Abstract
Regenerative medicine (RM) is an interdisciplinary field that aims to repair, replace or regenerate damaged or missing tissue or organs to function as close as possible to its physiological architecture and functions. Stem cells, which are undifferentiated cells retaining self-renewal potential, excessive proliferation and differentiation capacity into offspring or daughter cells that form different lineage cells of an organism, are considered as an important part of the RM approaches. They have been widely investigated in preclinical and clinical studies for therapeutic purposes. Extracellular vesicles (EVs) are the vital mediators that regulate the therapeutic effects of stem cells. Besides, they carry various types of cargo between cells which make them a significant contributor of intercellular communication. Given their role in physiological and pathological conditions in living cells, EVs are considered as a new therapeutic alternative solution for a variety of diseases in which there is a high unmet clinical need. This review aims to summarize and identify therapeutic potential of stem cells and EVs in diseases requiring acute emergency care such as trauma, heart diseases, stroke, acute respiratory distress syndrome and burn injury. Diseases that affect militaries or societies including acute radiation syndrome, sepsis and viral pandemics such as novel coronavirus disease 2019 are also discussed. Additionally, featuring and problematic issues that hamper clinical translation of stem cells and EVs are debated in a comparative manner with a futuristic perspective.
Graphical Abstract
Keywords: Stem cell therapy, Extracellular vesicle therapy, Acute emergency care, Trauma and critical care medicine
Introduction
Regenerative medicine (RM) is an emerging interdisciplinary field aiming to repair, replace or regenerate damaged or missing tissue or organs to function as close as possible to its physiological architecture and functions. There have been tremendous advancements in this evolving discipline in the past decades including small molecule drugs, cell and gene therapies, and tissue and organ engineering. However, the main focus of RM has been human cells particularly stem cells for years, which may be somatic, adult stem, embryo-derived or reprogrammed cells [1].
Stem cells, which are defined as undifferentiated cells retaining self-renewal potential, excessive proliferation and differentiation capacity into offspring or daughter cells that form different lineage cells of an organism, are considered among landmark steps in the evolution of cell-based RM approaches. Their distinctive characteristics make them an ideal source for replacing and/or regenerating damaged tissues [1, 2]. Briefly, they are classified according to their tissue of origin and differentiation ability. Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are considered as pluripotent, which means that they can differentiate into all cell types from ectodermal, endodermal, and mesodermal origin, whereas hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) are examples of multipotent cells that can differentiate into various cell types of a single germ layer [2].
The main goal of stem cell therapies can be summarized as the replacement of dysfunctional or dead cells and tissues with physiologically functioning cells, prevention of further damage, microenvironment modification of the tissue such as anti-inflammatory and immunosuppressive activity, and activation of self-regenerative and reparative mechanisms [2, 3]. For these reasons, they have been investigated extensively in various experimental studies and in phase-1/3 clinical trials of cancer, cardiovascular diseases, immune system disorders, neurological diseases, liver, lung, kidney, orthopedic, ocular, urological, skin diseases, etc. [3–5]. Although most preclinical studies have demonstrated encouraging results, translation of stem cell therapies into clinics and success in clinical trials have not been at the desired level yet. Except for a few well-established indications such as hematological cancers, stem cell therapies have not exactly improved patient outcomes and cured the disease. Unfortunately, evidence mostly coming from small, uncontrolled trials and a few well-controlled, randomized clinical studies have still been somewhat not fully satisfactory [6].
Extracellular vesicles (EVs) are small sized, lipid membrane enclosed, heterogenous membrane vesicles secreted from all cell types, and they comprise three subgroups according to their biogenesis namely exosomes, microvesicles and apoptotic bodies. Briefly, exosomes are 40-120 nm sized vesicles resulting from intraluminal budding of multivesicular bodies and fusion of these multivesicular bodies with cell membrane via the endolysosomal pathway. Microvesicles are 50-1000 nm sized vesicles secreted from cell surface by budding of the cell membrane. Apoptotic bodies, which are out of scope of this review, are 50-2000 nm sized vesicles and released from the cell surface through outward budding of apoptotic cell membrane [7]. There are various isolation methods for EVs with their inherent principles, advantages and disadvantages, which are reviewed in detail elsewhere [8]. In addition, lack of individual markers for EV subtypes and their overlapping characteristics regarding size, density and composition make the nomenclature problematic. In this context, the term “extracellular vesicle” is suggested for use as a generic nomenclature to describe vesicles isolated from body fluids and cell cultures by the International Society for Extracellular Vesicles [9]. However, nomenclature issue is still a matter of debate in the scientific community and exosomes, microvesicles and EVs have been frequently used interchangeably in the biomedical literature since years [10]. Therefore, we chose not to strictly distinguish them and preferred to use the definition “extracellular vesicle” in this review unless otherwise stated in the related article.
EVs carry various types of cargo between cells which make them vital mediators of intercellular communication. EV cargo encapsulated within a lipid membrane consists diverse combinations of proteins, lipids, peptides, carbohydrates and nucleic acids including DNA, mRNA, microRNA (miRNA) and long noncoding RNA, allowing transmission of biological signals between cells. They represent the native characteristics of donor cell resulting with de novo gene expression, posttranslational modification or new transcript translation in the recipient cell [11]. Not only they regulate the physiological processes in cells, they are tightly linked to various disease pathogenesis. EVs are secreted by all cell types in the organism and can be detected in all body fluids, which make them an attractive source for biomarker investigations. Because they carry cargo between cells, they can be ideal candidates to be utilized as carriers for drug delivery [7, 12]. In addition, their inherent role in pathological conditions highlight their potential role as novel targets for therapeutic interventions as cell-free therapies in various diseases [7, 11, 12].
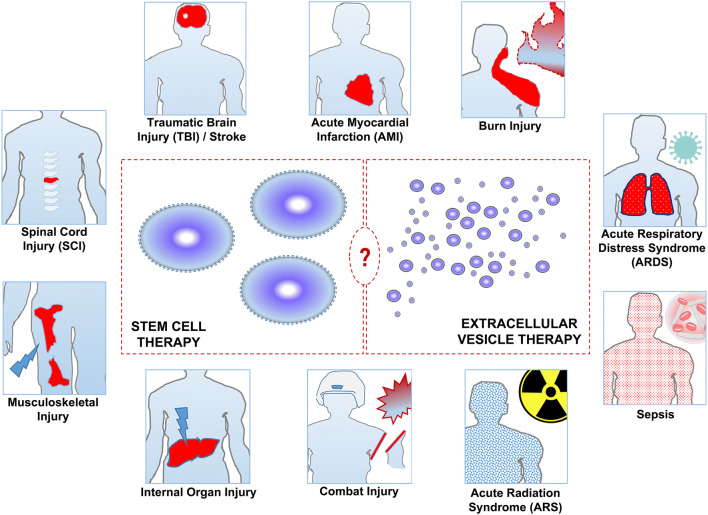
Given the prolonged human life and survival expectancy, the number of people requiring emergency care and emergency department admissions are increasing all over the world [13, 14]. These mostly include trauma patients, cardiovascular disease, neurological disease patients and patients suffering from organ failures such as acute respiratory failure or thermal burns. In addition, biological or chemical threats causing mass casualty situations such as pandemics, sepsis, and radiation related incidents are significant issues for communities, militaries and local medical systems. Although RM approaches including stem cell applications and EV based therapies are in their infancy hitherto, they promise as novel therapeutic targets from a futuristic perspective (Fig. 1). In this context, this review aims to summarize and discuss the therapeutic potential of stem cells and EVs in patients suffering from diseases requiring acute emergency care.
Fig. 1.
Main pathological conditions requiring acute emergency care that can benefit from stem cell therapies or extracellular vesicle therapies in the future
Trauma
Trauma related injury is a significant cause of mortality and disability all over the world. Despite the reductions in mortality from cancer and cardiovascular diseases in the last years, death rates from trauma have remained constant. However, there have been significant achievements in the management of trauma patients including timely prehospital care, rapid diagnostic tests, hemorrhage control, transfusion and surgical approaches. Traumatic brain injury (TBI), spinal cord injury (SCI), chest trauma, abdominal trauma and musculoskeletal injuries are among subtypes of traumatic injuries. The most common causes of death from traumatic injuries include TBI and hemorrhagic shock [15, 16]. Besides being an important civilian health issue, trauma related injuries are also a significant concern for militaries in time of war [17].
In general, trauma causes structural damage to tissues, disrupts perfusion and provokes inflammation subsequently resulting with irreversible tissue damage, loss of organ function and finally death. Host defense response, which involves local and systemic release of acute phase proteins, pro-inflammatory cytokines, metabolites and hormonal mediators, plays major role in the clinical outcomes including mortality after trauma [18]. Optimal healing in a tissue after a traumatic injury can be provided by the reversal to preinjury situation and function without scarring and is associated with the nature and degree of the traumatic injury, intrinsic biological activity and regenerative capacity of the affected tissues. Theoretically, optimal healing can be accomplished by optimizing the healing process through augmenting patient biology. Due to their abilities to modulate inflammation, cell death, vascular dysregulation and tissue damage, which also underlies the complex and heterogeneous pathogenesis of traumatic injury, stem cell therapies may hold a promise for promoting organ and tissue repair after a traumatic process including civilian as well as military populations [19–21]. For these purposes, the therapeutic potential of stem cells have been widely investigated in various preclinical models of trauma such as TBI, SCI, musculoskeletal injury, circulatory-pulmonary tissue injury, skin injury, postinjury organ failure and ocular-auditory injuries with encouraging results [19–24]. MSCs from various tissue sources and donor types have been the most investigated cell type in animal models of trauma. Their beneficial effects in trauma can be summarized as migration and integration into the site of injury and respond to immunostimulatory molecules defined as damage-associated molecular patterns. Besides, they generate an anti-inflammatory and pro-regenerative microenvironment through secreting factors that activate the growth and differentiation of adjacent cells, stimulate angiogenic activity, regulate functions of endothelial cells and fibroblasts, and inhibit fibrosis [25]. A previous systematic review and meta-analysis study investigating the efficacy of MSC transplantation in animal models of TBI suggested that MSCs might have beneficial effects on locomotor recovery [26]. Likewise, systemic and local administration of allogeneic bone marrow (BM)-MSCs promoted fracture healing in rats [27]. Percutaneous intraspinal injection of autologous neurogenically-induced BM-MSCs provided clinical benefits in paraplegic dogs lacking deep pain perception after spinal trauma in a study performed by our research team previously [28]. Our research group also demonstrated that MSC infusion is associated with improved local inflammation and histological findings in rats subjected to polytrauma model comprising bone fracture and liver trauma [29].
Clinical data of stem cell therapies in trauma patients mostly come from TBI and SCI patients. A phase-1 clinical trial investigating autologous BM-derived mononuclear cell (BM-MNC) infusion in pediatric TBI patients indicated that harvesting and infusion of stem cells is safe in children with no infusion related toxicity or death [30]. A retrospective analysis of this study with age- and severity-matched control group demonstrated that stem cell therapy is associated with lower treatment intensity required to manage TBI in children [31]. Another phase-1/2a trial conducted by the same group evaluated the safety and efficacy of autologous BM-MNC infusion in adult TBI patients and demonstrated the safety and feasibility of cell treatment. There was a potential signal of treatment effect regarding structural preservation and down-regulation of inflammatory biomarkers after cell infusion [32]. Autologous BM-MSC therapy was also proven to be safe and effective when administrated through lumbar puncture in the subacute stage of TBI [33]. There are also ongoing clinical trials evaluating the therapeutic effects of stem cells in TBI patients in which the results are highly anticipated and briefly summarized in the related papers [34, 35].
There have been numerous clinical studies testing stem cells for neuronal repair in SCI patients. Human ESC-derived oligodendrocyte progenitor cells were transplanted to SCI patients into the site of injury in a phase-1 trial sponsored by Geron Corp., but the study was halted. Although complete results have not been published, it was announced that there were no safety issues regarding cell treatment. The study has been in progress under the direction of another company [4, 36]. Besides, BM-MSCs have been investigated in many clinical trials of SCI with diverse study designs and outcomes. Although no adverse reactions or side effects were reported, favorable outcomes were limited in these studies compared to expectations. Among these studies, few of them appeared to encourage cell therapy in SCI patients. There are also ongoing clinical trials which will probably improve our knowledge about the clinical effects of stem cells in SCI treatment [37].
