Abstract
Background
Older adults with chronic kidney disease (CKD) are at heightened risk for polypharmacy. We examined potentially inappropriate prescribing in this population and whether introducing pharmacists into the ambulatory kidney care model was associated with improved prescribing practices.
Methods
Retrospective cohort study using linked administrative databases. We included patients with an eGFR ≤30 mL/min/1.73 m2 ≥66 years of age followed in multidisciplinary kidney clinics in Ontario, Canada (n = 25,016 from 28 centres). The primary outcome was the absence of a statin prescription or the receipt of a potentially inappropriate prescription defined by the American Geriatric Society Beers Criteria® and a modified Delphi panel that identified key drugs of concern in CKD. We calculated the crude cumulative incidence and incidence rate for the primary outcome and used change-point regression to determine if a change occurred following pharmacist introduction.
Results
There were 6,007 (24%) and 16,497 patients (66%) not prescribed a statin and with ≥1 potentially inappropriate prescription, respectively. The rate of potentially inappropriate prescribing was 125.6 per 100 person-years and was higher in more recent years. The change-point regression analysis included 2,275 patients from two centres. No immediate change was detected at pharmacist introduction, but potentially inappropriate prescribing was increasing pre-pharmacist introduction, and this rising trend was reversed post-pharmacist introduction. The incidence of potentially inappropriate prescribing still remained high post-pharmacist introduction.
Conclusions
Potentially inappropriate prescribing practices were common. Incorporating pharmacists into the kidney care model may improve prescribing practices. The role of pharmacists in the ambulatory kidney care team warrants further investigation in a randomized controlled trial.
Introduction
Older adults (≥65 years) with advanced chronic kidney disease (CKD) (estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2) are a growing patient population [1]. The risk of adverse drug reactions is increased in this population due to polypharmacy as well as altered drug pharmacokinetics and pharmacodynamics caused by age-related changes, altered nutritional state, and reduced kidney clearance [2–4]. Therefore, dose adjustment and enhanced monitoring or complete discontinuation and avoidance are required for several medications in order to prevent drug accumulation that may lead to toxicity [2, 5–9]. The prevalence of potentially inappropriate prescribing in patients with CKD in the ambulatory setting varies from 13% to 96%, depending on the patient population and how CKD and inappropriate prescribing are defined [10–13]. There are limited data on how best to reduce potentially inappropriate prescribing in patients with advanced CKD. Studies suggest that involving pharmacists in the care of patients with CKD improves prescribing practices [10]. However, most studies have focused on prescribing in the acute care hospital setting or hemodialysis population, or have involved recommendations from a community pharmacist and have not examined the impact of pharmacists as part of the ambulatory kidney care team [10, 14–16]. With this in mind, we conducted a retrospective cohort study to examine potentially inappropriate prescribing in older patients with advanced CKD followed in multidisciplinary kidney clinics and examined if inappropriate prescribing was reduced following the introduction of pharmacists into the kidney clinics. We anticipated a high rate of inappropriate prescribing and that introducing pharmacists into the kidney clinics would be associated with improved prescribing practices.
Materials and methods
Design and setting
We conducted a retrospective cohort study using administrative healthcare databases linked via unique encoded identifiers and analyzed at ICES in Ontario, Canada. The study was conducted according to a pre-specified protocol. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board. The reporting of this study follows the Reporting of Studies Conducted Using Observational Routinely Collected Health Data (RECORD) guidelines for observational studies (Appendix A in S1 File) [17].
We included patients followed in multidisciplinary kidney clinics across the province of Ontario, Canada. Adults (≥18 years of age) with advanced CKD (eGFR <30 mL/min/1.73 m2) are referred for care in the clinics at the discretion of their treating nephrologist. The care provided in multidisciplinary clinics across the province of Ontario is not standardized; therefore, the role and availability of interdisciplinary healthcare professionals may vary across clinics. The care team may include the following healthcare professionals: nephrologist, nurse practitioner, nurse, dietitian, social worker, pharmacist and diabetes nurse educator. The focus of multidisciplinary kidney clinics is to provide education, manage CKD complications, prevent CKD progression, and prepare patients for kidney replacement therapy. Pharmacists may see patients at each clinic visit to perform medication reconciliation and provide education. Any identified medication concerns would be discussed with the treating nephrologist, which may result in changes to the medication regimen by the nephrologist or a written or verbal communication with the physician prescribing any drug(s) of concern. Pharmacists also serve as a resource for nephrologists regarding drug dosing and drug interactions when prescribing new medications.
Data sources
Details regarding databases used for the study are outlined in Appendix B in S1 File. Patients followed in multidisciplinary kidney clinics were identified using the Ontario Renal Reporting System (ORRS). The Ontario Health Insurance Plan (OHIP) database, the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) and ORRS were used to identify patients with a prior history of maintenance dialysis, or a history of a kidney transplant (exclusion criteria). Baseline laboratory data were determined using the Ontario Laboratory Information System (OLIS). Serum creatinine concentrations from OLIS, which were all measured using the isotope dilution mass spectroscopy–traceable enzymatic method, were used to calculate eGFR using the Chronic Kidney Disease Epidemiology (CKD-EPI) equation [18]. Demographics and vital status information were obtained from the Ontario Registered Persons Database. Diagnostic and procedure information from all hospitalizations were determined using the CIHI-DAD and CIHI-Same Day Surgery database. Diagnostic information from emergency room visits was determined using the CIHI-National Ambulatory Care Reporting System (NACRS). Medication data were obtained from the Ontario Drug Benefit Plan database, which contains highly accurate records of all outpatient prescriptions dispensed to patients ≥65 years [19]. Whenever possible, we defined patient characteristics and outcomes using validated codes (Appendix C in S1 File).
