Abstract
Parasitic diseases have been known to cause pulmonary vascular lesions. Schistosomiasis is the most common parasitic disease associated with pulmonary arterial hypertension, although other trematodes have been implicated. Systematic evaluation of and interest in this problem have been rekindled because of the current availability of pulmonary arterial hypertension treatment.
Keywords: Parasitic disease, pulmonary arterial hypertension, review, schistosomiasis
Schistosomiasis
Schistosomiasis (or bilharzia, bilharziosis or snail fever) is a parasitic disease caused by trematode flatworms of the genus Schistosoma. The parasites were first identified in 1851 by Theodor Bilharz, a German pathologist working in Egypt.
There are 24 species of Schistosoma, and they can be split into four distinct groups on the basis of the compatibility of species with particular snail host genera and geographical distribution, as well as common egg morphologies. Among these species, six cause disease in humans, with the three main species being Schistosoma mansoni, Schistosoma japonicum and Schistosoma haematobium [1]. Some species also infect wild animals and domestic stock (e.g. S. japonicum), and others are primarily veterinary pathogens (Schistosoma bovis, Schistosoma mattheii and Schistosoma curassoni) [2].
There is currently no vaccine available, and primary treatment for human schistosomiasis is the drug praziquantel, which is usually effective in a single dose. However, praziquantel does not prevent re-infection, is inactive against juvenile schistosomes and has only a limited effect on already developed liver and spleen lesions. Praziquantel is also limited by the emergence of schistosome phenotypes that are resistant to this drug [3]. Alternative treatments include oxamniquine [4,5].
In this review, we will concentrate on pulmonary and inflammatory manifestations caused by Schistosoma infection, even though the lung is a mandatory step in the parasite cycle. These manifestations can be acute or chronic, depending on the phase of the cycle. Morbidity resulting from chronic manifestations is particularly severe and should be prevented whenever possible.
Life cycle of schistosomes
Schistosomes have a vertebrate–invertebrate life cycle, in which humans are a definitive host. The first step in the cycle is infection of a freshwater snail by the miracidium, a small, free-living larva that swims and penetrates specific snail inter-mediate hosts within 1–24 h. In the snail, miracidia transform into the next larval stage, cercariae, which subsequently emerge from the snail. The cercariae remain swimming in fresh water using a whip-like tail (for a maximum of 48 h) at speeds of about 4 m/h, and can penetrate the skin of people working or bathing in the infected water in 3–5 min. Cercariae have the ability to attach to human skin and, through secretion of enzymes, break down skin proteins and invade the new host. After penetration, they may remain in the skin for 1–2 days. After infecting humans, cercariae shed their tails and become schistosomula, which migrate in the venous circulation, pass through the lungs, and home to their target organ, where they mature and unite. A pair of worms, male and female, attaches to the superior mesenteric veins (S. mansoni), the inferior mesenteric and superior haemorrhoidal veins (S. japonicum) or the vesical plexus and veins draining the ureters (S. haematobium). About 4–6 weeks after infection, schistosomes begin to produce eggs, and this continues for the parasite’s entire lifespan, usually 3–5 years (about 300 eggs per day for S. mansoni and up to 3000 eggs per day for S. japonicum). Eggs are excreted in the faeces (S. mansoni and S. japonicum) or urine (S. haematobium). Under optimal conditions, the eggs hatch within 30 min, releasing miracidia. Nevertheless, many of the eggs remain within the host, resulting in focal granulomatous reactions. Eggs laid by S. mansoni and S. japonicum remain in the tissues within the hepatic portal system, resulting in pre-hepatic fibrosis and portal hypertension.
Chronic portal hypertension can result in the opening of portocaval shunts, allowing eggs to migrate from the portal system to the pulmonary parenchyma. Within the lungs, the eggs induce an immune response, which similarly results in severe granulomas. However, a unique pathology of pulmonary arterial vascular remodelling is also manifested, resulting in clinical pulmonary arterial hypertension (PAH) [1,6–8].
Epidemiology
Schistosomiasis affects over 200 million people worldwide, resulting in more than 250 000 deaths and up to 4.5 million disability-adjusted years lost annually [9–15]. The chronic morbidity of schistosomiasis includes anaemia, fatigue, undernutrition and chronic pain. Schistosomiasis is endemic in 74 countries, including throughout Africa, Brazil, the Middle East and Southeast Asia. Schistosomiasis is the third most common parasitic disease worldwide after malaria and amoebiasis.
Cercariae are found in fresh water in endemic regions, resulting in human infection in the context of daily activities, including farming, walking, bathing and swimming. Despite the availability of antihelminthic agents such as praziquantel, endemic areas can have case-prevalence rates in excess of 50%. Treated individuals are re-infected upon re-exposure, making the disease difficult to completely eradicate. Socio-economic development projects such as the construction of dams, natural disasters, including flooding, and poor sanitary conditions all combine to result in high prevalence rates within underdeveloped and developing regions [16].
Of all people infected with Schistosoma worldwide, approximately 10% of those chronically infected with S. mansoni develop hepatosplenic disease, resulting in pre-portal fibrosis and portocaval shunting, including oesophageal varicies [17]. Approximately 10–20% of those with hepatosplenic disease, or 2–5 million people worldwide, develop PAH, a progressive and fatal illness [18]. This high prevalence makes schistosomiasis one of the most common causes of PAH worldwide [19], resulting in considerable mortality and morbidity, although the precise disease burden is unknown.
Clinical manifestations of Schistosoma infection
Patients initially infected with Schistosoma develop a maculo-papular rash at the site of parasite penetration within hours of infection [20]. This reaction lasts for several days and then spontaneously resolves as the cercariae transform into schistosomula and move into the systemic venous system. The rash, also called cercarial dermatitis, occurs more commonly in people infected for the first time than in chronically infected and re-infected individuals.
As the parasite embolizes through the systemic venous circulation to the pulmonary arterioles, it causes a generalized inflammatory response in the host, termed Katayama fever. This self-limiting syndrome is characterized by fevers, chills, shortness of breath and a dry cough [21]. Physical examination may reveal tender hepatomegaly and splenomegaly [20]. Chest radiographs may show a patchy alveolar infiltrate. Laboratory studies reveal peripheral eosinophilia. Katayama fever begins 2–12 weeks after primary infection, and can last for several weeks, before spontaneous resolution as the parasite passes through the lungs and migrates through the systemic arterial circulation to its target organ.
The symptoms of chronic Schistosoma infection depend on the specific parasite and the severity of disease. Patients with intestinal S. mansoni or S. japonicum infection can have abdominal pain, diarrhoea and iron-deficiency anaemia, with the resulting constitutional symptoms including weight loss, undernutrition and chronic fatigue. Patients with hepatosplenic disease have the symptoms of intestinal Schistosoma infection plus the symptoms resulting from pre-portal fibrosis and portal hypertension, including splenomegaly and the formation of oesophageal and haemorrhoidal varicies with resultant bleeding (potentially massive and fatal). However, because the disease remains pre-portal, cirrhosis does not generally result. Patients with chronic S. haematobium infection have symptoms resulting from chronic inflammation of the venous plexus around the bladder, including haematuria, fibrosis with ureteral obstruction and nephrolithiasis, and squamous cell carcinoma of the transitional epithelium of the bladder [22].
In patients with chronic Schistosoma infection, eggs can embolize throughout the body, with focal granulomatous reactions and symptoms that depend on the precise location of deposition. One of the most sensitive locations is the central nervous system, with the consequence of focal or generalized tonic–clonic seizures or focal neurological deficits [20,23].
