Abstract
Epigallocatechin gallate (EGCG) is the main component of green tea, which has been proven to inhibit a variety of viruses, including influenza A virus. However, the mechanism of EGCG against influenza virus remains to be further explored. The mechanism of EGCG against influenza virus was studied. The results showed that EGCG significantly increased the levels of HBD3 mRNA and protein, while the levels of phosphorylation of (p)-p38 MAPK, ERK and JNK after EGCG treatment were significantly up-regulated. p38 MAPK, ERK and JNK inhibitors significantly inhibited the expression of HBD3 induced by EGCG. On the other hand, EGCG significantly inhibited the expression of HA and NP proteins in influenza A virus H1N1, but attenuated the anti-influenza A virus effect of EGCG after silencing HBD3. Thus, the anti-influenza virus effect of EGCG is related to the induction of HBD3 expression. In addition, the expression of EGCG-induced HBD3 is related to the p38 MAPK, ERK and JNK signaling pathways. The research data show that EGCG can induce HBD3 expression through p38 MAPK, ERK and JNK signaling pathway to inhibit the replication of influenza A virus H1N1, providing a new and effective candidate drug for influenza virus.
Keywords: EGCG, β-defensin 3, H1N1, MAPK signaling pathway
Introduction
Influenza A virus (IAV) is a single negative chain RNA virus belonging to the positive myxovirus family, which is the main pathogen of seasonal influenza. Seasonal influenza kills 290,000 to 650,000 patients every year (1-3). Influenza vaccine and antiviral drugs are the main strategies for the prevention and treatment of influenza virus. However, at present, the influenza virus vaccine is only aimed at the corresponding subtypes of strains, and the anti-influenza virus drugs amantadine and neuraminidase inhibitors have serious drug resistance (4,5). Therefore, it is urgent to find a long-term and effective new strategy against influenza virus.
β-defensin 3 is a class of antibacterial peptides produced by epithelial cells (6,7). It has been found that β-defensin 3 enhances the resistance ability against influenza virus (8). MDCK cells transfected with β-defensin 3 plasmid also showed resistance to influenza virus (9). Respiratory epithelial cells are the first line of defense against influenza virus, so inducing β-defensin 3 in respiratory epithelial cells may become a new strategy against influenza virus. MAPKs are a key family of proteins involved in regulating gene expression in response to extracellular stimuli and activating a variety of cellular responses, including cell proliferation, differentiation and apoptosis (10). According to the literature, the expression and regulation of human β-defensin 3 in human lung epithelial cells are dependent on MAPKs ERK1/2 and JNK (11). Epigallocatechin gallate (EGCG) is the most abundant catechin in green tea, which has a variety of biological functions, including anti-tumor, anti-inflammatory and immunomodulatory effects (12). Recently, it has been found that EGCG also has anti-influenza virus effect. Previous studies have shown that EGCG mainly prevents the virus from entering the cell by destroying the viral envelope (13). EGCG can also play an antiviral role by inducing the expression of antiviral protein (14). However, it has not been clarified at this stage whether EGCG can play an antiviral role by inducing the expression of β-defensin 3 in respiratory epithelial cells and the mechanism of inducing the expression of β-defensin 3.
Therefore, in the present study, bronchial epithelial cells (BEAS-2B) were used as a model to explore the mechanism of β-defensin 3 expression induced by EGCG and the relationship between β-defensin 3 expression and anti-influenza A virus. The results confirmed that EGCG pretreatment against influenza A virus is related to the induction of the expression of β-defentin 3. EGCG induces the expression of β-defense 3 through the p38 MAPK, ERK, JNK signaling pathway to inhibit the replication of influenza A virus H1N1 in BEAS-2B cells. We elucidated part of the mechanism of EGCG against influenza A virus.
Materials and methods
Cells, virus and reagents
BEAS-2B cells were purchased from Kunming Cell Bank of the Chinese Academy of Sciences (Kunming, China) and cultured in KCB medium at 37˚C with 5% CO2. Madin-Darby canine kidney (MDCK) cells were purchased from Kunming Cell Library of the Chinese Academy of Sciences (Kunming, China), and cultured in Dulbecco's modified Eagle's medium (DMEM), with 10% v/v fetal bovine serum both from (Gibco; Thermo Fisher Scientific, Inc.). Penicillin (100 U/ml), and 100 μg/ml strepsin both from (HyClone; GE Healthcare Life Sciences) at 37˚C in 5% CO2. Influenza A virus (A/PR/8/34 H1N1, PR8) in this experiment was cultured in the allantoic cavity of chicken embryo at 9 days of age for 48 h, and the Reed-Muench method was used to determine the TCID50 of the virus in MDCK cells. The study was approved by the Ethics Committee of The Affiliated Hospital of Guizhou Medical university (Guiyang, China).
