Abstract
A new coronavirus (SARS-CoV-2) has determined a pneumonia outbreak in China (Wuhan, Hubei Province) in December 2019, called COVID-19 disease. In addition to the person-to person transmission dynamic of the novel respiratory virus, it has been recently studied the role of environmental factors in accelerate SARS-CoV-2 spread and its lethality. The time being, air pollution has been identified as the largest environmental cause of disease and premature death in the world. It affects body's immunity, making people more vulnerable to pathogens. The hypothesis that air pollution, resulting from a combination of factors such as meteorological data, level of industrialization as well as regional topography, can acts both as a carrier of the infection and as a worsening factor of the health impact of COVID-19 disease, has been raised recently. With this review, we want to provide an update state of art relating the role of air pollution, in particular PM2.5, PM10 and NO2, in COVID-19 spread and lethality. The Authors, who first investigated this association, often used different research methods or not all include confounding factors whenever possible. In addition, to date incidence data are underestimated in all countries and to a lesser extent also mortality data. For this reason, the cases included in the reviewed studies cannot be considered conclusive. Although it determines important limitations for direct comparison of results, and more studies are needed to strengthen scientific evidences and support firm conclusions, major findings are consistent, highlighting the important contribution of PM2.5 and NO2 as triggering of the COVID-19 spread and lethality, and with a less extent also PM10, although the potential effect of airborne virus exposure it has not been still demonstrated.
Keywords: Air pollution, Particulate matter, Nitrogen dioxide, COVID-19, Pandemic
1. Introduction
A new coronavirus (SARS-CoV-2) has determined a pneumonia outbreak in China (Wuhan, Hubei Province) in December 2019, called COVID-19 disease. The scientific community has come together to implement pharmaceutical and non-pharmaceutical intervention measures designed to contain SARS-CoV-2 global spread. Nevertheless, on March 11th, 2020, WHO's Director-General announced that COVID-19 can be characterized as a pandemic.
SARS-CoV-2 is primarily transmitted from person-to-person through close contact (approximately 2 m), by aerosol respiratory droplets smaller than 5 μm in diameter (https://www.who.int/). Indoor environments might be especially hazardous because of their reduced ventilation (Morawska et al., 2020), lack ultraviolet light which rapidly inactivates the virus and because it can become less diluted than it would in outdoor environments (Schuit et al., 2020). It is also known how the virus can survive and being infectious in aerosols for hours and on surface up to days (van Doremalen et al., 2020), similarly with transmission dynamics known for SARS-CoV-1, associated with nosocomial spread and super-spreading events (Chen et al., 2020a,b). Beyond the causality, it is uncertain even if certain demographics of the population are more vulnerable to SARS-CoV-2 infection. Based on recent reports, male gender, advancing age and co-morbidities seem to be correlated with death and severe illness (Harris et al., 2020). Furthermore, COVID-19 seems to be associated with an increasing rate of thromboembolic events in hospitalized patients (Llitjos et al., 2020).
Mechanisms of social and economic interactions are additionally supposed to be involved in the diffusion dynamic of COVID-19 in the diverse parts of the world or of the same country, such as the living conditions, the health-related behaviour (Khalatbari-Soltani et al., 2020), the commercial exchanges (Bontempi, 2020a) or the migration scale index (H. Chen et al., 2020). It seems that these diffusion dynamics have particularly affected the COVID-19 spread at the early stage.
Among the environmental parameters, some climate condition such as temperature, humidity, sunlight and wind revealed a reduction of the COVID-19 spread (S. Chen et al., 2020; Coccia, 2020a), and air pollution seems to have a role in airborne transmission of SARS-CoV-2 and severity of COVID-19 (Domingo et al., 2020). Nevertheless, to better understand COVID-19's diffusion patterns, an interdisciplinary, multidimensional approach should be encourage in order to develop firm conclusions (Bontempi et al., 2020).
Air pollution has been identified as the largest environmental cause of disease and premature death in the world (GBD, 2018). Ambient particulate matter (PM) induces its pro-inflammatory and thrombogenic effects through the generation of oxidative stress by its chemical compounds and metals (Li et al., 2008; Signorelli et al., 2019). The recent identification of environmentally persistent free radicals (EPFRs) in the PM, resulting from a mixture of combustion sources, theorize its role in the increase of disease severity of lower respiratory tract infections (LRTI) (Jaligama et al., 2017). Scientific evidences support that short- and long-term exposures to ambient air pollutants are associated with a broad of adverse health outcomes (Ferrante and Conti, 2017; Fiore et al., 2019), such as higher mortality rates, greater hospital admissions and increased outpatient visits (Bremner et al., 1999; Cohen et al., 2017; Dehghani et al., 2017; Dockery et al., 1993). It has markedly detrimental consequences on asthma, bronchitis, pneumonia and COPD (Dick et al., 2014; Perng and Chen, 2017; Raji et al., 2020; Vignal et al., 2017; Yarahmadi et al., 2018), where bacteria and viruses are the most accepted causative factors that harm airway stability, driving to infection exacerbation. Furthermore, air pollution represents an aggravating factor for infection diseases caused by some viral infections (Domingo and Rovira, 2020), such as respiratory syncytial virus (RSV), influenza A and B, para influenza virus type 3, pneumonia and influenza-like illness (Carugno et al., 2018; Croft et al., 2020; Fukuda et al., 2011; Huang et al., 2016; Huh et al., 2020; Liang et al., 2014; Lin et al., 2005; Silva et al., 2014; Somayaji et al., 2020). It determines an increase in the rate of hospitalizations and access to emergency department visits. Studies related to the epidemic of SARS-CoV coronavirus identified in November 2002 from the Guangdong province of southern China, reported similar associations (Cai et al., 2007; Cui et al., 2003; Kan et al., 2005).
Several Authors suggest that outdoor air pollution, resulting from a combination of factors such as meteorological data, level of industrialization as well as regional topography, could operate both as a carrier of the infection and as a worsening factor of the COVID-19 severity (Conticini et al., 2020; Frontera et al., 2020; Isaifan, 2020; Martelletti and Martelletti, 2020). This association is getting stronger thanks to the results of the numerous studies that have been launched all over the world, and summarized with this review. Most of the reviewed studies support that chronic exposure to air pollution might led people more susceptible to COVID-19 disease, leading to widespread COVID-19 spread and lethality. Nevertheless, as suggested by Bontempi (2020b), the potential effect of airborne virus exposure due to PM10 remain unclear.
With this review, we want to provide an updated state of art of the recently epidemiological studies dealing with understanding the role of air pollution, in particular PM2.5, PM10 and NO2, in COVID-19 spread and lethality.