Schistosomiasis-associated PAH
PAH is one of the pulmonary manifestations in schistosomiasis, particularly in its hepatosplenic presentation. Portal hypertension almost invariably precedes PAH in schistosomiasis, establishing venous shunts between the portal and systemic circulation, allowing the passage of schistosome eggs from the liver to the lungs [24]. In one recent series of 65 patients, the incidence of PAH (pulmonary artery systolic pressure >40 mmHg) in patients with hepatosplenic disease referred to a gastroenterology clinic was 18.5% on echocardiography, and PAH was confirmed in 7.7% by right heart catheterization [25] (Loureiro et al., American Heart Association, 2004, Abstract No. 2659, pp. III-572). Other reports from two pulmonary referral centres in Brazil demonstrated that, among all the patients, 30% had symptoms of PAH associated with schistosomiasis [26]. Analysis of chest radiographs revealed that among 115 schistosomiasis patients, 84 had radiographs compatible with cardiopulmonary abnormalities, including 11 suggestive of PAH [27]. Portopulmonary hypertension and PAH associated with schistosomiasis have also been described in Africa [28].
Patients who develop schistosomiasis-associated PAH have the signs and symptoms of this condition, primarily resulting from progressive right heart failure. Initial symptoms include dyspnoea, fatigue and exercise intolerance. As the disease progresses, patients can experience chest pain from right ventricular angina and syncope caused by from depressed cardiac output and low systolic blood pressure. Physical examination may reveal a prominent pulmonic component second heart sound (P2), right ventricular heave and digital clubbing. Patients with frank right heart failure manifest cyanosis and peripheral oedema or anasarca. Radiographs may reveal cardiomegaly, particularly dilatation of the right ventricle and right atrium, and enlarged pulmonary trunk and arteries, with pruning of the distal vasculature (Fig. 1). Electrocardiography typically shows right ventricular hypertrophy or strain and right atrial enlargement, and may also reveal a right bundle branch block. Echocardiography demonstrates right ventricular dilatation, potentially compressing the left ventricle with septal bowing, usually accompanied by right atrial dilation, tricuspid valve regurgitation and an increased pressure gradient across the tricuspid valve. Finally, right heart catheterization (when available) is used to confirm the diagnosis of PAH in the absence of an elevated pulmonary artery occlusion pressure.
FIG. 1.
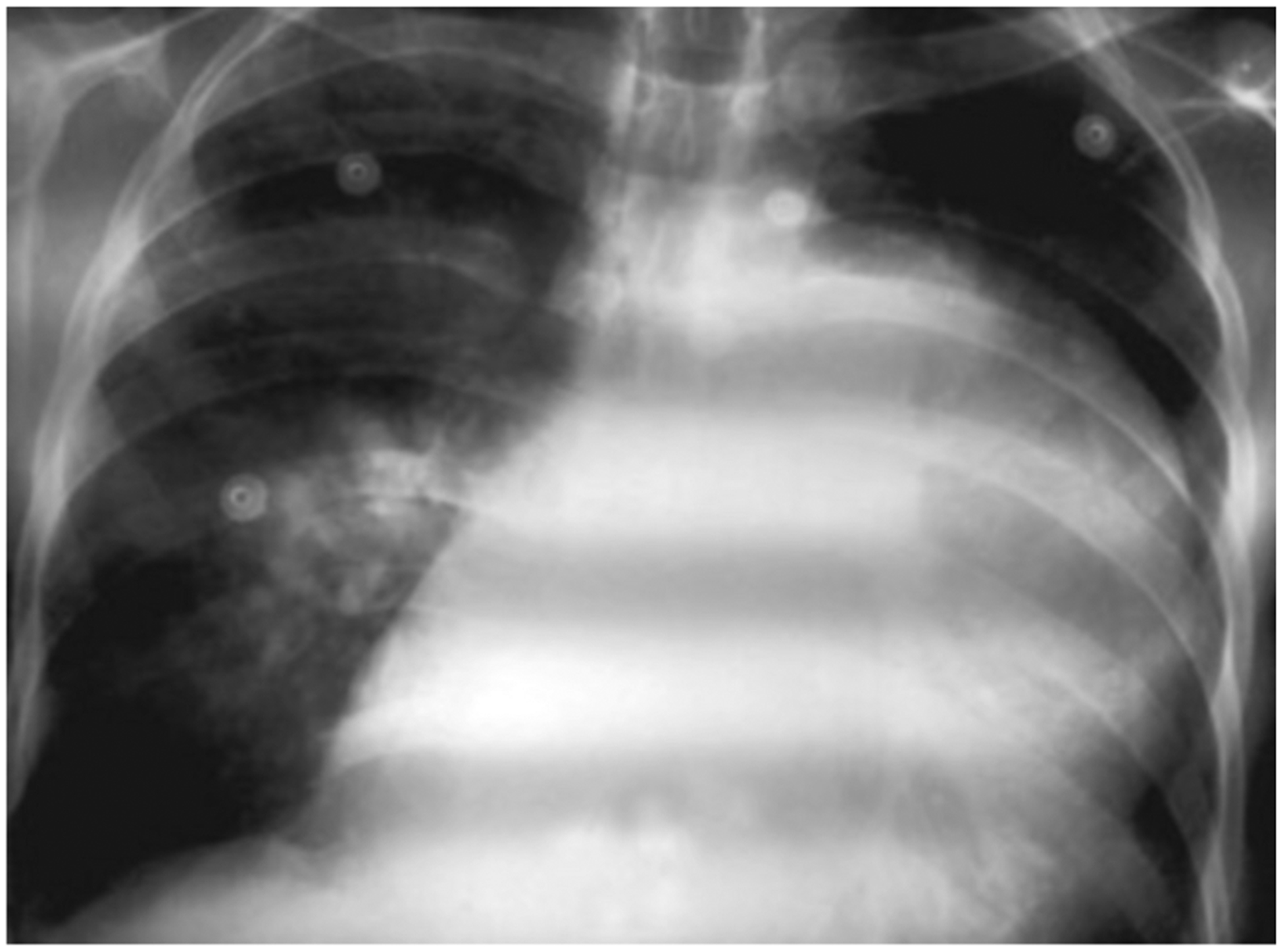
A chest radiograph of a 52-year-old man with schistosomiasis-associated pulmonary arterial hypertension, resulting in right ventricular hypertrophy and a dilated pulmonary trunk. Reproduced with permission from Safwat, PVRI Review 2009; 1: 139 [134].
Specific prognostic data from patients with schistosomiasis-associated PAH arelacking, but the prognosis is probably similar to that with other forms of PAH, including idiopathic PAH (IPAH). Patients with untreated IPAH usually die within 3–5 years after initial presentation, the lifespan being <1 year in those with severe symptoms [29]. It is of note that patients with varicial bleeding as a consequence of schistosomiasis portal hypertension who receive surgical portocaval shunt therapy can have poor short-term outcomes, because of the development of PAH [30].
Histopathology of schistosomiasis-associated PAH
The pulmonary histopathology of schistosomiasis-associated PAH has similarities to and differences from other forms of PAH that are more common in the developed world, most classically IPAH. The pathology of IPAH includes focal alterations in all layers of the arterial vasculature, including the intima, media and adventitia. However, the precise distribution of lesions is not known, as both abnormal and normal vasculature are often seen concurrently even in the same specimen. Examples of vascular lesions seen at autopsy in a patient who died of schistosomiasis-associated PAH are shown in Fig. 2.
