Abstract
Expression of miR-409-5p in gestational diabetes mellitus (GDM) and its relationship with insulin resistance were explore. One hundred and forty-nine pregnant women who underwent antenatal examination in Taizhou First People's Hospital were divided into a GDM group and a control group according to whether they had GDM or not. Serum miR-409-5p expression of the two groups was detected, and the levels of glycosylated hemoglobin (HbAlc) and other GDM-related biochemical indicators were measured. Fasting plasma glucose (FPG) was determined by glucose oxidase method, fasting insulin (FINS) was detected by radioimmunoassay, and homeostasis model assessment of insulin resistance (HOMA-IR) was calculated. The relationship between miR-409-5p and other biochemical indicators and insulin resistance was analyzed, and logistic multivariate regression was employed to analyze the risk factors of GDM. miR-409-5p was highly expressed in the serum of GDM patients. HbAlc, FPG, FINS, and HOMA-IR in pregnant women in the GDM group were markedly higher than those in the control group. The serum miR-409-5p in GDM pregnant women showed a positive correlation with HbAlc, FPG, FINS, and HOMA-IR (P<0.05). The insulin resistance group presented remarkably higher serum miR-409-5p level than the non-insulin resistance group. Moreover, it was found that elevated miR-409-5p, FINS, and HOMA-IR were all independent risk factors for the onset of GDM. miR-409-5p is highly expressed in the serum of patients with GDM, and it is positively correlated with insulin resistance index of GDM patients, which may be a potential target for clinical diagnosis and treatment of GDM.
Keywords: miR-409-5p, gestational diabetes mellitus, insulin resistance
Introduction
Gestational diabetes mellitus (GDM), as one of the most frequent complications in pregnancy, is also one of the common threats to the life and safety of mothers and infants (1). It can easily lead to premature delivery, malformation and even miscarriage and dystocia of pregnant women (2). In recent years, the incidence of GDM has increased, which brings a heavy burden on families and society (3). However, except some studies (4,5) indicating that insulin resistance during pregnancy may trigger GDM, its pathogenesis remains poorly understood. The complex pathogenesis of GDM results in a lack of effective GDM screening markers during pregnancy, which is unfavorable for the diagnosis of GDM (6).
As a kind of non-coding micro-RNA, the post-transcriptional level of miRNA is closely related to the pathophysiological processes of many diseases (7). Studies (8,9) in recent years have shown that miRNA is one of the essential biological regulators in insulin resistance, and many miRNAs have been proved to be effective diagnostic markers for GDM. For example, a study (10) pointed out that miR-330-3p was upregulated in GDM and was related to the patient's insulin resistance. Other studies have further explored the role of miRNA in GDM. Some scholars (11) found that miR-494 protected the pancreatic β-cell function of GDM by targeting PTEN. Among them, miR-409-5p has been proven to play a role as an oncogene in a variety of tumors. For example, a study (12) found that miR-409-5p was up-regulated in breast cancer, and inhibiting its expression can significantly suppress the growth of breast cancer cells. While recent evidence (13) revealed that miR-409-5p is associated with the occurrence of diabetes. However, the relationship between miR-409-5p and GDM has not been studied yet.
Therefore in this study, we explored the expression of miR-409-5p in GDM and its relationship with insulin resistance, so as to provide more possibilities for the clinical diagnosis and mechanism research of GDM.
Patients and methods
General information
A total of 149 pregnant women, including 76 patients with GDM (GDM group) and 73 normal pregnant women (control group), with an average age of 27.02±3.17 years, who underwent antenatal examination in the hospital from April 2015 to January 2017 were selected as the study participants. Inclusion criteria: The GDM group included patients who were diagnosed and met the diagnostic criteria of GDM (14). Glucose screening is currently recommended at 24-28 weeks of gestation (15), so patients diagnosed during their pregnancy were included. Exclusion criteria: Patients with diabetes before pregnancy; patients with other complications during pregnancy; patients with multiple pregnancies; patients with malignant tumors; patients with severe infectious diseases; patients who had used glucocorticoids; patients with severe hepatic and renal dysfunction; patients who refused to participate in the study. The patients and their families agreed to participate in the study and signed an informed consent form. This study was approved by the Ethics Committee of Taizhou First People's Hospital (Taizhou, China) (TZFPHPH201503A), and conformed to the Declaration of Helsinki.