Effect of EGCG on BEAS-2B cell activity
Different concentrations of EGCG (Solarbio Life Science; SE8120) (6.25, 12.5, 25, 50, 100 μg/ml) were added to the monolayer BEAS-2B cells in 96-well plates. Five wells were repeated for each concentration and cultured for 48 h. Cell viability was determined by CCK8 kit (Dojindo Molecular Technologies Inc.,) and absorbance was detected at 450 nm by BioTek Instruments, Inc.
Intervention of EGCG on BEAS-2B cells
Different concentrations of EGCG (0, 6.25, 12.5, 25 and 50 μg/ml) were added to the single-layer BEAS-2B cells in the 6-well plate and cultured for 24 h. EGCG of 50 μg/ml was added to the single-layer BEAS-2B cells in the 6-well plate and cultured for 0, 2, 4, 12, 24 and 48 h. One hour before adding 50 μg/ml EGCG, 20 μM SB203580, 20 μM U1026 or 10 μM SP600125 dissolved in dimethyl sulfone was added to inhibit the MAPK signaling pathway.
Transfection of silenced HBD3 in BEAS-2B cells
When 50% of the bottom area of the confocal dish was covered by cells, the culture solution was discarded. After rinsing 3 times with 1X PBS, serum-free DMEM basal medium was added at 2 ml/well. After culture for 12 h, 120 μl ribo FECTCP buffer, 5 μl, 20 μM siRNA and 12 μl ribo FECTCP reagent was taken. HBD3, sequence 5'-TGTGGAAATGCCTTCTTAA-3' and control siRNA (NC), article number: N Control_05815, transfection reagent and primer design were provided by Guangzhou RiboBio Co., Ltd. The mixture was placed at room temperature for 15 min to form a transfection complex, then mixed with 1,863 μl of serum-free DMEM medium (confocal dish 1 well system). Then cells were added and incubated at 37˚C for 24 h.
Virus infection of BEAS-2B cells
At 24 h after transfection, the original culture solution was removed and rinsed 3 times with 1X PBS. The experimental groups were control, IAV, EGCG + IAV, EGCG + siNC and EGCG + IAV + siHBD3 group. In IAV, EGCG + IAV and EGCG + IAV + siHBD3 group, 100X TCID50 H1N1 collected after chicken embryo culture was added, and 50 μg/ml EGGC was added to EGCG + IAV group, EGCG + siNC group and EGCG + IAV + siHBD3 group for 12 h culture. After washing twice with PBS, the medium was changed to KCB and cultured for 12 h.
RNA extraction and real-time fluorescence quantitative PCR
Total RNA was extracted using total RNA extraction kit (Tiangen Biotech Co., Ltd.,) and tested on NanoDrop 2000 (Thermo Fisher Scientific, Inc.) UV spectrophotometer for RNA purity and concentration. cDNA was synthesized using PrimeScriptTM RT kit with gDNA Eraser (Takara Bio, Inc.). Quantitative PCR was performed on CFX96 real-time fluorescent quantitative PCR instrument (Bio-Rad Laboratories, Inc.). Primer sequence-β-defensin 3 forward: 5'-TTATTGCAGAGT CAGAGGCGG-3', and reverse: 5'-CGAGCACTTGCCGAT CTGTT-3'. GAPDH forward, 5'-AGAAGGCTGGGGCTCAT TTG-3' and reverse 5'-AGGGGCCATCCACAGTCTTC-3'. The expression of β-defensin 3 mRNA was calculated using the 2-ΔΔCq method.
Western blot analysis
Cells were washed with PBS at 37˚C and RIPA lysate buffer was added into the cells and centrifuged at 4˚C for 5 min at 14,800 x g to obtain complete cell lysates. Protein samples (20 μg) were electrophoresed using SDS polyacrylamide gel (Bio-Rad Laboratories, Inc.) and the target protein molecules were transferred to a polyvinylidene fluoride membrane. Polyvinylidene fluoride membrane was sealed for 2 h. Then the corresponding resistance p-ERK (cat. no. 4370; 1:1,000), and ERK (cat. no. 4695; 1:1,000) (both from Cell Signaling Technology, Inc.), p-JNK (Abcam; 1:1,000), JNK (Abcam; cat. no. ab179461; 1:1,000), p-p38 lightning MAPK (Cell Signaling Technology, Inc.; cat. no. 9211; 1:1,000), p38 lightning MAPK (Cell Signaling Technology, Inc.; cat. no. 9212; 1:1,000), NP (BIOSS; cat. no. bs-4976R; 1:1,000) and GAPDH (Boster Biological Technology; cat. no. M00227-5; 1:700) were added and incubated overnight at 4˚C. After washing, horseradish peroxidase-labeled secondary antibodies (Boster Biological Technology; cat. no. BA1054; 1:5,000) were added for incubation and exposure in the gel imaging system.
Cytokine detection
The supernatant of cell culture was taken and the content of β-defensin 3 was detected by human β-defensin 3 ELISA kit (Wuhan Gene-beauty Organism).
