Abstract
This study evaluates the impact of a 6-month care management intervention for 206 children diagnosed with comorbid attention deficit hyperactivity disorder (ADHD) from a sample of 321 five- to 12-year-old children recruited for treatment of behavior problems in 8 pediatric primary care offices. Practices were cluster-randomized to Doctor Office Collaboration Care (DOCC) or Enhanced Usual Care (EUC). Chart reviews documented higher rates of service delivery, prescription of medication for ADHD, and titration in DOCC (vs EUC). Based on complex conditional models, DOCC showed greater acute improvement in individualized ADHD treatment goals and follow-up improvements in quality of life and ADHD and oppositional defiant disorder goals. Medication use had a significant effect on acute and follow-up ADHD symptom reduction and quality of life. Medication continuity was associated with some long-term gains. A collaborative care intervention for behavior problems that incorporated treatment guidelines for ADHD in primary care was more effective than psychoeducation and facilitated referral to community treatment.
Keywords: collaborative care, integrated care, pediatric care integration, clinical trials, treatment of ADHD
Introduction
Pediatric primary care is a major venue for the delivery of mental or behavioral health services1,2 that can enhance service access, acceptability, quality, and benefit.3–5 Several research studies have evaluated the use of clinical guidelines recommended for the management of attention deficit hyperactivity disorder (ADHD) in primary care (American Academy of Pediatrics [AAP]6 and American Academy of Child and Adolescent Psychiatry [AACAP]),7 which have just been updated.8 Informed by empirical studies and provider experiences,9–14 these guidelines emphasize several key tasks (eg, collection of parent and teacher ratings, prescription of an appropriate medication and/or evidence-based behavior therapy, titration, monitoring of response and side effects during follow-up visits). Many of these methods are included in treatment studies of ADHD in the general literature.15
Large-scale surveys of compliance with these guidelines in primary care providers (PCPs) have documented variable rates of certain elements, such as the use of formal diagnostic criteria16 or rating scales and delivery of parent training.17 More recently, Visser et al18 reported high rates of medication treatment, especially stimulants in the past week (74%), but modest rates of behavior therapy for children diagnosed with ADHD (47%) in the past year. A chart review study confirmed higher rates of medication use (93%), but lower rates for psychosocial treatment (13%) and a modest rate of initial follow-up (47%).19 A second chart review study20 also noted a high rate of medication adjustment in the first year (73%), but only 45% of the children had their first physician contact in the first month, medication covered only 60% of the year, and few parent (12%) and teacher (8%) Vanderbilt assessment forms were collected to inform treatment outcome. These studies highlight generally high levels of implementation for most guidelines (eg, medication),21,22 but some remain more modest (eg, use of rating scales).
Comprehensive intervention models have helped PCPs apply these guidelines for ADHD and identify factors that enhance delivery. For example, Epstein and colleagues23 compared the effects of collaborative consultation (eg, packaged medications, dose recommendations by a child psychiatrist, a titration report, rating scales were scored for PCP), relative to a control condition. Pediatrician selfreports documented greater use of evidence-based guidelines but no group difference in ADHD symptoms. A second trial found that a quality improvement intervention using these and other methods enhanced specific components of medication care (eg, days to first contact after medication, number of teacher scales collected) relative to controls, which were also related to lower ADHD symptom scores.24 Two uncontrolled quality improvement studies by the same team25,26 examined an expanded collaborative intervention (eg, training, office flow management, scripts for assessing response, patient report cards). The intervention showed gains in PCPs’ use of rating scales and monitoring of medication response,23 use of titration, and follow-ups during medication management.3 Finally, one randomized trial compared collaborative care and decision-making support alone (basic arm) or with motivational enhancement and parent management by the care manger (enhanced arm).27 Among those with diagnosed ADHD, there were greater improvements at 12 months in parent-reported ADHD symptoms or social skills in the enhanced arm. Other reports have documented PCP decision-making, treatment planning, and family interactions that promote ADHD care.28–30
These improvements highlight the benefits of programs that incorporate consultation, infrastructure support, and technology, and the need to rigorously evaluate the use of treatment guidelines for ADHD to confirm and extend outcomes in pediatric primary care. For example, few primary care studies have examined whether medication has incremental benefits beyond psychosocial intervention. Studies of medicated samples may not have the opportunity to examine the role of concurrent mental health services and the separate effects of medication on ADHD symptoms. It is plausible that other services may contribute similarly or additively to improvements in ADHD symptoms.31 This may be especially relevant in samples where the child’s ADHD is not the only problem being treated (eg, oppositional or defiant behavior). Furthermore, it is important to include medication prescription or utilization data based on the medical record and long-term outcome data in order to understand if medication use or continuity is related to clinical improvement.24 To further document improvement in ADHD- and oppositional defiant disorder (ODD)-related outcomes, we examine a novel outcome measure that captured individualized treatment targets related to a child’s ADHD or ODD and progress toward reaching these goals,32 pediatric quality of life, and overall clinical global functioning. Finally, some studies have reported small samples, significant reductions in sample size across time, or the lack of an experimental design, which may raise questions about the representativeness of the sample on which final analyses are based.25 Attention to these details would enhance both the clinical relevance and empirical rigor of interventions designed to enhance the quality of community care for children with ADHD.
This intervention study seeks to extend prior work examining the effects of collaborative care for children with behavior problems (n = 321) that was delivered by care managers (CMs) in coordination with the child’s PCP in community pediatrics practices. In the initial publication of this cluster-randomized trial, 8 pediatric practices were randomized to Doctor Office Collaborative Care (DOCC) or Enhanced Usual Care (EUC). DOCC (vs EUC) was associated with higher rates of treatment initiation and completion, improvements in behavioral and emotional problems, and parental stress,33 and greater satisfaction. In this report, we evaluate outcomes for the subset of children who were diagnosed with comorbid ADHD (n = 206). Specifically, we evaluate the use and impact of a concurrent medication management protocol based on prior AAP guidelines in DOCC, relative to a referral to their PCP for services and an optional facilitated referral to a local mental health provider in EUC. We hypothesized that the collaborative care ADHD protocol in DOCC would yield higher rates of PCP services, medication use, and dose titration, greater clinical improvement in symptoms of ADHD, and greater improvement in other clinical problems and functional impairment, than EUC.
Methods
Settings and Providers
Study sites included 7 Children’s Community Pediatric practices and 1 general academic pediatric practice affiliated with Children’s Hospital of Pittsburgh. Sites were randomized to 1 of 2 conditions, stratified by prior research participation (no/yes) and level of patient diversity (low vs high) before randomization. The study was approved by the institutional review board at the University of Pittsburgh. All PCPs and parents or legal guardians provided written informed consent and children provided assent. Pediatric care providers included pediatricians (n = 67), certified nurse practitioners (n = 6), and a physician assistant (n = 1). Ages ranged from 29 to 69, and most were female (57%) and white (82%). All but 2 PCPs were specialty-certified. Four master’s- level clinicians (MSW) served as CMs and delivered the treatment protocol. CMs were trained and received ongoing clinical supervision from a senior clinician and the study child/adolescent psychiatrist. Each CM was assigned to 2 practices (one in each condition) and worked 2 days/week per practice.