Increasing line of evidence suggests that beneficial effects of stem cell therapies are mediated through EVs and their miRNAs released from the transplanted cells. This evidence raises the possibility that instead of cell transplantation, EVs can be used for the treatment of several traumatic injuries as cell-free agents. Evidence demonstrating that severe traumatic injury is associated with elevation of circulating procoagulant and proinflammatory EVs underlies the significance of EVs in trauma patients [38]. However, our understanding about their therapeutic role in trauma comes from animal model studies. For example, a recent study showed that administration of exosomes derived from human BM-MSCs improve functional recovery of injured animals after TBI [39]. Likewise, exosomes from BM-MSCs improved fracture healing in a rat model of femoral nonunion through enhancing osteogenesis and angiogenesis [40]. Due to encouraging preclinical data coming from several works, they have been suggested as an alternative cell-free treatment in several subtypes of trauma including bone fractures [41] and neurotraumas [42, 43]. The mechanisms underlying the beneficial effects of EVs in trauma may be the modulation of immune system and systemic inflammatory response that occurs in the acute phase of trauma. Besides, increased angiogenesis and vascular density, prevention of cell death, transfer of their cargo between cells, stimulating endogenous reparative mechanisms might mediate their therapeutic functions [43]. However, lack of clinical data especially in polytrauma patients hampers to make a clinical judgement about possible therapeutic applications of EVs in traumatic injuries.
Heart Diseases
Coronary heart diseases including acute myocardial infarction (AMI) and the leading heart failure (HF) are the main causes of mortality all over the world and constitute a major problem of global health causing frequent acute hospital admissions and hospitalizations. Although long term survival of AMI and HF patients has improved thanks to improvements in coronary interventions, medical treatments and surgical therapies, prognosis is still poor and admission to emergency departments, hospitalization rates and economic costs are high [44, 45]. The main pathophysiological mechanism of poor outcomes in AMI and HF patients is the lack of adequate cardiomyocyte renewal capacity in the failing heart and formation of a fibrotic scar tissue in the infarcted myocardium with progressive cardiomyocyte death subsequently resulting with pump failure. Besides, none of the current treatment strategies except heart transplantation can reverse these mechanisms back and induce a true regeneration in the heart [46, 47].
In recent decades, cell-based therapies including skeletal myoblasts, BM-derived stem cells, MSCs, cardiac progenitor cells and PSCs have been investigated in order to generate new and functional myocardial tissue and/or activate endogenous repair mechanisms in the heart. After encouraging preclinical studies with small and large animal models, the regenerative potential of these cells have been tested in multiple clinical trials with miscellaneous results and heterogenous outcomes which may be attributed to diversities in study designs such as cell types, cell preparation techniques, delivery route, dose, timing of application, study endpoints and follow-up methods of patients. In brief, there have been no safety issues regarding these cell therapies, but their efficacy has mostly been neutral or at most marginally positive outcomes [46–48].
Among adult tissue sources, MSCs preferably obtained from BM or adipose tissue might be promising due to their high secretory profile and paracrine effects. In addition, cardiac cells derived from PSCs may provide superior benefits when compared to other cell types although comparative studies are needed to support this hypothesis. It should also be kept in mind that the use of PSCs are restricted due to ethical issues and/or teratoma formation risk although they have a robust differentiation potential than other cell types [48, 49]. Evidence mostly coming from large animal models also points to arrhythmogenic potential of cardiomyocytes derived from PSCs [50]. A recent phase-1 study investigated the safety of fibrin patch embedded cardiac progenitor cells derived from human ESCs in six severe HF patients. The results of the study demonstrated short- and medium-term safety and technical feasibility of the surgical procedure pioneering future efficacy studies [51].
Initially, the hypothesis that the transplanted stem cells reach their target tissue, differentiate into cardiomyocytes, engraft into the host myocardium electromechanically and improve cardiac functions was thought to be the main mechanism for therapeutic effects of stem cells. However, subsequent researches demonstrated that cardiac differentiation and engraftment of stem cells into the myocardial tissue are very rare [52, 53]. The recognition that stem cells secrete a variety of EVs that act in a paracrine manner and these EVs mediate the beneficial effects of stem cells has shifted the stem cell paradigm into a new concept defined as “paracrine hypothesis” [54]. Exosomes, specifically secreted from PSCs and MSCs, were shown to exert regenerative effects in the heart and vasculature by modulating apoptosis, inflammation, fibrosis and angiogenesis [55]. Besides, beneficial effects of these secretomes released from other stem cell populations such as cardiac and endothelial progenitor cells (EPCs) in the cardiovascular system were demonstrated. Accordingly, they have been suggested as a potential cell-free therapy to regenerate the diseased heart instead of stem cell applications [54, 55].
Cardioprotective effects of EVs and exosomes have been investigated in several preclinical models including MI [56–58] and chronic HF [59, 60] rendering the potential of these cell-free agents as therapeutic biological medications. Besides, a recent study compared the cardiac regenerative effects of intramyocardially-injected iPSCs and iPSC-derived EVs in a murine MI model and demonstrated that iPSC-derived EVs are safer than iPSC injection in regard to teratoma formation. On the other hand, iPSC-derived EVs exhibited superior cardiac repair effects than iPSCs in terms of left ventricular function, angiogenesis, reduction of apoptosis and hypertrophy [58]. An immunocompetent mouse MI model study to investigate the immunological effects of EVs obtained from human cardiac progenitor cells demonstrated that intramyocardial delivery of these EVs does not induce an allogenic immune response and likely to have a systemic anti-inflammatory effect. However, the hypothesis that whether the systemic delivery of EVs would trigger similar anti-inflammatory effects with positive outcomes on heart functions needs to be tested in future studies which might intensify and accelerate the clinical translation of therapeutic EV applications [61].
Accumulating evidence suggests that cardioprotective effects of EVs are mostly mediated through specific miRNAs [62]. They carry information and mediate the cross-talk between cells in tissue of interest such as cardiomyoctes, stem cells, endothelial cells, smooth muscle cells, fibroblasts and others to modulate cellular changes and disease phenotypes. There are numerous cardiac-related miRNAs in the heart, which are secreted from various sources of cells with varying functions such as enhanced cardiomyocyte survival and functions, attenuation of cardiac fibrosis, induction of angiogenesis, inhibition of apoptosis and oxidative stress, and regulation of sarcomeric genes and ion channel/automaticity genes [62–65].
Stroke
Stroke is a serious neurological condition with high mortality and disability rates worldwide [66]. Ischemic stroke, which occurs suddenly due to thromboembolic occlusion of a major artery that supplies blood to the brain, represents the most common type and requires restoration of blood flow immediately. Thrombolytic agents and endovascular mechanical thrombectomy are the only treatment options to achieve recanalization with their limited therapeutic time window and side effects such as risk of hemorrhage [67]. On the other hand, hemorrhagic stroke occurs secondary to rupture and bleeding of a vessel in the brain and requires immediate surgery to remove clots and blood and decrease intracranial pressure [68]. Irrespective of stroke origin, the integrity of neurovascular unit, which includes numerous cellular components and tissue-related proteins, is impaired in the early times of stroke leading to disruption of blood-brain barrier (BBB) and ischemic cell damage. From a pathophysiological perspective, a series of molecular and cellular pathways are activated during this process such as inflammation, apoptosis and oxidative stress-related pathways subsequently resulting with neuroinflammation, neurodegeneration and irreversible tissue injury. The aforementioned therapies can improve neurological functions and mortality in the acute phase of stroke but their therapeutic effects are limited in the subacute and chronic stages [69].
It is known that some stroke patients show spontaneous recovery owing to limited plasticity and remodeling capacity of the brain. Therefore, activation of intrinsic reparative processes in the brain may promise as a therapeutic strategy for stroke theoretically. There is also increasing evidence that stem cells, particularly neural stem cells located in the brain niches, contribute to remodeling and recovery processes by activating angiogenesis, neurogenesis and neuroprotection after stroke, subsequently provoking improved neurological outcomes [69, 70]. In this context, stem cell therapies with their wider therapeutic window may represent a new treatment paradigm to ameliorate the subacute and chronic phases of stroke. Beyond stem cells, exosomes are known to regulate intercellular communication between neurovascular system and other system cells and contribute to brain repair processes after stroke putting forward them as promising cell-free agents in stroke therapy [42, 70, 71].
The regenerative potential of various types of stem cells, with different sources, dosages, delivery routes, application times and end-points has been investigated in preclinical animal models and human clinical trials with the expectation that these cells would successfully engraft into the damaged brain tissue, differentiate into functional neuronal and vascular system cells and promote full recovery after stroke. A recently published systematic review of 76 studies testing stem cells in rodent ischemic stroke models and 4 randomized human clinical trials encompassing ischemic stroke patients treated with autologous stem cells with at least one year follow-up period demonstrated that stem cell therapies show beneficial effects in terms of behavior and histological outcomes in rodents. Pooled data of 4 human clinical trials failed to show functional recovery although there were some improvements in terms of neurological outcomes [72]. Likewise, translational deficiency was observed from animal models to clinical studies in a systematic review and meta-analysis study investigating the efficacy of MSCs in subacute or chronic ischemic stroke [73].
Therapeutic applications of EVs for stroke may confer some pros when compared to stem cell applications, which are discussed in detail in the subsequent sections of this paper and related review articles. Emerging evidence indicates that the neurorestorative effects of stem cells are mediated through release of exosomes and their miRNA cargo instead of integration into neural networks [42, 70, 71]. Besides, exosomes mediate dynamic cross-talk between neural system cells and endothelial cells [42]. Comparative studies of stem cells and EVs in stroke treatment mostly come from animal models. A previously published study demonstrated that MSC-derived EVs have similar functional tissue regeneration capacity as MSCs in mice subjected to stroke [74]. Another rat stroke model study indicated that MSC-derived EVs possess better rehabilitation effects than MSC treatment in stroke repair which might be due to higher BBB permeability of EVs compared to MSCs [75]. In a recent study, systemic application of MSC-derived exosomes loaded with miRNA-124 was shown to stimulate cortical neural progenitors to obtain neuronal identity and ameliorate ischemic injury by cortical neurogenesis in mice subjected to stroke suggesting a great potential for clinical translation [76]. Accordingly, a phase 1-2 clinical trial (NCT03384433) aims to investigate the safety and efficacy of allogenic BM-MSC-derived exosomes genetically enriched with miRNA-124 in ischemic stroke patients. The primary endpoint is safety including treatment-related adverse events in 12 months such as deteriorating or recurrent stroke, brain edema, seizures and hemorrhagic transformation. The secondary endpoint is efficacy measured by the improvement in the modified Rankin Score at 12 months. The results of the study have not been posted yet and are awaited.
Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS), in other words acute respiratory failure, is a life-threatening acute lung injury characterized by diffuse alveolar damage and hypoxemia in the lungs with high morbidity and mortality rates [77]. Among several inciting events such as toxic substance inhalation, trauma, burns and pneumonia, sepsis is the most common cause of ARDS. A complex interaction between the immune system and the alveolar-capillary barrier, and widespread uncontrolled inflammation in the lungs underlies the pathophysiology of the disease. Treatment of ARDS patients relies on supportive strategies such as mechanical ventilation, fluid management, neuromuscular blockade and prone positioning, because there is no specific pharmacological therapy proven to be effective and reduce mortality in this patient group [78, 79]. In fact, numerous pharmacological agents such as surfactant, nitric oxide, corticosteroids, antifungals, phosphodiesterase inhibitors, antioxidants and immune modulating agents have been attempted in acute lung injury and/or ARDS with unfavorable outcomes and no effect in mortality [80–82]. Therefore, novel therapies for treating or preventing ARDS are highly needed.
Stem cells yield substantial promise as a novel treatment strategy for ARDS patients due to their differentiation abilities to various cells, immune system modulation and anti-inflammatory characteristics [83]. For this purpose, various type of stem cells such as MSCs, EPCs, ESCs, iPSCs have been investigated in preclinical models with encouraging results. Among all cell types, MSCs obtained from different sources mostly BM and umbilical cord (UC) have attracted much more attention than other cell types. MSCs have been shown to modulate anti-inflammatory and antiapoptotic pathways, ameliorate epithelial and endothelial cell recovery, and increase microbial and alveolar fluid clearance in several animal models of ARDS. These beneficial effects of MSCs resulting with improved lung and distal organ functions and survival have moved MSC therapies to a clinical stage [79, 83, 84].
A previous case report of an ARDS patient demonstrated short term beneficial effects of UC-MSC treatment although the patient could not survive in the long-term period [85]. Likewise, allogeneic BM-MSCs were demonstrated to improve hemodynamic status and multiorgan failure of two severe ARDS patients who did not respond to treatments such as mechanic ventilation and extracorporeal membrane oxygenation. Besides, clinical improvement of these patients was shown to be mediated through reduction of several pulmonary and systemic inflammatory markers [86]. A previous phase-1 trial of allogeneic adipose tissue-derived MSC treatment in ARDS patients demonstrated safety of systemic infusion with limited efficacy [87]. According to the results of another phase-1 dose escalation trial, allogeneic BM-MSC infusion was well-tolerated and there were no infusion related adverse events in moderate to severe ARDS patients [88]. Subsequently performed phase-2a trial by the same group with a single intravenous infusion dose (10x106 cells/kg) of allogeneic BM-MSCs demonstrated safety of the treatment. When it comes to efficacy, there were no differences between the placebo and cell-treated groups in terms of clinical outcomes. However, there was a tendency for improvement in oxygenation index in cell-treated group. According to biomarker measurements, endothelial injury was significantly improved in cell-treated patients, and MSC viability after thawing emerged as a potentially important factor for the efficacy of cell treatment [89].
