Abstract
Objectives:
To measure parents’ perceptions of child vulnerability, as a precursor to developing a population-scale mechanism to mitigate harm after.
Study design.
Participants were parents of infants aged 2–5 months. PPCV was assessed with an adapted version of the Vulnerable Baby Scale.25 The Scale was included in the script for a larger study of telephone follow-up for two NBS samples (carrier status for cystic fibrosis or sickle cell). A comparison sample was added using a paper survey with well-baby visits to an urban/suburban clinic.
Results.
Sample sizes consisted of 288 parents in the CF group, 426 in the SCH group, and 79 in the clinic comparison group. PPCV was higher in the SC group than CF group (p< 0.0001), and both were higher than the clinic comparison group (p< 0.0001). PPCV was inversely correlated with parental age (P < .002) and lower health literacy (p < 0.015, SCH group only).
Conclusions.
Increased PPCV appears to be a bona fide complication of incidental NBS findings, and health care professionals should be alert to the possibility. From a public health perspective, we recommend routine follow-up after incidental findings, to mitigate psychosocial harm.
Keywords: communication, genetic screening, incidental findings
Newborn blood screening (NBS) is one of the best examples of successful bench to bedside research. Over 6,000 infants in the United States are diagnosed annually with dozens of potentially fatal, relatively rare, conditions.1 As whole genome sequencing moves towards a reality for disease identification in the newborn period, it has garnered the interest of parents, clinicians, researchers and industry leaders as a public health screening tool.2, 3 Despite the lives NBS saves and the promise the future holds, there are still concerns about the psychosocial implications of incidental findings, such as false positive results and carrier statuses, that accompany NBS. These concerns from policy experts and investigators alike do not diminish the value of scientific advancement, but do encourage us to be thoughtful about how new technologies are implemented in NBS policy and practice.
Vulnerable Child Syndrome is a commonly mentioned complication after NBS,4–8 although much of the VCS literature focuses on health conditions rather than test results. Originally described by Green and Solnit in 1964,9 VCS includes a suite of parental behavioral and psychological issues following a perceived health threat to a child. Despite recovery from illness, VCS families may develop common symptoms, including parental overprotection, separation difficulties, poor school performance, challenges with limit setting, and preoccupation with somatic complaints like abdominal pain or headaches.10–13 VCS and the resulting overconcern contribute to increased utilization of health care services11–16 and increased dissatisfaction with health services rendered.16
The original description referred to a history of “life-threatening illness,”9 and risk for VCS is correlated with severity of the child’s original illness.9, 11, 12, 17–20 However, VCS has also been associated with relatively benign conditions like feeding difficulties,21, 22 gastroenteritis,23 croup,24 and jaundice.25–27 Other risk factors for VCS include the child being the first born,28 having a history of prematurity,18, 28, 29 or being a product of a high-risk pregnancy or delivery10, 12. VCS is also thought to be partially modulated by other parent and child experiences,13 including postpartum depression.30, 31
VCS has been observed after false-positive NBS for metabolic disorders,5, 6 newborn hearing screening,8 and infant screening for type 1 diabetes risk.7 VCS may also occur after NBS for cystic fibrosis (CF) identifies heterozygous or “carrier” status, as observed in a modest sample in previous research.4, we suspect VCS will continue to be mentioned as a complication in policy discussions, given the expansion of molecular genetic methods in NBS and elsewhere. The chief opportunity for this research is afforded by incidental finding of carrier status for CF or sickle cell hemoglobinopathy. A VCS measure was included in the Wisconsin Project on Improvement of Communication Process and Outcomes after Newborn Screening,32–41 but the VCS results were provocative enough that we postponed this report until we had a comparison sample from a more general pediatric population.
METHODS
Diagnosis of VCS depends on clinical judgment, but in research there have been a variety of methods to operationalize the concept.4–7, 9–31, 42–44 Prior investigations have assessed “parental perception of child vulnerability” as with Forsyth’s Child Vulnerability Scale.15 The first iteration of this tool asked for 5-point responses to 12 statements such as “In general my child seems less healthy than other children” and “I often think about calling the doctor about my child.” This was revised to 8 items with a 4-point response scale15 and later validated.21 Kerruish and colleagues developed the Vulnerable Baby Scale (VB Scale) by modifying Forsyth’s survey statements to be appropriate for young infants.25 We made slight modifications to Kerruish’s text to fit with our study (Table I; available at www.jpeds.com).
The current report compares analyses of VB scale data from three parallel groups of parents of infants between 2 and 5 months of age. In the first two groups, the infants had been identified by NBS as carriers for SCH or CF. The third group is referred to as the “clinic-comparison group,” and consisted of parents who presented for a 2 or 4 month well check at a primary care clinic. Institutional Review Board approvals were obtained separately for the NBS groups and clinic comparison group.
