revealed that MALAT1 is upregulated in DVT tissues. Furthermore, MALAT1 was able to regulate the biological behaviors of EPCs, such as proliferation, migration, cell cycle arrest and apoptosis. In addition, the Wnt/β-catenin signaling pathway is a promising downstream target of MALAT1 in DVT. The changes of biological behaviors in EPCs caused by silenced MALAT1 were reversed by inhibition of the Wnt/β-catenin signaling pathway. In summary, the data indicated the roles of MALAT1 in the pathogenesis of DVP, and the MALAT1/Wnt/β-catenin axis could be a novel therapeutic target for the treatment of this disease.
Materials and methods
Clinical specimens
A total of 20 blood samples were obtained from patients with DVT and healthy donors (age, 45-70 years; 10 males and 10 females) at the First Affiliated Hospital of Jinzhou Medical University (Jinzhou, China) between May 2015 and April 2017. None of the patients received treatment prior to enrollment. The present study was conducted according to the Declaration of Helsinki and approved by the Review Committee of the First Affiliated Hospital of Jinzhou Medical University (approval no. 20152874). Written informed consent was signed by each patient. Samples were snap-frozen using liquid nitrogen and immediately stored at -80˚C until further use.
Cell culture
For the isolation of mononuclear cells (MNCs), ~100 ml of circulating blood was obtained from each patient with DVT or healthy controls using BD Vacutainer EDTA tube (BD Biosciences) and immediately stored in the dark. The samples were processed following collection as follows: MNCs were extracted by density gradient centrifugation using Biocoll (Biochrom, Ltd.) at 5,003 x g at 4˚C for 20 min and washed three times using PBS. Isolated cells were plated on culture dishes that were pre-coated with human fibronectin (Sigma-Aldrich; Merck KGaA) and cultured in endothelial cell growth medium (GE Healthcare Life Sciences) supplemented with bovine brain extract (12 mg/ml), human epidermal growth factor (10 ng/ml), human insulin-like growth factor-1 (50 ng/ml), hydrocortisone (1 mg/ml) and streptomycin (100 µg/ml) and penicillin (100 U/ml; GE Healthcare Life Sciences) at 37˚C. Heparin (10 U/ml; Tocris Bioscience) was used to avoid platelet coagulation and cells were maintained in a humidified 5% CO2 atmosphere at 37˚C. After 3 days, floating cells were aspirated and the culture medium was replaced. EPC colonies formed after 1-2 weeks of culture. Medium was replenished every day for the first 7 days and every other day for the following 7 days. The medium was then replenished every 2-3 days. A total of two batches of cells were used in the subsequent experiments.
Cell transfection
To establish a MALAT1 knockdown model, small interfering RNA (siRNA) sequences targeting MALAT1 (si-MALAT1; 5'-GUACAUUCGUGGAGACUAGC-3' and 3'-ACCAUGUAAGCACCUCUGAU-5') and negative control (si-NC; 5'-GCUACAUUCUGGAGACAUA-3' and 3'-CGAUGUAAGACCUCUGUAU-5') were purchased from GenePharma Co. Ltd. Following annealing, siRNA were integrated into lentiviral pU6-Luc-Puro vectors (GenePharma Co., Ltd.). Furthermore, to establish a MALAT1 overexpression model, wild-type MALAT1 (LV-MALAT1) or mutant (LV-NC) fragments were amplified using PCR and integrated into pcDNA3.1 vectors (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were transfected with corresponding lentiviral vectors or controls. A total of 10 nM plasmids or the aforementioned siRNA were used for transfection. Up- or downregulation of MALAT1 was determined using reverse transcription-quantitative PCR (RT-qPCR). The Wnt/β-catenin signaling inhibitor XAV939 (10 nM; cat. no. ab120897; Abcam) was used to treat si-MALAT1-transfected cells. All transfections were performed using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). At 8 h post-transfection, endothelial cell growth medium was replenished with fresh culture medium containing 10% FBS. Cells were cultured for 24 h post-transfection and subjected to further analysis.