FIG. 2.
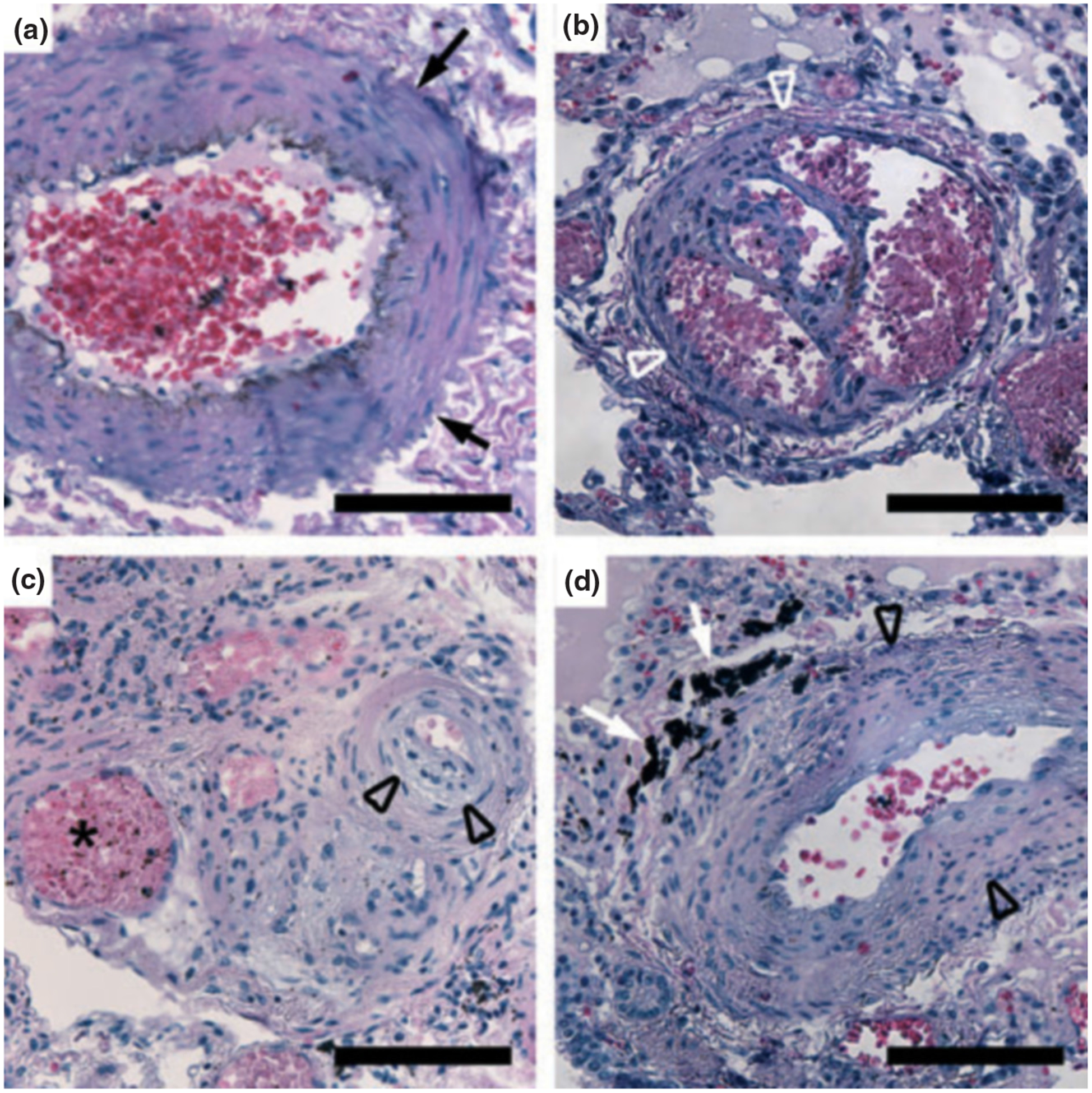
Representative pulmonary pathology from a patient who died of schistosomiasis-associated pulmonary arterial hypertension. (a) Medial hypertrophy (arrows). (b) Plexiform lesion (white arrowheads). (c) Eccentric intimal thickening (black arrowheads) and a dilated or angiomatoid lesion (star). (d) Pigment (white arrows) adjacent to intimal thickening (black arrowheads). All scale bars are 100 μm.
There are four types of intimal lesion: concentric thickening, eccentric thickening, plexiform, and dilated or angiomatoid [31]. Lesions with concentric thickening have an onion skin appearance, with multiple layers. Eccentric lesions may result from old or chronic thrombus that has become largely incorporated into the vessel wall. Plexiform lesions are focal proliferations of endothelial cells resulting in focal tortuous passages. Dilated or angiomatous lesions may be located downstream of plexiform lesions resulting from the focal turbulence in blood flow.
The vascular media can be thickened in association with or in locations distinct from the endothelial lesions. Most severe forms of PAH, including that associated with schistosomiasis, are probably inflammatory in nature [32], with an inflammatory infiltrate often being seen around the adventitia of affected vasculature. Remarkably, there is evidence that this infiltrate, and potentially many of the cells forming the vascular media and intimal lesions, are derived from the circulatory system [33,34].
Most of the molecular studies on PAH have been performed in IPAH and connective tissue disease-associated PAH. Many of the pathological lesions manifest as an imbalance between the proliferation and death of vascular cell elements [35]. Elements of abnormal cell proliferation include clonality, loss of tumour suppression, and germline mutations in familial disease, specifically in the bone morphogenic protein receptor type 2 (BMPR-2). Pathological endothelial cells also display resistance to normal cell death through apoptosis [36]. Remarkably, these characteristics are all highly suggestive of neoplasia [37–39]. It is unclear at this time what the roles of BMPR-2, clonality and dysregulated proliferation are in schistosomiasis-associated PAH.
The pathology of schistosomiasis-associated PAH is unique, in that a dark pigment is commonly seen adjacent to vascular lesions (see example in Fig. 2d). The aetiology of this pigment is not known, but may represent either debris left from the parasite or remnants of the host response. Whole ova surrounded by granulomatous inflammation are rarely seen.
Schistosomiasis, inflammation and PAH
The pathology associated with schistosomiasis is mostly attributed to the intense granulomatous inflammation and subsequent fibrosis induced by parasite eggs that become trapped in host organs.
The primary response is caused by migrating parasites in the circulatory system, and shows characteristics of a type 1 T-cell response (Th1)—production of proinflammatory cytokines such as interleukin (IL)-1 [6,7,40], IL-12 [41–44], inter-feron-γ (INF-γ) [6,45], transforming growth factor-β (TGF-β) [42,46] and tumour necrosis factor-α (TNF-α) [7,41]. They may modulate the release of chemokines such as CXCL2, CXCL5, CXCL9, CXCL10, CXCL11, CXCL22, CCL3, CCL7, CX3CR1, RANTES and lymphotactin (XCL1) [2].
The secondary response is caused by schistosome eggs and egg-derived antigens, which are potent and independent inducers of the type 2 T-cell response (Th2). Transition to the Th2 response occurs approximately 8 weeks post-transfection, and is characterized by secretion of IL-4 [2,43,47], IL-5 [48], IL-10 [49,50] and IL-13 [45,51,52].