Although stem cells promise an emerging role for ARDS treatment, it is known that secretion of soluble mediators acting in a paracrine manner such as EVs, which are also highly abundant in the conditioned medium of stem cells, mediate the therapeutic effects of these cells. Besides, EVs secreted from various type of cells are closely linked with the pathogenesis of ARDS. Therefore, in recent years there has been a growing interest in exploring the effects of EVs specifically derived from MSCs in ARDS treatment. The biological rationale for the therapeutic use of these cell-free agents is comparable to stem cells including immune modulation and anti-inflammatory effects, promoting alveolar epithelium and endothelium repair, alveolar fluid clearance improvement, antimicrobial effects and inhibition of lung fibrosis [90, 91]. Preclinical studies investigating EVs in ARDS as a therapeutic approach are in their infancy yet but several encouraging reports indicate that EVs derived from stem cells confer similar beneficial effects when compared to administration of stem cells themselves [90–92].
Management and treatment of ARDS has attracted much more interest than previous times because of the pandemic of novel coronavirus disease 2019 (COVID-19) pneumonia, which has spread worldwide in a very short time. Clinical course of this viral infection may progress to ARDS and death, and there is no effective pharmacological therapy or vaccine yet. Increased inflammatory situation with cytokine activation named as cytokine storm underlies the pathogenetic mechanism of infection. Due to close intersection between the disease pathogenesis and mechanism of action of stem cells regarding immune modulation and anti-inflammatory activity, stem cells and/or their secretomes have been considered as a possible therapeutic agent for COVID-19 pneumonia [93, 94]. A recent case study of a severe COVID-19 pneumonia patient reported improved clinical course and inflammatory biomarker levels after human UC-MSC infusion [95]. Likewise, clinical grade MSC infusion was reported to be safe and effective in a pilot study including seven COVID-19 pneumonia patients. Laboratory tests of these patients demonstrated improved inflammatory status and according to in-vitro tests infused MSCs were not infected with the virus [96]. In addition to these reports, there are several registered clinical studies to test stem cells, specifically MSCs obtained from diverse sources, in COVID-19 pneumonia patients. Among these recorded studies, two of them (ChiCTR2000030484 and NCT04276987) aim to investigate therapeutic potential of exosomes [97, 98]. The results of these studies are highly anticipated.
In parallel with ARDS, treatment of sepsis and septic shock is an unmet medical need. Despite intensive efforts to decrease morbidity and mortality associated with sepsis, there is no specific therapy yet and management includes symptomatic approaches. In addition, it is the most common cause of ARDS. Altered immune homeostasis with a hyper-inflammatory response and subsequent immune suppression are the main pathophysiological processes during the initiation and progression of the disease [99]. MSCs have been supposed to have beneficial effects in sepsis through different ways depending on the origin such as bacterial or viral sepsis. Their immune and inflammation modulating capacity, antibacterial actions and organ protective effects, which have been demonstrated in various animal models of sepsis with varying outcomes, yield MSC therapies as an attractive cure for sepsis and septic shock [100]. A recent pilot clinical trial showed that single dose MSC administration was safe and well-tolerated in neutropenic patients with septic shock. Although MSC therapy was associated with improved outcomes such as faster hemodynamic stabilization, vasodepressor withdrawal, improvement in respiratory failure and decrease in neutropenic period, it did not prevent death from the sepsis related organ failure [101]. However, a meta-analysis study evaluating the efficacy of MSC therapies in animal models of sepsis demonstrated lower mortality rates underlining the potential therapeutic effects of MSCs in sepsis and the need for future studies [102]. On the other hand, EVs as cell-free agents and/or drug carriers may have therapeutic functions in sepsis. Animal model studies are promising but the need for further preclinical and clinical data comes to the fore [103].
Acute Radiation Syndrome
Radiation-related nuclear accidents or weapons have been a growing concern worldwide since the past century due to the fact that they are associated with high morbidity and mortality rates in affected societies [104]. Acute radiation syndrome (ARS) refers to a wide spectrum of clinical conditions occurring in stages during hours to weeks after a large portion of the body is exposed to a high dosage of ionizing radiation thereby altering organ and tissue functions at varying levels. Although BM toxicity and myelosuppression have been considered as the major causes of morbidity and mortality, ARS is also accompanied by gastrointestinal failure, neurological damage and multiorgan dysfunction. From a pathophysiological point of view, acute ionizing radiation exposure disrupts physiological recovery of cellular systems through the depletion of radiosensitive stem cells and genotoxic damage [105, 106]. It is also a challenging issue for health care providers to identify and appropriately treat highly exposed victims in a real-life situation where a large number of community are exposed to high doses of radiation and exceed the limited capacity of local medical systems [105]. Management of ARS generally includes prophylaxis and therapy of infections, detoxification, parenteral nutrition, topical and surgical therapies for damaged skin, correction of secondary toxic metabolic disturbances, and BM transplantation including growth factors and HSC transplantation in selected patients [107]. Given that exposure to high doses can be fatal in hours to weeks, palliative care may also be required. Prognosis mostly depends on recovery of the BM and cell transplantation should only be considered if growth factors fail to reconstitute the hematopoietic system [106].
Beyond the preclinical animal model studies demonstrating favorable effects of stem cell therapies in ARS [108–110], our understanding about the clinical outcome of stem cell transplantation in ARS patients mostly comes from previous tragic nuclear accidents and natural disasters. Clinical data of these victims concluded that the survival benefit of cell transplantation is limited probably due to late administration time, graft rejection, accompanying organ failures and inhomogeneous radiation exposures [111, 112]. Therefore, HSC transplantation is only recommended if severe aplasia persists despite cytokine treatment in ARS patients [106, 113]. Despite these limitations, stem cells have an immense potential to combat the early and late complications of radiation exposure. Besides taking a role in hematopoietic system reconstitution, they can also impact the recovery of neurological, pulmonary, gastrointestinal system organs and cutaneous wounds, which are highly affected after radiation exposure in acute and long term. MSCs isolated from the BM and UC have been at the forefront of investigations [111, 112]. Local autologous BM-MSC administration was associated with a favorable clinical outcome and no recurrence of radiation inflammatory waves during eight month follow-up of a patient suffering from ARS with very severe radiation burns [114]. Currently, there is not any stem cell product approved by the regulatory authorities for radiation injuries to use in the context of a mass casualty incident. However, intramuscular injection of human placenta-derived PLX-R18 cells developed by Pluristem Therapeutics, Inc. was associated with improved survival in mice after radiation injury suggesting that PLX-R18 cells may be beneficial for ARS treatment [115]. Similarly, CLT-008 cells derived from myeloid progenitors and developed by Cellerant Therapeutics, Inc. improved survival in irradiated mice suffering from hematopoietic and gastrointestinal subsyndromes of ARS even cell treatment initiated days after irradiation [116]. These two “off-the-shelf” products developed by different companies appear to be promising for future clinical applications of ARS after a mass casualty radiological or nuclear incident.
Our knowledge about the therapeutic role of EVs in ARS is limited yet and entirely origins from preclinical studies. However, there is a growing body of evidence that these cell-free agents play role in radiation-induced genomic instability and bystander effects suggesting a possible pathogenetic effect of these molecules in radiation injury [117–119]. Exosomes derived from BM-MSCs and endometrial regenerative cells were demonstrated to increase proliferation of thymidine-incorporated HSCs in a previous experimental work [120]. Human neural stem cell-derived microvesicles were able to reverse or prevent radiation-induced cognitive dysfunction in brain-irradiated mice suggesting a possible therapeutic effect of microvesicles for radiation-induced injury in the brain [121]. Culturing macrophages with exosomes derived from lipopolysaccharide-primed MSCs was shown to improve survival through hematopoietic system recovery in a mouse model of ARS [122]. In the light of these data, it might be reasonable to suggest that EVs may be an attractive approach in ARS treatment with the help of future studies as discussed in a workshop held in France in July 2015 cosponsored by National Institute of Allergy and Infectious Diseases and Institut de Radioprotection et de Sûreté Nucléaire [111].
Burn Injury
Burns exert significant consequences in the community including death, disability, economic and social loss. A significant portion of burn cases occur in low-to middle-income countries. There are various types of burns according to the origin, but thermal burns are the most common type of burn injuries [123]. In addition, thermal burns are a significant cause of morbidity during war time accounting approximately 5% to 20% of conventional war casualties [124]. Appropriate wound healing of the damaged skin constitutes an important part of the healing process in acute full-thickness burns. Skin grafts and skin substitute products containing somatic cells are widely used for this purpose with their inherent limitations such as lack of enough skin to cover the burns and/or effectiveness. Considering the challenges of available therapies and systemic consequences of burns, it is evident that RM approaches to treat acute burn injuries are essential [20].
Cutaneous tissue maintains robust regeneration capacity and various stem cell types such as interfollicular epidermal stem cells, hair follicle stem cells, sebaceous gland stem cells, melanocyte stem cells and neural progenitor cells that reside in different compartments of the skin [125]. Skin wound healing includes coordinated complex series of overlapping events occurring in four phases namely hemostasis, inflammation, proliferation and remodeling. Stem cells of the skin and other organs play role at all these stages in a coordinated fashion to regenerate the skin. However, several factors influence this process such as the degree and size of burn, patient related conditions such as age, immune status and comorbid situations, and materials covering the burn wounds [125, 126]. In this context, autologous or allogenic stem cell therapies might emerge as a promising and effective treatment strategy both for wound healing and systemic effects of the burns such as inflammation, hypermetabolism and immune suppression. Various stem cell types from different sources, especially MSCs have been investigated for this purpose [127]. A phase-1 clinical trial (NCT02104713) collaborated by United States Department of Defense aims to investigate the safety of allogenic MSC application to second degree burn wounds. In addition, the study aims to investigate maximum safe dose that will be used in phase-2 investigations. The estimated study completion date is February 2020 and the results have not been posted yet. Another phase 1-2 clinical trial (NCT01443689) aimed to investigate the safety and efficacy of allogeneic human UC-MSCs and human cord blood MNCs in patients with acute moderate-severe full thickness burn but the results have not been published although the estimated study completion date was July 2013. In addition to these studies, there are several clinical trials evaluating therapeutic effects of stem cells in cutaneous burn injury patients recorded in the National Institute of Health clinical trials website with various study protocols, cell types and outcomes, in which the results are highly anticipated.
EVs and their related miRNAs are known to modulate therapeutic effects of stem cells. Therefore, they have been suggested as alternative cell-free agents for the treatment of burn injuries. Similar to stem cells, various EVs have been demonstrated to play role in the pathophysiological processes of burn wound healing at all stages. Their therapeutic role has also been tested successfully in various animal model studies, being MSC-derived exosomes or EVs the prevailing source of origin [128, 129]. A phase-1 clinical trial (NCT02565264) aiming to investigate the beneficial effect of plasma-derived exosomes in cutaneous wound patients with ulcer is currently in enrollment status. Although the study protocol does not specifically include acute burn injury patients, the results of this study may shed light on our knowledge about possible therapeutic potential of EVs in cutaneous burn injury patients.
Outstanding Issues for Clinical Applications
RM holds an immense potential for a variety of diseases in which there is a high unmet clinical need. Despite the relatively slow rate of translational success from laboratory to clinics, expectations, optimism and excitement surrounding this field remain great. RM involves cell and gene therapies, and tissue engineering applications, however stem cells have been at the center of interest for years because of their biological potential [1, 3, 130]. On the other hand, secretomes of cells namely EVs have attracted great attention as therapeutics in recent years and have been suggested as alternative to stem cell therapies as cell-free agents [7, 12]. There are many challenges that need to be addressed in order to improve translation of stem cell therapies and EVs into clinical practice with sustainable and clinically significant benefits. However, it is obvious that both stem cell therapies and EV therapies are in their infancy ages with their inherent and/or similar pros and cons which are briefly summarized in Table 1.
Table1.