Immunofluorescence detection of influenza virus replication in BEAS-2B cells
When the BEAS-2B cells were fused to 80%, the cells were washed with PBS twice, fixed with 4% paraformaldehyde for 30 min, and then treated with 0.2% triton for 2 min. After blocking with 5% BSA, rabbit anti-NP antibody (Boster Biological Technology) and rabbit anti-HA antibody (Cell Signaling Technology, Inc.) were added at 4˚C overnight. After washing with PBS, fluorescence labeled secondary antibody (Boster Biological Technology) was added avoiding light for 30 min and photographed under a confocal microscope.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA), with Bonferroni multiple comparison post hoc test, or Student's t-test. Statistical calculations were carried out using the GraphPad InStat3 software (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of EGCG on cell activity
Different concentrations of EGCG (0, 6.25, 12.5, 25, 50, 100 μg/ml) were applied to BEAS-2B cells for 48 h, and CCK8 kit was used to detect cell activity. The results showed that the activity of BEAS-2B cells was significantly inhibited only by 100 μg/ml of EGCG compared with the control group, and the remaining concentrations were not significantly different from those in the control group (Fig. 1). Therefore, EGCG of up to 50 μg/ml was selected for the next experiment.
Figure 1.
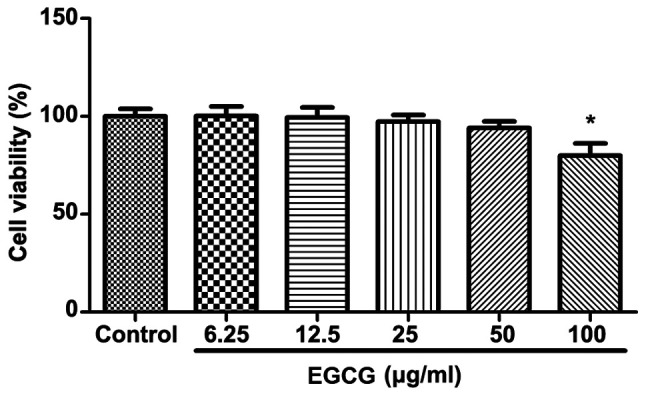
Toxicity test of EGCG in BEAS-2B cells. BEAS-2B were treated with EGCG at various concentrations (0, 6.25, 12.5, 25, 50 and 100 μg/ml) for 48 h. Cell viability was analyzed by CCK-8 assay. Data are shown as means ± SD in five parallel experiments. *P<0.05, when compared with the control without EGCG. The data are representative of three independent experiments. EGCG, Epigallocatechin gallate; SD, standard deviation.
Expression of β-defensin 3 in BEAS-2B cells induced by EGCG
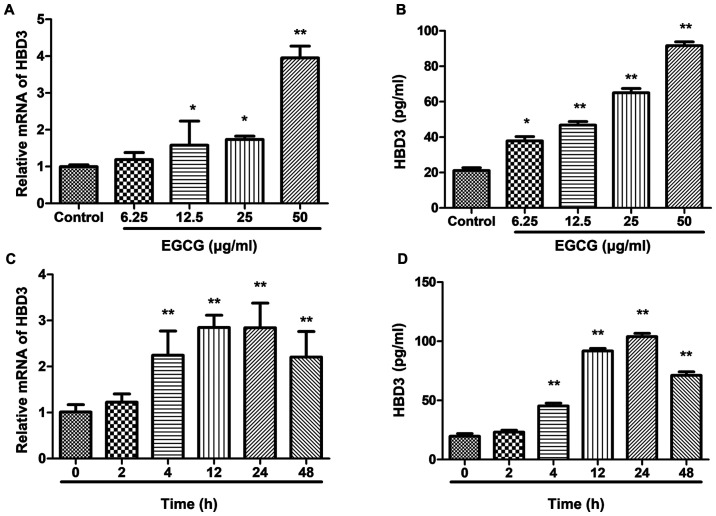
After confirming that EGCG at concentrations of up to 50 μg/ml had no effect on cell activity, the time-dose relationship was studied between EGCG-induced expression of β-defensin 3 in BEAS-2B cells. After the intervention of different concentrations of EGCG in BEAS-2B cells, the expression level of β-defensin 3 mRNA was detected by real-time fluorescence quantitative PCR and the protein expression level of β-defensin 3 was detected by ELISA. The results showed that the expression of β-defensin 3 in BEAS-2B cells was up-regulated in a dose-dependent manner, with the highest concentration of 50 μg/ml (Fig. 2A and B). When EGCG was applied to BEAS-2B cells at a concentration of 50 μg/ml, the expression of β-defense 3 mRNA and protein in BEAS-2B cells at different time periods was up-regulated in a time-dependent manner and reached its peak at 24 h (Fig. 2C and D).
Figure 2.