Study cohort
We included patients with active follow-up in multidisciplinary kidney clinics from April 1, 2011 to March 31, 2017. The first clinic visit date within this time period was the cohort entry date (index date). We excluded individuals less than 66 years of age, without an eGFR measurement in the year prior to the index date, or with a history of dialysis or kidney transplant. To ensure only patients with advanced CKD were included in the cohort, patients with an eGFR >30 mL/min/1.73 m2 (based on the most recent value prior to the index date) were excluded. We assessed comorbidities in the 5 years prior to the index date and albuminuria and eGFR (taking the most recent value) in the year prior to the index date. Baseline medication use was determined based on prescriptions dispensed in the 120 days prior to the index date.
Potentially inappropriate prescribing
Potentially inappropriate prescribing was defined by the absence of a statin prescription from a patient’s index date to the end of follow-up or receipt of a potentially inappropriate prescription at any point from the index date to the end of the follow-up. Patients were followed until they experienced a censoring event (discharge or withdrawal from the multidisciplinary kidney clinic, death, kidney transplant, or maintenance dialysis initiation) or maximum follow-up occurred (March 31, 2018). The absence of a statin prescription was considered potentially inappropriate given that the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline For Lipid Management in CKD recommends that all adults ≥50 years of age with an eGFR <60 mL/min/1.73 m2 be treated with a statin or statin/ezetimibe combination (Grade 1A evidence) [20]. Potentially inappropriate prescriptions (see Appendix D in S1 File) were defined by the American Geriatric Society Beers Criteria® [5], (medications contraindicated or to be prescribed with caution in older persons), and a modified Delphi panel that identified key medications of concern in CKD [21]. Additional medications of concern in CKD and kidney dosing guidelines were obtained from the Compendium of Pharmaceuticals and Specialties (CPS) and Micromedex [22, 23]. Potentially inappropriate prescriptions were further classified into the following categories: medications of concern in CKD, medications of concern in older patients, medications recommended to be avoided in patients with an eGFR <15 mL/min/1.73 m2 (examined in patients with a baseline eGFR <15 mL/min/1.73 m2, n = 5,689), and medications with clear dosing guidelines dispensed above the recommended dose for an eGFR <30 mL/min/1.73 m2 (Appendix E in S1 File). Non-steroidal anti-inflammatory medications were not examined due to the common non-prescription use of these medications, which is not captured in our databases.
Statistical analysis
We determined the crude cumulative incidence of potentially inappropriate prescribing by dividing the total number of patients with one or more potentially inappropriate prescriptions (fill date on or after the index date) or with absence of a statin prescription throughout the follow-up by the total number of patients. The potentially inappropriate prescribing rate per 100 person-years was calculated by dividing the total person-years of potentially inappropriate prescribing (based on time supplied ≥1 potentially inappropriate prescription or time with no statin prescription) by the total person-years of follow-up. Potentially inappropriate prescribing rates were stratified by age (66-<80 and ≥80), sex, and index year and incidence rate ratios were calculated for each subgroup of interest. Rates per 100 person-years were calculated for each potentially inappropriate prescription category. The crude cumulative incidence for each potentially inappropriate medication of interest was calculated (total number of patients with ≥1 prescription for each medication of interest divided by the total number of patients) to determine the most commonly prescribed medications.
We used change-point regression with monthly intervals to determine if there was a difference in the risk of potentially inappropriate prescribing before and after pharmacist introduction into multidisciplinary kidney clinics. Change-point regression analysis allows for an intervention effect to be studied over time, accounting for prior trends in the outcome, seasonality, and correlation between time points. It also allows the assessment of whether an intervention has an immediate effect in the outcome of interest or if there is an effect over time post-intervention [24]. There were two centres that had introduced a pharmacist into the clinic early in the accrual period, providing sufficient pre- and post-pharmacist data. The first centre had introduced a pharmacist in November 2013; providing a pre-pharmacist time period from April 1, 2011 to October 31, 2013 and post-pharmacist time period from November 1, 2013 to March 31, 2018. The second centre had introduced a pharmacist in May 2014; providing a pre-pharmacist time period from April 1, 2011 to April 30, 2014 and post-pharmacist time period from May 1, 2014 to March 31, 2018. Data were pooled from the two centres and arranged into monthly intervals relative to the pharmacist start date. Absolute standardized differences were used to compare baseline characteristics pre- and post-pharmacist introduction; a value ≥0.1 was considered a significant imbalance between the two time periods. The proportion of patients with potentially inappropriate prescribing during each monthly interval was calculated. Change in the risk of potentially inappropriate prescribing was estimated using linear regression. We tested for the presence of autocorrelation using the Durbin-Watson statistic and if there was evidence of autocorrelation, we included an autocorrelation term at lag 1 in the model. A sensitivity analysis examining the impact of pharmacist introduction on the mean number of potentially inappropriate prescriptions per patient during each monthly interval was performed (absence of statin prescription excluded from this analysis). We conducted all analyses using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
Once all exclusion criteria were applied, 25,016 patients from 28 multidisciplinary kidney clinics were included (S1 Fig). The mean (standard deviation, SD) age was 78 (7.4) years and 56% were male. Patients were on a mean (SD) of 10 (4.8) medications at baseline. The mean (SD) baseline eGFR was 19.9 (6.0) mL/min/1.73 m2; 23% of the cohort had an eGFR <15 mL/min/1.73 m2 (Table 1).
