Abstract
Objective
Fever is a very common reason for emergency consultation during pregnancy, and may be associated with maternal, obstetrical and/or fetal adverse outcomes. The aim of this study was to determine the etiologies and to analyze the maternal or fetal complications of fever in pregnancy.
Study design
A retrospective single center study including all patients who consulted for fever above 38 °C during pregnancy in the gynecological emergency ward from August 2016 to July 2017.
Results
A total of 100 pregnant women who consulted for fever were included. The etiologies were common viral infections (37 %), influenza (21 %), pyelonephritis (11 %), viral gastroenteritis (6%), chorioamnionitis (5%), other (5%). The etiology was unknown for 15 %. Fever was confirmed during consultation in 45/100 patients (45 %). Among patients with confirmed fever, 21/45 (47 %) were hospitalized with a median stay of 3 days [IQR 2–4] and 10/45(22 %) developed fetal or maternal complications. Probabilistic antibiotics were delivered for 34/45, 76 % patients. Only 14/45, 31 % had confirmed bacterial infections. Of the 32 patients with confirmed fever who had no etiologic diagnosis at the initial work-up in the emergency room, 19/32, 59 % received presumptive treatment with amoxicillin against Listeria monocytogenes. None had confirmed listeriosis, and all were probably common viral infections. Among all patients, the complications rate was 13 % and 22 % in the subgroup with fever confirmed at presentation.
Conclusions
This study quantifies the main etiologies and complications of fever during pregnancy. A challenge is to reduce excessive antibiotic use by improving rapid diagnosis of bacterial and viral infections. Prospective studies are needed to target patients at risk of complications in an optimal way and to study new management strategies.
Keywords: Fever, Pregnancy, Influenza, Etiology, Complications, Antibiotics
1. Introduction
Fever is one of the most frequent reasons for emergency consultation during pregnancy and may be associated with significant adverse outcomes, these being maternal (sepsis, organ damages) obstetrical (miscarriage, preterm birth, chorioamnionitis) or fetal (malformations, fetal demise). However, only one study including mainly second and third trimester pregnant women, evaluated causes of acute undifferentiated fever and 25 % of women had no identified cause [1]. Moreover, nearly 12 % (IC95 8.6–16.8) of patients with fever during pregnancy required hospitalization in intensive care unit and it has been shown that bacteremia is complicated by fetal loss in 10 % of cases [2,3]. Published studies usually focus on a single etiology, such as influenza, or pyelonephritis, and some studies focus on fever as a symptom during labor [[4], [5], [6]]. To our knowledge, etiologies and complications of fever during pregnancy have never been studied.
There is no recommendation about fever in pregnant women, but usual care for undifferentiated fever is to introduce probabilistic antibiotic against listeria monocytogenes, responsible for overuse of antibiotics. Improving knowledge about etiology and management of fever in pregnant women could modify antibiotics prescription, which can have consequences on public health [7]. The importance of correctly orienting diagnosis and care is underscored by the current COVID-19 epidemic.
The aim of this study was to determine etiology, antibiotics prescription and maternal or fetal complications in women consulting for fever during pregnancy.
