Abstract
FOXP3+ regulatory T cells (Tregs) constitute a critical barrier that enforces tolerance to both the self-peptidome and the extended-self peptidome to ensure tissue-specific resistance to autoimmune, allergic, and other inflammatory disorders. Here, we review intuitive models regarding how T cell antigen receptor (TCR) specificity and antigen recognition efficiency shape the Treg and conventional T cell (Tcon) repertoires to adaptively regulate T cell maintenance, tissue-residency, phenotypic stability, and immune function in peripheral tissues. Three zones of TCR recognition efficiency are considered, including Tcon recognition of specific low-efficiency self MHC-ligands, Treg recognition of intermediate-efficiency agonistic self MHC-ligands, and Tcon recognition of cross-reactive high-efficiency agonistic foreign MHC-ligands. These respective zones of TCR recognition efficiency are key to understanding how tissue-resident immune networks integrate the antigenic complexity of local environments to provide adaptive decisions setting the balance of suppressive and immunogenic responses. Importantly, deficiencies in the Treg repertoire appear to be an important cause of chronic inflammatory disease. Deficiencies may include global deficiencies in Treg numbers or function, subtle ‘holes in the Treg repertoire’ in tissue-resident Treg populations, or simply Treg insufficiencies that are unable to counter an overwhelming molecular mimicry stimulus. Tolerogenic vaccination and Treg-based immunotherapy are two therapeutic modalities meant to restore dominance of Treg networks to reverse chronic inflammatory disease. Studies of these therapeutic modalities in a preclinical setting have provided insight into the Treg niche, including the concept that intermediate-efficiency TCR signaling, high IFN-β concentrations, and low IL-2 concentrations favor Treg responses and active dominant mechanisms of immune tolerance. Overall, the purpose here is to assimilate new and established concepts regarding how cognate TCR specificity of the Treg repertoire and the contingent cytokine networks provide a foundation for understanding Treg suppressive strategy.
Keywords: FOXP3+ Tregs, Immunological tolerance, T cell antigen receptor, Tolerogenic vaccination, Clonotypic specificity
1. Introduction:
1.1. FOXP3+ Treg-based mechanisms of self-tolerance.
CD4+ CD25high FOXP3+ Tregs represent the main regulatory subset that suppresses excessive inflammatory responses and thereby protects the host from chronic autoimmune and allergic disorders [1–9]. FOXP3+ Tregs have also been implicated as beneficial mediators in broad categories of chronic inflammatory disorders including metabolic diseases [10], cardiovascular diseases [11], connective tissue diseases [12–14], musculoskeletal diseases [15], and neurodegenerative diseases [16–19] such as Alzheimer’s Disease, Parkinsonism, and Amyotrophic Lateral Sclerosis, among many other inflammatory disease categories. Conversely, excessive activity of Tregs has been implicated in cancer and chronic infectious disease [20–22].
FOXP3+ Tregs represent approximately 4–12% of circulating CD4+ T cells [23]. The FOXP3+ Treg lineage is defined in mice and humans by the stable, constitutive cell-lineage-specific expression of the master transcription factor FOXP3 [24–26]. A central criteria of the Treg lineage includes expression of FOXP3 in both blastogenic and quiescent phases, because some human Tcons express FOXP3 transiently in the activated phase but downregulate FOXP3 during quiescence [27–30]. For example, Tregs are often demarcated by a CD25high CD127low FOXP3+ phenotype, but this phenotype marks activated human T cells in general and does not distinguish Treg versus activated Tcon subsets after in vitro blastogenesis or during inflammation in vivo. Given that human Tregs and activated Tcons show extensive overlap in phenotype, the distinction of Tregs and Tcons requires an immunologically quiescent host and is thereby dependent upon physiologic context.
In addition to CD4+ FOXP3+ Tregs, a minor population of CD8+ lineage Tregs (0.1 – 0.5% of peripheral blood T cells) also express a CD25+ CD127low FOXP3+ phenotype [23]. These CD8+ Tregs exist in both mice and humans and mediate regulatory functions similar to that of the CD4+ FOXP3+ Treg counterparts. Several FOXP3negative regulatory subsets also mediate important regulatory functions, including IL-10-producing type 1 Tregs (Tr1 cells), IL-35 producing iTr35 cells, FOXA1+ regulatory T cells, TGF-β-producing Th3 cells, myeloid-derived suppressor cells, NKT regulatory cells, regulatory dendritic cells (DCs), regulatory γδ T cells (γδ Tregs), and regulatory ILCs (innate lymphoid cells), and regulatory macrophages (e.g., tumor-associated macrophages [31–38]. With the potential exception of regulatory DCs [39], the FOXP3+ Treg subset is the only regulatory subset that is considered indispensable for immune homeostasis and required to preclude lethal systemic autoimmunity. Genetic deficiency of FOXP3 results in a lack of Tregs, and this deficiency of Tregs causes the fatal systemic autoimmune diseases IPEX (immunodysregulation polyendocrinopathy enteropathy X-linked) in humans and scurfy in mice [40–42]. FOXP3 is primarily operative in Tregs because Treg-specific deletion of Foxp3 in adult mice leads to systemic lethal autoimmune disease [43–45].
Tregs arise from both intrathymic and extrathymic tissues and migrate into secondary lymphoid tissues. A significant contingent of Tregs reside as tissue-resident inhabitants of peripheral nonlymphoid tissues where these Tregs mediate tolerogenic immune function but also mediate specialized non-immune roles in tissue homeostasis [46–49]. These Tregs generally establish long-term residency in their respective tissues [50]. Thymic (tTregs) (i.e., also referred to as natural Tregs or nTregs) arise during thymic ontogeny upon recognition of self-peptides presented via MHCII [51, 52]. Peripheral Tregs (pTregs) differentiate de novo from naïve T cell precursors in secondary lymphoid organs and peripheral tissues upon recognition of tissue-specific self-antigens or foreign environmental antigens (e.g., extended self) [53, 54]. Peripheral pTregs might also differentiate from antigen-experienced Tcons, but permissible paths of differentiation are constrained by varying degrees of subset-specific plasticity and the depth of commitment of a particular Tcon cell to a conventional T cell lineage. To date, the field lacks convincing physiological data that committed antigen-experienced Tcons trans-differentiate into FOXP3+ Tregs, although antigen-experienced Tcons can differentiate into IL-10 producing Tr1 cells that suppress autoimmunity [55]. The degree to which Tcons can morph into Tregs and vice versa is an area of active inquiry [56]. Induced Tregs (iTregs) are FOXP3+ Tregs induced in vitro during culture with a TCR agonist and TGF-β [57]. Together, tTregs and pTregs mediate active dominant tolerance to universal and tissue-specific self-antigens to prevent autoimmune disease [58]. Conversely, pTregs specific for innocuous foreign antigens, particularly those that are present in early-life environments and chronically thereafter, are thought to establish tolerance to ‘extended self’ and thereby prevent allergic diseases against gut microbiota, food antigens, respiratory allergens, and other common environmental antigens that chronically permeate self-tissues [59]. In experimental settings, iTregs act similarly to tTregs and pTregs to control pathogenic inflammation [60, 61]. Although iTregs are generically considered to have less stable FOXP3 expression, issues of stability vary depending on the experimental setting [62].
To date, the most reliable FOXP3+ Treg marker is stable FOXP3 expression within quiescent environments in association with selective demethylation of a CpG-rich domain in the Treg-specific demethylated region (TSDR), which is a conserved non-coding sequence within the first intron of the Foxp3 gene locus [63–65]. Demethylation of the TSDR is actively engraved during early stages of thymic Treg development and is subsequently maintained in response to TGF-β. TSDR demethylation enables binding of multiple transcription factors and enhancers that stabilize active transcription of the Foxp3 gene thereby ensuring stable constitutive expression of the tTreg phenotype coupled with maintenance of robust tTreg-mediated suppressive activity. Due to TSDR demethylation, tTregs are considered more stable than pTregs, which are considered more stable than iTregs. However, additional research is needed to understand tTreg instability under strong destabilizing activation conditions as well as iTreg stability when iTregs are repeatedly boosted by multiple stabilizing in vitro activations with DCs, antigen, and TGF-β. Another important future research direction is to devise an effective feasible means to stabilize primary Treg responses in vivo in the aftermath of Treg immunotherapy or tolerogenic vaccination. The issue of Treg stability is critical, because FOXP3+ Tregs represent a nonredundant checkpoint for maintenance of self-tolerance, and destabilization of FOXP3+ Tregs may be a central consideration in the etiology and progression of autoimmune disease [66].
CD4+ CD25high FOXP3+ Tregs, rather than CD4+ CD25low FOXP3+ Tregs, represent the critical immunosuppressive population [67–69]. For example, the anti-mouse CD25 mAb PC61 selectively eliminates CD25high Tregs and abrogates FOXP3+ Treg-mediated immune suppression but does not impair CD25low Tregs [67, 70, 71]. The CD25high subset is defined by constitutively high expression of CD25, which is the transmembrane IL2Rα low-affinity receptor. CD25 pairs with the intermediate-affinity IL2Rβ (CD122)/ common gamma-chain IL2Rγ (CD132) complex to form the high-affinity IL-2 receptor [72]. The CD4+ CD25low FOXP3+ subset is prevalent, consisting of approximately 20–50% of the FOXP3+ Treg population, but this Treg subset lacks strong suppressive activity and may transition into Tcon subsets [73, 74]. Immunosuppressive FOXP3+ Tregs require IL-2 signaling because mice lacking IL-2 receptor chains CD25, CD122, or the IL-2 receptor signaling molecule STAT5 have deficiencies in FOXP3+ Tregs and exhibit systemic autoimmunity [75–83].
In murine experimental systems, activated CD25high FOXP3+ Tregs represent the key operative cell type responsible for the adoptive transfer of tolerance from tolerant donors to naïve recipients. In such adoptive transfer systems [61, 84, 85], Tregs can be used prophylactically to prevent the subsequent induction of autoimmune disease and can be used therapeutically to reverse ongoing chronic autoimmune disease. These adoptive transfer studies serve as the experimental foundation for contemporary efforts to use human Tregs in cell-based autologous immunotherapies [86–90].
1.2. FOXP3+ Tregs preserve tolerance to self and extended-self to inhibit autoimmunity and allergic disease.
The engagement of cognate TCR on Tregs is the critical event that selectively initiates tailored suppressive programs, each of which has a multiplicity of regulatory actions on local APCs, Tcons, and Tregs to favor active tolerance [33]. These mechanisms include Treg-derived soluble mediators such as TGF-β, IL-35, and IL-10 that facilitate Treg differentiation and effector function. Tregs also express cell-surface CD39 and CD73 ectoenzymes that control purinergic signaling via catabolism of ATP/ ADP to AMP and then AMP to adenosine respectively, thereby converting an ATP-dominant proinflammatory environment to an adenosine-dominant anti-inflammatory environment [91]. Tregs express cell surface checkpoint regulators (CTLA-4, PD-1, LAG3, GITR, TIM-3) that impose T cell anergy and maintain localized Tcon cell populations in a sub-responsive state. Activated Tregs also produce cytotoxic proteins such as perforin and granzyme B that directly kill target cells including Tcons and immunogenic APCs. Treg-mediated sequestration of IL-2 prevents neighboring Tcons from accessing a cytokine needed for survival, growth, and effector function. Tregs also downregulate APC-derived costimulatory molecules and sequester MHC/ peptide complexes from APC to inhibit cognate APC/ Tcon stimulatory responses [92, 93]. Tregs elicit differentiation of ‘regulatory’ DCs that in turn promote the differentiation of naïve T cells into new Tregs. By mechanisms that are incompletely resolved, Tregs selectively orchestrate these diverse suppressive mechanisms to curtail inflammatory responses and impose harmony in local tissues.
Tregs express clonotypic self-reactive TCR specific for cognate self-peptides, which elicit Treg-mediated tolerogenic responses that prevent autoimmune disease [94]. Tregs also express clonotypic TCR for foreign-peptides of the ‘extended-self’ peptidomes, which is postulated to elicit Treg-mediated tolerogenic responses that prevent allergic disease [3, 95]. The concept of an ‘extended-self’ peptidome is a useful means to consider foreign environmental antigens that are chronically present during life, that persistently permeate self-tissues, and that are immunologically ‘accepted’ as ‘self’ by mechanisms of active tolerance and/ or passive immunological ignorance. Hence, ‘extended self’ should be considered herein as a conceptual construct intended to explain allergic disease. The postulate is that Tregs enforce tolerance to foreign environmental peptides of the extended-self peptidome, otherwise the immune system would wage a chronic inflammatory war against innocuous foreign peptides of the environment [96]. Thus, the consideration is that foreign peptides representing ‘extended-self’ are treated immunologically as ‘self’ in accordance with the concept that these foreign peptides are constitutively present in self-tissues such as the lung and gut mucosal tissues, particularly during early-life imprinting of immune tolerance [95]. Chronic recognition of ever-present self-peptides or ubiquitous environmental peptides as agonistic TCR ligands in conjunction with the FOXP3 transcriptional program is believed to account for the constitutively activated CD25high phenotype of Tregs [97]. The defining functional hallmark of Tregs is the functional coupling of TCR specificity for agonistic self, chronic activation in response to self, and immune suppression of reactions against self (Figure 1). Some Treg clones also express clonotypic TCR specific for foreign peptides derived from infectious pathogens and control excessive inflammatory responses during infectious disease, thereby limiting disease when the immune response is a primary mediator of immunopathogenesis and tissue injury [98–100].
Figure 1: The modus operandi of CD4+ FOXP3+ CD25high Tregs:
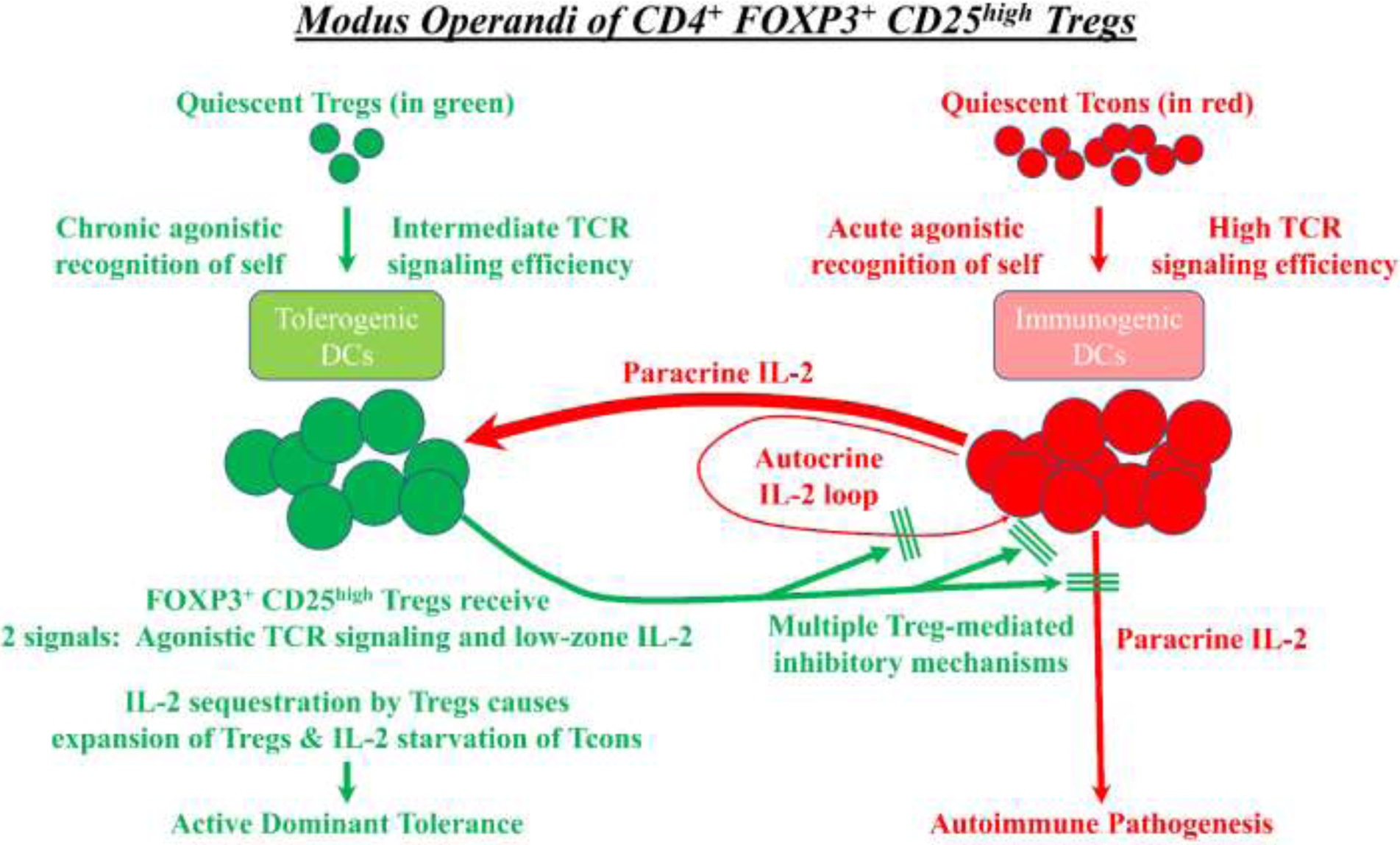
The defining hallmark of FOXP3+ Tregs is the functional coupling of TCR specificity for agonistic self, chronic activation in response to self, and suppression of Tcon-mediated reactions against self. Quiescent Tregs (green) are selected in the thymus and periphery to recognize self-MHC ligands as intermediate-efficiency agonistic ligands, which is postulated to maintain viability of FOXP3+ Tregs and elicit high surface expression of CD25. Rogue Tcons (red) that have the potential to mediate autoimmune pathogenesis recognize agonistic self-MHC ligands and produce IL-2, which is a source of paracrine IL-2 for Tregs. In this environment, Tregs receive two signals including agonistic TCR ligation and paracrine IL-2 stimulation. Due to their superlative CD25 expression, Tregs monopolize the local IL-2 supply to drive Treg growth while depriving Tcons of the IL-2 needed for effector function and survival. Aside from sequestering IL-2, activated effector Tregs use multiple direct and indirect mechanisms to block Tcon-mediated autoimmune pathogenesis.