Family Recruitment
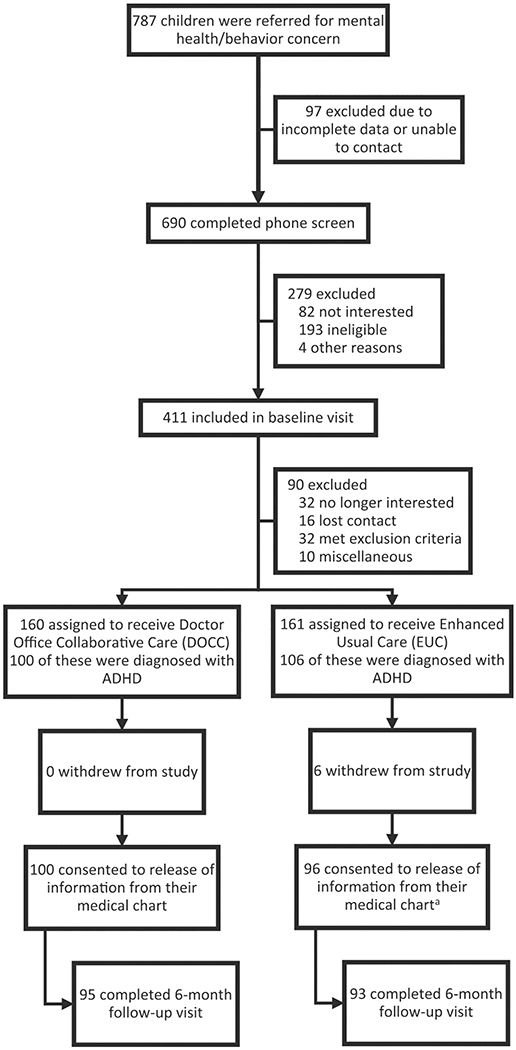
Study screening, recruitment, assessment, interventions, and data collection procedures have been described for the parent study33; such information for this subsample of children with ADHD is depicted in the CONSORT diagram (Figure 1). Briefly, the CM conducted a screen with caregivers of children 5 to 12 years old referred by their PCPs or who self-referred due to mental health concerns. Any child who met a modest clinical cutoff (≥6 or 75th percentile) on the externalizing subscale of the Pediatric Symptom Checklist (PSC-17)34 was invited for intake assessment. At intake, parents and children completed self-report forms and participated in a diagnostic/clinical interview based on the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, fourth edition) criteria35 to identify formal diagnoses. The interview was used to determine if the child met an exclusion due to diagnosis (eg, bipolar disorder, psychosis), ongoing treatment (eg, antidepressant medication), or need for a higher level of care (eg, suicidal ideation). Of 787 referred children, 321 were randomized to DOCC or EUC. Of those randomized, 206 met criteria for ADHD with 106 in DOCC and 100 in EUC. Randomization status was revealed after baseline assessment.
Figure 1.
Flow of participants in the intervention trial.
Sample Description
The mean age of the 206 participants was 8.0 ± 1.9 years. Most participants were male (69%) and Caucasian (70%). Most participants had only a diagnosis of ADHD (69%); however, 31% were also diagnosed with a disruptive behavior disorder, and 15% were also diagnosed with an anxiety disorder. At baseline, participants had moderate ADHD symptoms (mean Vanderbilt ADHD Diagnostic Parent Rating Scale [VADPRS] of 34.4 ± 9.4) and moderate impairment (mean Pediatric Quality of Life [PedsQL] of 72.4 ± 12.2). Fifteen percent of all participants were receiving medication for ADHD at intake.
Treatment Conditions
In both DOCC and EUC, the CM contacted parents by phone after intake to identify individualized goals, review evaluation findings, and specify treatment recommendations. Both parents and PCPs received a written evaluation summary.32
DOCC
Children and parents received on-site services in coordination with the PCP. To address behavior problems, the CM delivered content modules from an evidence-based mental health treatment for behavior problems adapted for delivery in primary care.33 The content included strategies to promote engagement (eg, goal setting), self-management (eg, emotion regulation), and behavior change (training in parenting or social skills) in caregivers and their children. To address ADHD, PCPs received a 1-hour didactic training by the study child and adolescent psychiatrist in an ADHD care management module based on AAP guidelines6 and then met with the practice CM to coordinate these procedures. The CM obtained parent and teacher Vanderbilt ADHD rating scales at baseline. The CM met separately with the caregiver and child to provide psychoeducation about ADHD and medication treatment, and to review ADHD medication options, potential side effects and common myths about them, and strategies to address barriers to medication use. Caregivers interested in medication were scheduled for a consultation with the PCP to address questions and receive a prescription, if warranted. During intervention, the CM monitored adherence and response by collecting parent and teacher versions of the Vanderbilt, including the side effects rating scale, and arranged an initial (by 1-month) and follow-up (by 4-month) PCP appointment. The CM also taught behavioral strategies to manage ADHD with caregivers (eg, homework structure, simple instructions) and ADHD “survival skills” with children (eg, taking notes) in 3 to 4 sessions.
EUC
Families received a referral to a mental health provider who was known to the CM, located in the area, accepted the child’s insurance, and had experience with children. Thus, children in EUC could receive services for ADHD from their PCP and/or a community mental health provider.
Assessment Procedures
Two bachelor’s-level research associates who were unaware of the child’s treatment condition administered assessments. Assessments were done at baseline (0), 6, 12, and 18 months. A CM who was uninvolved with the practice reviewed and coded the details of any medication-related procedures from each participant’s medical record after the acute treatment period ended. Of the 206 children, 188 (91%) completed the 6-month, 185 (90%) completed the 12-month, and 176 (85%) completed the 18-month assessments. Completion rates did not differ significantly by condition at 6-month (95% in DOCC vs 88% in EUC; P = .07), 12-month (93% in DOCC vs 87% in EUC; P = .14), or 18-month (89% in DOCC vs 82% in EUC; P = .16) assessments.
Assessment Measures
Clinical Effectiveness Outcomes
Parents completed the VADPRS36,37 to measure child symptom severity on 2 scales derived from 18 ADHD items and 8 ODD items. ADHD and ODD items are rated on 4-point scales ranging from never (0) to very often (3). A total for each scale score is derived by summing the component item scores. The ADHD total score has a clinical cutoff of 18 or higher. Cases at or above the cutoff for ADHD at baseline (197/206 = 96%) were coded at later time points (6, 12, 18) for ADHD remission (1 = yes, 0 = no). The VADPRS was also collected in DOCC during medication treatment to monitor and modify the treatment regimen. The Vanderbilt ADHD Diagnostic Teacher Rating Scale36,38 was also collected as part of the DOCC condition at both baseline and during medication treatment (see below). This version includes a combined oppositional/conduct factor.
Parents reported their children’s health quality of life using the PedsQL measure. This measure includes 23 items rated on 5-point scales reflecting a child’s level of functional difficulties (0 = never; 4 = almost always). A “total mean” score is derived by taking the average of 4 mean quality of life scores (higher = better) from subsections concerning physical, emotional, social, and school functioning.
Parents were interviewed using the Individualized Goal Attainment Ratings (IGAR) form to identify treatment goals for up to 4 primary individualized prob- lems.39 At pretreatment, each problem was described narratively and then the specific frequency or intensity of the problem was specified. Next, specific behavioral anchors were obtained to define the level of improvement on each goal that would reflect goal resolution or achievement. Once these endpoints were operationalized, behavioral anchors for 3 intermediate levels of progress were specified, yielding a total of 5 levels of goal improvement (eg, 1 = pretreatment severity/no goal improvement, 3 = modest improvement; 5 = expected/acceptable level of improvement). All goals were also coded into a specific content area (eg, ADHD, ODD). During later assessments (6, 12, 18), parents were asked to rate the level of improvement for each goal. Mean scores for each content area were then derived from all content-related goals.
