Abstract
Transforming growth factor β1 (TGF-β1) can promote the proliferation and differentiation of intervertebral disc cells and participates in its repair process. However, whether TGF-β1 engages in the process of disc degeneration has not yet been fully elucidated. The present study aimed to investigate the function of high-dose TGF-β1 on the metabolism of nucleus pulposus cells (NPCs). TGF-β1 levels in human degenerative intervertebral disc tissues and tumor necrosis factor (TNF)-α-induced degenerative NPCs were analyzed. Furthermore, NPCs were treated with TGF-β1 and inhibitors of TGF-β1 receptors [ALK tyrosine kinase receptor (ALK) 1 and ALK5] to determine the effect of the receptors in the mediation of NPC degeneration. The NPC state was determined by the components of secretory collagen I/II, tissue inhibitor of metalloproteinase-3 (TIMP-3) and matrix metalloproteinase (MMP)-13. The mRNA expression of Smad1/2/3/5/8, the downstream gene of TGF-β1 mediated by ALK, was also measured. Results showed that TGF-β1 and ALK1 were positively associated with the degree of degeneration of NP or NPCs in vitro, but negatively associated with ALK5. Furthermore, high-doses of TGF-β1 suppressed collagen II, but enhanced collagen I, TIMP-3, MMP-13, ALK1/5 and Smad1/2/3/5/8 expression. ALK5 inhibition induced the suppression of Smad2/3 and aggravated high-dose TGF-β1-induced NPC degeneration, as shown by the reduction in collagen II and increase in collagen I, TIMP-3 and MMP-13. By contrast, ALK1 inhibition resulted in Smad1/5/8 suppression and alleviated high-dose TGF-β1-induced NPC degeneration. Taken together, it was concluded that high-doses of TGF-β1 contributed to the degeneration of NPCs via the upregulation of ALK1 and Smad1/5/8.
Keywords: nucleus pulposus cells, intervertebral disc degeneration, transforming growth factor-β1, ALK tyrosine kinase receptor 1/5, Smad
Introduction
Human intervertebral disc degeneration (IDD) refers to the loss of typical structure and function of intervertebral disc tissue, gradual disappearance of the nucleus pulposus (NP) tissue, blurring and disappearing of the boundary between NP and fibrous rings, accompanied by degenerated NP cells (NPCs) (1). Various types of pathological damage and aging of physiological functions lead to the degenerative changes of the intervertebral discs and imbalance in the spine. Once the progression of IDD begins, it is difficult to reverse (2). Degenerative changes of the disc mainly occur in the NP of the inner tissue of the intervertebral disc. The NP is composed of NPCs and the extracellular matrix (ECM). The former is derived from mesenchymal stem cells and functions to maintain the healthy metabolism of the NP. Concurrently, NPC activity is also affected by the physical structure and material composition of the ECM (3).
Several functional growth factors and their receptors have been found in normal and degenerative disc tissues, including insulin-like growth factor-1, basic fibroblast growth factor and platelet-derived growth factor (4). In recent years, transforming growth factor (TGF)-β is a cytokine that has been confirmed to be closely associated with IDD and plays an essential role in the development, growth and maintenance of disc tissue (5). The TGF-β superfamily includes TGF-βs (TGF-β1, 2, 3), activins and inhibins, growth differentiation factors, bone morphogenetic proteins and Nodal. Among the TGF-β superfamily, TGF-β1 is closely related to the development and maturation of chondrocytes, as well as the maintenance of chondrocytes in an undifferentiated state (6). It was reported that TGF-β1 can promote the proliferation and differentiation of cartilage-like NPCs and participate in the process of its damage repair (7). However, previous studies have also demonstrated that TGF-β1 and TGF-β1 receptor expression increased with the degree of degeneration of intervertebral disc tissue compared with the normal control disc tissue (8,9). Chen et al (10) also reported increased TGF-β1 levels within IDD. An excess of TGF-β activation exacerbates IDD, and suppression of the excessive TGF-β1 accumulation can prevent IDD development. Therefore, it was observed that TGF-β1 has a dual effect on the development of IDD. However, how excess TGF-β1 mediates IDD and NPC function has not been fully elucidated.
In the TGF-β signaling pathway, its receptor ALK tyrosine kinase receptor (ALK) 1/5 was reported to mediate Smad, playing a vital role in the homeostasis of the ECM of the NP (11-13). The present study hypothesized that high-dose TGF-β1 regulates NPC degeneration via the TGF-β receptors ALK1 and ALK5 and the downstream Smad. Through the inhibition of different TGF receptors, the present study aimed to explore the role of high-dose TGF-β1 in the development of NPC degeneration to provide a scientific basis for clinical prevention and treatment of IDD.
Materials and methods
Ethics statement
All the lumbar intervertebral disc tissues were obtained with consent from the patients or their families, and the project was approved by the Ethics Committee of The Second Affiliated Hospital of Soochow University.