2. The antigen-specific landscape of the T cell repertoire is the foundation of self-nonself discrimination (Figure 2):
Figure 2: The antigen-dependent selection of the T cell repertoire:
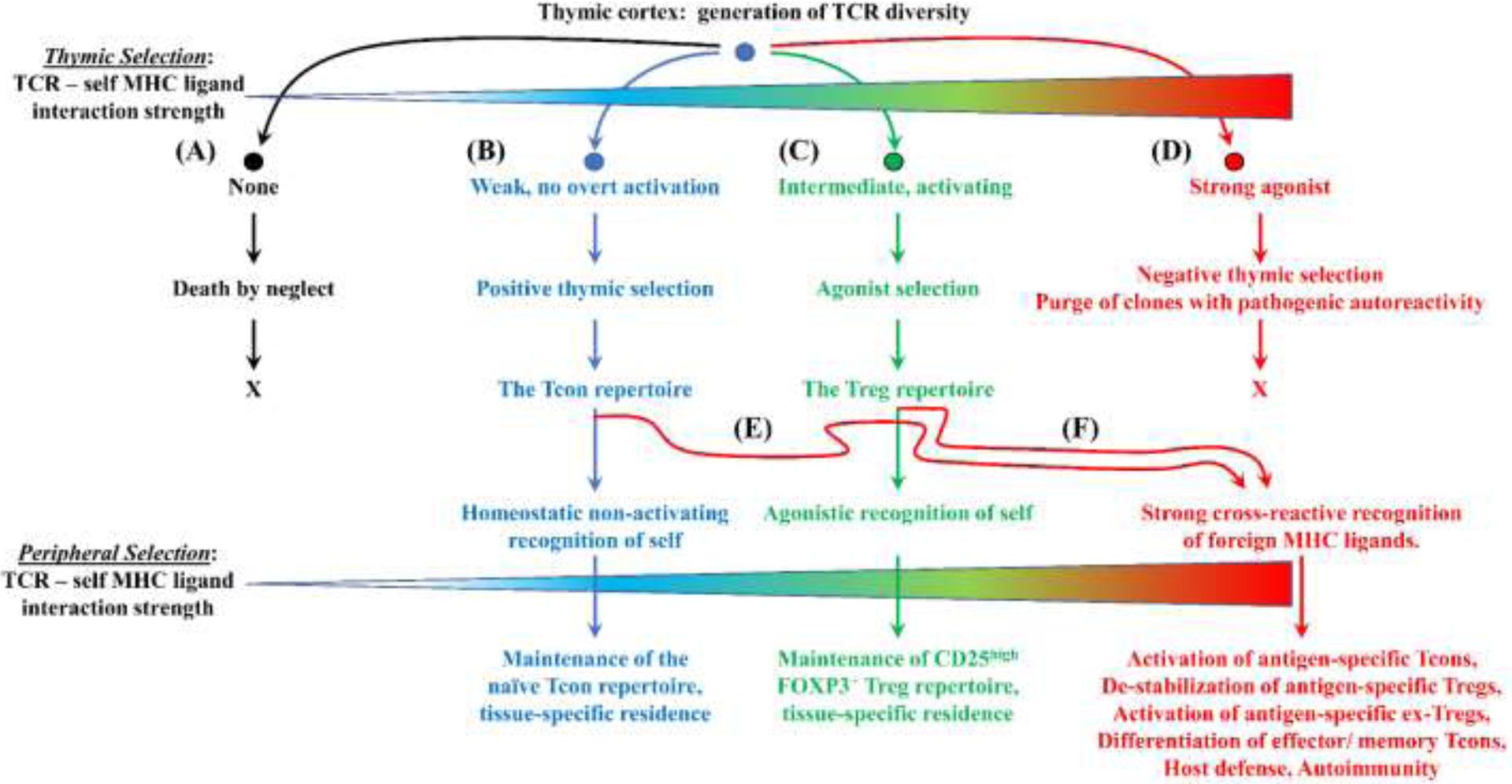
A quantitative continuum conceptualized by four zones of TCR ligation efficiencies shape the emerging T cell repertoire during thymic selection with each zone associated with a unique fate. (A) Death by neglect: Thymocytes that lack recognition of any self-MHC/ peptide complex die in the thymus due to the lack of homeostatic signaling. (B) Positive thymic selection: Thymocytes that experience relatively weak, non-activating TCR engagement receive requisite homeostatic signaling and emerge from the thymus as the mature Tcon repertoire. These Tcons continue to recognize positively-selecting self MHC/ peptide ligands in peripheral tissues as requisite survival signals. Many Tcons are thought to establish tissue residence at sites where these clones detect high concentrations of cognate self MHC/ peptide complexes. (C) Agonist selection: Thymocytes that experience intermediate-efficiency agonistic TCR engagement receive requisite survival signaling and emerge from the thymus as the mature Treg repertoire. These Tregs continue to recognize cognate self MHC/ peptide ligands in peripheral tissues as requisite survival signals. Many Tregs are thought to establish tissue residence at sites where these clones detect high concentrations of cognate self MHC/ peptide complexes. (D) Negative thymic selection: Thymocytes that recognize self-MHC/ peptide complex as high-efficiency agonistic ligands die in the thymus due to excess agonistic signaling. (E-F) Tcons and Tregs may cross-react and recognize foreign antigens as strong agonists and contribute to host defense.
Quantitative and qualitative parameters of TCR signaling efficiency control the antigenic niche that drives T cell differentiation in thymic environments [101–107]. These thymic recognition events are integrated to influence lineage fate decisions that commit differentiation of thymocyte precursors into the Treg versus Tcon lineages. TCR recognition efficiency is often used as a nondescript term to indicate parameters of affinity or avidity. Our perspective on TCR signaling efficiency is congruent with most pharmacological receptor systems, in which affinity refers to the strength of ligand-receptor binding interactions, and efficacy refers to ligand-receptor signaling activity. Affinity is a quantitative parameter of receptor occupancy dependent upon ligand concentration. Efficacy determines whether receptor occupancy translates into receptor signaling. Efficacy is a quantitative variable for a diverse set of receptor ligands but is a qualitative parameter for an individual ligand. That is, a given ligand that occupies a given receptor may be an antagonist, a mixed partial agonist/ antagonist, or a full agonist that will reliably stimulate no response, an intermediate response, or a full response, respectively, by that receptor. Although a set of ligands may differ in efficacy ranging from antagonists to agonists, the efficacy of a single ligand is relatively constant for a given ligand-receptor pair. We previously argued that, among MHC-ligands that have sufficient affinity to mediate TCR binding, the efficacy of a MHC-ligand is a key qualitative parameter determining the efficiency of TCR interactions and outcomes of thymic and peripheral selection [108]. Affinity and avidity are contingent upon ligand concentration, which would be highly variable in vivo, and a set-point based on variable quantitative concentrations of an MHC-ligand would be problematic as the foundation for self-nonself discrimination. However, the qualitative parameter of efficacy, which reflects the qualitative signaling activity of a given MHC-ligand, would provide a more reliable and valid foundation to faithfully interface thymic selection with accuracy in peripheral self-nonself discrimination, despite variations in ligand concentrations in different tissues.
During thymic ontogeny, CD4+ CD8+ double positive thymocytes use randomly-assembled clonotypic TCR to survey MHCII/ peptide and MHCI/ peptide ligands on thymic epithelial cells and DCs in the cortical and medullary regions of the thymus [109]. Thymocytes that lack effective recognition of any MHC/ peptide ligand are useless for the prospect of host defense and will die (i.e., death by neglect) due to the lack of requisite homeostatic TCR signaling (Figure 2A). Conversely, thymocytes that recognize cognate self-MHC ligands with strong efficacy pose an inherent danger for autoimmune disease and are purged from the repertoire via negative thymic selection (Figure 2D). Thymocytes that recognize cognate MHCII/ peptide and MHCI/ peptide ligands, respectively, will commit to the major CD4 or CD8 lineages. Within the CD4 T cell compartment, a qualitative and quantitative continuum of TCR signaling intensities distinguish the cues for differentiation of the Tcon and Treg repertoires. Thymocytes destined to seed the MHCII-restricted Tcon repertoire largely experience low TCR signaling efficiencies that are not overtly activating but rather provide homeostatic signaling necessary for thymocyte survival (Figure 2B). Thymocytes destined to seed the MHCII-restricted tTreg repertoire experience intermediate TCR signaling efficiencies that surpass agonistic activation thresholds but are below those that drive negative thymic selection (Figure 2C) [110]. We presume that the TCR recognition efficiencies that delineate Tcon and Treg thymic lineage fates reflects quantitative transitions rather than qualitative demarcations. Particularly for TCR signaling efficiencies within the Tcon/ Treg transition zone, the lineage fate of thymocytes is likely influenced by many other cues including the cytokine, adhesion, and costimulatory microenvironments that concur with relevant TCR antigen recognition events. Overall, a quantitative selectional model based on TCR signaling efficiency provides a simple model for selection of the T cell repertoire and an intuitive mechanism of self/ nonself discrimination.
The fundamental concept of host defense is based on the principal of TCR cross-reactivity [108, 111–114]. The CD4+ and CD8+ Tcon repertoire, in which each clone is selected to recognize allelic self-MHC glycoproteins complexed to cognate but non-activating self-peptides, is ideally optimized to cross-react with the same self-MHC glycoprotein complexed with a foreign peptide. Even though cross-reactivity may be rare between a random T cell clone and a random MHC-restricted ligand (0.01 – 0.0001% probability), the immense diversity of the Tcon repertoire ensures a substantial number of Tcon clones will cross-react to mediate host defense [115]. Thus, Tcons are selected to recognize self-MHC ligands as cognate non-activating ligands and are optimized to cross-react and recognize foreign-MHC ligands as strong activating ligands to drive clonal expansion and differentiation of effector/ memory functionalities (Figure 2E). Conversely, T cells that differentiate into Tregs recognize cognate self-MHC complexes as activating ligands [116]. Tregs may also cross-react more strongly with the same self-MHC glycoprotein complexed with a foreign peptide (Figure 2F) [117, 118]. However, the Treg phenotype is malleable, and in the presence of strongly activating MHC-foreign peptide ligands, many Tregs may differentiate into ex-Tregs and contribute to pro-inflammatory responses of the activated Tcon repertoire [119]. Alternatively, stable Tregs cross-reactive with foreign epitopes of infectious pathogens may modulate the intensity of active inflammatory responses to inhibit immunopathogenesis [104].
3. The antigen-specificity of the T cell repertoire determines tissue-specific residence (Figure 3):
Figure 3: T cell tissue-specific residency strategized by TCR specificity and antigen recognition efficiency.
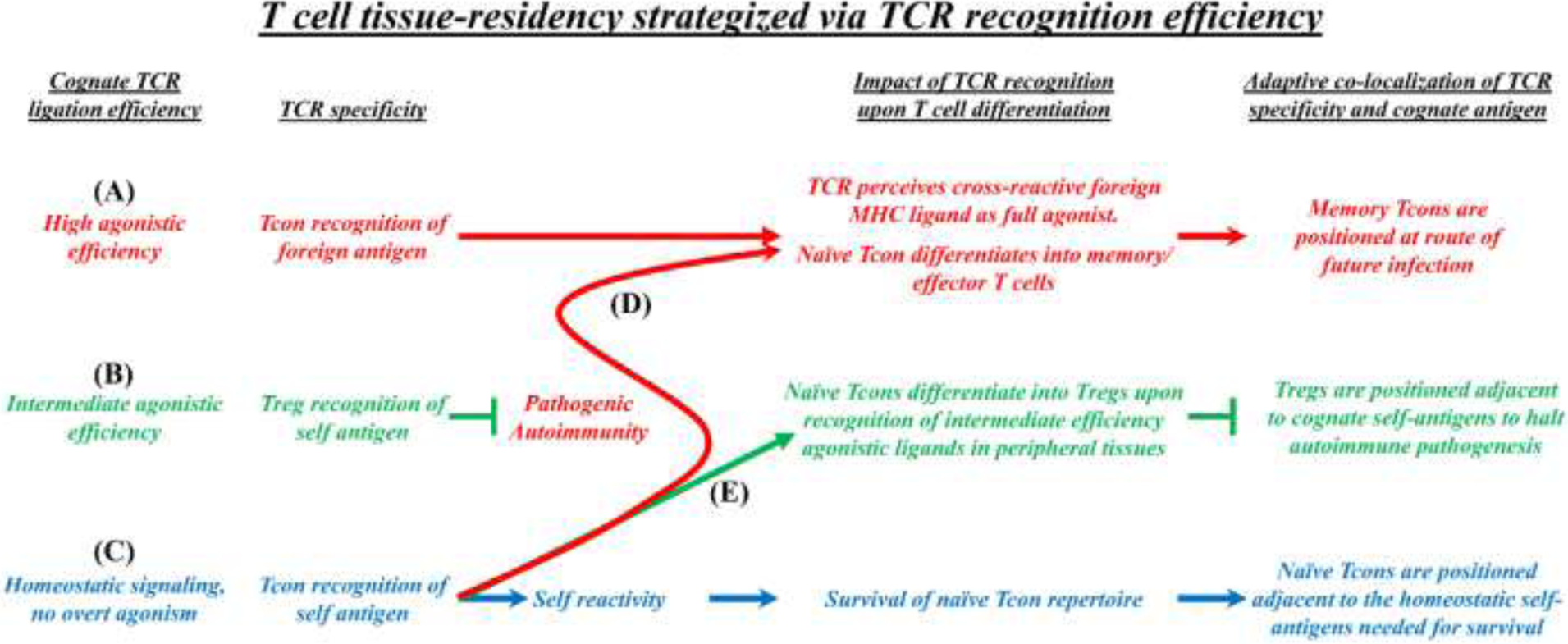
TCR specificity and antigen recognition efficiency are thought to guide T cell homing, tissue residency, and function in peripheral tissues. (A, D) Tcon recognition of high-efficiency agonistic antigens is hypothesized to maintain immunogenic sentinel memory T cells at sites that will likely become routes of future infection. (B) Treg recognition of intermediate-efficiency agonistic self-antigens is hypothesized to provide homeostatic survival signals that guide homing and survival while maintaining phenotypic and functional stability of tissue-resident Tregs. (C) Tcon recognition of peripheral self-antigens as low-efficiency non-agonistic ligands (i.e. the positive-selecting MHC-ligands of positive thymic selection) is hypothesized to provide homeostatic survival signals that guide homing and survival of the Tcon repertoire. (E) Inflammatory conditions may amplify a low-efficiency homeostatic TCR recognition into an intermediate TCR efficiency signaling mode that favors naïve Tcon differentiation into Tregs to counter epitope spread to tissue-specific self-antigens.
We would like to propose that three zones of TCR recognition efficiency also guide TCR-dependent mechanisms of homing and tissue-residence of the respective T cell subsets to optimally position clones for adaptive tissue-specific responses. Upon TCR engagement, T cells exhibit gene expression programs that control survival and metabolic strategy together with tissue-specific homing receptors as part of overall differentiation programs enabling naïve, effector-memory, or tissue-resident memory subsets with the propensity for tissue residency in peripheral nonlymphoid tissues [120–123]. An attractive concept is that naïve T cells that home to the vicinity of cognate positively-selecting self-antigens in peripheral tissues would be ideally positioned to differentiate into pTregs to protect tissues if neighboring pathogenic Tcons wage an inflammatory war against those cognate self-antigens in local tissues. Tissue-resident Tregs localized to the vicinity of cognate self-antigens would be ideally positioned to inhibit pathogenic Tcons that target those cognate self-antigens in local self-tissues. Lastly, memory Tcons that remain in previously infected tissues are optimally localized to provide memory responses against future repeat exposures of the same foreign antigen in that same tissue, given that infectious pathogens often stage repeat infections via the same route of invasion (e.g., respiratory viruses). Co-localization of T cells and their cognate self-antigens provides an adaptive ‘guard’ function to stage protective responses that can be optimally mobilized when needed against autoimmune and infectious threats.
The Tcon repertoire during homeostasis:
The naïve Tcon repertoire that was derived via low-efficiency, positively-selecting self-MHC ligands in the thymus will continue to recognize the same cognate self-MHC ligands in the periphery, and these TCR interactions provide homeostatic survival signals that maintain naïve Tcon viability [124]. Based on the tissue locations of the relevant cognate self-peptides and mechanisms of TCR-guided tissue migration and retention, individual Tcon clonotypes are postulated to establish tissue-specific residence in tissues bearing high concentrations of the relevant cognate self-peptides that are presented by resident APCs (Figure 3C). Because abnormal states such as inflammation or lymphopenia may alter TCR signaling intensity, the naïve Tcon repertoire may perceive their positively-selecting non-activating self-ligands instead as intermediate-efficiency agonistic self-MHC ligands in various disease states (Figure 3E). For example, changes in the relative expression of CD4 or CD8 coreceptor affects T cell perception of a MHC-ligand as either an agonist or antagonist [125, 126]. Thus, tissue-resident naïve Tcons may serve as a seed population for pTregs if naive T cell recognition of self transforms from a low-efficiency mode to an intermediate-efficiency mode, given that agonistic intermediate-efficiency self-ligands favor Treg differentiation [127–130]. Via this perspective, the naïve tissue-resident Tcon repertoire is ideally placed to differentiate into protective pTreg populations that maintain self-tolerance in local tissues during inflammation. This potential mechanism of de novo Treg differentiation during local inflammatory responses may represent a significant obstacle for ‘epitope spread’ [131] and may thereby preclude recruitment and differentiation of clonotypically-diverse naïve Tcons as autoimmune effector T cells during inflammatory events in peripheral tissues. Indeed, induction of diverse self-specific Tregs during inflammation may explain why epitope spread may be of limited importance in some autoimmune conditions [132].
The Treg repertoire:
The nascent Treg repertoire was selected to recognize agonistic intermediate-efficiency self-MHC ligands as positively-selecting ligands in the thymus, and the mature Treg repertoire will continue to recognize the same cognate self-MHC ligands in the periphery [102, 105, 133–136]. These Treg-TCR interactions are believed to guide TCR-dependent migratory/ tissue retention responses to localize Tregs as tissue-specific residents in those tissues that express those cognate self-antigens (Figure 3B) [13, 50, 128, 137]. Because self-antigen is more efficiently recognized by Tregs than Tcons, constant self-antigen recognition events help maintain tolerance in peripheral tissues [116]. These TCR/ self-MHC ligand interactions are thought to provide critical survival signals that sustain Treg viability. The chronic recognition of intermediate-efficiency agonistic TCR ligands may also preserve Treg functional and phenotypic stability as an anergic (lack IL-2 production capacity), CD25high, FOXP3+ regulatory population [138]. These mechanisms place the tissue-resident Treg population adjacent to tissue-specific self-antigens so that the resident Treg population is positioned to counter-balance Tcon-mediated autoimmune responses during inflammation within that tissue. Co-localization of tissue-specific antigen-reactive Tregs with the tissue-specific self-antigens may also represent a critical barrier for ‘epitope spread’ by precluding Treg-targeted self-antigens from driving clonotypic diversification of an autoreactive, pathogenic Tcon repertoire.