Participants
The SCH and CF groups were recruited as part of the Wisconsin Project on Improvement of Communication Process and Outcomes after Newborn Screening,32–41 which was conducted from 2008 to 2012 in collaboration with Wisconsin’s NBS laboratory. For the SCH group, infants had an NBS result showing fetal, adult, and sickle hemoglobin (the “FAS” result). For the CF group, infants’ NBS result showed elevated immunoreactive trypsinogen and a single mutation in the CFTR gene, followed by a normal result on the infant’s sweat chloride testing.
Recruiting procedures have been detailed elsewhere, including design elements meant to mitigate recruitment bias.32, 41 In brief, NBS results were assessed until we identified 1669 infants with SCH carrier status and 800 infants with CF results. Then, ten types of exclusion criteria were applied from NBS records and a call to the primary care provider (PCP): (1) more than one abnormality found on NBS, (2) NBS was a repeat specimen, (3) gestational age under 35 weeks, (4) calendar age at collection was more than 180 days, (5) a PCP could not be identified, (6) the infant spent more than 5 days in hospital, (7) the infant was re-hospitalized after discharge, (8) the infant was being evaluated for another serious medical condition, (9) the parent(s) reportedly needed a language interpreter, and (10) infants in the CF group had a positive sweat chloride test.
As described elsewhere,32, 41 consent occurred over a 5-stage process that was carefully designed to mitigate distress for parents who had forgotten about the NBS results or were never informed. Parents were offered the chance to decline the research aspect of the project but still discuss the NBS result with us.
Clinic comparison group
When PPCV data from the NBS groups were higher than expected, we sought permission from a local primary care clinic to gather a comparison sample. The clinic served a diverse population across an urban and suburban region. The clinic’s desk staff were given a stack of large envelopes that each contained a printed survey packet (described below). The desk staff were asked to give the envelope to parents who were presenting to the pediatrics and family medicine groups for a 2- or 4-month well check, as identified on the clinic schedule. On the cover of the envelope was printed a message describing the research, assuring parents that they were not obligated to participate, and they could withdraw at any time. All returned envelopes were opened later and abstracted to the study database, regardless of how complete they were.
Data collection
NBS groups
Trained nurses or a genetic counselor were scheduled to telephone the parents when their infants were between 3 and 5 months old, to allow for at least one well-baby visit.
As detailed elsewhere,32, 41 callers followed a standardized script that was initially designed for clinical follow-up, and then structured to facilitate research data collection. The core clinical topics in the script were verifying receipt of the NBS result, checking for misunderstandings, and providing initial counseling. Embedded in the script was an adapted version of the VB Scale (Table 1).25 Health literacy was evaluated with a three-item screening tool adapted from Chew et al.45
Table 1.
Vulnerable Baby Scale*
| Question | Options for reply |
|---|---|
| 1. How often do you check on baby while he/she is asleep at night? | Not at all /Rarely / 1–2 times each night / Several times each night / Every half hour or so |
| 2. If baby was awake and playing, what’s the longest you would leave baby alone without being able to hear him/her? | Not at all / 5 minutes / 15 minutes / half an hour / an hour or longer |
| 3. If a friend came over to visit and they had a cold, would you… | Not allow them in the house / Not allow them in the same room as the baby / Allow them in the same room but ask them not to hold the baby / Make them wash their hands before picking up baby/ Allow them to pick up the baby as they are |
| 4. How often does baby seem to get stomach pains or other pains? | On a scale of 1 to 5, 5 being “All of the time,” 1 being “Not at all.” |
| 5. How concerned are you that baby is not as healthy as he/she should be? | On a scale of 1 to 5, 5 being “I think of it all of the time,” 1 being “Not concerned at all.” |
| 6. When you compare baby ‘s health to that of other babies do you think he/she is … | On a scale of 1 to 5, 5 being “A lot less healthy,” 1 being “a lot more healthy.” |
| 7. How often do you find yourself worrying that your baby may become seriously ill? | On a scale of 1 to 5, 5 being “I think of it all the time,” 1 being “Not concerned at all” |
| 8. How often do you find yourself worrying about crib death or SIDS? | On a scale of 1 to 5, 5 being “I think of it all the time,” 1 being “Not concerned at all” |
| 9. If you left the baby with someone else how likely would you be to make contact with that person while you were away? | On a scale of 1 to 5, 5 being “ Yes, definitely,” 1 being “No, not at all” |
| 10. In the last two weeks how often have been in contact with a doctor or nurse about baby, not including well-baby checks or shots? | Not at all / Once / Once each week / Twice per week /Daily or more |
Adapted from Kerruish25
To ensure that the dataset reflected multiracial diversity, we asked an open-ended question, “How would you describe your race or ethnicity?” We then abstracted responses as closely as possible into one or more binary fields for each of the standard NIH categories.46 For example, if a parent described his/her ethnicity as “mixed Latino and White” then the database fields for Hispanic and White were flagged (per NIH protocol, Spaniards were classified as Hispanic, and Brazilians as White).