Table 1. Baseline characteristics.
| Characteristic | All patients N = 25,016 |
|---|---|
| Demographics | |
| Age, mean (SD) | 78 (7.4) |
| Sex (male), n (%) | 14,000 (56.0) |
| Rurala, n (%) | 2,913 (11.6) |
| Index Year, n (%) | |
| 2011 | 5,611 (22.4) |
| 2012 | 2,033 (8.1) |
| 2013 | 3,737 (14.9) |
| 2014 | 4,786 (19.1) |
| 2015 | 4,712 (18.8) |
| 2016 | 3,387 (13.5) |
| 2017 | 750 (3.0) |
| Comorbiditiesb | |
| Atrial fibrillation, n (%) | 3,689 (14.7) |
| Chronic obstructive pulmonary disease, n (%) | 2,160 (8.6) |
| Congestive heart failure, n (%) | 5,519 (22.1) |
| Diabetes, n (%) | 16,077 (64.3) |
| Hypertension, n (%) | 23,659 (94.6) |
| Myocardial infarction, n (%) | 2,336 (9.3) |
| Peripheral vascular disease, n (%) | 1,060 (4.2) |
| Kidney Functionc | |
| Serum creatinine (μmol/L), mean (SD) | 254.2 (97.2) |
| eGFR (mL/min/1.73 m2), mean (SD) | 19.9 (6.0) |
| eGFR <15 (mL/min/1.73 m2), n (%) | 5,689 (22.7) |
| Urine albumin creatinine ratio (mg/mmol), mean (SD)d | 96.6 (157.6) |
| Medication Usee | |
| Number of prescribed medications, mean (SD) | 10 (4.8) |
| Colchicine, n (%) | ≤5 (0.0) |
| Lithium, n (%) | 53 (0.2) |
| Spironolactone, n (%) | 1,653 (6.6) |
| Methotrexate, n (%) | 88 (0.4) |
| Fibrates, n (%) | 556 (2.2) |
| Glyburide, n (%) | 662 (2.6) |
| Metformin, n (%) | 2,319 (9.3) |
| Sodium glucose transporter-2 inhibitors, n (%) | 14 (0.1) |
| Ciprofloxacin, n (%) | 1,497 (6.0) |
| Levofloxacin, n (%) | 462 (1.8) |
| Nitrofurantoin, n (%) | 605 (2.4) |
| Baclofen, n (%) | 124 (0.5) |
| Valacyclovir or acyclovir, n (%) | 143 (0.6) |
| Digoxin, n (%) | 0 (0.0) |
| Pregabalin, n (%) | 645 (2.6) |
| Gabapentin, n (%) | 931 (3.7) |
| Morphine, n (%) | 211 (0.8) |
| Codeine, n (%) | 2,533 (10.1) |
| Duloxetine | 17 (0.1) |
| Peripheral alpha-blockers, n (%) | 3,974 (15.9) |
| Alpha agonists, n (%) | 468 (1.9) |
| Tricyclic anti-depressants, n (%) | 791 (3.2) |
| Paroxetine, n (%) | 276 (1.1) |
| Benzodiazepines, n (%) | 2,936 (11.7) |
| Proton pump inhibitors, n (%) | 10,471 (41.9) |
| Metoclopramide, n (%) | 128 (0.5) |
| Skeletal muscle relaxants, n (%) | 0 (0.0) |
| First generation antihistamines, n (%) | 8 (0.0) |
| Anti-arrhythmic drugs, n (%) | 950 (3.8) |
| Anti-psychotics, n (%) | 253 (1.0) |
| Direct oral anticoagulants, n (%) | 533 (2.1) |
| Statins, n (%) | 17,595 (70.3) |
aRural is defined as residing in a location with a population of ≤10,000 individuals.
bComorbidities in the 5 years prior to index date were considered.
cLaboratory measurements in the year prior to index date were considered, using the most recent value. eGFR was determined using the CKD-EPI equation.
dMissing values, n = 7,986 (32%)
ePrescriptions dispensed in the 120 days prior to index date were considered.
*In accordance with ICES privacy policies, cell sizes less than or equal to five cannot be reported.
Potentially inappropriate prescribing
The cumulative incidence of potentially inappropriate prescribing was 22,504 out of 25,016 (90%) patients over a median (interquartile range, IQR) follow-up of 2.0 (1.1 to 3.2) years [absence of a statin prescription: 6,007 (24%); ≥1 potentially inappropriate prescription: 16,497 (66%)]. The overall rate of potentially inappropriate prescribing was 125.6 per 100 person-years, calculated by dividing 72,453 total person years of potentially inappropriate prescribing by 57,707 total person years of follow-up. The potentially inappropriate prescribing rate did not differ by age category (incidence rate ratio, IRR, 0.99, 95% confidence interval, CI, 0.98 to 1.01), but was higher in female patients (IRR 1.13, 95% CI 1.11 to 1.14) and in more recent index years. The most commonly prescribed potentially inappropriate prescription category was medications of concern in older patients (74.2 per 100 person-years), followed by medications of concern in CKD (21.1 per 100 person-years). Medications to be avoided at an eGFR <15 mL/min/1.73 m2 and medications dispensed above the recommended dose for an eGFR <30 mL/min/1.73 m2 were prescribed at low rates (3.4 and 0.1 per 100 person-years, respectively) (Table 2).