The Tcon repertoire in host defense:
Naïve Tcons that interface with strongly efficacious foreign MHC-peptide ligands differentiate into CD44high effector/ memory Tcons, and these TCR recognition events guide migration of Tcons from secondary lymphoid organs into local tissues bearing those foreign antigens (Figure 3A, D) followed by retention of antigen-ligated Tcons as tissue-resident memory T cells [139, 140]. That is, Tcon recognition of fully agonistic high-efficiency cognate foreign-MHC ligands is hypothesized to guide TCR-dependent migratory patterns and tissue-specific residence such that activated Tcons are strategically positioned locally to contain the infectious agent during ongoing and future infection of that tissue [141]. Memory Tcon and B cell subsets may form ectopic lymphoid structures in local tissues if foreign antigens exhibit recurrent or chronic exposure in those tissues [142]. The coherence of Tcon TCR specificity strategically positioned in routes of likely infectious disease invasion is highly adaptive for prevention of future infection. However, this adaptation may be problematic in autoimmune disease, because ectopic lymphoid structures may form adjacent to target tissues bearing high concentrations of the relevant auto-antigens to facilitate local re-activation of autoreactive pathogenic Tcons [143–145].
The interesting aspect of this model is that three pivotal T cell subsets (naïve Tcons, Tregs, and effector/ memory Tcons) are postulated to exhibit TCR-dependent homing to locales bearing high concentrations of the respective cognate antigens so that these subsets are optimally positioned to preempt autoimmune responses while promoting host defense. In antigen-bearing tissues, these three zones of TCR recognition efficiency may be cross-integrated based on T cell subset frequencies, the antigenic composition of the local environment, and the respective frequencies/ durations of the relative TCR/ MHC-ligand interactions. TCR-guided migration may reflect a classical model of immune surveillance whereby T cells migrate from blood into peripheral tissues based on tethering, rolling, and diapedesis across post-capillary endothelium, based on antigen-independent interactions of T cells with selectins, integrins, and chemokines. Once within the tissue, T cells that perceive cognate MHCII/ peptides on local immature DCs are thought to upregulate retention signals that maintain antigen-ligated T cells in that tissue whereas non-ligated clonotypes exit and traffic to secondary lymphoid tissues and then recirculate to the blood, peripheral tissues, and secondary lymphoid organs as part of immune surveillance and the T cell search for cognate antigens [146]. A related possibility is that cognate MHCII/ peptide complexes presented on post-capillary endothelium may direct particular clonotypes into antigen-bearing tissues [147]. In addition, MHCII/ peptide complexes on exosomes, upon release by tissue-resident DCs, may help guide antigen-specific clonotypes into antigen-bearing tissues. Tissue residence and co-localization of T cell clonotypes with tissue concentrations of cognate antigen may be facilitated by many mechanisms as a core aspect of T cell biology.
4. APCs integrate ‘antigenic complexity’ to determine immune outcomes (Figure 4):
Figure 4: Antigen complexity theory:
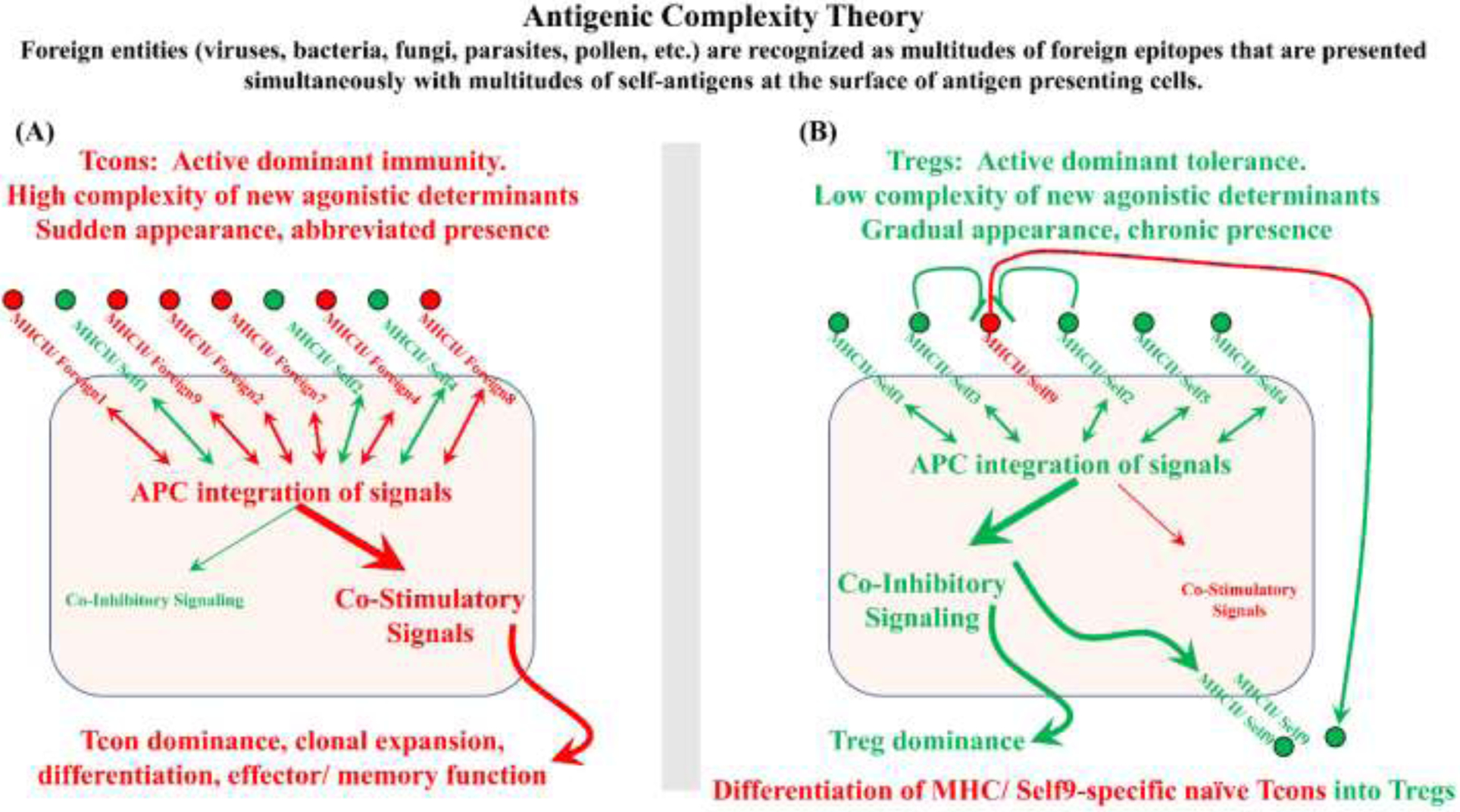
Most foreign pathogens are antigenically complex entities that present MHC-peptide ligands that are highly diverse and strongly-agonistic to collectively stimulate the robust activation of a diverse polyclonal Tcon repertoire. Rather than reflecting independent binary decisions between a single APC and a single Tcon, antigen complexity theory postulates that the immune system integrates decisions encompassing inputs from local T cell and APC populations to provide a consensus output. According to this postulated mechanism, Tcons or Tregs recognize cognate MHC-ligands on the surface of an APC and provide stimulatory or inhibitory feedback signals to the APC. The APC in turn collectively integrates the diverse signals and provides consensus costimulatory or tolerogenic signaling to the T cells in direct contact with that APC or to T cells in the local environment via cytokine production. (A) High diversity of strong agonistic peptides drives high frequencies of activated Tcons that provide collective feedback to the APC to drive robust costimulatory signaling by APCs, thereby overcoming inhibitory inputs from the Treg population. (B) Conversely, in the presence of low frequencies of strong agonistic peptides, the low frequencies of activated Tcons do not surpass thresholds requisite for costimulatory signaling by APCs, and dominant inhibitory inputs from the Treg population drive tolerogenic Treg responses. When Tregs are dominant, tolerogenic APCs and local Tregs mediate bystander inhibition of activated Tcons and mediate de novo differentiation of naïve Tcons into Tregs (i.e., infectious tolerance).
The antigenic complexity model is an extension of the original Bretscher and Cohn two-signal model of T cell activation [148, 149], which postulated that two independent but concurrent antigen recognition events were needed to produce a positive immunogenic output. This two-signal model is distinct from the contemporary two-signal model wherein signal 1 is a cognate TCR stimulus and signal 2 is a generic co-stimulatory signal. The Bretscher and Cohn two-signal model serves as the foundation of the antigen complexity model, which is designed to incorporate the full complexity of self and nonself antigens presented by APCs [126, 150, 151]. That is, immune decisions are not simple binary decisions per an isolated interaction of a single T cell and a single APC. Rather, the immune system is thought to integrate ‘antigen complexity’ of a local tissue to provide orchestrated responses among many T cell clonotypes and APCs during recognition of complex arrays of self and foreign antigens. This integrated response is not simply an averaging response, because regulatory mechanisms must persist in inflammatory environments. An integrated fenestrated mechanism must enable Tcon responses to foreign antigens while simultaneously inhibiting Tcon responses to self-antigens.
A central tenet of the ‘antigen complexity theory’ is that infectious agents are complex entities that produce multitudes of qualitatively diverse foreign epitopes (Figure 4A, Table 1). Even small viral pathogens have multiple viral proteins, each of which may contain multiple foreign epitopes presented on host MHC molecules. Bacterial pathogens likely give rise to many thousands of foreign epitopes that are presented on host MHC glycoproteins. A pathogenic infection thereby results in the sudden appearance of multitudes of foreign strongly-agonistic MHC ligands. In contrast, uninfected tissues are marked chronically by multitudes of self-MHC ligands that are largely recognized by the local Treg repertoire, with only a few self-MHC ligands recognized as strong efficacious ligands by autoreactive Tcons with the potential to mediate autoimmune disease (Figure 4B, Table 1). Thus, the distinguishing features of infection are that infectious pathogens are often marked by a sudden, time-delimited appearance of highly complex MHC-ligands (i.e., high T cell frequencies and high clonotypic diversity of strongly efficacious MHC-ligands). Conversely, homeostatic non-infected environments may be marked by a gradual appearance and chronic presence of at most a few isolated strongly efficacious self MHC-ligands amidst a sea of cognate intermediate-efficiency self MHC-ligands that are constantly surveyed by the Treg repertoire allowing Tregs to dominate the recognition events in the non-infected microenvironment.
Table 1.
Antigen Complexity Theory
| Immunogenic responses/Host defense | Tolerogenic responses/ Self tolerance |
|---|---|
| TCR recognition of high-efficiency agonists by Tcons | TCR recognition of intermediate efficiency agonists by Tregs |
| Sudden appearance of antigen | Gradual appearance of antigen |
| High diversity of new agonistic determinants | Low diversity of new agonistic determinants |
| Abbreviated presence/ rapid immune clearance of antigen. | Chronic presence of antigen |
Foreign epitopes are not recognized in isolation. Rather, foreign entities (viruses, bacteria, fungi, parasites, pollen, etc.) are recognized by an integrated process including multitudes of foreign epitopes that are presented simultaneously at the surface of individual APCs to polyclonal T cell populations. Antigenic complexity of local environments is measured by the clonal diversity of Tcons perceiving of high-efficiency TCR signaling relative to the clonal diversity of Tregs perceiving intermediate-efficiency TCR signaling.
Integration of diverse antigenic signaling is thought to occur at two levels; clonotypic T cells integrate signals from a surface population of TCRs and subsequently DCs integrate signals from a clonotypically diverse population of T cells that dock onto a particular DC. According to this model, clonotypically diverse T cells simultaneously dock onto individual DCs over time to survey MHC-ligands and sequester relevant cognate MHC-ligands in the confines of an immunological synapse [93, 152] . TCR signals must be integrated by an individual T cell clone because a given T clone may simultaneously recognize qualitatively distinct MHC-ligands. For example, the 2D2 TCR recognizes Myelin Oligodendrocyte MOG35–55/ I-Ab as an intermediate-efficiency ligand but also recognizes Neurofilament Medium NFM13–37/ I-Ab as a high-efficiency ligand [129, 153]. The central concept is that each T cell integrates the quality and quantity of TCR signaling to provide feedback signals to the DC. If a T cell perceives integrated TCR signals that surpass a given threshold, then the T cell will express signaling molecules (e.g., CD40L) to regulate activation of the APC [130]. Diverse T cell clonotypes are thereby postulated to gauge perceptions of cognate antigens presented in a local environment by tissue-resident DCs and respectively provide feedback signals across an immunological synapse back to that DC. These signals are in turn integrated by DCs to set the balance of costimulatory or coinhibitory signaling back to antigen-engaged T cells, thereby controlling the scope and nature of the local immune response. Tissue-resident DCs are thereby well situated to provide a level of integration needed to balance tolerogenic versus immunogenic responses.
In environments marked by a novel appearance of highly diverse, highly agonistic ligands, such as an infected tissue, a suprathreshold of T cells express CD40L and other activating signals to drive DCs to express high levels of costimulatory molecules that in turn drives a dominant immunogenic response, which dominates until the infectious source of the antigenic stimulus is cleared from the system. Conversely, in homeostatic environments marked by the gradual appearance of a just few high-efficiency agonistic ligands, most engaged T cells are Tregs which continue to see their cognate self-antigens whereas a minority are pathogenic autoimmune Tcons that perceive fully agonistic MHC-ligands. In this case, the supermajority of Tregs suppresses DC activation and enforces active dominant tolerance, which results in the suppression of pathogenic Tcons. Overall, APCs are thought to be critical integrators of the local antigen-engaged Treg and Tcon repertoires, and these integrative events may be the critical parameters controlling the dominance of either tolerogenic or immunogenic outcomes in local tissues, respectively.
5. IL-2 integrates the balance of Tregs and Tcons to determine immune outcomes (Figure 5):
Figure 5: Antigenic complexity and IL-2 dependent control of the Tcon/ Treg balance.
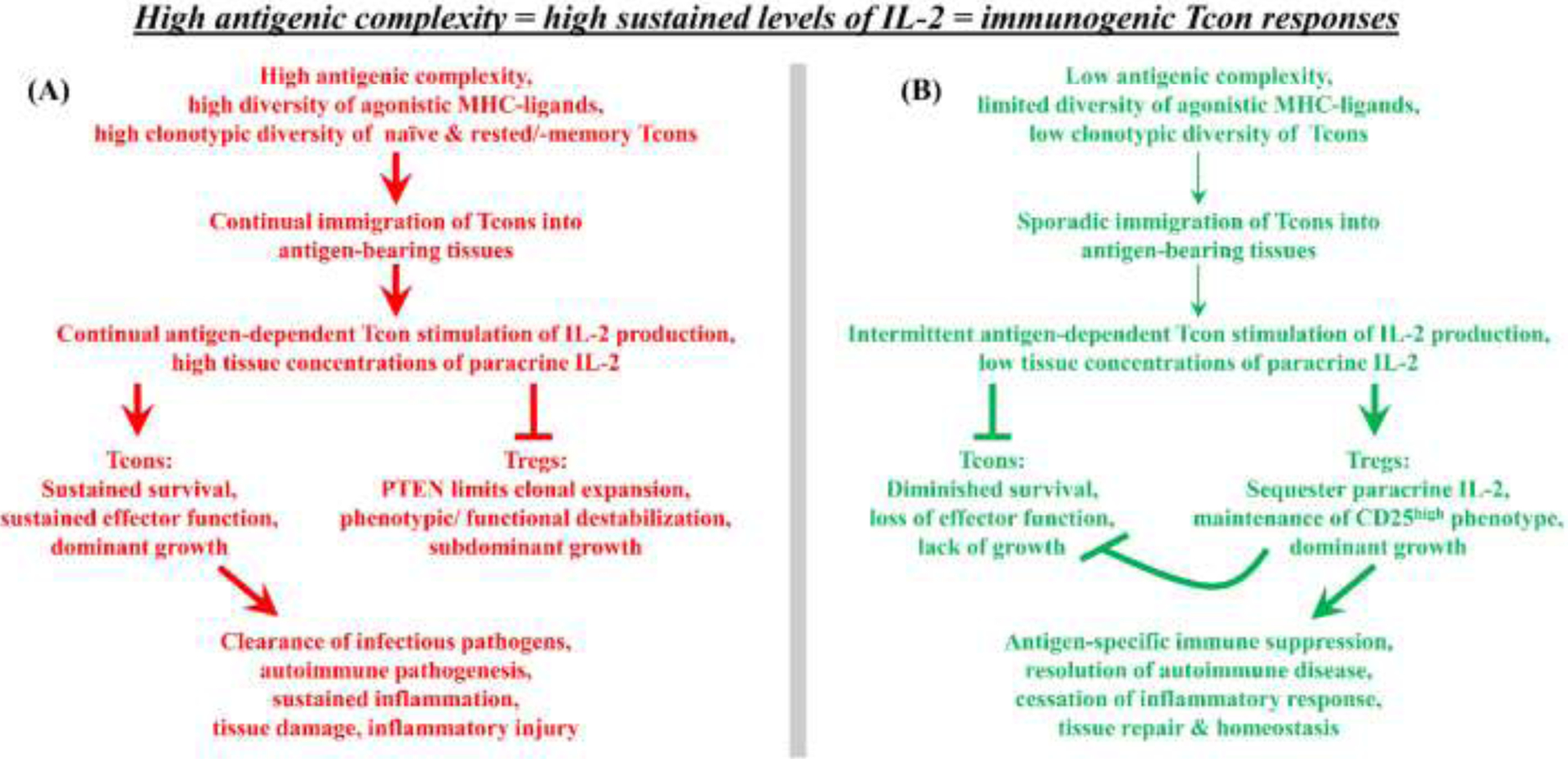
Naïve Tcons and rested memory/ effector Tcons respond to agonistic antigen by producing IL-2, whereas chronically activated Tcons and Tregs lack capacity for antigen-stimulated IL-2 production. (A) Given persistence of high antigenic complexity in a local tissue, newly immigrating naïve and rested effector/ memory Tcons represent the main sources for the local supply of paracrine IL-2 needed to sustain pre-existing populations of tissue-resident effector Tcons and Tregs. In the presence of high antigenic complexity, the sustained consistent immigration of new Tcons will provide an abundant supply of IL-2 to establish high-zone IL-2 concentrations ensuring Tcon dominance and immunogenic responses. (B) In the presence of low antigenic complexity, the sporadic inconsistent immigration of new Tcons will provide a limited supply of IL-2 to establish low-zone IL-2 concentrations ensuring Treg dominance and tolerogenic responses.
The differential regulation of IL-2 production and IL-2 responsiveness also balances Treg-mediated suppression with Tcon-mediated immunity, based on the following tenets. TCR engagement, cellular activation, and the FOXP3 transcriptional program elicits higher CD25 expression on Tregs compared to Tcons, which engenders Tregs with superior responsiveness to low doses of IL-2. Tregs however lack IL-2 production capacity but require IL-2 for survival, which is supplied as paracrine IL-2 derived from antigen-stimulated Tcons [154, 155]. Thus, Tregs require two signals to maintain tissue dominance including; (a) chronic recognition of self-antigen in combination with the FOXP3 transcriptional program to maintain superlative CD25 expression and (b) Tcon-derived paracrine IL-2 to maintain Treg survival and function. This mechanism ensures that the depth and diversity of Treg repertoire in local tissues is proportional to the degree of Tcon-mediated inflammation in that tissue. Treg numbers and function will be optimal during smoldering inflammation due to the presence of paracrine IL-2 whereas Treg numbers will be more limited in noninflammatory quiescent environments due to the lack of paracrine IL-2. This mechanism thereby scales Treg-mediated defenses in proportion to the autoimmune threat in that tissue.