The Clinical Global Impression-Improvement scale (CGI-I)40 was completed by an independent evaluator to assess overall level of functional improvement at 6- and 12-month assessment using a 7-point scale (1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6 = much worse, 7 = very much worse).40,41 The evaluator is instructed to rate total improvement in overall illness or dysfunction since baseline. Treatment responders are classified as those with an improvement rating of a 1 or 2. The CGI-I score had high interrater agreement (r = 0.92, P < .001).
Processes of Care During Acute Study Treatment
The CMs recorded the following elements of services documented in the medical record during the acute study treatment period (ie, within 6 months of baseline): (1) Did the child receive any services from the PCP or other staff? (2) Did the child receive any services for ADHD from the PCP or other staff? (3) Was a medication prescribed for ADHD by the PCP or other practice staff? (4) If a medication was prescribed, what was the name, dose, and administration schedule of the medication? (5) Was there medication titration (a change in the total daily dose of medication)? (6) Was there a switch of medication (a change of active ingredient, such as methylphenidate to amphetamine salt)? (7) Was there evidence of any concurrent medication (the use of 2 or more medications with different action mechanisms, such as methylphenidate and atomoxetine)? (8) If medication was prescribed, did the child have at least one office visit with the PCP or other practice staff within the first month following prescription? (9) If medication was prescribed, did the child have at least one additional office visit with the PCP or other practice staff within the first 4 months following prescription? (10) Was medication prescribed for any other mental or behavioral health problem by the PCP or other practice staff? In the DOCC condition only, we also recorded the number of completed parent and teacher Vanderbilt forms, and parent-completed side effects forms obtained by the CM, including the ADHD scores on these measures.
Parent-Reported Medication Use
The VADPRS includes a question asking if the child received any medication during the past 6 months. For comparison purposes with the medical chart review, we used this VADPRS medication item. We also used this item to represent medication use at 12 and 18 months.
Medication Use Over Time
Medication use documented during acute treatment (medical record at month 6) and at the 12- and 18-month assessment (parent report) were aggregated to code the continuity of medication use over time. Cases with complete data (n = 159) were coded in 2 different ways. A “pure” coding system included 4 groups: no medication use at all (n = 44; 31.4%), medication use during the acute study treatment period but not afterward (n = 12; 8.6%), no medication use during the acute study treatment period but consistent medication use after (n = 13; 9.3%), medication use consistently during the study (n = 71; 50.7%), and 19 additional cases omitted due to inconsistencies in medication use during the 2 maintenance assessments (12 and 18 month). Alternatively, a “broad” coding system included those 19 cases in either an expanded group with medication use during the acute study treatment period but less than 2 of the maintenance assessments (11 additional cases for a total of n = 23) or an expanded group with no medication use during the acute study treatment period but at least some medication use after (8 additional cases for a total of n = 21).
Data Analysis
Analyses were done using Statistical Package for the Social Sciences (SPSS; version 24) and Hierarchical Linear Models (HLM; version 7).42,43 We first examined DOCC and EUC participants at baseline to assess the balance across experimental groups. Medication care processes in DOCC and EUC conditions were compared using t tests for dimensional variables and χ2 tests for categorical variables (ie, without nesting by practices) since there were no significant within-group practice differences on any process variables within each condition.
To conduct longitudinal analyses for outcomes with baseline through follow-up data, we used a piecewise growth curve modeling approach44 with an intercept representing baseline levels of functioning and 2 linear slope factors representing change over time. Preliminary intraclass correlation coefficient (ICC) analyses revealed minimal (ie, <2%) variance in outcomes accounted for by higher level nesting, so these models were 2-level with time nested within families. We used full maximum likelihood estimate.
The level-1 equations for the unconditional models were Yti = π0i + π1i(response) + π0i(maintenance) + eti, where Yti is the observed outcome score at time t for participant i. The “response” slope is the change from baseline through posttreatment. The “maintenance” slope is the change over any 6 months of the follow-up. Condition was later entered as a level-2 variable (referred to as the “Simple Conditional Model”). The response coefficient for condition shows the change in outcome from baseline to posttreatment due to condition. The maintenance coefficient shows the change for each 6-month period after acute treatment due to condition.
To examine medication use and its interaction with condition, medication was entered into a separate set of models as a level-2 predictor along with condition and their interaction (“Complex Conditional Model”).45–47 Condition, medication use, and their interaction were modeled and tested for both response and maintenance. Point-in-time contrasts at 6 months were interpreted from the intercept. Point-in-time contrasts at 12 months and at 18 months were later obtained by recoding time to reset the intercept.
For longitudinal analyses for outcomes with only post-treatment and follow-up data, we used a linear HLM model with an intercept at 6 months and a single slope over the 3 time points (6, 12, 18). Preliminary ICC analyses revealed nonminimal variance (up to 19.1%) embedded within 4 levels of nesting: time within families within PCPs within practices. Next, we added condition as a level-4 variable predicting both slope and intercept (“Simple Conditional Model”). Point-in-time contrasts were obtained in the same manner as noted earlier.
To examine the effects of medication use as well as possible medication use by condition interaction, models were built incrementally. Medication use was first entered as a level-2 (family) predictor of both slope and intercept into a separate series of longitudinal models with no level-4 (practice) predictor. This provided main effects of medication use that could not be derived from models including condition in level-4. Only later was condition added at level-4 to add C X M of slope, C X M of intercept, main effect of condition on slope, and main effect of condition on intercept. The results of all models for each outcome were amalgamated to provide (separately for intercept and slope) main effect of medication use, main effect of condition, and their interaction. Together, these 6 effects are the “Complex Conditional Model.”45–47 As before, point-in-time contrasts at 6 months were taken from the intercept. Recoding the intercept produced point-in-time contrasts at 12 months and at 18 months.
Medication Use Over Time
Further HLM analyses examined the relationship of medication use over the entire study with primary outcomes. Condition was not included due to reduced sample size. The 4 medication groups were represented by dummy codes. Goodness- of-fit tests from deviance statistics comparing models with and without medication group served as omnibus tests. Three Vanderbilt measures (ADHD, ODD, and ADHD remission) and quality of life were examined; IGAR ADHD and IGAR ODD were not examined due to reduced sample sizes from some families not reporting goals for specific content areas.
Results
Baseline Equivalence
Child demographics, child clinical characteristics, child outcomes, and family background characteristics were examined at baseline for differences by condition (see Table 1). There were no significant differences for any of the 15 tested comparisons except that more parents in DOCC (vs EUC) had completed some college education (84.8% vs 70.5%; P = .01).
Table 1.
