Abstract
Vaginitis, also known as vulvovaginitis, is an inflammation of the vagina and vulva and a common disease in females. It is thought to be caused by vaginal dysbiosis and improved by probiotics. Bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC) are the major types of vaginal infections. The present systematic review and meta-analysis aimed to clarify the efficacy of probiotics in the treatment of common vaginal infections in non-pregnant females. Literature on randomized controlled trials and two-armed prospective studies on any intervention with probiotics published until December 24th, 2018 was searched in the PubMed, Cochrane and EMBASE databases. The outcomes of interest were recurrence rate, cure rate, remission rate and normal vaginal flora restoration. Finally, a total of 30 studies on bacterial vaginosis (BV) and/or VVC were included and stratified into 3 study types based on treatment design as follows: Type I, antibiotic/probiotics vs. antibiotics/antifungals (22 studies); Type II, probiotics vs. placebo (5 studies); Type III, probiotics vs. antibiotics (3 studies). The type I studies comprised 1,788 non-pregnant females and had the highest inter-study comparability in post-treatment follow-up design and meta-analysis outcome data. Probiotics interventions were significantly associated with a lower recurrence rate of vaginitis [pooled odds ratio (OR)=0.27, 95% CI: 0.18-0.41, P<0.001] and higher cure/remission rate (pooled OR=2.28, 95% CI: 1.20-4.32, P=0.011). However, a significant increase in normal vaginal flora after probiotic treatment was observed only in BV (pooled OR=4.55, 95% CI: 1.44-14.35, P=0.01). In addition, supportive but heterogeneous results were obtained from the 6-month follow-up data of Type-I studies, different infection types and supplementary analysis of Type-II studies. In conclusion, probiotics have a significant short-term effect in the treatment of common vaginal infections in non-pregnant females. In order to evaluate the long-term effects of probiotics in common vaginal infections, it is worthwhile to perform higher-quality clinical trials in the future.
Keywords: probiotics, Lactobacillus, vaginitis, bacterial vaginosis, vulvovaginal candidiasis, meta-analysis
Introduction
Vaginal infections of bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC) are common in females, accounting for almost 80% of all cases of vaginitis also known as vulvovaginitis, is an inflammation of the vagina and vulva. Symptoms may include itching, burning, pain, discharge and a bad odor (1,2). While BV is generally regarded as a mild disease, it has been indicated to be associated with the occurrence of endometritis and pelvic inflammatory disease in females without clinical symptoms of BV and may lead to spontaneous abortion, premature rupture of the membranes, and premature delivery during pregnancy (2,3). VVC results from overgrowth of one or more types of yeast organism (e.g., Candida albicans) that normally inhabit the vaginal mucosa in small numbers, and symptoms include external dysuria, pruritus, redness and flocculant vaginal discharge (2,4). In most cases, standard treatments with antibiotics or anti-fungals are effective for BV and VVC. However, the use of antibiotics may cause physiological and non-physiological changes in patients, and interfere with the balance of the normal vaginal microbiota. Thus, the common side-effects of antibiotic treatment are characterized by reduction or depletion of the Lactobacillus species and the excessive growth of Candida species. In addition, excessive use of antibiotics frequently causes the emergence of resistant strains.
Probiotics are defined as ‘live microorganisms when administered in adequate amounts confer a health benefit to the host’ (5). Over the past 2 decades, accumulating evidence has indicated that the intestinal and urogenital microflora has a central role in maintaining the health of human beings (5). In addition, the use of beneficial bacteria to improve dysbiosis by replacing pathogenic bacteria or augmenting normal microflora has been gradually accepted and proven useful in conditions including necrotizing enterocolitis and antibiotic-resistant infections (5). The intestinal, vaginal and urethral microflora have an important role in maintaining health and preventing gynecologic infections in females, and the use of probiotics has been extended to the treatment of refractory cases of female urogenital infections (5).
The use of probiotics has been examined in a number of studies over the past 2 decades as a method of treating and reducing the risk and recurrence rate of gynecologic infections in females, particularly in whom standard treatments are not effective. Probiotics may protect the vagina from pathogen colonization through a number of mechanisms, including blocking potential sites of attachment, production of microbiocidal substances, e.g. hydrogen peroxide, maintenance of a low pH and induction of anti-inflammatory cytokine responses in epithelial cells (3-5). The most common probiotics used in female patients are of the Lactobacillus species (3-5).
While numerous clinical trials have been performed to determine the effectiveness of probiotics for the treatment of vaginal infections, the results have generally been inconsistent, with certain studies suggesting an excellent response and other indicating no effect. Meta-analyses have also provided inconsistent results. A meta-analysis by Huang et al (3) from 2014 indicated that probiotic supplementation improves the cure rate for BV. Other previously published systematic reviews have suggested that the use of probiotics remains controversial in preventing BV and VVC in adult females due to evidence limitations (4,6,7). Potential bias on the benefits of probiotics cannot be ruled out, as the majority of evidence came from small-scale studies, heterogeneous populations, different lengths of follow-up and inhomogeneous treatment designs among the study. Similar views were also expressed by a recently published systemic review by Hanson et al (4) from 2016 with a focus on urogenital infections in non-pregnant females, highlighting the requirement of carefully-planned study stratification upon meta-analysis.
The purpose of the present study was to perform a meta-analysis of randomized controlled trials (RCTs) and two-armed prospective studies identified by a thorough systematic review and meta-analysis of adequately-selected literature to determine the effect of probiotics for the treatment of common vaginal infections in non-pregnant adult females.
Materials and methods
Literature search strategy and inclusion criteria
The present systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (8). On December 24th, 2018, the Pubmed, Cochrane and EMBASE databases were searched for all studies published previously using the following key words: ‘Probiotics’, ‘Lactobacillus’, ‘urogenital infections’, ‘bacterial vaginosis’, ‘vulvovaginitis’, ‘vaginitis’ and ‘candidiasis’. The search strategy was (probiotics or Lactobacillus) and (vaginosis or vulvovaginal candidiasis or vaginitis or vulvovaginitis or urogenital infections). Articles of interest were also hand-searched for potentially relevant studies. Searches were performed by 2 independent reviewers (HSJ and JYC) and any disagreements were resolved by a third reviewer (TRY). Inclusion criteria for the analysis were as follows: i) RCTs and two-armed prospective studies; ii) studies including females with a current or history of gynecologic infections of BV and/or VCC; iii) studies that examined probiotic treatment vs. non-probiotics treatment (control) with or without antibiotics; iv) studies that provided quantitative data of the outcomes of interest; and v) full-text articles published in English or Chinese. Exclusion criteria were as follows: i) Retrospective studies, cohort studies, case series, letters, comments, editorials, case reports, proceedings, personal communications and one-arm studies; ii) studies on pediatric patients, pregnant females or males; iii) studies on healthy females with/without a history of recurrent urogenital infections. Studies designed to examine Lactobacillus treatment in combination with estriol, probiotic agents containing an unknown number of Lactobacilli or a mixture of multiple types of non-Lactobacillus bacteria were also excluded.
Data extraction
The following information/data was extracted from studies that met the inclusion criteria: Name of the first author, year of publication, study design, number of participants in each group, participants' age, type of infection, type of interventions, probiotic agents, probiotic administration, length of follow-up period and major outcomes (recurrence rate, cure/remission rate and/or the rate of restoring normal vaginal flora).
Quality assessment
The quality of the RCTs included was assessed using the Cochrane ‘assessing risk of bias’ table, which consists of 6 domains (random sequence generation, allocation concealment, blinding of patients and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting risk) (9). The quality of non-RCTs was assessed using a Cochrane risk of bias assessment tool for non-randomized studies of interventions (ACROBAT-NRSI) (10). This tool assesses 7 sources of bias associated with confounding, selection of participants, measurement of interventions, departures from intended interventions (10), missing data, measurement of outcomes and selection of the reported result.