Although Th2-mediated pathology is ultimately detrimental to the host, it is also clear that granulomas serve an important host-protective function during infection. In chronically infected hosts, schistosome eggs provide a chronic antigenic stimulus for the immune response [52]. If these antigens are not sequestered or neutralized effectively, they may damage host tissues, the liver being particu larly sensitive [52–54]. CD4+ T-cells are essential for granuloma formation, and early studies examining the respective roles of Th1-associated and Th2-associated cytokines showed that the granulomatous response evolves from an early Th1-type to a sustained and dominant Th2-type cytokine response [55–57]. Granuloma formation therefore occurs in an environment that is initially proinflammatory and type 1-like, but switches rapidly to one that is predominantly type 2-like.
Interestingly, recent reports have suggested that inflammation plays an important role in pulmonary hypertension. T-cells, B-cells, mast cells, macrophages and dendritic cells, as well as inflammatory cytokines and chemokines, were identified in the lungs of patients with PAH, in remodelled small pulmonary arteries including plexiform lesions. Several cytokines, as well as receptors, are reported to be increased in PAH patients [58–62], and cytokine antagonist therapy has been tested with the experimental monocrotaline model of PAH, including antagonists to IL-1 [63], RANTES [64], CCL2 [65] and CX3CR1 [66].
Little is known about the direct effects of Schistosoma infection on the pulmonary vasculature, and more studies need to be performed. A recent publication correlated cytokines with pulmonary remodelling, and provided some new insights on the topic [59]. Interestingly, some of the cytokines were seen to be increased in a time-dependent manner, in parallel with vascular changes and plexiform lesion formation, whereas right ventricular hypertrophy was not observed. Moreover, a significant positive correlation between the number of muscularized small peripheral vessels and the lung egg burden was reported [59].
As inflammation is the main feature of Schistosoma infection and plays a role in the pulmonary vasculature as well, correlation of different cytokines with schistomiasis and PAH is characterized below.
IL-1, a profibrotic cytokine secreted by monocytes and macrophages, is released in the early stages of Schistosoma infection [40]. On the other hand, an IL-1 antagonist was shown to lower pulmonary artery pressure in acute respiratory distress syndrome associated with PAH in a piglet model [67], whereas an IL-1 receptor antagonist reduced pulmonary artery pressure and right heart hypertrophy in monocrotaline-treated rats [63].
IL-4 is one of the major Th2 cytokines that stimulates proliferation of activated B-cells and controls the release of other cytokines. Its important role was demonstrated in many studies in which IL-4 levels increased after infection [68–70]. Moreover, administration of IL-4 with schistosome eggs led to increased granuloma size, whereas anti-IL-4 treatment or IL-4 knockdown suppressed granuloma formation and hepatic fibrosis [43,47,51]. Concerning the lungs, the role of IL-4 role is mostly clearly shown for asthma [71,72]. The role of IL-4 in PAH is less clear, but IL-4 can potentiate pathogenic autoreactive B-cells, which are present in the plexiform lesions in some models and may produce anti-endothelial antibodies [73,74]. In a recent study, it was demonstrated that IL-4 levels increase in a time-dependent manner after Schistosoma infection in a murine model of PAH [59]. It was also reported that the severity of pulmonary arterial remodelling was correlated with the levels of IL-4 in bronchoalveolar lavage fluid [75].
IL-5 was reported to participate in the maturation of eosinophils and in tissue eosinophilia associated with Schistosoma infection [48]. It has been suggested that different variants of the IL-5 gene region may modulate the immune response to Schistosoma infection [69]. With respect to PAH, the effect of IL-5 may be similar to that of IL-4, and the severity of pulmonary arterial remodelling correlates with the levels of IL-5 in bronchoalveolar lavage fluid in a murine model [75].
IL-6 acts as both a proinflammatory and an anti-inflamma-tory cytokine, and is secreted by T-cells and macrophages to stimulate the immune response. It was reported that Schistosoma infection induces IL-6 secretion in vitro in human and mouse lung microvascular endothelial cells, as well as in vivo in the pulmonary microvasculature in mice [76]. IL-6 correlates with the development and progression of pulmonary vascular remodelling [59], and has been shown to be proproliferative [77,78].
A major function of IL-10 is to limit and ultimately terminate inflammatory responses, effectively controlling immune responses and tolerance in vivo. The levels of IL-10 have been demonstrated to be elevated in schistosomiasis patientsand to be associated with the inhibition of fibrosis, owing to IL-10’s antifibrotic and anti-inflammatory effects [50,53,68,70,79]. In mice infected with Schistosoma, IL-10 levels increase in a time-dependent manner, in parallel with vascular remodelling [59]. Additionally, when overexpressed, IL-10 signals via haem oxygenase-1. IL-10 has been reported to reduce mean pulmonary artery pressure and the remodelling effect of monocrotaline-induced PAH, as well to inhibit the proliferation of cultured human pulmonary artery smooth muscle cells (PASMCs) [80].
IL-13 is known to induce physiological changes in parasitized organs that eliminate the offending organisms or their products. IL-13 has been reported to be induced after Schistosoma infection [45,51,52,59,69], and several studies performed in mice using IL-13 suppression [81] and inhibition [82], demonstrated decreased inflammation after Schistosoma infection. In the lung, depletion of IL-13 significantly ameliorated pulmonary arterial muscularization in mice exposed to inhaled Aspergillus [75] and was reported to prevent emphysema and pulmonary inflammation. Moreover, IL-13 stimulates PASMC migration, which is probably significant in vascular remodelling [59].
The IFN-γ level is elevated after Schistosoma infection [68,68,70]. IFN-γ is associated with protection against fibrosis [79]. IFN-γ was reported to induce human PASMCs to release endothelin-1, which is a potent vasoconstrictor and comitogen for vascular smooth muscle [83]. IFN-γ produced at high levels in inflammation acts on endothelial cells to modulate the evolution of the inflammatory response and endothelial damage [84].
TGF-β is a multifunctional cytokine that controls proliferation, cellular differentiation [85,86] and other functions in many cells. It is known to play a role in immunity [50,87], cancer [86,87], heart disease and fibrosis [86]. In schistosomiasis, TGF-β suppresses hepatic inflammation, whereas TGF-β blockade significantly increases the production ofIL-4, IL-6, IL_17, TNF-α and IFN-γ [46,50], as well as neutrophilia [50]. It has been reported that TGF-β levels are elevated in PAH patients [88]. Moreover, genetic mutations in members of the TGF-β receptor superfamily, mostly BMPR-2, are observed in patients with familial PAH and are associated with changes in the structure of the lung vasculature [89–93]. Additionally, TNF-α, IL-1β and IL-10 levels correlate with pulmonary vascular changes in Schistoma-infected mice [59].
Pulmonary Vascular Diseases Associated with Other Parasitic Diseases
Wuchereria bancrofti (Filaria) is a parasitic filarial nematode worm spread by mosquitoes. It largely affects areas across the broad equatorial belt (Africa, Turkey, India, Southeast Asia, Philippines, Oceanic Islands, Australia and parts of South America). If the W. bacrofti infection is left untreated, it can develop into a chronic disease called elephantiasis [94,95].
Filarial worms are known to cause PAH in animals, and it has been shown that this disease may also cause human PAH [96–99]. Many studies in dogs suggest that Dirofilaria immitus (heartworm) is the cause of pulmonary diseases, and its effect on pulmonary arterial pressure has been noted [100–103], as well as its effect on lesion formation [102]. It has also been demonstrated that chronic mild interstitial lung disease persists in tropical pulmonary eosinophilia [104]. W. bancrofti can also cause acute or refractory bronchial asthma [105]. Current therapy for filariasis includes treatment with albendazole, ivermectin, doxycline and diethylcarbamazine [106–110].