2. Methods
2.1. Study population
We conducted a retrospective single center cohort study, including all pregnant women who presented for fever over one year. Inclusion criteria were: any pregnant women consulting between August 1, 2016 and July 31, 2017 to the gynecological emergency department of the Louis Mourier hospital (Assistance Publique des Hopitaux de Paris), a tertiary care center, with a temperature greater than or equal to 38 °C (100.4 °F) at home or at the emergency department. Among all consultations, use of antipyretics before the consultation could not be comprehensively collected. We chose to include all patients who had fever, even if fever was not confirmed in the Emergency room (ER). Because this group was usually not described in available literature, the population was then analyzed in two groups: patients whose fever was confirmed in the ER vs patients whose fever was not confirmed in the ER (fever only at home). Exclusion criteria were incomplete medical records, outcome of pregnancy not known or in progress at the time of the study and opposition by the patient to use of her medical data. We chose not to include pregnancy with unknown outcome in order to because the % of complications was an important outcome of our study. We wished to be the most exhaustive on outcome and avoid diagnostic errors.Clinical features, laboratory findings, prescription and outcomes were collected from electronic medical records (Diamm©, Villers les Nancy, France / Stare©, France / Carestream©, Rochester, United-Sate) with secure software (Redcap©, Vanderbilt University, Nashville, United State). Cold Season was defined as the period most sensitive for common viral infection, that is from October to March. [[8]]
2.2. Variables collected: determination of etiology
The management of fever during pregnancy in our gynecological emergency department consists in performing systematically a first line biological work-up including blood tests with hemogram, plasma C-reactive protein (CRP), and bacteriological samples with urine culture (UC), PCR enterovirus in the blood, vaginal swab and hemocultures for Listeria Monocytogene. Second line examination depends on maternal symptoms (ex: PCR influenza if flu-like syndrome, renal ultrasound if lumbar pain, chest x-ray if chest pain or dyspnea). When fever is not confirmed at presentation, diagnostic testing is decided by the physician. Hospitalization was not systematic in our center,but was largely carried out in case of confirmed fever without certainty of diagnosis, in order to monitor a possible Listeria. Similarly, antibiotic therapy against Listeria monocytogene was largely recommended in the absence of certainty of diagnosis.
2.3. Categorization of etiologies
Etiologies were divided in two different group, according the certainty of diagnosis. Diagnosis criteria were decided by CE and OP. All etiologies were than classified by CE. In the rare cases of difficulty in classifying etiologies, cases were reviewed by JS.
-
•
Certainty of diagnosis
-
-
Intra uterine infection: fever in the context of premature rupture of membranes, associated with at least one supplementary criteria among the following : persistent fetal tachycardia, painful uterine contractions, spontaneous labor or purulent amniotic fluid defined as the French guidelines [9].
-
-
Acute pyelonephritis: lumbar pain and positive urine culture without other most likely diagnosis
-
-
Influenza: nasopharyngeal PCR positive for influenza A/B
-
-
Listeriosis: Positive hemoculture for Listeria monocytogene
-
-
Other: proof of diagnosis explaining the fever, with etiology not classifiable in categories mentioned above
-
•
Probability of diagnosis (only in the absence of a certain diagnosis)
-
-
Common viral infection: Flu-like symptoms ie. chills, headache, myalgia, asthenia, coughing without other most likely diagnosis
-
-
Viral acute gastroenteritis: compatible symptoms (intestinal disorder, nausea/vomiting, abdominal pain) without any other most likely diagnosis
-
-
Unknown: no etiology found, eg. no symptoms or work-up compatible with any of the diagnoses cited above.
One diagnosis was assigned to every consultation, if two diagnoses were possible, the one with the higher probability was selected.
2.4. Statistical analysis and ethical committee
Continuous data were presented as medians and 25th to 75th percentiles. Categorical data were presented as counts and percentages. For the description of outcomes, the 95 % confidence interval was estimated. Fisher exact test or Chi-square were performed for the categorical data as appropriate. Statistical analysis was performed using R software (version 3.5.1)
This study was approved by the Institutional Review Board (IRB status number 00,006,477) number 2018-032.
3. Results
3.1. Diagnoses
Over the study period of one year, 3315 patients were followed for their pregnancy, and 121 consulted for fever at home or at the emergency departments, i.e. 3.6 % (95 % confidence interval [3; 4.3]. For 21 of these patients, medical charts were considered as incomplete and were excluded (Fig. 1 ). We compared characteristics of patients according to the confirmation of fever at presentation (N = 45; 45 %; 95 %CI 35–55) vs fever reported only at home (N = 55 ; 55 % 95 %CI 45–65) (Fig. 1).
Fig. 1.