Statistical analysis
Outcome measures for the meta-analysis were recurrence rate, cure and/or remission rate and restoration rate of normal flora. The odds ratios (ORs) with 95% CIs were calculated for each individual study and for all the studies combined. ORs of <1 for recurrence and ORs of >1 for cure and/or remission rate and normal flora restoration rate indicated that the probiotic group was favored. By contrast, ORs of >1 for recurrence and ORs of <1 for cure and/or remission rate and normal flora restoration rate indicated the control group was favored. OR=1 indicated that the probiotic and control groups had comparable outcomes. A χ2-based test of homogeneity was performed and the inconsistency index (I2) and Q-statistics were determined. A random effect model (DerSimonian-Laird method) was considered for the meta-analysis if either the Q statistic of P<0.10 or I2 value of >50% were derived; otherwise, a fixed effect model (Mantel-Haenszel method) was considered for the meta-analysis (11). Heterogeneity determined using the I2 statistic was defined as follows: 0-24%, no heterogeneity; 25-49%, moderate heterogeneity; 50-74%, high heterogeneity; and 75-100%, extreme heterogeneity. When the number of studies included in a meta-analysis is small, heterogeneity tests have low statistical power (12) and in this situation, a random-effects model of analysis is used (13). The National Research Council recommends the use of random-effects approaches for meta-analysis and the exploration of sources of variation in study results (14). Pooled effects were calculated and a 2-sided P<0.05 was considered to indicate statistical significance. Sensitivity analysis was performed using the leave-one-out approach to test the validity and robustness of the major results (12). All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat).
Results
Literature search
A flow diagram of the study selection process is provided in Fig. 1. A total of 771 articles were identified by database- and hand-searching with duplicates removed. After screening by title and abstract, 682 articles were excluded based on inclusion and exclusion criteria. The full text of the remaining 89 articles was reviewed and 59 were further excluded for reasons presented in Fig. 1. The remaining 30 articles were included in the qualitative synthesis, including 20 studies for BV alone or with other pathogens (15-34), 10 studies for VVC alone (31,35-43) and 1 study for BV/VVC (44).
Figure 1.

Flow diagram of study selection in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of the reviewed studies
Studies were categorized into three types based on treatment design (Table I): Type I, antibiotics plus Lactobacillus (probiotic) vs. antibiotic with or without placebo (control; n=22) (15,17,18,20,22-26,29,31,32,35-41); type II, Lactobacillus (probiotic) vs. placebo (control; no antibiotics; n=5) (19,21,27,33,34); and type III, Lactobacillus (probiotic) vs. antibiotic (control; n=3) (16,28,30). A summary of the patients' characteristics and interventions for the treatment of BV and/or VVC is provided in Table I. The age range of the female patients included in the analysis was between 18 to 50 years. Table II presents a summary of the outcomes of the studies included. Table III provides a summary of the type of probiotic and the route and dose of administration for the treatment of vaginitis. Probiotic species included L. rhamnosus BMX54, L. fermentum, L. plantarum, L. gasseri, L. plantarum, L. acidophilus, L. brevis CD2, L. salivarius subsp. Salicinius, L. delbrueckii subsp. lactis, L. reuteri, P. acidilactici, L. casei rhamnosus, L. reuteris, B. bifidum, B. longum, L. crispatus and Lactobacillus GG either alone or in various combinations depending on the infection being treated. The route of administration included oral capsule, vaginal tablet and vaginal capsule (Table III).
Table I.
Summary of patient characteristics and interventions for the treatment of bacterial vaginosis.
| A, Type I, antibiotics plus Lactobacillus (probiotic) vs. antibiotic with or without placebo (control) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Disease | |||||||||
| First author (publication year) | Study design | Grouping | Number of patients | Age (years) | Diagnosis | Diagnostic standard | Intervention | Follow-up time | (Refs.) |
| Laue (2018) | RCT | Probiotic | 18 | 32.6 | BV | Amsel criteria | Metronidazole plus Lactobacillus | 4 weeks | (23) |
| Control | 18 | 39 | Metronidazole plus placebo | ||||||
| Davar (2016) | RCT | Probiotic | 28 | 32.3 | VVC | Symptoms and positive culture | Fluconazole plus probiotic tablet | 6 months | (36) |
| Control | 31 | 31.1 | Fluconazole plus placebo | ||||||
| Recine (2016) | Prospective | Probiotic | 125 | 29.3 | BV | ≥3 of Amsel criteriaa | MTZ plus Lactobacillus | 9 months | (32) |
| Control | 125 | 29.5 | MTZ plus placebo | ||||||
| Heczko (2015) | RCT | Probiotic | 73 | 18-50 | BV/AV | Clinical signs, NSb | MTZ plus Lactobacillus | NA | (20) |
| Control | 81 | MTZ plus placebo | |||||||
| Bradshaw (2012) | RCT | Probiotic | 140 | 27c | BV | NS 7-10 | MTZ plus clindamycin cream | 6 months | (17) |
| Control | 133 | 27c | or ≥3 of Amsel criteria and NS 4-10 | MTZ plus vaginal pessary containing Lactobacillus | |||||
| Clindamycin | 135 | 27c | MTZ plus placebo vaginal pessary | ||||||
| Nouraei (2012) | RCT | Probiotic | 45 | 18-40 | VVC | Symptoms and culture | Fluconazole plus probiotic | 5-7 days | (41) |
| Control | 45 | Fluconazole plus placebo | |||||||
| Ehrström (2010) | RCT | Probiotic | 60 | 18-45 | BV/VVC | ≥3 Amsel criteria | Antibiotics plus vaginal capsule containing Lactobacillus | 1 menstruation | (44) |
| Control | 35 | Antibiotics plus placebo vaginal capsule | |||||||
| Marcone (2010) | RCT | Probiotic | 24 | N/A | BV | Fulfilled all | MTZ plus Lactobacillus tablet | 12 months | (25) |
| Control | 25 | Amsel criteria | MTZ plus placebo tablet | ||||||
| Anukam (2009) | RCT | Probiotic | 19 | 18-50 | VVC | Positive culture | Fluconazole plus L. rhamnosus GR-1 | 3 months | (35) |
| Control | 7 | Fluconazole plus placebo capsule | |||||||
| Martinez (2009a) | RCT | Probiotic | 32 | N/A | BV | ≥3 Amsel criteria | Tinidazole plus Lactobacillus | 28 days | (26) |
| Control | 32 | or NS 7-10 | Tinidazole plus placebo capsule | ||||||
| Martinez (2009b) | RCT | Probiotic | 29 | 29.1±7.5 | VVC | Symptoms and positive culture | Fluconazole plus L. rhamnosus GR-1 and L. reuteri RC-14 | 4 wks | (43) |
| Control | 26 | 26.9±7.8 | Fluconazole plus placebo | ||||||
| Yang (2009) | RCT | Probiotic | 44 | 36 (range: 25-48) | VVC | Symptoms and microscopy | Clotrimazole plus Lactobacillus | 30 days | (42) |
| Control | 42 | Clotrimazole | |||||||
| Hua (2008) | RCT | Probiotic | 118 | 28.45 | VVC | Symptoms and microscopy | Miconazole plus Lactobacillus | 33-37 days | (38) |
| Control | 117 | Miconazole | |||||||