Clonorchis sinensis lives in the liver of humans, and is found mainly in the common bile duct and gall bladder, feeding on bile. C. sinensis is endemic in Japan, China, Taiwan and Southeast Asia. A few cases of PAH have been associated with this parasite [95].
Conclusions
Schistosomiasis is a common cause of PAH and a prototypical model for other inflammatory causes of PAH. The antihelminthic agent used for the treatment of schistosomiasis is praziquantel, but it appears to have little effect on patients with schistosomiasis-associated PAH after prolonged chronic infection [111]. The effects of more conventional PAH therapies on schistosomiasis have been very poorly investigated.
Phosphodiesterase (PDE) inhibitors are currently used to treat PAH [112–114], and the PDE5 inhibitors sildenafil and tadalafil are in clinical use [115–119]. PDE and its inhibitors have been reported to affect schistosomes [120–122], which could also be a promising targets. Small studies have suggested that PDE5 inhibitors can be effective in treating schistosomiasis-associated PAH (Loureiro et al., American Heart Association, 2004, Abstract No. 2659, pp. III-572) [123].
Recent reports have suggested that tyrosine kinase (TK) inhibitors could have anti-remodelling effects and also reverse the PAH changes [124–129]. Interestingly, effects of TKs on adult schistosomes have been reported, and some studies have suggested that TKs directly affect adult worms [130–133].
The pathogenic mechanism by which the combination of the parasite’s effect on the host and the host’s immune response to the parasite results in PAH remains largely unknown at this time. Further basic science and clinical research is necessary to determine elements of this process that could be amenable to future treatments for Schistosoma infection in general and schistoschomiasis-associated PAH specifically. In particular, agents directed at blocking or reversing inflammation caused by the parasite antigens and proliferation of vascular elements may be required for complete treatment of the disease.
Transparency Declaration
B. B. Graham is funded by a Parker B. Francis Fellowship, and B. B. Graham and R. M. Tuder are funded by the Cardiovascular Medical Research and Education Fund. All authors declare no conflict of interest.
References
- 1.Rollinson D A wake up call for urinary schistosomiasis: reconciling research effort with public health importance. Parasitology 2009; 136: 1593–1610. [DOI] [PubMed] [Google Scholar]
- 2.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol 2007; 85: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allam G Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology 2009; 214: 712–727. [DOI] [PubMed] [Google Scholar]
- 4.Casella ML, Fanni VS, Verndl DO, Basso MC, Mello LF, Glina S. Schistosomiasis mansoni of the bladder simulating bladder cancer: a case report. Rev Soc Bras Med Trop 2009; 42: 581–582. [DOI] [PubMed] [Google Scholar]
- 5.Araujo N, Mattos AC, Coelho PM, Katz N. Association of oxamniquine praziquantel and clonazepam in experimental Schistosomiasis mansoni. Mem Inst Oswaldo Cruz 2008; 103: 781–785. [DOI] [PubMed] [Google Scholar]
- 6.Caldas IR, Campi-Azevedo AC, Oliveira LF, Silveira AM, Oliveira RC, Gazzinelli G. Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection. Acta Trop 2008; 108: 109–117. [DOI] [PubMed] [Google Scholar]
- 7.He YX, Chen L, Ramaswamy K. Schistosoma mansoni, S. haematobium, and S. japonicum: early events associated with penetration and migration of schistosomula through human skin. Exp Parasitol 2002; 102: 99–108. [DOI] [PubMed] [Google Scholar]
- 8.Ross AG, Bartley PB, Sleigh AC et al. Schistosomiasis. N Engl J Med 2002; 346: 1212–1220. [DOI] [PubMed] [Google Scholar]
- 9.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop 2000; 77: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitsulo L, Loverde P, Engels D. Schistosomiasis. Nat Rev Microbiol 2004; 2: 12–13. [DOI] [PubMed] [Google Scholar]
- 11.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 2005; 365: 1561–1569. [DOI] [PubMed] [Google Scholar]
- 12.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop 2000; 77: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chitsulo L, Loverde P, Engels D. Schistosomiasis. Nat Rev Microbiol 2004; 2: 12–13. [DOI] [PubMed] [Google Scholar]
- 14.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 2005; 365: 1561–1569. [DOI] [PubMed] [Google Scholar]
- 15.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 2006; 6: 411–425. [DOI] [PubMed] [Google Scholar]
- 16.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 2006; 6: 411–425. [DOI] [PubMed] [Google Scholar]
- 17.Warren KS. Hepatosplenic schistosomiasis: a great neglected disease of the liver. Gut 1978; 19: 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Cleva R, Herman P, Pugliese V et al. Prevalence of pulmonary hypertension in patients with hepatosplenic mansonic schistosomiasis—prospective study. Hepatogastroenterology 2003; 50: 28–30. [PubMed] [Google Scholar]
- 19.Butrous G, Ghofrani HA, Grimminger F. Pulmonary vascular disease in the developing world. Circulation 2008; 118: 1758–1766. [DOI] [PubMed] [Google Scholar]
- 20.Ross AG, Bartley PB, Sleigh AC et al. Schistosomiasis. N Engl J Med 2002; 346: 1212–1220. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz E Pulmonary schistosomiasis. Clin Chest Med 2002; 23: 433–443. [DOI] [PubMed] [Google Scholar]
- 22.Thomas JE, Bassett MT, Sigola LB, Taylor P. Relationship between bladder cancer incidence, Schistosoma haematobium infection, and geographical region in Zimbabwe. Trans R Soc Trop Med Hyg 1990; 84: 551–553. [DOI] [PubMed] [Google Scholar]
- 23.Fowler R, Lee C, Keystone JS. The role of corticosteroids in the treatment of cerebral schistosomiasis caused by Schistosoma mansoni: case report and discussion. Am J Trop Med Hyg 1999; 61: 47–50. [DOI] [PubMed] [Google Scholar]
- 24.Chaves E The pathology of the arterial pulmonary vasculature in Manson’s schistosomiasis. Dis Chest 1966; 50: 72–77. [DOI] [PubMed] [Google Scholar]
- 25.Lapa M, Dias B, Jardim C et al. Cardiopulmonary manifestations of hepatosplenic schistosomiasis. Circulation 2009; 119: 1518–1523. [DOI] [PubMed] [Google Scholar]
- 26.Lapa MS, Ferreira EV, Jardim C, Martins BC, Arakaki JS, Souza R. Clinical characteristics of pulmonary hypertension patients in two reference centers in the city of Sao Paulo. Rev Assoc Med Bras 2006; 52: 139–143. [DOI] [PubMed] [Google Scholar]
- 27.Rocha RL, Pedroso ER, Rocha MO, Lambertucci JR, Greco DB, Ferreira CE. Chronic pulmonary form of Schistosomiasis mansoni. Clinico-radiologic evaluation. Rev Soc Bras Med Trop 1990; 23: 83–89. [DOI] [PubMed] [Google Scholar]
- 28.Ramanampamonjy RM, Razafimahefa SH, Rajaonarivelo P, Rajaona HR. Portopulmonary hypertension due to schistosomiasis in two Malagasy patients. Bull Soc Pathol Exot 2007; 100: 28–29. [PubMed] [Google Scholar]