Advantages and disadvantages of stem cell and extracellular vesicle therapies
| Advantages | Disadvantages | |
|---|---|---|
| Stem cells |
-Living cells -Potency to differentiate and/or regenerate into the tissue of interest. -Ability to be reprogrammed into pluripotent cells -Well-defined isolation, characterization and expansion procedures -Potential for bioengineering and/or conditioning -Fabrication as “off-the-shelf” products in large quantities -Availability of clinical trials -Approved by federal agencies for the treatment of certain diseases |
-Risk of malign transformation -Trap in lungs and elimination from the vasculature -Risk for vascular obstruction -Minimal homing, migration and differentiation capacity -Risk for immune rejection -Insufficient therapeutic effects in clinical trials -Lack of optimal dosage, route of administration, and timing -Necessity for fabrication under GMP conditions -Risk for altered viability during cryopreservation |
| Extracellular vesicles |
-Cell-free agents -Minimal risk of malign transformation -Minimal risk of trap in lungs -Ability to pass blood-brain barrier -Minimal risk of vascular obstruction -Non-immunogenic profile -Secreted by all cell types -Detected in all body fluids -Availability as biomarkers -Ideal candidates for drug delivery -Potential for bioengineering and/or conditioning -Fabrication as “off-the-shelf” products in large quantities -Presence of databases to provide information about their composition and functions -Demonstrated efficacy in case studies of certain diseases |
-Inability to differentiate into any cell -Lack of understanding of mechanism of action -Systemic and diverse effects of miRNAs -Lack of standardization regarding nomenclature -Risk of tumor growth, autoimmunity, neurodegenerative diseases, prion diseases or viral infections -Lack of standardization for fabrication procedures -Very short half-life in the blood after application -Lack of well-designed clinical trials -Lack of optimal dosage, route of administration, and timing -Necessity for fabrication under GMP conditions |
Autologous versus Allogeneic Source
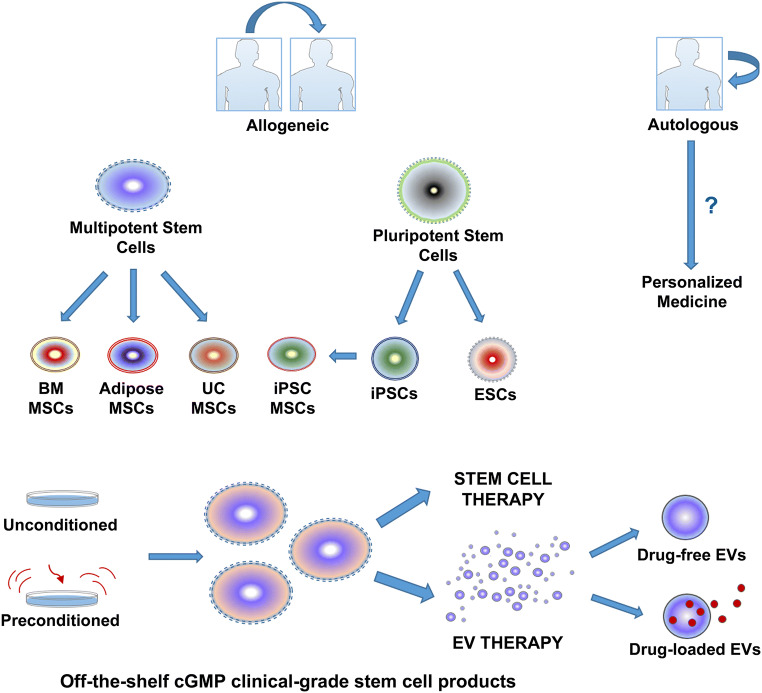
Bioprocessing of stem cells for transplantation relies on successful isolation from the donors and this can be achieved by either an autologous or allogeneic source. In autologous transplantation, stem cells are obtained from the patients’ own tissue which does not carry a risk for immune rejection but need sufficient time in order to obtain high quality cells with adequate numbers. In addition, isolation procedure involves an invasive and painful procedure and obtaining cells with high quality may be problematic because of impaired stem cell functions in patients with comorbid diseases and genetic alterations. This limitation might be specifically evident in aged and frail patients. On the other hand, allogeneic stem cells are isolated from healthy individuals and expanded in large numbers and efficient quality. They can be stored and administered as “off-the-shelf” products, which make them an attractive option for acute emergency conditions and mass casualty settings and enable diverse and multiple dosing strategies (Fig. 2). However, allogeneic cell transplantation requires immunological matching between host and donor [131].
Fig. 2.
Scheme demonstrating the major options and sources of stem cells and extracellular vesicle products for potential therapeutics.
It is reasonable to question which stem cell source is superior to another. For example, comparison of autologous and allogeneic stem cells in large animal models of ischemic heart disease showed similar effect size [132]. Likewise, allogeneic and autologous BM-MSCs compared in randomized clinical trials demonstrated no significant difference in terms of safety and improvement in left ventricular ejection fraction in ischemic cardiomyopathy and nonischemic HF patients [133, 134]. A preclinical meta-analysis study of stem cells for experimental stroke showed that autologous cells were better than allogeneic cells to ameliorate infarct volume, whereas allogeneic cells were better for improving functional outcomes [135]. According to another meta-analysis study investigating MSCs in locomotor recovery of rat models of SCI, cell source was a significant predictor of improved outcome as autologous and allogeneic cells performed better than xenogeneic and syngeneic cell sources [136].
Such comparative studies have not been performed with regards to EV therapies yet. Although first clinical studies tested autologous dendritic cell-derived exosomes in cancer patients [137, 138], the field has shifted to allogeneic sources and most companies are developing allogeneic products because of difficulties of harvesting autologous EVs in sufficient quantities [11, 139]. In this context, a previous clinical case of a Graft-versus-host disease patient refractory to steroid therapy indicated promising outcomes after allogeneic MSC-derived EV administration [140].
Stem Cell Type
Stem cells are a group of heterogeneous cells that have the ability to self-renew and differentiate into mature cells through asymmetric cell division [3]. Among all stem cell types, MSCs are numerically the most used cell type in preclinical and clinical settings. They are considered as the ideal cell type for transplantation. This may be due to their pleiotropic properties such as antiapoptotic, antifibrotic, anti-inflammatory and angiogenic activities, growth factor production, and immune system modulation in addition to their differentiation capacity to various cells of lineages. Their immune privileged abilities also render MSCs an ideal source for allogeneic applications [4, 5].
MSCs can be obtained from various sources of tissues in the body including but not limited to BM, adipose tissue and UC. When compared among themselves, adipose tissue-derived MSCs seem to be ahead because of higher number of cells per gram of tissue, higher cell proliferation rate compared to others and technical convenience to obtain cells [141]. In the light of these advantages, it is reasonable to suggest that adipose MSCs may be an ideal source for acute emergency conditions as well as autologous applications. However, tissue source of MSCs may be a challenging task for translational activity. For example, a recent study demonstrated that MSCs obtained from adipose tissue are more efficient than those obtained from UC and endometrial tissue in terms of in-vitro and in-vivo angiogenic activity [142]. Likewise, neuro-regenerative potential of MSCs derived from human BM, adipose tissue and Wharton’s jelly was shown to be non-equal in a recent study. Although BM-MSCs were inefficient than other cell types in terms of neurotrophic growth factor secretion and gene expression, secretome of all cell lines had pronounced neurotrophic potential regarding composition and in-vitro paracrine activity [143].
PSCs, namely ESCs and iPSCs, hold the potential to differentiate into all cells in the organism and are considered as the future of cell transplantation. However, they possess two problematic issues which slow down their clinical translation. Despite the fact that ESCs are obtained from an embryo, there remain ethical problems and restrictions with their use. In fact, iPSCs were generated to overcome ethical issues surrounding ESCs but both of these cell types also carry a risk for teratoma formation which limit their translation into clinics [1–3]. Preclinical data suggest that MSCs derived from iPSCs have superior effects than BM-MSCs to attenuate lung disease in rats [144].
The first human clinical trial using iPSCs focused on macular degeneration, but the trial was suspended because of legal issues after the first patient was treated with autologous iPSC-derived retinal pigment epithelium cell sheet. In addition, genetic alterations were detected in the iPSCs of the second patient and transplantation was canceled. There was no safety concern including tumor formation and immune rejection at 25 months of follow-up of the treated patient [145]. However, the study group revised the study protocol and announced that they implanted iPSC-derived retinal pigment epithelial cells obtained from an allogeneic donor to a man first time [146].
When it comes to acute emergency situations such as critical organ injuries including MI, stroke, trauma, or mass casualty settings including radiation injury, burns, pandemics and combat injuries, iPSC biobanking can be an alternative solution to obtain sufficient numbers of cells in short times. Biobanking of allogeneic stem cells may also help to reduce costs, by eliminating the need to prepare limited doses of cells for each patient. However, immunogenicity still remains as a drawback tried to be solved through donors who are homozygous at human leukocyte antigen alleles namely “super donors”. Fortunately, encouraging efforts have come from the United States, European Union, and Japan in recent years in order to expedite approval processes for translation of stem cell therapies into the clinics including PSCs [147, 148].
Dosage, Route of Administration and Timing
Significant limitations exist while considering the optimal dose, transplantation route and timing of administration for stem cell therapies and EV-based therapeutics. For example, a dose of four million cells is considered as effective for intravenous transplantation in a stroke rat weighing 250 g, which corresponds to 840 million cells in a 75 kg man suffering from stroke. If cells are transplanted through stereotaxic route, 200k cells in a 250 g rat is considered as effective dose, which is equivalent to 56 million cells in a 75 kg stroke patient [149]. However, most of the clinical trials investigating stem cells in stroke patients use cells below these doses and vary among each other [150]. It should also be kept in mind that higher number of cell transplantation does not mean more improved outcomes. For instance, the lowest number of transendocardially injected MSCs were associated with the greatest improvements in left ventricular volume and ejection fraction in a dose escalation study of ischemic cardiomyopathy patients [133].
There are a wide range of transplantation routes for stem cells and EVs varying according to disease characteristics but the optimal route for a specific disease is not fully known yet. According to a previous rat study of ventilator-induced lung injury, intratracheal MSC application was found to be as effective as intravenous MSC therapy [151]. On the other hand, MSC transplantation through intravenous route was found to be more effective compared to intraperitoneal route for improving liver injury in a rat polytrauma model [29]. Although intravenous injection is the most common way of transplantation, it is evident that stem cells are trapped in lungs and eliminated from circulation, which is named as “pulmonary first-pass effect”. In a previous rat TBI model study, a significant proportion of MSCs were localized into the lungs at 48 hours of intravenous infusion and less than 4% of the cells reached the arterial system. Moreover, this study demonstrated that very few proportion of cells (0.0005%) reached into the cerebral tissue [152]. However, this problem might be achieved through multiple infusions instead of single bolus administration [5, 153].
Despite the fact that EVs can easily cross the BBB and they do not carry a risk for vascular obstruction, they promise for systemic applications in neurological diseases such as stroke and TBI compared to stem cells [42, 70, 71, 75]. A recent study found that biodistribution of EVs can be affected by varying dosages, routes of injection, and cellular origin of EVs [154]. Organ specific applications such as intracoronary route for cardiac diseases or intratracheal and inhalation route for lung diseases may be an alternative solution regarding the limitations of systemic infusions. In addition, topically applied allogeneic MSC-derived EVs are being planned to be tested in a phase-1/2a trial of a rare genetic skin disorder (NCT04173650) [139]. Combination of stem cells and EVs with tissue engineering modalities such as scaffold-based technologies, cell sheets and injectable biomaterials might be promising strategies for local or percutaneous applications specifically in cardiac diseases [155, 156].
Optimal time window for application is not well-defined, however it may depend on disease characteristics and availability of donors. For example, delivery of stem cells at early times may be beneficial to decrease proinflammatory processes which occur at the first days of TBI [35]. Likewise, a clinical study testing allogeneic multipotent adult progenitor cells in acute stroke patients showed that patients treated before 36 hours were more likely to benefit from cell therapy [157]. One potential limitation of early treatment is the harsh microenvironment at the injury region surrounded by toxic and inflammatory cells that may attenuate the therapeutic effects of cells. In addition, if cell treatment is planned at early times as in such diseases exemplified in this paper, use of autologous sources may not be possible because of time needed for harvesting procedure [20, 35].
Methodological and Interpretative Issues
Beside the invaluable contribution of animal model studies to understand disease pathogenesis, and stem cell and EV biology, an important aspect is the ability to test the efficacy of the products before moving to clinical trials. However, it is evident that translational success obtained from clinical trials has been modest despite the encouraging data coming from animal models [158]. Several reasons exist for this discrepancy mostly regarding methodological and interpretative issues observed in preclinical and clinical studies. Lack of animal models that exactly match phenotypically and physiologically with the related human disease with similar organ size is a significant limitation. It is known that cellular functions of stem cells such as homing and niche activities, intercellular contact, cytokine and growth factor secretion may differ among various species [158, 159]. Likewise, biological functions of EVs obtained from MSCs of different species such as human and murine may vary [160].