2. Method
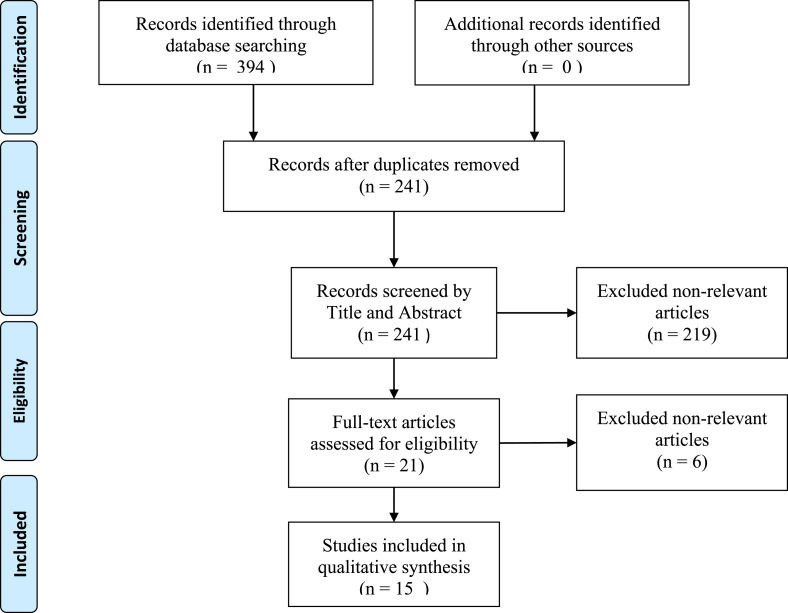
We have conducted a systematic review of the literature concerning the relationship between some air pollutants, PM2.5, PM10 and NO2, and COVID-19 outbreak. The research was performed in compliance with the PRISMA criteria, Preferred Reporting Items for Systematic Reviews and Meta-Analyses, and the Flow Diagram is showed in Fig. 1 . The research was conducted between April 2020 and July 6th, 2020 in PubMed database. It was used the Advanced Search Builder and the keywords were searched in [Title OR Abstract]. We have filtered only research articles published in English language and selected the following keywords: Air pollution and COVID-19 or SARS-COV-2; Particulate matter or PM and COVID-19 or SARS-COV-2; Nitrogen dioxide or NO2 and COVID-19 or SARS-COV-2.
Fig. 1.
PRISMA Flow Diagram of identification, screening and inclusion of studies.
We choose as inclusion criteria all the available epidemiological studies aimed to identify any temporal and spatial association between reported COVID-19 cases and/or deaths and air pollution data related to PM2.5, PM10 and NO2, thus excluding any Letter, Opinion, Commentary, Review or non-relevant articles. We obtained a total of 13 eligible published research articles in their final version and 2 paper in its preprint version. For some of them we chose to include only principal findings that clearly fit the aim this review.
3. Particulate Matter and COVID-19
Atmospheric particulate matter (PM) is originated by a wide range of anthropogenic and natural sources (Kim, 2013). It consists of a heterogeneous mixture of solid and liquid particles suspended in air that varies continuously in size and chemical composition, including nitrates, sulphates, elemental and organic carbon, organic compounds, biological compounds and metals (WHO, 2003).
It has been associated with increased respiratory morbidity and mortality (Liu et al., 2019), especially in susceptible people, due to cardiorespiratory events, including asthma, chronic obstructive pulmonary disease, and thrombosis (Li et al., 2008; Rhee et al., 2019). In vitro and in vivo studies highlighted its role in the exacerbation of respiratory viral infections (Becker and Soukup, 1999). Recently, the research group of Setti et al. (2020) gave first preliminary evidence that SARS-CoV-2 RNA can be present on outdoor particulate matter, thus suggesting that, in conditions of atmospheric stability and high concentrations of PM, it could represent a potential early indicator of COVID-19, although it does not give information regarding COVID-19 progression or severity.
Several observations report a significant association between ambient concentrations of PM2.5 (Adhikari and Yin, 2020; Bashir et al., 2020; Fattorini and Regoli, 2020; Frontera et al., 2020; Jiang et al., 2020; Li et al., 2020; Vasquez-Apestegui et al., 2020; Wu et al., 2020; Yao et al., 2020; Zhu et al., 2020; Zoran et al., 2020a) and PM10 (Bashir et al., 2020; Coccia, 2020b; Fattorini and Regoli, 2020; Jiang et al., 2020; Li et al., 2020; Yao et al., 2020; Zhu et al., 2020; Zoran et al., 2020a) with COVID-19 pandemic across the most affected countries: China, Italy and U.S.A (see Table 1 ).
Table 1.
Summary table reporting reviewed results on the association between COVID-19 cases/deaths and air pollution (PM2.5, PM10 and NO2).
| References | Period | Area of Study | Aim | Data analysis | PM2.5 | PM10 | NO2 |
|---|---|---|---|---|---|---|---|
| Zhu et al. (2020) | From Jan 23rd to Feb 29th | 120 cities of China | Temporal association between daily confirmed cases and air pollution (PM2.5, PM10 and NO2) | Generalized Additive Model (GAM) | A 10-μg/m3 PM2.5 increase (lag0–14) was associated with a 2.24% increase of daily confirmed new cases | A 10-μg/m3 PM10 increase (lag0–14) was associated with a 1.76% increase of daily confirmed new cases | A 10-μg/m3 NO2 increase (lag0–14) was associated with a 6.94% increase in daily confirmed new cases |
| Jiang et al. (2020) | From Jan 25th to Feb 29th | Wuhan, XiaoGan and HuangGang (China) | Temporal association between daily confirmed cases and air pollution (PM2.5, PM10 and NO2) | Multivariate Poisson regression | Wuhan (RR = 1.036, CI:1.032–1.039); XiaoGan (RR = 1.059, CI = 1.046–1.072); HuangGang (RR = 1.144, CI = 1.12–1.169) | Wuhan (RR = 0.964, CI: 0.961–0.967); XiaoGan (RR = 0.961, CI = 0.950–0.972); HuangGang (RR = 0.915, CI = 0.896–0.934) | Wuhan (RR = 1.056, CI: 1.053–1.059); XiaoGan (RR = 1.115, CI = 1.095–1.136); HuangGang (no association found) |
| Li et al. (2020) | From Jan 26th to Feb 29th in 2020 | Wuhan and XiaoGan | Temporal association between daily confirmed cases and air pollution PM2.5, PM10 and NO2) | Simple linear regression | Wuhan (R2 = 0.174, p < 0.05); XiaoGan (R2 = 0.23, p < 0.01). | Wuhan (R2 = 0.105; p > 0.05); XiaoGan (R2 = 0.158, p < 0.05). | Wuhan (R2 = 0.329, p < 0.001); XiaoGan (R2 = 0.158, p < 0.05). |
| Yao et al. (2020) | Data up to March 22nd | 49 cities of China | Spatial association between fatality rate and air pollution (PM2.5 and PM10) | Multiple linear regression | χ2 = 15.25, p = 0.004; A 10 μg/m3 increase in PM2.5 was associated with a 0.24% (0.01%–0.48%) increase in fatality rate | χ2 = 13.53, p = 0.009; A 10 μg/m3 increase in PM10 was associated with a 0.26% (0.00%–0.51%) increase in fatality rate | / |
| Ogen (2020) | Data up to the end of Feb | 66 administrative regions in Italy, Spain, France and Germany | Spatial association between deaths counts and air pollution (NO2) | Descriptive analysis: percentage of deaths in three NO2 μmol/m2concentration range (0–50; 50–100; 100–300) | / | / | 83% of fatality cases are associated with NO2 > 100 μmol/m2 |
| Zoran et al. (2020a) | From Jan 1st to Apr 30th | Milan (Italy) | Temporal association between total cases, daily confirmed cases and total deaths and air pollution (PM2.5 and PM10) | Pearson coefficient correlation | R = −0.39; R = 0.25; R = −0.53 | R = −0.30; R = 0.35; R = −0.49 | / |
| Zoran et al. (2020b) | From Jan 1st to Apr 30th | Milan (Italy) | Temporal association between total cases, daily confirmed cases and total deaths and air pollution (NO2) | Pearson coefficient correlation | / | / | R = −0.55; R = −0.35; R = −0.58 |