Effects of EGCG on the expression of HBD3 in BEAS-2B cells. Real-time RT-PCR assay for HBD3 mRNA stimulated and ELISA assay for protein stimulated with EGCG. BEAS-2B cells were incubated with 0, 6.25, 12.5, 25, and 50 μg/ml EGCG for 24 h. The expression of HBD3 was quantified by SYBR-Green Real-time RT-PCR. GAPDH mRNA was used as an internal control. Data are the means ± SEM of three separate experiments. mRNA expression of HBD3 is significantly higher at 50 μg/ml EGCG than the control without EGCG. Detection of HBD3 mRNA expression level by real-time RT-PCR and protein expression level by ELISA in BEAS-2B cells after 50 μg/ml EGCG stimulation for different times (0, 2, 4, 12, 24 and 48 h). Data are shown as means ± SEM of three separate experiments. The expression of HBD3 mRNA is significantly higher at 24 h than the control at 0 h (*P<0.05, **P<0.01). The data are representative of three independent experiments: (A) Effects of different concentrations of EGCG on HBD3 mRNA expression; (B) Effects of different concentrations of EGCG on HBD3 protein expression; (C) HBD3 mRNA expression at different time points; (D) HBD3 protein expression at different time points. EGCG, Epigallocatechin gallate; SEM, standard error of the mean; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ELISA, enzyme-linked immunosorbent assay.
Expression of phosphorylation protein in MAPK signaling pathway induced by EGCG in BEAS-2B cells
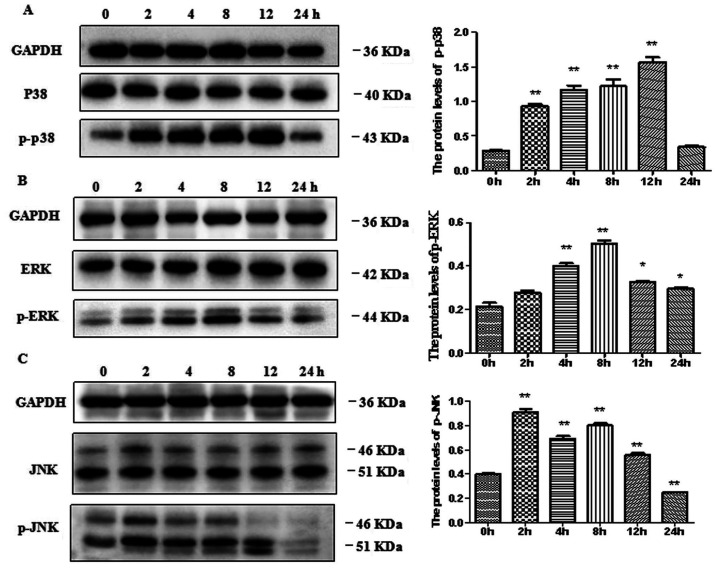
It was confirmed that β-defensin 3 was most strongly induced by 50 μg/ml EGCG, so the signal pathway of β-defensin 3 expression in BEAS-2B cells was determined by 50 μg/ml EGCG. BEAS-2B cells were treated with EGCG for 0, 2, 4, 8, 12 and 24 h. The effects of EGCG on the activation of p38 MAPK, ERK and JNK in BEAS-2B cells were determined. The results showed that EGCG did not affect the levels of total p38 MAPK, ERK and JNK protein over time, but significantly increased the levels of phosphorylated p38 MAPK, p-ERK and p-JNK (Fig. 3).
Figure 3.
Effects of EGCG on the expressions of (A) p-p38, (B) p-ERK and (C) p-JNK proteins in BEAS-2B cells. BEAS-2B cells were treated with 50 µg/ml EGCG for the indicated times. At each time, whole-cell lysates were prepared and used for p-p38 MAPK, p38 MAPK, p-ERK, ERK, p-JNK, JNK, or GAPDH western with respective antibodies. Data are shown as means ± SEM of three separate experiments. The treatment with 50 μg/ml EGCG triggered the phosphorylation (activation) of ERK, p38 MAPK, and JNK (*P<0.05, **P<0.01). The data are representative of three independent experiments. EGCG, Epigallocatechin gallate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SEM, standard error of the mean.
Inhibition of MAPK signaling pathway induces expression of β-defensin 3 mRNA and protein in BEAS-2B cells induced by EGCG
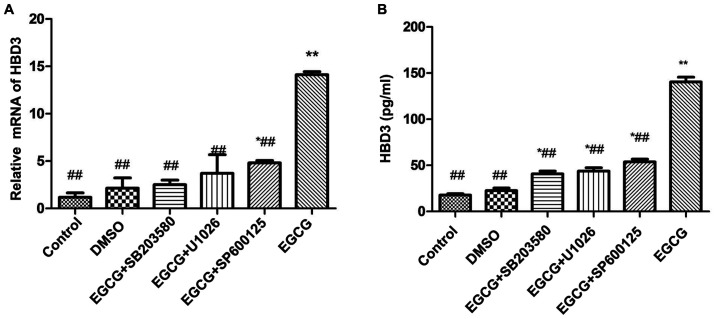
The expression of β-defensin 3 induced by p38 MAPK, ERK and JNK signaling pathway in EGCG was further detected using 20 μM SB203580, 20 μM U1026 or 10 μM SP600125 in the pretreatment of the BEAS-2B cells for 1 h and then 50 μg/ml EGCG was added to the cells. Pretreatment with 20 μM SB203580, 20 μM U1026 and 10 μM SP600125 significantly inhibited the expression of β-defensin 3 mRNA and protein induced by EGCG (Fig. 4).