Table 2. Potentially inappropriate prescribing rates.
| N | Total person years of potentially inappropriate prescribing | Total person years of follow up | Potentially inappropriate prescribing rate per 100 person-years | IRR (95% CI) | IRR p value | |
|---|---|---|---|---|---|---|
| Total cohort | 25,016 | 72,453 | 57,707 | 125.6 | ||
| Age (years) | ||||||
| 66-<80 | 14,490 | 43,534 | 34,576 | 125.9 | Reference | 0.35 |
| ≥80 | 10,526 | 28,919 | 23,131 | 125.0 | 0.99 (0.98, 1.01) | |
| Sex | ||||||
| Male | 14,000 | 37,315 | 31,431 | 118.7 | Reference | <0.0001 |
| Female | 11,016 | 35,138 | 26,276 | 133.7 | 1.13 (1.11, 1.14) | |
| Index year | ||||||
| 2011 | 5,611 | 25,630 | 20,865 | 122.8 | Reference | |
| 2012 | 2,033 | 6,470 | 5,198 | 124.5 | 1.01 (0.99, 1.04) | 0.34 |
| 2013 | 3,737 | 11,344 | 8,835 | 128.4 | 1.05 (1.02, 1.07) | <0.0001 |
| 2014 | 4,786 | 12,516 | 9,997 | 125.2 | 1.02 (1.00, 1.04) | 0.08 |
| 2015 | 4,712 | 10,205 | 7,945 | 128.5 | 1.05 (1.02, 1.07) | 0.0001 |
| 2016 | 3,387 | 5,425 | 4,206 | 129.0 | 1.05 (1.02, 1.08) | 0.001 |
| 2017 | 750 | 864 | 662 | 130.4 | 1.06 (0.99, 1.14) | 0.08 |
| Potentially inappropriate prescription categories | ||||||
| Medications of concern in CKD | 25,016 | 12,156 | 57,707 | 21.1 | ||
| Medications of concern in older patients | 25,016 | 42,808 | 57,707 | 74.2 | ||
| Medications to be avoided at an eGFR <15 mL/min/1.73 m2a | 5,689 | 336 | 9,791 | 3.4 | ||
| Medications dispensed above the recommended dose for an eGFR <30 mL/min/1.73 m2 | 25,016 | 47 | 57,707 | 0.1 | ||
aExamined in sub-group of patients with a baseline eGFR <15 mL/min/1.73 m2.
Abbreviations: CKD, chronic kidney disease, eGFR: estimated glomerular filtration rate, IRR: incidence rate ratio.
Potentially inappropriate medications with the highest cumulative incidence (determined by ≥1 prescription per patient) were proton pump inhibitors (PPI) (consecutive use >8 weeks) (40%), codeine (30%), peripheral alpha-blockers (23%), ciprofloxacin (any dose) (21%), and benzodiazepines (21%). With respect to indications for peripheral alpha-blocker use, 636 (11%) patients with an alpha-blocker prescription had a prior diagnosis of benign prostatic hyperplasia within five years prior to the first prescription. Pregabalin and gabapentin (commonly prescribed for neuropathic pain) were prescribed to 1,835 (7%) and 2,135 (9%) patients, respectively. Among those prescribed pregabalin, 264 (14%) filled at least one prescription with a dose >150 mg per day. Among those prescribed gabapentin, 504 (23%) filled at least one prescription with a dose >700 mg per day. The cumulative incidence for each medication in the categories of medications recommended to be avoided in patients with an eGFR <15 mL/min/1.73 m2 and medications dispensed above the recommended dose for an eGFR <30 mL/min/1.73 m2 are detailed in S1 and S2 Tables, respectively. Primary care physicians were responsible for most potentially inappropriate prescriptions, but were also responsible for most statin prescriptions (Table 3).
Table 3. Medical specialty of prescribers.
| Physician specialty | Potentially inappropriate prescriptions (%) | Potentially inappropriate prescriptions for medications of concern in CKD (%) | Statin prescriptions (%) |
|---|---|---|---|
| Primary care | 75.6 | 72.9 | 77.3 |
| Nephrology | 9.8 | 8.9 | 9.6 |
| Other | 8.6 | 11.8 | 7.5 |
| Missing | 6.0 | 6.4 | 5.6 |
Abbreviations: CKD: chronic kidney disease.
Impact of pharmacists in multidisciplinary kidney clinics on potentially inappropriate prescribing
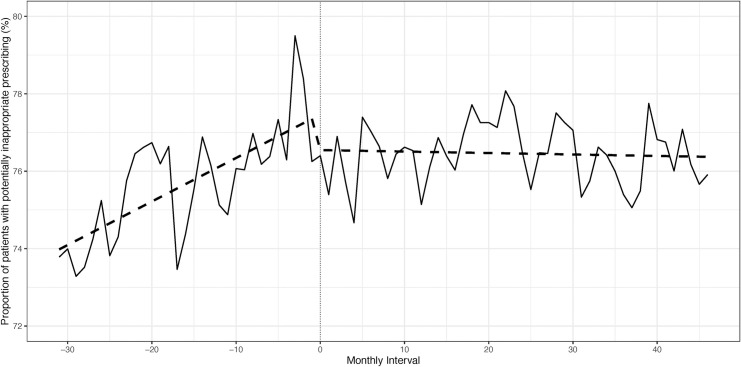
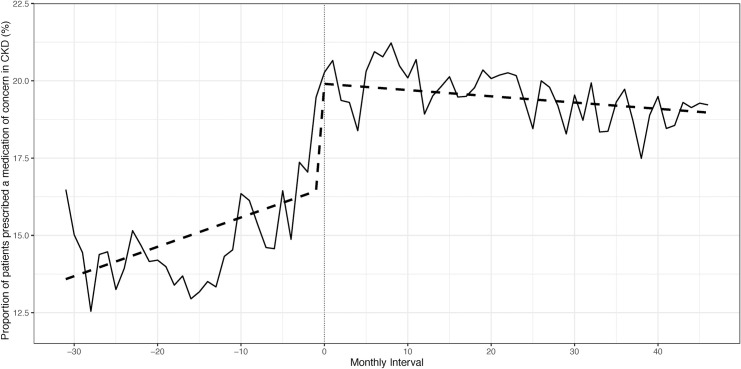
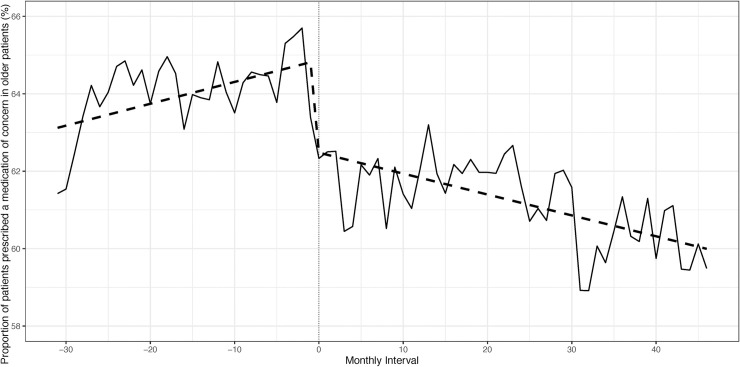
There were 2,275 patients from two centres included in the change point regression analysis. There were minor differences in baseline characteristics pre- and post-pharmacist introduction (S3 Table). No change in polypharmacy was observed from the first to last study interval (mean number of medications = 10). The proportion of patients with potentially inappropriate prescribing was compared pre- and post-pharmacist introduction. No immediate change at pharmacist introduction was detected (p = 0.14), but the slope pre-pharmacist introduction was positive, indicating a rising proportion of individuals with potentially inappropriate prescribing over the months prior to pharmacist introduction (p<0.001). The slope changed to negative post-pharmacist introduction, indicating that the rise in potentially inappropriate prescribing was reversed and a slight decline over the months post-pharmacist