Publication bias is a significant problem for both preclinical and clinical studies, which may result with inaccurate interpretation of efficacy and decelerate the translational process [161, 162]. The most relevant example of this effect comes from animal stroke studies leading to overstatement of efficacy [161]. In fact, registration to public databases such as ClinicalTrials.gov are encouraged to reduce bias in clinical studies in line with the Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects, but publication and reporting of results are not at desired levels [162, 163]. Similarly, there are now public based platforms for preclinical studies including PreclinicalTrials and Animal Study Registry in order to reduce bias. However, it should be noted that neutral or negative results do not impress researchers, sponsors, authors, peer reviewers and editors, and such papers are most likely to be rejected by the journals [163].
Diversities in study protocols, outcomes, patient populations and interpretation may be another significant hurdle of clinical translational in RM therapies. For instance, according to an analysis of autologous BM-derived stem cell clinical studies for ischemic heart disease, a significant proportion of studies contain factual discrepancies regarding the enhancement in ejection fraction [164]. Fortunately, strategies for standardization of clinical trials of stem cells and EVs are being developed [165, 166]. Such efforts are also being performed to standardize design, organization and reporting of in-vitro research and animal model studies [167, 168].
Strategies to Improve Efficacy
Stem cells tend to lose their biological functions after isolation and long term culture in-vitro. Furthermore, when they are injected into the body, they challenge with a harsh microenvironment accompanying death signals because of the inadequate interaction between the cells and surrounding extracellular matrix (ECM). Therefore, various strategies have been developed in order to enhance survival, engraftment rate, immunosuppressive, immunomodulatory and regenerative functions of stem cells, and improve their efficacy. Preconditioning with physical, chemical and biological factors, genetic modification, and optimization of culture conditions are the prevailing strategies [169]. Combinatory approaches using tissue engineering and biotechnology applications might also enhance the therapeutic activity of stem cells. For example, in a study performed by our research group, encapsulation of BM-MSCs in platelet rich plasma-derived fibrin microbeads yielded accelerated regeneration period with better myofiber orientation in volumetric muscle loss injury of rats [170]. Furthermore, MSCs seeded on decellularized bovine small intestinal submucosa contributed healing process of a critical-sized full-thickness skin defect in rodents [171].
Similar approaches can also have beneficial effects on EVs such as increased production in numbers or improved functions [172]. For instance, a TBI rat model demonstrated that cultivating human MSCs in three-dimensional collagen scaffolds yielded enhanced exosome number and therapeutic outcomes compared to exosomes derived from MSCs cultured in two-dimensional conventional conditions [39]. Besides, overexpression of specific miRNAs and transcription factors, or gene editing may be a feasible approach to improve beneficial effects of EVs. MSCs overexpressing particular miRNAs were shown to have neuroprotective and cardioprotective effects in animal models [172]. Indeed, cultivation of macrophages with exosomes obtained from lipopolysaccharide-treated MSCs increased secretion of cytokines and expression of growth factors. These macrophages improved survival in mouse with ARS [122].
Genetically modified cell lines such as NT2N, CTX0E03 and SB623 have been widely investigated in preclinical and clinical studies of stroke. There were no safety concerns in clinical trials of these cell lines but genetic stability including ectopic tissue formation and tumorigenesis are potential caveats before moving into clinics [69]. On the other hand, genetically engineered EVs in contrast to naive EVs may allow for production of more potent and disease-specific subset of EVs with specific therapeutic effects. These engineered EVs may be translated into novel strategies for effective use in clinics and may be the treatment choice of future [91]. However, genetic modification of EVs by using viral capsids may provoke an adverse immunological reaction, which can be avoided by using chemically synthesized peptides [42]. For example, modification of MSC-derived exosome surface by a functional chemically synthesized cyclopeptide provided more efficiently targeting of ischemic region compared to unmodified exosomes in a mouse model of cerebral ischemia [173]. Furthermore, genetically modified allogeneic MSC-derived exosomes enriched with miRNA-124 are being tested in acute ischemic stroke patients in a phase-1/2 clinical study (NCT03384433).
Extracellular Vesicles and Looking Forward
The recognition that homing and migratory effects of stem cells are limited, and paracrine mediators secreted from cells constitute their major regenerative functions has highlighted a new perspective into RM therapeutics. Subsequently, there has been a shift of interest from cell transplantation to conditioned medium and/or EV-based therapeutics. Conditioned medium from BM-MSCs overexpressing Akt mediated cardioprotective effects both in-vitro and in-vivo [174]. Multidimensional protein identification technology and cytokine antibody array analysis of the conditioned medium of MSCs derived from ESCs demonstrated various gene products associated with cardiovascular biology, bone development and hematopoiesis suggesting a therapeutic potency of conditioned medium without cell transplantation [175]. Moreover, microvesicles derived from human MSCs were as effective as their parent stem cells in mice suffering from severe bacterial pneumonia [176].
Cell-free therapies may have several advantages in contrast to cell applications. There is a thrombogenic potential of stem cells in the vasculature as well as arrythmia in the heart, ossification and calcification. In addition, cryopreservation may decrease viability of stem cells. On the other hand, cell-free applications do not require robust and expensive strategies for isolation and expansion, which make them an attractive source in acute conditions or military applications. In general, there are two types of cell-free products namely conditioned medium concentrates, which also contain EVs, and EVs free from soluble factors such as growth factors [177]. At this point, one can ask whether conditioned medium or EVs have more therapeutic potential. Although it is difficult to give a definite answer, a previous study of hyperoxia-induced bronchopulmonary dysplasia demonstrated that EV deficient conditioned medium has no therapeutic effect, whereas both EVs and conditioned medium containing EVs improved lung inflammation and morphologic alterations [178]. In addition, utilization of EVs as biomarkers and their abilities as drug delivery systems put EVs a step forward in RM [7, 12].
When compared to stem cells, EV transplantation seem to be less risky than live stem cells. They cannot replicate and the risk of transformation into malign cells is less. Due to the fact that they are smaller in size, the risk of elimination in the vasculature is less and they can easily pass the BBB, a property which makes them an ideal source for drug carrying and/or transplantation in neurological diseases. Besides, they do not evoke an immune response after transplantation, so there is no need for immunosuppression [7, 12, 42]. They can also display systemic beneficial effects even in local applications as demonstrated in a recent mouse MI model study [61]. Although they have similar functional effects, their content such as mRNA, miRNA and proteins may vary compared to their parent cells. For example, comparative analyses of adipose tissue-derived MSCs and their EVs demonstrated diverse genetic cargo including mRNA and miRNA, and protein contents that play role in angiogenesis, adipogenesis, apoptosis, regulation of inflammation, blood coagulation and ECM remodeling [179, 180]. Moreover, protein levels and surface markers also differ between EVs and their parent cells [181, 182]. A rat MI model study showed superior beneficial effects of MSC-derived exosomes in contrast to MSCs in cardiac repair. This superiority was attributed to differences in expression profiles of several miRNAs from that of MSCs detected through miRNA sequence analysis, raising the possibility that EVs can be used alone and are superior to MSCs in promoting cardiac repair [183]. A recent cutaneous wound model study reported that intradermal injection of EVs derived from adipose and BM-MSCs were superior to stem cell injection in-vivo. Furthermore, adipose MSC-derived EVs enhanced wound closure better than their BM-derived counterparts suggesting diverse therapeutic effects of EVs obtained from different sources in an organism [184]. At this point, it is reasonable to speculate that one size does not fit all in EV-based therapies.
Among diverse cell sources, EVs derived from MSCs are the most investigated type of EVs compared to other cell sources such as PSCs. For example, comparison of exosomes obtained from iPSC-derived MSCs and synovial membrane-derived MSCs yielded greater therapeutic effect of iPSC-derived MSC exosomes in an osteoarthritis model [185]. However, EVs derived from PSCs may be tumorigenic despite their high regenerative capacity because they carry the characteristics of their parent cells. On the other hand, superior safety profile regarding teratoma formation and therapeutic effect of iPSC-derived EVs in contrast to iPSCs were shown in terms of cardiac repair [58]. Given their inherent role in pathological processes and systemic effects, EV applications can induce spreading of tumor growth, autoimmunity, neurodegenerative diseases, prion diseases or viral infections because they are able to transfer their contents to recipient cells. Exosomes derived from MSCs of multiple myeloma patients had decreased tumor suppressor miRNA-15a expression levels compared to normal MSC-derived exosomes. Besides, exosomes derived from multiple myeloma patients expressed higher levels of oncogenic proteins, cytokines, and adhesion molecules, and promoted tumor growth, whereas exosomes of normal MSCs inhibited the growth of multiple myeloma cells [186]. Actually, it should be kept in mind that this feature of EVs makes them highly suitable candidates for drug delivery, particularly therapeutic nucleic acid delivery. However, risk of tumorigenesis remains as a concern because of systemic and diverse effects of their cargo though their acellular nature [7].
The mechanism of action of EVs has not been well-understood yet but it is thought that they perform their functions through miRNAs. The proportion of miRNAs accounts for less than 1% of total RNA cargo in EVs but they are considered as the leading molecules in regulating functions of EVs. It is also evident from multiple knockdown experiments that diverse miRNAs might take role for the same effect in different tissues. Indisputably, there may be contribution of other components such as proteins and lipids, which are highly abundant in the EV cargo [177]. On the other hand, the content and function of EVs may depend on the metabolic properties of the donors and/or conditioned medium, which might make donor selection and manufacturing process more problematic [166, 177, 187]. In addition, systemic effects of miRNAs should be kept in mind if systemic therapy is the preferred route. For example, miRNA-126 regulates brain-heart interaction after ischemic stroke [188]. miRNAs involved in cancer pathogenesis should also be kept in mind [189]. These limitations highlight the significance of tissue specific applications. Furthermore, short half-life, off-target effects and insufficient endocytosis remain as major limitations of EVs that need to be improved during clinical translation process [154, 190].
There exist significant limitations while considering the isolation, characterization, tracking procedures and clinical grade production of EV-based therapeutics. There are various isolation and characterization methods of EVs such as ultracentrifugation, density gradient, filtration, size exclusion chromatography, precipitation, magnetic based capture or combinatory methods, while ultracentrifugation is the most commonly used technique for isolation according to a worldwide survey. Techniques for characterization also vary but Western blotting is the most preferred method [191]. However, it is not surprising that the other techniques are also widely used in different studies all over the world [8, 190, 191]. On the other hand, efforts to standardize these procedures and confusions regarding the nomenclature are ongoing [9]. Likewise, databases built up to provide information about the composition and functions of EVs such as ExoCarta, Evpedia, and Vesiclepedia will likely to contribute to clinical translation process [190]. When it comes to clinical-grade large scale production, EVs must be produced and stored under current Good Manufacturing Practice (cGMP) conditions in order to meet market demands. This includes a strict workflow including manufacturing through bioreactors, quality analyses and screening, and preservation in appropriate conditions in order to maintain the stability and integrity of EVs. Similar strict production flows are also relevant for clinical-grade stem cell production but EVs have a simplified cold chain process when compared to stem cells. Because of the acellular nature of EVs, lower risk of spontaneous DNA transformation might be an advantage during cryopreservation [192].
Despite limitations discussed above, EVs have moved to clinical studies rapidly. Results coming from oncological patients were encouraging in terms of safety and shed light on future studies [137, 138]. Moreover, administration of EVs derived from UC-MSCs was safe and ameliorated the inflammatory immune status and improved the kidney functions in a clinical study [193]. Clinical case report of a Graft-versus-host disease patient also demonstrated improved outcomes after EV therapy [140]. In addition, several clinical trials have been designed and conducted in order to investigate therapeutic potential of EVs in various diseases including cancer, type 1 diabetes, pleural effusion, ulcers, ischemic stroke, bronchopulmonary dysplasia, etc. However, there is an inevitable need for well-designed, well-conducted clinical trials in order to expedite clinical translation of EV-based therapeutics for acute emergency and/or mass casualty situations.
Conclusions
RM with personalized biologics are the future of medicinal sciences. Although they are not considered as a drug or pharmacologic agent yet, stem cells promise a potential for the treatment of various diseases with unmet clinical needs. On the other hand, their secretomes namely EVs have been the new therapeutic target due to the fact that they are the prominent components that regulate functions of stem cells. Both stem cells and EVs have demonstrated their therapeutic potential in preclinical models of various diseases but there are many limitations and caveats that need to be considered and improved during the translational process. They are also in their infancy ages and well-designed clinical trials will help to identify their therapeutic activity in human beings. This review aimed to summarize and understand the therapeutic potential of stem cells and EVs in diseases requiring acute emergency care such as trauma, heart diseases, stroke, ARDS and burn injury. Diseases that affect militaries or societies including ARS, sepsis, and viral pandemics such as COVID-19 have also been discussed. In addition, featuring and problematic issues which hamper clinical translation of stem cells and EVs have been debated with a futuristic perspective. Their advantages and disadvantages have been discussed in a comparative manner by keeping in mind the need for future studies and with the belief that these RM therapies will help to prolong human life and improve quality of life in the near future.