| Bontempi (2020b) | From Feb 10th to March 12th | 7 provinces of Lombardy, Italy; 6 provinces of Piedmont, Italy; | Spatial description of PM10 exceedances versus COVID-19 cases | Descriptive analysis: Number of days of PM10 exceeding 50 μg/m3 and COVID-19 incidence | / | Lombardy: PM10 exceeding between 0 and 8, COVID-19 incidence % between 0,03 and 0,49. Piedmont: PM10 exceeding between 3 and 12, COVID-19 incidence % between 0,01 and 0,03. | / |
| Coccia (2020b) | Data up to April 7th | 55 Italian Provinces | Spatial association between confirmed cases and air pollution (PM10) | Hierarchical multiple regression model | / | COVID-19 in North Italy has a high association with air pollution of cities measured with days exceeding the limits set for PM10 | / |
| Fattorini and Regoli (2020) | Data up to April 27th | 71 Italian provinces | Spatial association between total confirmed cases and air pollution (PM2.5, PM10 and NO2) | Pearson regression coefficient analysis | R2 = 0.340; p < 0.01 | R2 = 0.267; p < 0.01 | R2 = 0.247; p < 0.01 |
| Frontera et al. (2020) | Data up to 31st March | Italian regions | Spatial association between total confirmed cases and air pollution (PM2.5) | Pearson regression coefficient analysis | R2 = 0.64; p < 0.01 | / | / |
| Frontera et al. (2020) | Data up to 31st March | Italian regions | Spatial association between deaths and air pollution (PM2.5) | Pearson regression coefficient analysis | R2 = 0.53; p < 0.05 | / | / |
| Wu et al. (2020) | Data up to April 04th | 3000 counties in the U.S.A. | Prediction of risk of COVID-19 deaths in the long-term average exposure to fine particulate matter (PM2.5) | Zero-inflated negative binomia models | Long-term exposure increase of 1 μg/m3 in PM2.5 is associated with a 15% increase in the COVID-19 death rate. | / | / |
| Adhikari and Yin (2020) | From March 1st to Apr 20th | Queens county, New York (U.S.A) | Temporal association between daily confirmed cases and total deaths and air pollution (PM2.5) | Negative binomial regression model | Estimate on cases values = −0.4029 (CI%: 0.6478–0.6896); Estimate on deaths value = −0.1151 (CI%: 0.7966–0.9971) | / | / |
| Bashir et al. (2020) | From March 4th to April 24th | California | Association between confirmed cases and air pollution (PM2.5, PM10 and NO2) | Spearman and Kendall correlation tests | Kendall r (−0.359); Spearman r (−0.453) | Kendall r (−0.287); Spearman r (−0.375) | Kendall r (−0.514); Spearman r (−0.736) |
| Bashir et al. (2020) | From March 4th to April 24th | California | Association between deaths and air pollution (PM2.5, PM10 and NO2) | Spearman and Kendall correlation tests | Kendall r (−0.339); Spearman r (−0.429) | Kendall r (−0.267); Spearman r (−0.350) | Kendall r (−0.485); Spearman r (−0.731) |
| Vasquez-Apestegui et al. (2020) | Data up to June 12th | 24 districts of Lima, Perù | Spatial association between total confirmed cases and air pollution (PM2.5) | Multivariate regression model | Crude coefficient = 0.083, p < 0.05 | / | / |
| Vasquez-Apestegui et al. (2020) | Data up to June 12th | 24 districts of Lima, Perù | Spatial association between deaths and air pollution (PM2.5) | Multivariate regression model | Crude coefficient = 0.0016, p < 0.01 | / | / |
| Vasquez-Apestegui et al. (2020) | Data up to June 12th | 24 districts of Lima, Perù | Spatial association between case fatality rate and air pollution (PM2.5) | Multivariate regression model | Crude coefficient = −0.014, p > 0.05 | / | / |
First evidences on the temporal association between air pollution and COVID-19 were reported in China, where the outbreak was first identified. Zhu et al. (2020) explored the relationship between particulate matter and the viral infection caused by the novel coronavirus in 120 cities in China. The Authors included over 58,000 (70%) of daily-confirmed new cases in the whole of China between January 23rd, 2020 and February 29th, 2020. They applied a Generalized Additive Model (GAM) to examine the effects of meteorological factors and air pollution on COVID-19 incidence, applying a moving-average approach to capture the cumulative lag effect of ambient air pollution and considering population size and density as potential confounders. They observed that the effect of PM2.5 on daily confirmed cases was greater than PM10. In particular they found that a 10-μg/m3 increase (lag0–14) in PM2.5 and PM10 was associated with a 2.24% (95% CI: 1.02 to 3.46) and 1.76% (95% CI: 0.89 to 2.63) increase in the daily counts of COVID-19 confirmed cases, respectively.
Jiang et al. (2020) focused their attention on three most affected cities of China, Wuhan, XiaoGan and HuangGang, collecting data of daily cases and ambient air pollutant from Jan 25th to Feb 29th. The Authors, by applying a multivariate Poisson regression revealed a significant temporal association between PM2.5 increased and COVID-19 incidence in all the considered cities, especially in HuangGang (Wuhan RR = 1.036, CI: 1.032–1.039; XiaoGan RR = 1.059, CI = 1.046–1.072; HuangGang RR = 1.144, CI = 1.12–1.169). Conversely, an increase in PM10 concentrations was associated with a decrease of COVID-19 incidence. These results were partially confirmed by findings of Li et al. (2020), who conducted a simple linear regression to compare COVID-19 incidence with PM concentrations in Wuhan and XiaoGan from Jan 26th to Feb 29th in 2020. They found that an increase in PM2.5 was correlated with an increase of COVID-19 incidence in both cities (Wuhan: R2 = 0.174, p < 0.05; XiaoGan: R2 = 0.23, p < 0.01), while for PM10 only in XiaoGan (R2 = 0.158, p < 0.05). The spatial distribution of particulate matter and case fatality rate (CFR) of COVID-19 was studied by Yao et al. (2020) in 49 cities of China, including Wuhan, collecting data up to March 22nd. First, they found a significantly positive global spatial auto-correlation of COVID-19 CFR (Global Moran's index I = 0.16, p < 0.0001), highlighting a high CFR clustering located in Hubei Province. With a multiple linear regression, they adjusted their results for several effect modifiers and confounder factors such temperature, relative humidity, gross domestic product (GDP) per capita, hospital beds per capita, local indicators of spatial association (LISA) map values, city size and population or proportion of people older than 65 years. It was found that for every 10 μg/m3 increase in PM2.5 and PM10, the CFR increased by 0.24% (0.01%–0.48%) and 0.26% (0.00%–0.51%) respectively, and the risk estimates increased to 0.61% (0.09%–1.12%) and 0.33% (0.03%–0.64%) with every 10 μg/m3 increase in average concentrations of PM2.5 and PM10 in 2015–2019, respectively.