| Larsson (2008) | RCT | Probiotic | 50 | 34.3 | BV | Amsel criteria | Clindamycin plus Lactobacillus | 6 months | (22) |
| Control | 50 | Clindamycin plus placebo | |||||||
| Marcone (2008) | RCT | Probiotics | 42 | 18-40 | BV | Fulfilled all | MTZ plus Lactobacillus tablet | 1 month | (24) |
| Control | 42 | Amsel criteria | MTZ plus placebo tablet | ||||||
| Petricevic (2008) | RCT | Probiotics | 83 | 32.6 | BV | NS 7-10 | Clindamycin plus Lactobacillus | 1 month | (29) |
| Control | 88 | Clindamycin plus placebo | |||||||
| Ma (2007) | RCT | Probiotics | 54 | 26 | VVC | Symptoms and microscopy | Miconazole plus Lactobacillus | NA | (39) |
| Control | 54 | Miconazole | |||||||
| Mai (2007) | RCT | Probiotics | 85 | 30.1 (range: 20-47) | VVC | Symptoms and microscopy | Clotrimazole plus Lactobacillus | 30 days | (40) |
| Control | 84 | Clotrimazole | |||||||
| Anukam (2006a) | RCT | Probiotic | 65 | 18-44 | BV | NS 7-10 and positive BV Blue test | MTZ plus Lactobacillus tablet | 30 days | (15) |
| Control | 60 | MTZ plus placebo tablet | |||||||
| Han (2006) | RCT | Probiotic | 86 | 37 (range: 19-48) | VVC | Symptoms and microscopy | Clotrimazole plus lactobacillus capsule | 30 days | (37) |
| Control | 90 | Clotrimazole | |||||||
| Lin (2006) | RCT | Probiotic | 32 | 30 (range: 20-44) | Trichomonial | Microscopy | MNZ plus lactobacillus capsule | 30 days | (31) |
| Control | 30 | Vaginitis | |||||||
| Probiotic | 53 | VVC | Microscopy | Cretrozole plus lactobacillus capsule | |||||
| Control | 52 | ||||||||
| Probiotic | 59 | BV | Amsel criteria | MNZ plus Lactobacillus capsule | |||||
| Control | 51 | ||||||||
| Eriksson (2005) | RCT | Probiotics | 91 | 32c | BV | ≥3 Amsel criteria | Clindamycin plus tampons containing Lactobacillus | NA | (18) |
| Control | 96 | 32c | Clindamycin plus placebo tampons | ||||||
| B, Type II studies: Lactobacillus (probiotic) vs. placebo (control) | |||||||||
| Disease | |||||||||
| First author (publication year) | Study design | Grouping | Number of patients | Age (years) | Diagnosis | Diagnostic standard | Intervention | Follow-up time | (Refs.) |
| Vicariotto (2014) | RCT | Probiotic | 24 | 34.7 | BV | ≥3 of Amsel criteria | Lactobacillus | 56 days | (33) |
| Control | 10 | or NS 7-10 | Placebo tablet | ||||||
| Vujic (2013) | RCT | Probiotic | 395 | N/A | BV and | ≥3 Amsel criteria | Lactobacillus capsule | 44.16 days | (34) |
| Control | 149 | Other vaginal infections | or NS 7-10 | Placebo capsule | |||||
| Hemalatha (2012) | RCT | Probiotic | 34 | N/A | BV | NS 7-10 | Lactobacillus | 9 days | (21) |
| Control | 27 | Placebo tablet | |||||||
| Mastromarino (2009) | RCT | Probiotics | 18 | 33 | BV | NS 7-10 | Vaginal tablets containing Lactobacillus | 2 weeks | (27) |
| Control | 16 | 35 | Placebo vaginal tablets | ||||||
| Hallen (1992) | RCT | Probiotics | 28 | 24 | BV | ≥3 Amsel criteria | Vaginal tablets containing Lactobacillus | 7-10 days; 20-40 days | (19) |
| Control | 29 | Placebo vaginal tablets | |||||||
| C, Type III studies: Lactobacillus (probiotic) vs. antibiotics (control) | |||||||||
| Disease | |||||||||
| First author (publication year) | Study design | Grouping | Number of patients | Age (years) | Diagnosis | Diagnostic standard | Intervention | Follow-up time | (Refs.) |
| Ling (2013) | RCT | Probiotic | 25 | N/A | BV | Amsel criteria and NS | Intravaginal Lactobacillus | 30 days | (30) |
| Control | 30 | MTZ | |||||||
| Anukam (2006b) | RCT | Probiotic | 20 | N/A | BV | NS7-10 and positive BV Blue test | Lactobacillus capsule | 30 days | (16) |
| Control | 20 | (Symptomatic) | MTZ | ||||||
| Parent (1996) | RCT | Probiotics | 16 | 31.1 | BV | ≥3 Amsel criteria | Lactobacillus tablet | 28 days | (28) |
| Control | 16 | 34.4 | MTZ plus placebo tablet | ||||||
aAmsel criteria: i) presence of a characteristic homogeneous grey discharge; ii) vaginal fluid pH of >4.5; iii) positive amine odor test; iv) the identification of ‘clue cells’ by microscopic examination of vaginal fluid mixed with saline. bNugent score: 10-point scale based on the presence or absence of Lactobacillus morphotypes under oil immersion (x1,000 magnification). A score of 0-3 was interpreted as consistent with a normal Gram-positive rod-dominated microbiota; a score of 4-6 as intermediate; a score of 7-10 was considered consistent with BV-like conditions in which the samples were dominated by small Gram-negative and Gram-variable straight and curved rods. cMedian of age. AV, aerobic vaginitis; BV, bacterial vaginosis; MTZ, metronidazole; NS, Nugent score; RCT, randomized controlled trial; TMP-SMX, trimethoprim-sulfamethoxazole; VVC, vulvovaginal candidiasis.
Table II.
Summary of the outcomes in the meta-analysis.
| A, Type I | |||||||
|---|---|---|---|---|---|---|---|
| First author (year) | Disease type | Patients (n) | Intervention | Recurrence | Cure/remission | Restored normal flora | (Refs.) |
| Laue (2018) | BV | 18 | Probiotic | 16(100) | (23) | ||
| 18 | Control | 13 (76.5) | |||||
| Davar (2016) | VVC | 28 | Probiotic | 2 (7.2) | (36) | ||
| 31 | Control | 11 (35.5) | |||||
| Recine (2016) | BV | 125 | Probiotic | 2 mo: 113 (90.4) 6 mo: 106 (74.6) 9 mo: 118 (79.7) | (32) | ||
| 125 | Control | 2 mo: 99 (79.2) 6 mo: 36 (25.4) 9 mo: 30 (20.3) | |||||
| Heczko (2015) | BV/AV | 73 | Probiotic | 33 (45.2) | (20) | ||
| 81 | Control | 38 (47.0) | |||||
| Bradshaw (2012) | BV | 140 | Clindamycin | 42(30) | 92 (65.7) | (17) | |
| 133 | Probiotic | 37 (27.8) | 63 (47.4) | ||||
| 135 | Control | 36 (26.7) | 63 (46.7) | ||||
| Nouraei (2012) | VVC | 45 | Probiotic | 42 (93.3) | (41) | ||
| 45 | Control | 37 (82.2) | |||||
| Ehrström (2010) | BV/VVC | 60 | Probiotic | 1 mo: 13 (22.4) | 1 mo: 47(78) 2 mo: 23 (38.1) 6 mo: 35 (58.4) | (44) | |
| 35 | Control | 1 mo: 10 (29.4) 2 mo: 13 (38.1) 6 mo: 20 (56.6) | 1 mo: 25(71) | ||||
| Marcone (2010) | BV | 24 | Probiotic | 6 mo: 18(74) | (25) | ||
| 12 mo: 16(69) | |||||||
| 25 | Control | 6 mo: 24(96) 12 mo: 23(91) | |||||
| Anukam (2009) | VVC | 19 | Probiotic | 15(79) | (35) | ||
| 7 | Control | 3(43) | |||||
| Martinez (2009a) | BV | 32 | Probiotic | 4 (12.5) | 28 (87.5) | 24(75) | (26) |
| 32 | Control | 15 (46.9) | 16(50) | 11 (34.4) | |||
| Martinez (2009b) | VVC | 29 | Probiotic | 3 (10.3) | (43) | ||
| 26 | Control | 10 (38.5) | |||||
| Yang (2009) | VVC | 44 | Probiotic | 3 (7.1) | 42 (92.86) | (42) | |
| 42 | Control | 7 (16.7) | 38 (83.33) | ||||
| Hua (2008) | VVC | 118 | Probiotic | 4 (4.8) | 83 (70.34) | (38) | |
| 117 | Control | 11 (13.9) | 79 (67.52) | ||||
| Larsson (2008) | BV | 50 | Probiotics | 24 (64.9) | (22) | ||
| 50 | Control | 18 (46.2) | |||||
| Marcone (2008) | BV | 42 | Probiotics | 1 mon: 22(96) 6 mon: 23(98) | 30 d: 37(88) 90 d: 37(88) 180 d: 35(83) | (24) | |
| 42 | Control | 1 mon: 21(91) 6 mo: 17(74) | 30 d: 34(81) 90 d: 30(71) 180 d: 28(67) | ||||
| Petricevic (2008) | BV | 83 | Probiotics | 1 mon: 83(100) | 69 (83.1) | (29) | |