Detection of miR-409-5p expression by RT-PCR
Fasting venous blood samples were taken from all the patients, centrifuged at 4˚C for 10 min at 1,500 x g, and the obtained serum (3 ml) was processed for detection. The total RNA in serum was extracted with TRIzol reagent (Thermo Fisher Scientific, Inc.), and its purity and concentration were detected by ultraviolet spectrophotometer. Then 5 µg of the total RNA was taken for reverse transcription of cDNA according to the instructions of the reverse transcription kit (TransGen Biotech). The amplification system of miR-409-5p (PCR kit, TransGen Biotech) was as follows: cDNA: 1 µl, upstream and downstream primers (concentrations: 10 µmol/l): 0.4 µl each, 2X TransTaq® Tip Green qPCR SuperMix: 10 µl, Passive Reference Dye (50X): 0.4 µl, and finally ddH2O was added to a total volume of 20 µl. Amplification conditions: PCR reaction conditions: pre-denaturation at 94˚C for 45 sec, denaturation at 94˚C for 10 sec, annealing at 60˚C for 45 sec, totaling 40 cycles. Three replicate wells were set for each sample and the experiment was performed three times. With U6 as the internal reference, 2-∆∆ct was employed to analyze the data. Primer sequences: miR-409-5p: F, 5'-AGGTTACCC GAGCAACTTTG-3', R, 5'-GTGTCGTGGAGTCGGCAA-3'; U6: F, 5'-GCTTCGGCAGCACATATACTAAAAAT-3', R, 5'-CGCTTCACGAATTTGCGTGTCAT-3'.
Detection of other biochemical indicators
The plasma triacylglycerol (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were detected by automatic standard routine enzymatic methods (Abbott Aeroset 2000, Abbott Corp.). The level of glycosylated hemoglobin (HbAlc) was determined by an automatic biochemical analyzer. Fasting plasma glucose (FPG) was measured by glucose oxidase method, and fasting insulin (FINS) was tested by radioimmunoassay. The homeostatic model assessment (HOMA) was calculated by the formulate of HOMA-insulin resistance (HOMA-IR) index (16) = (FBG x FINS)/22.5.
Statistical methods
The experimental data in this study was statistically analyzed using SPSS 19.0. The counting data were verified by the chi-square test, while the measurement data were expressed as mean ± standard deviation. The inter-group comparison was performed by the independent t-test. GraphPad Prism 6 software was adopted to draw the required illustrations of this experiment, and Pearson was employed for correlation analysis. Receiver operating characteristic (ROC) curve was used to analyze the diagnostic value of miR-409-5p in GDM. P<0.05 indicates a statistically significant difference.
Results
Patient information
There were no significant differences between the two groups in terms of age, body mass index (BMI) or gravidity (P>0.05), but there were differences in TG, TC, and HDL-C (P<0.001) (Table I).