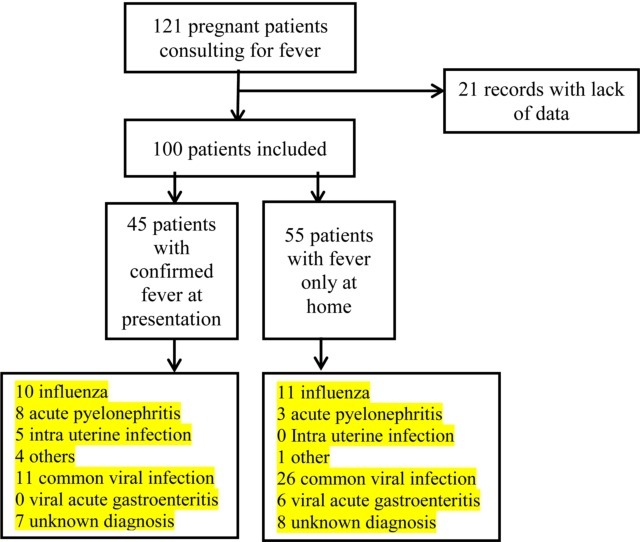
Flow chart. Women presenting for fever in pregnancy in the emergency department between 08/01/2016 and 07/31/2017.
Median age of patients was 30 years [IQR 26–32]. No patient had preexisting diabetes, immunodeficiency or immunosuppressive therapy and they were all seronegative for HIV, hepatitis C and syphilis. Most consultations took place during cold season (73 % from october to march). Characteristics did not differ between the two groups (Table 1 ) except for the period of consultation (winter was more frequent in the group with no confirmed fever, p < 0.005).
Table 1.
Characteristic of the 100 included pregnant women according the fever status at presentation (confirmed vs only at home).
| All patients (n = 100) |
Fever at presentation (n = 45) |
Fever only at home (n = 55) |
P Value* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||||
| Demographics | Age | 0.1 | ||||||||
|
17 | 17 | 11 | 24 | 6 | 11 | ||||
|
66 | 66 | 25 | 56 | 41 | 74 | ||||
|
17 | 17 | 9 | 20 | 8 | 15 | ||||
| Parity | 0.86 | |||||||||
|
50 | 50 | 21 | 47 | 29 | 53 | ||||
|
27 | 27 | 13 | 29 | 14 | 25 | ||||
|
23 | 23 | 11 | 24 | 12 | 22 | ||||
| Pregnancy | Type of gestation | |||||||||
|
95 | 95 | 42 | 93 | 53 | 96 | 0.65 | |||
|
5 | 5 | 3 | 7 | 2 | 4 | ||||
| Immunized for rubella | 93 | 93 | 41 | 91 | 52 | 95 | 0.69 | |||
| Immunized for toxoplasmosis | 43 | 43 | 22 | 49 | 20 | 36 | 0.22 | |||
| Hepatitis B | ||||||||||
|
78 | 78 | 34 | 76 | 44 | 80 | 0.63 | |||
|
20 | 20 | 10 | 22 | 10 | 28 | 0.62 | |||
|
2 | 2 | 1 | 2 | 1 | 2 | 1 | |||
|
0 | 0 | 0 | 0 | 0 | 0 | 1 | |||
| Diabetes | 19 | 19 | 9 | 20 | 10 | 18 | 1 | |||
| Consultation | Gestational age | |||||||||
|
12 | 12 | 4 | 9 | 8 | 14 | 0.53 | |||
|
36 | 36 | 19 | 42 | 18 | 33 | 0.4 | |||
|
19 | 19 | 8 | 18 | 11 | 20 | 0.8 | |||
|
32 | 32 | 14 | 31 | 18 | 33 | 1 | |||
| Month of consultation | ||||||||||
|
73 | 73 | 24 | 53 | 49 | 89 | <0.005 | |||
|
27 | 27 | 21 | 47 | 6 | 11 | <0.005 | |||
| Antibiotic therapy before consultation | 5 | 5 | 2 | 4 | 3 | 5 | 1 | |||
Concerning the etiologies of fever, 42 patients (42 % ; 95 %CI 32–52) had a certain diagnosis: 21 influenza (21 % ; 95 %CI 13–29), 11 acute pyelonephritis (11 % ; 95 %CI 5–17), 5 intra uterine infection (5% ; 95 %CI 1–9), 5 others (5% ; 95 %CI 1–10) (detailed in Table 2 ), and 58 patients (58 % ; 95 %CI 48–67) had only a uncertain diagnosis : 37 common viral infection (37 % ; 95 %CI 28–46), including 2 cases of rhinovirus diagnosed on multiplex PCR, 6 viral acute gastroenteritis (6% ; 95 %CI 1–11) and 15 unknown (15 % ; 95 %CI 8–22). (Table 2). Certain diagnosis was more often reported in women with confirmed fever at presentation than among women who reported fever only at home (60 % vs 27 %, p = 0.001). No case of listeria was diagnosed. Common viral infection are defined as association of fever with one or more flu-like symptoms (chills, headache, myalgia, asthenia, coughing), whereas in case of no symptoms associated to fever, we classified as unknown diagnosis (N = 15).