Reagents
Dulbecco's modified Eagle's medium/F12 (DMEM/F12), fetal bovine serum (FBS), type II collagenase, trypsin, penicillin-streptomycin, tumor necrosis factor (TNF)-α and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (Merck KGaA). Phosphate buffered saline (PBS), goat serum, Elite ABC reagent, ECL substrate (cat. no. P0018S), and radioimmunoprecipitation assay (RIPA) lysate buffer (1:500; cat. no. A0277) were purchased from Beyotime Institute of Biotechnology. Primary antibodies against TGF-β1 (cat. no. sc-130348; 1:1,000), collagen-II (Col-II; cat. no. sc-52658; 1:1,000), ALK1 (cat. no. sc-101556; 1:1,000), GAPDH (cat. no. sc-47724; 1:3,000), and the secondary antibody m-immunoglobulin G (IgG)κ binding protein conjugated to horseradish peroxidase (cat. no. sc-516102; 1:500) were purchased from Santa Cruz Biotechnology, Inc. Primary antibody against ALK5 (cat. no. ab31013; 1:1,000) and Alexa Fluor 568 (cat. no. ab175473; 1:1,000) were from Abcam. TRIzol reagent, RETROscript™ reverse transcription kit and SYBR™ Green master mix were purchased from Invitrogen (Thermo Fisher Scientific, Inc.). The ALK5 inhibitor SB431542 (SB; 100 nM; cat. no. s1067) was purchased from Selleck Chemicals. The ALK1 inhibitor San 78-130 (San; 100 nM; cat. no. CS-0020876) was purchased from AbaChemScene, LLC.
NP cell isolation and culture
NP tissues were collected from 18 patients during May 2019 (10 male, 8 female; age range, 43 to 65 years) who underwent spinal surgery for disc herniation in The Second Affiliated Hospital of Soochow University (Suzhou, China). Specimens were divided into four groups based on the Pfirrmann (14) disc magnetic resonance imaging (MRI) score: i) 2#, 3 samples; ii) 3#, 5 samples; iii) 4#, 4 samples; and iv) 5#, 6 samples. Specimens were washed three times with sterile PBS. Subsequently, they were cut into small pieces and sequentially digested with 0.25% trypsin and type II collagenase for 6 h. The NP cell pellets were obtained after filtration and seeded (1x105 per ml) in DMEM/F12 medium containing 10% FBS and 1% penicillin-streptomycin. NP cells were cultured in an incubator at 37˚C with 95% humidity and 5% CO2. A TNF-α-induced NPC degeneration model was used as previously described (15) to investigate TGF-β1 expression in degenerated NPCs in vitro. NPCs were treated with TGF-β1 or ALK1/5 inhibitor for 72 h at 37˚C. For the co-treated group, NPCs were treated with TGF-β1 combined with ALK1/5 inhibitor for 72 h at 37˚C.
Immunohistochemical (IHC) staining
The NP tissue was fixed with 4% formaldehyde at room temperature, rehydrated in descending alcohol series, embedded in paraffin and cut into 5-µm-thick slices. Sections were dewaxed, hydrated, heated in citric acid at 100˚C and blocked with 10% goat serum for 1 h at room temperature. Sections were incubated with TGF-β1 primary antibody overnight at 4˚C. The next day, sections were incubated with biotinylated IgG Elite ABC reagent at room temperature for 1 h, developed with 3,3'-diaminobenzidine and counterstained with hematoxylin at room temperature for 5 min. The positive area was imaged by a light confocal microscope (magnification, x400).
Western blotting (WB)
Total protein of NP tissue or NPCs was extracted with RIPA lysate buffer at a low temperature, and the protein concentration was determined by bicinchoninic acid spectrophotometry. Equal amounts of protein (50 µg/lane) were separated using 10% SDS-PAGE. Proteins were then transferred to a nitrocellulose membrane and blocked with 5% skimmed milk for 2 h at room temperature. Subsequently, membranes were incubated with diluted primary antibodies against collagen II, ALK1, ALK5 and GAPDH overnight at 4˚C. Following washing, the membranes were incubated with the secondary antibody for 1 h at room temperature. The brands were exposed using ECL substrate. Finally, band gray value analysis was performed with a gel image processing system (VILBER FUSION FX5; Vilber Lourmat).