Table I.
Patient information.
| Factors | GDM group n=76 | Control group n=73 | t/χ2 | P-value |
|---|---|---|---|---|
| Age, years | 0.001 | 0.970 | ||
| ≤27 | 45 (59.21) | 43 (58.90) | ||
| >27 | 31 (40.79) | 30 (41.10) | ||
| Pre-pregnancy BMI, kg/m2 | 0.003 | 0.954 | ||
| ≤23 | 42 (55.26) | 40 (54.79) | ||
| >23 | 34 (44.74) | 33 (45.21) | ||
| Educational level | 0.001 | 0.975 | ||
| Below junior high school | 21 (27.63) | 20 (27.40) | ||
| Junior high school or above | 55 (72.37) | 53 (72.60) | ||
| Place of residence | 0.016 | 0.901 | ||
| Rural | 32 (42.11) | 30 (41.10) | ||
| Urban | 44 (57.89) | 43 (58.90) | ||
| Gravidity, times | 0.002 | 0.961 | ||
| ≤1 | 57 (75.00) | 55 (75.34) | ||
| >1 | 19 (25.00) | 18 (24.66) | ||
| Gestational week of delivery | 38.21±2.01 | 38.45±2.08 | 0.716 | 0.475 |
| TC (mmol/l) | 5.93±0.47 | 5.45±0.41 | 6.632 | <0.001 |
| TG (mmol/l) | 2.81±0.54 | 1.97±0.32 | 11.49 | <0.001 |
| LDL-C (mmol/l) | 2.74±0.48 | 2.63±0.43 | 1.471 | 0.143 |
| HDL-C (mmol/l) | 1.29±0.26 | 1.67±0.37 | 7.277 | <0.001 |
GDM, gestational diabetes mellitus; BMI, body mass index; TC, total cholesterol; TG, triacylglycerol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Expression and diagnostic value of miR-409-5p in the two groups
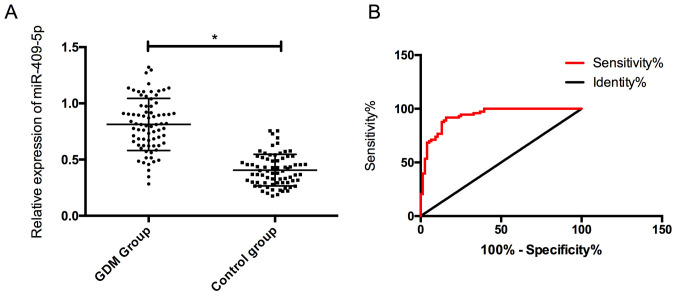
The expression of serum miR-409-5p in the GDM group was (0.78±0.24), which was noticeably higher than (0.42±0.14) in the control group, and the difference was statistically significant (P<0.05). In addition, it was found that the the ROC curve of miR-409-5p in the diagnosis of GDM was 0.933, indicating a high diagnostic value (Fig. 1).
Figure 1.
Expression and diagnostic value of miR-409-5p in the two groups. (A) miR-409-5p expression of pregnant women in the two groups. (B) The diagnostic ROC curve of miR-409-5p for GDM. *P<0.05. ROC, receiver operating characteristic; GDM, gestational diabetes mellitus.
Comparison of other biochemical indicators between the two groups
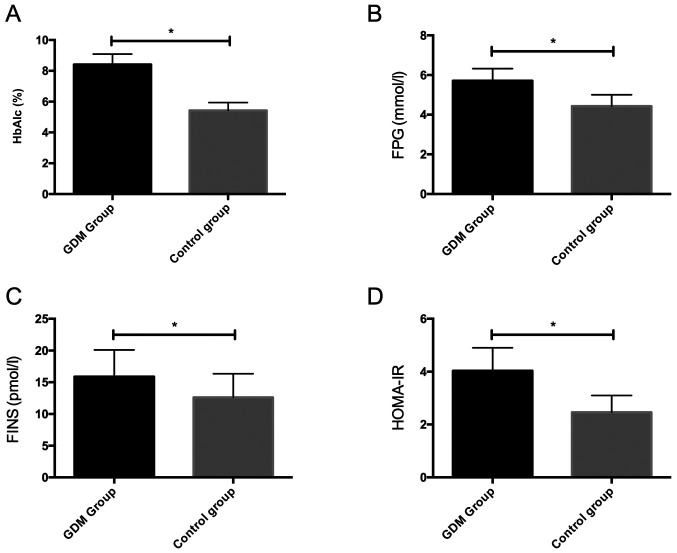
We detected HbAlc, FPG and FINS of the two groups of pregnant women, and calculated HOMA-IR. The results showed that HbAlc, FPG, FINS and HOMA-IR in the GDM group were remarkably higher than those in the control group, with statistically significant differences (P<0.05) (Fig. 2).
Figure 2.
Comparison of biochemical indicators between the two groups. (A) Comparison of serum HbAlc between the two groups. (B) Comparison of serum FPG between the two groups. (C) Comparison of serum FINS between the two groups. (D) Comparison of HOMA-IR between the two groups. *P<0.05. HbAlc, glycosylated hemoglobin; FPG, fasting plasma glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance.