Calls were digitally audio-recorded, and transcribed without names or other identifying information. Interviewers kept written notes during the call, and both notes and transcripts were abstracted for fixed answers and other fields in the project database.
Clinic comparison group
The paper survey instrument included our adapted version of Kerruish’s VB scale25 (Table 1). Also included were the open-ended race/ethnicity questions and the Chew health literacy questions.45 Several other questions were added to confirm the diverse nature of the sample, because we knew in advance that the clinic-comparison sample size would be smaller than the SCH and CF groups.
Statistical Analyses
Analyses were done as applicable for the nature of each variable (t-test, correlation, the Wilcoxon rank-sum test, or logistic and ordinal modeling) using JMP software (SAS Institute, Cary, NC).
Missing data were addressed with 2 approaches. For the main analysis, records were excluded if a VB scale item was missing. However, in the NBS study we were aware of anecdotes where the VB scale would be interrupted because the parent was growing impatient to get back to the NBS result. We therefore conducted a secondary analysis with prorated scores from any parent who answered at least 7 of the 10 VB scale items. A third analytic approach was added post hoc, as will be described in the Results section.
RESULTS
As reported elsewhere,41 the NBS study’s final sample consisted of 426 in the SCH group and 288 in the CF group (respective participation rates 34.8% and 49.6% of eligible parents). For the primary care clinic sample it unfortunately is not possible to know how many parents were approached by the desk staff, but based on the printed supply and the clinic schedule we estimated a ratio of about 20% of eligible parents were approached and half of those envelopes were returned. Of these surveys, 7.1% were returned too incomplete for analysis, and there was no way to discern if the parent chose to stop or if the survey had been interrupted by the arrival of the clinic provider. The final sample size for the comparison group was 79.
Characteristics of the 3 groups are given in Table 2, with the CF and SCH columns adapted from previous reports.41
Table 2.
Participant Characteristics
| SCH carrier group | CF carrier group | Clinic comparison group | ||||||
|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | |||
| Gest age at birth, weeks | 38.9 | 1.3 | 39.1* | 1.2 | 39.1 | (1.8) | ||
| Baby’s age at interview (days) | 107.1 | 23.9 | 110.8 | 25.8 | 101 | (16.3) | ||
| Parent’s age (years) | 25.8 | (5.9) | 28.7 | (5.6) | 30 | (5.3) | ||
| Categorical data | n | (%) | n | (%) | n | (%) | ||
| Screen positive for health literacy problem | 150 | (37.7) | 104 | (36.5) | 35 | (44.3) | ||
| Parent race data* | ||||||||
| Race-included | Black-included | 280 | (65.7) | 20 | (6.9) | 5 | 6.3 | |
| White-included | 87 | (20.4) | 250 | (86.8) | 56 | 70.9 | ||
| Hispanic-included | 29 | (6.8) | 8 | (2.8) | 13 | 16.5 | ||
| Other-included | 14 | (3.3) | 7 | (2.4) | 7 | 8.9 | ||
| Race-only | Black-only | 265 | (62.2) | 16 | (5.6) | 3 | 3.8 | |
| White-only | 72 | (16.9) | 246 | (85.4) | 49 | 62 | ||
| Hispanic-only | 19 | (4.5) | 5 | (1.7) | 10 | 12.7 | ||
| Other-only | 7 | (16.4) | 4 | (1.4) | 5 | 6.3 | ||
| Multiracial unspecified | 5 | (1.2) | 1 | (0.4) | 1 | 1.3 | ||
| Not asked or answered | 36 | (8.4) | 9 | (3.1) | 4 | 5.1 | ||
| Infant race data* | ||||||||
| Race-included | Black-included | 330 | (77.5) | 24 | (8.3) | 9 | 11.4 | |
| White-included | 82 | (19.3) | 255 | (88.5) | 54 | 68.4 | ||
| Hispanic-included | 38 | (8.9) | 14 | (4.9) | 10 | 12.7 | ||
| Other-included | 23 | (5.4) | 11 | (3.8) | 6 | 7.6 | ||
| Race-only | Black-only | 249 | (58.5) | 14 | (4.9) | 3 | 3.8 | |
| White-only | 13 | (3.1) | 235 | (81.6) | 48 | 60.8 | ||
| Hispanic-only | 15 | (3.5) | 4 | (1.4) | 7 | 8.9 | ||
| Other-only | 2 | (0.5) | 4 | (1.4) | 5 | 6.3 | ||
| Multiracial unspecified | 52 | (12.0) | 7 | (2.4) | 1 | 1.3 | ||
| Not asked or answered | 14 | (3.2) | 6 | (2.1) | 8 | 10.1 | ||
Columns for race data do not sum to 100% because data are not mutually exclusive
VB scale scores
The distributions of PPCV data are depicted in the Figure, with histogram columns connected into lines for ease of comparison.