Immunofluorescence (IF) staining
NPCs (1x105 per ml) were seeded in six-well plates. Prior to staining, cells were fixed with 4% paraformaldehyde for 15 min at room temperature and then treated with 0.1% Triton-X for 15 min. Subsequently, 5% BSA was used to block NPCs for 1 h at room temperature. The cells were washed and incubated with TGF-β1 primary antibody overnight at 4˚C. Following incubation with Alexa Fluor 568-conjugated secondary antibody for 1 h in the dark, the staining intensity of NPCs was determined using an inverted fluorescence microscope.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA of NPCs was extracted using TRIzol reagent according to the manufacturer's instructions. RNA was reverse transcribed into cDNA with RETROscript at 25˚C for 5 min and then at 42˚C for 1 h. qPCR analysis of Smad1/2/3/5/8, collagen I/II, tissue inhibitor of metalloproteinase-3 (TIMP-3), matrix metalloproteinase-13 (MMP-13) and GAPDH mRNA expression was performed using SYBR Green master mix with the following reaction conditions: 95˚C for 5 sec; followed by 45 cycles of 92˚C for 15 sec, 58˚C for 8 sec and 60˚C for 30 sec; and a final extension at 72˚C for 5 min. mRNA levels were normalized to GAPDH expression and calculated using the 2-∆∆Cq method (16). The primers used for qPCR are listed in Table I.
Table I.
Primer of the genes for RT-qPCR.
| Gene | Primer sequences (5'→3') |
|---|---|
| Collagen II | F: TGGACGATCAGGCGAAACC |
| R: GCTGCGGATGCTCTCAATCT | |
| Collagen I | F: GAGGGCCAAGACGAAGACATC |
| R: CAGATCACGTCATCGCACAAC | |
| TIMP-3 | F: CATGTGCAGTACATCCATACGG |
| R: CATCATAGACGCGACCTGTCA | |
| MMP-13 | F: ACTGAGAGGCTCCGAGAAATG |
| R: GAACCCCGCATCTTGGCTT | |
| Smad1 | F: AGAGACTTCTTGGGTGGAAACA |
| R: ATGGTGACACAGTTACTCGGT | |
| Smad2 | F: CGTCCATCTTGCCATTCACG |
| R: CTCAAGCTCATCTAATCGTCCTG | |
| Smad3 | F: TGGACGCAGGTTCTCCAAAC |
| R: CCGGCTCGCAGTAGGTAAC | |
| Smad5 | F: CCAGCAGTAAAGCGATTGTTGG |
| R: GGGGTAAGCCTTTTCTGTGAG | |
| Smad8 | F: CTAGGCTGGAAGCAAGGAGAT |
| R: GGGGAATCGTGACGCATTT | |
| GAPDH | F: ACAACTTTGGTATCGTGGAAGG |
| R: GCCATCACGCCACAGTTTC |
F, forward; R, reverse; TIMP-3, tissue inhibitor of metalloproteinase-3; MMP-13, matrix metalloproteinase 13.
Statistical analysis
Data are presented as the mean ± SD. GraphPad Version 8.4 (GraphPad Software, Inc.) was used to perform statistical analysis and generate graphs. Differences between two groups were analyzed using unpaired Student's t-test. Comparison between multiple groups was performed using one-way ANOVA followed by Bonferroni as the post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Increased expression of TGF-β1 in degenerated human NP tissues
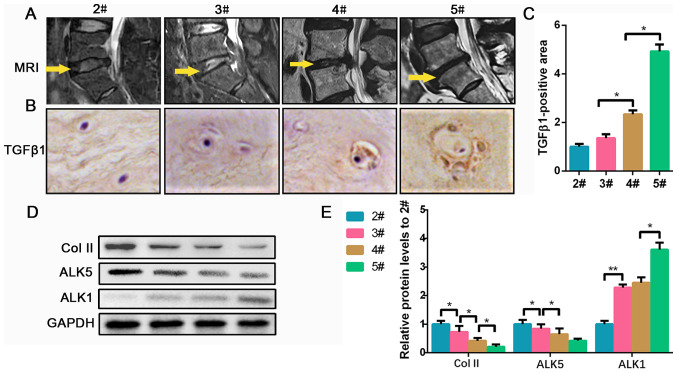
To investigate the levels of TGF-β1 in degenerated human NP tissues, 18 NP samples were collected from patients undergoing disc herniation operation in our hospital. As shown in the MRI in Fig. 1A, the operation section of 2# is higher compared with the others, and the section of 3# is brighter and higher compared with 4# and 5#, indicating that the disc of 2# contained a higher level of water and ECM compared with the other groups. Furthermore, the IHC results indicated that the NPCs tend to be hypertrophic and polynuclear in severely degenerated conditions, and the expression of TGF-β1 increased according to the level of NP degeneration (Fig. 1B and C). Collagen II is an important component secreted by NPCs in the ECM of the NP, which was found to be decreased, most likely due to the degeneration of NPCs (17). ALKs (ALK1-7) exert kinase activity and belong to the type I receptor of the TGF-β superfamily; they can activate Smad1/2/3/5/8, collectively known as receptor-regulated Smad protein (15). ALK5-Smad2/3 and ALK1-Smad1/5/8 signaling pathways play opposite roles in regulating the homeostasis of chondrocytes (18). Therefore, the expression of ALK1 and ALK5 in degenerated NP tissues was investigated. Collagen II protein gradually decreased in groups 2# to 5#. For the ALK5 levels, no significance was found between 4# and 5#, but 4# was significantly less than 2/3#. Meanwhile, ALK1 protein gradually increased as the disc degeneration grade increased (Fig. 1D and E). Collectively, the data suggested that TGF-β1 accumulates at higher levels as the NP tissue becomes increasingly degraded, which is accompanied with a reduction in ALK5 and an increase in ALK1 expression.