Correlation of serum miR-409-5p with HbAlc, FPG, FINS and HOMA-IR in pregnant women with GDM
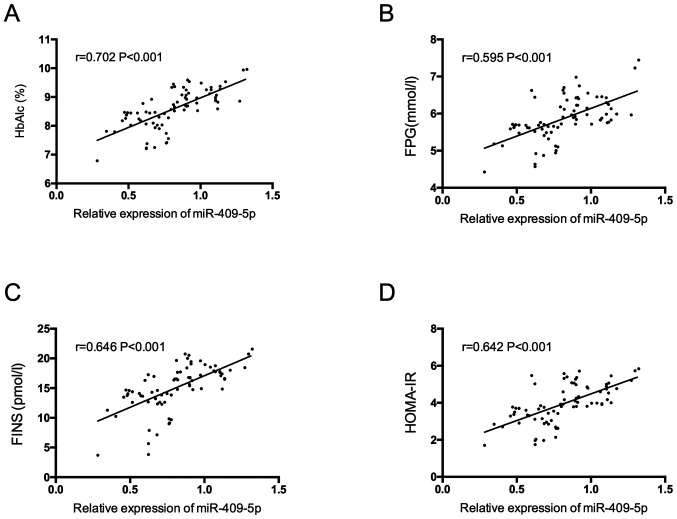
miR-409-5p was positively correlated with HbAlc, FPG, FINS and HOMA-IR in pregnant women with GDM (P<0.05) (Fig. 3).
Figure 3.
Correlation of serum miR-409-5p with HbAlc, FPG, FINS and HOMA-IR in pregnant women with GDM. (A) The relationship between miR-409-5p and HbAlc in pregnant women with GDM. (B) The relationship between miR-409-5p and FPG in pregnant women with GDM. (C) The relationship between miR-409-5p and FINS in pregnant women with GDM. (D) The relationship between serum miR-409-5p and HOMA-IR in pregnant women with GDM. HbAlc, glycosylated hemoglobin; FPG, fasting plasma glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance; GDM, gestational diabetes mellitus.
Expression of miR-409-5p in patients with different insulin resistance
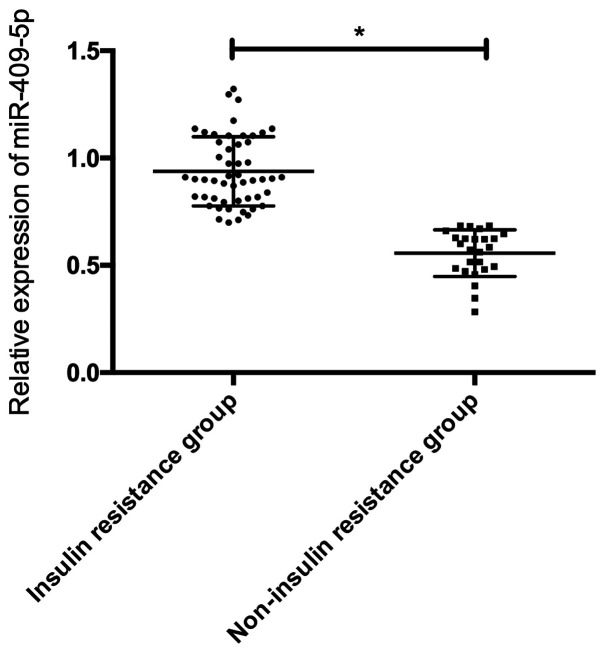
According to the insulin resistance index, the GDM patients were divided into 51 cases of insulin resistance group (HOMA-IR ≥1.66) and 25 cases of non-insulin resistance group (HOMA-IR <1.66) to compare the serum miR-409-5p expression in the two groups. The results showed that serum miR-409-5p level in the insulin resistance group was dramatically higher than that in the non-insulin resistance group, and the difference was statistically significant (P<0.05) (Fig. 4).
Figure 4.
Expression of miR-409-5p in patients with different insulin resistance. *P<0.05.
Multivariate analysis of the occurrence of GDM
Based on the above results, we found that the occurrence of GDM was associated with serum miR-409-5p, HbAlc, FPG, FINS, and HOMA-IR. These factors were listed as independent variables and assigned values, and took GDM as the dependent variable for multivariate analysis by logistic regression analysis (Table II). The results revealed that the increase of miR-409-5p, FINS and HOMA-IR were all independent risk factors for GDM.
Table II.
Multivariate analysis.
| Factors | β | S.E | Wald | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| miR-409-5p | 0.343 | 0.157 | 5.442 | 1.465 | 1.008-1.871 | <0.05 |
| HbAlc | 0.113 | 0.508 | 0.294 | 1.061 | 0.742-1.674 | 0.153 |
| FPG | 0.228 | 0.384 | 0.341 | 1.249 | 0.903-1.714 | 0.129 |
| FINS | 0.421 | 0.128 | 6.237 | 1.745 | 1.166-2.354 | <0.05 |
| HOMA-IR | 0.509 | 0.086 | 8.626 | 2.432 | 1.505-3.774 | <0.05 |
HbAlc, glycosylated hemoglobin; FPG, fasting plasma glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance; SE, standard error; OR, odds ratio.