introduction was observed (p<0.001). However, the incidence of potentially inappropriate prescribing still remained high (Fig 1, S4 Table). When the category of medications of concern in CKD was examined, a rising trend of potentially inappropriate prescriptions was seen pre-pharmacist introduction (p = 0.003), which continued immediately post-pharmacist introduction (p<0.001), but a significant decline was then noted post-pharmacist introduction (p = 0.003) (Fig 2, S5 Table). For medications of concern in older patients, potentially inappropriate prescriptions were increasing pre-pharmacist introduction (p = 0.024), and an immediate (p<0.001), sustained decline was noted post-pharmacist introduction (p = 0.0003) (Fig 3, S6 Table). A sensitivity analysis that examined the mean number of potentially inappropriate prescriptions per patient also showed that a rising trend of potentially inappropriate prescriptions was reversed post-pharmacist introduction (S2 Fig, S7 Table).
Fig 1. Proportion of patients with potentially inappropriate prescribing pre- and post-pharmacist introduction.
Fig 2. Proportion of patients prescribed a medication of concern in CKD pre- and post-pharmacist introduction.
Fig 3. Proportion of patients prescribed a medication of concern in older patients pre- and post-pharmacist introduction.
Discussion
In this retrospective cohort study of 25,016 older patients with advanced CKD, we found that polypharmacy was common (mean of 10 medications per patient) and that potentially inappropriate prescribing occurred in 90% of patients at some point over the follow-up (almost one quarter due to the absence of a statin prescription). Pharmacist presence in multidisciplinary kidney clinics was associated with a significant reduction in potentially inappropriate prescribing; suggesting that the inclusion of pharmacists as part of the ambulatory kidney care team improves prescribing practices in a population that is at high risk for adverse drug reactions. However, it should be noted that a very modest reduction was observed, and potentially inappropriate prescribing still remained common post pharmacist introduction.
Our findings are similar to prior studies examining prescribing in patients with kidney disease [10, 25]. The reported prevalence of potentially inappropriate prescribing in patients with CKD is quite variable, depending on the patient population studied and how potentially inappropriate prescribing is defined [10]. We found a higher potentially inappropriate prescribing rate in more recent years, which is consistent with prior studies [26]. We also found a higher potentially inappropriate prescribing rate in women, which has been previously reported [27]. Medications of concern in older patients were prescribed at the highest rate, primarily driven by chronic PPI prescriptions. Chronic PPI use was the most common potentially inappropriate prescribing practice (40%), which is not surprising given the dramatic increase in long-term PPI prescribing over the past two decades and the fact that PPIs are the second most commonly prescribed drug in Canada [26, 28]. Prolonged PPI administration is of concern due to the association with an increased risk of Clostridium difficile colitis, pneumonia, fractures and, more recently, CKD [29–33]. The high frequency of chronic PPI use suggests that clinicians are not attempting to de-prescribe PPIs after a course of at least 4 weeks and no further symptoms, as recommended by guidelines [34, 35]. Peripheral alpha-blockers, also of concern in older patients, were commonly prescribed. This may be due to the high prevalence of resistant hypertension in patients with advanced CKD [36], which often requires the addition of less desirable anti-hypertensive medications to achieve blood pressure targets. The low prevalence of benign prostatic hyperplasia found in patients prescribed these agents suggests that they were primarily prescribed for hypertension. While alpha-blocker use may be appropriate in certain older patients, prescribers must be aware of the heightened risk for orthostatic hypotension causing falls [5, 37]. The opioid codeine, which is a high-risk medication in older patients, was commonly prescribed. Codeine requires dose adjustment in CKD and has an unpredictable response depending on the rate of drug metabolism [38, 39]. Of further concern is the frequent prescribing of benzodiazepines and, to a lesser degree, gapabentin and pregabalin, which are all associated with an increased risk of death when co-administered with opioids [40–43].