Table 2.
Etiologies of the 100 included pregnant women according to the fever status at presentation (confirmed vs only at home).
| All patients (n = 100) | Fever at presentation (n = 45) | Fever only at home n = 55) | ||
|---|---|---|---|---|
| Certain diagnosis | Influenza | 21 (21 %) | 10 (22 %) | 11 (20 %) |
| Acute pyelonephritis | 11 (11 %) | 8 (18 %) | 3 (6%) | |
| Intra uterine infection | 5 (5%) | 5 (11 %) | 0 (0%) | |
| Other | 5 (5%) | 4 (9%)1 | 1 (2%)2 | |
| Uncertain diagnosis | Common viral infection | 37 (37 %) | 11 (25 %) | 26 (47 %) |
| Viral acute gastroenteritis | 6 (6%) | 0 (0%) | 6 (10 %) | |
| Unknown diagnosis | 15 (15 %) | 7 (15 %) | 8 (15 %) |
0.002 p-value of the Fisher exact test between the two groups according the confirmed fever status for all etiologies.
One herpes simplex meningitis, one drug (misoprostol) intoxication, one dental abscess, one pregnancy gingivitis.
One acute cystitis (This patient had burning micturition with temperature of 38° never confirmed at emergency, no lumbar pain, negative urine culture, and CRP was negative with normal white count cells.).
Intrauterine infections occurred in patients who all had premature rupture of membranes: three at term and one at 22 W G in a context of known cervical incompetency. The last one occurred in a case of prolonged rupture of membranes: PPROM was diagnosed at 17 W G and intra uterine infection at 31 W G.All patients were in labor except two, at term, for which induce of labor was necessary.
3.2. Hospitalization and complications
In the group with confirmed fever at presentation, 46 % (21 of 45 patients; 95 %CI 32–62) were hospitalized, for a median of 3 days [IQR 2–4] and 22 % (10 of 45 patients; 95 %CI 10–34) presented maternal or fetal complications (Table 3 ). The fetal complications were: 2 (4%) early miscarriage, 2 (4%) late miscarriage, 2 (4%) preterm delivery at 31 W G and 26 W G, 1 (2%) stillbirth. The maternal complications were: 1 (2%) hospitalization for painful uterine contractions without preterm labor, 3 (6%) severe sepsis, 1 additional patient (2%) who required hospitalization in intensive care unit.
Table 3.
Complications and hospitalization of the 100 included pregnant women according to fever status at presentation (confirmed vs only at home).
| All patients (n = 100) | Fever at presentation (n = 45) | Fever only at home (n = 55) | P Value* | ||
|---|---|---|---|---|---|
| Hospitalization | Rate | 25 (25 %) | 21 (46 %) | 4 (7%) | < 0.001 |
| Median duration [IQR] | 3 [2–4] | 3 [2–4] | 2,5 [1.75−3] | ||
| Fetal complications | Prematurity3 | 2 | 2 | 0 | 0.2 |
| Late miscarriage2 | 2 | 2 | 0 | 0.2 | |
| IUFD | 2 | 1 | 1 | 1 | |
| Early miscarriage2 | 4 | 2 | 2 | 1 | |
| Total | 10 (10 %) | 7 (15 %) | 3 (5%) | 0.1 | |
| Maternal complications | Intensive care | 1 | 1 | 0 | 0.45 |
| Severe sepsis | 3 | 3 | 0 | 0.08 | |
| Threatened preterm birth | 1 | 1 | 0 | 0.45 | |
| Total | 5 (5%) | 5 (11 %) | 0 (0%) | 0.01 | |
| Total complications | 13 (13 %)1 | 10 (22 %)1 | 3 (5%)1 | 0.01 | |
2 patients presented maternal and fetal complications simultaneously. One late miscarriage and one IUFD (misoprostol intoxication) had a severe sepsis.