Baseline Characteristics of Children and Families.
| Condition |
||||
|---|---|---|---|---|
| Variable | All Cases (N = 206) | DOCC (N = 100) | EUC (N = 106) | Pa |
| Child characteristics | ||||
| Age in years | 8.0 ± 1.9b | 7.8 ± 1.8 | 8.2 ± 1.9 | .15 |
| Gender (% male) | 142 (68.9) | 72 (72.0) | 70 (66.0) | .36 |
| Minority status (% minority)c | 59 (28.9) | 28 (28.0) | 31 (29.8) | .78 |
| Primary diagnosis (%) | ||||
| ADHD only | 142 (68.9) | 73 (73.0) | 69 (65.1) | .22 |
| ADHD and DBD(s)d | 64 (31.1) | 27 (27.0) | 37 (34.9) | |
| Secondary diagnosis (%)e | ||||
| Anxiety disordersf | 30 (14.6) | 11 (11.0) | 19 (17.9) | .16 |
| Tic disordersg | 3 (1.5) | 3 (3.0) | 0 (0.0) | .11h |
| Elimination disordersi | 16 (7.8) | 7 (7.0) | 9 (8.5) | .69 |
| VADPRS: ADHD total | 34.4 ± 9.4 | 34.1 ± 9.8 | 34.6 ± 9.1 | .70 |
| PedsQL: tota1 mean | 72.4 ± 12.2 | 71.8 ± l2.5 | 73.1 ± l2.0 | .46 |
| VADPRS: ADHD medication (%) | 32 (15.6) | 17 (17.2) | 15 (14.2) | .55 |
| Family characteristics | ||||
| Marital status (% married) | 124 (61.1) | 64 (65.3) | 60 (57.1) | .23 |
| Number of children living in home | 1.6 ± 1.1 | 1.5 ± 1.1 | 1.7 ± 1.1 | .38 |
| Welfare: any social assistance (%) | 94 (46.1) | 44 (44.4) | 50 (47.6) | .65 |
| Parent education (% some college) | 158 (77.5) | 84 (84.8) | 74 (70.5) | .01 |
| Family income (1–5 scale) | 3.4 + 1.5 | 3.5 + 1.5 | 3.3 + 1.6 | .38 |
Abbreviations: DOCC, Doctor Office Collaborative Care; EUC, Enhanced Usual Care; ADHD, attention deficit hyperactivity disorder; DBD, disruptive behavioral disorder; VADPRS, Vanderbilt ADHD Diagnostic Parent Rating Scale; PedsQL, Pediatric Quality of Life.
Pearson χ2 test, Fischer’s exact test when expected cell size <5.
Plus-minus values are mean ± SD.
Minority status was reported by the participants.
DBDs include oppositional defiant disorder, conduct disorder, and DBD not otherwise specified.
Secondary diagnosis refers to an allowable diagnosis that was rated as less severe than the primary disorder of interest.
Anxiety disorders include separation anxiety disorder, social phobia, generalized anxiety disorder, or anxiety disorder not otherwise specified.
Tic disorders include Tourette’s disorder, transient tic disorder, and tic disorder not otherwise specified.
Expected cell size <5.
Elimination disorders include enuresis and encopresis.
Processes of Care During the 6 Months of Acute Treatment
DOCC Versus EUC Effects
Table 2 presents the results for the chart review that documented the types of services received and medications prescribed by the PCP or other practice staff during acute treatment. DOCC (vs EUC) had significantly higher rates of receiving any service. DOCC also had higher rates of specific services to address the child’s ADHD and any medication prescribed for ADHD. There was a high level of agreement between this medication use variable (chart review) and the parent-reported Vanderbilt item reflecting any medication use at 6 months (κ = 0.83, P < .001).
Table 2.
Treatment Condition Effects on Processes of Care.
| Process of Care Variables | Na | DOCC (%) | EUC (%) | χ2 | Pb |
|---|---|---|---|---|---|
| Any services by PCP or other staff | 195 | 95 (95.0) | 77 (81.1) | 9.1 | .003 |
| Any services delivered for ADHD by PCP | 195 | 86 (86.0) | 58 (61.1) | 15.7 | <.001 |
| Any medication prescribed for ADHD | 195 | 76 (76.0) | 43 (45.3) | 19.4 | <.001 |
| Care parameters for those receiving medication for ADHD | |||||
| Titration of ADHD medication | 119 | 58 (76.3) | 22 (51.2) | 7.9 | .005 |
| Switch of ADHD medication | 119 | 21 (27.6) | 10 (23.3) | 0.3 | .60 |
| Any concurrent use of ADHD medicationsc | 119 | 5 (6.6) | 0 (0) | 3.0 | .16 |
| Any adherence information noted in record | 119 | 27 (35.5) | 19 (44.2) | 0.9 | .35 |
| Was there a visit <1 month after starting medication | 119 | 44 (57.9) | 24 (55.8) | 0.5 | .83 |
| Was there a visit <4 months after starting medication | 119 | 58 (76.3) | 28 (65.1) | 1.7 | .19 |
| Any meds prescribed for other mental health problemsc | 195 | 3 (3.0) | 2 (2.1) | 0.2 | .69 |
Abbreviations: DOCC, Doctor Office Collaborative Care; EUC, Enhanced Usual Care; PCP, primary care provider; ADHD, attention deficit hyperactivity disorder.
For full sample (n = 195), n for DOCC and EUC were 100 and 95, respectively. For those receiving medication (n = 119), n for DOCC and EUC were 76 and 43, respectively.
Pearson χ2 test, Fischer’s exact test when expected cell size <5.
Expected cell size <5.
Nearly 98% of those who received medication for ADHD were prescribed a stimulant. The most common medications (stimulants or otherwise) used in DOCC and EUC at any point during treatment were methylphenidate (82%, 74%), amphetamine salt (11%, 12%), and atomoxetine (4%, 0%), respectively. There were no significant differences in the specific medications used in the 2 conditions and in the proportion of cases who switched from stimulant to nonstimulant (or vice versa).
For those participants who received medication for ADHD (n = 119), chart review showed a significantly higher rate of dose titration in DOCC than EUC. The mean number of dose changes was 1.49 ± 1.3 in DOCC, and 0.98 ± 1.2 in EUC (P = .04). Other medication prescription patterns (ie, availability of adherence information, switch to a new ADHD medication, concurrent use of ADHD medications) did not differ significantly between conditions. Rates of having any type of follow-up visit within 1 month or within 4 months of starting medication also did not differ between conditions. Finally, the mean number of days that medication was taken was 120.2 for DOCC (SD = 55.0; range = 1–185) and 128.8 for EUC (SD = 56.1; range = 11–185). This reflects a mean of 66% and 70% of the 6-month time period (P = .51). There were no differences in medication being prescribed for other mental health problems. All children who received medication had been diagnosed with ADHD.
DOCC Condition Only
A majority of cases (54/100 = 54%) had teacher Vanderbilt forms completed within approximately 1 month of enrollment to constitute a baseline in keeping with standard practice for teacher- reported behavioral ratings. These baseline teacher reports had a mean of 29.0 (SD = 11.3) with 46 of 54 (85.2%) above the clinical cutoff for ADHD (≥18).
After baseline, a total of 228 parent and 135 teacher Vanderbilt forms were completed to monitor DOCC treatment. We computed the percentage of children who had at least 1 nonbaseline parent (79/100, 79%) and at least 1 nonbaseline teacher (48/100, 48%) rating scale. A total of 70% and 60% of these patients had more than 1 nonbaseline parent and teacher form, respectively. Averaged across all nonbaseline forms completed during treatment for each case, the mean ADHD scale (and SD) for the parent Vanderbilt forms was 22.4 (SD = 8.8). For teacher Vanderbilt forms, the mean was 23.5 (SD = 10.5).
In terms of side effects, 64 of 79 cases (81%) with parent-reported Vanderbilt data during treatment also had at least 1 parent-reported side effects rating form; 80% had 2 or more forms. The mean number of completed side effects forms was 2.6 (SD = 1.2; range = 1–6). The mean number of side effects reported across all available forms was 2.6 (SD = 1.6; range = 0–7). Only 4 of 64 cases (6.3%) with side effects rating forms reported no side effects of any kind. The most common side effects reported were appetite change (68.8%), irritability (67.2%), and trouble sleeping (53.1%). The rates for other side effects varied widely (4.7% to 43.8%).