Discussion
GDM is considered to be a severe glucose intolerance during pregnancy. In recent years, increasing number of women have been diagnosed with GDM, which has become a common public health problem (17). For many patients, untimely diagnosis and treatment will inevitably bring severe adverse effects on the mother and fetus (18). As one of the mechanisms of GDM, insulin resistance has been reported in previous research (19) indicating that many pregnant women with GDM have more severe insulin resistance than non-pregnant women, the possible mechanism of action, however, remains unknown.
It is well-established that miRNAs play a vital part in the occurrence and progression of many diseases, including diabetes (20,21). For example, research (22) identified that the serum miR-132 expression in patients with GDM was decreased, and it may be used as a biomarker for the diagnosis of GDM. In this study, we found that the expression of miR-409-5p was up-regulated in the serum of GDM patients, and ROC analysis exhibited that it had a high diagnostic value for GDM. Previous studies on miR-409-5p mainly focused on its role in tumors. For example, a study (23) revealed that miR-409 inhibited the invasion and metastasis of tumor cells by directly targeting the radixin in gastric cancer. Another study (24) showed that miR-409 inhibited the development of non-small cell lung cancer (NSCLC) by directly targeting SPIN1. In our study, for the first time it was found that miR-409-5p was up-regulated in GDM, which provides certain ideas and directions for follow-up research.
To further analyze the relationship between miR-409-5p and GDM patients, we tested other relevant biochemical indicators of GDM and calculated HOMA-IR. The results demonstrated that compared with normal pregnant women, the HbAlc, FPG, FINS and HOMA-IR were significantly increased in GDM patients, and correlation analysis revealed a positive correlation between miR-409-5p and serum HbAlc, FPG, FINS and HOMA-IR. As is know, insulin resistance is an important index for evaluating islet cell function in diabetes mellitus (25), our study found there was a positive correlation between miR-409-5p and insulin resistance index in patients with GDM. Subsequently, we compared the expression of miR-409-5p in patients with different insulin resistance, and noted that the serum miR-409-5p level in patients with insulin resistance was significantly higher than that without, which validated the previous idea on the one hand, and on the other hand, it suggested that miR-409-5p may affect the function of islet cells, thus affecting the pathogenesis of GDM. However, more basic experiments and clinical studies are needed to confirm this hypothesis. Many studies have explored the effect of miRNA on islet function. For example, evidence (26) found that miR-375 was highly expressed in islet cells, which could inhibit glucose-induced insulin secretion. Another study (27) conducted animal experiments and also pointed out that miR-29 was highly expressed in diabetic rats and could induce insulin resistance. Finally, we analyzed the causes of GDM, and the results showed that the increase of miR-409-5p, FINS, and HOMA-IR were all independent risk factors for GDM. Studies in the past (28,29) indicated that insulin resistance may be one of the causes of GDM, but the possible effect of miR-409-5p in GDM has not been studied yet. The present study is the first that found miR-409-5p may be an independent risk factor for the occurrence of GDM, which we speculate may be related to the association between miR-409-5p and insulin resistance, but more basic experiments are yet to be performed for confirmation.