The IL-2 signaling pathways of Tregs and Tcons also differ due to the differential expression of the PI(3)K inhibitor PTEN, which is preferentially expressed in Tregs rather than Tcons [156–159]. In that IL-2 signaling is translated via parallel activation of the PI(3)K and JAK/STAT signaling cascades, PTEN represents a partial brake on the IL-2 signaling cascade. In Tregs, IL-2 stimulation acts in synergy with the FOXP3 transcriptional program to elicit high levels of PTEN expression, which inhibits the activation of PI(3)K and thereby limits IL-2 signaling intensity. In Tcons, TCR or IL-2 stimulation downregulates PTEN expression, activates PI(3)K, and thereby enables full IL-2 signaling intensity. When IL-2 is limiting, antigen-stimulated Tregs dominate because superior CD25 expression and higher IL-2 potency enables Tregs to sequester the available IL-2, which in turn stimulates Tregs unilaterally through the JAK/STAT signaling pathway to mediate survival and expansion. When IL-2 is abundant, antigen-stimulated Tcons dominate because the responsive PI(3)K and JAK/STAT signaling pathways confer superior clonal Tcon expansion compared to Tregs which bear PTEN-repressed PI(3)K pathways. In pharmacological terms, Tregs engage IL-2 with superior potency, whereas Tcons engage IL-2 with superior efficacy.
Rested and chronically-activated Tcon subsets are also distinguished by differential regulation of IL-2 production [160–172]. Stimulation of Tcons with optimal antigen and costimulation drives a phasic bout of IL-2 production that is followed by a post-activation refractory phase wherein restimulation with optimal antigen plus costimulation results in little or no IL-2 production. Thus, naïve and rested effector/ memory Tcons exhibit robust IL-2 production when stimulated with immunogenic antigen, whereas chronically-activated Tcons lack IL-2 production capacity when restimulated with immunogenic antigen, and like Tregs, must depend upon paracrine sources of IL-2 for survival and maintenance of effector function. Indeed, the post-activation refractory period of activated Tcons is prolonged by exposure to IL-2, whereas exposure of rested Tcons to IL-2 augments subsequent responsiveness to restimulation with antigen or IL-2 [170]. Post-activation refractoriness appears to be a special attribute of the IL-2 pathway rather than other T cell growth factors such as IL-4 [171]. Although blastogenic Tcons exhibit a refractory phase defined by the lack of IL-2 production capacity, activated Tcons nonetheless fully recover antigen-stimulated IL-2 production capacity when these lymphoblasts revert to a quiescent rested phenotype.
The consideration is that antigenic complexity may be translated via localized IL-2 production to control tolerogenic versus immunogenic outcomes. The main concept is that high or low antigenic complexity, reflecting the presence or absence of an active infection, respectively, will proportionally determine the relative numbers of immigrating Tcons capable of IL-2 production. The immigration rate of rested/ naïve Tcons that enter, activate, and produce IL-2 determines local IL-2 concentrations available to competing populations of tissue-resident Tregs and effector Tcons, both of which require paracrine IL-2. During an active infection marked by high levels of antigenic complexity (Figure 5A), high frequencies of immigrating Tcons will be restimulated upon entering antigen-bearing local tissues and will produce high IL-2 concentrations to favor pre-established effector Tcon populations over PTEN-limited Treg populations. Conversely, in noninfected tissues (i.e., low antigenic complexity, Figure 5B), the occasional immigration of a naïve Tcon with pathogenic autoimmune potential will be quantitatively limited in frequency, and re-stimulation with autoantigen in the local tissue will stimulate limited IL-2 concentrations which will be sequestered by local Tregs. Thus, in non-infected tissues, newly-immigrating and established effector Tcons would be starved of the IL-2 needed for survival, thereby terminating any autoimmune threat. Even in inflamed tissues, clearance of the infection (i.e., the antigenic stimulus) will result in the gradual attenuation of IL-2 concentrations due to cessation of antigen-driven IL-2 production and the emergence of a dominant Treg response. Thus, during resolution of inflammation, the high IL-2 sensitivity of the CD25high Treg phenotype will help restore tissue homeostasis.
6. Thwarting immune tolerance: the molecular mimicry hypothesis of autoimmune pathogenesis.
Molecular mimicry is a widely-accepted hypothesis used to explain etiology of autoimmune disease and is exemplified here to portray how molecular mimicry may confer susceptibility to Multiple Sclerosis (MS). Most normal individuals have circulating naïve myelin-specific clonotypes [173–175], but these autoreactive T cells normally lack pathogenic activity due to limitations imposed by a variety of mechanisms, including ‘immunological ignorance’ and Treg-mediated tissue-specific regional tolerance. Approximately 33% of Tcons are estimated to have pathogenic potential that is constrained by Tregs [176]. Active and passive inhibitory mechanisms presumably preempt clonal expansion and effector T cell differentiation and thereby preclude CNS autoimmune disease. Nonetheless, myelin-reactive naïve clonotypes may be primed at sites distant from regional Treg-mediated defenses of the CNS via a cross-reaction with foreign epitopes from infectious pathogens in peripheral immunogenic environments. Thus, stochastic TCR cross-reactivity during infectious illness, essentially a rolling of the dice, may prime expansion and differentiation of myelin-reactive clonotypes into effector T cells that have pathogenic activity capable of invading the CNS and mediating MS.
Yet, the intersection of molecular mimicry and peripheral T cell autoreactivity may not be the primary culprit that confers susceptibility to autoimmune disease. Mimicry appears to be a common mechanism of immune evasion due to a strong evolutionary drive for infectious pathogens to mimic self-peptides as camouflage [177, 178], and peripheral T cells with autoreactive potential appear ubiquitous [176, 179]. But infectious disease is only rarely accompanied by autoimmune disease. According to this perspective, insufficient numbers, function, or stability of tissue-resident Tregs may represent a breach or a ‘hole in the Treg repertoire’ as a key immunodeficiency that seeds tissue-specific autoimmune disease [94, 180]. Alternatively, the magnitude of the autoimmune attack may overwhelm a normal repertoire of tissue-resident Tregs. Of these three variables (i.e., the mimicry antigen, the Tcon repertoire, and the Treg repertoire), special consideration should be given to strengthening the Treg repertoire via tolerogenic vaccination or adoptive Treg-based immunotherapy.
7. Tolerogenic vaccines: a new generation of disease-specific interventions for inflammatory disease.
7.1. General considerations:
Tolerogenic vaccines are meant to unilaterally expand Tregs without expanding pathogenic Tcon repertoires as interventions for chronic autoimmune disease. Tolerogenic vaccines represent diverse technologies that are rationally designed based on our emerging knowledge of the Treg niche, including the antigenic, cytokine, and APC niches that comprise preferential Treg microenvironments [181–184]. Given that Tregs may be numerically or functionally deficient in autoimmune disease [185], the overriding question is whether new tolerogenic vaccine technologies can effectively reverse potential Treg deficiencies to remedy autoimmune disease. Tolerogenic vaccines intended for the treatment of MS will need to restore CNS-specific immune tolerance by eliciting CNS-specific Tregs to inhibit autoimmunity and ensure long-term remission.
Because tolerogenic vaccines are autoantigen-specific, these interventions preserve host defense and avoid severe opportunistic infections associated with broad-spectrum immunosuppression. As opposed to conventional immunogenic vaccines that are preventative and drive humoral immunity, tolerogenic vaccines exhibit therapeutic action by reversing pre-established autoimmune disease via the direct inactivation of pre-existing pathogenic effector/ memory T cells together with induction and/or expansion of suppressive Tregs to maintain long-lasting immune tolerance. Examples of tolerogenic vaccines include the following technologies; (a) peptide-based vaccines which are composed of naked myelin peptides or proteins delivered via various routes with or without various adjuvants, (b) nucleic acid-based vaccines comprised of genetic or viral vectors, (c) nanoparticles embedded with autoantigens and immunomodulatory agents such as cyclosporin or rapamycin, and (d) cell-based passive vaccines that utilize infusion of leukocytes including tolerogenic DCs or polyclonal Tregs to induce autoantigen-specific tolerance [186]. Currently, tolerogenic vaccines are being vetted in early phase clinical trials, but tolerogenic vaccines have not yet been approved as clinical interventions for autoimmune disease.
7.2. Tolerogenic vaccines and infectious tolerance (Figure 6):
Figure 6: Tolerogenic vaccination: bridging holes in the Treg repertoire to enhance bystander inhibition and infectious tolerance.
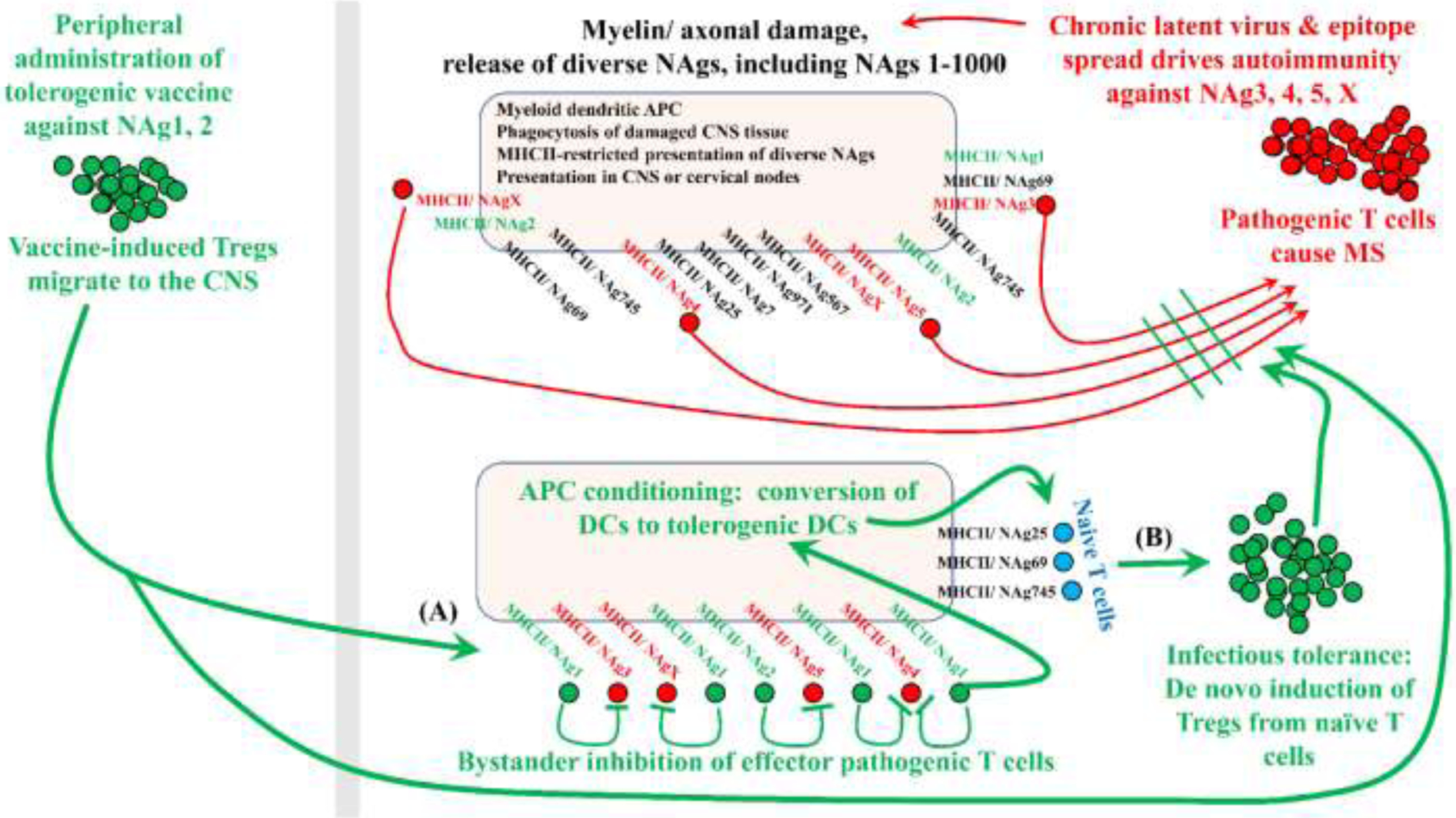
Tolerogenic vaccination must target pathogenic T cell clonotypes without knowledge of the pathogenic epitopes. For example, if pathogenesis in MS is driven by NAg3, 4, 5, and X, then CNS-derived DCs will present these pathogenic NAgs together with myriads of other NAgs because CNS tissue destruction releases diverse NAgs including endogenous versions of the vaccine NAgs. As a result, local DCs will present diverse NAgs including the unknown pathogenic NAgs and the known vaccine-specific NAgs (i.e., not derived from the vaccine per se but derived from damaged myelin). Upon tolerogenic vaccination with nonpathogenic NAg1 and NAg2, peripheral DCs will drive expansion of NAg1/2-specific Tregs, which will migrate throughout the body and home into tissues bearing high endogenous concentrations of NAg1 and NAg2. DCs presenting damage-associated CNS NAg3, 4, 5, and X represent the interface of vaccine-generated Tregs with pathogenic Tcons. This interface is the checkpoint that re-establishes tolerance (A) by reinforcing bystander inhibition of pathogenic Tcons and (B) by eliciting infectious tolerance via de novo differentiation of naïve tissue-resident Tcons into Tregs.
A central issue underlying tolerogenic vaccination is that the etiological antigens responsible for most autoimmune diseases are not known with any precision [187]. Thus, the prospect of directly targeting pathogenic T cells lacks feasibility. Not only do we lack knowledge of self-protein targets in autoimmune disease, the specific pathogenic epitopes likely exhibit substantial inter-patient variability due to the polymorphic variability of the MHC genes. Also, due to potential mechanisms of epitope spread, the causative pathogenic epitopes may diversify during disease progression and may exist as a complicated array of pathogenic epitopes unique to each patient. For example, MS might be initiated against an epitope of MOG. As CNS damage progresses, other distinct epitopes of MOG and additional myelin proteins may prime additional pathogenic clonotypes to diversify encephalitogenic responses to multiple myelin and axonal antigens. The challenge of tolerogenic vaccination is to reverse years or decades of pathogenic T cell diversification, with the prospect that autoimmunity may be mediated by multitudes of autoreactive clonotypes with the caveat that the diverse pathogenic repertoire may be unique to each patient.
Due to uncertainties regarding identity of pathogenic epitopes compounded by epitope spread, a central question is whether tolerogenic vaccines that include tissue-specific self-antigens evoke tolerogenic mechanisms that curtail disparate unknown pathogenic epitopes that are not included in the vaccine. Central to this consideration is the concept that vaccine-induced Tregs likely interface directly with pathogenic T cells, simply because both regulatory and pathogenic T cells share CNS-specificity. In consideration of a tolerogenic vaccine for MS, vaccine-induced neuroantigen (NAg)-specific Tregs are predicted to home into the CNS and CNS-draining lymphatics during MS where these Tregs interface with DCs that are presenting damage-associated CNS tissue antigens. The same DCs thereby present both endogenous CNS-derived mimics of the vaccine NAgs adjacent to the unknown diverse pathogenic NAgs. By this mechanism, vaccine-induced Tregs dock onto the same APC surface adjacent to the pathogenic T cells that mediate autoimmunity, along with naïve tissue-resident T cells. Thus, DCs presenting damage-associated myelin antigens become the nexus for cross-regulation of the respective Treg and Tcon subsets and the intersection of tolerogenic vaccination with unknown pathogenic autoimmune antigens.
Two tolerogenic vaccine mechanisms include bystander suppression (Figure 6A) and infectious tolerance (Figure 6B) [188]. Bystander suppression implies the array of mechanisms by which an existing Treg clonotype inhibits terminally-differentiated, clonotypically-distinct pathogenic effector/ memory Tcons. Bystander suppression is considered a passive mechanism of tolerance in that the mechanism confers the passive loss of autoreactive T cells. Conversely, infectious tolerance implies the array of mechanisms by which existing Treg clonotypes recruit new Treg clonotypes to diversify the CNS-specific Treg response. Infectious tolerance includes mechanisms by which existing Tregs elicit de novo differentiation of clonotypically-distinct naïve T cells into nascent Tregs. Infectious tolerance represents a formative mechanism, because Tregs have the potential to recruit clonotypically diverse arrays of nascent Tregs when relevant Treg epitopes are presented concurrently with other damage-associated CNS antigens on the same regulatory DC. Infectious tolerance is considered an active dominant mechanism that enables the clonotypic diversification of a tolerogenic Treg response that parallels ‘epitope spread’, but in a suppressive mode [189–194].
7.3. Tolerogenic adjuvants:
Tolerogenic vaccines must reliably induce tolerance without inadvertently sensitizing autoantigen-specific immune responses that exacerbate autoimmunity. Many tolerogenic vaccine platforms thereby incorporate tolerogenic adjuvants to ensure safety and enhance efficacy by skewing the vaccine response toward tolerogenic outcomes. Tolerogenic adjuvants may be co-delivered with autoantigens in nanoparticles, microparticles, liposomes, among many other formulations. Tolerogenic adjuvants used in preclinical models include vitamin D, IL-10, TGF-β, retinoic acid, rapamycin, and aryl hydrocarbon receptor ligands [195–197]. A related vaccine strategy includes modalities that target vaccine autoantigens to specific APC subsets in the presence or absence of tolerogenic adjuvants. Vaccine autoantigens may be targeted to particular APC by fusion to cytokines, antibodies, or other biologics that bind subset-specific receptors on APC and induce receptor mediated endocytosis leading to enhanced antigen presentation by the targeted APC [198–201]. An additional criterion is that tolerogenic vaccines must be effective in inflammatory environments, because tolerogenic vaccines are intended for therapeutic use during ongoing inflammatory autoimmune disease. Many preclinical vaccine strategies rely on steady-state homeostatic environments to drive tolerance and prevent disease induction. However, these strategies are typically useful only as a pretreatment before the onset of autoimmunity and generally lack effectiveness in treating established inflammatory disease. Thus, tolerogenic vaccines contingent upon a rested steady-state environment will likely lack efficacy in disease states marked by the presence of systemic smoldering inflammation. Many tolerogenic vaccine platforms are currently in development and efforts should be directed to developing vaccine platforms that induce robust long-lasting tolerance even in inflammatory environments.