Figure 4.
Effects of EGCG on the expression of HBD3 in BEAS-2B cells. (A) Real-time RT-PCR assay for the upregulated expression of HBD3 mRNA treated with inhibitors for intracellular pathways. The cells were treated with inhibitors for intracellular pathways in a series of experiments. BEAS-2B cells were pre-incubated with SB203580 (an inhibitor of p38 MAPK), U1026 (an inhibitor of ERK), and SP600125 (an inhibitor of JNK) for 1 h and then treated with 50 µg/ml EGCG for 12 h. SB203580 (an inhibitor of p38 MAPK), U1026 (an inhibitor of ERK), and SP600125 (an inhibitor of JNK) completely inhibited the upregulated expression of HBD3 by EGCG (*P<0.05, **P<0.01, compared with the control group; #P<0.05, ##P<0.01 compared with the EGCG group). The data are representative of three independent experiments. (B) Competitive ELISA for the quantification of HBD3 secretion by cultured BEAS-2B cells after EGCG and inhibitor treatments. The cell were preincubated with SB203580, U1026, and SP600125 for 1 h and then treated with 50 μg/ml EGCG for 12 h. Serial dilutions of synthetic mature HBD3 peptide were used to generate a standard curve, and the HBD3 peptide concentration of each experimental group was considered. In accordance with the RT-PCR result, SB203580, U1026, and SP600125 completely inhibited the secretion of HBD3 by EGCG (*P<0.05, **P<0.01, compared with the control group; ##P<0.01, compared with the EGCG group). The data are representative of three independent experiments. EGCG, Epigallocatechin gallate.
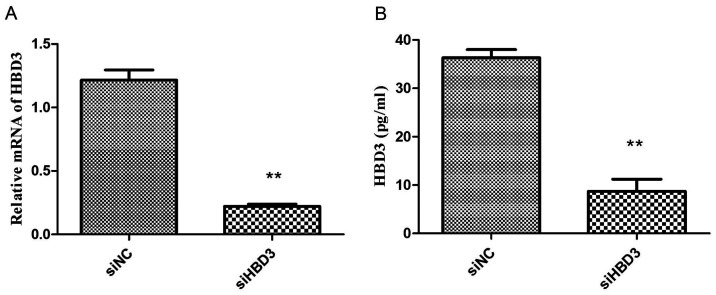
Expression of HBD3 mRNA and protein in BEAS-2B cells after silent transfection qPCR and ELISA were used to detect the expression of HBD3 mRNA and protein in BEAS-2B cells of siNC and siHBD3. The results showed that compared with the siNC group, the expression of HBD3 mRNA and protein in the siHBD3 group was significantly reduced (P<0.01) (Fig. 5). This suggested the successful silencing of the HBD3 gene of BEAS-2B cells.
Figure 5.
The levels of HBD3 mRNA and protein. Confirmation of suppression after transfection with siRNA-HBD3 in BEAS-2B cells using real-time RT-PCR and ELISA analysis. **P<0.01, compared with the siRNA-NC group. The data are representative of three independent experiments. (A) Expression level of HBD3 mRNA. (B) Expression level of HBD3 protein.
EGCG induces BEAS-2B cells against influenza A virus H1N1
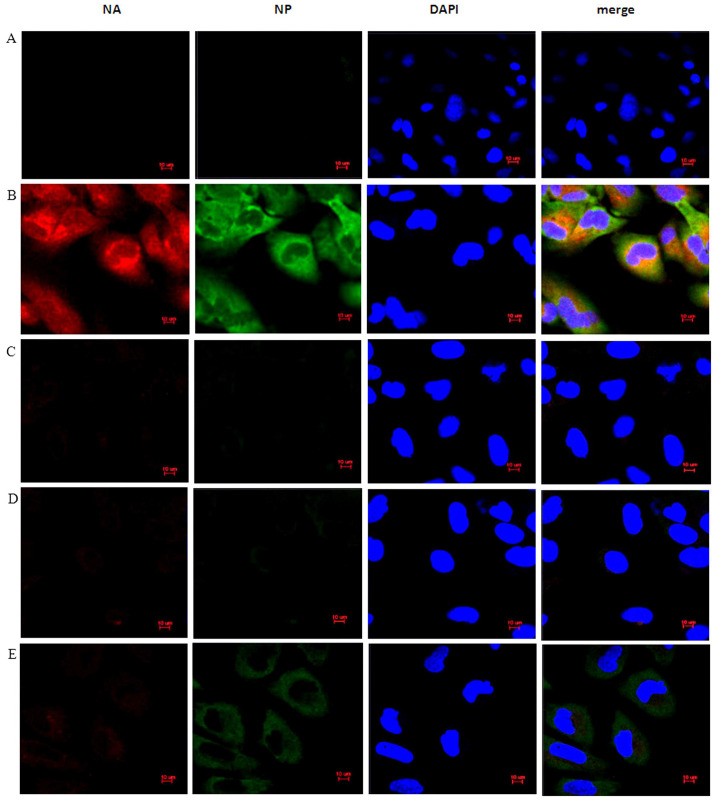
The above study confirmed that EGCG can induce the expression of β-defensin 3 in BEAS-2B cells. Whether EGCG anti-influenza virus H1N1 was related to the induction of β-defensin 3 expression was explored. Immunofluorescence was used to detect the expression of HA and NP proteins in BEAS-2B cells. The results showed that compared with the IAV group (Fig. 6B), the expression of HA and NP in the EGCG + IAV group (Fig. 6C) was significantly inhibited, but the inhibition of the expression of HA and NP in the EGCG + IAV + siHBD3 group (Fig. 6E) was significantly lower than that in the EGCG + IAV group (Fig. 6C), indicating that silencing HBD3 inhibited the antiviral effect of EGCG (Fig. 6).