Absolutely contraindicated medications or doses clearly outside the recommended range for eGFR were fortunately prescribed at relatively low rates. Fluoroquinolones were the most common medication class dispensed above recommended doses and were commonly prescribed. Antibiotics have been previously reported as medications at high risk of inappropriate prescribing in patients with CKD. Interestingly, automated eGFR reporting has not been found to improve antibiotic prescribing practices [44, 45]. Patients with kidney failure (eGFR <15 mL/min/1.73 m2) filled prescriptions for medications contraindicated at very low levels of kidney function, such as metformin, fibrates, baclofen and glyburide. These types of prescriptions are most concerning because they place patients at highest risk for serious adverse drug reactions.
Nearly 25% of the cohort was not prescribed a statin despite CKD guidelines providing a strong recommendation to prescribe these agents to all patients with CKD above the age of 50 [20]. One potential reason for the lack of a statin prescription could be side effects prompting discontinuation. Unfortunately, this information was not available in our databases. However, the reported prevalence of statin-related side effects ranges from 1–10% [46], which is much lower than 25%. One other reason may be therapeutic nihilism on the part of prescribers. Our cohort consisted of older patients with advanced CKD; a patient population typically excluded from cardiovascular therapeutic trials [47, 48].
When prescribing over multiple monthly intervals was compared, the introduction of pharmacists into multidisciplinary kidney clinics was associated with a reduction in potentially inappropriate prescribing, and a sustained effect was noted. A significant, immediate reduction in potentially inappropriate prescribing at the time of pharmacist introduction was not observed in all analyses. We did not necessarily anticipate an immediate impact given that first-time visits with a pharmacist occurred at the time of routine clinic visits and would therefore be expected to occur over several months. Consistent with our findings, a beneficial impact of pharmacists on prescribing practices has been previously demonstrated in other clinical settings and patient populations with kidney disease [10]. Although not examined in this study, reducing potentially inappropriate prescribing is clinically important since this should reduce adverse drug reactions, pill burden, and costs for the patient as well as the healthcare system in jurisdictions with publically funded drug plans [49, 50]. It is however important to note that even with the introduction of pharmacists, we still found that the incidence of potentially inappropriate prescribing remained high, suggesting that a multi-faceted intervention is needed to address this issue. A successful intervention would likely need to incorporate primary care physicians since we found that they were responsible for most potentially inappropriate prescriptions and most statin prescriptions.
The generalizability of our findings is increased by the inclusion of a large, multi-centre cohort with universal drug coverage. However, our study has limitations worth noting. Our databases lacked information on why potentially inappropriate prescribing occurred; individual patient tolerability of potentially inappropriate medications, side effects and any therapeutic benefits were also not available. Our list of potentially inappropriate prescribing practices was based on expert opinion and published guidelines; however, we acknowledge that the evidence to support the avoidance or dose reduction of many included drugs is limited. Also, the best equation to estimate kidney function for the purposes of drug adjustment or avoidance continues to be controversial. The National Kidney Disease Education Program indicates that equations which express results in mL/min/1.73 m2 or mL/min are both appropriate for this purpose. In this study, we estimated the glomerular filtration rate using the CKD-EPI equation, which when <30 mL/min/1.73 m2, would also generally identify a patient with a Cockcroft–Gault creatinine clearance <30 mL/min [51]. Our finding that pharmacist presence in multidisciplinary kidney clinics was associated with improved prescribing practices may not be causal since the intervention was not randomly assigned. Other factors occurring at the same time that pharmacists were introduced may have led to the observed reduction in potentially inappropriate prescribing. However, the finding is strengthened by the fact that the reduction in potentially inappropriate prescribing was noted across two centres that each introduced pharmacists at different time periods (November 2013 and May 2014). Lastly, the role and practice of pharmacists at each clinic were not standardized.
Conclusions
Potentially inappropriate prescribing was common in this cohort of older patients with advanced CKD. Our findings demonstrate the important need to improve outpatient-prescribing practices in this high-risk population and that including pharmacists in the delivery of ambulatory kidney care might improve prescribing. The role and impact of pharmacists as part of the ambulatory kidney care team warrants further investigation in a randomized controlled trial.
Supporting information
(TIFF)
(TIFF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The research was conducted by members of the ICES Kidney, Dialysis and Transplantation team, at the ICES Western facility. Parts of this material are based on data and information compiled and provided by the Ontario Ministry of Health and Long-Term Care (MOHLTC), Canadian Institute for Health Information (CIHI) and Cancer Care Ontario (CCO). The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed in the material are those of the authors, and not necessarily those of CIHI. Parts of this material are based on data and information provided by CCO. The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred. We thank IMS Brogan Inc. for use of their Drug Information Database.
Data Availability
The data set from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at https://www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the programs may rely upon coding templates or macros that are unique to ICES.
Funding Statement
This study was conducted with the support of Cancer Care Ontario through funding provided by the Government of Ontario (awarded to AOM). The sponsor had no role in the study design, conduct, data analysis or manuscript preparation. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). This study was completed at the ICES Western site, where core funding is provided by the Academic Medical Organization of Southwestern Ontario, the Schulich School of Medicine and Dentistry, Western University, and the Lawson Health Research Institute. Amber O. Molnar receives salary support from the KRESCENT Foundation and the McMaster Department of Medicine. Manish M Sood is supported by the Jindal Research Chair for the Prevention of Kidney Disease.