| 88 | Control | 1 mon: 35 (39.8) | 31(35.2) | ||||
| Ma (2007) | VVC | 54 | Probiotics | 46 (85.2) | (39) | ||
| 54 | Control | 38 (70.4) | |||||
| Mai (2007) | VVC | 85 | Probiotics | 5 (5.9) | 80 (94.1) | (40) | |
| 84 | Control | 13 (15.5) | 70 (83.3) | ||||
| Anukam (2006a) | BV | 65 | Probiotic | 0 (0) | 8(12) | 57(88) | (15) |
| 60 | Control | 17(28) | 19(32) | 24(40) | |||
| Han (2006) | VVC | 86 | Probiotic | 3 (3.9) | 74 (96.10) | (37) | |
| 90 | Control | 9 (13.0) | 60 (86.96) | ||||
| Lin (2006) | VVC | 53 | Probiotic | 2 (3.8) | 52 (98.1) | (31) | |
| 52 | Control | 13 (25.0) | 49 (94.2) | ||||
| Lin (2006) | BV | 59 | Probiotic | 1 (1.7) | 58 (98.3) | (31) | |
| 51 | Control | 12 (23.5) | 47 (92.2) | ||||
| Eriksson (2005) | BV | 91 | Probiotics | 52 (56.8) | (18) | ||
| 96 | Control | 58 (60.2) | |||||
| B, Type II | |||||||
| First author (year) | Disease type | Patients (n) | Intervention | Recurrence | Cure/remission | Restored normal flora | (Refs.) |
| Vicariotto (2014) | BV | 24 | Probiotic | Day 28: 2 (8.3) Day 56: 4 (16.7) | Day 28: 22 (91.7) Day 56: 20 (83.3) | (33) | |
| 10 | Control | Day 28: 8(80) Day 56: 9(90) | Day 28: 2(20) Day 56: 1(10) | ||||
| Vujic (2013) | BV and other infection | 395 | Probiotic | 1.5 mo: 243 (61.5) 3 mo: 202 (51.1) | (34) | ||
| 149 | Control | 1.5 mo: 40 (26.8) 3 mo: 31 (20.8) | |||||
| Hemalatha (2012) | BV | 34 | Probiotic | 7(21) | 11(32) | (21) | |
| 27 | Control | 7(26) | 7(26) | ||||
| Mastromarino (2009) | BV | 18 | Probiotics | 11(61) | 9(50) | (27) | |
| 16 | Control | 3 (18.75) | 1 (6.25) | ||||
| Hallen (1992) | BV | 28 | Probiotics | 7-10 d: 16 (57.1) 20-40 d: 0 (0) | (19) | ||
| 29 | Control | 7-10 d: 3 (10.3) 20-40 d: 0 (0) | |||||
| C, Type III | |||||||
| First author (year) | Disease type | Patients (n) | Intervention | Recurrence | Cure/remission | Restored normal flora | (Refs.) |
| Ling (2013) | BV | 25 | Probiotic | (30) | |||
| 30 | Control | ||||||
| Anukam (2006b) | BV | 20 | Probiotic | 2(10) | 15(75) | 11(55) | (16) |
| 20 | Control | 9(45) | 9(45) | 6(30) | |||
| Parent (1996) | BV | 16 | Probiotics | 14 (87.5) | (28) | ||
| 16 | Control | 4 (22.2) | |||||
Values are expressed as n for patients' number, n (%) for recurrence, cure/remission, and restored normal flora. mo, months; d, days; BV, bacterial vaginosis; VVC, vulvovaginal candidiasis; AV, aerobic vaginitis; Ref., reference.
Table III.
Types of probiotics and route and dose of administration of probiotics for treatment.
| A, Disease type, BV | ||||||
|---|---|---|---|---|---|---|
| First author (year) | Probiotic regimen | Brand | Dosage and duration | Route of administration | Length of follow-up period | (Refs.) |
| Laue (2018) | Lactobacillus | Verum | 125 g yoghurt containing (besides L. delbrueckii ssp. bulgaricus and S. thermophilus) living strains L. crispatus LbV 88, L. gasseri LbV 150N, L. jensenii LbV 116 and L. rhamnosus LbV96, each 1x107 cfu/ml; placebo was 125 g chemically acidified milk. Twice daily for 4wks | Oral | 4 wks | (23) |
| Recine (2016) | L. rhamnosus BMX54 | NORMOGIN | Once a day for 10 d, twice a week for 15 d and once every 5 d for 7 mo as maintenance therapy | Vaginal tablet | 9 mo | (32) |
| Heczko (2015) | L. fermentum, L. plantarum, and L. gasseri | prOVag | One capsule daily for 10 d perimenstrually | Oral | Approximately 4 menstrual periods | (20) |
| Vicariotto (2014) | L. fermentum plus L. plantarum | N/A | Once a day for 7 nights, followed by 1 tablet every 3 nights for 3 wks and 1 tablet per wk | Vaginal tablet | 56 d | (33) |
| Bradshaw (2012) | L. acidophilus | N/A | 12 nights | Vaginal tablet | 6 mo | (17) |
| Hemalatha (2012) | L. brevis CD2, L. salivarius subsp. Salicinius, L. plantarum | Florisia | 8 nights | Vaginal tablet | 9 d | (21) |
| Ling (2013) | L. delbrueckii subsp. lactis | N/A | 10 d | Vaginal capsule | 30 d | (30) |
| Vujic (2013) | L. rhamnosus and L. reuteri | Lactogyn | Twice daily | Oral | 6 mo | (34) |
| Ehrström (2010) | L gasseri, L. fermentum, L. casei subsp. rhamnosus and P. acidilactici | N/A | 2 capsules daily for 5 d | Vaginal capsule | 6 mo | (44) |
| Marcone (2010) | L. rhamnosus | Normogin | Once a week for 6 mo of a capsule containing 40 mg of L. rhamnosus (N40000 CFU; Normogin), beginning 8 d after MTZ discontinued | Oral | 12 mo | (25) |
| Martinez (2009a) | GR-1, RC-14 | 2 Capsule daily for the following 4 wks | Vaginal capsule | 28 d | (26) | |
| Mastromarino (2009) | L. brevis, L. salivarius subsp. salicinius, and L. plantarum | N/A | Daily for 7 d | Vaginal tablet | 2 wks | (27) |
| Larsson (2008) | L. gasseri and L. rhamnosus | EcoVag® | 10 d during 3 menstrual cycles | Vaginal capsule | 6 mo | (22) |
| Marcone (2008) | L. rhamnosus | N/A | Once a week at bedtime for 2 mo starting 1 wk after the last antibiotic administration | Vaginal tablet | (24) | |
| Petricevic (2008) | L. caseirhamnosus | N/A | 7 d | Vaginal capsule | 1 mo | (29) |
| Anukam (2006a) | L. rhamnosus and L. reuteri | N/A | 1-30 d | Vaginal tablet | 30 d | (15) |
| Anukam (2006b) | L. rhamnosus and L. reuteri | N/A | 2 capsules for 5 d | Vaginal tablet | 30 d | (16) |
| Eriksson (2005) | L. gasseri, L. caseivarrhamnosus and L. fermentum | Medipharm AB | During the following menstruation | Tampon containing lactobacilli | After the second menstrual period (~1 mo) | (18) |
| Parent (1996) | L. acidophilus | Gynoflor | 1-2 tablets daily for 6 d | Vaginal tablet | 28 d | (28) |
| Hallen (1992) | L. acidophilus (Vivag) | Vigag | Twice daily for 6 d | Vaginal capsule | 7-10 d, 20-40 d | (19) |
| Lin (2006) | Lactobacillus capsule | Once daily for 7 d | Vaginal capsule | 30 d | (31) | |
| B, Disease type, VVC | ||||||
| First author (year) | Probiotic regimen | Brand | Dosage and duration | Route of administration | Length of follow-up period | (Refs.) |
| Davar (2016) | L. acidophilus, B. bifidum and B. longum | Pro-Digest | Twice daily for 10 d | Oral | 6 mo | (36) |
| Anukam (2009) | L. rhamnosus and L. reuteri | N/A | Once daily for 3 mo | Oral | 3 mo | (35) |
| Martinez (2009b) | L. rhamnosus and L. reuteri | N/A | Once daily for 28 d | Oral | 4 wks | (43) |
| Nouraei (2012) | protexin | 20 capsules within an interval of 72 h (3 d) | Oral | 5-7 d | (41) | |
| Yang (2009) | Lactobacillus capsule | Ding-jun-sheng | Once daily for 10 d | Vaginal capsule | 30 d | (42) |
| Hua (2008) | Lactobacillus capsule | Ding-jun-sheng | Once daily for 10 d | Vaginal capsule | 33-37 d | (38) |
| Ma (2007) | Lactobacillus capsule | N/A | 0.5 g once daily for 7 d | Vaginal capsule | N/A | (39) |
| Mai (2007) | Lactobacillus capsule | Ding-jun-sheng | 0.25 g capsule, once daily for 10 d | Vaginal capsule | 30 d | (40) |
| Han (2006) | Lactobacillus capsule | Ding-jun-sheng | Once daily for 10 d | Vaginal capsule | 30 d | (37) |
| Lin (2006) | Lactobacillus capsule | N/A | Once daily for 7 d | Vaginal capsule | 30 d | (31) |
d, day; mo, month; wk, week; N/A, not available; Ref., reference.