Figure 1.
TGF-β1 expression is increased in degenerated discs. (A) Representative MRI of the patients from Pfirrmann grade 2 to 5. The yellow arrow indicates the surgical segment. (B) Representative images of immunohistochemical targets TGF-β1 (magnification, x400) and (C) quantification analysis. (D) Protein expression of Col II, ALK5 and ALK1 was determined by western blotting and (E) semi-quantified. Data are presented as the mean ± SD of three independent experiments. *P<0.05, **P<0.01. TGF-β1, transforming growth factor β1; Col II, collagen II; MRI, magnetic resonance imaging; ALK, ALK tyrosine kinase receptor.
TGF-β1 is increased in TNF-α-treated human NPCs
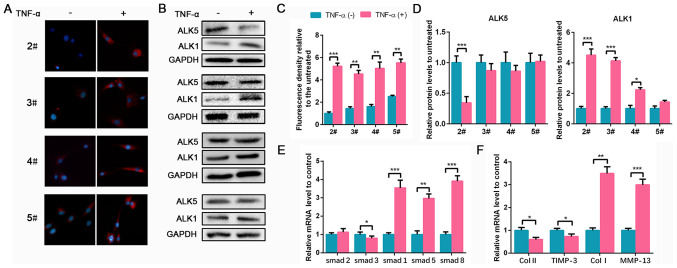
To investigate TGF-β1 expression in degenerated NPCs in vitro, a TNF-α-induced NPC degeneration model was used as previously described (19). NPCs were isolated from 2# to 5# degenerated NP tissues. Following treatment with TNF-α, TGF-β1 expression markedly increased compared with non-treatment cells in each group (Fig. 2A and C). However, no significant differences in ALK5 expression was found before and after TNF-α treatment in 3#, 4# and 5# groups. ALK1 expression in groups 2#, 3# and 4# significantly increased following treatment with TNF-α, consistent with results observed in NP tissues (Fig. 2B and D). Since the ALK5 content of NPCs from groups 3# to 5# did not significantly differ following TNF-α treatment, to better reflect the changing trend of ALK5, 2# NPCs with lower levels of degeneration were used in subsequent experiments. Additionally, Smad1/2/3/5/8 mRNA expression levels in NPCs were evaluated. TNF-α induced the decrease of Smad3 and significantly upregulated Smad1/5/8 levels compared with the controls (Fig. 2E). ECM mRNA expression was also analyzed. Collagen II and TIMP-3 decreased following TNF-α treatment, whereas a marked increase of collagen I and MMP-13 expression was observed (Fig. 2F). Therefore, these results indicated that TNF-α induced NPC degeneration and upregulated TGF-β1, ALK1 and Smad1/5/8 expression.
Figure 2.
TGF-β1 is upregulated in TNF-α-treated NPCs. NPCs isolated from specimens in groups 2# to 5# were treated with 50 ng/ml TNF-α for 24 h. (A) TGF-β1 IF staining of NPCs from groups 2# to 5# (magnification, x400). (B) Protein expression of ALK5 and ALK1 in NPCs from groups 2# to 5# was determined by WB. (C) The quantification of IF. (D) The semi-quantification of WB. (E and F) The mRNA expression levels of smad1/2/3/5/8, Col II, TIMP-3, Col I and MMP-13 in NPCs from group 2# were determined using reverse transcription-quantitative PCR. Data are presented as the mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001. TGF-β1, transforming growth factor β1; TNF-α, tumor necrosis factor α; NPC, nucleus pulposus cell; IF, immunofluorescence; ALK, ALK tyrosine kinase receptor; WB, western blotting; Col, collagen; TIMP-3, tissue inhibitor of metalloproteinase-3; MMP-13, matrix metalloproteinase 13.