References
- 1.Stevens LA, Viswanathan G, Weiner DE. Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Advances in chronic kidney disease. 2010; 17:293–301. 10.1053/j.ackd.2010.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallieni M, Cancarini G. Drugs in the elderly with chronic kidney disease: beware of potentially inappropriate medications. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2015; 30:342–4. [DOI] [PubMed] [Google Scholar]
- 3.Bailie GR, Eisele G, Liu L, Roys E, Kiser M, Finkelstein F, et al. Patterns of medication use in the RRI-CKD study: focus on medications with cardiovascular effects. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2005; 20:1110–5. [DOI] [PubMed] [Google Scholar]
- 4.Jones SA, Bhandari S. The prevalence of potentially inappropriate medication prescribing in elderly patients with chronic kidney disease. Postgraduate medical journal. 2013; 89:247–50. 10.1136/postgradmedj-2012-130889 [DOI] [PubMed] [Google Scholar]
- 5.American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society. 2015; 63:2227–46. 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 6.Aronoff G. R. BWM, Berns J.S., Brier M.E., Nishaminy K., Mueller B. A., Pasko D. A., et al. Drug Prescribing in Renal Failure, 5th Edition. 2007.
- 7.Matzke GR, Aronoff GR, Atkinson AJ Jr., Bennett WM, Decker BS, Eckardt KU, et al. Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney international. 2011; 80:1122–37. 10.1038/ki.2011.322 [DOI] [PubMed] [Google Scholar]
- 8.Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. American family physician. 2007; 75:1487–96. [PubMed] [Google Scholar]
- 9.Ponticelli C, Sala G, Glassock RJ. Drug management in the elderly adult with chronic kidney disease: a review for the primary care physician. Mayo Clinic proceedings. 2015; 90:633–45. 10.1016/j.mayocp.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 10.Tesfaye WH, Castelino RL, Wimmer BC, Zaidi STR. Inappropriate prescribing in chronic kidney disease: A systematic review of prevalence, associated clinical outcomes and impact of interventions. International journal of clinical practice. 2017; 71. [DOI] [PubMed] [Google Scholar]
- 11.Breton G, Froissart M, Janus N, Launay-Vacher V, Berr C, Tzourio C, et al. Inappropriate drug use and mortality in community-dwelling elderly with impaired kidney function—the Three-City population-based study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2011; 26:2852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang F, O'Hare AM, Miao Y, Steinman MA. Use of Renally Inappropriate Medications in Older Veterans: A National Study. Journal of the American Geriatrics Society. 2015; 63:2290–7. 10.1111/jgs.13790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papaioannou A, Clarke JA, Campbell G, Bedard M. Assessment of adherence to renal dosing guidelines in long-term care facilities. Journal of the American Geriatrics Society. 2000; 48:1470–3. 10.1111/j.1532-5415.2000.tb02639.x [DOI] [PubMed] [Google Scholar]
- 14.Salgado TM, Moles R, Benrimoj SI, Fernandez-Llimos F. Pharmacists' interventions in the management of patients with chronic kidney disease: a systematic review. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2012; 27:276–92. [DOI] [PubMed] [Google Scholar]
- 15.Stemer G, Lemmens-Gruber R. Clinical pharmacy activities in chronic kidney disease and end-stage renal disease patients: a systematic literature review. BMC nephrology. 2011; 12:35 10.1186/1471-2369-12-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Raiisi F, Stewart D, Fernandez-Llimos F, Salgado TM, Mohamed MF, Cunningham S. Clinical pharmacy practice in the care of Chronic Kidney Disease patients: a systematic review. International journal of clinical pharmacy. 2019; 41:630–66. 10.1007/s11096-019-00816-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholls SG, Quach P, von Elm E, Guttmann A, Moher D, Petersen I, et al. The REporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) Statement: Methods for Arriving at Consensus and Developing Reporting Guidelines. PloS one. 2015; 10:e0125620 10.1371/journal.pone.0125620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009; 150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy AR, O'Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. The Canadian journal of clinical pharmacology = Journal canadien de pharmacologie clinique. 2003; 10:67–71. [PubMed] [Google Scholar]
- 20.Wanner C, Tonelli M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney international. 2014; 85:1303–9. 10.1038/ki.2014.31 [DOI] [PubMed] [Google Scholar]
- 21.Taji L, Battistella M, Grill AK, Cunningham J, Hemmelgarn BL, Quinn KM, et al. Medications Used Routinely in Primary Care to be Dose-Adjusted or Avoided in People With Chronic Kidney Disease: Results of a Modified Delphi Study. The Annals of pharmacotherapy. 2020:1060028019897371. [DOI] [PubMed] [Google Scholar]
- 22.Compendium of Pharmaceuticals and Specialties (CPS 2019) 2019. Available from: http://www.pharmacists.ca/products-services/compendium-of-pharmaceuticals-and-specialties/.
- 23.IBM Micromedex [Internet]. 2019 [cited December 12, 2018]. Available from: http://www.micromedexsolutions.com/micromedex2/librarian/ssl/true.
- 24.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. Journal of clinical pharmacy and therapeutics. 2002; 27:299–309. 10.1046/j.1365-2710.2002.00430.x [DOI] [PubMed] [Google Scholar]
- 25.Schmidt IM, Hubner S, Nadal J, Titze S, Schmid M, Barthlein B, et al. Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: the German Chronic Kidney Disease study. Clinical kidney journal. 2019; 12:663–72. 10.1093/ckj/sfz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriarty F, Hardy C, Bennett K, Smith SM, Fahey T. Trends and interaction of polypharmacy and potentially inappropriate prescribing in primary care over 15 years in Ireland: a repeated cross-sectional study. BMJ open. 2015; 5:e008656 10.1136/bmjopen-2015-008656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assiri GA, Shebl NA, Mahmoud MA, Aloudah N, Grant E, Aljadhey H, et al. What is the epidemiology of medication errors, error-related adverse events and risk factors for errors in adults managed in community care contexts? A systematic review of the international literature. BMJ open. 2018; 8:e019101 10.1136/bmjopen-2017-019101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Top Drug Classes [Internet]. Canadian Institute for Health Information. 2017. Available from: http://www.cihi.ca/en/health-spending/2018/prescribed-drug-spending-in-canada.