Meta-analysis
The detailed treatment outcomes of all studies reviewed are summarized in Table II. The majority of studies adopted a type I treatment design for BV and/or VVC infections and those with 1- and/or 6-months follow-up data were included in the meta-analysis. These comprised of a total of 21 articles (10 articles on BV, 9 studies on VVC and 2 on BV/VVC) (15,17,18,20,22-26,29,31,32,35-41). The total number of patients evaluated in the 21 type I studies was 1,788 (probiotic test group, n=910; control group, n=878). These type I studies were the major focus of the present meta-analysis, while type II and III studies were analyzed separately for supplementation.
With respect to recurrence at 1 month after treatment, 9 studies [2 on BV alone (15,26), 5 on VVC alone (37,38,40,42,43) and 2 on BV/VVC (31,44)] with complete quantitative data were included in the present meta-analysis. A total of 1,220 patients were evaluated (probiotic test group, n=631; control group, n=589). There was no heterogeneity present among all 9 studies or those on either BV or VVC (total: Q=11.82, I2=24%; BV: Q=2.14, I2=7%; VVC: Q=1.86, I2=0%; Fig. 2A). The analysis indicated that patients in the probiotic group had a significantly lower recurrence rate than those in the control group (pooled OR=0.27, 95% CI: 0.18-0.41; Fig. 2A). A favorable outcome associated with the probiotics group was also observed when analyzing BV and VVC individually (BV: Pooled OR=0.10, 95% CI: 0.04-0.26; VVC: Pooled OR=0.27, 95% CI: 0.16-0.45; all P<0.001; Fig. 2A). However, there was no significant difference in the recurrence rate between the probiotic and control groups at 6 months after treatment (Fig. 2A).
Figure 2.
Forest plots for antibiotic plus Lactobacillus vs. antibiotic plus placebo (type I study) in the treatment of bacterial vaginosis and vulvovaginal candidiasis: (A) 1-month and 6-month recurrence rate; (B) 1-month and 6-month cure or remission rate; (C) restoration of normal flora after 1 month of follow-up. BV, bacterial vaginosis; VVC, vulvovaginal candidiasis; df, degrees of freedom.
With respect to cure or remission after treatments, a total of 12 studies were included. These comprised 12 studies with 1-month follow-up results [6 for BV alone (15,18,23,24,26,29), 4 for VVC alone (37,38,40,42) and 2 for BV/VVC (31,44)] and 2 studies (22,24) with 6-month follow-up for BV alone. In the 12 studies with 1-month follow-up outcomes, 1,643 patients in total were evaluated (probiotic test group, n=836; control group, n=807). There was moderate to high heterogeneity among the 12 studies with 1-month follow-up (total: Q=52.69, I2=77%; BV: Q=47.02, I2=87. %; VVC: Q=5.45, I2=27%), as well as between studies with 6-month follow-up (Q=1.70, I2=40%). The analysis indicated that probiotic treatment was favorable among all studies and those focusing on VVC alone 1 month after treatment (total: Pooled OR=2.28, 95% CI: 1.21-4.32, P=0.011; VVC: Pooled OR=1.72, 95% CI: 1.13-2.64, P=0.012), as well as 6 months after treatment of BV (pooled OR=2.58, 95% CI: 1.07-6.23, P=0.036; Fig. 2B). However, there was no significant difference in the cure rate at 1 month for BV (pooled OR=2.59; 95% CI: 0.76-8.85; P=0.129; Fig. 2B).
With respect to restoration of the normal flora, 4 studies (15,24,26,29) had complete quantitative data at 1 month and 4 studies (17,24,25,32) at 6 months after treatments for BV, and were included in the analysis. High heterogeneity existed among the studies on the restoration of normal flora at 1 month and 6 months after treatment (1 month: Q=17.28, I2=83%; 6 months: Q=47.86, I2=94%). The analysis indicated that patients in the probiotic group had a significantly higher rate of normal flora restoration at 1 month after treatment (pooled OR=4.55, 95% CI: 1.44-14.36, P=0.010). However, there were no differences in the normal flora restoration rate between the two groups at 6 months after treatment (Fig. 2C).
Additional analyses were performed for type II (19,21,27,33,34) or type III (16,28) studies that had at least one follow-up outcome. These studies all focused on BV and had varied heterogeneity (Recurrence: Q=7.98; I2=87%; Cure or remission: Q=1.94; I2=0%; Restored normal flora: Q=4.37; I2=54% for type II and Cure or remission: Q=2.58; I2=61%; for type III). Patients with BV given type II treatments in the probiotic group were indicated to have a higher cure or remission rate and normal flora restoration rate than those in the control group (cure/remission rate: Pooled OR=12.44, 95% CI: 4.86-31.89, P<0.001; normal flora restoration rate: Pooled OR=3.32, 95% CI: 1.11-9.97, P=0.033). In BV patients given type III treatments, the probiotic group had a higher cure/remission rate than the control group (cure/remission rate: Pooled OR=8.39, 95% CI: 1.32-53.23, P=0.024; Table IV).
Table IV.
Extra meta-analysis for recurrence, cure or remission, and restored normal flora for patients with BV given type II and type III treatment.
| Outcome/study [Author name (year) (Refs.)] | Treatment type | Time-point | Odds ratio | Lower limit | Upper limit | Z-value | P-value | Heterogeneity test |
|---|---|---|---|---|---|---|---|---|
| Recurrence | Q=7.977, df=1, P=0.005, I2=87.46% | |||||||
| Vicariotto (2014) (33) | BV-Type II | 1 mon | 0.023 | 0.003 | 0.189 | -3.498 | <0.001 | |
| Hemalatha (2012) (21) | BV-Type II | 9 d | 0.757 | 0.230 | 2.492 | -0.459 | 0.646 | |
| Combined | 0.147 | 0.005 | 4.529 | -1.096 | 0.273 | |||
| Cure or remission | Q=1.939, df=2, P=0.379, I2=0% | |||||||
| Vicariotto (2014) (33) | BV-Type II | 1 mon | 44.019 | 5.280 | 366.956 | 3.498 | <0.001 | |
| Mastromarino (2009) (27) | BV-Type II | 14 d | 6.806 | 1.412 | 32.809 | 2.390 | 0.017 | |
| Hallen (1992) (19) | BV-Type II | 40 d | 11.560 | 2.821 | 47.366 | 3.401 | 0.001 | |
| Combined | 12.444 | 4.856 | 31.888 | 5.251 | <0.001 | |||
| Restored normal flora | Q=4.366, df=2, P=0.113, I2=54.19% | |||||||
| Vujic (2013) (34) | BV-Type II | 3 mon | 3.983 | 1.603 | 9.895 | 2.977 | 0.003 | |
| Hemalatha (2012) (21) | BV-Type II | 9 d | 1.339 | 0.436 | 4.113 | 0.510 | 0.610 | |
| Mastromarino (2009) (27) | BV-Type II | 14 d | 15.000 | 1.621 | 138.821 | 2.385 | 0.017 | |
| Combined | 3.319 | 1.105 | 9.974 | 2.137 | 0.033 | |||
| Restored normal flora | ||||||||
| Anukam (2006b) (16) | BV-Type III | 1 mon | 2.852 | 0.777 | 10.467 | 1.580 | 0.114 | Not assessed |
| Recurrence | ||||||||
| Anukam (2006b) (16) | BV-Type III | 1 mon | 0.136 | 0.025 | 0.748 | -2.294 | 0.022 | Not assessed |
| Cure or remission | Q=2.577, df=1, P=0.108, I2=61.19% | |||||||
| Anukam (2006b) (16) | BV-Type III | 1 mon | 3.667 | 0.958 | 14.028 | 1.898 | 0.058 | |
| Parent (1996) (28) | BV-Type III | 28d | 24.532 | 3.693 | 162.947 | 3.312 | 0.001 | |
| Combined | 8.393 | 1.323 | 53.227 | 2.257 | 0.024 |
df, degrees of freedom; mon, months; d, days; BV, bacterial vaginosis.