Means for PPCV data are shown in Table 3, for three parallel analyses. The top row depicts the main analysis, of parents who completed the entire VB scale. PPCV was significantly greater for the SCH group than the CF group, which in turn was greater than for the clinic comparison group (both p < 0.0001 on t-test). The second row depicts the “at least 7 items completed” analysis described in the Methods section, prorated for comparison with the 10-item data in the top row. The significant differences were maintained (SCH group > CF group > clinic comparison group, both p < 0.0001 on t-test).
Table 3.
Vulnerable baby scores*
| Analytic approach | SCH carrier group | CF carrier group | Clinic comparison group | |||
|---|---|---|---|---|---|---|
| mean | (SD) | mean | (SD) | mean | (SD) | |
| Entire survey completed | 38.4 | (4.8) | 36.5 | (3.5) | 31.8 | (2.9) |
| At least 7 items completed (prorated) | 38.3 | (3.9) | 36.3 | (3.7) | 31.8 | (2.9) |
| Without item 9 (prorated) | 37.4 | (3.7) | 35.8 | (3.2) | 33.7 | (2.5) |
Each mean is significantly different from the other 2 means in its row (p < 0.0001).
The bottom row of Table 3 presents an ad hoc analysis that was added within the first few weeks of the NBS study, when we realized from many parent comments that there was an applicability problem with question #9: If you left the baby with someone else how likely would you be to make contact with that person while you were away?” In their responses, many parents mentioned that they would use their mobile phone to text or telephone the babysitter. We inferred that the increased availability of mobile phones might lead to a secular trend that would artefactually seem like an increase in VCS since earlier studies.15, 21, 25–27, 43 Nevertheless, we decided to continue asking the question and analyze the PPCV data both with and without the babysitter question. We also carried forward this analytic approach for the clinic comparison group. Removal of item #9 did not change the significant differences (SCH group > CF group > clinic comparison group, both p < 0.0001 on t-test).
Characteristics associated with perception of vulnerability
Inferential analyses were conducted in aggregate and for each of the three groups. In the SCH group, the screen for health literacy problems was associated with a slightly higher PPCV (28.8 versus 27.7, p < 0.015 on t-test). PPCV data in both groups were correlated with younger parental age (for SCH group, r = − 0.17, p < 0.002; for the CF group, r = − 0.20, p < 0.002).
Race/ethnicity factors had a variety of associations with PPCV, but many factors covaried. With stepwise regression, PPCV data in the CF group were inversely associated with black-race-only for the infant (O.R. 0.23, p < 0.001). For the SCH group, VB scale data were inversely associated with white-race-only for the parent (O.R. 0.33, p < 0.001).
Within the clinic comparison group, no significant associations were detected.
Individual items from the VB scale
As mentioned above, our anecdotal experiences with item #9 led us to analyze data with and without that item. We then decided to report responses to all of the individual items, in order to document the individual attitudes within the PPCV construct. The resulting analyses are listed in Table 4. Although the data are nonparametric, the inter-group differences are qualitatively analogous to those of the summary scores.
Table 4.
Responses to individual questions in the vulnerable baby scale
| SCH carrier group | CF carrier group | Clinic comparison group | ||||
|---|---|---|---|---|---|---|
| Individual items | median | p vs. CF carrier | median | p vs. Clinic comp. | median | p vs. SCH carrier |
| 1. Check while asleep | 4 | < 0.0001 | 3 | NS | 3 | < 0.0001 |
| 2. Leave out of earshot | 5 | NS | 5 | < 0.0001 | 1 | < 0.0001 |
| 3. Friend with a cold | 3 | < 0.0001 | 3 | NS | 3 | < 0.0001 |
| 4. Stomach pains | 1 | < 0.0001 | 2 | 0.0002 | 4 | < 0.0001 |
| 5. Concern not healthy | 1 | NS | 1 | < 0.0001 | 5 | < 0.0001 |
| 6. Worse than others | 2 | < 0.006 | 2 | < 0.0001 | 4 | < 0.0001 |
| 7. Worry will become ill | 2 | NS | 2 | < 0.0001 | 4 | < 0.0001 |
| 8. Think about SIDS | 2 | NS | 2 | < 0.0001 | 4 | < 0.0001 |
| 9. Contact babysitter | 5 | < 0.0001 | 5 | < 0.0001 | 1 | < 0.0001 |
| 10. Contact doctor/nurse | 1 | < 0.03 | 1 | < 0.0001 | 2 | < 0.0001 |
DISCUSSION
Many child health professionals encounter families with high PPCV. These families often improve, but some family members develop persistent VCS symptoms or long-term issues with the health care system. VCS is especially regrettable when NBS identifies carrier status, because carrier results are incidental findings during the effort to reduce disease morbidity and mortality, and have limited health implications. When we evaluated parents of carrier infants, the PPCV data were considerably worse than we were expecting based on previous samples.21, 25 Our clinic comparison group also had worse PPCV than previous reports, but significantly less so than our two NBS groups. Thus, increased PPCV (and likely some cases of VCS) appears to be a bona fide complication of carrier identification after NBS.