- 29.Huntsberry AM, Linnebur SA, Fixen DR, Saba LM, Saseen JJ. Effects of Chronic Proton-Pump Inhibitor Use on Kidney Function in Older Adults. The Senior care pharmacist. 2019; 34:325–33. [PubMed] [Google Scholar]
- 30.Jaynes M, Kumar AB. The risks of long-term use of proton pump inhibitors: a critical review. Therapeutic advances in drug safety. 2019; 10:2042098618809927 10.1177/2042098618809927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clinic proceedings. 2018; 93:240–6. 10.1016/j.mayocp.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 32.Wijarnpreecha K, Thongprayoon C, Chesdachai S, Panjawatanana P, Ungprasert P, Cheungpasitporn W. Associations of Proton-Pump Inhibitors and H2 Receptor Antagonists with Chronic Kidney Disease: A Meta-Analysis. Digestive diseases and sciences. 2017; 62:2821–7. 10.1007/s10620-017-4725-5 [DOI] [PubMed] [Google Scholar]
- 33.Thong BKS, Ima-Nirwana S, Chin KY. Proton Pump Inhibitors and Fracture Risk: A Review of Current Evidence and Mechanisms Involved. International journal of environmental research and public health. 2019; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrell B, Pottie K, Thompson W, Boghossian T, Pizzola L, Rashid FJ, et al. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Canadian family physician Medecin de famille canadien. 2017; 63:354–64. [PMC free article] [PubMed] [Google Scholar]
- 35.Peiffer-Smadja N, Bauvois A, Chilles M, Gramont B, Maatoug R, Bismut M, et al. The French Society of Internal Medicine's Top-5 List of Recommendations: a National Web-Based Survey. Journal of general internal medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamrahian SM, Falkner B. Hypertension in Chronic Kidney Disease. Advances in experimental medicine and biology. 2017; 956:307–25. 10.1007/5584_2016_84 [DOI] [PubMed] [Google Scholar]
- 37.Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. The American journal of medicine. 2007; 120:841–7. 10.1016/j.amjmed.2007.02.023 [DOI] [PubMed] [Google Scholar]
- 38.Chau DL, Walker V, Pai L, Cho LM. Opiates and elderly: use and side effects. Clinical interventions in aging. 2008; 3:273–8. 10.2147/cia.s1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davison SN. Clinical Pharmacology Considerations in Pain Management in Patients with Advanced Kidney Failure. C linical journal of the American Society of Nephrology: CJASN. 2019; 14:917–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomes T, Greaves S, van den Brink W, Antoniou T, Mamdani MM, Paterson JM, et al. Pregabalin and the Risk for Opioid-Related Death: A Nested Case-Control Study. Annals of internal medicine. 2018; 169:732–4. 10.7326/M18-1136 [DOI] [PubMed] [Google Scholar]
- 41.Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case-control study. PLoS medicine. 2017; 14:e1002396 10.1371/journal.pmed.1002396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gressler LE, Martin BC, Hudson TJ, Painter JT. Relationship between concomitant benzodiazepine-opioid use and adverse outcomes among US veterans. Pain. 2018; 159:451–9. 10.1097/j.pain.0000000000001111 [DOI] [PubMed] [Google Scholar]
- 43.Hawkins EJ, Goldberg SB, Malte CA, Saxon AJ. New Coprescription of Opioids and Benzodiazepines and Mortality Among Veteran Affairs Patients With Posttraumatic Stress Disorder. The Journal of clinical psychiatry. 2019; 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farag A, Garg AX, Li L, Jain AK. Dosing errors in prescribed antibiotics for older persons with CKD: a retrospective time series analysis. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2014; 63:422–8. [DOI] [PubMed] [Google Scholar]
- 45.Zhu JXG, Nash DM, McArthur E, Farag A, Garg AX, Jain AK. Nephrology comanagement and the quality of antibiotic prescribing in primary care for patients with chronic kidney disease: a retrospective cross-sectional study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2019; 34:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramkumar S, Raghunath A, Raghunath S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiologica Sinica. 2016; 32:631–9. 10.6515/acs20160611a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006; 296:1377–84. 10.1001/jama.296.11.1377 [DOI] [PubMed] [Google Scholar]
- 48.Konstantinidis I, Nadkarni GN, Yacoub R, Saha A, Simoes P, Parikh CR, et al. Representation of Patients With Kidney Disease in Trials of Cardiovascular Interventions: An Updated Systematic Review. JAMA Intern Med. 2015:1–3. [DOI] [PubMed] [Google Scholar]
- 49.Harrison SL, Kouladjian O'Donnell L, Milte R, Dyer SM, Gnanamanickam ES, Bradley C, et al. Costs of potentially inappropriate medication use in residential aged care facilities. BMC geriatrics. 2018; 18:9 10.1186/s12877-018-0704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan SG, Hunt J, Rioux J, Proulx J, Weymann D, Tannenbaum C. Frequency and cost of potentially inappropriate prescribing for older adults: a cross-sectional study. CMAJ open. 2016; 4:E346–51. 10.9778/cmajo.20150131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Institute of Diabetes and Digestive and Kidney Diseases. CKD & Drug Dosing: Information for Providers. 2015. Available from: https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-education-outreach/ckd-drug-dosing-providers