Quality assessment
The risk of bias assessment for individual studies is provided in Fig. 3, including the potential risk of individual studies (Fig. 3A and B) and the overall risk (Fig. 3C and D). Overall, the studies had a low risk of attrition bias and reporting bias, and low or unclear risk of selection bias and detection bias. Furthermore, 3 studies had a high risk of performance bias due to improper blinding of participants and researchers.
Figure 3.

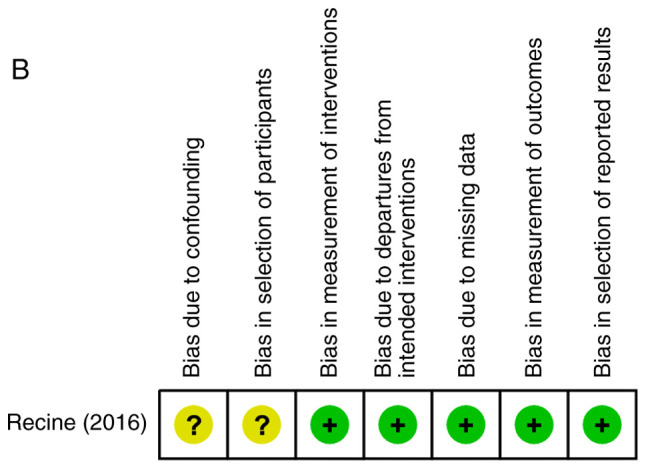

Quality assessment of included studies. Risk of bias summary of (A) randomized controlled trials and (B) non-randomized controlled trials. Risk of bias graph of (C) randomized controlled trials and (D) non-randomized controlled trials.
Sensitivity analysis
Sensitivity analyses were performed on the major results using the leave-one-out approach, in which the meta-analysis was performed with each study removed in turn (Table V). The direction of combined estimates on recurrence rates and cure/remission rates at 1 month and normal flora restoration rates at 6 months did not vary markedly with the removal of the studies, indicating that the meta-analysis had good reliability and supported that there was no or little inter-study heterogeneity. However, for normal flora restoration rates at 1 month, the study of Marcone et al (24) from 2008 may have had a disproportionate effect on the pooled OR, as the difference became more significant and greater when this study was not included in the meta-analysis, while the three other studies had no such effect.
Table V.
Sensitivity analysis.
| A, Recurrence at 1 month | ||||||
|---|---|---|---|---|---|---|
| Statistics with study removed | ||||||
| Author name (year) | Odds ratio | Lower limit | Upper limit | Z-value | P-value | (Refs.) |
| Ehrström (2010) | 0.214 | 0.135 | 0.342 | -6.478 | <0.001 | (44) |
| Martinez (2009a) | 0.287 | 0.184 | 0.447 | -5.516 | <0.001 | (26) |
| Martinez (2009b) | 0.279 | 0.180 | 0.432 | -5.720 | <0.001 | (43) |
| Yang (2009) | 0.260 | 0.168 | 0.403 | -6.023 | <0.001 | (42) |
| Hua (2008) | 0.264 | 0.169 | 0.412 | -5.848 | <0.001 | (38) |
| Mai (2007) | 0.258 | 0.164 | 0.407 | -5.848 | <0.001 | (40) |
| Anukam (2006a) | 0.286 | 0.187 | 0.436 | -5.809 | <0.001 | (15) |
| Han (2006) | 0.269 | 0.173 | 0.420 | -5.797 | <0.001 | (37) |
| Lin (2006) | 0.288 | 0.188 | 0.441 | -5.718 | <0.001 | (31) |
| Lin (2006) | 0.288 | 0.186 | 0.444 | -5.622 | <0.001 | (31) |
| B, Cure or remission at 1 month | ||||||
| Statistics with study removed | ||||||
| Author name (year) | Odds ratio | Lower limit | Upper limit | Z-value | P-value | (Refs.) |
| Laue (2018) | 2.165 | 1.131 | 4.146 | 2.330 | 0.020 | (23) |
| Ehrström (2010) | 2.416 | 1.197 | 4.879 | 2.461 | 0.014 | (44) |
| Martinez (2009a) | 2.062 | 1.069 | 3.979 | 2.158 | 0.031 | (26) |
| Yang (2009) | 2.271 | 1.150 | 4.486 | 2.361 | 0.018 | (42) |
| Hua (2008) | 2.521 | 1.209 | 5.256 | 2.467 | 0.014 | (38) |
| Marcone (2008) | 2.286 | 1.179 | 4.431 | 2.447 | 0.014 | (24) |
| Petricevic (2008) | 1.818 | 1.047 | 3.155 | 2.123 | 0.034 | (29) |
| Mai (2007) | 2.224 | 1.117 | 4.427 | 2.275 | 0.023 | (40) |
| Han (2006) | 2.764 | 1.523 | 5.015 | 3.345 | 0.001 | (37) |
| Lin (2006) | 2.197 | 1.114 | 4.333 | 2.271 | 0.023 | (31) |
| Lin (2006) | 2.199 | 1.136 | 4.259 | 2.337 | 0.019 | (31) |
| Eriksson (2005) | 2.252 | 1.160 | 4.372 | 2.398 | 0.016 | (18) |
| Anukam (2006a) | 2.578 | 1.280 | 5.193 | 2.651 | 0.008 | (15) |
| C, Restoration of normal flora at 1 month | ||||||
| Statistics with study removed | ||||||
| Author name (year) | Odds ratio | Lower limit | Upper limit | Z-value | P-value | (Refs.) |
| Martinez (2009a) | 4.121 | 0.853 | 19.905 | 1.762 | 0.078 | (26) |
| Marcone (2008) | 8.705 | 5.274 | 14.368 | 8.464 | <0.001 | (24) |
| Petricevic (2008) | 3.442 | 0.646 | 18.335 | 1.448 | 0.148 | (29) |
| Anukam (2006a) | 3.284 | 0.692 | 15.591 | 1.496 | 0.135 | (15) |
| D, Restoration of normal flora at 6 months | ||||||
| Statistics with study removed | ||||||
| Author name (year) | Odds ratio | Lower limit | Upper limit | Z-value | P-value | (Refs.) |
| Recine (2016) | 0.536 | 0.195 | 1.477 | -1.205 | 0.228 | (32) |
| Bradshaw (2012) | 0.861 | 0.062 | 11.885 | -0.112 | 0.911 | (17) |
| Marcone (2010) | 1.599 | 0.293 | 8.737 | 0.542 | 0.588 | (25) |
| Marcone (2008) | 1.312 | 0.197 | 8.715 | 0.281 | 0.779 | (24) |
BV, bacterial vaginosis.
Discussion
The overall summary of the qualitative analysis of the 30 studies suggests that probiotic treatments are useful for managing common vaginal infections, particularly BV and VVC. However, patient populations, treatment protocols, endpoints and follow-up time-points exhibited a marked variation. The results of the meta-analysis indicated that probiotics as a supplement of antibiotic/anti-fungal treatments (as observed in type I studies) reduced the recurrence rate and increased the cure/remission rate in non-pregnant adult females at 1 month after treatment. With less evident data, the normal bacterial flora restoration rate was also increased by probiotic-supplemented treatments in BV. The short-term benefits of probiotics were further supported by individual analysis of BV and VVC, although probiotics supplementary to standard treatments did not increase the cure/remission rate in BV and the post-treatment normal bacterial restoration rate in VVC was lacking. However, observations at 6 months post-treatment were less frequently reported. In line with the results demonstrated by probiotic-supplemented treatments, probiotics alone without antibiotics may have clinical benefits in promoting the cure/remission rate and normal flora restoration rates in BV.