Telephone and paper methods were used in the NBS and comparison groups respectively, but both approaches have also been reported in the literature.4, 7, 15–28, 42–44 The comparison group was smaller than the two NBS groups because this ad hoc collection was not budgeted in our grants, but there were enough participants to allow statistical significance. The NBS groups’ recruiting methods were designed to mitigate bias,32, 35, 41 but we recognize that some parents’ voices may not have been represented. Even so, the effect sizes were strong enough that significance would have been maintained despite substantial increases in response rate with limited PPCV. Further study may be needed to discern how modest numeric differences in PPCV relate to the number of children with clinical differences in VCS.
Our experience with this analysis has convinced us of a serious need for more research into PPCV and VCS in the general population. Previous reports suggest that PPCV may be elevated in between 3–10% of parents in the general community.15, 21, 25, 43, 44 If PPCV has increased broadly (as suggested by our small comparison group), there may be society-scale effects on child development, health care and expenditures. Further research may also help clinicians and health systems to identify families where PPCV has increased to worrisome levels, and has actually led to psychological problems and unnecessary utilization. On the other hand, we may need to re-calibrate the PPCV construct. For example, the convenience of mobile phones may have influenced VB scale item #9, and it is difficult for us to judge whether texting a babysitter reflects unnecessary anxiety. The significant differences for item #9 seemed to parallel the overall differences, including items such as perception of health or desire for health care visits.
However, this report was not originally intended to address the ongoing debate about VCS and measurement. Instead, we intended to implement a straightforward set of questions in a real-world public health setting. Given that success, the next step is to consider implications for clinical care and NBS policy.
The clinical implication is that health care providers should be aware of the possibility for increased PPCV and VCS after NBS. Some parents may be at greater risk. Younger parents in both NBS groups had higher PPCV. Lower health literacy may also be a risk factor, although we are unsure why this association was limited to our SCH group. Racial/ethnic disparities in PPCV were hard to interpret succinctly, and call for further investigation.
Clinical awareness may not be enough, because there may be problems with providers’ communication after NBS.37 We therefore recommend that NBS programs conduct follow-up and provide skilled counseling as a public health measure for families of infants with incidental and false-positive findings.
Routine follow-up after incidental findings would be consistent with what we have called a “safety approach” to ethical, legal, and social implications (ELSIs).37, 41 In a safety approach to ELSIs, NBS programs assume responsibility for incidental findings and the resulting psychosocial complications. A safety approach contrasts with ELSI scholarship grounded in questions about whether certain screening tests should be implemented. We anticipate continued expansion regardless of ELSIs, because NBS is so dominated by disease advocacy groups and attractive technological advances. We believe that the next step for NBS and bioethicists is to collaborate on mitigating VCS and other psychosocial complications. Anyone worried about the cost of follow-up programs should consider how the cost-benefits of expanding NBS outweigh the modest personnel costs of telephone counselors.
Our results may be relevant for the longstanding debate about withholding carrier results from parents, as has been explored in literature too extensive to cite here. We acknowledge that commentators who favor withholding may cite our data in their arguments. However, we have argued for a population-scale mechanism for follow-up after carrier identification, to ensure “more good than harm.”41, 47, 48
Some critics might argue that NBS policy does not need to be concerned about VCS, because our analysis only measured PPCV. In our view, however, the precise incidence of VCS is not so relevant as the fact that VCS is at least partially iatrogenic. Families with risk for VCS after incidental NBS findings are paying part of the price for other infants’ early identification.
In summary, increased PPCV and risk for VCS are bona fide risks of incidental findings after NBS identifies carrier status for CF or SCH. Health care professionals should be aware of this risk, but we also recommend public health follow-up for safety reasons. There is a need for re-investigation of PPCV, VCS, health care, and child development.
Supplementary Material
Figure 1.