- 29.D’Alonzo GE, Barst RJ, Ayres SM et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 30.de Cleva R, Herman P, Pugliese V, Zilberstein B, Saad WA, Gama-Rodrigues JJ. Fatal pulmonary hypertension after distal splenorenal shunt in schistosomal portal hypertension. World J Gastroenterol 2004; 10: 1836–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuder RM. Pathology of pulmonary arterial hypertension. Semin Respir Crit Care Med 2009; 30: 376–385. [DOI] [PubMed] [Google Scholar]
- 32.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J 2003; 22: 358–363. [DOI] [PubMed] [Google Scholar]
- 33.Frid MG, Brunetti JA, Burke DL et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 2006; 168: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majka SM, Skokan M, Wheeler L et al. Evidence for cell fusion is absent in vascular lesions associated with pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2008; 295: L1028–L1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuder RM. Pathology of pulmonary arterial hypertension. Semin Respir Crit Care Med 2009; 30: 376–385. [DOI] [PubMed] [Google Scholar]
- 36.Masri FA, Xu W, Comhair SA et al. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2007; 293: L548–L554. [DOI] [PubMed] [Google Scholar]
- 37.Nichols WC, Koller DL, Slovis B et al. Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31–32. Nat Genet 1997; 15: 277–280. [DOI] [PubMed] [Google Scholar]
- 38.Deng ZM, Morse JH, Slager SL et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 2000; 67: 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuder RM, Radisavljevic Z, Shroyer KR, Polak JM, Voelkel NF. Monoclonal endothelial cells in appetite suppressant-associated pulmonary hypertension. Am J Respir Crit Care Med 1998; 158: 1999–2001. [DOI] [PubMed] [Google Scholar]
- 40.El RR, Wagih A, Salem R, Mahana N, El DM, Tallima H. Impact of interleukin-1 and interleukin-6 in murine primary schistosomiasis. Int Immunopharmacol 2006; 6: 1100–1108. [DOI] [PubMed] [Google Scholar]
- 41.Duraes FV, Carvalho NB, Melo TT, Oliveira SC, Fonseca CT. IL-12 and TNF-alpha production by dendritic cells stimulated with Schistosoma mansoni schistosomula tegument is TLR 4 and My D88 dependent. Immunol Lett 2009; 125: 72–77. [DOI] [PubMed] [Google Scholar]
- 42.Othman AA, Shoheib ZS, Saied EM, Soliman RH. Congenital exposure to Schistosoma mansoni infection: impact on the future immune response and the disease outcome. Immunobiology 2010; 215: 101–112. [DOI] [PubMed] [Google Scholar]
- 43.Pearce EJ, Cheever A, Leonard S et al. Schistosoma mansoni in IL-4-deficient mice. Int Immunol 1996; 8: 435–444. [DOI] [PubMed] [Google Scholar]
- 44.Wynn TA, Cheever AW, Jankovic D et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature 1995; 376: 594–596. [DOI] [PubMed] [Google Scholar]
- 45.Dessein A, Kouriba B, Eboumbou C et al. Interleukin-13 in the skin and interferon-gamma in the liver are key players in immune protection in human schistosomiasis. Immunol Rev 2004; 201: 180–190. [DOI] [PubMed] [Google Scholar]
- 46.Tallima H, Salah M, Guirguis FR, El RR. Transforming growth factor-beta and Th17 responses in resistance to primary murine Schistosomiasis mansoni. Cytokine 2009; 48: 239–245. [DOI] [PubMed] [Google Scholar]
- 47.King CL, Malhotra I, Jia X. Schistosoma mansoni: protective immunity in IL-4-deficient mice. Exp Parasitol 1996; 84: 245–252. [DOI] [PubMed] [Google Scholar]
- 48.Magalhaes ES, Paiva CN, Souza HS et al. Macrophage migration inhibitory factor is critical to interleukin-5-driven eosinophilopoiesis and tissue eosinophilia triggered by Schistosoma mansoni infection. FASEB J 2009; 23: 1262–1271. [DOI] [PubMed] [Google Scholar]
- 49.Abbas OM, Abdel-Rahman MH, Omar NA, Badran HM, Amir EM. Interleukin-10 promoter polymorphisms in hepatitis C patients with and without Schistosoma mansoni co-infection. Liver Int 2009; 29: 1422–1430. [DOI] [PubMed] [Google Scholar]
- 50.Herbert DR, Orekov T, Perkins C, Finkelman FD. IL-10 and TGF-beta redundantly protect against severe liver injury and mortality during acute schistosomiasis. J Immunol 2008; 181: 7214–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol 2000; 164: 2585–2591. [DOI] [PubMed] [Google Scholar]
- 52.Wynn TA. IL-13 effector functions. Annu Rev Immunol 2003; 21: 425–456. [DOI] [PubMed] [Google Scholar]
- 53.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol 2000; 164: 6406–6416. [DOI] [PubMed] [Google Scholar]
- 54.Cheever AW, Poindexter RW, Wynn TA. Egg laying is delayed but worm fecundity is normal in SCID mice infected with Schistosoma japonicum and S. mansoni with or without recombinant tumor necrosis factor alpha treatment. Infect Immun 1999; 67: 2201–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vella AT, Pearce EJ. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0-like stage. J Immunol 1992; 148: 2283–2290. [PubMed] [Google Scholar]
- 56.Wynn TA, Eltoum I, Cheever AW, Lewis FA, Gause WC, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol 1993; 151: 1430–1440. [PubMed] [Google Scholar]
- 57.Everts B, Perona-Wright G, Smits HH et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med 2009; 206: 1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balabanian K, Foussat A, Dorfmuller P et al. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med 2002; 165: 1419–1425. [DOI] [PubMed] [Google Scholar]
- 59.Crosby A, Jones FM, Southwood M et al. Pulmonary vascular remodeling correlates with lung eggs and cytokines in murine schistosomiasis. Am J Respir Crit Care Med 2010; 181: 279–288. [DOI] [PubMed] [Google Scholar]
- 60.Dorfmuller P, Zarka V, Durand-Gasselin I et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2002; 165: 534–539. [DOI] [PubMed] [Google Scholar]
- 61.Humbert M, Monti G, Brenot F et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995; 151: 1628–1631. [DOI] [PubMed] [Google Scholar]
- 62.Perros F, Dorfmuller P, Souza R et al. Fractalkine-induced smooth muscle cell proliferation in pulmonary hypertension. Eur Respir J 2007; 29: 937–943. [DOI] [PubMed] [Google Scholar]
- 63.Voelkel NF, Tuder RM, Bridges J, Arend WP. Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am J Respir Cell Mol Biol 1994; 11: 664–675. [DOI] [PubMed] [Google Scholar]
- 64.Song E, Zou H, Yao Y et al. Early application of Met-RANTES ameliorates chronic allograft nephropathy. Kidney Int 2002; 61: 676–685. [DOI] [PubMed] [Google Scholar]
- 65.Sanchez O, Marcos E, Perros F et al. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2007; 176: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 66.Feng L, Chen S, Garcia GE et al. Prevention of crescentic glomeru-lonephritis by immunoneutralization of the fractalkine receptor CX3CR1 rapid communication. Kidney Int 1999; 56: 612–620. [DOI] [PubMed] [Google Scholar]