To the best of our knowledge, the present meta-analysis was the first to review and analyze the effect of probiotics in common vaginal infections reported by RCTs or appropriately-controlled studies. Furthermore, only few studies have evaluated the benefits of probiotics in vaginal infection stratified by treatment regimen. The quantitative data of the present study are supported by conclusions from two published systemic reviews, which examined the overall effect of probiotics in females with urogenital infections qualitatively. In 2009, Abad and Safdar (6) identified 25 studies that used Lactobacillus-containing probiotics to either prevent or treat a urogenital infection [BV, VVC and urinary tract infections (UTI)]. Of the 25 studies, 18 used Lactobacillus preparations for the treatment or prevention of urogenital infections and 7 focused solely on vaginal colonization (6). Of the 18 studies, only 8 studies included patients with BV, 4 included patients with VVC, 5 included patients with UTI and 1 was on multiple infections (6). Overall, Lactobacilli were beneficial for the treatment of BV, while no clear benefit was observed for VVC or UTI (6). A more recent systematic review published in 2016 investigated probiotics for the treatment and prevention of urogenital infections in females (4). A total of 20 studies (published from 2008 to 2015) were identified, with 14 examining BV, 2 examining VCC, 3 examining UTI and 1 examining human papillomavirus (HPV) (4). While the studies reviewed by Hanson et al (4) in 2016 were heterogeneous with respect to study type, design, intervention and outcomes and varied in quality (4 of good quality, 9 of fair and 7 of poor quality), the authors still made to the conclusion that the use of probiotics may be effective for the treatment and prevention of BV, recurrent candidiasis or UTI, as well as HPV lesions. In the current review, an analysis of quantitative outcomes from a total of 1,788 patients with common vaginal infections was presented, with focus on BV and VVC that are most directly impacted by an imbalanced microflora/dysbiosis.
One prior meta-analysis examined the use of probiotics for treating BV. In a meta-analysis published in 2014, Huang et al (3) indicated that the use of probiotic supplementation significantly improved the cure rate in adult females with BV [risk ratio (RR)=1.53; 95% CI: 1.19-1.97]. When only 9 high-quality studies were included in the analysis, the RR increased slightly to 1.60 (95% CI: 1.16-2.22) (3). Of note, when a subgroup analysis was performed, a single treatment with probiotics may only be effective for short-term follow-up (≤1 month) but not for long-term follow-up (>1 month) (3), which was consistent with the present result that no difference between two groups in recurrence rate and cure/remission rates was determined at 6 months after the treatment. In a meta-analysis by Huang et al (3) from 2014, the eligible articles were searched up to May 2013 and the studies included in the meta-analysis were also heterogeneous. In the present meta-analysis, the literature search was further updated to December 24th, 2018, and studies all except one RCT analyzed in the previous study by Huang et al (3) from 2014 were included. This particular RCT was excluded from the present study due to its study design for healthy females with a history of BV (45); furthermore, it had different follow-up time-points from other studies analyzed in the present study and was deemed unsuitable for analysis of post-treatment outcomes.
A recent meta-analysis study suggested that, although probiotics appeared effective in treating VVC, relevant studies were not sufficient in number (5-7 studies included for each analysis) or of comparable quality (7). In the present study, which focused on common vaginal infections as a whole, only studies with comparable treatment designs and study follow-up schedules were included in the meta-analysis. Furthermore, the major results of the present study were based on >10 RCTs or prospective studies with control arms. In 2006, Falagas et al (46) reported on several clinical trials on VVC that support the effectiveness of Lactobacilli administered either orally or intravaginally in decreasing colonization of C. albicans or preventing vaginal candidiasis. However, most of the relevant clinical trials had methodological problems, including small sample size, no control group (single-arm) and included females without confirmed recurrent VVC. All of the studies on VVC reviewed in the present meta-analysis were designed to compare Lactobacillus capsule-supplemented anti-fungal treatments (probiotic group) with anti-fungal agents alone (control group). Despite the follow-up period ranging from <1 week to 6 months among the studies included, only those with comparable follow-up schedules were included in the present meta-analysis. The outcome supports the effectiveness of Lactobacilli in decreasing the recurrence rate and improving the cure rate.
The primary limitation of the present study has already been mentioned-the large heterogeneity between studies with respect to the patient population, type of treatment, probiotic strains and outcome follow-ups. However, it was sought to overcome this by carefully-planned stratification based on treatment design and follow-up schedules. The major results on short-term benefits of combined therapy of antibiotics/anti-fungals with probiotics was further confirmed by the sensitivity test. By contrast, the limited sample size and heterogeneous study design prevented us from a reliable subgroup analysis of long-term benefits and of probiotics treatment alone without antibiotic/anti-fungal agents.
In conclusion, the results of the present study confirm the results of other reports in a quantitative manner, namely that probiotics as a supplement to conventional pharmacological treatments are effective in the short term for the treatment of common vaginal infections in non-pregnant adult females. However, high-quality evidence for the effectiveness of probiotics alone in recurrent or curative vaginal infections is limited. Further high-quality clinical trials are necessary to identify the most effective probiotic strains, the most effective treatment regimens (with or without antibiotics) and the subpopulations of females (e.g. pre-menopausal vs. post-menopausal) that may benefit the most from probiotics.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
HSJ conceived and designed the current study, defined the content of the research, conducted literature research, performed statistical analysis and prepared and edited the manuscript. TRY is the guarantor of study integrity, designed the current study, defined the content of the research and reviewed the manuscript. JYC conducted literature research, acquired data and performed statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
References
- 1.Kent HL. Epidemiology of vaginitis. Am J Obstet Gynecol. 1991;165:1168–1176. doi: 10.1016/s0002-9378(12)90722-x. [DOI] [PubMed] [Google Scholar]
- 2.Mills BB. Vaginitis: Beyond the Basics. Obstet Gynecol Clin North Am. 2017;44:159–177. doi: 10.1016/j.ogc.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Huang H, Song L, Zhao W. Effects of probiotics for the treatment of bacterial vaginosis in adult women: A meta-analysis of randomized clinical trials. Arch Gynecol Obstet. 2014;289:1225–1234. doi: 10.1007/s00404-013-3117-0. [DOI] [PubMed] [Google Scholar]
- 4.Hanson L, VandeVusse L, Jermè M, Abad CL, Safdar N. Probiotics for treatment and prevention of urogenital infections in women: A systematic review. J Midwifery Womens Health. 2016;61:339–355. doi: 10.1111/jmwh.12472. [DOI] [PubMed] [Google Scholar]
- 5.Reid G. Probiotic use in an infectious disease setting. Expert Rev Anti Infect Ther. 2017;15:449–455. doi: 10.1080/14787210.2017.1300061. [DOI] [PubMed] [Google Scholar]
- 6.Abad CL, Safdar N. The role of Lactobacillus probiotics in the treatment or prevention of urogenital infections-a systematic review. J Chemother. 2009;21:243–252. doi: 10.1179/joc.2009.21.3.243. [DOI] [PubMed] [Google Scholar]
- 7.Xie HY, Feng D, Wei DM, Mei L, Chen H, Wang X, Fang F. Probiotics for vulvovaginal candidiasis in non-pregnant women. Cochrane Database Syst Rev. 2017;11(CD010496) doi: 10.1002/14651858.CD010496.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37:1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- 10. Sterne JAC, Higgins JPT, Reeves BC on behalf of the development group for ACROBAT- NRSI. A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT- NRSI), Version 1.0.0, 24 September 2014. http://www.riskofbias.info. [Google Scholar]