Miscarriage are considered as “early” before 14 W G, and “late” between 14 W G and 23 W G.
P-value of the Fisher exact test between the two groups according the confirmed fever status.
Early miscarriage occurred at 11 and 6 W G, one with a common viral infection and the other with positive influenza PCR. Late miscarriage concerned two patients. The first occurred at 19 W G, following hospitalization for fever, contraction and bleeding related to a subchorionic hematoma. The other was the intra uterine infection at 22 W G cited above.
One preterm delivery was the intra uterine infection at 31 W G with PPROM cited above. The other preterm delivery occurred in a patient hospitalized at 26 W G for bleeding and uterine contraction with a temperature of 38 °C (100.4 °F) without evidence for intra uterine infection. The patient underwent a caesarean section for non-reassuring fetal heart rate and spontaneous labor, two days after hospitalization. Bacteriologic sample after birth was negative.
The stillbirth occurred after voluntary intoxication with 10 intra vaginal misoprostol tablets at 21 W G. The patient presented a severe case of hyperthermia at 40.7 °C (105.3 °F). No other etiology for fever was found.
One patient required intensive care unit hospitalization because of herpetic meningitis. She consulted at 38 W G with fever (38.6 °C/101.5 °F), chills, emesis, headache, and signs of confusion. PCR HSV in the cerebrospinal fluid was positive. A caesarean section was decided at 38 W G following clinical deterioration with hemodynamic instability. The patient delivered a 3200 g male infant, PCR HSV on the newborn was negative and he had a normal evolution. Maternal hospitalization was complicated by pneumonia and she was able to leave the intensive care unit after 14 days.
Both hospitalizations and complications were less frequent in the group with no confirmed fever at presentation (Fisher’s exact test p < 0.001 and p = 0.01 respectively). Among them, 3 of 55 (5% ; 95 %CI 0–11) had complications : miscarriage at 7 and 8 W G and a fetal demise at 15 W G with no other cause. The patient consulted for fever at 38° at home, not confirmed at presentation, associated with headache and chest pain. Induction of labor was performed, and she delivered without complications or fever; the fetus had no apparent malformation. Among patients with no fever confirmed, 4 out of 55 (7%; 95 %CI 0.2–14) were hospitalized, for a median of 2,5 days [IQR 1.75−3].
We also compared the rate of hospitalization and complications according to the fact that diagnosis was certain or not. Hospitalization rate was lower among patients for which the diagnosis was uncertain, 7/58 (12 %; 95 %CI 4–20) than among patients for which diagnosis was certain : 18/42 hospitalizations, 43 %, p < 0.001). Rate of complication did not differ : 8/58 (14 % (95 %CI 5–22) for uncertain diagnosis vs 5/42 complications, 12 %, for patients with certainty of diagnosis p = 1.0).
3.3. Biological results
Among all patients, 43 % (43/100) had blood exams with C-reactive protein (CRP), 29/45 of women with fever in the ER and 14/55 of women with fever only at home. CRP levels was not significantly different in the group with complications than in the group without complications. (29.7 mg/L vs 45.8 mg/L, p = 0.221). (Fig. 2 ). Moreover, 25 % patients (2/8) in the group with complications had negative CRP (<6 mg/L) and 9% patients (3/35) had negative CRP in the group without complications. The negative predictive value of a positive CRP for an adverse outcome was 60 %.
Fig. 2.