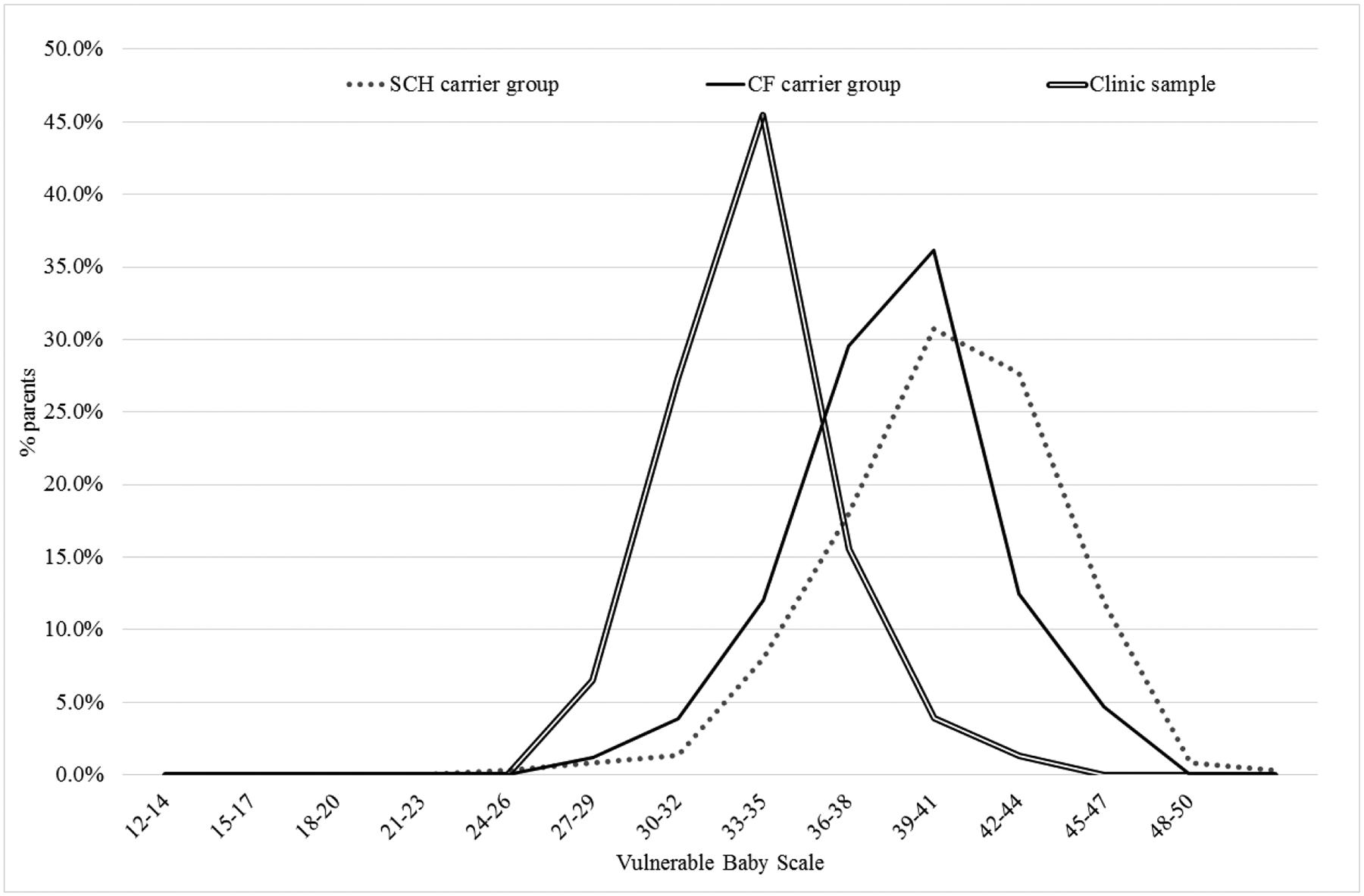
Distributions of scores for Vulnerable Baby Scale
Acknowledgments
M.F. and A.L. were supported by the National Institutes of Health (R01 HL086691 and HL086691-02S1). The authors declare no conflicts of interest.