7.4. Tolerogenic vaccines as tools to understand the Treg niche:
The study of tolerogenic vaccines and Treg-based immunotherapy has been invaluable for delineating the Treg niche (Table 2). For example, the study of GMCSF-antigen tolerogenic vaccines (i.e., single-chain granulocyte-macrophage colony-stimulating factor - antigen fusion proteins) showed that intermediate-efficiency self-antigens represented the key parameter for induction of tolerance [129, 130]. The study of IFN-β-based vaccines revealed that high concentrations of IFN-β were pivotal in amplifying Treg responses in vitro and in vivo [202–204]. The study of long-term cultures of murine Tregs showed that low IL-2 concentrations favored immunosuppressive Tregs whereas high IL-2 concentrations favored Tcons [61]. Collectively, these studies provided evidence that the Treg niche is favored by intermediate-efficiency agonistic self-antigens, high concentrations of IFN-β, and low concentrations of IL-2.
Table 2.
Experimental contributions to the understanding of the Treg niche
| Experimental modality | Contributing knowledge |
|---|---|
| GMCSF-NAg tolerogenic vaccine | An intermediate-efficiency antigenic domain favors Treg dominance in vivo. |
| IFN-β + NAg in Alum tolerogenic vaccine | High-zone IFN-β signaling favors Treg dominance in vivo. |
| IFN-β + NAg induction of murine Tregs | High-zone IFN-β signaling favors Treg dominance in vitro. |
| Continuous stable lines of murine Tregs | Low-zone IL-2 signaling favors Treg dominance in vitro. |
Zones of antigenic and cytokine signaling appear operative for definition of the Treg niche, including intermediate-efficiency TCR signaling (i.e., intermediate quality of TCR signaling efficacy), high-zone IFN-β signaling (i.e., contingent upon high IFN-β concentrations), and low-zone IL-2 signaling (i.e., contingent upon limiting concentrations of IL-2).
8. GMCSF-based tolerogenic vaccines: evidence that intermediate-efficiency TCR signaling defines a Treg niche.
8.1. GMCSF-NAg tolerogenic vaccines: the contemporary model:
The study of GMCSF-NAg tolerogenic vaccines has revealed a strategy to directly target covalently-tethered NAg to tolerogenic DCs and thereby target NAg for presentation to Tregs, based on the simple subcutaneous injection of GMCSF-NAg in saline [129, 130, 181, 203, 205–207]. The model predicts that the GM-CSF domain of GMCSF-NAg exhibits high affinity interactions with GM-CSF (i.e., CD116/ CD131) receptors on myeloid cells to stimulate DC differentiation concurrent with upregulation of MHCII antigen processing and presentation activity. The GM-CSF domain also efficiently targets the NAg domain for enhanced presentation by these DCs. Because NAgs have been subject to central and peripheral tolerance, the MHCII/ NAg complexes on these DCs are predominantly recognized as intermediate-efficiency MHCII-ligands by the Treg repertoire, which converts these DCs to a tolerogenic phenotype and drives clonal expansion of Tregs to inhibit concurrent and future encephalitogenic responses [129, 130]. As a parallel mechanism, presentation of MHCII/ NAg by these GM-CSF-conditioned DCs to pathogenic MOG-specific effector T cells stimulates production of nitric oxide and other mechanisms of bystander suppression to deplete the NAg-specific Tcon repertoire and inhibit ongoing pathogenesis [207].
8.2. The experimental foundation for GMCSF-NAg tolerogenic vaccines.
The study of GMCSF-NAg tolerogenic vaccines was initiated in the monophasic Lewis rat model of EAE [205]. A fusion protein comprised of rat GM-CSF and the encephalitogenic 69–87 peptide of myelin basic protein (MBP69–87) (i.e., the NAg) suppressed EAE in Lewis rats when administered as a prophylactic or therapeutic vaccine. The GMCSF-MBP69–87 fusion protein exhibited exquisite antigen targeting to DCs and was 1000-fold more potent than the MBP69–87 peptide in assays measuring antigenic proliferation of MBP-specific T cell clones. GM-CSF did not potentiate antigenic responses when GM-CSF and MBP69–87 were added as separate molecules, and the potentiated antigenic responses of GMCSF-NAg were inhibited by free GM-CSF but not by M-CSF. Similar studies of other cytokine-NAg fusion proteins including MCSF-NAg, IL4-NAg, IL16-NAg, and IL2-NAg showed that cytokine-NAg fusion proteins targeted the NAg domain for enhanced presentation by particular APC subsets but GMCSF-NAg nonetheless exhibited the most robust tolerogenic activity as measured by inhibition of EAE [203, 205, 208, 209]. This finding highlights a potentially special role of DCs in immunological tolerance [39].
GMCSF-NAg vaccines were also tolerogenic in murine models of EAE [129, 130, 181, 203, 206, 207]. In the chronic C57BL/6 model of EAE, a murine GMCSF-MOG fusion protein comprised of GM-CSF and the MOG35–55 peptide blocked the development and progression of EAE when administered (subcutaneously in saline) before active induction of EAE or when administered after the onset of EAE in both active and passive models of EAE [203, 206, 207]. GMCSF-MOG was an effective therapeutic in an aggressive B cell-deficient model of EAE, which discounted any potential role of anti-GM-CSF antibodies in the resolution of EAE [207]. In the relapsing-remitting SJL model of murine EAE, a GMCSF-Proteolipid Protein (PLP)139–151 fusion protein abrogated the subsequent induction of EAE [206]. In both Lewis rat and mouse EAE models, the single-chain fusion protein GMCSF-NAg elicited tolerance to EAE whereas control formulations including a mixture of “GMCSF + NAg”, GM-CSF alone, NAg alone, or saline had no effect [203, 205, 206]. Therefore, covalent linkage of GM-CSF and NAg domains was required for tolerogenic activity. The tolerogenic activity of GMCSF-NAg reflected the action of CD25+ Tregs, because treatment of mice with the Treg-depleting anti-CD25 mAb PC61 reversed tolerance [129]. In conclusion, these data showed that GMCSF-NAg fusion proteins are efficacious tolerogens in multiple models of rat and murine EAE.
8.3. The efficiency of TCR engagement determines the Treg/ Tcon balance:
The GMCSF-NAg platform is unique in that GMCSF-NAg elicits robust systemic induction of FOXP3+ Tregs in TCR transgenic (2D2), FOXP3-reporter (FIG) mice (2D2-FIG) mice, which have a transgenic T cell repertoire that recognizes MOG35–55 as an intermediate-efficiency ligand and NFM13–37 as a high-efficiency ligand [153]. A single subcutaneous or intravenous vaccination of the intermediate-efficiency GMCSF-MOG vaccine in saline elicited a major population of FOXP3+ Tregs (20–40% of circulating CD4+ T cells) that appeared within 3–4 days, was sustained over several weeks, expressed canonical Treg markers, and was present systemically at high frequencies in the blood, spleen, and lymph nodes [129, 130]. Covalent linkage of GM-CSF with MOG35–55 was required for Treg induction, and repeated booster vaccinations with GMCSF-MOG elicited FOXP3 expression in over 40% of all circulating T cells. The ability of the vaccine to induce Tregs was dependent upon the efficiency of T cell antigen recognition, because vaccination of 2D2-FIG or Ovalbumin (OVA)323–339-specific transgenic OTII mice (OTII-FIG) mice with the high-efficiency ligands GMCSF-NFM or GMCSF-OVA, respectively, did not elicit Tregs. Instead the high-efficiency GMCSF-NFM vaccine induced a robust Tcon memory response. In conclusion, these findings indicate that the GM-CSF domain was critical for DC conditioning and antigenic targeting whereas the NAg domain of the GMCSF-NAg tolerogenic vaccine established the balance between regulatory and conventional T cell responses.
8.4. GMCSF-NAg fusion proteins mediate tolerogenic activity in inflammatory environments:
The GMCSF-NAg platform is also unique among the broad class of tolerogenic vaccines because GMCSF-NAg elicited tolerance in both quiescent and inflammatory environments. That is, GMCSF-MOG was an effective tolerogen even when injected subcutaneously in saline adjacent to an encephalitogenic Complete Freund’s Adjuvant (CFA)/ MOG35–55 emulsion in C57BL/6 mice [203, 207]). GMCSF-MOG and GMCSF-PLP were also effective tolerogens when directly emulsified within an encephalitogenic CFA/ NAg emulsion in C57BL/6 and SJL models of EAE, respectively [207]. GMCSF-MOG also exhibited profound inhibitory activity when directly included in a CFA/ MOG35–55 emulsion in B cell-deficient mice. As noted for tolerance induction [207], GMCSF-MOG also elicited high levels of circulating Tregs in 2D2-FIG mice when administered in immunogenic adjuvants such as CFA or Alum [129]. In conclusion, these data show that GMCSF-NAg elicited dominant tolerogenic regulation of EAE even in robustly pro-inflammatory environments.
An important question in vaccinology is whether tolerogenic vaccines are sub-dominant when co-administered with immunogenic vaccines. A standard assumption is that a tolerogenic vaccine such as GMCSF-MOG would be subdominant when co-administered with an immunogenic vaccine, such as GMCSF-NFM. However, this was not the case [130]. Rather, the combined GMCSF-MOG/ GMCSF-NFM vaccine elicited percentages and numbers of circulating Tregs that were intermediate compared to vaccination with GMCSF-MOG or GMCSF-NFM alone. GMCSF-MOG also caused an anergic phenotype among circulating CD3+ CD4+ MOG-specific Tcons including an enduring diminution of TCR expression on a per cell basis and persistent expression of CD44 [129, 130]. Importantly, the tolerogenic GMCSF-MOG vaccine was dominant compared to the immunogenic GMCSF-NFM vaccine for induction of anergy in Tcons because a combined GMCSF-MOG/ GMCSF-NFM vaccine, like the GMCSF-MOG vaccine, elicited a long-term TCRlow CD44high CD62high Tcon phenotype whereas GMCSF-NFM lacked this activity [130]. Thus, a tolerogenic vaccine targeting NAg to DCs robustly altered both Treg and Tcon repertoires by mechanisms that were partially or completely dominant for the MOG-specific Treg and Tcon repertoires, respectively.
These studies also implicated CD40L as an integrative node because tolerogenic or immunogenic vaccines were demarcated by deficient or robust CD40L induction and the presence or absence of Treg induction, respectively. Mechanistic involvement of CD40L was shown in experiments wherein in vivo administration of an anti-CD40 agonist mAb inhibited the Treg-inductive activity of GMCSF-MOG. This study thereby supported the concept that T cells tally intermediate and high efficiency TCR signaling events to balance the expression of CD40L as part of an integrative system controlling adaptive output.
8.5. Why GM-CSF?
Although GM-CSF is widely noted as a pro-inflammatory cytokine, an extensive literature supports the concept that GM-CSF has tolerogenic activity in many disease models. For example, administration of exogenous GM-CSF inhibits experimental autoimmune myasthenia gravis, experimental autoimmune thyroiditis, type I diabetes, and graft versus host disease via the induction of ‘tolerogenic DCs’ and regulatory T cell subsets [210–219]. As noted above, the study of GMCSF-NAg fusion proteins showed that GM-CSF conditioning of DCs, GM-CSF-mediated targeting of NAg to those conditioned DCs, and the consequent presentation of intermediate-efficiency NAgs to a NAg-specific T cell repertoire resulted in severe diminution of the Tcon repertoire and dominant outgrowth of the Treg repertoire [130]. Because the antigenic domain of GMCSF-antigen fusion proteins was a pivotal parameter in determining the Treg/ Tcon balance, GM-CSF may be considered an immune ‘amplifier’ that enhances the intrinsic activity of a local set of antigens.
9. IFN-β-based tolerogenic vaccines: High concentrations of IFN-β favor a Treg niche:
9.1. The experimental foundation for IFN-β-based tolerogenic vaccines.
Consistent with the use of IFN-β as a leading first-option therapeutic for MS for over 2 decades and the strong therapeutic activity of IFN-β in multiple models of EAE, studies from our laboratory [202–204] implicated IFN-β as a formative cytokine in the FOXP3+ Treg lineage and as a potent Treg-biasing tolerogenic adjuvant. Initial studies determined that IFN-β-NAg fusion proteins were effective prophylactic and therapeutic vaccines that suppressed multiple rodent models of EAE [202–204]. Interestingly, vaccines comprised of the IFNβ-NAg fusion proteins or “IFN-β + NAg” as separate molecules suppressed the acute monophasic model of EAE in Lewis rats [202]. However, IFN-β-NAg fusion proteins required covalently-linked IFN-β and NAg domains to inhibit EAE in chronic C57BL/6 and SJL mouse models of EAE [203, 204]. The interpretation was that unlinked IFN-β and NAg domains were sufficient to inhibit acute EAE (i.e. the rat model) whereas covalently-linked domains were more robust tolerogens, which were required to mitigate chronic EAE (i.e., the mouse models) [204].
A second-generation IFN-β-based tolerogenic vaccine incorporated three components; IFN-β, NAg, and the Alum adjuvant [204]. Given the requirement for covalent linkage in murine EAE models [203, 204], the vaccine platform was based on the concept that IFN-β and the NAg would bind Alum, which would provide indirect noncovalent linkage of IFN-β and NAg needed for tolerogenic activity. The “IFN-β + NAg in Alum” tolerogenic vaccine inhibited EAE in C57BL/6 mice in both pre-treatment and therapeutic regimens by a mechanism that was dependent upon IFN-β and the Alum adjuvant. Prophylactic treatment with the “IFN-β + NAg in Alum” vaccine inhibited the subsequent induction of EAE. The tolerogenic activity of this vaccine was reversed by an anti-CD25 Treg-depleting mAb, which restored full disease susceptibility. This finding implicated vaccine-induced CD25high Tregs as the operative subset responsible for inhibition of EAE. Vaccination with “IFN-β + MOG in Alum” impaired subsequent encephalitogenic sensitization with MOG35–55 emulsified in CFA and thereby inhibited clonal expansion of MOG-specific effector T cells. IFN-β/Alum-based vaccination exhibited hallmarks of infectious tolerance, because “IFN-β + OVA in Alum” vaccination inhibited EAE elicited by “OVA + MOG in CFA” but not by “MOG in CFA”. The interpretation was that tolerogenic vaccination with “IFN-β + OVA in Alum” elicited OVA-specific Tregs that inhibited encephalitogenic sensitization, but only when OVA323–339 and the encephalitogenic MOG3–55 peptides were mixed in the same CFA emulsion to ensure co-presentation of the two antigens on the same DCs [204].
IFN-β also exhibited Treg-inductive activity in both in vivo and in vitro models [204]. “IFN-β + MOG in Alum” vaccination of 2D2-FIG mice elicited elevated numbers and percentages of FOXP3+ T cells in blood and secondary lymphoid organs. Repeated boosters of the vaccine elicited an activated CD44high CD25+ FOXP3+ Treg population. The combination of IFN-β and MOG35–55 (in the absence of Alum) synergistically elicited suppressive FOXP3+ Tregs in vitro by a mechanism that was neutralized by an anti-TGF-β mAb. IFN-β-induced Tregs that were induced in vitro suppressed EAE when these Tregs were adoptively transferred in actively-challenged recipients. Overall, these findings indicated that “IFN-β + NAg in Alum” vaccination elicited NAg-specific, suppressive FOXP3+ CD25+ Tregs that inhibited EAE. These data are consistent with the concept that IFN-β has an activity spectrum that favors suppressive FOXP3+ Tregs [220–223]. The mechanisms by which IFN-β elicits Treg dominance are unknown, and both direct and indirect mechanisms may be relevant. IFN-β has also been shown to promote a FOXP3negative FOXA1+ regulatory T cell subset that resolves CNS inflammation at least in part through PD-L1-mediated killing of activated T cells [31, 224, 225]. Possibly, FOXA1+ and FOXP3+ Treg subsets act cooperatively to control CNS autoimmunity.
9.2. Why would IFN-β inhibit adaptive immunity?
As the prototypic driver of innate immunity, why would IFN-β inhibit adaptive immunity? Several overlapping possibilities deserve consideration. First, the IFN-β/ Treg axis may have evolved to concurrently inhibit viral dissemination via IFN-β while also inhibiting excessive anti-viral immunopathogenesis via Tregs as complementary mechanisms to preserve tissue integrity. Second, the IFN-β/ Treg axis may inhibit or kill infected leukocytes and thereby prevent leukocyte emigration away from virally infected tissues to prevent the systemic spread of the virus. Third, one might surmise that Tregs may represent an evolutionary magnet for infectious agents because immunosuppressive activity of virally-infected Tregs might provide a protected niche for an infectious agent. The idea is that viral pathogens or other infectious agents may specifically target Tregs as part of a protected infectious niche and thereby establish chronic infection under the cover of strong immunosuppression. The evolutionary patch for this immune system vulnerability may be a specialized IFN-β/ Treg axis that affords heightened innate defenses for an immunosuppressive Treg lineage that otherwise would be a favored niche for infectious agents.
10. Continuous stable lines of murine FOXP3+ Tregs: Low concentrations of IL-2 constitute a Treg niche.
FOXP3+ Tregs represent a promising platform for cell-based interventions and tissue-specific therapy to treat autoimmune disease and other inflammatory disorders [87–90, 226–228]. Contemporary Treg immunotherapy would be advanced by new technologies that enable long-term expansion and activation of stable polyclonal, antigen-specific FOXP3+ Tregs in cell culture. To realize this goal, we studied whether anti-CD25 mAbs such as PC61 differentially affected the IL-2 dependent growth and survival of mouse Treg and Tcon subsets in vitro. These studies revealed that the inhibitory the PC61 anti-CD25 mAb partially inhibited IL-2 dependent responses by CD25high Tregs but strongly blocked IL-2 dependent growth by Tcons. This differential inhibition resulted in the predominant outgrowth of Tregs and the establishment of highly-enriched, continuous Treg lines, presumably because higher levels of cell surface CD25 on Tregs conferred partial resistance to the inhibitory mAb [61]. Although the PC61 anti-CD25 mAb is used to deplete murine FOXP3+ Tregs in vivo by a mechanism of antibody-dependent cytotoxicity (ADCC) [70], PC61 lacked cytotoxic activity in vitro in T cell maintenance and activation cultures, due to the absence of ADCC-competent macrophages. This study revealed a ‘Treg window’ that was operationally defined by relatively low IL-2 concentrations coupled with high concentrations of an anti-CD25 mAb. These conditions enabled dominant survival and expansion of murine Tregs that stably expressed high levels FOXP3 over several months of continuous propagation without any indication of phenotypic instability.