ALK5 suppression aggravates high-dose TGF-β1-induced NPC degeneration
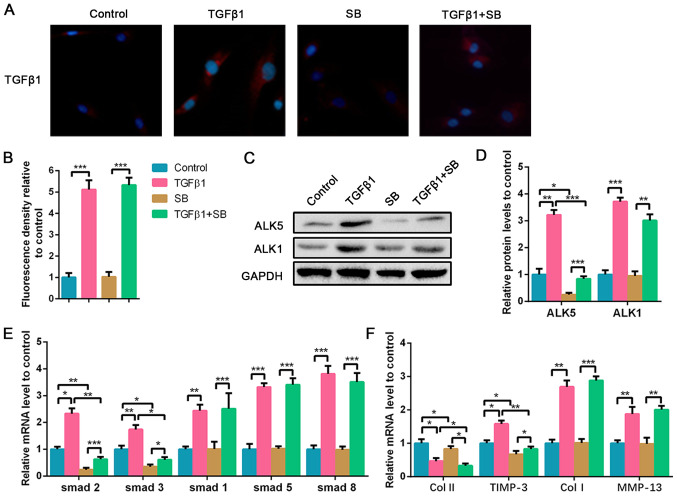
Due to the upregulation of TGF-β1 in the degenerated NP tissues, high-dose TGF-β1 was used to stimulate the NPCs, and the ALK5 inhibitor was also used to explore the effect of ALK5 on NPC degeneration. As shown in Fig. 3A and B, exogenous TGF-β1 treatment significantly increased TGF-β1 expression in NPCs, whereas the ALK5 inhibitor SB did not affect TGF-β1 expression. To evaluate the effect of TGF-β1 treatment on NPCs and the suppressed efficiency of SB, ALK5 protein expression in NPCs was evaluated. Results showed that TGF-β1 stimulation upregulated ALK5 expression and the supplement of SB was sufficient to inhibit ALK5 protein expression compared with the control group. ALK1 protein expression was also analyzed. TGF-β1 promoted ALK1 expression as well; however, SB did not affect ALK1 levels (Fig. 3C and D). Additionally, it was demonstrated that TGF-β1 increased Smad1/2/3/5/8 mRNA expression. However, SB significantly downregulated Smad2 and Smad3 expression with or without the presence of TGF-β1 stimulation. The mRNA levels of Smad1, Smad5 and Smad8 increased following TGF-β1 stimulation, however, SB did not affect their expression (Fig. 3E). High-dose TGF-β1 decreased collagen II expression, and increased TIMP-3, collagen I and MMP-13 expression compared with the control (Fig. 3F). ALK5 plays a positive role in the mediation of cell viability by regulating the Smad2/3 pathway (20). Following inhibition of ALK5 expression, collagen II expression was further decreased compared with the TGF-β1 treated group. TIMP-3 expression also decreased following suppression of ALK5, whereas no significant differences were found in collagen I and MMP-13 mRNA expression (Fig. 3F). The results suggested that high-dose TGF-β1 promoted degeneration of NPCs via suppression of collagen II and the promotion of collagen I and MMP-13 expression, whereas ALK5 inhibition aggravated the reduction of collagen II and decreased TIMP-3 expression.
Figure 3.
Inhibition of ALK5 aggravates high-dose TGF-β1-induced degeneration of NPCs. NPCs were treated with 5 nM TGF-β1 or 100 nM SB for 72 h. For the co-treated group, NPCs were treated with 5 nM TGF-β1 combined with 100 nM SB for 72 h. (A) Immunofluorescence staining of TGF-β1 (magnification, x400) and (B) quantification analysis. (C) Protein expression of ALK5 and ALK1 was determined by western blotting and (D) semi-quantified. (E and F) The mRNA expression levels of smad1/2/3/5/8, Col II, TIMP-3, Col I and MMP-13 in NPCs were determined using reverse transcription-quantitative PCR. Data are presented as the mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001. TGF-β1, transforming growth factor β1; NPC, nucleus pulposus cell; ALK, ALK tyrosine kinase receptor; Col, collagen; TIMP-3, tissue inhibitor of metalloproteinase-3; MMP-13, matrix metalloproteinase 13; SB, SB525334.
ALK1 suppression reverses high-dose TGF-β1-induced NPC degeneration
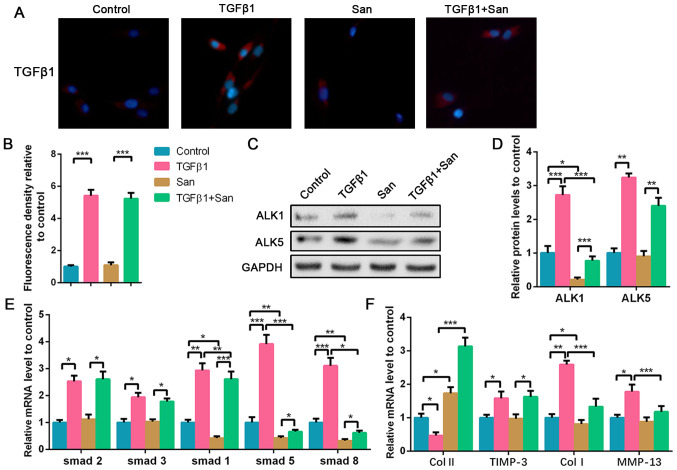
To explore the effect of ALK1 in high-dose TGF-β1-treated NPCs, ALK1 inhibitor was co-cultured with TGF-β1. As aforementioned, exogenous TGF-β1 upregulated TGF-β1 expression in NPCs, which was not affected by San supplement (Fig. 4A and B). WB results indicated that San significantly decreased ALK1 protein expression either with or without TGF-β1 treatment. ALK5 protein was upregulated following TGF-β1 treatment and was not affected by San (Fig. 4C and D). As an ALK1 suppressor, San treatment also led to the suppression of Smad1, Smad5 and Smad8, but had no effect on Smad2 and Smad3 (Fig. 4E). Additionally, ALK1 suppression reversed high-dose TGF-β1-induced NPC degeneration via the upregulation of collagen II and suppression of collagen I and MMP-13 mRNA expression compared with the TGF-β1 treated group (Fig. 4F). Therefore, it was hypothesized that high-dose TGF-β1 degraded NPCs via the activation of ALK1, which subsequently leads to the upregulation of Smad1/5/8 expression.