Figure 6.
NA, NP Immunofluorescence expression in BEAS-2B cells, x100. Zeiss AxioSkop fluorescence microscope (x100) shows NA and NP protein (red and green) antibody immunofluorescence stained cytoplasm and nuclear localization on BEAS-2B cells. When it was co-stained with DAPI, the nucleus was blue. A total of 50 μg/ml EGCG was used for treatment for 12 h before BEAS-2B cells infected with 100X TCID50 H1N1. After incubation with H1N1 for 1 h, culture for 12 h. The confocal microscope was used to observe the expression and localization of NA and NP proteins. These data represent three independent experiments. (A) Control group. (B) IAV group. (C) EGCG + IAV group. (D) EGCG + IAV + siNC group. (E) EGCG + IAV + siHBD3 group.
Discussion
In this study, EGCG was used to intervene in BEAS-2B cells, and the results showed that EGCG significantly up-regulated the expression levels of β-defensin 3 mRNA and protein in BEAS-2B cells. Therefore, it was confirmed that EGCG can induce the production of HBD3 in BEAS-2B cells, and EGCG stimulates the up-regulation of HBD3 mRNA and protein expression in a concentration- and time-dependent manner. Gan et al (14) have shown that the expression levels of β-defensin 2 mRNA and protein in 16HBECs cells are in a concentration- and time-dependent relationship with paeoniflorin (PF) stimulation, which is similar to the trend in this study, but the concentration and the length of time were different, which related to the experimental subjects and the different types of drugs and defensins. β-defensin 3 has strong antiviral (9) and antibacterial activity (15). HBD3 can be induced by viruses, bacteria, microbial products, toll-like receptors, epidermal cell growth factors, and pro-inflammatory factors (16). Although the exact mechanism of action is not yet clear, it is currently believed that HBD3 works through ion channels (17).
The mechanism of EGCG-induced β-defensin 3 expression in BEAS-2B cells was explored. MAPKs in mammals include c-Jun NH2 terminal kinase (JNK), p38 MAPK and extracellular signal-regulated kinase (ERK). These enzymes are serine-threonine protein kinases that can regulate various cellular activities, including proliferation, differentiation, apoptosis, survival, inflammation and innate immunity (18-20). It has been confirmed that EGCG can regulate the activation of p38 MAPK, ERK, and JNK signaling pathways in colon cancer cells (21). ERK and JNK-dependent HBD3 expression has been confirmed in Helicobacter pylori infected gastric epithelial cells (22). In this study, it was found that EGCG can significantly increase the level of phosphorylated (p)-p38 MAPK, ERK and JNK, and the results show that EGCG can activate the p38 MAPK, ERK and JNK signaling pathways. On the other hand, p38 MAPK, ERK, or JNK inhibitors were used to inhibit the signaling pathway. p38 MAPK, ERK, or JNK inhibitors significantly reduced the expression level of β-defensin 3 in BEAS-2B cells induced by EGCG. The results in this study were similar to other studies which suggested that the expression of β-defensin 3 is also regulated by p38 MAPK, ERK and JNK signaling pathways (14,23,24). Therefore, it is suggested in this study that EGCG can induce β-defensin 3 expression in BEAS-2B cells through p38 MAPK, ERK and JNK signaling pathways. In addition, the expression of β-defensin 3 mRNA and protein peaked at 12 or 24 h under EGCG stimulation, while the activation peak of MAPK pathway was 8 or 12 h, but the expression at 24 h was significantly reduced. The reason may be that since β-defensin 3 was downstream of MAPK pathway, when MAPKs start to be highly expressed at 8 h, the downstream β-defensin 3 was gradually activated to express. At 12 h, β-defensin 3 expression began to increase sharply, and although the expression of MAPKs was reduced at 24 h, the gradual reactions persisted. β-defensin 3 continued to maintain an expression peak, and the expression level was slightly higher than at 12 h.