Clinical Outcomes Over Time
Simple Conditional Models
Initial analyses were run in HLM models that included only treatment condition as a predictor of outcome. Table 3 (panel A) shows the results for outcomes with baseline through follow-up data. The acute effect of condition was also examined in the “response” component of the piecewise HLM simple conditional models. DOCC (vs EUC) showed greater improvements in ADHD and ODD symptom severity, and quality of life from baseline to 6-month (ie, the response slope) that were then maintained from 6- to 18-month assessments (ie, the maintenance slope was not significantly different from 0). Point-in-time contrasts showed that DOCC cases showed significant improvement in ODD symptoms and quality of life at 12 months and at 18 months; DOCC cases only tended to show improved ADHD symptoms at both time points.
Table 3.
Simple Conditional HLM Results for Longitudinal Outcomes.
| Panel A: Longitudinal Outcomes With Baseline through Follow-up Dataa | ||||||||
| Slope |
Point-in-Time |
|||||||
| Response |
Maintenance |
12 Months |
18 Months |
|||||
| Coeff | P | Coeff | P | Coeff | P | Coeff | P | |
| VADPRS: ADHD total | −3.31 | .02 | 1.18 | .08 | −4.17 | .08 | −6.01 | .10 |
| VADPRS: ODD total | −1.79 | .01 | 0.54 | .14 | −2.59 | .03 | −3.67 | .03 |
| PedsQL: total mean | 4.11 | .005 | −0.75 | .39 | 6.18 | .03 | 9.91 | .02 |
| Panel B: Longitudinal Outcomes with Post-treatment and Follow-up Datab | ||||||||
| Slope |
Point-in-Time |
|||||||
| Overall |
6 Months |
12 Months |
18 Months |
|||||
| Coeff (OR) | P | Coeff (OR) | P | Coeff (OR) | P | Coeff (OR) | P | |
| IGAR ADHD | −0.14 | .21 | 0.69 | .01 | 0.55 | .01 | 0.41 | .08 |
| IGAR ODD | −0.21 | .08 | 0.72 | .004 | 0.51 | .01 | 0.30 | .15 |
| CGI-I response (≤2) | (0.66) | .38 | (2.14) | .05 | (1.41) | .35 | —c | —c |
| VADPRS: ADHD remission (<18)d | (0.77) | .38 | (2.50) | .07 | (1.94) | .11 | (1.50) | .42 |
Abbreviations: HLM, hierarchical linear models; Coeff, coefficient; VADPRS, Vanderbilt ADHD Diagnostic Parent Rating Scale; ADHD, attention deficit hyperactivity disorder; ODD, oppositional defiant disorder; PedsQL, Pediatric Quality of Life; OR, odds ratio; IGAR, Individualized Goal Achievement Rating; CGI-I, Clinical Global Impression Scale-Improvement.
Two-level HLM model sets with piecewise slope.
Four-level HLM models with single slope.
CGI-I was not administered at 18 months.
Nine participants with baseline scores of <18 on VADPRS were not included.
Table 3 (panel B) shows the results for the outcomes with post-treatment and follow-up data that reflect achievement of treatment goals or clinical response. Condition did not significantly predict the slope over all 3 time points for any of the 4 outcomes. However, point- in-time contrasts showed that DOCC (vs EUC) at 6 months and at 12 months had significantly greater achievement in individualized ADHD and ODD goals, and at 6 months, only in overall treatment response. The increase in the ADHD remission rate for DOCC (vs EUC) only approached significance at 6 months.
Complex Conditional Models
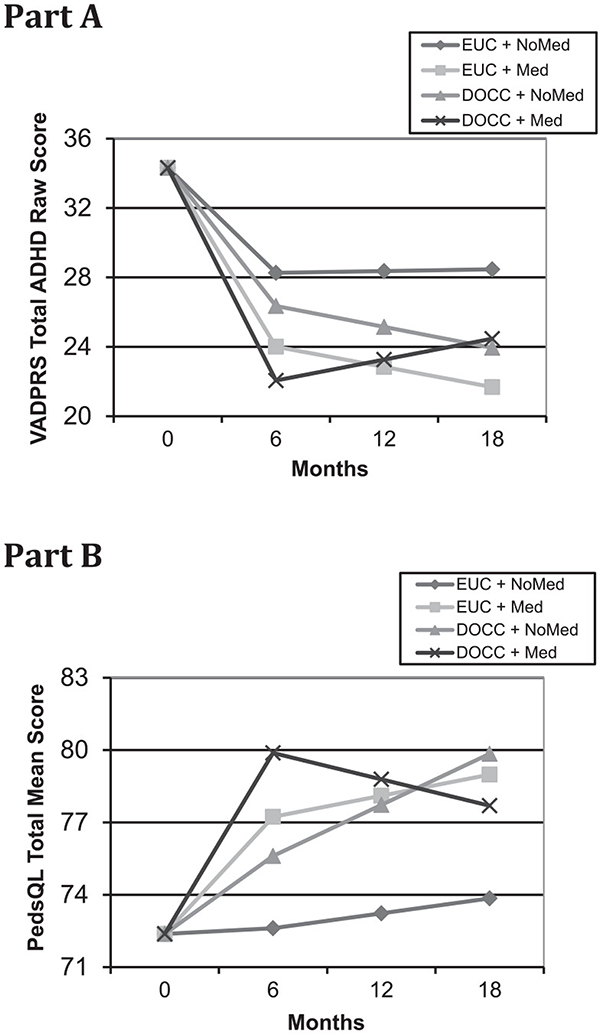
Additional analyses used HLM models to examine the main effects of condition and medication use as well as their interaction. Medication use data were available for 119 cases that were medicated during acute treatment and 76 cases that were not. Table 4 (Panel A) presents the results for those outcomes with baseline through follow-up data. ADHD symptom severity decreased significantly more during treatment for cases receiving medication (vs not). During later time points, this difference was significantly affected by a condition by medication use interaction in the maintenance slope whereby DOCC cases that used medication did not fully maintain earlier gains (see Figure 2, Part A). In this model, there were no significant effects or interactions during response or maintenance for ODD symptom severity. The coefficient for the effect of condition on the response slope for ODD symptom severity (−2.04) is slightly larger than the same coefficient in the simple conditional models (−1.79), but it is no longer significant (P = .11) in the complex conditional model due to including the additional (and nonsignificant) medication use and interaction parameters. Quality of life increased significantly more during treatment for cases receiving medication (vs not), and this difference was maintained during later time points. Medication use was associated with significantly higher quality of life for point-in-time contrasts at 12 months and 18 months. The DOCC (vs EUC) condition was also associated with higher quality of life at 12 months and 18 months, despite a nonsignificant response slope for condition. This seeming incongruity is explained by a trend (P = .06) condition by medication use interaction effect in the maintenance slope (see Figure 2, Part B) that is very similar in nature to the one seen with the ADHD symptom severity score.
Table 4.