RT-qPCR
RT-qPCR was performed to evaluate the levels of MALAT1, proliferating cell nuclear antigen (PCNA), segment polarity protein dishevelled homolog DVL-2 (DVL2), GSK3β, cyclin D1 and β-catenin. Total RNA from clinical samples or cells was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The concentration of extracted RNA was measured using a NanoDrop™ 1000 spectrophotometer (Thermo Fisher Scientific, Inc.). The quality of RNA was determined with an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.). Subsequently, cDNA was synthesized using a PrimeScript™ RT Reagent kit (Takara Biotechnology Co., Ltd.), and qPCR was performed using a SYBR Green PCR Master Mix (Takara Biotechnology Co., Ltd.) according to the manufacturer's protocol. β-actin was used as the internal reference gene. The temperature protocol of reverse transcription was as follows: 42˚C for 45 min, 99˚C for 5 min and 5˚C for 5 min. The following primer pairs were used for qPCR: MALAT1 forward, 5'-CTAAGGTCAAGAGAAGTGTCAG-3' and reverse, 5'-AAGACCTCGACACCATCGTTAC-3'; PCNA forward, 5'-CATGGTGAAACCCCGTCTCTACTA-3' and reverse, 5'-GAGCACTTAGGCAATTTTGGTGAT-3'; DVL2 forward, 5'-CATCCAGCCAATTGACCCTG-3' and reverse, 5'-AGGGATGGTGATCTTGAGCC-3'; GSK3β forward, 5'-GGAACTCCAACAAGGGAGCA-3' and reverse, 5'-TTCGGGGTCGGAAGACCTTA-3'; cyclin D1 forward, 5'-TGAACTACCTGGACCGCT-3' and reverse, 5'-GCCTCTGGCATTTTGGAG-3'; β-catenin forward, 5'-AGTTCCTTACCGTCCCCAAG-3' and reverse, 5'-CAGACACGCCTGTTTCGAAT-3' and β-actin forward, 5'-GCACCACACCTTCTACAATG-3' and reverse, 5'-TGCTTGCTGATCCACATCTG-3'. The following thermocycling conditions were used for the PCR: Initial denaturation at 95˚C for 5 min; 45 cycles of 95˚C for 15 sec, 60˚C for 20 sec and 72˚C for 10 sec, followed by 72˚C for 5 min. The relative expression of each gene was calculated using 2-∆∆Cq method (24).
Western blot analysis
Total protein was extracted from tissues or cells using RIPA buffer (Beyotime Institute of Biotechnology). Protein concentration was measured using a BCA assay (Beyotime Institute of Biotechnology). Equal amounts (30 µg) of protein samples were separated using 10% SDS-PAGE gel and then transferred onto a PVDF membrane (EMD Millipore). Subsequently, the membranes were blocked in TBS containing 5% skimmed milk at room temperature for 2 h and incubated with the following primary antibodies: PCNA (1:2,000; cat. no. 2586; Cell Signaling Technology, Inc.), DVL2 (1:1,000; cat. no. 3216; Cell Signaling Technology, Inc.), GSK3β (1:1,000; cat. no. 9832; Cell Signaling Technology, Inc.), cyclin D1 (1:1,000; cat. no. 2978; Cell Signaling Technology, Inc.), β-catenin (1:1,000; cat. no. 2698; Cell Signaling Technology, Inc.) and β-actin (1:1,000; cat. no. 3700; Cell Signaling Technology, Inc.) overnight at 4˚C. Membranes were then incubated in horseradish peroxidase-conjugated secondary antibodies (1:5,000; cat. no. sc-2371 or 1:10,000; cat. no. sc-2004; Santa Cruz Biotechnology Inc.) at room temperature for 1 h. Bands were visualized using an ECL protein detection kit (Pierce; Thermo Fisher Scientific, Inc). Protein blots were quantified by densitometric analysis using ImageJ software (v1.48; National Institutes of Health).
MTT assay
Following transfection for 24 h, cells were harvested and a total of 2x104 cells were seeded onto a 96-well plate. Cell proliferation was examined using an MTT assay (Sigma-Aldrich; Merck KGaA) at days 1, 2, 3 and 4 post-inoculation. Briefly, 20 µl of MTT solution was added into each well followed by incubation at 37˚C for 4 h. Dimethyl sulfoxide was subsequently used to dissolve formazan. The absorbance was read at a wavelength of 450 nm and measured using a microplate reader (Bio-Rad Laboratories, Inc.).
Transwell assay
The migratory abilities of cells were examined using a Transwell assay. For the migration assay, a total of 1x105 cells in serum-free endothelial cell growth medium were seeded into the upper chamber of Transwell plates (BD Biosciences) with 8-µm pore size. Subsequently, 500 µl of culture medium supplemented with 10% FBS was added into the lower chamber. Following overnight incubation at 37˚C, non-migratory cells were removed using a cotton swab, whereas the migrated cells in the lower chamber were fixed using 4% paraformaldehyde at room temperature for 30 min and stained with 0.5% crystal violet at room temperature for 10 min. The numbers of migratory cells were counted in five randomly selected fields using an inverted light microscope (magnification, x200; Olympus Corporation).
Cell cycle and apoptosis analysis
Cells were plated onto a six-well plate at a density of 3x105 cells/well. Cells were then collected using low-speed centrifugation (1,000 x g at 4˚C for 5 min. Cell pellets were rinsed and resuspended in PBS, fixed with 70% pre-chilled ethanol at room temperature for 15 min and stored at 4˚C for two days. Cells were lysed prior to flow cytometry, centrifuged at 10,000 x g at room temperature for 5 min and then resuspended using propidium iodide (PI; Sigma-Aldrich; Merck KGaA) staining buffer containing 50 µl/ml of PI with 250 µl/ml RNase A. To evaluate cell apoptosis, suspended cells were incubated in the dark at 4˚C for 30 mins and stained with 5 µl Annexin V-FITC (JingMei Biotech Co., Ltd.). Both cell cycle distribution and the number of apoptopic cells were analysed using a flow cytometer (BD Biosciences) and FlowJo software (version 7.6; FlowJo LLC).