Continuous FOXP3+ Treg lines were derived from MOG35–55-specific 2D2 transgenic mice, OVA323–339-specific transgenic OTII mice, and mitogen-stimulated FIG mice without physical purification or genetic modification. The FOXP3+ Treg lineage was stable and dominant in vitro when PC61 was included in culture during the IL-2 expansion phase. These antigen-specific and polyclonal lines contained approximately 90% - 99% FOXP3high Tregs and were maintained indefinitely (e.g., 3 months duration) without attenuation or loss of the Treg phenotype [61]. A reiterative activation-rest strategy was used for the continuous propagation and expansion of stable FOXP3+ Treg lines with one cycle comprised of a 3-day activation culture followed by several 3-day rest cultures in IL-2 and PC61. During the resting phase, cell numbers were static or were modestly expanded (~2 fold) over the course of a week. During activation with mitogen/ antigen and irradiated DCs, Tregs required TGF-β to maintain FOXP3 expression during exponential expansion. During a 3-day activation culture and the subsequent 3-day passage in IL-2/ PC61activation, Treg numbers expanded 10–25-fold. A long-term Treg line derived from transgenic 2D2 mice exhibited suppressive activity in both prophylactic and therapeutic adoptive transfer studies of EAE. In the therapeutic model, blastogenic FOXP3+ Tregs were prepared by activation for 3 days with DCs, Con-A, MOG35–55, IL-2, and TGF-β, and approximately 97% of T cells expressed high levels of FOXP3. Adoptive transfer of these activated Tregs on day 15 during severe EAE significantly alleviated disease. These data provided evidence that blastogenic MOG-specific Tregs mitigate EAE when administered in therapeutic and prophylactic treatment regimens.
In conclusion, low IL-2 concentrations coupled with high PC61 concentrations constrained IL-2 signaling to a low-intensity range that enabled dominance of suppressive CD25high FOXP3+ Tregs in vitro [61]. These findings provide a significant advance in our understanding of the cytokine ‘Treg niche’ which exists within the confines of a low IL-2 concentration range that is unique to CD25high FOXP3+ Tregs. These findings represent a significant technical advance that may provide a foundation for clinical development of feasible antigen-specific Treg-based immunotherapies with the potential to treat broad classes of human inflammatory disease. These data parallel the emerging field of low-dose IL-2 therapy which has beneficial activity for induction of FOXP3+ Tregs and alleviation of autoimmune inflammatory disease [229]. The use of an anti-CD25 mAb has qualitative advantages because the combination of an anti-CD25 mAb and low IL-2 concentrations provides a consistent clamp on IL-2 signaling and a stringent window for selective Treg responses.
11. Conclusions:
The stratification of TCR recognition efficiencies by which TCRs on FOXP3+ Tregs recognize self and extended- self MHC-ligands are centrally important for understanding the modus operandi of Tregs, including thymic and peripheral selection, maintenance of the peripheral Treg repertoire, tissue-residency, phenotypic stability, and suppressive function. The stratification of TCR recognition efficiencies that distinguish the Treg and Tcon repertoire, in combination with antigen complexity theory, provides a foundation to understand how DCs may integrate signals from tissue-resident Treg and Tcon repertoires to provide adaptive tolerogenic and immunogenic responses. Differential regulation of IL-2 production and IL-2 responsive pathways among the Treg and Tcon repertoires provides an understanding of the relative sources and sinks for paracrine IL-2 in a local tissue, which in combination with antigen complexity theory may also underlie adaptive mechanisms controlling the balance of tolerogenic and immunogenic outcomes. The study of tolerogenic vaccination and continuous lines of stable murine Tregs provide insight into the Treg niche, including evidence that zones of antigenic and cytokine signaling appear operative for definition of the Treg niche, including an intermediate-efficiency zone of TCR signaling, a high concentration zone of IFN-β signaling, and a low concentration zone of IL-2 signaling (Table 2). The Treg field is reaching an inflection point where advances in basic scientific understanding fuel clinical applicability and vice versa.
Highlights:
TCR recognition efficiencies shape the repertoire of CD4+ CD25high FOXP3+ regulatory T cells.
The Treg antigenic niche controls tissue-residency, stability, and regulatory function in peripheral tissues.
Tolerogenic vaccination and Treg-based immunotherapy reverse chronic autoimmune disease.
Tregs mediate tolerance by mechanisms of bystander suppression and infectious tolerance.
Intermediate-efficiency MHC-ligands, high-zone IFN-β, and low-zone IL-2 favor Treg responses.
Funding:
Research from the MDM laboratory reviewed here was supported by the NIH grants R01-NS072150 and R01-AI126398 (MDM), the Harriet and John Wooten Laboratory for Alzheimer’s and Neurodegenerative Disease Research (MDM), the Brody Brothers Endowment Fund (MDM), and by the NIH S10-OD021615 instrumentation grant.
Abbreviations:
- APC
Antigen Presenting Cell
- CNS
Central Nervous System
- CFA
Complete Freund’s Adjuvant
- Tcon
Conventional T Cell
- DC
Dendritic Cell
- EAE
Experimental Autoimmune Encephalomyelitis
- FOXP3
Forkhead Box P3
- Tregs
FOXP3+ Regulatory T Cells
- GMCSF
Granulocyte-Macrophage Colony Stimulating Factor
- IFN-β
Interferon-β
- IL-2
Interleukin-2
- CD25 Or IL2Rα
Interleukin-2 Receptor-Alpha
- MHC
Major Histocompatibility Complex
- MHCI or MHCII
Major Histocompatibility Complex Class I or Class II
- MBP
Myelin Basic Protein Peptide 69–87
- MOG
Myelin Oligodendrocyte Glycoprotein Peptide 35–55
- MS
Multiple Sclerosis
- Nag
Neuroantigen
- NFM
Neurofilament Medium Peptide 13–37
- OVA
Ovalbumin Peptide 323–339
- PLP
Proteolipid Protein Peptide 139–151
- TCR
T Cell Antigen Receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Competing Interest: The authors declare that no competing financial interests or personal relationships influenced the content of this review.
References
- [1].Hall BM, CD4+CD25+ T Regulatory Cells in Transplantation Tolerance: 25 Years On, Transplantation, 100 (2016) 2533–2547. [DOI] [PubMed] [Google Scholar]
- [2].Bacchetta R, Barzaghi F, Roncarolo MG, From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation, Ann. N. Y. Acad. Sci, (2016). [DOI] [PubMed] [Google Scholar]
- [3].Noval Rivas M, Chatila TA, Regulatory T cells in allergic diseases, J. Allergy Clin. Immunol, 138 (2016) 639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alessandrini A, Turka LA, FOXP3-positive regulatory T cells and kidney allograft tolerance, Am. J. Kidney Dis, 69 (2017) 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Danikowski KM, Jayaraman S, Prabhakar BS, Regulatory T cells in multiple sclerosis and myasthenia gravis, J. Neuroinflammation, 14 (2017) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Martin-Orozco E, Norte-Munoz M, Martinez-Garcia J, Regulatory T Cells in Allergy and Asthma, Front Pediatr, 5 (2017) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pereira LMS, Gomes STM, Ishak R, Vallinoto ACR, Regulatory T Cell and Forkhead Box Protein 3 as Modulators of Immune Homeostasis, Front. Immunol, 8 (2017) 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Campbell C, Rudensky A, Roles of Regulatory T Cells in Tissue Pathophysiology and Metabolism, Cell Metab, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mohr A, Atif M, Balderas R, Gorochov G, Miyara M, The role of FOXP3(+) regulatory T cells in human autoimmune and inflammatory diseases, Clin. Exp. Immunol, 197 (2019) 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Becker M, Levings MK, Daniel C, Adipose-tissue regulatory T cells: Critical players in adipose-immune crosstalk, Eur. J. Immunol, 47 (2017) 1867–1874. [DOI] [PubMed] [Google Scholar]
- [11].Albany CJ, Trevelin SC, Giganti G, Lombardi G, Scotta C, Getting to the Heart of the Matter: The Role of Regulatory T-Cells (Tregs) in Cardiovascular Disease (CVD) and Atherosclerosis, Front. Immunol, 10 (2019) 2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Peng X, Moore MW, Peng H, Sun H, Gan Y, Homer RJ, Herzog EL, CD4+CD25+FoxP3+ Regulatory Tregs inhibit fibrocyte recruitment and fibrosis via suppression of FGF-9 production in the TGF-beta1 exposed murine lung, Front. Pharmacol, 5 (2014) 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ali N, Rosenblum MD, Regulatory T cells in skin, Immunology, 152 (2017) 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Frantz C, Auffray C, Avouac J, Allanore Y, Regulatory T Cells in Systemic Sclerosis, Front. Immunol, 9 (2018) 2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Villalta SA, Rosenthal W, Martinez L, Kaur A, Sparwasser T, Tidball JG, Margeta M, Spencer MJ, Bluestone JA, Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy, Sci. Transl. Med, 6 (2014) 258ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].He F, Balling R, The role of regulatory T cells in neurodegenerative diseases, Wiley Interdiscip. Rev. Syst. Biol. Med, 5 (2013) 153–180. [DOI] [PubMed] [Google Scholar]
- [17].Gonzalez H, Pacheco R, T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases, J. Neuroinflammation, 11 (2014) 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Negi N, Das BK, CNS: Not an immunoprivilaged site anymore but a virtual secondary lymphoid organ, Int. Rev. Immunol, 37 (2018) 57–68. [DOI] [PubMed] [Google Scholar]
- [19].Thonhoff JR, Simpson EP, Appel SH, Neuroinflammatory mechanisms in amyotrophic lateral sclerosis pathogenesis, Curr. Opin. Neurol, 31 (2018) 635–639. [DOI] [PubMed] [Google Scholar]
- [20].Belkaid Y, Tarbell K, Regulatory T cells in the control of host-microorganism interactions, Annu. Rev. Immunol, 27 (2009) 551–589. [DOI] [PubMed] [Google Scholar]
- [21].Boer MC, Joosten SA, Ottenhoff TH, Regulatory T-Cells at the Interface between Human Host and Pathogens in Infectious Diseases and Vaccination, Front. Immunol, 6 (2015) 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wing JB, Tanaka A, Sakaguchi S, Human FOXP3(+) Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer, Immunity, 50 (2019) 302–316. [DOI] [PubMed] [Google Scholar]
- [23].Churlaud G, Pitoiset F, Jebbawi F, Lorenzon R, Bellier B, Rosenzwajg M, Klatzmann D, Human and Mouse CD8(+)CD25(+)FOXP3(+) Regulatory T Cells at Steady State and during Interleukin-2 Therapy, Front. Immunol, 6 (2015) 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F, Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse, Nat. Genet, 27 (2001) 68–73. [DOI] [PubMed] [Google Scholar]
- [25].Deng G, Song X, Greene MI, FoxP3 in Treg cell biology: a molecular and structural perspective, Clin. Exp. Immunol, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ramsdell F, Rudensky AY, Foxp3: a genetic foundation for regulatory T cell differentiation and function, Nat. Immunol, 21 (2020) 708–709. [DOI] [PubMed] [Google Scholar]
- [27].Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF, Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells, J. Clin. Invest, 112 (2003) 1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE, Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells, Eur. J. Immunol, 37 (2007) 129–138. [DOI] [PubMed] [Google Scholar]
- [29].McMurchy AN, Gillies J, Gizzi MC, Riba M, Garcia-Manteiga JM, Cittaro D, Lazarevic D, Di Nunzio S, Piras IS, Bulfone A, Roncarolo MG, Stupka E, Bacchetta R, Levings MK, A novel function for FOXP3 in humans: intrinsic regulation of conventional T cells, Blood, 121 (2013) 1265–1275. [DOI] [PubMed] [Google Scholar]
- [30].Voss K, Lake C, Luthers CR, Lott NM, Dorjbal B, Arjunaraja S, Bauman BM, Soltis AR, Sukumar G, Dalgard CL, Snow AL, FOXP3 protects conventional human T cells from premature restimulation-induced cell death, Cell. Mol. Immunol, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu Y, Carlsson R, Comabella M, Wang J, Kosicki M, Carrion B, Hasan M, Wu X, Montalban X, Dziegiel MH, Sellebjerg F, Sorensen PS, Helin K, Issazadeh-Navikas S, FoxA1 directs the lineage and immunosuppressive properties of a novel regulatory T cell population in EAE and MS, Nat. Med, 20 (2014) 272–282. [DOI] [PubMed] [Google Scholar]
- [32].Liu J, Cao X, Regulatory dendritic cells in autoimmunity: A comprehensive review, J. Autoimmun, 63 (2015) 1–12. [DOI] [PubMed] [Google Scholar]
- [33].Zhao H, Liao X, Kang Y, Tregs: Where We Are and What Comes Next?, Front. Immunol, 8 (2017) 1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kunicki MA, Amaya Hernandez LC, Davis KL, Bacchetta R, Roncarolo MG, Identity and Diversity of Human Peripheral Th and T Regulatory Cells Defined by Single-Cell Mass Cytometry, J. Immunol, 200 (2018) 336–346. [DOI] [PubMed] [Google Scholar]
- [35].Veglia F, Perego M, Gabrilovich D, Myeloid-derived suppressor cells coming of age, Nat. Immunol, 19 (2018) 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, Callahan DJ, Sun Z, Sun T, Tabib T, Pennathur A, Corry DB, Luketich JD, Lafyatis R, Chen W, Poholek AC, Bruno TC, Workman CJ, Vignali DAA, Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion, Nat. Immunol, 20 (2019) 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].DeNardo DG, Ruffell B, Macrophages as regulators of tumour immunity and immunotherapy, Nat. Rev. Immunol, 19 (2019) 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sullivan JA, Tomita Y, Jankowska-Gan E, Lema DA, Arvedson MP, Nair A, Bracamonte-Baran W, Zhou Y, Meyer KK, Zhong W, Sawant DV, Szymczak-Workman AL, Zhang Q, Workman CJ, Hong S, Vignali DAA, Burlingham WJ, Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance, Cell reports, 30 (2020) 1039–1051.e1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D, Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity, J. Exp. Med, 206 (2009) 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD, The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3, Nat. Genet, 27 (2001) 20–21. [DOI] [PubMed] [Google Scholar]
- [41].van der Vliet HJ, Nieuwenhuis EE, IPEX as a result of mutations in FOXP3, Clin. Dev. Immunol, 2007 (2007) 89017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ramsdell F, Ziegler SF, FOXP3 and scurfy: how it all began, Nat. Rev. Immunol, 14 (2014) 343–349. [DOI] [PubMed] [Google Scholar]
- [43].Williams LM, Rudensky AY, Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3, Nat. Immunol, 8 (2007) 277–284. [DOI] [PubMed] [Google Scholar]
- [44].Zheng Y, Rudensky AY, Foxp3 in control of the regulatory T cell lineage, Nat. Immunol, 8 (2007) 457–462. [DOI] [PubMed] [Google Scholar]
- [45].Kim J, Lahl K, Hori S, Loddenkemper C, Chaudhry A, deRoos P, Rudensky A, Sparwasser T, Cutting edge: depletion of Foxp3+ cells leads to induction of autoimmunity by specific ablation of regulatory T cells in genetically targeted mice, J. Immunol, 183 (2009) 7631–7634. [DOI] [PubMed] [Google Scholar]
- [46].Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, Yang SH, Anthony BA, Sverdrup FM, Krow-Lucal E, MacKenzie TC, Johnson DS, Meyer EH, Löhr A, Hsu A, Koo J, Liao W, Gupta R, Debbaneh MG, Butler D, Huynh M, Levin EC, Leon A, Hoffman WY, McGrath MH, Alvarado MD, Ludwig CH, Truong HA, Maurano MM, Gratz IK, Abbas AK, Rosenblum MD, Memory regulatory T cells reside in human skin, J. Clin. Invest, 124 (2014) 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Panduro M, Benoist C, Mathis D, Tissue Tregs, Annu. Rev. Immunol, 34 (2016) 609–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sharma A, Rudra D, Emerging Functions of Regulatory T Cells in Tissue Homeostasis, Front. Immunol, 9 (2018) 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McIntyre LL, Greilach SA, Othy S, Sears-Kraxberger I, Wi B, Ayala-Angulo J, Vu E, Pham Q, Silva J, Dang K, Rezk F, Steward O, Cahalan MD, Lane TE, Walsh CM, Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant, Neurobiol. Dis, (2020) 104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhou X, Tang J, Cao H, Fan H, Li B, Tissue resident regulatory T cells: novel therapeutic targets for human disease, Cell. Mol. Immunol, 12 (2015) 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DA, Ziegler SF, Regulatory T cells: recommendations to simplify the nomenclature, Nat. Immunol, 14 (2013) 307–308. [DOI] [PubMed] [Google Scholar]
- [52].Karimi S, Chattopadhyay S, Chakraborty NG, Manipulation of regulatory T cells and antigen-specific cytotoxic T lymphocyte-based tumour immunotherapy, Immunology, 144 (2015) 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H, Inducing and expanding regulatory T cell populations by foreign antigen, Nat. Immunol, 6 (2005) 1219–1227. [DOI] [PubMed] [Google Scholar]
- [54].Pyzik M, Piccirillo CA, TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets, J. Leukoc. Biol, 82 (2007) 335–346. [DOI] [PubMed] [Google Scholar]
- [55].Umeshappa CS, Singha S, Blanco J, Shao K, Nanjundappa RH, Yamanouchi J, Parés A, Serra P, Yang Y, Santamaria P, Suppression of a broad spectrum of liver autoimmune pathologies by single peptide-MHC-based nanomedicines, Nature communications, 10 (2019) 2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Omenetti S, Pizarro TT, The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome, Front. Immunol, 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM, Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3, J. Exp. Med, 198 (2003) 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, Li SH, Simpson PM, Chatila TA, Williams CB, A central role for induced regulatory T cells in tolerance induction in experimental colitis, J. Immunol, 182 (2009) 3461–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hong SW, Kim KS, Surh CD, Beyond Hygiene: Commensal Microbiota and Allergic Diseases, Immune Netw, 17 (2017) 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nikolouli E, Hardtke-Wolenski M, Hapke M, Beckstette M, Geffers R, Floess S, Jaeckel E, Huehn J, Alloantigen-Induced Regulatory T Cells Generated in Presence of Vitamin C Display Enhanced Stability of Foxp3 Expression and Promote Skin Allograft Acceptance, Front. Immunol, 8 (2017) 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wilkinson DS, Ghosh D, Nickle RA, Moorman CD, Mannie MD, Partial CD25 antagonism enables dominance of antigen-inducible CD25high FOXP3+ regulatory T cells as a basis for a Treg-based adoptive immunotherapy, Front. Immunol, 10.3389/fimmu.2017.01782 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Schmitt EG, Williams CB, Generation and function of induced regulatory T cells, Front. Immunol, 4 (2013) 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J, DNA methylation controls Foxp3 gene expression, Eur. J. Immunol, 38 (2008) 1654–1663. [DOI] [PubMed] [Google Scholar]