Complex Conditional HLM Results for Longitudinal Outcomes.
| Panel A: Longitudinal Outcomes with Baseline through Follow-up Dataa | ||||||||
| Slope |
Point-in-Time |
|||||||
| Response |
Maintenance |
12 Months |
18 Months |
|||||
| Coeff | P | Coeff | P | Coeff | P | Coeff | P | |
| Condition | ||||||||
| VADPRS: ADHD total | −1.91 | .41 | −1.30 | .27 | −6.37 | .08 | −9.34 | .09 |
| VADPRS: ODD total | −2.04 | .11 | 0.99 | .17 | −2.02 | .25 | −3.23 | .23 |
| PedsQL: total mean | 2.99 | .26 | 1.50 | .31 | 10.20 | .02 | 13.80 | .03 |
| Medication Use | ||||||||
| VADPRS: ADHD total | −4.26 | .03 | −1.27 | .20 | −11.17 | .001 | −17.17 | .001 |
| VADPRS: ODD total | −0.66 | .52 | 0.10 | .86 | −1.10 | .50 | −1.70 | .48 |
| PedsQL: total mean | 4.62 | .03 | 0.25 | .86 | 9.18 | .02 | 14.51 | .01 |
| Condition × Medication Use interaction | ||||||||
| VADPRS: ADHD total | −0.04 | .99 | 3.67 | .01 | 7.31 | .13 | 11.11 | .13 |
| VADPRS: ODD total | 0.65 | .68 | −0.54 | .51 | 0.07 | .98 | 0.50 | .89 |
| PedsQL: total mean | −0.35 | .92 | −3.47 | .06 | −9.62 | .09 | −11.95 | .14 |
| Panel B: Longitudinal Outcomes Without Baseline Post-treatment and Follow-up Datab | ||||||||
| Slope |
Point-in-Time |
|||||||
| Overall |
6 Months |
12 Months |
18 Months |
|||||
| Coeff (OR) | P | Coeff (OR) | P | Coeff (OR) | P | Coeff (OR) | P | |
| Condition | ||||||||
| IGAR ADHD | −0.20 | .39 | 0.93 | .01 | 0.73 | .003 | 0.53 | .09 |
| IGAR ODD | −0.24 | .38 | 0.78 | .06 | 0.55 | .05 | 0.31 | .38 |
| CGI-I response (≤2) | (0.73) | .73 | (1.78) | .42 | (1.31) | .69 | —c | —c |
| VADPRS: ADHD remission (<18)b | (0.66) | .44 | (4.10) | .11 | (2.71) | .11 | (1.77) | .46 |
| Medication Use | ||||||||
| IGAR ADHD | −0.20 | .18 | 0.42 | .07 | 0.23 | .24 | 0.03 | .91 |
| IGAR ODD | −0.12 | .42 | 0.10 | .65 | −0.02 | .88 | −0.15 | .52 |
| CGI-I response (≤2) | (0.76) | .60 | (1.85) | .15 | (1.41) | .40 | —c | —c |
| VADPRS: ADHD remission (<18)b | (0.53) | .07 | (2.83) | .05 | (1.49) | .28 | (0.79) | .63 |
| Condition × Medication Use interaction | ||||||||
| IGAR ADHD | 0.16 | .59 | −0.72 | .08 | −0.56 | .12 | −0.40 | .44 |
| IGAR ODD | 0.05 | .86 | −0.19 | .65 | −0.14 | .67 | −0.08 | .86 |
| CGI-I response (≤2) | (0.95) | .96 | (1.07) | .94 | (1.01) | .99 | —c | —c |
| VADPRS: ADHD remission (<18)d | (1.55) | .51 | (0.34) | .27 | (0.52) | .37 | (0.83) | .85 |
Abbreviations: HLM, hierarchical linear models; Coeff, coefficient; VADPRS, Vanderbilt ADHD Diagnostic Parent Rating Scale; ADHD, attention deficit hyperactivity disorder; ODD, oppositional defiant disorder; PedsQL, Pediatric Quality of Life; OR, odds ratio; IGAR, Individualized Goal Achievement Rating; CGI-I, Clinical Global Impression Scale-Improvement.
Two-level HLM model sets with piecewise slope.
Four-level HLM models with single slope.
CGI-I not administered at 18 months.
Nine participants with baseline scores of <18 on VADPRS were not included.
Figure 2.
Hierarchical linear models (HLM) estimations of condition by medication status interactions in maintenance phase for Vanderbilt Total ADHD raw scale scores [Part A] or Pediatric Quailty of Life total scale scores [Part B].
Table 4 (panel B) shows the results for those outcomes with post-treatment and follow-up data that reflect achievement of treatment goals or clinical response. There were no significant differences in overall slope for the main effect of condition or medication use, or their interaction for any of the 4 outcomes, though the effect of medication use approached significance. Individualized ADHD goals were significantly improved for DOCC (vs EUC) in point-in-time contrasts at 6 months and at 12 months, but the difference for medication use only approached significance at 6 months. Individualized ODD goals were significantly improved for DOCC (vs EUC) at 12 months, but not for medication use. Overall treatment response was not significantly different for condition or medication use at any time point. Receiving medication for ADHD (vs not) was significantly related to higher ADHD remission rates at 6 months.
Medication Use Over Time
HLM analyses were run to predict primary outcomes at 18 months by the 4 medication continuity groups without the inclusion of condition in the models due to reduced sample size. Because results were equivalent using the “pure” codes and the “broad” codes, we reported only the “pure” codes. The omnibus test for the ADHD symptom severity scale was P < .01. In terms of specific follow-up group comparisons that were significant, cases receiving medication during acute treatment only (P < .01) or consistently over time (p < .01) had significantly lower ADHD scale scores than those who did not receive any medication. The omnibus test for quality of life was P < .01. In terms of specific group comparisons that were significant, cases with consistent use of medication over time were significantly higher than cases who did not receive any medication (P = .04) and cases receiving medication only after acute treatment (P = .04); cases that received medication during treatment but not afterward were significantly higher than cases receiving medication only after acute treatment (P < .01). The 4 groups did not differ in ODD symptom severity ratings or ADHD remission rates.
Relationship of Stratification Variables to Outcomes
Analyses revealing significant condition differences were rerun to examine the relationship of each outcome to the 2 stratification variables. Each variable was run in separate complex conditional models using outcomes resulting in significant results with simple conditional models. There were no significant interaction effects between condition and either stratification variable, and there were no significant main effects for either stratification variable.
Discussion
We evaluated a simplified collaborative care model for children with modest behavior problems (DOCC) that included a care management regimen to target their comorbid ADHD. For the 206 (64.2%) children who met diagnostic criteria for ADHD, DOCC included psychosocial skills training and medication management showed improvements on some care process and outcome measures relative to EUC. Some acute improvements were maintained through 1-year follow-up. Medication continuity across time was associated with some gains in long-term ADHD outcomes and quality of life. These findings are discussed in the context of the broader primary care literature, the study’s limitations, and suggestions for enhancing the impact of collaborative care models.
After the 6-month intervention, medical chart reviews showed higher rates of PCP-delivered ADHD services, medication prescriptions for ADHD, and medication titration in DOCC (vs EUC). PCPs were very responsive to the 1-hour training by meeting with families to provide psychoeducation and engaging in shared decision-making about a possible medication trial, which led 76% of parents in DOCC to choose this option. Our DOCC titration rate for medication (76%) is not only significantly higher than for EUC but also parallels the rate for an enhanced collaborative model (72%)27 and is somewhat higher than rates found in a collaborative consultation program (68%)23 and routine practice (63%).20 However, there were no condition differences in other medication parameters (eg, any adherence information recorded, a 1- or 4-month follow-up visit). Notably, more than one half and two thirds of cases that received medication had a visit by 1 or 4 months, respectively. One of the challenges to documenting these visits in DOCC was that families who attended psychosocial visits could convey information about the child’s medication use to the CM without being scheduled for a formal PCP visit. Furthermore, collection of parent and teacher Vanderbilt scales to monitor treatment response was variable, as has been noted in other large-scale studies.19,23,25
In terms of the simple conditional models, DOCC (vs EUC) cases showed a greater reduction in ADHD symptoms, more improvement in individualized ADHD-related treatment goals, and a trend toward a higher rate of ADHD remission. These findings add to the large body of evidence documenting significant improvements in parent- and teacher-reported ADHD symptoms after comprehensive training or quality improvement programs for children with ADHD in community pediatric practices.19,20,23–27 In addition, DOCC cases showed a greater improvement in ODD symptoms and individualized ODD treatment targets, better quality of life, and greater overall functional improvement. Such gains in comorbid clinical symptoms and healthier functioning are worth noting given that broader clinical improvements are not always found and may vary based on the level of exposure to other psychosocial inter- ventions.24,25 In DOCC, virtually all caregivers and children received at least some psychosocial intervention.