Statistical analysis
Data were presented as the mean ± standard deviation and analysed using SPSS 17.0 software (SPSS, Inc.). Any significant difference between groups was analysed using Student's t-test or one-way ANOVA followed by Student-Newman-Keuls test. P<0.05 was considered to indicate a statistically significant difference.
Results
MALAT1 is upregulated in DVT samples
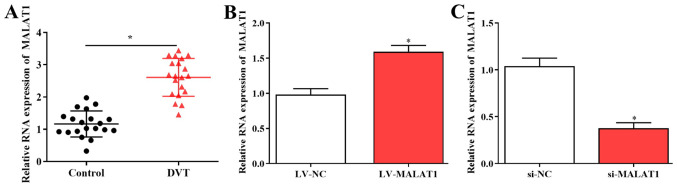
The expression of MALAT1 was evaluated in 20 DVT samples and healthy controls using RT-qPCR. The results revealed that expression of MALAT1 was significantly increased in DVT tissues compared with controls (Fig. 1A). To study the role of MALAT1 in DVT, EPCs isolated from patients were transfected with LV-MALAT1, si-MALAT1 or control vectors, and transfection efficiencies were measured using RT-qPCR (Fig. 1B and C).
Figure 1.
MALAT1 is upregulated in DVT tissue samples. (A) Levels of MALAT1 were examined in 20 DVT tissues and healthy controls using RT-qPCR. Isolated endothelial progenitor cells were transfected with (B) LV-MALAT1, (C) si-MALAT1 and corresponding control vectors. Transfection efficiencies were confirmed using RT-qPCR. *P<0.05. DVT, deep vein thrombosis; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; MALAT1, metastasis associated lung adenocarcinoma transcript 1; LV-MALAT1, wild-type MALAT1; si-MALAT1, small interfering RNA targeting MALAT1; NC, negative control.
MALAT1 regulates the biological behaviors of EPCs
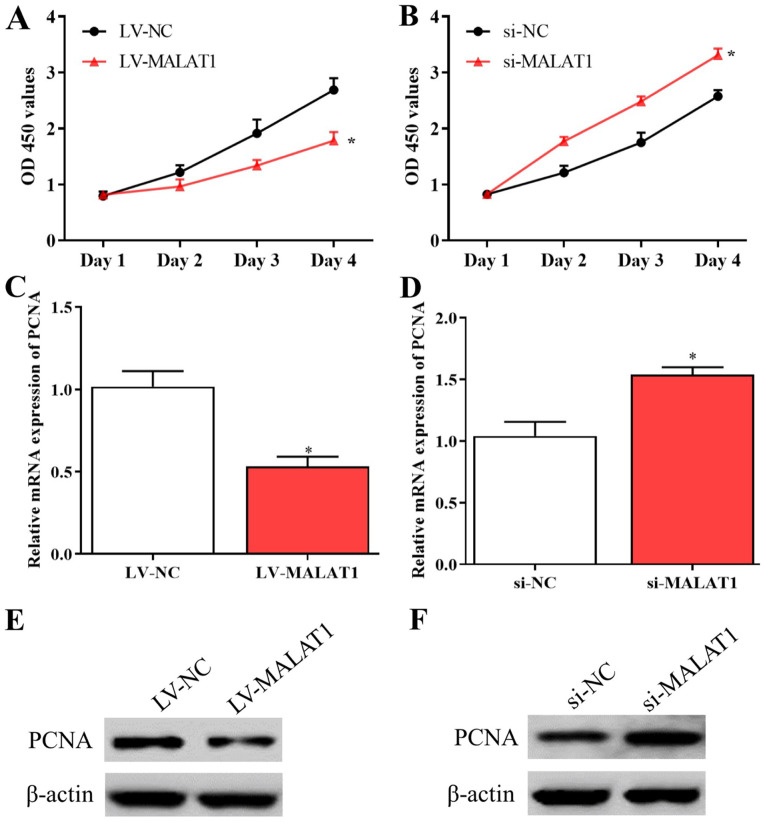
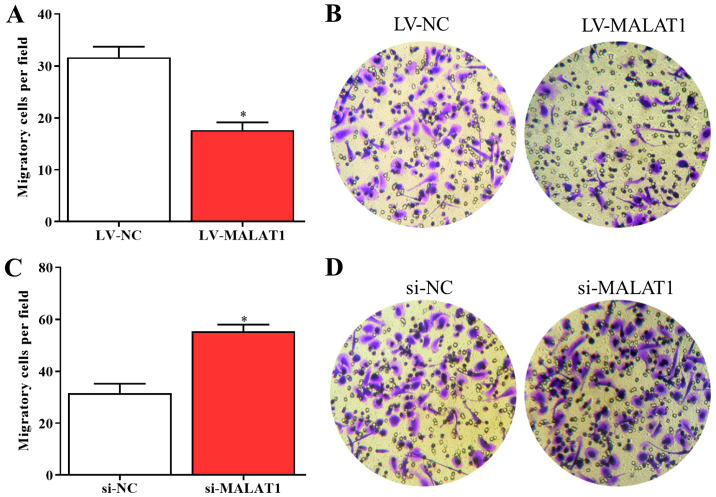
Further experiments were conducted to explore the effects of MALAT1 on the proliferation and migration of EPCs. The results of the MTT assay suggested that the proliferative activity of EPCs was inhibited by overexpressed MALAT1 and enhanced by silenced MALAT1 compared with negative controls (Fig. 2A and B). Consistent with these data, the levels of PCNA significantly decreased in EPCs overexpressing MALAT1 and significantly increased in cells with silenced MALAT1 compared with corresponding negative controls (Fig. 2C-F). In addition, the results of the Transwell assay indicated that the migration of LV-MALAT1-transfected EPCs significantly decreased whereas the migratory abilities of EPCs significantly increased by silenced MALAT1 compared with negative controls (Fig. 3A-D). The results revealed that the growth and migration of EPCs could be inhibited by upregulated MALAT1 and enhanced by downregulated MALAT1.