Compliance with Ethical Standards
Consent for Publication
This manuscript has been approved by all authors. The authors are alone responsible for the content and writing of the paper.
Disclosure of Interest
Y.M.E. is the founder and shareholder of Biovalda Health Technologies, Inc. (Ankara, Turkey). A.E.E. and Y.M.E. have patent applications in relation to regenerative biomaterials. The authors declare no competing financial interests in relation to this particular article.
Footnotes
This article belongs to the Topical Collection Special Issue on Exosomes and Microvesicles: from Stem Cell Biology to Translation in Human Diseases
Guest Editor: Giovanni Camussi
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cossu G, Birchall M, Brown T, De Coppi P, Culme-Seymour E, Gibbon S, et al. Lancet Commission: stem cells and regenerative medicine. Lancet. 2018;391(10123):883–910. doi: 10.1016/S0140-6736(17)31366-1. [DOI] [PubMed] [Google Scholar]
- 2.Glotzbach JP, Wong VW, Gurtner GC, Longaker MT. Regenerative medicine. Current Problems in Surgery. 2011;48(3):148–212. doi: 10.1067/j.cpsurg.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. International Journal of Cell Biology. 2016;2016:6940283. doi: 10.1155/2016/6940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Kabat M, Bobkov I, Kumar S, Grumet M. Trends in mesenchymal stem cell clinical trials 2004-2018: is efficacy optimal in a narrow dose range? Stem Cells Translational Medicine. 2020;9(1):17–27. doi: 10.1002/sctm.19-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks PW, Witten CM, Califf RM. Clarifying stem-cell therapy’s benefits and risks. The New England Journal of Medicine. 2017;376(11):1007–1009. doi: 10.1056/NEJMp1613723. [DOI] [PubMed] [Google Scholar]
- 7.Ela S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nature Reviews. Drug Discovery. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 8.Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of Extracellular vesicles: general methodologies and latest trends. BioMed Research International. 2018;2018:8545347. doi: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witwer KW, Thery C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. Journal of Extracellular Vesicles. 2019;8(1):1648167. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiklander, O. P. B., Brennan, M. A., Lotvall, J., Breakefield, X. O., & El Andaloussi, S. (2019). Advances in therapeutic applications of extracellular vesicles. Science Translational Medicine, 11(492). [DOI] [PMC free article] [PubMed]
- 12.Melling GE, Carollo E, Conlon R, Simpson JC, Carter DRF. The challenges and possibilities of extracellular vesicles as therapeutic vehicles. European Journal of Pharmaceutics and Biopharmaceutics. 2019;144:50–56. doi: 10.1016/j.ejpb.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Lowthian JA, Curtis AJ, Jolley DJ, Stoelwinder JU, McNeil JJ, Cameron PA. Demand at the emergency department front door: 10-year trends in presentations. The Medical Journal of Australia. 2012;196:128–132. doi: 10.5694/mja11.10955. [DOI] [PubMed] [Google Scholar]
- 14.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scandinavian Journal of Public Health. 2011;39(7 Suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 15.Tisherman SA, Stein DM. ICU management of trauma patients. Critical Care Medicine. 2018;46(12):1991–1997. doi: 10.1097/CCM.0000000000003407. [DOI] [PubMed] [Google Scholar]
- 16.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, et al. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Annals of Surgery. 2015;261(3):586–590. doi: 10.1097/SLA.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritenour AE, Blackbourne LH, Kelly JF, McLaughlin DF, Pearse LA, Holcomb JB, et al. Incidence of primary blast injury in US military overseas contingency operations: a retrospective study. Annals of Surgery. 2010;251(6):1140–1144. doi: 10.1097/SLA.0b013e3181e01270. [DOI] [PubMed] [Google Scholar]
- 18.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36(6):691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 19.Thurairajah, K., Broadhead, M. L., & Balogh, Z. J. (2017). Trauma and stem cells: biology and potential therapeutic implications. International Journal of Molecular Science, 18(3). [DOI] [PMC free article] [PubMed]
- 20.Pati S, Pilia M, Grimsley JM, Karanikas AT, Oyeniyi B, Holcomb JB, et al. Cellular therapies in trauma and critical care medicine: forging new frontiers. Shock. 2015;44(6):505–523. doi: 10.1097/SHK.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christopherson GT, Nesti LJ. Stem cell applications in military medicine. Stem Cell Research & Therapy. 2011;2(5):40. doi: 10.1186/scrt81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weston NM, Sun D. The potential of stem cells in treatment of traumatic brain injury. Current Neurology and Neuroscience Reports. 2018;18(1):1. doi: 10.1007/s11910-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha B, Krishna Kumar H, Borgohain MP, Thummer RP. Prospective applications of induced pluripotent stem cells in military medicine. Medical Journal, Armed Forces India. 2018;74(4):313–320. doi: 10.1016/j.mjafi.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ude CC, Miskon A, Idrus RBH, Abu Bakar MB. Application of stem cells in tissue engineering for defense medicine. Military Medical Research. 2018;5(1):7. doi: 10.1186/s40779-018-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber B, Lackner I, Haffner-Luntzer M, Palmer A, Pressmar J, Scharffetter-Kochanek K, et al. Modeling trauma in rats: similarities to humans and potential pitfalls to consider. Journal of Translational Medicine. 2019;17(1):305. doi: 10.1186/s12967-019-2052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng W, Sun J, Sheng C, Wang Z, Wang Y, Zhang C, et al. Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Research & Therapy. 2015;6:47. doi: 10.1186/s13287-015-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S, Xu L, Zhang Y, Sun Y, Li G. Systemic and local administration of allogeneic bone marrow-derived mesenchymal stem cells promotes fracture healing in rats. Cell Transplantation. 2015;24(12):2643–2655. doi: 10.3727/096368915X687219. [DOI] [PubMed] [Google Scholar]
- 28.Besalti O, Aktas Z, Can P, Akpinar E, Elcin AE, Elcin YM. The use of autologous neurogenically-induced bone marrow-derived mesenchymal stem cells for the treatment of paraplegic dogs without nociception due to spinal trauma. The Journal of Veterinary Medical Science. 2016;78(9):1465–1473. doi: 10.1292/jvms.15-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanriverdi AK, Polat O, Elcin AE, Ahlat O, Gurman G, Gunalp M, et al. Mesenchymal stem cell transplantation in polytrauma: evaluation of bone and liver healing response in an experimental rat model. European Journal of Trauma and Emergency Surgery. 2020;46(1):53–64. doi: 10.1007/s00068-019-01101-9. [DOI] [PubMed] [Google Scholar]
- 30.Cox CS, Jr, Baumgartner JE, Harting MT, Worth LL, Walker PA, Shah SK, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011;68(3):588–600. doi: 10.1227/NEU.0b013e318207734c. [DOI] [PubMed] [Google Scholar]
- 31.Liao GP, Harting MT, Hetz RA, Walker PA, Shah SK, Corkins CJ, et al. Autologous bone marrow mononuclear cells reduce therapeutic intensity for severe traumatic brain injury in children. Pediatric Critical Care Medicine. 2015;16(3):245–255. doi: 10.1097/PCC.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox CS, Jr, Hetz RA, Liao GP, Aertker BM, Ewing-Cobbs L, Juranek J, et al. Treatment of severe adult traumatic brain injury using bone marrow mononuclear cells. Stem Cells. 2017;35(4):1065–1079. doi: 10.1002/stem.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian C, Wang X, Wang X, Wang L, Wang X, Wu S, et al. Autologous bone marrow mesenchymal stem cell therapy in the subacute stage of traumatic brain injury by lumbar puncture. Experimental and Clinical Transplantation. 2013;11(2):176–181. doi: 10.6002/ect.2012.0053. [DOI] [PubMed] [Google Scholar]
- 34.Willing, A. E., Das, M., Howell, M., Mohapatra, S. S., & Mohapatra, S. (2020). Potential of mesenchymal stem cells alone, or in combination, to treat traumatic brain injury. CNS Neuroscience & Therapeutics 26(6), 616–627. [DOI] [PMC free article] [PubMed]
- 35.Schneider, C. M., Jackson, M. L., Bedi, S. S., & Cox Jr., C. S. (2019). Stem cells for traumatic brain injury. Principles of Regenerative Medicine (pp. 369–389). Elsevier.
- 36.Frantz S. Embryonic stem cell pioneer Geron exits field, cuts losses. Nature Biotechnology. 2012;30(1):12–13. doi: 10.1038/nbt0112-12. [DOI] [PubMed] [Google Scholar]
- 37.Cofano, F., Boido, M., Monticelli, M., Zenga, F., Ducati, A., Vercelli, A., et al. (2019). Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. International Journal of Molecular Science, 20(11). [DOI] [PMC free article] [PubMed]
- 38.Kuravi SJ, Yates CM, Foster M, Harrison P, Hazeldine J, Hampson P, et al. Changes in the pattern of plasma extracellular vesicles after severe trauma. PLoS ONE. 2017;12(8):e0183640. doi: 10.1371/journal.pone.0183640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochemistry International. 2017;111:69–81. doi: 10.1016/j.neuint.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Jiao G, Ren S, Zhang X, Li C, Wu W, et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Research & Therapy. 2020;11(1):38. doi: 10.1186/s13287-020-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao, Z. C., Lu, J., Wang, S. Z., Wu, H., Zhang, Y. T., & Xu, S. G. (2017). Stem cell-derived exosomes: a promising strategy for fracture healing. Cell Proliferation, 50(5). [DOI] [PMC free article] [PubMed]
- 42.Zhang ZG, Buller B, Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nature Reviews. Neurology. 2019;15(4):193–203. doi: 10.1038/s41582-018-0126-4. [DOI] [PubMed] [Google Scholar]
- 43.Yates AG, Anthony DC, Ruitenberg MJ, Couch Y. Systemic immune response to traumatic cns injuries-are extracellular vesicles the missing link? Frontiers in Immunology. 2019;10:2723. doi: 10.3389/fimmu.2019.02723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. Journal of the American College of Cardiology. 2014;63(12):1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 45.Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. European Journal of Heart Failure. 2015;17(9):884–892. doi: 10.1002/ejhf.319. [DOI] [PubMed] [Google Scholar]
- 46.Silvestre JS, Menasche P. The evolution of the stem cell theory for heart failure. EBioMedicine. 2015;2(12):1871–1879. doi: 10.1016/j.ebiom.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cambria E, Pasqualini FS, Wolint P, Gunter J, Steiger J, Bopp A, et al. Translational cardiac stem cell therapy: advancing from first-generation to next-generation cell types. NPJ Regenerative Medicine. 2017;2:17. doi: 10.1038/s41536-017-0024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menasche P. Cell therapy trials for heart regeneration - lessons learned and future directions. Nature Reviews. Cardiology. 2018;15(11):659–671. doi: 10.1038/s41569-018-0013-0. [DOI] [PubMed] [Google Scholar]
- 49.Murry CE, MacLellan WR. Stem cells and the heart-the road ahead. Science. 2020;367(6480):854–855. doi: 10.1126/science.aaz3650. [DOI] [PubMed] [Google Scholar]
- 50.Romagnuolo R, Masoudpour H, Porta-Sanchez A, Qiang B, Barry J, Laskary A, et al. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Reports. 2019;12(5):967–981. doi: 10.1016/j.stemcr.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Parouchev A, et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. Journal of the American College of Cardiology. 2018;71(4):429–438. doi: 10.1016/j.jacc.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 52.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 53.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 54.Amosse J, Martinez MC, Le Lay S. Extracellular vesicles and cardiovascular disease therapy. Stem Cell Investigation. 2017;4:102. doi: 10.21037/sci.2017.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung JH, Fu X, Yang PC. Exosomes generated from iPSC-derivatives: new direction for stem cell therapy in human heart diseases. Circulation Research. 2017;120(2):407–417. doi: 10.1161/CIRCRESAHA.116.309307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barile L, Milano G, Vassalli G. Beneficial effects of exosomes secreted by cardiac-derived progenitor cells and other cell types in myocardial ischemia. Stem Cell Investigation. 2017;4:93. doi: 10.21037/sci.2017.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research. 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, Kedracka-Krok S, Samanta A, et al. Induced Pluripotent Stem Cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circulation Research. 2018;122(2):296–309. doi: 10.1161/CIRCRESAHA.117.311769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El Harane N, Kervadec A, Bellamy V, Pidial L, Neametallas HJ, Perier MC, et al. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. European Heart Journal. 2018;39(20):1835–1847. doi: 10.1093/eurheartj/ehy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kervadec A, Bellamy V, El Harane N, Arakelian L, Vanneaux V, Cacciapuoti I, et al. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. The Journal of Heart and Lung Transplantation. 2016;35(6):795–807. doi: 10.1016/j.healun.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 61.Harane, N. E., Correa, B. L., Gomez, I., Hocine, H. R., Vilar, J., Desgres, M., et al. (2020). Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovascular Research. 10.1093/cvr/cvaa028 [DOI] [PubMed]