Some studies describe the association between air pollution and COVID-19 across Italy, the second country of the world where the infection spread significantly at the beginning of the pandemic, and suddenly has reached many other European countries. The 28th of July Italy recorded more than 245,000 total confirmed cases and 35,107 deaths (WHO, 2020), most of which were distributed in the regions of Northern Italy, especially the Lombardy. It is recognized as one the most air polluted areas of Europe (EEA, 2019), where the frequent PM10 annual exceedances of the WHO threshold of 20 μg/m3 are responsible for 302 attributable deaths per year, corresponding to attributable community rates of 13 deaths per 100,000 inhabitants per year (Baccini et al., 2011). Bontempi (2020b)focused the attention on two of the most affected regions of Northern Italy, Lombardy and Piedmont. The Authors, based on PM10 daily exceedances and COVID-19 confirmed cases on March 12th, thus before the Italian sanitary crisis, observed that PM10 concentration was exceeded only few times among the Lombard cities that at the beginning of the epidemic were most affected. On the contrary, among some Piedmont cities suffering of severe PM10 pollution events, COVID-19 incidence was lower. Based on their results, the Authors concluded that COVID-19 diffusion by airborne PM10 is hard to demonstrate, nevertheless, several research article revealed how PM, in particular PM2.5, could had a role in accelerate and vast diffusion of COVID-19 in Northern Italy. For example, Coccia (2020b), by analyzed data on 55 Italian province capitals, and data of infected individuals up to April 7th, 2020, revealed a relationship between air pollution of cities measured with days exceeding the limits set for PM10 in previous years and COVID-19 diffusion. In particular, cities with more than 100 days of PM10 exceedances showed a very high average number of infected individual (about 3600 infected individuals on 7th April 2020), whereas cities having less than 100 days of PM10 exceedances, showed a lower average number of infected (about 1000 infected individuals).
Frontera et al. (2020) gave also evidences on the role of PM2.5 as a contributing factor of COVID-19 outbreak in Northern Italy, where mortality was found significantly higher than less polluted Italian regions. By collecting data up to March 31st for all Italian regions and performing a Pearson correlation analysis, they found a strong positive association both with the total number of confirmed cases (R = 0.64) and deaths (R = 0.53), other than with hospitalized cases (R = 0.62).
The Italian situation was further highlighted by the study of Fattorini and Regoli (2020) in 71 Italian provinces. They explored the spatial association between air pollution and COVID-19 cases with data up to April 27th. By applying the Pearson regression coefficient analysis, they revealed a positive association both with PM2.5 and PM10 (R2 = 0.340, p < 0.01 and R2 = 0.267, p < 0.01 respectively).
A focus on the most affected city of Italy, Milan, was conducted by Zoran et al. (2020a). This city is located in the Po valley basin, known hotspot for atmospheric pollution at the continental scale (EEA, 2019). The Authors performed a temporal association between COVID-19 (Total cases, Daily New positive cases and Total Deaths) and particulate matter from Jan 1st and Apr 30th by applying a Person correlation. In accordance with other studied, they found a positive association between daily confirmed cases and PM2.5 (R = 0.25) and PM10 (R = 0.35), although they did not consider any delay time from infection to COVID-19 onset. Nevertheless, they found a negative association between total cases and total deaths and particulate matter, but the assumption of a temporal linear correlation may be inaccurate because the above mentioned variables could have more complex nonlinear relationships.
To date, the U.S.A. have more than 4 million confirmed cases and 145 thousand deaths (WHO, 2020). Here, ambient concentrations of PM 2.5 and O3 were found responsible to cause between 130,000 and 340,000 premature deaths (Fann et al., 2012).
The association between air pollutants and COVID-19 cases and deaths was studied by Bashir et al. (2020) in the state of California from March 4th to April 24th, corresponding to the beginning of the COVID-19 outbreak in U.S.A. Based on their significant correlation found, the Authors state that a limited human exposure to these pollutants will contribute to defeating COVID-19. This conclusion seems unclear, because they found a negative correlation with PM2.5 and PM10 by applying both the Kendall rank correlation and Spearman's one and it is not clear if they normalized COVID-19 cases by population size and if they performed a day by day association or a spatial association across the country.
A focus on the Queen county, New York (U.S.A.), was provided by Adhikari and Yin (2020). They retrieved data of PM 2.5 daily concentrations from two ground monitoring stations and collected data of confirmed COVID-19 cases and numbers of related deaths from USAFacts in the period from 1 March to April 20, 2020. The Authors elaborated their data with a negative binomial regression model and considered the cumulative lag effect of PM2.5 on disease outcomes over the past 21 days. They found a significant negative association among PM2.5 and new daily confirmed COVID-19 cases (−0.4029, CI%: 0.6478–0.6896) and deaths (−0.1151 (CI%: 0.7966–0.9971). Low PM concentrations in this area of study (mean = 4.73 μg/m3) are likely to have played a less central role in the spread of infection than in other areas such as Italy, where PM2.5 monthly concentrations reached values higher than 30 μg/m3 (Fattorini and Regoli, 2020; Frontera et al., 2020), or in China where PM2.5 monthly concentrations reached values higher than 40 μg/m3 (Zhu et al., 2020; Jiang et al., 2020). As said by the Authors, other gaseous pollutants, such as NO2 and SO2 could have influenced transmission and pathogenesis of COVID-19.
In the United States, Wu et al. (2020) investigated whether long-term average exposure to fine particulate matter (PM2.5) increases the risk of COVID-19 deaths, by considering approximately 3000 counties in the United States (98% of the population). With an exposure prediction model, the Authors calculated the county level long-term exposure to PM2.5, averaged for 2000 to 2016, and collected COVID-19 deaths counts up to April 04th, 2020. They conducted a strong and robust statistical analysis with zero-inflated negative binomial mixed models, adjusting their results by several potential confounders such as sociodemographic, socioeconomic, behavioural and meteorological factors. They found that a small long-term exposure increase of only 1 μg/m3 in PM2.5 is associated with a 15% increase in the COVID-19 death rate, 95% confidence interval (CI) (5%, 25%).
Vasquez-Apestegui et al. (2020), recently reported first evidences on the spatial relationship between particulate matter and COVID-19 outbreak from Latin America. The Authors described the situation occurred in 24 districts of Lima, located in the second most affected country of Latin America, Peru. In particular, by applying a multivariate regression model, they evaluated the association between the population exposure to PM2.5 concentrations in the previous years (2010–2016) and cases, deaths and case-fatality rates of COVID-19 with data up to June 12th. A significant association has been found both with cases and deaths (Crude coefficient 0.083 with p < 0.05 and 0.0016 with p < 0.01 respectively) but not with case fatality rate.