Figure 2.
MALAT1 affects the proliferation of endothelial progenitor cells. Proliferative activity of cells with (A) overexpressed and (B) silenced MALAT1 were examined using an MTT assay. Expression levels of PCNA mRNA in cells transfected with (C) LV-MALAT1 and (D) si-MALAT1. Expression levels of PCNA protein in cells transfected with (E) LV-MALAT1 and (F) si-MALAT1. *P<0.05 vs. si-NC at day 4. OD, optical density; PCNA, proliferating cell nuclear antigen; MALAT1, metastasis associated lung adenocarcinoma transcript 1; LV-MALAT1, wild-type MALAT1; si-MALAT1, small interfering RNA targeting MALAT1; NC, negative control.
Figure 3.
MALAT1 affects EPC migration (A) The migratory ability of EPCs was inhibited by overexpressed MALAT1. (B) A Transwell assay was performed to evaluate cell migration. (C) The migration of EPCs transfected with si-MALAT1 was enhanced by silenced MALAT1 compared with the control. (D) Cells transfected with si-MALAT1 or si-NC were subjected to Transwell assay. *P<0.05. EPCs, endothelial progenitor cells; MALAT1, metastasis associated lung adenocarcinoma transcript 1; LV-MALAT1, wild-type MALAT1; si-MALAT1, small interfering RNA targeting MALAT1; NC, negative control.
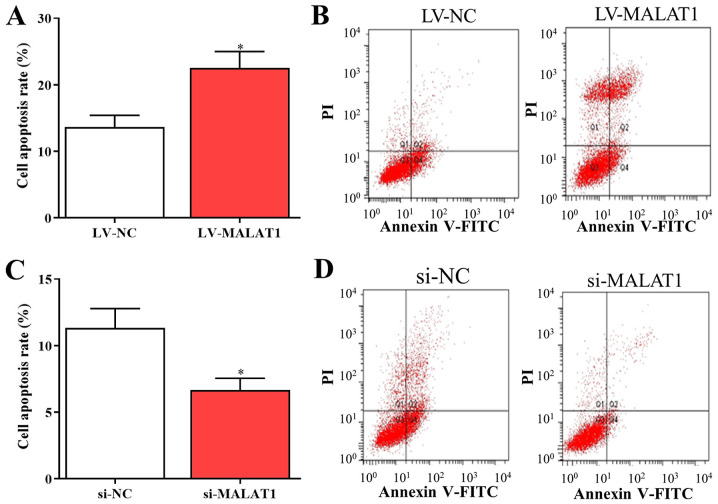
MALAT1 affects cell cycle arrest and apoptosis in EPCs
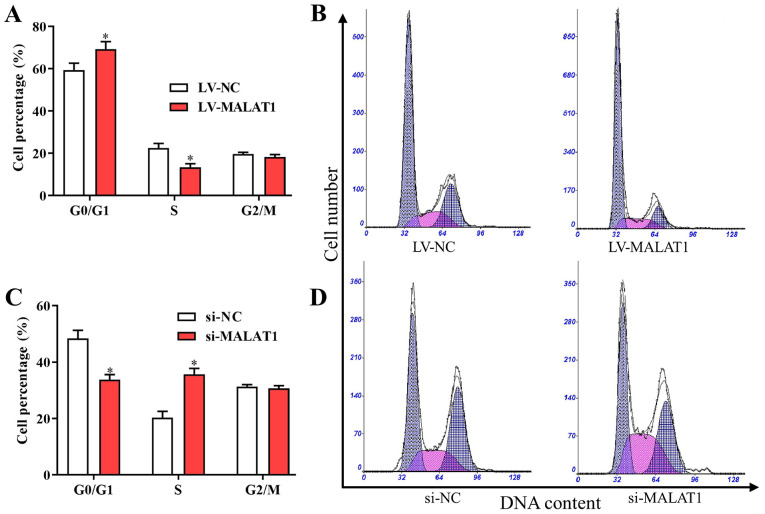
Based on the aforementioned findings, MALAT1 was associated with the proliferation and metastasis of EPCs in vitro. Furthermore, to investigate the potential effects of MALAT1 on the cell cycle and apoptosis, the cell cycle distribution and apoptosis of EPCs transfected with LV- MALAT1 or si-MALAT1 were also examined. The results indicated that the number of cells in G0/G1 was increased and the number of cells in S phase was decreased following transfection with LV-MALAT1 (Fig. 4A and B). In comparison, the percentage of cells in the G0/G1 phase significantly decreased, while cells in the S phase significantly increased in cells with silenced MALAT1 compared with negative controls (Fig. 4C and D). Additionally, flow cytometry data indicated that overexpressed MALAT1 induced apoptosis of EPCs (Fig. 5A and B), while cell apoptosis was significantly reduced by silenced MALAT1 compared with negative controls (Fig. 5C and D). These findings indicated that the upregulation of MALAT1 was able to arrest the cell cycle in the G0/G1 phase to promote cell apoptosis, and vice versa.