- 62.Rezaie J, Rahbarghazi R, Pezeshki M, Mazhar M, Yekani F, Khaksar M, et al. Cardioprotective role of extracellular vesicles: a highlight on exosome beneficial effects in cardiovascular diseases. Journal of Cellular Physiology. 2019;234(12):21732–21745. doi: 10.1002/jcp.28894. [DOI] [PubMed] [Google Scholar]
- 63.Moghaddam AS, Afshari JT, Esmaeili SA, Saburi E, Joneidi Z, Momtazi-Borojeni AA. Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis. 2019;285:1–9. doi: 10.1016/j.atherosclerosis.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 64.Yang PC. Induced Pluripotent Stem Cell (iPSC)-derived exosomes for precision medicine in heart failure. Circulation Research. 2018;122(5):661–663. doi: 10.1161/CIRCRESAHA.118.312657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovascular Pathology. 2015;24(4):199–206. doi: 10.1016/j.carpath.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 66.Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45(1):315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henderson SJ, Weitz JI, Kim PY. Fibrinolysis: strategies to enhance the treatment of acute ischemic stroke. Journal of Thrombosis and Haemostasis. 2018;16(10):1932–1940. doi: 10.1111/jth.14215. [DOI] [PubMed] [Google Scholar]
- 68.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Progress in Neurobiology. 2017;158:94–131. doi: 10.1016/j.pneurobio.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang ZG, Chopp M. Exosomes in stroke pathogenesis and therapy. The Journal of Clinical Investigation. 2016;126(4):1190–1197. doi: 10.1172/JCI81133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen J, Chopp M. Exosome therapy for stroke. Stroke. 2018;49(5):1083–1090. doi: 10.1161/STROKEAHA.117.018292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Meyer J, Pryck J, Hachimi-Idrissi S. Stem cell therapy for ischemic stroke: from bench to bedside. International Journal of Critical Care and Emergency Medicine. 2018;4:058. [Google Scholar]
- 73.Lalu, M. M., Montroy, J., Dowlatshahi, D., Hutton, B., Juneau, P., Wesch, N., et al. (2019). From the lab to patients: a systematic review and meta-analysis of mesenchymal stem cell therapy for stroke. Translational Stroke Research 11(3), 345–364. [DOI] [PubMed]
- 74.Doeppner TR, Herz J, Gorgens A, Schlechter J, Ludwig AK, Radtke S, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Translational Medicine. 2015;4(10):1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moon GJ, Sung JH, Kim DH, Kim EH, Cho YH, Son JP, et al. Application of mesenchymal stem cell-derived extracellular vesicles for stroke: biodistribution and MicroRNA study. Translational Stroke Research. 2019;10(5):509–521. doi: 10.1007/s12975-018-0668-1. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Molecular Therapy--Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. The New England Journal of Medicine. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 78.Bein T, Grasso S, Moerer O, Quintel M, Guerin C, Deja M, et al. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Medicine. 2016;42(5):699–711. doi: 10.1007/s00134-016-4325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cruz FF, Weiss DJ, Rocco PR. Prospects and progress in cell therapy for acute respiratory distress syndrome. Expert Opinion on Biological Therapy. 2016;16(11):1353–1360. doi: 10.1080/14712598.2016.1218845. [DOI] [PubMed] [Google Scholar]
- 80.Silva PL, Pelosi P, Rocco PRM. Personalized pharmacological therapy for ARDS: a light at the end of the tunnel. Expert Opinion on Investigational Drugs. 2020;29(1):49–61. doi: 10.1080/13543784.2020.1699531. [DOI] [PubMed] [Google Scholar]
- 81.Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Current Medicinal Chemistry. 2008;15(19):1911–1924. doi: 10.2174/092986708785132942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131(3):913–920. doi: 10.1378/chest.06-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayes M, Curley G, Ansari B, Laffey JG. Clinical review: Stem cell therapies for acute lung injury/acute respiratory distress syndrome - hope or hype? Critical Care. 2012;16(2):205. doi: 10.1186/cc10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lopes-Pacheco M, Robba C, Rocco PRM, Pelosi P. Current understanding of the therapeutic benefits of mesenchymal stem cells in acute respiratory distress syndrome. Cell Biology and Toxicology. 2020;36(1):83–102. doi: 10.1007/s10565-019-09493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang Y, Park SH, Huh JW, Lim CM, Koh Y, Hong SB. Intratracheal administration of umbilical cord blood-derived mesenchymal stem cells in a patient with acute respiratory distress syndrome. Journal of Korean Medical Science. 2014;29(3):438–440. doi: 10.3346/jkms.2014.29.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simonson OE, Mougiakakos D, Heldring N, Bassi G, Johansson HJ, Dalen M, et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Translational Medicine. 2015;4(10):1199–1213. doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respiratory Research. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. The Lancet Respiratory Medicine. 2015;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. The Lancet Respiratory Medicine. 2019;7(2):154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu A, Zhang X, He H, Zhou L, Naito Y, Sugita S, et al. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opinion on Biological Therapy. 2020;20(2):125–140. doi: 10.1080/14712598.2020.1689954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shah TG, Predescu D, Predescu S. Mesenchymal stem cells-derived extracellular vesicles in acute respiratory distress syndrome: a review of current literature and potential future treatment options. Clinical and Translational Medicine. 2019;8(1):25. doi: 10.1186/s40169-019-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee JH, Park J, Lee JW. Therapeutic use of mesenchymal stem cell-derived extracellular vesicles in acute lung injury. Transfusion. 2019;59(S1):876–883. doi: 10.1111/trf.14838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shetty AK. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)-induced pneumonia. Aging and Disease. 2020;11(2):462–464. doi: 10.14336/AD.2020.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bari, E., Ferrarotti, I., Saracino, L., Perteghella, S., Torre, M. L., & Corsico, A. G. (2020). Mesenchymal stromal cell secretome for severe COVID-19 infections: premises for the therapeutic use. Cells, 9(4). [DOI] [PMC free article] [PubMed]
- 95.Liang B, Chen J, Li T, Wu H, Yang W, Li Y, et al. (2020) Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. Medicine (Baltimore), 99(31), e21429 [DOI] [PMC free article] [PubMed]
- 96.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Öztürk, S., Elçin, A. E., & Elçin, Y. M. (2020). Mesenchymal stem cells for coronavirus (COVID-19)-induced pneumonia: revisiting the paracrine hypothesis with new hopes? Aging and Disease 11(3), 477–479. [DOI] [PMC free article] [PubMed]
- 98.Golchin, A., Seyedjafari, E., & Ardeshirylajimi, A. (2020). Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Reviews and Reports 16(3), 427–433. [DOI] [PMC free article] [PubMed]
- 99.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nature Reviews. Immunology. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Laroye C, Gibot S, Reppel L, Bensoussan D. Concise review: mesenchymal stromal/stem cells: a new treatment for sepsis and septic shock? Stem Cells. 2017;35(12):2331–2339. doi: 10.1002/stem.2695. [DOI] [PubMed] [Google Scholar]
- 101.Galstyan G, Makarova P, Parovichnikova E, Kuzmina L, Troitskaya V. The results of the single center pilot randomized russian clinical trial of mesenchymal stromal cells in severe neutropenic patients with septic shock (RUMCESS) International Journal of Blood Research and Disorders. 2018;5:033. [Google Scholar]
- 102.Sun XY, Ding XF, Liang HY, Zhang XJ, Liu SH, Bing H, et al. Efficacy of mesenchymal stem cell therapy for sepsis: a meta-analysis of preclinical studies. Stem Cell Research & Therapy. 2020;11(1):214. doi: 10.1186/s13287-020-01730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu J, Wang Y, Li L. Functional significance of exosomes applied in sepsis: a novel approach to therapy. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2017;1863(1):292–297. doi: 10.1016/j.bbadis.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 104.Coleman CN, Hrdina C, Bader JL, Norwood A, Hayhurst R, Forsha J, et al. Medical response to a radiologic/nuclear event: integrated plan from the Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services. Annals of Emergency Medicine. 2009;53(2):213–222. doi: 10.1016/j.annemergmed.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 105.Weisdorf D, Apperley J, Courmelon P, Gorin N-C, Wingard J, Chao N. Radiation emergencies: evaluation, management, and transplantation. Biology of Blood and Marrow Transplantation. 2007;13:103–106. [Google Scholar]
- 106.Heslet L, Bay C, Nepper-Christensen S. Acute radiation syndrome (ARS) - treatment of the reduced host defense. International Journal of General Medicine. 2012;5:105–115. doi: 10.2147/IJGM.S22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mettler FA, Jr, Gus'kova AK, Gusev I. Health effects in those with acute radiation sickness from the Chernobyl accident. Health Physics. 2007;93(5):462–469. doi: 10.1097/01.HP.0000278843.27969.74. [DOI] [PubMed] [Google Scholar]
- 108.Hu KX, Sun QY, Guo M, Ai HS. The radiation protection and therapy effects of mesenchymal stem cells in mice with acute radiation injury. The British Journal of Radiology. 2010;83(985):52–58. doi: 10.1259/bjr/61042310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, Eggert K, Gehling UM, Rudolph C, et al. Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PLoS ONE. 2011;6(1):e14486. doi: 10.1371/journal.pone.0014486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang X, Balakrishnan I, Torok-Storb B, Pillai MM. Marrow Stromal cell infusion rescues hematopoiesis in lethally irradiated mice despite rapid clearance after infusion. Advances in Hematology. 2012;2012:142530. doi: 10.1155/2012/142530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DiCarlo AL, Tamarat R, Rios CI, Benderitter M, Czarniecki CW, Allio TC, et al. Cellular therapies for treatment of radiation injury: report from a NIH/NIAID and IRSN workshop. Radiation Research. 2017;188(2):e54–e75. doi: 10.1667/RR14810.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rios C, Jourdain JR, DiCarlo AL. Cellular therapies for treatment of radiation injury after a mass casualty incident. Radiation Research. 2017;188(2):242–245. doi: 10.1667/RR14835.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gourmelon P, Benderitter M, Bertho JM, Huet C, Gorin NC, De Revel P. European consensus on the medical management of acute radiation syndrome and analysis of the radiation accidents in Belgium and Senegal. Health Physics. 2010;98(6):825–832. doi: 10.1097/HP.0b013e3181ce64d4. [DOI] [PubMed] [Google Scholar]
- 114.Bey E, Prat M, Duhamel P, Benderitter M, Brachet M, Trompier F, et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair and Regeneration. 2010;18(1):50–58. doi: 10.1111/j.1524-475X.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 115.Gaberman E, Pinzur L, Levdansky L, Tsirlin M, Netzer N, Aberman Z, et al. Mitigation of lethal radiation syndrome in mice by intramuscular injection of 3D cultured adherent human placental stromal cells. PLoS ONE. 2013;8(6):e66549. doi: 10.1371/journal.pone.0066549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singh VK, Christensen J, Fatanmi OO, Gille D, Ducey EJ, Wise SY, et al. Myeloid progenitors: a radiation countermeasure that is effective when initiated days after irradiation. Radiation Research. 2012;177(6):781–791. doi: 10.1667/rr2894.1. [DOI] [PubMed] [Google Scholar]
- 117.Rastogi S, Hwang A, Chan J, Wang JYJ. Extracellular vesicles transfer nuclear Abl-dependent and radiation-induced miR-34c into unirradiated cells to cause bystander effects. Molecular Biology of the Cell. 2018;29(18):2228–2242. doi: 10.1091/mbc.E18-02-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Al-Mayah AH, Irons SL, Pink RC, Carter DR, Kadhim MA. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiation Research. 2012;177(5):539–545. doi: 10.1667/rr2868.1. [DOI] [PubMed] [Google Scholar]
- 119.Szatmari T, Kis D, Bogdandi EN, Benedek A, Bright S, Bowler D, et al. Extracellular vesicles mediate radiation-induced systemic bystander signals in the bone marrow and spleen. Frontiers in Immunology. 2017;8:347. doi: 10.3389/fimmu.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bogin, V., Ichim, T.E. (2015) Endometrial regenerative cells and exosomes thereof for treatment of radiation exposure. Regenerative Medicine. Springer (pp. 33-7).