Figure 4.
Inhibition of ALK1 alleviates high-dose TGF-β1-induced NPC degeneration. NPCs were treated with 5 nM TGF-β1 or 100 nM San for 72 h. For the co-treated group, NPCs were treated with 5 nM TGF-β1 combined with 100 nM San for 72 h. (A) Immunofluorescence staining of TGF-β1 (magnification, x400) and (B) quantification analysis. (C) Protein expression of ALK5 and ALK1 was determined by western blotting and (D) semi-quantified. (E and F) The mRNA expression levels of smad1/2/3/5/8, Col II, TIMP-3, Col I and MMP-13 in NPCs were determined using reverse transcription-quantitative PCR. Data are presented as the mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001. TGF-β1, transforming growth factor β1; NPC, nucleus pulposus cell; ALK, ALK tyrosine kinase receptor; Col, collagen; TIMP-3, tissue inhibitor of metalloproteinase-3; MMP-13, matrix metalloproteinase 13; San, San 78-130.
Discussion
The causes of IDD have not yet been fully elucidated, but the fundamental pathological changes in the process of degeneration are now generally evident. The main manifestations are the gradual reduction of normal NPCs, inflammatory cell infiltration, ECM and water loss, secondary intervertebral disc fibrosis and endplate calcification (21). The TGF-β pathway is requisite for the physiological growth and development of the intervertebral disc (22,23). However, TGF-β and its receptors perform different biological functions in various tissues. The level of secretion, and the distribution and expression of receptors also play an essential role in their function. TGF-β1 can promote ECM synthesis in the early stages of disc degeneration, and repair and protect the disc. Yang et al (24) found that TGF-β1 prevented the overexpression of MMP-3 caused by TNF-α in NPCs. Additionally, TGF-β1 exerted anti-inflammatory effects by inhibition of the NF-κB pathway and promoted collagen II and aggrecan expression in degraded intervertebral discs (25). However, the accumulation of TGF-β1 increases the risk of disc degeneration as the disease develops, accelerating the process of degeneration in the middle and late stages of the degeneration (26). In the present study, TGF-β1 was widely expressed both in the highest degenerated NP tissue and in TNF-α-induced degenerated NPCs in vitro, indicating that TGF-β1 is associated with the development of NP degeneration.
The present study found that TGF-β1 accumulates in degenerated NP and confirmed that high-dose TGF-β1 contributed to the degenerated phenotype of NPCs, with an upregulation in ALK1/5 and Smad1/2/3/5/8. It was reported that TGF-β upregulation can increase the activation of type I receptors (ALK5 or ALK1), and further activate Smad2/3 or Smad1/5/8(27). Kwon et al (28) also illustrated that both Smad2/3 and Smad1/5/8 were upregulated in degenerative bovine NPCs, and activated Smad1/5/8 could inhibit Smad2/3 expression, leading to further IDD. Accumulating evidence has indicated that TGF-β can stimulate ECM production via Smad2/3 overexpression (29,30). TIMP-3 has a specific protective effect on cartilage, and the TGF-β/Smad2/3 pathway can enhance the expression of TIMP-3(31), thereby explaining the protective effect on the ECM. However, activated ALK1 can mediate Smad1/5/8 phosphorylation and has the opposite effect in numerous tissues (32,33). The TGF-β/Smad1/5/8 signaling pathway produces the mineralization marker MMP-13 and causes chondrocyte hypertrophy (34). Hence, suppression of ALK1 and the downstream downregulation of Smad1/5/8 could exert a positive effect on NPCs. Most tissue and organ fibrotic diseases where the pathological basis includes the excessive deposition of collagen I are closely related to the overexpression of TGF-β (35). Therefore, the present study observed high-dose TGF-β1-induced overexpression of collagen I, which was also inhibited by the suppression of ALK1.