Figure 4.
MALAT1 affects cell cycle arrest in endothelial progenitor cells. (A and C) The (A) cell cycle distribution and (B) DNA content of EPCs transfected with LV-MALAT1 and LV-NC. The (C) cell cycle distribution and (D) DNA content in cells transfected with si-MALAT1 and si-NC. *P<0.05 vs. LV-NC. MALAT1, metastasis associated lung adenocarcinoma transcript 1; LV-MALAT1, wild-type MALAT1; si-MALAT1, small interfering RNA targeting MALAT1; NC, negative control.
Figure 5.
MALAT1 affects EPC apoptosis. (A) Bar graph and (B) flow cytometry plots showing overexpressed MALAT1 significantly increased EPC apoptosis. (C) Bar graph and (D) flow cytometry plots showing EPC apoptosis was significantly reduced by silenced MALAT1. *P<0.05. EPC, endothelial progenitor cells; PI, propidium iodide; MALAT1, metastasis associated lung adenocarcinoma transcript 1; LV-MALAT1, wild-type MALAT1; si-MALAT1, small interfering RNA targeting MALAT1; NC, negative control.
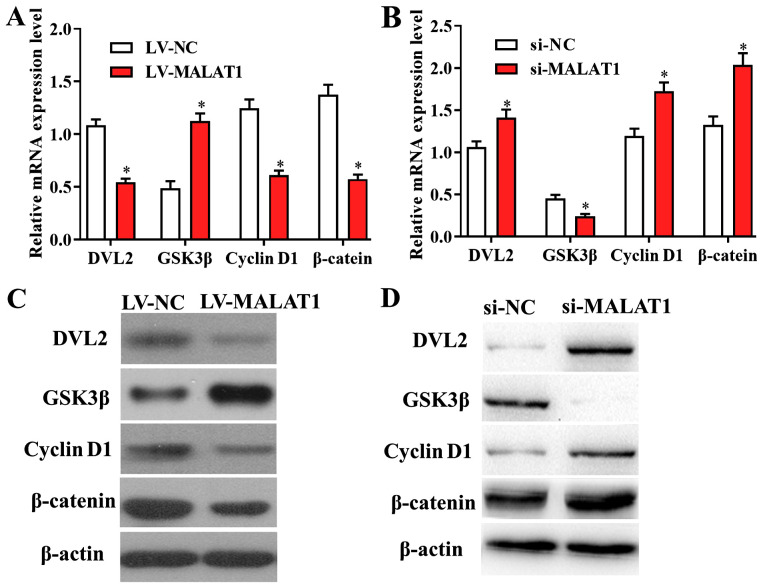
The Wnt/β-catenin signaling pathway is a potential downstream target of MALAT1 in DVT
Further experiments were performed to investigate whether MALAT1 modulates the proliferation and migration of EPCs though targeting its downstream signaling pathways. RT-qPCR results revealed that the levels of genes involved in Wnt/β-catenin signalling were altered in cells transfected with LV-MALAT1 compared with negative controls, suggesting that overexpressed MALAT1 significantly suppressed the activity of the Wnt/β-catenin pathway, and vice versa (Fig. 6A and B). In addition, these findings were confirmed by western blot analysis, as the protein levels of Wnt/β-catenin-associated genes were also affected by overexpressed or silenced MALAT1 in EPCs (Fig. 6C and D). The data suggested that the Wnt/β-catenin signalling pathway is a potential downstream target of MALAT1 in DVT.
Figure 6.
The Wnt/β-catenin signaling pathway is a potential target of MALAT1 in deep vein thrombosis. mRNA levels of Wnt/β-catenin-associated genes were affected by (A) LV-MALAT1 and (B) si-MALAT1 transfection, indicating overexpressed MALAT1 significantly altered the activity of the Wnt/β-catenin pathway, and vice versa. Western blot results confirmed that the protein levels of Wnt/β-catenin-related genes were also affected by (C) overexpressed or (D) silenced MALAT1 in endothelial progenitor cells. *P<0.05. MALAT1, metastasis associated lung adenocarcinoma transcript 1; LV-MALAT1, wild-type MALAT1; si-MALAT1, small interfering RNA targeting MALAT1; NC, negative control; DVL2, segment polarity protein dishevelled homolog DVL-2; GSK3β, glycogen synthase kinase 3β.