- 67.Chada M, Nogel S, Schmidt AM et al. Anakinra (IL-1R antagonist) lowers pulmonary artery pressure in a neonatal surfactant depleted piglet model. Pediatr Pulmonol 2008; 43: 851–857. [DOI] [PubMed] [Google Scholar]
- 68.Mutapi F, Winborn G, Midzi N, Taylor M, Mduluza T, Maizels RM. Cytokine responses to Schistosoma haematobium in a Zimbabwean population: contrasting profiles for IFN-gamma, IL-4, IL-5 and IL-10 with age. BMC Infect Dis 2007; 7: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ellis MK, Zhao ZZ, Chen HG, Montgomery GW, Li YS, McManus DP. Analysis of the 5q31 33 locus shows an association between single nucleotide polymorphism variants in the IL-5 gene and symptomatic infection with the human blood fluke, Schistosoma japonicum. J Immunol 2007; 179: 8366–8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Kady IM, Lotfy M, Badra G, El-Masry S, Waked I. Interleukin (IL)-4, IL-10, IL-18 and IFN-gamma cytokines pattern in patients with combined hepatitis C virus and Schistosoma mansoni infections. Scand J Immunol 2005; 61: 87–91. [DOI] [PubMed] [Google Scholar]
- 71.Corry DB, Kheradmand F. Toward a comprehensive understanding of allergic lung disease. Trans Am Clin Climatol Assoc 2009; 120: 33–48. [PMC free article] [PubMed] [Google Scholar]
- 72.Tachdjian R, Mathias C, Al KS et al. Pathogenicity of a disease-associated human IL-4 receptor allele in experimental asthma. J Exp Med 2009; 206: 2191–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J 2005; 26: 1110–1118. [DOI] [PubMed] [Google Scholar]
- 74.Ulrich S, Taraseviciene-Stewart L, Huber LC, Speich R, Voelkel N. Peripheral blood B lymphocytes derived from patients with idiopathic pulmonary arterial hypertension express a different RNA pattern compared with healthy controls: a cross sectional study. Respir Res 2008; 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daley E, Emson C, Guignabert C et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 2008; 205: 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Angeli V, Faveeuw C, Delerive P et al. Schistosoma mansoni induces the synthesis of IL-6 in pulmonary microvascular endothelial cells: role of IL-6 in the control of lung eosinophilia during infection. Eur J Immunol 2001; 31: 2751–2761. [DOI] [PubMed] [Google Scholar]
- 77.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 2009; 104: 236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyata M, Sakuma F, Yoshimura A, Ishikawa H, Nishimaki T, Kasukawa R. Pulmonary hypertension in rats. 2. Role of interleukin-6. Int Arch Allergy Immunol 1995; 108: 287–291. [DOI] [PubMed] [Google Scholar]
- 79.Arnaud V, Li J, Wang Y et al. Regulatory role of interleukin-10 and interferon-gamma in severe hepatic central and peripheral fibrosis in humans infected with Schistosoma japonicum. J Infect Dis 2008; 198: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ito T, Okada T, Miyashita H et al. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res 2007; 101: 734–741. [DOI] [PubMed] [Google Scholar]
- 81.Mentink-Kane MM, Cheever AW, Thompson RW et al. IL-13 receptor alpha 2 down-modulates granulomatous inflammation and prolongs host survival in schistosomiasis. Proc Natl Acad Sci USA 2004; 101: 586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest 1999; 104: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wort SJ, Woods M, Warner TD, Evans TW, Mitchell JA. Cyclooxy-genase-2 acts as an endogenous brake on endothelin-1 release by human pulmonary artery smooth muscle cells: implications for pulmonary hypertension. Mol Pharmacol 2002; 62: 1147–1153. [DOI] [PubMed] [Google Scholar]
- 84.Lombardi A, Cantini G, Piscitelli E et al. A new mechanism involving ERK contributes to rosiglitazone inhibition of tumor necrosis factor-alpha and interferon-gamma inflammatory effects in human endothelial cells. Arterioscler Thromb Vasc Biol 2008; 28: 718–724. [DOI] [PubMed] [Google Scholar]
- 85.Eickelberg O, Seeger W. Pulmonary hypertension: pathophysiology, genetics and functional genomics. Internist (Berl) 2005; 46: 759–768. [DOI] [PubMed] [Google Scholar]
- 86.Kolosionek E, Savai R, Ghofrani HA et al. Expression and activity of phosphodiesterase isoforms during epithelial mesenchymal transition: the role of phosphodiesterase 4. Mol Biol Cell 2009; 20: 4751–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diener KR, Need EF, Buchanan G, Hayball JD. TGF-beta signalling and immunity in prostate tumourigenesis. Expert Opin Ther Targets 2010; 14: 179–192. [DOI] [PubMed] [Google Scholar]
- 88.Selimovic N, Bergh CH, Andersson B, Sakiniene E, Carlsten H, Rundqvist B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J 2009; 34: 662–668. [DOI] [PubMed] [Google Scholar]
- 89.Upton PD, Morrell NW. TGF-beta and BMPR-II pharmacology—implications for pulmonary vascular diseases. Curr Opin Pharmacol 2009; 9: 274–280. [DOI] [PubMed] [Google Scholar]
- 90.Eickelberg O, Morty RE. Transforming growth factor beta/bone morphogenic protein signaling in pulmonary arterial hypertension: remodeling revisited. Trends Cardiovasc Med 2007; 17: 263–269. [DOI] [PubMed] [Google Scholar]
- 91.Machado RD, Eickelberg O, Elliott CG et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 1 (suppl): S32–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang H, Li W, Zhang W et al. Novel promoter and exon mutations of the BMPR2 gene in Chinese patients with pulmonary arterial hypertension. Eur J Hum Genet 2009; 17: 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davies RJ, Morrell NW. Molecular mechanisms of pulmonary arterial hypertension: role of mutations in the bone morphogenetic protein type II receptor. Chest 2008; 134: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 94.Laurence BR. The global dispersal of Bancroftian filariasis. Parasitol Today 1989; 5: 260–264. [DOI] [PubMed] [Google Scholar]
- 95.Butrous G, Ghofrani HA, Grimminger F. Pulmonary vascular disease in the developing world. Circulation 2008; 118: 1758–1766. [DOI] [PubMed] [Google Scholar]
- 96.Obeyesekere I, de Soysa N. ‘Primary’ pulmonary hypertension, eosinophilia, and filariasis in Ceylon. Br Heart J 1970; 32: 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Franco-Paredes C, Rouphael N, Mendez J et al. Cardiac manifestations of parasitic infections part 3: pericardial and miscellaneous cardiopulmonary manifestations. Clin Cardiol 2007; 30: 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Madiyono B, Kusmana D, Sonityo OW, Rachman OJ. Pulmonary hypertension, hypereosinophilia and filariasis. Paediatr Indones 1978; 18: 51–57. [DOI] [PubMed] [Google Scholar]
- 99.Obeyesekere I, Peiris D. Pulmonary hypertension and filariasis. Br Heart J 1974; 36: 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Atkins CE, Keene BW, McGuirk SM, Sato T. Acute effect of hydralazine administration on pulmonary artery hemodynamics in dogs with chronic heartworm disease. Am J Vet Res 1994; 55: 262–269. [PubMed] [Google Scholar]