These simple models also documented some benefits for DOCC (vs EUC) at 12 and 18 months (ie, 6 and 12 months after intervention, respectively). DOCC showed greater improvement in individualized goals related to both ADHD and ODD, ODD symptom severity, and quality of life at 12 months. Furthermore, the latter 2 improvements were also found at 18 months. Such follow-up improvements in multiple clinical and quality of life domains provide some support for the maintenance of the broad benefits of DOCC.
In our complex conditional models, the main effects of DOCC were no longer apparent in terms of initial response, but it was associated with greater improvement in individualized ADHD targets at 6 months. At follow-up, DOCC cases showed better quality of life at 12 and 18 months, and greater improvements in individualized ADHD and ODD targets at 12 months. In contrast, medication use had a significant effect on ADHD symptom reduction and quality of life in terms of initial response, and on ADHD remission rate after treatment. Medication use also was associated with greater ADHD symptom improvement and quality of life at both 12- and 18-month follow-ups. There were few significant condition X medication interactions, suggesting that condition and medication generally had separate effects across outcomes. DOCC and medication use seemed to have greater effects on individualized targets and ADHD symptoms or remission, respectively, whereas both were related to enhanced quality of life.
At the same time, there were few improvements on key outcomes at follow-up (eg, ADHD remission). Such results are similar to those of the Multimodality Treatment of ADHD study (MTA),9 which found that when interventions were not maintained, the initial results disappeared by 16 months. The results from the MTA and this study emphasize the importance of maintaining care for children with ADHD since efficacious acute treatments are not curative and the long-term outcomes show continued problems and a lack of sustained treatment.10,48 Our findings showing the superiority of receiving consistent medication across the acute and follow-up periods relative to some of the other medication use subgroups is consistent with that observation. Of course, other strategies such as physician-parent shared decision-making during treatment planning and the use of other educational and medication support strategies may facilitate the use and maintenance of medication for ADHD.28
The limitations of this study should be noted. First, efficient and timely collection of teacher rating scales was not possible, so our outcomes are based on parent reports as reported in some other studies.24,49 And, we were only modestly successful in collecting teacher and parent Vanderbilt scales to monitor medication response, also noted by other researchers.20,25 The use of a web-based portal for data collection and intervention for ADHD (eg, MyADHDportal. com) is a notable advance in the collection of parent and teacher outcomes.50 Furthermore, comprehensive chart reviews could not be conducted for those participants in EUC who received medication or therapy outside of their pediatrician’s office. Although the study sought to obtain treatment summary report data from outside providers, only 45% (48/106) returned these forms in EUC whereas 100% were completed by the CMs in DOCC, which precluded any group comparisons on specialty care services (eg, use of medication, dose of treatment). It is also worth noting that the comparison condition (EUC) was not treatment as usual (TAU) as it augmented their usual care (eg, PCPs and parents received assessment summaries, families received facilitated referrals to local providers). This design feature was required by the practices to enhance acceptability, but it likely made for a very conservative test of the benefits of DOCC.
The use of collaborative or integrated models in primary care employing embedded behavioral health providers has been recommended and reported since 1980.51 These models have been applied to an array of conditions and incorporated a range of providers (nurses, social workers, psychologists). Despite the added benefits of these collaborative models, provider availability and cost has limited their implementation in pediatric primary care. This study’s use of master’s level CMs should reduce the cost of implementing a collaborative model in pediatric primary care; indeed, some cost analysis data from this trial support this claim.52 Since care coordination has been shown to improve clinical outcomes of other chronic illnesses in pediatric primary care,53 care management can be adapted and cross-trained to facilitate implementation of evidence-based guidelines to address a full range of pediatric behavioral health patient problems.
Acknowledgments
We thank Dr. Joel Greenhouse for statistical consultation, Dr. Kelly Kelleher for editorial feedback, Dr. Abigail Schlesinger for psychiatric consultation, and Kevin Rumbarger for his expertise in preparing this document, and to Dr. Brooke Molina for her input on the analyses.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIMH Grant #063272 to the first author.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Clinical Trial Registration
Effectiveness of Collaborative Services in Primary Care for Treating Children With Behavior Disorders (SKIP); http://www.clinicaltrials.gov/ct2/show/record/NCT00600470; NCT00600470.
References
- 1.Kolko DJ. Options for the delivery of mental health services In: Mclnerny TK, ed. American Academy of Pediatrics Textbook of Pediatric Care. Elk Grove Village, IL: American Academy of Pediatrics; 2009. [Google Scholar]
- 2.Kolko DJ, Perrin E. The integration of behavioral health services in pediatric primary care: services, science, and suggestions. J Clin Child Adolesc Psychol 2014;43:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Havens JF. Another reason to love integrated behavioral health: notes from the north. J Am Acad Child Adolesc Psychiatry. 2017;56:458–459. [DOI] [PubMed] [Google Scholar]
- 4.Sarvet B The need for practice transformation in children’s mental health care. J Am Acad Child Adolesc Psychiatry. 2017;56:460–461. [DOI] [PubMed] [Google Scholar]
- 5.Asarnow J, Rozenman M, Wiblin J, Zeltzer L. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a metaanalysis. JAMA Pediatr. 2015;169:929–937. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics, Subcommittee on Attention-Deficit/Hyperactivity Disorder and Committee on Quality Improvement. Clinical practice guidelines: treatment of the school-aged child with attention-deficit/ hyperactivity disorder. Pediatrics. 2001;108:1033–1044.11581465 [Google Scholar]
- 7.Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. [DOI] [PubMed] [Google Scholar]
- 8.Wolraich ML, Hagan JF, Allan C, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144:e20192528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46:989–1002. [DOI] [PubMed] [Google Scholar]
- 10.Molina BSG, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics. 2004;113:762–769. [DOI] [PubMed] [Google Scholar]
- 12.A 14-month randomized clinical trial of treatment strategies for attention deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal treatment study of children with ADHD. Arch Gen Psychiatry. 1999;56:1073–1086. [DOI] [PubMed] [Google Scholar]
- 13.Moderators and mediators of treatment response for children with attention-deficit/hyperactivity disorder: the multimodal treatment study of children with attention-deficit/hyperac- tivity disorder. Arch Gen Psychiatry. 1999;56:1088–1096. [DOI] [PubMed] [Google Scholar]
- 14.Leslie L, Weckerly J, Plemmons D, Landsverk J, Eastman S. Implementing the American Academy of Pediatrics atten- tion-deficit/hyperactivity disorder diagnostic guidelines in primary care settings. Pediatrics. 2004;114:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans S, Sarno Owens J, Wymbs B, Ray A. Evidence- based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47:157–198. [DOI] [PubMed] [Google Scholar]
- 16.Chan E, Hopkins MR, Perrin JM, Herrerias C, Homer CJ. Diagnostic practices for attention deficit hyperactivity disorder: a national survey of primary care physicians. AmbulPediatr 2005;5:201–208. [DOI] [PubMed] [Google Scholar]