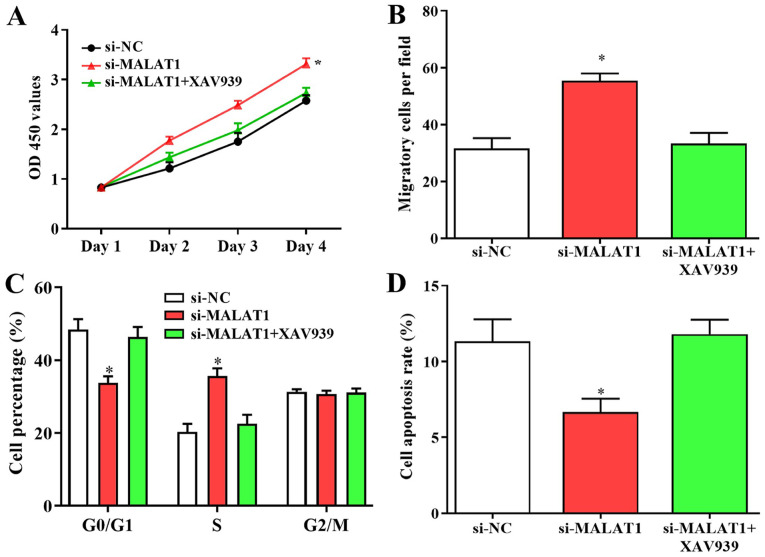
Wnt/β-catenin signaling is involved in the growth and migration of EPCs
Since the Wnt/β-catenin pathway may be a novel target of MALAT1 in DVT, its effects on the growth and migration of EPCs were also investigated. Enhanced cell proliferation and migration caused by downregulated MALAT1 was suppressed following treatment with the Wnt/β-catenin inhibitor XAV939 (Fig. 7A and B). Furthermore, the number of cells in G0/G1 phase was increased and the number of cells in S phase was decreased following XAV939 treatment, and suppressed apoptosis in cells transfected with si-MALAT1 were reversed by Wnt/β-catenin inhibitor transfection (Fig. 7C and D). The results revealed that changes in biological behaviors of EPCs caused by silenced MALAT1 were suppressed by the inhibition of Wnt/β-catenin signalling, suggesting that the Wnt/β-catenin signalling pathway may serve a role in MALAT1-modulated growth and the migration of EPCs.
Figure 7.
Wnt/β-catenin signaling is involved in the growth and migration of endothelial progenitor cells. Enhanced cell (A) proliferation and (B) migration caused by silenced MALAT1 was suppressed following treatment with the Wnt/β-catenin inhibitor XAV939. (C and D) (C) The shift of cells from G0/G1 to S stage and (D) suppressed apoptosis in cells transfected with si-MALAT1 were reversed by XAV939 treatment. *P<0.05 vs. si-NC. OD, optical density; MALAT1, metastasis associated lung adenocarcinoma transcript 1; LV-MALAT1, wild-type MALAT1; si-MALAT1, small interfering RNA targeting MALAT1; NC, negative control.
Discussion
In the present study, the detailed expression profile and potential functions of MALAT1 in DVT were elucidated. The results indicated that MALAT1 was upregulated in DVT samples compared with healthy controls. Additionally, MALAT1 was able to regulate the biological behaviors of EPCs, including proliferation, migration, cell cycle arrest and apoptosis. The growth and migration of EPCs was inhibited by upregulated MALAT1 and enhanced by downregulated MALAT1. Furthermore, upregulation of MALAT1 was able to arrest cell cycle in the G0/G1 phase to promote cell apoptosis, and vice versa. Consistent with the present findings, MALAT1 is involved in the growth and migration of various types of cells in numerous diseases, including esophageal squamous cell carcinoma, pancreatic cancer and colorectal cancer (25-27).