All these studies highlight the role of PM in triggers of the COVID-19 disease, and how government measures targeting to sustainable growth, such as the reduction of urban and industrial emissions, could have a positive impact on the prevention of health outcomes, reducing mortality rate as well the burden on health care systems.
4. Nitrogen dioxide (NO2) and COVID-19
Nitrogen dioxide is a nasty-smelling gas formed by reaction in the atmosphere of nitrogen oxides (NOx) with other chemicals. NOx is naturally produced in atmosphere by lightning (Kang et al., 2019), volcanoes, oceans and biological decay (Thurston, 2017). The major outdoor anthropogenic sources of NOx are primarily emissions from transportation and fuel combustion, in particular, in urban areas they comes from vehicle exhaust gases and domestic heating (Grange et al., 2019; Maawa et al., 2020).
The nitrogen dioxide has mainly effect on the respiratory system, because an increase of the outdoor concentration of NO2 may significantly increase the risk of respiratory tract infection. This phenomenon is particularly evident in children, as they are more susceptible to NO2-induced lung damage. Hence, viral infection becomes more common after exposure to NO2 (Zhu et al., 2020), Furthermore, NO2 is associated with other several health effects such as elevated risks for asthma, allergic rhinitis and eczema in children (To et al., 2020), increase of outpatient visits and hospitalizations due to bronchitis and asthma exacerbation (Bahrami Asl et al., 2018; Kowalska et al., 2020), increase of chronic obstructive pulmonary disease (COPD) (Ghanbari Ghozikali et al., 2016; Pfeffer et al., 2019) and increase of pulmonary heart disease - related mortality (Chen et al., 2019).
A recent study explored the possible role of NO2 in interference in Angiotensin converting enzyme 2 (ACE2). The expression of ACE2 is high on lung alveolar epithelial cells and it is the human cell receptor of virus agent of COVID-19 (Alifano et al., 2020).
First observations report an association between ambient concentrations of NO2 and COVID-19 pandemic across Europe, China and U.S.A (Bashir et al., 2020; Fattorini and Regoli, 2020; Jiang et al., 2020; Li et al., 2020; Ogen, 2020; Zhu et al., 2020; Zoran et al., 2020b). Conversely to the other papers, findings of Zoran et al. (2020b) and Bashir et al. (2020) provides different findings, reporting no association or a negative one between NO2 and daily deaths counts.
In China, Zhu et al. (2020), by applying the same method explained for PM, observed that a 10-μg/m3 increase (lag0–14) in NO2 is associated with a 6.94% (95% CI: 2.38–11.51) increase in the daily counts of COVID-19 confirmed cases in 120 cities of China. These findings are confirmed by Jiang et al. (2020) and Li et a. (2020), who applied the same method described for PM. Jiang et al. (2020), revealed a significant positive association between NO2 and COVID-19 both in Wuhan and XiaoGan (Wuhan RR = 1.056, CI:1.053–1.059; XiaoGan RR = 1.115, CI = 1.095–1.136), but did not found any significant association in HuangGang. Li et al. (2020) found a significant linear correlation both in Wuhan (R2 = 0.329, p < 0.001) and XiaoGan (R2 = 0.158, p < 0.05).
Ogen (2020) presented evidences on the relationship between exposure to NO2 (including the months of January and February 2020 shortly before the COVID-19 spread in Europe) and novel coronavirus fatality in the most affected European countries, concluding that long-term exposure to NO2 may be a potential contributor to mortality caused by SARS-CoV-2. He collected data concerning the number of fatality cases from 66 administrative regions in Italy, Spain, France and Germany and correlated mortality with tropospheric NO2 concentrations measured by the Sentinel-5 Precursor space-borne satellite. The major tropospheric NO2 hotspot identified was located in the Northern Italy. In all European regions considered, gas concentrations ranged between 177.1 and 293.7 μmol/m2, with airflows directed downwards. Results showed that out of the 4443 fatality cases by March 19, 2020, 3487 (78%) were in five regions located in north Italy and central Spain. Furthermore, by analysing mortality trends, it was revealed that the highest percentage of deaths occurred in regions where the maximum NO2 concentration was above 100 μmol/m2 (83%), with a significant decrease where the maximum concentration was between 50 and 100 μmol/m2 (15.5%), and below 50 μmol/m2 (1.5%). The methodology used by Ogen (2020) cannot support a long-term exposure investigation. Surely, a validation of the satellite measure with those of the ground ’ones, the adjustment of the results according to the different population size of each country, could have made their results more robust. Nevertheless, the study provide new insights for future investigation.
The Italian situation was further studied by Fattorini and Regoli (2020), who collected data of COVID-19 incidence up to April 27th from 71 Italian provinces. They revealed a strong spatial correlation with NO2 mean levels concentrations (2016–2019) (Pearson coefficient: R2 = 0.247, p < 0.01), confirming the Northern Italy being a hotspot of NO2, in addition to urbanized cities of central and southern Italy, such as Rome and Naples.
A focus on the temporal association between ground levels of NO2 and COVD-19 cases (Total cases, Daily New positive cases and Total Deaths) was performed by Zoran et al. (2020b) for the city of Milan (Italy), in the period pre- and post-lockdown measures. The Authors found NO2 negative correlated with all the considered epidemiological data, but the methodology used has some limitations, as the delay time from infection to the COVID-19 onset or COVID-19 death was not considered, as well the significant reduction of air pollution due to lockdown measures since mid-March.
In U.S.A., the association was also studied by Bashir et al. (2020) for the state of California. As discussed above for PM, the Authors found a negative correlation also between NO2 levels and COVID-19 cases and mortality. Nevertheless, they stated that this pollutant contributes to the spread of the disease.
Based on these scientific evidences, in addition to confirming that exposure to NO2 is harmful to human health and increases the risk of incurring respiratory diseases, it can be stated that exposure to NO2 may be one of the most important trigger for the spread and fatality caused by the COVID-19 disease.
5. Conclusion
The scientific evidences collected in the literature highlight the important contribution of chronic exposure to air pollution on the COVID-19 spread and lethality, although the potential effect of airborne virus exposure it has not been still demonstrated. In particular, it seems that PM2.5 and NO2 are more closely correlated to COVID-19 than PM10. The lower correlation of PM10 with COVID-19 incidence and mortality can be due to the impossibility of particulate matter greater than 5 μm to reach type II alveolar cells, where is located the cell entry receptor (ACE2) for SARS-CoV-2. Nevertheless, differences between countries, such as the implementation of different lockdown restrictions, stage of infection, topographic, socio-demographic and socio-economic characteristics, level of air pollution and meteorological factors, may have contributed to obtain some contrasting finding.
Although most of the revised studies support the relationship between air pollution and COVID-19, the manifold limitations of this review are the small number of papers collected and the great diversity of methodologies used, sometimes lacking in some parts, which makes the results difficult to compare. The Authors, who first investigated this association, although with great effort and rapidity of analysis dictated by a global emergency, sometimes do not include all confounding factors whenever possible, such as control policy, urbanization rate, availability of medical resources, population size, weather, lifestyles, sociodemographic and socioeconomic variables. In addition, to date incidence data are underestimated in all countries, and to a lesser extent mortality data. For this reason, the cases included in the considered studies cannot be considered conclusive.