- 11. Deeks JJ, Higgins JPT and DG A: Chapter 10: Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Higgins JPT, Thomas J, Chandler J, et al. (eds.) Cochrane, 2019. [Google Scholar]
- 12.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. 1998;17:841–856. doi: 10.1002/(sici)1097-0258(19980430)17:8<841::aid-sim781>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Takkouche B, Cadarso-Suárez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol. 1999;150:206–215. doi: 10.1093/oxfordjournals.aje.a009981. [DOI] [PubMed] [Google Scholar]
- 14. National Research Council: Combining Information: Statistical issues and opportunities for research. The National Academies Press, Washington, DC, 1992. https://doi.org/10.17226/20865. [Google Scholar]
- 15.Anukam K, Osazuwa E, Ahonkhai I, Ngwu M, Osemene G, Bruce AW, Reid G. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: Randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450–1454. doi: 10.1016/j.micinf.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Anukam KC, Osazuwa E, Osemene GI, Ehigiagbe F, Bruce AW, Reid G. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect. 2006;8:2772–2776. doi: 10.1016/j.micinf.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Bradshaw CS, Pirotta M, De Guingand D, Hocking JS, Morton AN, Garland SM, Fehler G, Morrow A, Walker S, Vodstrcil LA, Fairley CK. Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: Randomised placebo-controlled double-blind trial. PLoS One. 2012;7(e34540) doi: 10.1371/journal.pone.0034540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksson K, Carlsson B, Forsum U, Larsson PG. A double-blind treatment study of bacterial vaginosis with normal vaginal lactobacilli after an open treatment with vaginal clindamycin ovules. Acta Derm Venereol. 2005;85:42–46. doi: 10.1080/00015550410022249. [DOI] [PubMed] [Google Scholar]
- 19.Hallen A, Jarstrand C, Påhlson C. Treatment of bacterial vaginosis with lactobacilli. Sex Transm Dis. 1992;19:146–148. doi: 10.1097/00007435-199205000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Heczko PB, Tomusiak A, Adamski P, Jakimiuk AJ, Stefanski G, Mikołajczyk-Cichońska A, Suda-Szczurek M, Strus M. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: A randomised, double-blind, placebo-controlled trial. BMC Womens Health. 2015;15(115) doi: 10.1186/s12905-015-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemalatha R, Mastromarino P, Ramalaxmi BA, Balakrishna NV, Sesikeran B. Effectiveness of vaginal tablets containing lactobacilli versus pH tablets on vaginal health and inflammatory cytokines: A randomized, double-blind study. Eur J Clin Microbiol Infect Dis. 2012;31:3097–3105. doi: 10.1007/s10096-012-1671-1. [DOI] [PubMed] [Google Scholar]
- 22.Larsson PG, Stray-Pedersen B, Ryttig KR, Larsen S. Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Womens Health. 2008;8(3) doi: 10.1186/1472-6874-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laue C, Papazova E, Liesegang A, Pannenbeckers A, Arendarski P, Linnerth B, Domig KJ, Kneifel W, Petricevic L, Schrezenmeir J. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women-a double-blind, randomised, controlled clinical pilot trial. Benef Microbes. 2018;9:35–50. doi: 10.3920/BM2017.0018. [DOI] [PubMed] [Google Scholar]
- 24.Marcone V, Calzolari E, Bertini M. Effectiveness of vaginal administration of Lactobacillus rhamnosus following conventional metronidazole therapy: How to lower the rate of bacterial vaginosis recurrences. New Microbiol. 2008;31:429–433. [PubMed] [Google Scholar]
- 25.Marcone V, Rocca G, Lichtner M, Calzolari E. Long-term vaginal administration of Lactobacillus rhamnosus as a complementary approach to management of bacterial vaginosis. Int J Gynaecol Obstet. 2010;110:223–226. doi: 10.1016/j.ijgo.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Martinez RC, Franceschini SA, Patta MC, Quintana SM, Gomes BC, De Martinis EC, Reid G. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55:133–138. doi: 10.1139/w08-102. [DOI] [PubMed] [Google Scholar]
- 27.Mastromarino P, Macchia S, Meggiorini L, Trinchieri V, Mosca L, Perluigi M, Midulla C. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin Microbiol Infect. 2009;15:67–74. doi: 10.1111/j.1469-0691.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- 28.Parent D, Bossens M, Bayot D, Kirkpatrick C, Graf F, Wilkinson FE, Kaiser RR. Therapy of bacterial vaginosis using exogenously-applied Lactobacilli acidophili and a low dose of estriol: A placebo-controlled multicentric clinical trial. Arzneimittelforschung. 1996;46:68–73. [PubMed] [Google Scholar]
- 29.Petricevic L, Witt A. The role of Lactobacillus casei rhamnosus Lcr35 in restoring the normal vaginal flora after antibiotic treatment of bacterial vaginosis. BJOG. 2008;115:1369–1374. doi: 10.1111/j.1471-0528.2008.01882.x. [DOI] [PubMed] [Google Scholar]
- 30.Ling Z, Liu X, Chen W, Luo Y, Yuan L, Xia Y, Nelson KE, Huang S, Zhang S, Wang Y, et al. The restoration of the vaginal microbiota after treatment for bacterial vaginosis with metronidazole or probiotics. Microb Ecol. 2013;65:773–780. doi: 10.1007/s00248-012-0154-3. [DOI] [PubMed] [Google Scholar]
- 31.Lin H, Meng XB. Clinical application of Lactasin capsules in vaginitis. J Practical Medicine. 2006;22:1927–1928. [Google Scholar]
- 32.Recine N, Palma E, Domenici L, Giorgini M, Imperiale L, Sassu C, Musella A, Marchetti C, Muzii L, Benedetti Panici P. Restoring vaginal microbiota: Biological control of bacterial vaginosis. A prospective case-control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Arch Gynecol Obstet. 2016;293:101–107. doi: 10.1007/s00404-015-3810-2. [DOI] [PubMed] [Google Scholar]
- 33.Vicariotto F, Mogna L, Del Piano M. Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: A pilot study. J Clin Gastroenterol. 2014;48 (Suppl 1):S106–S112. doi: 10.1097/MCG.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 34.Vujic G, Jajac Knez A, Despot Stefanovic V, Kuzmic Vrbanovic V. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: A double-blind, randomized, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2013;168:75–79. doi: 10.1016/j.ejogrb.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 35.Anukam KC, Duru MU, Eze CC, Egharevba J, Aiyebelehin A, Bruce A, Reid G. Oral use of probiotics as an adjunctive therapy to fluconazole in the treatment of yeast vaginitis: A study of Nigerian women in an outdoor clinic. Microbial Ecol Health Dis. 2009;21:72–77. [Google Scholar]
- 36.Davar R, Nokhostin F, Eftekhar M, Sekhavat L, Bashiri Zadeh M, Shamsi F. Comparing the recurrence of vulvovaginal candidiasis in patients undergoing prophylactic treatment with probiotic and placebo during the 6 months. Probiotics Antimicrob Proteins. 2016;8:130–133. doi: 10.1007/s12602-016-9218-x. [DOI] [PubMed] [Google Scholar]
- 37.Han YX, Zhao SW. Clinical observation of Ding JunSheng and clotrimazole vaginal tablet in the treatment ofvulvovaginal candidiasis. Anh Med J. 2006;27:528–529. [Google Scholar]
- 38.Hua Y, Lin M, Wang L, Xia L. Observation of curative effect of miconazole and Lactobacillus on vulvovaginal candidiasis treated. Chin J Microecol. 2008;20:386–387. [Google Scholar]
- 39.Ma L, Li L. Miconazole plus Lactasin capsules to treat vulvovaginal candidiasis (54 cases) Herald Med. 2007;26:1041–1042. [Google Scholar]
- 40.Mai XY. Analysis of probiotic Lactobacillus's effect on vulvovaginal candidiasis. Chin J Microecol. 2007;19:362–363. [Google Scholar]
- 41.Nouraei S, Amir Ali Akbari S, Jorjani M, Alavi Majd H, Afrakhteh M, Ghafoorian A, Tafazzoli Harandi H. Comparison between fluconazole with oral protexin combination and fluconazole in the treatment of vulvovaginal candidiasis. ISRN Obstet Gynecol. 2012;2012(375806) doi: 10.5402/2012/375806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Q, Zhao WF, Zheng JP. Clinical observat ion of mycobacterium lacticola preparation in treatment of vaginal vini candidiasis using clotrimazole effervescent tablets. Chin Hosp Pharm J. 2009;29:1377–1379. [Google Scholar]
- 43.Martinez RC, Franceschini SA, Patta MC, Quintana SM, Candido RC, Ferreira JC, De Martinis EC, Reid G. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14. Lett Appl Microbiol. 2009;48:269–274. doi: 10.1111/j.1472-765X.2008.02477.x. [DOI] [PubMed] [Google Scholar]
- 44.Ehrstrom S, Daroczy K, Rylander E, Samuelsson C, Johannesson U, Anzèn B, Påhlson C. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010;12:691–699. doi: 10.1016/j.micinf.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: A double-blind, randomized, placebo-controlled study. Am J Obstet Gynecol. 2010;203:120.e1–e6. doi: 10.1016/j.ajog.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: A review. J Antimicrob Chemother. 2006;58:266–272. doi: 10.1093/jac/dkl246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.