Abbreviations:
- SCH
sickle cell hemoglobinopathy
- CF
cystic fibrosis
- NBS
newborn screening
- VCS
vulnerable child syndrome
- PPCV
parental perception of child vulnerability
- VB Scale refers to our adaptation of Kerruish’s Vulnerable Baby Scale25
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing Statement: Deidentified individual participant data will not be made available
REFERENCES
- 1.Centers for Disease C, Prevention. CDC Grand Rounds: Newborn screening and improved outcomes. MMWR Morb Mortal Wkly Rep. 2012;61:390–3. [PubMed] [Google Scholar]
- 2.Joseph G, Chen F, Harris-Wai J, Puck JM, Young C, Koenig BA. Parental Views on Expanded Newborn Screening Using Whole-Genome Sequencing. Pediatrics. 2016;137 Suppl 1:S36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman JM, Cornel MC, Goldenberg AJ, Lister KJ, Senecal K, Vears DF, et al. Genomic newborn screening: public health policy considerations and recommendations. BMC Med Genomics. 2017;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavanagh L, Compton CJ, Tluczek A, Brown RL, Farrell PM. Long-term evaluation of genetic counseling following false-positive newborn screen for cystic fibrosis. Journal of genetic counseling. 2010;19:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurian EA, Kinnamon DD, Henry JJ, Waisbren SE. Expanded newborn screening for biochemical disorders: the effect of a false-positive result. Pediatrics. 2006;117:1915–21. [DOI] [PubMed] [Google Scholar]
- 6.Waisbren SE, Albers S, Amato S, Ampola M, Brewster TG, Demmer L, et al. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA : the journal of the American Medical Association. 2003;290:2564–72. [DOI] [PubMed] [Google Scholar]
- 7.Kerruish NJ, Campbell-Stokes PL, Gray A, Merriman TR, Robertson SP, Taylor BJ. Maternal psychological reaction to newborn genetic screening for type 1 diabetes. Pediatrics. 2007;120:e324–35. [DOI] [PubMed] [Google Scholar]
- 8.Poulakis Z, Barker M, Wake M. Six month impact of false positives in an Australian infant hearing screening programme. Arch Dis Child. 2003;88:20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green M, Solnit AJ. Reactions to the Threatened Loss of a Child: A Vulnerable Child Syndrome. Pediatric Management of the Dying Child, Part Iii. Pediatrics. 1964;34:58–66. [PubMed] [Google Scholar]
- 10.Boyce WT. The vulnerable child: new evidence, new approaches. Advances in pediatrics. 1992;39:1–33. [PubMed] [Google Scholar]
- 11.Spurrier NJ, Sawyer MG, Staugas R, Martin AJ, Kennedy D, Streiner DL. Association between parental perception of children’s vulnerability to illness and management of children’s asthma. Pediatr Pulmonol. 2000;29:88–93. [DOI] [PubMed] [Google Scholar]
- 12.Pearson SR, Boyce WT. Consultation with the specialist: the vulnerable child syndrome. Pediatr Rev. 2004;25:345–9. [DOI] [PubMed] [Google Scholar]
- 13.Thomasgard M, Metz WP. The vulnerable child syndrome revisited. Journal of developmental and behavioral pediatrics : JDBP. 1995;16:47–53. [PubMed] [Google Scholar]
- 14.Chambers PL, Mahabee-Gittens EM, Leonard AC. Vulnerable child syndrome, parental perception of child vulnerability, and emergency department usage. Pediatr Emerg Care. 2011;27:1009–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsyth BW, Horwitz SM, Leventhal JM, Burger J, Leaf PJ. The child vulnerability scale: an instrument to measure parental perceptions of child vulnerability. J Pediatr Psychol. 1996;21:89–101. [DOI] [PubMed] [Google Scholar]
- 16.Levy JC. Vulnerable children: parents’ perspectives and the use of medical care. Pediatrics. 1980;65:956–63. [PubMed] [Google Scholar]
- 17.Anthony KK, Gil KM, Schanberg LE. Brief report: Parental perceptions of child vulnerability in children with chronic illness. Journal of pediatric psychology. 2003;28:185–90. [DOI] [PubMed] [Google Scholar]
- 18.Perrin EC, West PD, Culley BS. Is my child normal yet? Correlates of vulnerability. Pediatrics. 1989;83:355–63. [PubMed] [Google Scholar]
- 19.Warschausky S, MacKenzie J, Roth RS, Bartlett RH. Maternal distress and perceptions of infant development following extracorporeal membrane oxygenation and conventional ventilation for persistent pulmonary hypertension. Child Care Health Dev. 1995;21:53–65. [DOI] [PubMed] [Google Scholar]
- 20.Allen EC, Manuel JC, Legault C, Naughton MJ, Pivor C, O’Shea TM. Perception of child vulnerability among mothers of former premature infants. Pediatrics. 2004;113:267–73. [DOI] [PubMed] [Google Scholar]
- 21.Forsyth BW, Canny PF. Perceptions of vulnerability 3 1/2 years after problems of feeding and crying behavior in early infancy. Pediatrics. 1991;88:757–63. [PubMed] [Google Scholar]
- 22.O’Connor ME, Szekely LJ. Frequent breastfeeding and food refusal associated with failure to thrive. A manifestation of the vulnerable child syndrome. Clinical pediatrics. 2001;40:27–33. [DOI] [PubMed] [Google Scholar]
- 23.Sigal J, Gagnon P. Effects of parents’ and pediatricians’ worry concerning severe gastroenteritis in early childhood on later disturbances in the child’s behavior. J Pediatr. 1975;87:809–14. [DOI] [PubMed] [Google Scholar]
- 24.Sigal JJ, Chagoya L, Villeneuve C, Mayerovitch J. Later psychosocial sequelae of early childhood illness (severe croup). Am J Psychiatry. 1973;130:786–9. [DOI] [PubMed] [Google Scholar]