EGCG has been proven to be resistant to influenza virus (13), but the role of EGCG in immune modulation against influenza virus remains to be further studied. Our experiments found that EGCG pretreatment can significantly inhibit the expression of HA and NP proteins of influenza virus in BEAS-2B cells. However, silencing HBD3 expression in cells will reduce the ability of EGCG to inhibit the expression of HA and NP of influenza virus. In the report of EGCG against influenza A virus, Colpitts and Schang pointed out that EGCG competitively binds to the sialic acid receptor on the surface of the virus, thereby inhibiting influenza disease (25). Kesic et al found that EGCG can upregulate the expression of Nrf-2 gene, the most important transcriptional regulator in the second phase of the cellular antioxidant pathway, to inhibit influenza virus (26). In a recent study, it was pointed out that EGCG can significantly down-regulate TLR4 and NF-κB protein levels and treat acute lung injury caused by swine influenza virus H9N2(27). These findings indicate that EGCG can fight influenza virus through a variety of mechanisms, but there are few reports on EGCG inhibiting influenza virus by regulating HBD3 secretion. However, our results confirm that EGCG can induce HBD3 expression in BEAS-2B cells and inhibit the expression of HA and NP in influenza A virus. Because HA plays an important role in the process of virus introduction into host cells, NP is a key protein for viral transcription and replication, it is speculated that EGCG can regulate the concentration of HBD3 produced by cells to inhibit influenza virus adsorption, invasion and replication, and then produce anti-influenza virus effects.
In conclusion, it has been shown in this study that EGCG can induce the expression of β-defensin 3 through p38 MAPK, ERK and JNK signaling pathways in BEAS-2B cells to inhibit influenza A virus H1N1, which indicates that EGCG may be an effective drug against influenza A virus.
Acknowledgements
Not applicable.
Funding
The study was supported by Baiyun Science and Technology Plan Project of Guiyang (grant no. [2019]33; ‘Research on the Mechanism of Epigallocatechin Gallate Inhibiting Influenza A Virus Based on NF-κB Signaling Pathway’) and the National Natural Science Foundation of China (grant no. 81260249; ‘Research on the Mechanism of Influenza A Virus Induces β-defensins Production in Respiratory Rpithelial Cells Based on NF-κB and p38 MAPK Signaling Pathways’).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QM conceived the study and wrote the manuscript. YJ interpreted and analyzed the data. LZ and ZZ designed the study and performed the experiment. TR was responsible for the analysis and discussion of the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The Affiliated Hospital of Guizhou Medical university (Guiyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Horimoto T, Kawaoka Y. Influenza: Lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- 2.Hause BM, Collin EA, Liu R, Huang B, Sheng Z, Lu W, Wang D, Nelson EA, Li F. Characterization of a novel influenza virus in cattle and Swine: Proposal for a new genus in the Orthomyxoviridae family. MBio. 2014;5:e00031–e14. doi: 10.1128/mBio.00031-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee VJ, Ho ZJM, Goh EH, Campbell H, Cohen C, Cozza V, Fitzner J, Jara J, Krishnan A, Bresee J. Advances in measuring influenza burden of disease. Influenza Other Respir Viruses. 2018;12:3–9. doi: 10.1111/irv.12533. WHO Working Group on Influenza Burden of Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mungall BA, Xu X, Klimov A. Surveillance of influenza isolates for susceptibility to neuraminidase inhibitors during the 2000-2002 influenza seasons. Virus Res. 2004;103:195–197. doi: 10.1016/j.virusres.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen JT, Smee DF, Barnard DL, Julander JG, Gross M, de Jong MD, Went GT. Efficacy of combined therapy with amantadine, oseltamivir, and ribavirin in vivo against susceptible and amantadine-resistant influenza A viruses. PLoS One. 2012;7(e31006) doi: 10.1371/journal.pone.0031006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pazgier M, Li X, Lu W, Lubkowski J. Human defensins: Synthe- sis and structural properties. Curr Pharm Des. 2007;13:3096–3118. doi: 10.2174/138161207782110381. [DOI] [PubMed] [Google Scholar]
- 7.Shah R, Chang TL. doi: 10.1021/bk-2012-1095.ch007. Defensins in viral infection. In: Small Wonders: Peptides for Disease Control. Rajasekaran K, Cary JW, Jaynes JM, Montesinos E (eds). Vol 1095. ACS Symposium Series, pp137-171, 2012. [DOI] [Google Scholar]