The growth and migration of EPCs involves a number of different pathways, and among these, the Wnt/β-catenin axis has been widely investigated. It serves essential roles in the development of various vascular diseases such as hypertensive heart disease (16-19). Furthermore, it is associated with the proliferation, differentiation and apoptosis of EPCs by regulating its downstream pathways including those of MYC and CDKN1A (20,21). Additionally, MALAT1 could regulate the growth and migration of numerous types of cells including neural stem cells and ovarian cancer cells, via the Wnt signaling pathway by affecting the levels of DVL2, GSK3β, β-catenin and cyclin D1 (22,23). In the present study, the data suggested that Wnt/β-catenin signaling is a novel downstream target of MALAT1 in DVT. The changes in biological behaviors in EPCs caused by silenced MALAT1 were reversed by the treatment with Wnt/β-catenin inhibitors. Enhanced cell proliferation and migration caused by downregulated MALAT1 was suppressed following treatment with Wnt/β-catenin inhibitor XAV939. Similarly, Wnt signaling can be regulated by other lncRNAs such as HOTAIR and LINC01197, which can affect cartilage damage and pancreatic adenocarcinoma cell proliferation (28,29). Consistent with the present findings, Wnt signaling blockage using XAV939 affected the growth and migration of numerous types of cells, such as lung adenocarcinoma A549 and breast cancer cells (30-32). Furthermore, the shift of cells from G0/G1 to S stage and suppressed apoptosis in cells transfected with si-MALAT1 were suppressed by Wnt/β-catenin inhibitors. However, there are some limitations to the present study. For example, a time-dependent model was observed for some indexes, but longer periods could be used till the maximum index value is reached. Additionally, the subcellular distribution of PCNA and β-catenin could be examined by immunostaining to investigate the nuclear expression of these genes. Furthermore, in vivo xenograft experiments should be performed to confirm the existing findings. Taken together, the MALAT1/Wnt/β-catenin axis could serve a role in the proliferation and migration of EPCs, contributing to the progression of DVT. This novel signaling pathway could be a potential therapeutic target for the treatment of patients with DVT.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BD and YD designed the study. BD, JW, SZ and XM performed the experiments and analyzed the data. BD and YD drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of The First Affiliated Hospital of Jinzhou Medical University (Jinzhou, China). Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sáez-Giménez B, Berastegui C, Loor K, López-Meseguer M, Monforte V, Bravo C, Santamaría A, Roman A. Deep vein thrombosis and pulmonary embolism after solid organ transplantation: An unresolved problem. Transplant Rev (Orlando) 2015;29:85–92. doi: 10.1016/j.trre.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Weitz JI, Eikelboom JW, Samama MM. New antithrombotic drugs: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141 (Suppl 2):e120S–e151S. doi: 10.1378/chest.11-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakogiannis C, Tousoulis D, Androulakis E, Briasoulis A, Papageorgiou N, Vogiatzi G, Kampoli AM, Charakida M, Siasos G, Latsios G, et al. Circulating endothelial progenitor cells as biomarkers for prediction of cardiovascular outcomes. Curr Med Chem. 2012;19:2597–2604. doi: 10.2174/092986712800492995. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Li X. Endothelial progenitor cells accelerate the resolution of deep vein thrombosis. Vascul Pharmacol. 2016;83:10–16. doi: 10.1016/j.vph.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Modarai B, Burnand KG, Sawyer B, Smith A. Endothelial progenitor cells are recruited into resolving venous thrombi. Circulation. 2005;111:2645–2653. doi: 10.1161/CIRCULATIONAHA.104.492678. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Li C, Li W, Kong L, Qian A, Hu N, Meng Q, Li X. MiR-150 enhances the motility of EPCs in vitro and promotes EPCs homing and thrombus resolving in vivo. Thromb Res. 2014;133:590–598. doi: 10.1016/j.thromres.2013.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Hsiao J, Yuan TY, Tsai MS, Lu CY, Lin YC, Lee ML, Lin SW, Chang FC, Liu Pimentel H, Olive C, et al. Upregulation of haploinsufficient gene expression in the brain by targeting a long non-coding RNA improves seizure phenotype in a model of Dravet syndrome. EBioMedicine. 2016;9:257–277. doi: 10.1016/j.ebiom.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holdt LM, Sass K, Gabel G, Bergert H, Thiery J, Teupser D. Expression of Chr9p21 genes CDKN2B (p15(INK4b)), CDKN2A (p16(INK4a), p14(ARF)) and MTAP in human atherosclerotic plaque. Atherosclerosis. 2011;214:264–270. doi: 10.1016/j.atherosclerosis.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Ballantyne M, Pinel K, Dakin R, Vesey A, Diver L, Mackenzie R, Garcia R, Welsh P, Sattar N, Hamilton G, et al. Smooth muscle enriched long noncoding RNA (SMILR) regulates cell proliferation. Circulation. 2016;133:2050–2065. doi: 10.1161/CIRCULATIONAHA.115.021019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallam T, Jones M, Thomas BJ, Wu X, Gilliland T, Qian K, Eskin A, Casero D, Zhang Z, Sandhu J, et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat Med. 2018;24:304–312. doi: 10.1038/nm.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulberdaa M, Scott E, Ballantyne M, Garcia R, Descamps B, Angelini GD, Brittan M, Hunter A, McBride M, McClure J, et al. A role for the long noncoding RNA SENCR in commitment and function of endothelial cells. Mol Ther. 2016;24:978–990. doi: 10.1038/mt.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii N, Ozaki K, Sato H, Mizuno H, Saito S, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, et al. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet. 2006;51:1087–1099. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 13.Vausort M, Wagner DR, Devaux Y. Long noncoding RNAs in patients with acute myocardial infarction. Circ Res. 2014;115:668–677. doi: 10.1161/CIRCRESAHA.115.303836. [DOI] [PubMed] [Google Scholar]