In conclusion, miR-409-5p is highly expressed in the serum of GDM patients, and it is positively correlated with insulin resistance index of GDM patients, which may be a potential target for clinical diagnosis and treatment of GMD. However, this study also has deficiencies. Relevant basic experiments in vivo and in vitro, which are absent in the present study, are needed to support our conclusions. As there are relatively few studies on miR-409-5p in GDM, our conclusions need to be further confirmed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CT conceived the study and drafted the manuscript. LW collected the general information and analyzed the data. HT and LG detected miR-409-5p expression using RT-PCR. SZ and XC were responsible for the detection of the biochemical indicators. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Taizhou First People's Hospital (Taizhou, China) (TZFPHPH201503A), and conformed to the Declaration of Helsinki. Patients who participated in this research signed an informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341–347. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan EP, Avalos G, O'Reilly MW, Dennedy MC, Gaffney G, Dunne F. Atlantic Diabetes in Pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia. 2016;59:873–873. doi: 10.1007/s00125-016-3888-5. Atlantic DIP collaborators: Erratum to. [DOI] [PubMed] [Google Scholar]
- 3.Lee KH, Han YJ, Chung JH, Kim MY, Ryu HM, Kim JH, Kwak DW, Kim SH, Yang S, Kim M. Treatment of gestational diabetes diagnosed by the IADPSG criteria decreases excessive fetal growth. Obstet Gynecol Sci. 2020;63:19–26. doi: 10.5468/ogs.2020.63.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Leng J, Li W, Zhang C, Feng L, Wang P, Chan JCN, Hu G, Yu Z, Yang X. Roles of insulin resistance and beta cell dysfunction in macrosomia among Chinese women with gestational diabetes mellitus. Prim Care Diabetes. 2018;12:565–573. doi: 10.1016/j.pcd.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaat N, Ignell C, Katsarou A, Berntorp K. Glucose homeostasis, beta cell function, and insulin resistance in relation to vitamin D status after gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2017;96:821–827. doi: 10.1111/aogs.13124. [DOI] [PubMed] [Google Scholar]
- 6.Bartolo S, Vambergue A, Deruelle P. Screening for gestational diabetes: Still many unsolved issues. J Gynecol Obstet Biol Reprod (Paris) 2016;45:105–111. doi: 10.1016/j.jgyn.2015.12.004. (In French) [DOI] [PubMed] [Google Scholar]
- 7.Pheiffer C, Dias S, Rheeder P, Adam S. Decreased expression of circulating miR-20a-5p in South African women with gestational diabetes mellitus. Mol Diagn Ther. 2018;22:345–352. doi: 10.1007/s40291-018-0325-0. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Xing W, Xie L. Regulation of microorganisms in diabetes. Int J Mol Sci. 2016;17(1729) doi: 10.3390/ijms17101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang XW, Qin QX. miR-335-5p induces insulin resistance and pancreatic islet β-cell secretion in gestational diabetes mellitus mice through VASH1-mediated TGF-β signaling pathway. J Cell Physiol. 2019;234:6654–6666. doi: 10.1002/jcp.27406. [DOI] [PubMed] [Google Scholar]
- 10.Sebastiani G, Guarino E, Grieco GE, Formichi C, Delli Poggi C, Ceccarelli E, Dotta F. Circulating microRNA (miRNA) expression profiling in plasma of patients with pestational diabetes mellitus reveals upregulation of miRNA miR-330-3p. Front Endocrinol. 2017;8(345) doi: 10.3389/fendo.2017.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y, Bai J, Liu P, Dong J, Tang Y, Zhou J, Han P, Xing J, Chen Y, Yu X. miR-494 protects pancreatic β-cell function by targeting PTEN in gestational diabetes mellitus. EXCLI J. 2017;16:1297–1307. doi: 10.17179/excli2017-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H, Xing H, Han W, Wang Y, Qi T, Song C, Xu Z, Li H, Huang Y. MicroRNA-409-5p is upregulated in breast cancer and its downregulation inhibits cancer development through downstream target of RSU1. Tumour Biol. 2017;39(1010428317701647) doi: 10.1177/1010428317701647. [DOI] [PubMed] [Google Scholar]