- 8.Jiang Y, Yang D, Li W, Wang B, Jiang Z, Li M. Antiviral activity of recombinant mouse β-defensin 3 against influenza A virus in vitro and in vivo. Antivir Chem Chemother. 2012;22:255–262. doi: 10.3851/IMP2077. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Wang Y, Kuang Y, Wang B, Li W, Gong T, Jiang Z, Yang D, Li M. Expression of mouse β-defensin-3 in MDCK cells and its anti-influenza-virus activity. Arch Virol. 2009;154:639–647. doi: 10.1007/s00705-009-0352-6. [DOI] [PubMed] [Google Scholar]
- 10.Yang SH, Sharrocks AD, Whitmarsh AJ. MAP kinase signalling cascades and transcriptional regulation. Gene. 2013;513:1–13. doi: 10.1016/j.gene.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Haarmann H, Steiner T, Schreiber F, Heinrich A, Zweigner J, N’Guessan PD, Slevogt H. The role and regulation of Moraxella catarrhalis-induced human beta-defensin 3 expression in human pulmonary epithelial cells. Biochem Biophys Res Commun. 2015;467:46–52. doi: 10.1016/j.bbrc.2015.09.126. [DOI] [PubMed] [Google Scholar]
- 12.Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: A literature review. Chin Med. 2010;5(13) doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim M, Kim SY, Lee HW, Shin JS, Kim P, Jung YS, Jeong HS, Hyun JK, Lee CK. Inhibition of influenza virus internalization by (-)-epigallocatechin-3-gallate. Antiviral Res. 2013;100:460–472. doi: 10.1016/j.antiviral.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Gan Y, Cui X, Ma T, Liu Y, Li A, Huang M. Paeoniflorin upregulates β-defensin-2 expression in human bronchial epithelial cell through the p38 MAPK, ERK, and NF-κB signaling pathways. Inflammation. 2014;37:1468–1475. doi: 10.1007/s10753-014-9872-7. [DOI] [PubMed] [Google Scholar]
- 15.Fusco A, Savio V, Cammarota M, Alfano A, Schiraldi C, Donnarumma G. Schiraldi C and Donnarumma G: Beta- defensin-2 and beta-defensin-3 reduce intestinal damage caused by Salmonella typhimurium modulating the expression of cytokines and enhancing the probiotic activity of Enterococcus faecium. J Immunol Res. 2017;2017(6976935) doi: 10.1155/2017/6976935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB Jr, Ganz T. Human beta-defensin-1: An antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reichel M, Heisig A, Heisig P, Kampf G. Skin bacteria after chlorhexidine exposure-is there a difference in response to human beta-Defensin-3? Eur J Clin Microbiol Infect Dis. 2010;29:623–632. doi: 10.1007/s10096-010-0904-4. [DOI] [PubMed] [Google Scholar]
- 18.Bezniakow N, Gos M, Obersztyn E. The RASopathies as an example of RAS/MAPK pathway disturbances - clinical presentation and molecular pathogenesis of selected syndromes. Dev Period Med. 2014;18:285–296. [PubMed] [Google Scholar]
- 19.Kono M, Tatsumi K, Imai AM, Saito K, Kuriyama T, Shirasawa H. Kuriyama T and Shirasawa H: Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: Involvement of p38 MAPK and ERK. Antiviral Res. 2008;77:150–152. doi: 10.1016/j.antiviral.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, He C, Pan H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–179. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Cerezo-Guisado MI, Zur R, Lorenzo MJ, Risco A, Martín-Serrano MA, Alvarez-Barrientos A, Cuenda A, Centeno F. Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG. Food Chem Toxicol. 2015;84:125–132. doi: 10.1016/j.fct.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Boughan PK, Argent RH, Body-Malapel M, Park JH, Ewings KE, Bowie AG, Ong SJ, Cook SJ, Sorensen OE, Manzo BA, et al. Nucleotide-binding oligomerization domain-1 and epidermal growth factor receptor: Critical regulators of beta-defensins during Helicobacter pylori infection. J Biol Chem. 2006;281:11637–11648. doi: 10.1074/jbc.M510275200. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Du X, Chen J, Hu L, Chen L. The induction expression of human β-defensins in gingival epithelial cells and fibroblasts. Arch Oral Biol. 2013;58:1415–1421. doi: 10.1016/j.archoralbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Paoletti I, Buommino E, Fusco A, Baudouin C, Msika P, Tufano MA, Baroni A, Donnarumma G. Patented natural avocado sugar modulates the HBD-2 and HBD-3 expression in human keratinocytes through toll-like receptor-2 and ERK/MAPK activation. Arch Dermatol Res. 2012;304:619–625. doi: 10.1007/s00403-012-1237-1. [DOI] [PubMed] [Google Scholar]
- 25.Colpitts CC, Schang LM. A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. J Virol. 2014;88:7806–7817. doi: 10.1128/JVI.00896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesic MJ, Simmons SO, Bauer R, Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic Biol Med. 2011;51:444–453. doi: 10.1016/j.freeradbiomed.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu MJ, Liu BJ, Wang CL, Wang GH, Tian Y, Wang SH, Li J, Li PY, Zhang RH, Wei D, et al. Epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor and effectively alleviates acute lung injury induced by H9N2 swine influenza virus. Int Immunopharmacol. 2017;52:24–33. doi: 10.1016/j.intimp.2017.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.