More studies are needed to better clarify the role of air pollution during the COVID-19 pandemic, particularly studies that consider the multiple-pollutants influences, or multidisciplinary studies, to strengthen scientific evidences and support firm conclusions, useful to implement pandemic application plans to adequately prevent new health emergencies.
For a long time we have known that reducing outdoor and indoor air pollution in cities or countries can have a significant effect on health almost immediately, and the benefits can far outweigh the costs. Surely, the health emergency that the world is experiencing right now highlights how environmental research is a fundamental reference point to improve the knowledge concerning diseases of infectious origin and how all the intellectual and economic resources are to be spent to accelerate actions aimed to implement environmental policies act to reduce air pollution and develop new urban planning interventions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors declare no conflict of interest. We have no funding to declare.
References
- Adhikari A., Yin J. Short-term effects of ambient ozone, PM2.5, and meteorological factors on COVID-19 confirmed cases and deaths in queens, New York. Int. J. Environ. Res. Publ. Health. 2020;17 doi: 10.3390/ijerph17114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alifano M., Alifano P., Forgez P., Iannelli A. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie. 2020 doi: 10.1016/j.biochi.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccini M., Biggeri A., Grillo P., Consonni D., Bertazzi P.A. Health impact assessment of fine particle pollution at the regional level. Am. J. Epidemiol. 2011;174:1396–1405. doi: 10.1093/aje/kwr256. [DOI] [PubMed] [Google Scholar]
- Bahrami Asl F., Leili M., Vaziri Y., Salahshour Arian S., Cristaldi A., Oliveri Conti G., Ferrante M. Health impacts quantification of ambient air pollutants using AirQ model approach in Hamadan, Iran. Environ. Res. 2018;161:114–121. doi: 10.1016/j.envres.2017.10.050. [DOI] [PubMed] [Google Scholar]
- Bashir M.F., Ma B.J., Bilal Komal B., Bashir M.A., Farooq T.H., Iqbal N., Bashir M. Correlation between environmental pollution indicators and COVID-19 pandemic: a brief study in Californian context. Environ. Res. 2020;187:109652. doi: 10.1016/j.envres.2020.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S., Soukup J.M. Exposure to urban air particulates alters the macrophage-mediated inflammatory response to respiratory viral infection. J. Toxicol. Environ. Health. 1999;57:445–457. doi: 10.1080/009841099157539. [DOI] [PubMed] [Google Scholar]
- Bontempi E. Commercial exchanges instead of air pollution as possible origin of COVID-19 initial diffusion phase in Italy: more efforts are necessary to address interdisciplinary research. Environ. Res. 2020;188:109775. doi: 10.1016/j.envres.2020.109775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): the case of Lombardy (Italy) Environ. Res. 2020;186:109639. doi: 10.1016/j.envres.2020.109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Vergalli S., Squazzoni F. Understanding COVID-19 diffusion requires an interdisciplinary, multi-dimensional approach. Environ. Res. 2020;188:109814. doi: 10.1016/j.envres.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner S.A., Anderson H.R., Atkinson R.W., McMichael A.J., Strachan D.P., Bland J.M., Bower J.S. Short-term associations between outdoor air pollution and mortality in London 1992-4. Occup. Environ. Med. 1999;56:237–244. doi: 10.1136/oem.56.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q.-C., Lu J., Xu Q.-F., Guo Q., Xu D.-Z., Sun Q.-W., Yang H., Zhao G.-M., Jiang Q.-W. Influence of meteorological factors and air pollution on the outbreak of severe acute respiratory syndrome. Publ. Health. 2007;121:258–265. doi: 10.1016/j.puhe.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carugno M., Dentali F., Mathieu G., Fontanella A., Mariani J., Bordini L., Milani G.P., Consonni D., Bonzini M., Bollati V., Pesatori A.C. PM10 exposure is associated with increased hospitalizations for respiratory syncytial virus bronchiolitis among infants in Lombardy, Italy. Environ. Res. 2018;166:452–457. doi: 10.1016/j.envres.2018.06.016. [DOI] [PubMed] [Google Scholar]
- Chen H., Chen Y., Lian Z., Wen L., Sun B., Wang P., Li X., Liu Q., Yu X., Lu Y., Qi Y., Zhao S., Zhang L., Yi X., Liu F., Pan G. Correlation between the migration scale index and the number of new confirmed coronavirus disease 2019 cases in China. Epidemiol. Infect. 2020;148:e99. doi: 10.1017/S0950268820001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Zeng J., Shi C., Liu R., Lu R., Mao S., Zhang L. Associations between short-term exposure to gaseous pollutants and pulmonary heart disease-related mortality among elderly people in Chengdu, China. Environ. Health. 2019;18 doi: 10.1186/s12940-019-0500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Prettner K., Kuhn M., Geldsetzer P., Wang C., Bärnighausen T., Bloom D.E. COVID-19 and climate: global evidence from 117 countries. MedRxiv Prepr. Serv. Health Sci. 2020 doi: 10.1101/2020.06.04.20121863. [DOI] [Google Scholar]
- Coccia M. Social Science Research Network; Rochester, NY: 2020. How High Wind Speed Can Reduce Negative Effects of Confirmed Cases and Total Deaths of COVID-19 Infection in Society (SSRN Scholarly Paper No. ID 3603380) [DOI] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138474. 138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.J., Brauer M., Burnett R., Anderson H.R., Frostad J., Estep K., Balakrishnan K., Brunekreef B., Dandona L., Dandona R., Feigin V., Freedman G., Hubbell B., Jobling A., Kan H., Knibbs L., Liu Y., Martin R., Morawska L., Pope C.A., Shin H., Straif K., Shaddick G., Thomas M., van Dingenen R., van Donkelaar A., Vos T., Murray C.J.L., Forouzanfar M.H. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet Lond. Engl. 2017;389 doi: 10.1016/S0140-6736(17)30505-6. 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. Barking Essex. 2020;114465 doi: 10.1016/j.envpol.2020.114465. 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft D.P., Zhang W., Lin S., Thurston S.W., Hopke P.K., van Wijngaarden E., Squizzato S., Masiol M., Utell M.J., Rich D.Q. Associations between source-specific particulate matter and respiratory infections in New York state adults. Environ. Sci. Technol. 2020;54:975–984. doi: 10.1021/acs.est.9b04295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Zhang Z.-F., Froines J., Zhao J., Wang H., Yu S.-Z., Detels R. Air pollution and case fatality of SARS in the People's Republic of China: an ecologic study. Environ. Health Glob. Access Sci. Source. 2003;2:15. doi: 10.1186/1476-069X-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani M., Keshtgar L., Javaheri M.R., Derakhshan Z., Oliveri Conti G., Zuccarello P., Ferrante M. The effects of air pollutants on the mortality rate of lung cancer and leukemia. Mol. Med. Rep. 2017;15:3390–3397. doi: 10.3892/mmr.2017.6387. [DOI] [PubMed] [Google Scholar]