In summary, the present study elucidated the degenerative effect of high-dose TGF-β1 on NPCs, which is mainly associated with the activation of ALK1 and Smad1/5/8 expression. High-dose TGF-β1 caused an increase in ALK1 and ALK5 expression, but the adverse effect of ALK1 was more pronounced than the protective effect of ALK5. The combination of high-dose TGF-β1 and ALK1 inhibitor had a robust protective impact on ECM stability. The present study provided a novel basis for future research on the association between these two. However, as it was difficult for us to obtain specimens without any degermation, no healthy disc samples were obtained from individuals without IDD, which is a limitation of the present study. Therefore, the relationship between TGF-β and IDD remains unclear, so we plan to confirm the experimental results in animals in the future so that we can have a control group without degeneration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
ZQ and YS designed the study and performed the experiments, ZQ and FZ collected the data, WC and TL analyzed the data, ZQ and YS prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of The Second Affiliated Hospital of Soochow University (approval no. AK2017-7K8J). Written informed consent was obtained from the patients and/or guardians.
Patient consent for publication
Patients or their guardians have provided written informed consents for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–261. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Zhou TY, Wu YG, Zhang YZ, Bao YW, Zhao Y. SIRT3 retards intervertebral disc degeneration by anti-oxidative stress by activating the SIRT3/FOXO3/SOD2 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:9180–9188. doi: 10.26355/eurrev_201911_19408. [DOI] [PubMed] [Google Scholar]
- 3.Sampara P, Banala RR, Vemuri SK, Av GR, Gpv S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: A review. Gene Ther. 2018;25:67–82. doi: 10.1038/s41434-018-0004-0. [DOI] [PubMed] [Google Scholar]
- 4.Vo NV, Hartman RA, Patil PR, Risbud MV, Kletsas D, Iatridis JC, Hoyland JA, Le Maitre CL, Sowa GA, Kang JD. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016;34:1289–1306. doi: 10.1002/jor.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley JW, Enomoto-Iwamoto M, Smith LJ, Mauck RL, Chan D, Lee J, Heyworth MF, An H, Zhang Y. Intervertebral disc development and disease-related genetic polymorphisms. Genes Dis. 2016;3:171–177. doi: 10.1016/j.gendis.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4(16009) doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, He X, Li Y, Feng J, Pang H, Wang J, Liu Q. Association of transforming growth factor-β1 with pathological grading of intervertebral disc degeneration. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:897–900. (In Chinese) [PubMed] [Google Scholar]
- 8.Tolonen J, Gronblad M, Vanharanta H, Virri J, Guyer RD, Rytomaa T, Karaharju EO. Growth factor expression in degenerated intervertebral disc tissue. An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J. 2006;15:588–596. doi: 10.1007/s00586-005-0930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolonen J, Gronblad M, Virri J, Seitsalo S, Rytomaa T, Karaharju E. Transforming growth factor beta receptor induction in herniated intervertebral disc tissue: An immunohistochemical study. Eur Spine J. 2001;10:172–176. doi: 10.1007/s005860000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Liu S, Ma K, Zhao L, Lin H, Shao Z. TGF-β signaling in intervertebral disc health and disease. Osteoarthritis Cartilage. 2019;27:1109–1117. doi: 10.1016/j.joca.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Uchiyama Y, Guttapalli A, Gajghate S, Mochida J, Shapiro IM, Risbud MV. SMAD3 functions as a transcriptional repressor of acid-sensing ion channel 3 (ASIC3) in nucleus pulposus cells of the intervertebral disc. J Bone Miner Res. 2008;23:1619–1628. doi: 10.1359/jbmr.080502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian Y, Yuan W, Li J, Wang H, Hunt MG, Liu C, Shapiro IM, Risbud MV. TGFβ regulates Galectin-3 expression through canonical Smad3 signaling pathway in nucleus pulposus cells: Implications in intervertebral disc degeneration. Matrix Biol. 2016;50:39–52. doi: 10.1016/j.matbio.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombier P, Clouet J, Boyer C, Ruel M, Bonin G, Lesoeur J, Moreau A, Fellah BH, Weiss P, Lescaudron L, et al. TGF-β1 and GDF5 act synergistically to drive the differentiation of human adipose stromal cells toward nucleus pulposus-like cells. Stem Cells. 2016;34:653–667. doi: 10.1002/stem.2249. [DOI] [PubMed] [Google Scholar]
- 14.Griffith JF, Wang YX, Antonio GE, Choi KC, Yu A, Ahuja AT, Leung PC. Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2007;32:E708–E712. doi: 10.1097/BRS.0b013e31815a59a0. [DOI] [PubMed] [Google Scholar]