- [64].Polansky JK, Schreiber L, Thelemann C, Ludwig L, Krüger M, Baumgrass R, Cording S, Floess S, Hamann A, Huehn J, Methylation matters: binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells, J. Mol. Med. (Berl.), 88 (2010) 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Toker A, Engelbert D, Garg G, Polansky JK, Floess S, Miyao T, Baron U, Düber S, Geffers R, Giehr P, Schallenberg S, Kretschmer K, Olek S, Walter J, Weiss S, Hori S, Hamann A, Huehn J, Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus, J. Immunol, 190 (2013) 3180–3188. [DOI] [PubMed] [Google Scholar]
- [66].Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, Bluestone JA, Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response, Immunity, 39 (2013) 949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shevach EM, McHugh RS, Piccirillo CA, Thornton AM, Control of T-cell activation by CD4+ CD25+ suppressor T cells, Immunol. Rev, 182 (2001) 58–67. [DOI] [PubMed] [Google Scholar]
- [68].Setoguchi R, Hori S, Takahashi T, Sakaguchi S, Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization, J. Exp. Med, 201 (2005) 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hotta-Iwamura C, Benck C, Coley WD, Liu Y, Zhao Y, Quiel JA, Tarbell KV, Low CD25 on autoreactive Tregs impairs tolerance via low dose IL-2 and antigen delivery, J. Autoimmun, 90 (2018) 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Setiady YY, Coccia JA, Park PU, In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes, Eur. J. Immunol, 40 (2010) 780–786. [DOI] [PubMed] [Google Scholar]
- [71].Ghosh D, Curtis AD 2nd, Wilkinson DS, Mannie MD, Depletion of CD4+ CD25+ regulatory T cells confers susceptibility to experimental autoimmune encephalomyelitis (EAE) in GM-CSF-deficient Csf2−/− mice, J. Leukoc. Biol, 100 (2016) 747–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Minami Y, Kono T, Miyazaki T, Taniguchi T, The IL-2 receptor complex: its structure, function, and target genes, Annu. Rev. Immunol, 11 (1993) 245–268. [DOI] [PubMed] [Google Scholar]
- [73].Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H, Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis, Nat. Med, 20 (2014) 62–68. [DOI] [PubMed] [Google Scholar]
- [74].Ferreira RC, Simons HZ, Thompson WS, Rainbow DB, Yang X, Cutler AJ, Oliveira J, Castro Dopico X, Smyth DJ, Savinykh N, Mashar M, Vyse TJ, Dunger DB, Baxendale H, Chandra A, Wallace C, Todd JA, Wicker LS, Pekalski ML, Cells with Treg-specific FOXP3 demethylation but low CD25 are prevalent in autoimmunity, J. Autoimmun, 84 (2017) 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. , Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta, Science, 268 (1995) 1472–1476. [DOI] [PubMed] [Google Scholar]
- [76].Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW, Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment, Immunity, 3 (1995) 521–530. [DOI] [PubMed] [Google Scholar]
- [77].Klebb G, Autenrieth IB, Haber H, Gillert E, Sadlack B, Smith KA, Horak I, Interleukin-2 is indispensable for development of immunological self-tolerance, Clin. Immunol. Immunopathol, 81 (1996) 282–286. [DOI] [PubMed] [Google Scholar]
- [78].Suzuki H, Hayakawa A, Bouchard D, Nakashima I, Mak TW, Normal thymic selection, superantigen-induced deletion and Fas-mediated apoptosis of T cells in IL-2 receptor beta chain-deficient mice, Int. Immunol, 9 (1997) 1367–1374. [DOI] [PubMed] [Google Scholar]
- [79].Sharfe N, Dadi HK, Shahar M, Roifman CM, Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor, Proc. Natl. Acad. Sci. U. S. A, 94 (1997) 3168–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Moriggl R, Topham DJ, Teglund S, Sexl V, McKay C, Wang D, Hoffmeyer A, van Deursen J, Sangster MY, Bunting KD, Grosveld GC, Ihle JN, Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells, Immunity, 10 (1999) 249–259. [DOI] [PubMed] [Google Scholar]
- [81].Barmeyer C, Horak I, Zeitz M, Fromm M, Schulzke JD, The interleukin-2-deficient mouse model, Pathobiology, 70 (2002) 139–142. [DOI] [PubMed] [Google Scholar]
- [82].Snow JW, Abraham N, Ma MC, Herndier BG, Pastuszak AW, Goldsmith MA, Loss of tolerance and autoimmunity affecting multiple organs in STAT5A/5B-deficient mice, J. Immunol, 171 (2003) 5042–5050. [DOI] [PubMed] [Google Scholar]
- [83].Goudy K, Aydin D, Barzaghi F, Gambineri E, Vignoli M, Ciullini Mannurita S, Doglioni C, Ponzoni M, Cicalese MP, Assanelli A, Tommasini A, Brigida I, Dellepiane RM, Martino S, Olek S, Aiuti A, Ciceri F, Roncarolo MG, Bacchetta R, Human IL2RA null mutation mediates immunodeficiency with lymphoproliferation and autoimmunity, Clin. Immunol, 146 (2013) 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kohm AP, Carpentier PA, Anger HA, Miller SD, Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis, J. Immunol, 169 (2002) 4712–4716. [DOI] [PubMed] [Google Scholar]
- [85].Stephens LA, Malpass KH, Anderton SM, Curing CNS autoimmune disease with myelin-reactive Foxp3+ Treg, Eur. J. Immunol, 39 (2009) 1108–1117. [DOI] [PubMed] [Google Scholar]
- [86].Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q, Type 1 diabetes immunotherapy using polyclonal regulatory T cells, Sci. Transl. Med, 7 (2015) 315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, Curtsinger J, Verneris MR, MacMillan ML, Levine BL, Riley JL, June CH, Le C, Weisdorf DJ, McGlave PB, Blazar BR, Wagner JE, Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect, Blood, 127 (2016) 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Elias S, Rudensky AY, Therapeutic use of regulatory T cells for graft-versus-host disease, Br. J. Haematol, 187 (2019) 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Raffin C, Vo LT, Bluestone JA, Treg cell-based therapies: challenges and perspectives, Nat. Rev. Immunol, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G, Past, Present, and Future of Regulatory T Cell Therapy in Transplantation and Autoimmunity, Front. Immunol, 10 (2019) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Silva-Vilches C, Ring S, Mahnke K, ATP and Its Metabolite Adenosine as Regulators of Dendritic Cell Activity, Front. Immunol, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Dhainaut M, Coquerelle C, Uzureau S, Denoeud J, Acolty V, Oldenhove G, Galuppo A, Sparwasser T, Thielemans K, Pays E, Yagita H, Borst J, Moser M, Thymus-derived regulatory T cells restrain pro-inflammatory Th1 responses by downregulating CD70 on dendritic cells, EMBO J, 34 (2015) 1336–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Akkaya B, Oya Y, Akkaya M, Al Souz J, Holstein AH, Kamenyeva O, Kabat J, Matsumura R, Dorward DW, Glass DD, Shevach EM, Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells, Nat. Immunol, 20 (2019) 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Pohar J, Simon Q, Fillatreau S, Antigen-Specificity in the Thymic Development and Peripheral Activity of CD4(+)FOXP3(+) T Regulatory Cells, Front. Immunol, 9 (2018) 1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mannie MD, Autoimmunity and asthma: The dirt on the hygiene hypothesis, Self/nonself, 1 (2010) 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Satitsuksanoa P, Jansen K, Globinska A, van de Veen W, Akdis M, Regulatory Immune Mechanisms in Tolerance to Food Allergy, Front. Immunol, 9 (2018) 2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ono M, Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes, Immunology, 160 (2020) 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Vahlenkamp TW, Tompkins MB, Tompkins WA, The role of CD4+CD25+ regulatory T cells in viral infections, Vet. Immunol. Immunopathol, 108 (2005) 219–225. [DOI] [PubMed] [Google Scholar]
- [99].Veiga-Parga T, Sehrawat S, Rouse BT, Role of regulatory T cells during virus infection, Immunol. Rev, 255 (2013) 182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Andargachew R, Martinez RJ, Kolawole EM, Evavold BD, CD4 T Cell Affinity Diversity Is Equally Maintained during Acute and Chronic Infection, J. Immunol, 201 (2018) 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].D’Cruz LM, Klein L, Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling, Nat. Immunol, 6 (2005) 1152–1159. [DOI] [PubMed] [Google Scholar]
- [102].Ribot J, Romagnoli P, van Meerwijk JP, Agonist ligands expressed by thymic epithelium enhance positive selection of regulatory T lymphocytes from precursors with a normally diverse TCR repertoire, J. Immunol, 177 (2006) 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ribot J, Enault G, Pilipenko S, Huchenq A, Calise M, Hudrisier D, Romagnoli P, van Meerwijk JP, Shaping of the autoreactive regulatory T cell repertoire by thymic cortical positive selection, J. Immunol, 179 (2007) 6741–6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Weissler KA, Caton AJ, The role of T-cell receptor recognition of peptide:MHC complexes in the formation and activity of Foxp3(+) regulatory T cells, Immunol. Rev, 259 (2014) 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kieback E, Hilgenberg E, Stervbo U, Lampropoulou V, Shen P, Bunse M, Jaimes Y, Boudinot P, Radbruch A, Klemm U, Kuhl AA, Liblau R, Hoevelmeyer N, Anderton SM, Uckert W, Fillatreau S, Thymus-Derived Regulatory T Cells Are Positively Selected on Natural Self-Antigen through Cognate Interactions of High Functional Avidity, Immunity, 44 (2016) 1114–1126. [DOI] [PubMed] [Google Scholar]
- [106].Kisielow P, How does the immune system learn to distinguish between good and evil? The first definitive studies of T cell central tolerance and positive selection, Immunogenetics, 71 (2019) 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yi J, Kawabe T, Sprent J, New insights on T-cell self-tolerance, Curr. Opin. Immunol, 63 (2019) 14–20. [DOI] [PubMed] [Google Scholar]
- [108].Mannie MD, A unified model for T cell antigen recognition and thymic selection of the T cell repertoire, J. Theor. Biol, 151 (1991) 169–192. [DOI] [PubMed] [Google Scholar]
- [109].Wang HX, Pan W, Zheng L, Zhong XP, Tan L, Liang Z, He J, Feng P, Zhao Y, Qiu YR, Thymic Epithelial Cells Contribute to Thymopoiesis and T Cell Development, Front. Immunol, 10 (2019) 3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sprouse ML, Shevchenko I, Scavuzzo MA, Joseph F, Lee T, Blum S, Borowiak M, Bettini ML, Bettini M, Cutting Edge: Low-Affinity TCRs Support Regulatory T Cell Function in Autoimmunity, J. Immunol, 200 (2018) 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cohn M, Degeneracy, mimicry and crossreactivity in immune recognition, Mol. Immunol, 42 (2005) 651–655. [DOI] [PubMed] [Google Scholar]
- [112].Colf LA, Bankovich AJ, Hanick NA, Bowerman NA, Jones LL, Kranz DM, Garcia KC, How a single T cell receptor recognizes both self and foreign MHC, Cell, 129 (2007) 135–146. [DOI] [PubMed] [Google Scholar]
- [113].Chen H, Chakraborty AK, Kardar M, How nonuniform contact profiles of T cell receptors modulate thymic selection outcomes, Phys Rev E, 97 (2018) 032413. [DOI] [PubMed] [Google Scholar]
- [114].Brzostek J, Gascoigne NRJ, Thymic Origins of T Cell Receptor Alloreactivity, Transplantation, 101 (2017) 1535–1541. [DOI] [PubMed] [Google Scholar]
- [115].Sewell AK, Why must T cells be cross-reactive?, Nat. Rev. Immunol, 12 (2012) 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA, T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse, J. Exp. Med, 208 (2011) 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Shafiani S, Dinh C, Ertelt JM, Moguche AO, Siddiqui I, Smigiel KS, Sharma P, Campbell DJ, Way SS, Urdahl KB, Pathogen-specific Treg cells expand early during mycobacterium tuberculosis infection but are later eliminated in response to Interleukin-12, Immunity, 38 (2013) 1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bedoya F, Cheng GS, Leibow A, Zakhary N, Weissler K, Garcia V, Aitken M, Kropf E, Garlick DS, Wherry EJ, Erikson J, Caton AJ, Viral antigen induces differentiation of Foxp3+ natural regulatory T cells in influenza virus-infected mice, J. Immunol, 190 (2013) 6115–6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Guo J, Zhou X, Regulatory T cells turn pathogenic, Cell. Mol. Immunol, 12 (2015) 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Shin H, Iwasaki A, Tissue-resident memory T cells, Immunol. Rev, 255 (2013) 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Brinkman C, Peske J, Engelhard V, Peripheral Tissue Homing Receptor Control of Naïve, Effector, and Memory CD8 T Cell Localization in Lymphoid and Non-Lymphoid Tissues, Front. Immunol, 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wong MT, Ong DE, Lim FS, Teng KW, McGovern N, Narayanan S, Ho WQ, Cerny D, Tan HK, Anicete R, Tan BK, Lim TK, Chan CY, Cheow PC, Lee SY, Takano A, Tan EH, Tam JK, Tan EY, Chan JK, Fink K, Bertoletti A, Ginhoux F, Curotto de Lafaille MA, Newell EW, A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures, Immunity, 45 (2016) 442–456. [DOI] [PubMed] [Google Scholar]
- [123].Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, Keitany GJ, Garza EN, Fraser KA, Moon JJ, Altemeier WA, Masopust D, Pepper M, Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma, Immunity, 44 (2016) 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Boyman O, Letourneau S, Krieg C, Sprent J, Homeostatic proliferation and survival of naive and memory T cells, Eur. J. Immunol, 39 (2009) 2088–2094. [DOI] [PubMed] [Google Scholar]
- [125].Jameson SC, Hogquist KA, Bevan MJ, Specificity and flexibility in thymic selection, Nature, 369 (1994) 750–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Mannie MD, Rosser JM, White GA, Autologous rat myelin basic protein is a partial agonist that is converted into a full antagonist upon blockade of CD4. Evidence for the integration of efficacious and nonefficacious signals during T cell antigen recognition, J. Immunol, 154 (1995) 2642–2654. [PubMed] [Google Scholar]
- [127].Gottschalk RA, Corse E, Allison JP, TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo, J. Exp. Med, 207 (2010) 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Gratz IK, Rosenblum MD, Maurano MM, Paw JS, Truong HA, Marshak-Rothstein A, Abbas AK, Cutting edge: Self-antigen controls the balance between effector and regulatory T cells in peripheral tissues, J. Immunol, 192 (2014) 1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Moorman CD, Curtis AD 2nd, Bastian AG, Elliott SE, Mannie MD, A GMCSF-Neuroantigen Tolerogenic Vaccine Elicits Systemic Lymphocytosis of CD4(+) CD25(high) FOXP3(+) Regulatory T Cells in Myelin-Specific TCR Transgenic Mice Contingent Upon Low-Efficiency T Cell Antigen Receptor Recognition, Front. Immunol, 9 (2018) 3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Moorman CD, Bastian AG, DeOca KB, Mannie MD, A GM-CSF-neuroantigen tolerogenic vaccine elicits inefficient antigen recognition events below the CD40L triggering threshold to expand CD4(+) CD25(+) FOXP3(+) Tregs that inhibit experimental autoimmune encephalomyelitis (EAE), J. Neuroinflammation, 17 (2020) 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Vanderlugt CL, Begolka WS, Neville KL, Katz-Levy Y, Howard LM, Eagar TN, Bluestone JA, Miller SD, The functional significance of epitope spreading and its regulation by co-stimulatory molecules, Immunol. Rev, 164 (1998) 63–72. [DOI] [PubMed] [Google Scholar]
- [132].Takacs K, Altmann DM, The case against epitope spread in experimental allergic encephalomyelitis, Immunol. Rev, 164 (1998) 101–110. [DOI] [PubMed] [Google Scholar]
- [133].Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ, Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide, Nat. Immunol, 2 (2001) 301–306. [DOI] [PubMed] [Google Scholar]
- [134].Lee HM, Bautista JL, Hsieh CS, Thymic and peripheral differentiation of regulatory T cells, Adv. Immunol, 112 (2011) 25–71. [DOI] [PubMed] [Google Scholar]
- [135].Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS, A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self, Immunity, 37 (2012) 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Richards DM, Delacher M, Goldfarb Y, Kagebein D, Hofer AC, Abramson J, Feuerer M, Treg Cell Differentiation: From Thymus to Peripheral Tissue, Prog. Mol. Biol. Transl. Sci, 136 (2015) 175–205. [DOI] [PubMed] [Google Scholar]
- [137].Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK, Response to self antigen imprints regulatory memory in tissues, Nature, 480 (2011) 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Levine AG, Arvey A, Jin W, Rudensky AY, Continuous requirement for the TCR in regulatory T cell function, Nat. Immunol, 15 (2014) 1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Schreiner D, King CG, CD4+ Memory T Cells at Home in the Tissue: Mechanisms for Health and Disease, Front. Immunol, 9 (2018) 2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].McLachlan JB, Jenkins MK, Migration and accumulation of effector CD4+ T cells in nonlymphoid tissues, Proceedings of the American Thoracic Society, 4 (2007) 439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Nguyen QP, Deng TZ, Witherden DA, Goldrath AW, Origins of CD4(+) circulating and tissue-resident memory T-cells, Immunology, 157 (2019) 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Pitzalis C, Jones GW, Bombardieri M, Jones SA, Ectopic lymphoid-like structures in infection, cancer and autoimmunity, Nat. Rev. Immunol, 14 (2014) 447–462. [DOI] [PubMed] [Google Scholar]
- [143].Manzo A, Bombardieri M, Humby F, Pitzalis C, Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling, Immunol. Rev, 233 (2010) 267–285. [DOI] [PubMed] [Google Scholar]
- [144].Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, Wucherpfennig K, Turley S, Carroll MC, Sobel RA, Bettelli E, Kuchroo VK, Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation, Immunity, 35 (2011) 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Lehmann-Horn K, Wang SZ, Sagan SA, Zamvil SS, von Büdingen HC, B cell repertoire expansion occurs in meningeal ectopic lymphoid tissue, JCI insight, 1 (2016) e87234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Muller WA, Getting leukocytes to the site of inflammation, Vet. Pathol, 50 (2013) 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Carman CV, Martinelli R, T Lymphocyte-Endothelial Interactions: Emerging Understanding of Trafficking and Antigen-Specific Immunity, Front. Immunol, 6 (2015) 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bretscher P, Cohn M, A theory of self-nonself discrimination, Science, 169 (1970) 1042–1049. [DOI] [PubMed] [Google Scholar]