- Dick S., Friend A., Dynes K., AlKandari F., Doust E., Cowie H., Ayres J.G., Turner S.W. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery D.W., Pope C.A., Xu X., Spengler J.D., Ware J.H., Fay M.E., Ferris B.G., Speizer F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Domingo J.L., Marquès M., Rovira J. Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. A review. Environ. Res. 2020;188:109861. doi: 10.1016/j.envres.2020.109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J.L., Rovira J. Effects of air pollutants on the transmission and severity of respiratory viral infections. Environ. Res. 2020;187:109650. doi: 10.1016/j.envres.2020.109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EEA . 2019. Air Quality in Europe 2019 — European Environment Agency [WWW Document]https://www.eea.europa.eu//publications/air-quality-in-europe-2019 URL. 7.28.20. [Google Scholar]
- Fann N., Lamson A.D., Anenberg S.C., Wesson K., Risley D., Hubbell B.J. Estimating the national public health burden associated with exposure to ambient PM 2.5 and ozone. Risk Anal. 2012;32:81–95. doi: 10.1111/j.1539-6924.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- Fattorini D., Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ. Pollut. Barking Essex 1987. 2020;264:114732. doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante M., Conti G.O. Environment and neurodegenerative diseases: an update on miRNA role. MicroRNA Shariqah United Arab Emir. 2017;6:157–165. doi: 10.2174/2211536606666170811151503. [DOI] [PubMed] [Google Scholar]
- Fiore M., Oliveri Conti G., Caltabiano R., Buffone A., Zuccarello P., Cormaci L., Cannizzaro M.A., Ferrante M. Role of emerging environmental risk factors in thyroid cancer: a brief review. Int. J. Environ. Res. Publ. Health. 2019;16 doi: 10.3390/ijerph16071185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera A., Martin C., Vlachos K., Sgubin G. Regional air pollution persistence links to COVID-19 infection zoning. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Hider P.N., Epton M.J., Jennings L.C., Kingham S.P. Including viral infection data supports an association between particulate pollution and respiratory admissions. Aust. N. Z. J. Publ. Health. 2011;35:163–169. doi: 10.1111/j.1753-6405.2010.00620.x. [DOI] [PubMed] [Google Scholar]
- GBD Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Lond. Engl. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari Ghozikali M., Heibati B., Naddafi K., Kloog I., Oliveri Conti G., Polosa R., Ferrante M. Evaluation of chronic obstructive pulmonary disease (COPD) attributed to atmospheric O3, NO2, and SO2 using air Q model (2011-2012 year) Environ. Res. 2016;144:99–105. doi: 10.1016/j.envres.2015.10.030. [DOI] [PubMed] [Google Scholar]
- Grange S.K., Farren N.J., Vaughan A.R., Rose R.A., Carslaw D.C. Strong temperature dependence for light-duty diesel vehicle NOx emissions. Environ. Sci. Technol. 2019;53:6587–6596. doi: 10.1021/acs.est.9b01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C., Carson G., Baillie J.K., Horby P., Nair H. An evidence-based framework for priority clinical research questions for COVID-19. J. Glob. Health. 2020;10 doi: 10.7189/jogh.10-011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Zhou L., Chen J., Chen K., Liu Y., Chen X., Tang F. Acute effects of air pollution on influenza-like illness in Nanjing, China: a population-based study. Chemosphere. 2016;147:180–187. doi: 10.1016/j.chemosphere.2015.12.082. [DOI] [PubMed] [Google Scholar]
- Huh K., Hong J., Jung J. Association of meteorological factors and atmospheric particulate matter with the incidence of pneumonia: an ecological study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020 doi: 10.1016/j.cmi.2020.03.006. [DOI] [PubMed] [Google Scholar]
- Isaifan R.J. The dramatic impact of Coronavirus outbreak on air quality: has it saved as much as it has killed so far? Glob. J. Environ. Sci. Manag. 2020;6:275–288. doi: 10.22034/gjesm.2020.03.01. [DOI] [Google Scholar]
- Jaligama S., Saravia J., You D., Yadav N., Lee G.I., Shrestha B., Cormier S.A. Regulatory T cells and IL10 suppress pulmonary host defense during early-life exposure to radical containing combustion derived ultrafine particulate matter. Respir. Res. 2017;18:15. doi: 10.1186/s12931-016-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Wu X.-J., Guan Y.-J. Effect of ambient air pollutants and meteorological variables on COVID-19 incidence. Infect. Control Hosp. Epidemiol. 2020:1–11. doi: 10.1017/ice.2020.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H.-D., Chen B.-H., Fu C.-W., Yu S.-Z., Mu L.-N. Relationship between ambient air pollution and daily mortality of SARS in Beijing. Biomed. Environ. Sci. BES. 2005;18:1–4. [PubMed] [Google Scholar]
- Kang D., Foley K.M., Mathur R., Roselle S.J., Pickering K.E., Allen D.J. Simulating lightning NO production in CMAQv5.2: performance evaluations. Geosci. Model Dev. (GMD) 2019;12:4409–4424. doi: 10.5194/gmd-12-4409-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalatbari-Soltani S., Cumming R.C., Delpierre C., Kelly-Irving M. Importance of collecting data on socioeconomic determinants from the early stage of the COVID-19 outbreak onwards. J. Epidemiol. Community Health. 2020;74:620–623. doi: 10.1136/jech-2020-214297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013 doi: 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Kowalska M., Skrzypek M., Kowalski M., Cyrys J. Effect of NOx and NO2 concentration increase in ambient air to daily bronchitis and asthma exacerbation, silesian voivodeship in Poland. Int. J. Environ. Res. Publ. Health. 2020;17 doi: 10.3390/ijerph17030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xu X.-L., Dai D.-W., Huang Z.-Y., Ma Z., Guan Y.-J. Air pollution and temperature are associated with increased COVID-19 incidence: a time series study. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;97:278–282. doi: 10.1016/j.ijid.2020.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Xia T., Nel A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Fang L., Pan H., Zhang K., Kan H., Brook J.R., Sun Q. PM2.5 in Beijing - temporal pattern and its association with influenza. Environ. Health Glob. Access Sci. Source. 2014;13:102. doi: 10.1186/1476-069X-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Stieb D.M., Chen Y. Coarse particulate matter and hospitalization for respiratory infections in children younger than 15 years in Toronto: a case-crossover analysis. Pediatrics. 2005;116:e235–240. doi: 10.1542/peds.2004-2012. [DOI] [PubMed] [Google Scholar]