- 25.Kerruish NJ, Settle K, Campbell-Stokes P, Taylor BJ. Vulnerable Baby Scale: development and piloting of a questionnaire to measure maternal perceptions of their baby’s vulnerability. Journal of paediatrics and child health. 2005;41:419–23. [DOI] [PubMed] [Google Scholar]
- 26.Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773–8. [PubMed] [Google Scholar]
- 27.Kemper KJ, Forsyth BW, McCarthy PL. Persistent perceptions of vulnerability following neonatal jaundice. American journal of diseases of children (1960). 1990;144:238–41. [DOI] [PubMed] [Google Scholar]
- 28.Culley BS, Perrin EC, Chaberski MJ. Parental perceptions of vulnerability of formerly premature infants. J Pediatr Health Care. 1989;3:237–45. [DOI] [PubMed] [Google Scholar]
- 29.Estroff DB, Yando R, Burke K, Snyder D. Perceptions of preschoolers’ vulnerability by mothers who had delivered preterm. Journal of pediatric psychology. 1994;19:709–21. [DOI] [PubMed] [Google Scholar]
- 30.Burger J, Horwitz SM, Forsyth BW, Leventhal JM, Leaf PJ. Psychological sequelae of medical complications during pregnancy. Pediatrics. 1993;91:566–71. [PubMed] [Google Scholar]
- 31.Field T, Estroff DB, Yando R, del Valle C, Malphurs J, Hart S. “Depressed” mothers’ perceptions of infant vulnerability are related to later development. Child Psychiatry Hum Dev. 1996;27:43–53. [DOI] [PubMed] [Google Scholar]
- 32.Farrell MH, Christopher SA, Tluczek A, Kennedy-Parker K, La Pean A, Eskra K, et al. Improving communication between doctors and parents after newborn screening. WMJ : official publication of the State Medical Society of Wisconsin. 2011;110:221–7. [PMC free article] [PubMed] [Google Scholar]
- 33.La Pean A, Collins JL, Christopher SA, Eskra KL, Roedl SJ, Tluczek A, et al. A qualitative secondary evaluation of statewide follow-up interviews for abnormal newborn screening results for cystic fibrosis and sickle cell hemoglobinopathy. Genetics in medicine : official journal of the American College of Medical Genetics. 2012;14:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradford L, Roedl SJ, Christopher SA, Farrell MH. Use of social support during communication about sickle cell carrier status. Patient education and counseling. 2012;88:203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christopher SA, Collins JL, Farrell MH. Effort required to contact primary care providers after newborn screening identifies sickle cell trait. Journal of the National Medical Association. 2012;104:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins JL, La Pean A, O’Tool F, Eskra KL, Roedl SJ, Tluczek A, et al. Factors that influence parents’ experiences with results disclosure after newborn screening identifies genetic carrier status for cystic fibrosis or sickle cell hemoglobinopathy. Patient education and counseling. 2013;90:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrell MH, Christopher SA. Frequency of high-quality communication behaviors used by primary care providers of heterozygous infants after newborn screening. Patient education and counseling. 2013;90:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.La Pean A, Farrell MH, Eskra KL, Farrell PM. Effects of immediate telephone follow-up with providers on sweat chloride test timing after cystic fibrosis newborn screening identifies a single mutation. The Journal of pediatrics. 2013;162:522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad NY, Farrell MH. Linguistic markers of emotion in mothers of sickle cell carrier infants: what are they and what do they mean? Patient education and counseling. 2014;94:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson R, Roedl SJ, Farrell MH. Internet Searching after Parents Receive Abnormal Newborn Screening Results. Journal of Communication in Healthcare: Strategies, Media and Engagement in Global Health. 2015;8:303–15. [Google Scholar]
- 41.Farrell MH, La Pean Kirschner A, Tluczek A, Farrell PM. Psychosocial outcomes of parents after newborn screening identifies genetic carriers. Submitted Manuscript. 2019. [Google Scholar]
- 42.Thomasgard M, Shonkoff JP, Metz WP, Edelbrock C. Parent-child relationship disorders. Part II. The vulnerable child syndrome and its relation to parental overprotection. Journal of developmental and behavioral pediatrics : JDBP. 1995;16:251–6. [PubMed] [Google Scholar]
- 43.Dogan DG, Ertem IO, Karaaslan T, Forsyth BW. Perception of vulnerability among mothers of healthy infants in a middle-income country. Child Care Health Dev. 2009;35:868–72. [DOI] [PubMed] [Google Scholar]
- 44.Gleason TR, Evans ME. Perceived vulnerability: a comparison of parents and children. J Child Health Care. 2004;8:279–87. [DOI] [PubMed] [Google Scholar]
- 45.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Family medicine. 2004;36:588–94. [PubMed] [Google Scholar]
- 46.National Institutes of Health. Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes. 2018. [Available from:https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html.
- 47.Farrell MH, Christopher SA, Kirschner AL, Roedl SJ, O’Tool FO, Ahmad NY, et al. Improving the quality of physician communication with rapid-throughput analysis and report cards. Patient education and counseling. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrell MH, Farrell PM. Newborn screening for cystic fibrosis: ensuring more good than harm. The Journal of pediatrics. 2003;143:707–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