- 121.Baulch JE, Acharya MM, Allen BD, Ru N, Chmielewski NN, Martirosian V, et al. Cranial grafting of stem cell-derived microvesicles improves cognition and reduces neuropathology in the irradiated brain. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(17):4836–4841. doi: 10.1073/pnas.1521668113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kink JA, Forsberg MH, Reshetylo S, Besharat S, Childs CJ, Pederson JD, et al. Macrophages educated with exosomes from primed mesenchymal stem cells treat acute radiation syndrome by promoting hematopoietic recovery. Biology of Blood and Marrow Transplantation. 2019;25(11):2124–2133. doi: 10.1016/j.bbmt.2019.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vivo C, Galeiras R, del Caz MD. Initial evaluation and management of the critical burn patient. Medicina Intensiva. 2016;40(1):49–59. doi: 10.1016/j.medin.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 124.Cancio LC, Horvath EE, Barillo DJ, Kopchinski BJ, Charter KR, Montalvo AE, et al. Burn support for Operation Iraqi Freedom and related operations, 2003 to 2004. The Journal of Burn Care & Rehabilitation. 2005;26(2):151–161. doi: 10.1097/01.bcr.0000155540.31879.fb. [DOI] [PubMed] [Google Scholar]
- 125.Ojeh N, Pastar I, Tomic-Canic M, Stojadinovic O. Stem cells in skin regeneration, wound healing, and their clinical applications. International Journal of Molecular Sciences. 2015;16(10):25476–25501. doi: 10.3390/ijms161025476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arno A, Smith AH, Blit PH, Shehab MA, Gauglitz GG, Jeschke MG. Stem cell therapy: a new treatment for burns? Pharmaceuticals (Basel) 2011;4(10):1355–1380. doi: 10.3390/ph4101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shpichka A, Butnaru D, Bezrukov EA, Sukhanov RB, Atala A, Burdukovskii V, et al. Skin tissue regeneration for burn injury. Stem Cell Research & Therapy. 2019;10(1):94. doi: 10.1186/s13287-019-1203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu P, Yang Q, Wang Q, Shi C, Wang D, Armato U, et al. Mesenchymal stromal cells-exosomes: a promising cell-free therapeutic tool for wound healing and cutaneous regeneration. Burns & Trauma. 2019;7:38. doi: 10.1186/s41038-019-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20(3):291–301. doi: 10.1016/j.jcyt.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 130.Mason C, Dunnill P. A brief definition of regenerative medicine. Regenerative Medicine. 2008;3(1):1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- 131.Ozturk S, Elcin YM. Cardiac stem cell characteristics in physiological and pathological conditions. Current Pharmaceutical Design. 2018;24(26):3101–3112. doi: 10.2174/1381612824666180903123817. [DOI] [PubMed] [Google Scholar]
- 132.Jansen Of Lorkeers SJ. Eding JE, Vesterinen HM, van der Spoel TI, Sena ES, Duckers HJ, et al. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: systematic review and meta-analysis of large animal studies. Circulation Research. 2015;116(1):80–86. doi: 10.1161/CIRCRESAHA.116.304872. [DOI] [PubMed] [Google Scholar]
- 133.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. Journal of the American College of Cardiology. 2017;69(5):526–537. doi: 10.1016/j.jacc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, et al. Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. International Journal of Stroke. 2012;7(7):582–588. doi: 10.1111/j.1747-4949.2012.00797.x. [DOI] [PubMed] [Google Scholar]
- 136.Oliveri RS, Bello S, Biering-Sorensen F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: systematic review with meta-analyses of rat models. Neurobiology of Disease. 2014;62:338–353. doi: 10.1016/j.nbd.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 137.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. Journal of Translational Medicine. 2005;3(1):10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. Journal of Translational Medicine. 2005;3(1):9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zipkin M. Exosome redux. Nature Biotechnology. 2019;37(12):1395–1400. doi: 10.1038/s41587-019-0326-5. [DOI] [PubMed] [Google Scholar]
- 140.Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 141.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 142.Lu H, Wang F, Mei H, Wang S, Cheng L. Human adipose mesenchymal stem cells show more efficient angiogenesis promotion on endothelial colony-forming cells than umbilical cord and endometrium. Stem Cells International. 2018;2018:7537589. doi: 10.1155/2018/7537589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Petrenko Y, Vackova I, Kekulova K, Chudickova M, Koci Z, Turnovcova K, et al. A comparative analysis of multipotent mesenchymal stromal cells derived from different sources, with a focus on neuroregenerative potential. Scientific Reports. 2020;10(1):4290. doi: 10.1038/s41598-020-61167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, et al. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. American Journal of Respiratory Cell and Molecular Biology. 2014;51(3):455–465. doi: 10.1165/rcmb.2013-0529OC. [DOI] [PubMed] [Google Scholar]
- 145.Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. The New England Journal of Medicine. 2017;376(11):1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 146.Cyranoski D. Japanese man is first to receive ‘reprogrammed’stem cells from another person. Nature. 2017;10:1038. [Google Scholar]
- 147.Glicksman MA. Induced pluripotent stem cells: the most versatile source for stem cell therapy. Clinical Therapeutics. 2018;40(7):1060–1065. doi: 10.1016/j.clinthera.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 148.Ortuno-Costela, M. D. C., Cerrada, V., Garcia-Lopez, M., & Gallardo, M. E. (2019). The challenge of bringing iPSCs to the patient. International Journal of Molecular Science, 20(24). [DOI] [PMC free article] [PubMed]
- 149.Diamandis T, Borlongan CV. One, two, three steps toward cell therapy for stroke. Stroke. 2015;46(2):588–591. doi: 10.1161/STROKEAHA.114.007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Borlongan CV. Concise review: stem cell therapy for stroke patients: are we there yet? Stem Cells Translational Medicine. 2019;8(9):983–988. doi: 10.1002/sctm.19-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Curley GF, Ansari B, Hayes M, Devaney J, Masterson C, Ryan A, et al. Effects of intratracheal mesenchymal stromal cell therapy during recovery and resolution after ventilator-induced lung injury. Anesthesiology. 2013;118(4):924–932. doi: 10.1097/ALN.0b013e318287ba08. [DOI] [PubMed] [Google Scholar]
- 152.Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, Dash PK, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. Journal of Neurosurgery. 2009;110(6):1189–1197. doi: 10.3171/2008.9.JNS08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells and Development. 2009;18(5):683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mager I, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. Journal of Extracellular Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dogan A, Elcin AE, Elcin YM. Translational applications of tissue engineering in cardiovascular medicine. Current Pharmaceutical Design. 2017;23(6):903–914. doi: 10.2174/1381612823666161111141954. [DOI] [PubMed] [Google Scholar]
- 156.Dogan A, Parmaksiz M, Elcin AE, Elcin YM. Extracellular matrix and regenerative therapies from the cardiac perspective. Stem Cell Reviews and Reports. 2016;12(2):202–213. doi: 10.1007/s12015-015-9641-5. [DOI] [PubMed] [Google Scholar]
- 157.Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurology. 2017;16(5):360–368. doi: 10.1016/S1474-4422(17)30046-7. [DOI] [PubMed] [Google Scholar]
- 158.Cibelli J, Emborg ME, Prockop DJ, Roberts M, Schatten G, Rao M, et al. Strategies for improving animal models for regenerative medicine. Cell Stem Cell. 2013;12(3):271–274. doi: 10.1016/j.stem.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O'Collins V, et al. Can animal models of disease reliably inform human studies? PLoS Medicine. 2010;7(3):e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cruz FF, Borg ZD, Goodwin M, Sokocevic D, Wagner DE, Coffey A, et al. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Translational Medicine. 2015;4(11):1302–1316. doi: 10.5966/sctm.2014-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biology. 2010;8(3):e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis. PLoS Med. 2009;6(9):e1000144. doi: 10.1371/journal.pmed.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jansen of Lorkeers SJ. Doevendans PA, Chamuleau SA. All preclinical trials should be registered in advance in an online registry. European Journal of Clinical Investigation. 2014;44(9):891–892. doi: 10.1111/eci.12299. [DOI] [PubMed] [Google Scholar]
- 164.Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun-Shin MJ, Jones S, et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ. 2014;348:g2688. doi: 10.1136/bmj.g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mathur A, Fernandez-Aviles F, Dimmeler S, Hauskeller C, Janssens S, Menasche P, et al. The consensus of the Task Force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for the treatment of acute myocardial infarction and heart failure: update 2016. European Heart Journal. 2017;38(39):2930–2935. doi: 10.1093/eurheartj/ehw640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. Journal of Extracellular Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Osborne, N., Avey, M. T., Anestidou, L., Ritskes-Hoitinga, M., & Griffin, G. (2018). Improving animal research reporting standards: HARRP, the first step of a unified approach by ICLAS to improve animal research reporting standards worldwide. EMBO Reports, 19(5). [DOI] [PMC free article] [PubMed]
- 168.Emmerich CH, Harris CM. Minimum information and quality standards for conducting, reporting, and organizing in vitro research. Handbook of Experimental Pharmacology. 2020;257:177–196. doi: 10.1007/164_2019_284. [DOI] [PubMed] [Google Scholar]
- 169.Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. Journal of Cellular and Molecular Medicine. 2018;22(3):1428–1442. doi: 10.1111/jcmm.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lalegul-Ulker O, Seker S, Elcin AE, Elcin YM. Encapsulation of bone marrow-MSCs in PRP-derived fibrin microbeads and preliminary evaluation in a volumetric muscle loss injury rat model: modular muscle tissue engineering. Artificial Cells Nanomedicines and Biotechnology. 2019;47(1):10–21. doi: 10.1080/21691401.2018.1540426. [DOI] [PubMed] [Google Scholar]
- 171.Parmaksiz M, Elcin AE, Elcin YM. Decellularization of bovine small intestinal submucosa and its use for the healing of a critical-sized full-thickness skin defect, alone and in combination with stem cells, in a small rodent model. Journal of Tissue Engineering and Regenerative Medicine. 2017;11(6):1754–1765. doi: 10.1002/term.2071. [DOI] [PubMed] [Google Scholar]
- 172.Joo, H. S., Suh, J. H., Lee, H. J., Bang, E. S., & Lee, J. M. (2020). Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. International Journal of Molecular Science, 21(3). [DOI] [PMC free article] [PubMed]
- 173.Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 174.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. The FASEB Journal. 2006;20(6):661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 175.Sze SK, de Kleijn DP, Lai RC, Khia Way Tan E, Zhao H, Yeo KS, et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Molecular & Cellular Proteomics. 2007;6(10):1680–1689. doi: 10.1074/mcp.M600393-MCP200. [DOI] [PubMed] [Google Scholar]
- 176.Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. American Journal of Respiratory and Critical Care Medicine. 2015;192(3):324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bogatcheva NV, Coleman ME. Conditioned medium of mesenchymal stromal cells: a new class of therapeutics. Biochemistry (Mosc) 2019;84(11):1375–1389. doi: 10.1134/S0006297919110129. [DOI] [PubMed] [Google Scholar]
- 178.Chaubey S, Thueson S, Ponnalagu D, Alam MA, Gheorghe CP, Aghai Z, et al. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Research & Therapy. 2018;9(1):173. doi: 10.1186/s13287-018-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, et al. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS ONE. 2017;12(3):e0174303. doi: 10.1371/journal.pone.0174303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O'Brien D, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, et al. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Scientific Reports. 2016;6:36120. doi: 10.1038/srep36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. Journal of Proteome Research. 2012;11(2):839–849. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 183.Shao L, Zhang Y, Lan B, Wang J, Zhang Z, Zhang L, et al. MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. BioMed Research International. 2017;2017:4150705. doi: 10.1155/2017/4150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pelizzo G, Avanzini MA, Icaro Cornaglia A, De Silvestri A, Mantelli M, Travaglino P, et al. Extracellular vesicles derived from mesenchymal cells: perspective treatment for cutaneous wound healing in pediatrics. Regenerative Medicine. 2018;13(4):385–394. doi: 10.2217/rme-2018-0001. [DOI] [PubMed] [Google Scholar]
- 185.Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Research & Therapy. 2017;8(1):64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. The Journal of Clinical Investigation. 2013;123(4):1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Meng Y, Eirin A, Zhu XY, O'Brien DR, Lerman A, van Wijnen AJ, et al. The metabolic syndrome modifies the mRNA expression profile of extracellular vesicles derived from porcine mesenchymal stem cells. Diabetology and Metabolic Syndrome. 2018;10:58. doi: 10.1186/s13098-018-0359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, et al. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Translational Stroke Research. 2017;8(4):374–385. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Van Roosbroeck K, Calin GA. Cancer Hallmarks and MicroRNAs: the therapeutic connection. Advances in Cancer Research. 2017;135:119–149. doi: 10.1016/bs.acr.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 190.Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Delivery. 2020;27(1):585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gardiner C, Di Vizio D, Sahoo S, Thery C, Witwer KW, Wauben M, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. Journal of Extracellular Vesicles. 2016;5:32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kusuma GD, Barabadi M, Tan JL, Morton DAV, Frith JE, Lim R. To protect and to preserve: novel preservation strategies for extracellular vesicles. Frontiers in Pharmacology. 2018;9:1199. doi: 10.3389/fphar.2018.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomaterials Research. 2016;20:21. doi: 10.1186/s40824-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]