- Liu C., Chen R., Sera F., Vicedo-Cabrera A.M., Guo Y., Tong S., Coelho M.S.Z.S., Saldiva P.H.N., Lavigne E., Matus P., Valdes Ortega N., Osorio Garcia S., Pascal M., Stafoggia M., Scortichini M., Hashizume M., Honda Y., Hurtado-Díaz M., Cruz J., Nunes B., Teixeira J.P., Kim H., Tobias A., Íñiguez C., Forsberg B., Åström C., Ragettli M.S., Guo Y.-L., Chen B.-Y., Bell M.L., Wright C.Y., Scovronick N., Garland R.M., Milojevic A., Kyselý J., Urban A., Orru H., Indermitte E., Jaakkola J.J.K., Ryti N.R.I., Katsouyanni K., Analitis A., Zanobetti A., Schwartz J., Chen J., Wu T., Cohen A., Gasparrini A., Kan H. Ambient particulate air pollution and daily mortality in 652 cities. N. Engl. J. Med. 2019;381:705–715. doi: 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llitjos J.-F., Leclerc M., Chochois C., Monsallier J.-M., Ramakers M., Auvray M., Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. JTH. 2020 doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maawa W.N., Mamat R., Najafi G., De Goey L.P.H. Performance, combustion, and emission characteristics of a CI engine fueled with emulsified diesel-biodiesel blends at different water contents. Fuel. 2020;267:117265. doi: 10.1016/j.fuel.2020.117265. [DOI] [Google Scholar]
- Martelletti L., Martelletti P. Air pollution and the novel covid-19 disease: a putative disease risk factor. Sn compr. Clin. Med. 2020:1–5. doi: 10.1007/s42399-020-00274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., Cao J., Dancer S., Floto A., Franchimon F., Haworth C., Hogeling J., Isaxon C., Jimenez J.L., Kurnitski J., Li Y., Loomans M., Marks G., Marr L.C., Mazzarella L., Melikov A.K., Miller S., Milton D.K., Nazaroff W., Nielsen P.V., Noakes C., Peccia J., Querol X., Sekhar C., Seppänen O., Tanabe S., Tellier R., Tham K.W., Wargocki P., Wierzbicka A., Yao M. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020;726:138605. doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng D.W., Chen P.K. The relationship between airway inflammation and exacerbation in chronic obstructive pulmonary disease. Tuberc. Respir. Dis. 2017;80:325–335. doi: 10.4046/trd.2017.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer P.E., Donaldson G.C., Mackay A.J., Wedzicha J.A. Increased chronic obstructive pulmonary disease exacerbations of likely viral etiology follow elevated ambient nitrogen oxides. Am. J. Respir. Crit. Care Med. 2019;199:581–591. doi: 10.1164/rccm.201712-2506OC. [DOI] [PubMed] [Google Scholar]
- Raji H., Riahi A., Borsi S.H., Masoumi K., Khanjani N., AhmadiAngali K., Goudarzi G., Dastoorpoor M. Acute effects of air pollution on hospital admissions for asthma, COPD, and bronchiectasis in ahvaz, Iran. Int. J. Chronic Obstr. Pulm. Dis. 2020;15:501. doi: 10.2147/COPD.S231317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J., Dominici F., Zanobetti A., Schwartz J., Wang Y., Di Q., Balmes J., Christiani D.C. Impact of long-term exposures to ambient PM2.5 and ozone on ARDS risk for older adults in the United States. Chest. 2019;156:71–79. doi: 10.1016/j.chest.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit M., Ratnesar-Shumate S., Yolitz J., Williams G., Weaver W., Green B., Miller D., Krause M., Beck K., Wood S., Holland B., Bohannon J., Freeburger D., Hooper I., Biryukov J., Altamura L.A., Wahl V., Hevey M., Dabisch P. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Pallavicini A., Ruscio M., Piscitelli P., Colao A., Miani A. Searching for SARS-COV-2 on particulate matter: a possible early indicator of COVID-19 epidemic recurrence. Int. J. Environ. Res. Publ. Health. 2020;17 doi: 10.3390/ijerph17092986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli S.S., Oliveri Conti G., Zanobetti A., Baccarelli A., Fiore M., Ferrante M. Effect of particulate matter-bound metals exposure on prothrombotic biomarkers: a systematic review. Environ. Res. 2019;177:108573. doi: 10.1016/j.envres.2019.108573. [DOI] [PubMed] [Google Scholar]
- Silva D.R., Viana V.P., Müller A.M., Livi F.P., Dalcin P. de T.R. Respiratory viral infections and effects of meteorological parameters and air pollution in adults with respiratory symptoms admitted to the emergency room. Influenza Other Respir. Viruses. 2014;8:42–52. doi: 10.1111/irv.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somayaji R., Neradilek M.B., Szpiro A.A., Lofy K.H., Jackson M.L., Goss C.H., Duchin J.S., Neuzil K.M., Ortiz J.R. Effects of air pollution and other environmental exposures on estimates of severe influenza illness. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2605.190599. Washington, USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G.D. In: International Encyclopedia of Public Health. second ed. Quah S.R., editor. Academic Press; Oxford: 2017. Outdoor air pollution: sources, atmospheric transport, and human health effects; pp. 367–377. [DOI] [Google Scholar]
- To T., Zhu J., Stieb D., Gray N., Fong I., Pinault L., Jerrett M., Robichaud A., Ménard R., van Donkelaar A., Martin R.V., Hystad P., Brook J.R., Dell S. Early life exposure to air pollution and incidence of childhood asthma, allergic rhinitis and eczema. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00913-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Apestegui V., Parras-Garrido E., Tapia V., Paz-Aparicio V.M., Rojas J.P., Sánchez-Ccoyllo O.R., Gonzales G.F. Association between air pollution in Lima and the high incidence of COVID-19: findings from a post hoc analysis. Res. Sq. 2020 doi: 10.21203/rs.3.rs-39404/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignal C., Pichavant M., Alleman L.Y., Djouina M., Dingreville F., Perdrix E., Waxin C., Ouali Alami A., Gower-Rousseau C., Desreumaux P., Body-Malapel M. Effects of urban coarse particles inhalation on oxidative and inflammatory parameters in the mouse lung and colon. Part. Fibre Toxicol. 2017;14 doi: 10.1186/s12989-017-0227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Coronavirus Disease (COVID-19) Situation Reports [WWW Document]https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports URL. 7.28.20. [Google Scholar]
- WHO . 2003. Health Effects of Particulate Matter. Policy Implications for Countries in Eastern Europe, Caucasus and Central Asia (2013) [Google Scholar]
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. Exposure to air pollution and COVID-19 mortality in the United States. medRxiv 2020. 2020 doi: 10.1101/2020.04.05.20054502. 04.05.20054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Pan J., Wang Weidong, Liu Z., Kan H., Qiu Y., Meng X., Wang Weibing. Association of particulate matter pollution and case fatality rate of COVID-19 in 49 Chinese cities. Sci. Total Environ. 2020;741:140396. doi: 10.1016/j.scitotenv.2020.140396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarahmadi M., Hadei M., Nazari S.S.H., Conti G.O., Alipour M.R., Ferrante M., Shahsavani A. Mortality assessment attributed to long-term exposure to fine particles in ambient air of the megacity of Tehran, Iran. Environ. Sci. Pollut. Res. Int. 2018;25:14254–14262. doi: 10.1007/s11356-018-1680-4. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan. Italy. Sci. Total Environ. 2020;738:139825. doi: 10.1016/j.scitotenv.2020.139825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N. Assessing the relationship between ground levels of ozone (O3) and nitrogen dioxide (NO2) with coronavirus (COVID-19) in Milan. Italy. Sci. Total Environ. 2020;740:140005. doi: 10.1016/j.scitotenv.2020.140005. [DOI] [PMC free article] [PubMed] [Google Scholar]