- 13.Massaro JD, Polli CD, Costa E Silva M, Alves CC, Passos GA, Sakamoto-Hojo ET, Rodrigues de Holanda Miranda W, Bispo Cezar NJ, Rassi DM, Crispim F, et al. Post-transcriptional markers associated with clinical complications in Type 1 and Type 2 diabetes mellitus. Mol Cell Endocrinol. 2019;490:1–14. doi: 10.1016/j.mce.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, et al. International Association of Diabetes and Pregnancy Study Groups Consensus Panel: International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demir E, Ozkan H, Seckin KD, Sahtiyancı B, Demir B, Tabak O, Kumbasar A, Uzun H. doi: 10.3390/biom9010024. Plasma zonulin levels as a non-invasive biomarker of intestinal permeability in women with gestational diabetes mellitus. Biomolecules: Jan 11, 2019 (Epub ahead of print). doi: 10.3390/biom9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Winden K, Montoro M, Korst LM, Ouzounian JG. A homeostatic model assessment of insulin resistance (HOMA-IR) relates to gestational diabetes, glycemic control [1K] Obstet Gynecol. 2017;129(112S) [Google Scholar]
- 17.Wei YM, Yan J, Yang HX. Identification of severe gestational diabetes mellitus after new criteria used in China. J Perinatol. 2016;36:90–94. doi: 10.1038/jp.2015.151. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Yang T, Miao J, Liu H, Wu K, Guo J, Chen J, Li T. Correlation between maternal and fetal insulin resistance in pregnant women with gestational diabetes mellitus. Clin Lab. 2018;64:945–953. doi: 10.7754/Clin.Lab.2018.171214. [DOI] [PubMed] [Google Scholar]
- 19.Ebert T, Hindricks J, Kralisch S, Lossner U, Jessnitzer B, Richter J, Blüher M, Stumvoll M, Fasshauer M. Serum levels of fractalkine are associated with markers of insulin resistance in gestational diabetes. Diabet Med. 2014;31:1014–1017. doi: 10.1111/dme.12451. [DOI] [PubMed] [Google Scholar]
- 20.Wander PL, Boyko EJ, Hevner K, Parikh VJ, Tadesse MG, Sorensen TK, Williams MA, Enquobahrie DA. Circulating early- and mid-pregnancy microRNAs and risk of gestational diabetes. Diabetes Res Clin Pract. 2017;132:1–9. doi: 10.1016/j.diabres.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Wang S, Li H, Wan J, Zhou Q, Zhou Y, Zhang C. microRNA-96 protects pancreatic β-cell function by targeting PAK1 in gestational diabetes mellitus. Biofactors. 2018;44:539–547. doi: 10.1002/biof.1461. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Xiang C, Zheng X. miR-132 serves as a diagnostic biomarker in gestational diabetes mellitus and its regulatory effect on trophoblast cell viability. Diagn Pathol. 2019;14(119) doi: 10.1186/s13000-019-0899-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng B, Liang L, Huang S, Zha R, Liu L, Jia D, Tian Q, Wang Q, Wang C, Long Z, et al. MicroRNA-409 suppresses tumour cell invasion and metastasis by directly targeting radixin in gastric cancers. Oncogene. 2012;31:4509–4516. doi: 10.1038/onc.2011.581. [DOI] [PubMed] [Google Scholar]
- 24.Song Q, Ji Q, Xiao J, Li F, Wang L, Chen Y, Xu Y, Jiao S. miR-409 inhibits human non-small-cell lung cancer progression by directly targeting SPIN1. Mol Ther Nucleic Acids. 2018;13:154–163. doi: 10.1016/j.omtn.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Retnakaran R, Ye C, Connelly PW, Hanley AJ, Sermer M, Zinman B. Serum apoA1 (Apolipoprotein A-1), insulin resistance, and the risk of gestational diabetes mellitus in human pregnancy - brief report. Arterioscler Thromb Vasc Biol. 2019;39:2192–2197. doi: 10.1161/ATVBAHA.119.313195. [DOI] [PubMed] [Google Scholar]
- 26.Kraus M, Greither T, Wenzel C, Bräuer-Hartmann D, Wabitsch M, Behre HM. Inhibition of adipogenic differentiation of human SGBS preadipocytes by androgen-regulated microRNA miR-375. Mol Cell Endocrinol. 2015;414:177–185. doi: 10.1016/j.mce.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Dooley J, Garcia-Perez JE, Sreenivasan J, Schlenner SM, Vangoitsenhoven R, Papadopoulou AS, Tian L, Schonefeldt S, Serneels L, Deroose C, et al. The microRNA-29 family dictates the balance between homeostatic and pathological glucose handling in diabetes and obesity. Diabetes. 2016;65:53–61. doi: 10.2337/db15-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersson-Hall U, Gustavsson C, Pedersen A, Malmodin D, Joelsson L, Holmäng A. Higher concentrations of BCAAs and 3-HIB are associated with insulin resistance in the transition from gestational diabetes to type 2 diabetes. J Diabetes Res. 2018;2018(4207067) doi: 10.1155/2018/4207067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J-Y, Wu G-M, Hou Z, Cao YM. Expression of C1q/TNF-related protein-3 (CTRP3) in serum of patients with gestational diabetes mellitus and its relationship with insulin resistance. Eur Rev Med Pharmacol Sci. 2017;21:5702–5710. doi: 10.26355/eurrev_201712_14016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.