- 101.Kondo M, Washizu M, Matsukura Y, Washizu T, Miyasaka K, Takata M. Pressure–flow relationship and longitudinal distribution of pulmonary vascular resistance in heartworm-infected dogs. J Vet Med Sci 2003; 65: 965–970. [DOI] [PubMed] [Google Scholar]
- 102.Sasaki Y, Kitagawa H, Hirano Y. Relationship between pulmonary arterial pressure and lesions in the pulmonary arteries and parenchyma, and cardiac valves in canine dirofilariasis. J Vet Med Sci 1992; 54: 739–744. [DOI] [PubMed] [Google Scholar]
- 103.Matsukura Y, Washizu M, Kondo M et al. Decreased pulmonary arterial endothelium-dependent relaxation in heartworm-infected dogs with pulmonary hypertension. Am J Vet Res 1997; 58: 171–174. [PubMed] [Google Scholar]
- 104.Vijayan VK. Tropical pulmonary eosinophilia: pathogenesis, diagnosis and management. Curr Opin Pulm Med 2007; 13: 428–433. [DOI] [PubMed] [Google Scholar]
- 105.Chitkara RK, Krishna G. Parasitic pulmonary eosinophilia. Semin Respir Crit Care Med 2006; 27: 171–184. [DOI] [PubMed] [Google Scholar]
- 106.Hooper PJ, Bradley MH, Biswas G, Ottesen EA. The Global Programme to Eliminate Lymphatic Filariasis: health impact during its first 8 years (2000–2007). Ann Trop Med Parasitol 2009; 103 (suppl 1): S17–S21. [DOI] [PubMed] [Google Scholar]
- 107.Hopkins AD, Molyneux DH. A decade of ivermectin–albendazole donation for lymphatic filariasis. Ann Trop Med Parasitol 2009; 103 (suppl 1): S3. [DOI] [PubMed] [Google Scholar]
- 108.Aswathy S, Beteena K, Leelamoni K. Mass drug administration against filariasis in India: perceptions and practices in a rural community in Kerala. Ann Trop Med Parasitol 2009; 103: 617–624. [DOI] [PubMed] [Google Scholar]
- 109.Coulibaly YI, Dembele B, Diallo AA et al. A randomized trial of doxycycline for Mansonella perstans infection. N Engl J Med 2009; 361: 1448–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Horton J The development of albendazole for lymphatic filariasis. Ann Trop Med Parasitol 2009; 103 (suppl 1): S33–S40. [DOI] [PubMed] [Google Scholar]
- 111.Morris W, Knauer CM. Cardiopulmonary manifestations of schistosomiasis. Semin Respir Infect 1997; 12: 159–170. [PubMed] [Google Scholar]
- 112.Schermuly RT, Pullamsetti SS, Kwapiszewska G et al. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation 2007; 115: 2331–2339. [DOI] [PubMed] [Google Scholar]
- 113.Dony E, Lai YJ, Dumitrascu R et al. Partial reversal of experimental pulmonary hypertension by phosphodiesterase-3/4 inhibition. Eur Respir J 2008; 31: 599–610. [DOI] [PubMed] [Google Scholar]
- 114.Archer SL, Michelakis ED. Phosphodiesterase Type 5 inhibitors for pulmonary hypertension. N Engl J Med 2009; 361: 1864–1871 [DOI] [PubMed] [Google Scholar]
- 115.Rosenzweig EB. Tadalafil for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother 2010; 11: 127–132. [DOI] [PubMed] [Google Scholar]
- 116.Singh TP. Clinical use of sildenafil in pulmonary artery hypertension. Expert Rev Respir Med 2010; 4: 13–19. [DOI] [PubMed] [Google Scholar]
- 117.Perez-Villa F, Farrero M, Sionis A, Castel A, Roig E. Therapy with sildenafil or bosentan decreases pulmonary vascular resistance in patients ineligible for heart transplantation because of severe pulmonary hypertension. J Heart Lung Transplant 2010; 29: 817–818. [DOI] [PubMed] [Google Scholar]
- 118.Apitz C, Reyes JT, Holtby H, Humpl T, Redington AN. Pharmacokinetic and hemodynamic responses to oral sildenafil during invasive testing in children with pulmonary hypertension. J Am Coll Cardiol 2010; 55: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 119.Guazzi M, Vicenzi M, Samaja M. Clinical use of phosphodiesterase-5 inhibitors in cardiopulmonary diseases: from experimental evidence to clinical application. G Ital Cardiol (Rome) 2009; 10: 725–737. [PubMed] [Google Scholar]
- 120.Matsuyama H, Takahashi H, Watanabe K, Fujimaki Y, Aoki Y. The involvement of cyclic adenosine monophosphate in the control of schistosome miracidium cilia. J Parasitol 2004; 90: 8–14. [DOI] [PubMed] [Google Scholar]
- 121.Reis LF, Ventura TG, Souza SO et al. Quantitative and qualitative interferences of pentoxifillyne on hepatic Schistosoma mansoni granulomas: effects on extracellular matrix and eosinophil population. Mem Inst Oswaldo Cruz 2001; 96 (suppl): 107–112. [DOI] [PubMed] [Google Scholar]
- 122.Brown JN, Smith TM. Phosphodiesterase activity in adult Schistosoma japonicum. Trans R Soc Trop Med Hyg 1977; 71: 356–357. [DOI] [PubMed] [Google Scholar]
- 123.Bandeira AP, Mendes AA, Santos-Filho P, Sa DT, Loureiro R. Clinical efficacy of oral sildenafil in severe pulmonary hypertension in patients with chronic pulmonary schistosomiasis. Circulation 2004; 110 (17 suppl III): 296. [Google Scholar]
- 124.Moreno-Vinasco L, Garcia JG. Receptor tyrosine kinase inhibitors in rodent pulmonary hypertension. Adv Exp Med Biol 2010; 661: 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McMurtry IF, Abe K, Ota H, Fagan KA, Oka M. Rho kinase-mediated vasoconstriction in pulmonary hypertension. Adv Exp Med Biol 2010; 661: 299–308. [DOI] [PubMed] [Google Scholar]
- 126.Chhina MK, Nargues W, Grant GM, Nathan SD. Evaluation of imatinib mesylate in the treatment of pulmonary arterial hypertension. Future Cardiol 2010; 6: 19–35. [DOI] [PubMed] [Google Scholar]
- 127.Klein M, Schermuly RT, Ellinghaus P et al. Combined tyrosine and serine/threonine kinase inhibition by sorafenib prevents progression of experimental pulmonary hypertension and myocardial remodeling. Circulation 2008; 118: 81–90. [DOI] [PubMed] [Google Scholar]
- 128.Grimminger F, Schermuly RT. PDGF receptor and its antagonists: role in treatment of PAH. Adv Exp Med Biol 2010; 661: 435–446. [DOI] [PubMed] [Google Scholar]
- 129.Tapper EB, Knowles D, Heffron T, Lawrence EC, Csete M. Portopulmonary hypertension: imatinib as a novel treatment and the Emory experience with this condition. Transplant Proc 2009; 41: 1969–1971. [DOI] [PubMed] [Google Scholar]
- 130.Ting-An W, Hong-Xiang Z. PTK-pathways and TGF-beta signaling pathways in schistosomes. J Basic Microbiol 2009; 49: 25–31. [DOI] [PubMed] [Google Scholar]
- 131.Dissous C, Ahier A, Khayath N. Protein tyrosine kinases as new potential targets against human schistosomiasis. Bioessays 2007; 29: 1281–1288. [DOI] [PubMed] [Google Scholar]
- 132.Vicogne J, Dissous C. Schistosoma mansoni receptor tyrosine kinases: towards new therapeutic targets. J Soc Biol 2003; 197: 367–373. [PubMed] [Google Scholar]
- 133.Beckmann S, Grevelding CG. Imatinib has a fatal impact on morphology, pairing stability and survival of adult Schistosoma mansoni in vitro. Int J Parasitol 2010; 40: 521–526. [DOI] [PubMed] [Google Scholar]
- 134.Safwat T Bilharzial pulmonary hypertension with aneurysmal pulmonary artery dilatation. PVRI Rev 2009; 1: 139. [Google Scholar]