- 15.Ten DP, Yamashita H, Ichijo H, Franzen P, Laiho M, Miyazono K, Heldin CH. Characterization of type I receptors for transforming growth factor-beta and activin. Science. 1994;264:101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Patil P, Niedernhofer LJ, Robbins PD, Lee J, Sowa G, Vo N. Cellular senescence in intervertebral disc aging and degeneration. Curr Mol Biol Rep. 2018;4:180–190. doi: 10.1007/s40610-018-0108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Kraan PM, Blaney DE, Blom A, van den Berg WB. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: Modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis Cartilage. 2009;17:1539–1545. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Wang XH, Hong X, Zhu L, Wang YT, Bao JP, Liu L, Wang F, Wu XT. Tumor necrosis factor alpha promotes the proliferation of human nucleus pulposus cells via nuclear factor-κB, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. Exp Biol Med (Maywood) 2015;240:411–417. doi: 10.1177/1535370214554533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Y, Tao H, Jin C, Liu Y, Lu X, Hu X, Wang X. Transforming growth factor-beta1 induces type II collagen and aggrecan expression via activation of extracellular signal-regulated kinase 1/2 and Smad2/3 signaling pathways. Mol Med Rep. 2015;12:5573–5579. doi: 10.3892/mmr.2015.4068. [DOI] [PubMed] [Google Scholar]
- 21.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88 (Suppl 2):S10–S14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 22.Peck SH, McKee KK, Tobias JW, Malhotra NR, Harfe BD, Smith LJ. Whole Transcriptome analysis of notochord-derived cells during embryonic formation of the nucleus pulposus. Sci Rep. 2017;7(10504) doi: 10.1038/s41598-017-10692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H, Shen J, Wang B, Wang M, Shu B, Chen D. TGF-β signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett. 2011;585:1209–1215. doi: 10.1016/j.febslet.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H, Gao F, Li X, Wang J, Liu H, Zheng Z. TGF-β1 antagonizes TNF-α induced up-regulation of matrix metalloproteinase 3 in nucleus pulposus cells: Role of the ERK1/2 pathway. Connect Tissue Res. 2015;56:461–468. doi: 10.3109/03008207.2015.1054030. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Cao C, Wu C, Yuan C, Gu Q, Shi Q, Zou J. TGF-β1 suppresses inflammation in cell therapy for intervertebral disc degeneration. Sci Rep. 2015;5(13254) doi: 10.1038/srep13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh K, Masuda K, Thonar EJ, An HS, Cs-Szabo G. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine (Phila Pa 1976) 2009;34:10–16. doi: 10.1097/BRS.0b013e31818e5ddd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehra A, Wrana JL. TGF-beta and the Smad signal transduction pathway. Biochem Cell Biol. 2002;80:605–622. doi: 10.1139/o02-161. [DOI] [PubMed] [Google Scholar]
- 28.Kwon YJ, Lee JW, Moon EJ, Chung YG, Kim OS, Kim HJ. Anabolic effects of Peniel. 2000, a peptide that regulates TGF-beta1 signaling on intervertebral disc degeneration. Spine (Phila Pa 1976) 2013;38:E49–E58. doi: 10.1097/BRS.0b013e31827aa896. [DOI] [PubMed] [Google Scholar]
- 29.Hu B, Xu C, Cao P, Tian Y, Zhang Y, Shi C, Xu J, Yuan W, Chen H. TGF-β stimulates expression of chondroitin polymerizing factor in nucleus pulposus cells through the Smad3, RhoA/ROCK1, and MAPK signaling pathways. J Cell Biochem. 2018;119:566–579. doi: 10.1002/jcb.26215. [DOI] [PubMed] [Google Scholar]
- 30.Chen MH, Sun JS, Liao SY, Tai PA, Li TC, Chen MH. Low-intensity pulsed ultrasound stimulates matrix metabolism of human annulus fibrosus cells mediated by transforming growth factor β1 and extracellular signal-regulated kinase pathway. Connect Tissue Res. 2015;56:219–227. doi: 10.3109/03008207.2015.1016609. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Zhu Y, Tao H, Jin C, Liu Y, Lu X, Hu X, Fan C. Interaction of ERK1/2 and Smad2/3 signaling pathways in TGF-β1-induced TIMP-3 expression in rat chondrocytes. Arch Biochem Biophys. 2014;564:229–236. doi: 10.1016/j.abb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Van Caam A, Madej W, Garcia DVA, Goumans MJ, Ten DP, Blaney DE, van der Kraan P. TGFβ1-induced SMAD2/3 and SMAD1/5 phosphorylation are both ALK5-kinase-dependent in primary chondrocytes and mediated by TAK1 kinase activity. Arthritis Res Ther. 2017;19(112) doi: 10.1186/s13075-017-1302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derynck R, Budi EH. Specificity, versatility, and control of TGF-β family signaling. Sci Signal. 2019;12(eaav5183) doi: 10.1126/scisignal.aav5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mariani E, Pulsatelli L, Facchini A. Signaling pathways in cartilage repair. Int J Mol Sci. 2014;15:8667–8698. doi: 10.3390/ijms15058667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenner DA, Rippe RA, Rhodes K, Trotter JF, Breindl M. Fibrogenesis and type I collagen gene regulation. J Lab Clin Med. 1994;124:755–760. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.