- 14.Arslan S, Berkan Ö, Lalem T, Özbilüm N, Göksel S, Korkmaz Ö, Çetin N, Devaux Y. Long non-coding RNAs in the atherosclerotic plaque. Atherosclerosis. 2017;266:176–181. doi: 10.1016/j.atherosclerosis.2017.10.012. Cardiolinc™ network. [DOI] [PubMed] [Google Scholar]
- 15.Zhuo Y, Zeng Q, Zhang P, Li G, Xie Q, Cheng Y. Functional polymorphism of lncRNA MALAT1 contributes to pulmonary arterial hypertension susceptibility in Chinese people. Clin Chem Lab Med. 2017;55:38–46. doi: 10.1515/cclm-2016-0056. [DOI] [PubMed] [Google Scholar]
- 16.Hermans KC, Blankesteijn WM. Wnt signaling in cardiac disease. Compr Physiol. 2015;5:1183–1209. doi: 10.1002/cphy.c140060. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Wang C, Wang C, Hong X, Miao J, Liao Y, Zhou L, Liu Y. An essential role for Wnt/β-catenin signaling in mediating hypertensive heart disease. Sci Rep. 2018;8(8996) doi: 10.1038/s41598-018-27064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen ED, Tian Y, Morrisey EE. Wnt signaling: An essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- 19.Gay A, Towler DA. Wnt signaling in cardiovascular disease: Opportunities and challenges. Curr Opin Lipidol. 2017;28:387–396. doi: 10.1097/MOL.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nayak G, Odaka Y, Prasad V, Solano AF, Yeo EJ, Vemaraju S, Molkentin JD, Trumpp A, Williams B, Rao S, Lang RN. Developmental vascular regression is regulated by a Wnt/β-catenin, MYC and CDKN1A pathway that controls cell proliferation and cell death. Development. 2018;145: pii(dev154898) doi: 10.1242/dev.154898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Y, Zhang S, Yu T, Du G, Zhang H, Yin Z. Wnt3a is critical for endothelial progenitor cell-mediated neural stem cell proliferation and differentiation. Mol Med Rep. 2016;14:2473–2482. doi: 10.3892/mmr.2016.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo C, Wang X, Chen LP, Li M, Li M, Hu YH, Ding WH, Wang X. Long non-coding RNA MALAT1 regulates ovarian cancer cell proliferation, migration and apoptosis through Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:3703–3712. doi: 10.26355/eurrev_201806_15249. [DOI] [PubMed] [Google Scholar]
- 23.Li GQ, Fang YX, Liu Y, Meng FR, Wu X, Zhang CW, Zhang Y, Liu D, Gao B. MALAT1-driven inhibition of Wnt signal impedes proliferation and inflammation in fibroblast-like synoviocytes through CTNNB1 promoter methylation in rheumatoid arthritis. Hum Gene Ther. 2019;30:1008–1022. doi: 10.1089/hum.2018.212. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Hu L, Wu Y, Tan D, Meng H, Wang K, Bai Y, Yang K. Up-regulation of long noncoding RNA MALAT1 contributes to proliferation and metastasis in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2015;34(7) doi: 10.1186/s13046-015-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Imanirad P, Jutooru I, Hedrick E, Jin UH, Rodrigues Hoffman A, Leal de Araujo J, Morpurgo B, Golovko A, Safe S. Role of metastasis-associated lung adenocarcinoma transcript-1 (MALAT-1) in pancreatic cancer. PLoS One. 2018;13(e0192264) doi: 10.1371/journal.pone.0192264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Zhang X, Hu X, Zhou W, Zhang P, Zhang J, Yang S, Liu Y. The effects of lncRNA MALAT1 on proliferation, invasion and migration in colorectal cancer through regulating SOX9. Mol Med. 2018;24(52) doi: 10.1186/s10020-018-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling J, Wang F, Liu C, Dong X, Xue Y, Jia X, Song W, Li Q. FOXO1-regulated lncRNA LINC01197 inhibits pancreatic adenocarcinoma cell proliferation by restraining Wnt/β-catenin signaling. J Exp Clin Cancer Res. 2019;38(179) doi: 10.1186/s13046-019-1174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W, He X, Chen Z, Fan D, Wang Y, Feng H, Zhang G, Lu A, Xiao L. LncRNA HOTAIR-mediated Wnt/β-catenin network modeling to predict and validate therapeutic targets for cartilage damage. BMC Bioinformatics. 2019;20(412) doi: 10.1186/s12859-019-2981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Zheng X, Han Y, Lv Y, Lan F, Zhao J. XAV939 inhibits the proliferation and migration of lung adenocarcinoma A549 cells through the WNT pathway. Oncol Lett. 2018;15:8973–8982. doi: 10.3892/ol.2018.8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilir B, Kucuk O, Moreno C. Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple-negative breast cancer cells. J Transl Med. 2013;11(280) doi: 10.1186/1479-5876-11-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bao R, Christova T, Song S, Angers S, Yan X, Attisano L. Inhibition of tankyrases induces Axin stabilization and blocks Wnt signalling in breast cancer cells. PLoS One. 2012;7(e48670) doi: 10.1371/journal.pone.0048670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.