- [149].Lafferty KJ, Gazda LS, Tolerance: a case of self/not-self discrimination maintained by clonal deletion?, Hum. Immunol, 52 (1997) 119–126. [DOI] [PubMed] [Google Scholar]
- [150].Mannie MD, Immune discrimination of self and nonself: a unified theory for the induction of self tolerance among thymocytes and mature peripheral T cells, Med. Hypotheses, 40 (1993) 105–112. [DOI] [PubMed] [Google Scholar]
- [151].Mannie MD, Immunological self/nonself discrimination: integration of self vs nonself during cognate T cell interactions with antigen-presenting cells, Immunol. Res, 19 (1999) 65–87. [DOI] [PubMed] [Google Scholar]
- [152].Patel DM, Arnold PY, White GA, Nardella JP, Mannie MD, Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition, J. Immunol, 163 (1999) 5201–5210. [PubMed] [Google Scholar]
- [153].Krishnamoorthy G, Saxena A, Mars LT, Domingues HS, Mentele R, Ben-Nun A, Lassmann H, Dornmair K, Kurschus FC, Liblau RS, Wekerle H, Myelin-specific T cells also recognize neuronal autoantigen in a transgenic mouse model of multiple sclerosis, Nat. Med, 15 (2009) 626–632. [DOI] [PubMed] [Google Scholar]
- [154].Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ, CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells, Nat. Immunol, 8 (2007) 1353–1362. [DOI] [PubMed] [Google Scholar]
- [155].Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, Gasteiger G, Feng Y, Fontenot JD, Rudensky AY, An essential role for the IL-2 receptor in Treg cell function, Nat. Immunol, 17 (2016) 1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA, Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells, J. Immunol, 172 (2004) 5287–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, Hou JZ, Negrin RS, Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells, Blood, 111 (2008) 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Huynh A, Zhang R, Turka LA, Signals and pathways controlling regulatory T cells, Immunol. Rev, 258 (2014) 117–131. [DOI] [PubMed] [Google Scholar]
- [159].Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, Bluestone JA, Turka LA, Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability, Nat. Immunol, 16 (2015) 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Wilde DB, Fitch FW, Antigen-reactive cloned helper T cells. I. Unresponsiveness to antigenic restimulation develops after stimulation of cloned helper T cells, J. Immunol, 132 (1984) 1632–1638. [PubMed] [Google Scholar]
- [161].Wilde DB, Prystowsky MB, Ely JM, Vogel SN, Dialynas DP, Fitch FW, Antigen-reactive cloned helper T cells. II. Exposure of murine cloned helper T cells to IL 2-containing supernatant induces unresponsiveness to antigenic restimulation and inhibits lymphokine production after antigenic stimulation, J. Immunol, 133 (1984) 636–641. [PubMed] [Google Scholar]
- [162].Otten G, Wilde DB, Prystowsky MB, Olshan JS, Rabin H, Henderson LE, Fitch FW, Cloned helper T lymphocytes exposed to interleukin 2 become unresponsive to antigen and concanavalin A but not to calcium ionophore and phorbol ester, Eur. J. Immunol, 16 (1986) 217–225. [DOI] [PubMed] [Google Scholar]
- [163].Radvanyi LG, Mills GB, Miller RG, Religation of the T cell receptor after primary activation of mature T cells inhibits proliferation and induces apoptotic cell death, J. Immunol, 150 (1993) 5704–5715. [PubMed] [Google Scholar]
- [164].Mannie MD, Truman H, Morrison-Plummer J, T-helper lymphocytes specific for myelin basic protein: activation-induced refractoriness of IL-2 production pathways augments an anti-CD4-mediated proliferative deficit, Cell. Immunol, 154 (1994) 484–497. [DOI] [PubMed] [Google Scholar]
- [165].Mannie MD, The post-activation refractory phase: a mechanism to measure antigenic complexity and ensure self-tolerance among mature peripheral T lymphocytes, Med. Hypotheses, 47 (1996) 467–470. [DOI] [PubMed] [Google Scholar]
- [166].Andris F, Van Mechelen M, De Mattia F, Baus E, Urbain J, Leo O, Induction of T cell unresponsiveness by anti-CD3 antibodies occurs independently of co-stimulatory functions, Eur. J. Immunol, 26 (1996) 1187–1195. [DOI] [PubMed] [Google Scholar]
- [167].Mannie MD, White GA, Lake KR, Nardella JP, Marinakis CA, McConnell TJ, T-helper lymphocytes specific for myelin basic protein: low-density activation prolongs a postactivation refractory phase marked by decreased pathogenicity and enhanced sensitivity to anergy, Cell. Immunol, 172 (1996) 108–117. [DOI] [PubMed] [Google Scholar]
- [168].Deeths MJ, Kedl RM, Mescher MF, CD8+ T cells become nonresponsive (anergic) following activation in the presence of costimulation, J. Immunol, 163 (1999) 102–110. [PubMed] [Google Scholar]
- [169].De Mattia F, Chomez S, Van Laethem F, Moulin V, Urbain J, Moser M, Leo O, Andris F, Antigen-experienced T cells undergo a transient phase of unresponsiveness following optimal stimulation, J. Immunol, 163 (1999) 5929–5936. [PubMed] [Google Scholar]
- [170].Norris MS, McConnell TJ, Mannie MD, Interleukin-2 promotes antigenic reactivity of rested T cells but prolongs the postactivational refractory phase of activated T cells, Cell. Immunol, 211 (2001) 51–60. [DOI] [PubMed] [Google Scholar]
- [171].Mannie MD, Fraser DJ, McConnell TJ, IL-4 responsive CD4+ T cells specific for myelin basic protein: IL-2 confers a prolonged postactivation refractory phase, Immunol. Cell Biol, 81 (2003) 8–19. [DOI] [PubMed] [Google Scholar]
- [172].Mescher MF, Popescu FE, Gerner M, Hammerbeck CD, Curtsinger JM, Activation-induced non-responsiveness (anergy) limits CD8 T cell responses to tumors, Semin. Cancer Biol, 17 (2007) 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Rohowsky-Kochan C, Molinaro D, Cook SD, Cytokine secretion profile of myelin basic protein-specific T cells in multiple sclerosis, Mult. Scler, 6 (2000) 69–77. [DOI] [PubMed] [Google Scholar]
- [174].Hellings N, Barée M, Verhoeven C, D’Hooghe MB, Medaer R, Bernard CC, Raus J, Stinissen P, T-cell reactivity to multiple myelin antigens in multiple sclerosis patients and healthy controls, J. Neurosci. Res, 63 (2001) 290–302. [DOI] [PubMed] [Google Scholar]
- [175].Cao Y, Goods BA, Raddassi K, Nepom GT, Kwok WW, Love JC, Hafler DA, Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis, Sci. Transl. Med, 7 (2015) 287ra274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Cebula A, Kuczma M, Szurek E, Pietrzak M, Savage N, Elhefnawy WR, Rempala G, Kraj P, Ignatowicz L, Dormant pathogenic CD4(+) T cells are prevalent in the peripheral repertoire of healthy mice, Nature communications, 10 (2019) 4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Cusick MF, Libbey JE, Fujinami RS, Molecular mimicry as a mechanism of autoimmune disease, Clin. Rev. Allergy Immunol, 42 (2012) 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Rojas M, Restrepo-Jimenez P, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramirez-Santana C, Leung PSC, Ansari AA, Gershwin ME, Anaya JM, Molecular mimicry and autoimmunity, J. Autoimmun, 95 (2018) 100–123. [DOI] [PubMed] [Google Scholar]
- [179].Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW, Autoreactive T cells in healthy individuals, J. Immunol, 172 (2004) 5967–5972. [DOI] [PubMed] [Google Scholar]
- [180].Long SA, Buckner JH, CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game, J. Immunol, 187 (2011) 2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Mannie MD, Curtis AD 2nd, Tolerogenic vaccines for Multiple sclerosis, Hum. Vaccin. Immunother, 9 (2013) 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Leventhal DS, Gilmore DC, Berger JM, Nishi S, Lee V, Malchow S, Kline DE, Kline J, Vander Griend DJ, Huang H, Socci ND, Savage PA, Dendritic Cells Coordinate the Development and Homeostasis of Organ-Specific Regulatory T Cells, Immunity, 44 (2016) 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Hasegawa H, Matsumoto T, Mechanisms of Tolerance Induction by Dendritic Cells In Vivo, Front. Immunol, 9 (2018) 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Kammona O, Kiparissides C, Recent Advances in Antigen-Specific Immunotherapies for the Treatment of Multiple Sclerosis, Brain Sci, 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Carbone F, De Rosa V, Carrieri PB, Montella S, Bruzzese D, Porcellini A, Procaccini C, La Cava A, Matarese G, Regulatory T cell proliferative potential is impaired in human autoimmune disease, Nat. Med, 20 (2014) 69–74. [DOI] [PubMed] [Google Scholar]
- [186].Willekens B, Cools N, Beyond the Magic Bullet: Current Progress of Therapeutic Vaccination in Multiple Sclerosis, CNS drugs, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Riedhammer C, Weissert R, Antigen Presentation, Autoantigens, and Immune Regulation in Multiple Sclerosis and Other Autoimmune Diseases, Front. Immunol, 6 (2015) 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Tang Q, Bluestone JA, The Foxp3+ regulatory T cell: a jack of all trades, master of regulation, Nat. Immunol, 9 (2008) 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Waldmann H, Adams E, Fairchild P, Cobbold S, Infectious tolerance and the long-term acceptance of transplanted tissue, Immunol. Rev, 212 (2006) 301–313. [DOI] [PubMed] [Google Scholar]
- [190].Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, Shevach EM, CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner, J. Exp. Med, 205 (2008) 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Selvaraj RK, Geiger TL, Mitigation of experimental allergic encephalomyelitis by TGF-beta induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance, J. Immunol, 180 (2008) 2830–2838. [DOI] [PubMed] [Google Scholar]
- [192].Kendal AR, Waldmann H, Infectious tolerance: therapeutic potential, Curr. Opin. Immunol, 22 (2010) 560–565. [DOI] [PubMed] [Google Scholar]
- [193].Kendal AR, Chen Y, Regateiro FS, Ma J, Adams E, Cobbold SP, Hori S, Waldmann H, Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance, J. Exp. Med, 208 (2011) 2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Gravano DM, Vignali DA, The battle against immunopathology: infectious tolerance mediated by regulatory T cells, Cell. Mol. Life Sci, 69 (2012) 1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Capurso NA, Look M, Jeanbart L, Nowyhed H, Abraham C, Craft J, Fahmy TM, Development of a nanoparticulate formulation of retinoic acid that suppresses Th17 cells and upregulates regulatory T cells, Self/nonself, 1 (2010) 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ, Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis, Proc. Natl. Acad. Sci. U. S. A, 109 (2012) 11270–11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Ben-Akiva E, Est Witte S, Meyer RA, Rhodes KR, Green JJ, Polymeric micro- and nanoparticles for immune modulation, Biomaterials science, 7 (2018) 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Legge KL, Gregg RK, Maldonado-Lopez R, Li L, Caprio JC, Moser M, Zaghouani H, On the role of dendritic cells in peripheral T cell tolerance and modulation of autoimmunity, J. Exp. Med, 196 (2002) 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Apostolopoulos V, Thalhammer T, Tzakos AG, Stojanovska L, Targeting antigens to dendritic cell receptors for vaccine development, Journal of drug delivery, 2013 (2013) 869718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Ring S, Maas M, Nettelbeck DM, Enk AH, Mahnke K, Targeting of autoantigens to DEC205(+) dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice, J. Immunol, 191 (2013) 2938–2947. [DOI] [PubMed] [Google Scholar]
- [201].Chappell CP, Giltiay NV, Dresch C, Clark EA, Controlling immune responses by targeting antigens to dendritic cell subsets and B cells, Int. Immunol, 26 (2014) 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Mannie MD, Abbott DJ, Blanchfield JL, Experimental autoimmune encephalomyelitis in Lewis rats: IFN-beta acts as a tolerogenic adjuvant for induction of neuroantigen-dependent tolerance, J. Immunol, 182 (2009) 5331–5341. [DOI] [PubMed] [Google Scholar]
- [203].Mannie MD, Blanchfield JL, Islam SM, Abbott DJ, Cytokine-neuroantigen fusion proteins as a new class of tolerogenic, therapeutic vaccines for treatment of inflammatory demyelinating disease in rodent models of multiple sclerosis, Front. Immunol, 3 (2012) 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Wang D, Ghosh D, Islam SM, Moorman CD, Thomason AE, Wilkinson DS, Mannie MD, IFN-beta Facilitates Neuroantigen-Dependent Induction of CD25+ FOXP3+ Regulatory T Cells That Suppress Experimental Autoimmune Encephalomyelitis, J. Immunol, 197 (2016) 2992–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Blanchfield JL, Mannie MD, A GMCSF-neuroantigen fusion protein is a potent tolerogen in experimental autoimmune encephalomyelitis (EAE) that is associated with efficient targeting of neuroantigen to APC, J. Leukoc. Biol, 87 (2010) 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Abbott DJ, Blanchfield JL, Martinson DA, Russell SC, Taslim N, Curtis AD, Mannie MD, Neuroantigen-specific, tolerogenic vaccines: GM-CSF is a fusion partner that facilitates tolerance rather than immunity to dominant self-epitopes of myelin in murine models of experimental autoimmune encephalomyelitis (EAE), BMC Immunol, 12 (2011) 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Islam SM, Curtis AD 2nd, Taslim N, Wilkinson DS, Mannie MD, GM-CSF-neuroantigen fusion proteins reverse experimental autoimmune encephalomyelitis and mediate tolerogenic activity in adjuvant-primed environments: association with inflammation-dependent, inhibitory antigen presentation, J. Immunol, 193 (2014) 2317–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Mannie MD, Abbott DJ, A fusion protein consisting of IL-16 and the encephalitogenic peptide of myelin basic protein constitutes an antigen-specific tolerogenic vaccine that inhibits experimental autoimmune encephalomyelitis, J. Immunol, 179 (2007) 1458–1465. [DOI] [PubMed] [Google Scholar]
- [209].Mannie MD, Clayson BA, Buskirk EJ, DeVine JL, Hernandez JJ, Abbott DJ, IL-2/neuroantigen fusion proteins as antigen-specific tolerogens in experimental autoimmune encephalomyelitis (EAE): correlation of T cell-mediated antigen presentation and tolerance induction, J. Immunol, 178 (2007) 2835–2843. [DOI] [PubMed] [Google Scholar]
- [210].Sheng JR, Li L, Ganesh BB, Vasu C, Prabhakar BS, Meriggioli MN, Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells, J. Immunol, 177 (2006) 5296–5306. [DOI] [PubMed] [Google Scholar]
- [211].Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS, GM-CSF-induced CD11c+CD8a--dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis, Int. Immunol, 21 (2009) 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Cheatem D, Ganesh BB, Gangi E, Vasu C, Prabhakar BS, Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function, Clin. Immunol, 131 (2009) 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Bhattacharya P, Gopisetty A, Ganesh BB, Sheng JR, Prabhakar BS, GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms, J. Leukoc. Biol, 89 (2011) 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Sheng JR, Muthusamy T, Prabhakar BS, Meriggioli MN, GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis, J. Neuroimmunol, 240–241 (2011) 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Gopisetty A, Bhattacharya P, Haddad C, Bruno JC Jr., Vasu C, Miele L, Prabhakar BS, OX40L/Jagged1 cosignaling by GM-CSF-induced bone marrow-derived dendritic cells is required for the expansion of functional regulatory T cells, J. Immunol, 190 (2013) 5516–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Bhattacharya P, Budnick I, Singh M, Thiruppathi M, Alharshawi K, Elshabrawy H, Holterman MJ, Prabhakar BS, Dual Role of GM-CSF as a Pro-Inflammatory and a Regulatory Cytokine: Implications for Immune Therapy, J. Interferon Cytokine Res, 35 (2015) 585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Bhattacharya P, Thiruppathi M, Elshabrawy HA, Alharshawi K, Kumar P, Prabhakar BS, GM-CSF: An immune modulatory cytokine that can suppress autoimmunity, Cytokine, 75 (2015) 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Hotta M, Yoshimura H, Satake A, Tsubokura Y, Ito T, Nomura S, GM-CSF therapy inhibits chronic graft-versus-host disease via expansion of regulatory T cells, Eur. J. Immunol, 49 (2019) 179–191. [DOI] [PubMed] [Google Scholar]
- [219].Park MY, Lim BG, Kim SY, Sohn HJ, Kim S, Kim TG, GM-CSF Promotes the Expansion and Differentiation of Cord Blood Myeloid-Derived Suppressor Cells, Which Attenuate Xenogeneic Graft-vs.-Host Disease, Front. Immunol, 10 (2019) 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Korporal M, Haas J, Balint B, Fritzsching B, Schwarz A, Moeller S, Fritz B, Suri-Payer E, Wildemann B, Interferon beta-induced restoration of regulatory T-cell function in multiple sclerosis is prompted by an increase in newly generated naive regulatory T cells, Arch. Neurol, 65 (2008) 1434–1439. [DOI] [PubMed] [Google Scholar]
- [221].Chen M, Chen G, Deng S, Liu X, Hutton GJ, Hong J, IFN-β induces the proliferation of CD4+CD25+Foxp3+ regulatory T cells through upregulation of GITRL on dendritic cells in the treatment of multiple sclerosis, J. Neuroimmunol, 242 (2012) 39–46. [DOI] [PubMed] [Google Scholar]
- [222].Piconese S, Pacella I, Timperi E, Barnaba V, Divergent effects of type-I interferons on regulatory T cells, Cytokine Growth Factor Rev, 26 (2015) 133–141. [DOI] [PubMed] [Google Scholar]
- [223].Metidji A, Rieder SA, Glass DD, Cremer I, Punkosdy GA, Shevach EM, IFN-α/β receptor signaling promotes regulatory T cell development and function under stress conditions, J. Immunol, 194 (2015) 4265–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Delgoffe GM, Vignali DA, A Fox of a different color: FoxA1 programs a new regulatory T cell subset, Nat. Med, 20 (2014) 236–237. [DOI] [PubMed] [Google Scholar]
- [225].Liu Y, Marin A, Ejlerskov P, Rasmussen LM, Prinz M, Issazadeh-Navikas S, Neuronal IFN-beta-induced PI3K/Akt-FoxA1 signalling is essential for generation of FoxA1(+)Treg cells, Nature communications, 8 (2017) 14709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Bluestone JA, Tang Q, Treg cells-the next frontier of cell therapy, Science, 362 (2018) 154–155. [DOI] [PubMed] [Google Scholar]
- [227].Esensten JH, Muller YD, Bluestone JA, Tang Q, Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: The next frontier, J. Allergy Clin. Immunol, 142 (2018) 1710–1718. [DOI] [PubMed] [Google Scholar]
- [228].Ferreira LMR, Muller YD, Bluestone JA, Tang Q, Next-generation regulatory T cell therapy, Nature reviews. Drug discovery, 18 (2019) 749–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Tahvildari M, Dana R, Low-Dose IL-2 Therapy in Transplantation, Autoimmunity, and Inflammatory Diseases, J. Immunol, 203 (2019) 2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]