- 17.Wolraich ML, Bard DE, Stein MT, Rushton JL, O’Connor KG. Pediatricians’ attitudes and practices on ADHD before and after the development of ADHD pediatric practice guidelines. JAtten Disord. 2010;13:563–572. [DOI] [PubMed] [Google Scholar]
- 18.Visser SN, Bitsko RH, Danielson ML, et al. Treatment ofatten- tion deficit/hyperactivity disorder among children with special health care needs. J Pediatr. 2015;166:1423–1430.e1-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein JN, Kelleher KJ, Baum R, et al. Variability in ADHD care in community-based pediatrics. Pediatrics. 2014;134:1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinkman WB, Baum R, Kelleher KJ, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry. 2016;55:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homer CJ, Horvitz L, Heinrich P, Forbes P, Lesneski C, Phillips J. Improving care for children with attention deficit hyperactivity disorder: assessing the impact of selfassessment and targeted training on practice performance. Ambul Pediatr 2004;4:436–441. [DOI] [PubMed] [Google Scholar]
- 22.Rushton JL, Fant KE, Clark SJ. Use of pediatric guidelines in the primary care of children with attention-deficit/ hyperactivity disorder. Pediatrics. 2004;114:23–28. [DOI] [PubMed] [Google Scholar]
- 23.Epstein JN, Rabiner D, Johnson DE, et al. Improving atten- tion-deficit/hyperactivity disorder treatment outcomes through use of a collaborative consultation treatment service by community-based pediatricians: a cluster randomized trial. Arch Pediatr Adolesc Med. 2007;161:835–840. [DOI] [PubMed] [Google Scholar]
- 24.Epstein JN, Kelleher KJ, Baum R, et al. Specific components of pediatricians’ medication-related care predict atten- tion-deficit/hyperactivity disorder symptom improvement. J Am Acad Child Adolesc Psychiatry. 2017;56:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein JN, Langberg JM, Lichtenstein PK, et al. Attention-deficit/hyperactivity disorder outcomes for children treated in community-based pediatric settings. Arch Pedatr Adolesc Med. 2010;164:160–165. [DOI] [PubMed] [Google Scholar]
- 26.Epstein JN, Langberg JM, Lichtenstein PK, Mainwaring BA, Luzader CP, Stark LJ. Community-wide intervention to improve the attention-deficit/hyperactivity disorder assessment and treatment practices of community physicians. Pediatrics. 2008;122:19–27. [DOI] [PubMed] [Google Scholar]
- 27.Silverstein M, Hironaka LK, Walter HJ, et al. Collaborative care for children with ADHD symptoms: a randomized comparative effectiveness trial. Pediatrics. 2015;135:e858–e867. [DOI] [PubMed] [Google Scholar]
- 28.Brinkman WB, Hartl J, Rawe LM, Sucharew H, Britto MT, Epstein JN. Physicians’ shared decision-making behaviors in attention-deficit/hyperactivity disorder care. Arch Pedatr Adolesc Med. 2011;165:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinkman WB, Epstein JN. Promoting productive interactions between parents and physicians in the treatment of children with attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2011;11:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinkman WB, Epstein JN. Treatment planning for children with attention-deficit/hyperactivity disorder: treatment utilization and family preferences. Patient Prefer Adherence. 2011;5:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelham WE Jr, Fabiano GA, Waxmonsky JG, et al. Treatment sequencing for childhood ADHD: a multiple-randomization study of adaptive medication and behavioral interventions. J Clin Child Adolesc Psychol. 2016;45:396–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolko DJ, Campo JV, Kilbourne AM, Hart J, Sakolsky D, Wisniewski S. Collaborative care outcomes for pediatric behavioral health problems: a cluster randomized trial. Pediatrics. 2014;133:e981–e992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolko DJ, Campo J, Kilbourne AM, Hart J, Sakolsky D, Wisniewski S. Collaborative care outcomes for pediatric behavioral health problems: a cluster randomized trial. Pediatrics. 2014;133:e981–e992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jellinek MS, Murphy J, Little M, Pagano ME, Comer DM, Kelleher KJ. Use of the pediatric symptom checklist to screen for psychosocial problems in pediatric primary care: a national feasibility study. Arch Pediatr Adolesc Med. 1999;153:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Diagnostic interview: Kiddie-SADS Present and Lifetime Version; 1996. https://www.researchgate.net/publication/285756826_Kiddie-SADS_-_Present_and_Lifetime_Version_K-SADS-PL.
- 36.Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley KB. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28:559–567. [DOI] [PubMed] [Google Scholar]
- 37.Bard D, Wolraich ML, Neas B, Doffing M, Beck L. The psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a community population. J Dev Behav Pediatr. 2013;34:72–82. [DOI] [PubMed] [Google Scholar]
- 38.Wolraich ML, Bard D, Neas B, Doffing M, Beck L. The psychometric properties of the Vanderbilt ADHD Diagnostic Teacher Rating Scale in a community population. J Dev Behav Pediatr. 2013;34:83–93. [DOI] [PubMed] [Google Scholar]
- 39.Kolko DJ, Campo JV, Kelleher K, Cheng Y. Improving access to care and clinical outcome for pediatric behavioral problems: a randomized trial of a nurse-administered intervention in primary care. J Dev Behav Pediatr. 2010;31:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guy W Clinical Global Impression Scale. Rockville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 41.National Institute of Mental Health. Clinical Global Impression (CGI). Psychopharmacol Bull. 1985;21:839–844. [Google Scholar]
- 42.Graham-Bermann S, Lynch S, Banyard VL, DeVoe ER, Halabu H. Community-based intervention for children exposed to intimate partner violence: an efficacy trial. J Consult Clin Psychol. 2007;75:199–209. [DOI] [PubMed] [Google Scholar]
- 43.Raudenbush S, Bryk A, Cheong YF, Congdon RT Jr. HLM 6: Hierarchical Linear and Nonlinear Modeling. Lincolnwood, IL: Scientific Software International; 2004. [Google Scholar]
- 44.Osgood DW, Smith GL. Applying hierarchical linear modeling to extended longitudinal evaluations. Eval Rev. 1995;19:3–38. [Google Scholar]
- 45.Beidas RS, Kendall PC. Training therapists in evidence- based practice: a critical review of studies from a sys- tems-contextual perspective. Clin Psychol (New York). 2010;17:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herschell AD, Kolko DJ, Baumann BL, Davis AC. The role of therapist training in the implementation of psychosocial treatments: a review and critique with recommendations. Clin Psychol Rev 2010;30:448–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glisson C, Schoenwald SK, Kelleher K, et al. Therapist turnover and new program sustainability in mental health clinics as a function of organizational culture, climate, and service structure. Adm Policy Ment Health. 2008;35:124–133. [DOI] [PubMed] [Google Scholar]
- 48.Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population- based perspective. J Dev Behav Pediatr. 2007;28:265–273. [DOI] [PubMed] [Google Scholar]
- 49.Silverstein M, Diaz-Linhart Y, Cabral H, et al. Efficacy of a maternal depression prevention strategy in head start: a randomized clinical trial. JAMA Psychiatry. 2017;74:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epstein JN, Kelleher KJ, Baum R, et al. Impact of a webportal intervention on community ADHD care and outcomes. Pediatrics. 2016;138:e20154240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeder CS, Gordon BN, Kanoy K, Ruth DK. Managing children’s behavior problems in pediatric practice. Adv Dev Behav Pediatr. 1983;4:25–86. [Google Scholar]
- 52.Yu H, Kolko DJ, Torres E. Collaborative mental health care for pediatric behavior disorders in primary care: does it reduce mental health care costs? Fam Syst Health. 2017;35:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooley WC, McAllister JW, Sherrieb K, Kuhlthau K. Improved outcomes associated with medical home implementation in pediatric primary care. Pediatrics. 2009;124:358–364. [DOI] [PubMed] [Google Scholar]