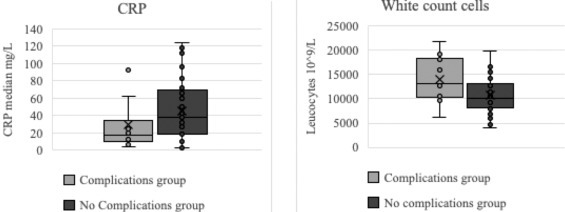
Comparative level of C-reactive protein (CRP) in mg/L and white count cells at the time of consultation for fever in the group with complications of pregnancy and the group without complications.
Among all patients, 58 % (58/100) had blood exams with white count cell (WCC), 37/45 of women with fever in the ER and 21/55 of women with fever only at home. WCC levels in the group with complications was significantly higher than in the group without complications. (14,070 × 109/L vs 10,720 × 109/L [IQR 8100; 13,063] p = 0.03) (Fig. 2).
3.4. Antibiotics prescription and maternal treatment
Among all included women, 45 % (45/100) received antibiotics and 29 % (29/100) received antibiotics targeting listeria (amoxicillin 2 g x 3 per days until result of blood cultures). Intra uterine infection and acute pyelonephritis were treated with appropriate antibiotics. Among 21 patients with influenza (PCR test positive), 15 received oseltamivir (75 mg x 2 per day for five days) and the other six patients did not receive oseltamivir, because symptoms occurred more than 48 h before the consultation. Three additional patients received oseltamivir although the PCR test was negative. Among the 32 patients with confirmed fever at presentation and no acute pyelonephritis or intrauterine infection, probabilistic antibiotic against Listeria monocytogene was prescribed for 19 patients (59 %, 19/32). No case of listeria was diagnosed. Prescription of antibiotics was more frequent among women with confirmed fever at presentation than among women who reported fever only at home (75 % vs 20 %, p < 0.001).
4. Discussion
Although fever in pregnancy is a common clinical issue, this is the first study to estimate the prevalence of different etiologies and to describe management and complications. The diagnosis remained uncertain in the majority of cases after the initial work-up (58 %). When fever was confirmed at presentation, 22 % of developed a maternal or fetal complication. When the diagnosis was uncertain at the end of the examination, the majority (59 %) received antibiotic therapy targeting Listeria monocytogenes. Uncertain diagnosis was not associated with length of hospital stay or a higher rate of complications. The large proportion of complications can probably be explained by the fact that patients consulting at the hospital emergency department are probably the most severe cases. Although advice is given to patients to come to the emergency department in case of temperature > 38 °C, it is likely that some of the least severe patients may have consulted their primary physicians first, and our study may thus be biased towards the most severe patients. Incidence of fever during pregnancy (i.e. 3.6 %) is probable underestimated, because some patients may have consulted elsewhere, or not.
Influenza was one of the most frequent etiologies in our series. The influenza test was generally performed using specific PCR, but in five cases, multiplex PCR was performed, detecting two positive cases for rhinoviruses. Two retrospective studies conducted during the 2009–2010 flu pandemic found in pregnant women with influenza-like symptoms, non-influenza respiratory viruses rates of 23 %, and influenza H1N1 rates between 31 and 42 %, similar to our results [10,11]. The multiplex PCR was not used on a routine basis because it is expensive and time-consuming, although this strategy could possibly avoid use of antibiotics and hospitalizations. Some studies have evaluated clinical and economic impact of multiplex respiratory virus assays, with different results, but this has never been assessed in a pregnant population [12]. Another alternative to the standard real-time influenza PCR for wich results is often delivered after several hours is to use a rapid molecular assay. A recent study highlight benefit on hospitalization and antibiotics consumption, but no economic impact was done [13]. Some other novel biological tests, such as RNA biosignature are promising, but need to be validated [14]. In this study, CRP seems not to be predictor for adverse outcome, unlike white count cell. However, the low number of patients and the retrospective design of our study requires careful interpretation of this results. Although there is a protocol for the management of fever in our emergency department, this one was not always respected. For example, CRP and hemogram was performed in only 58 % of the consultations and enterovirus PCR was never prescribed, although we now recommend performing it systematically in view of the fetal and neonatal risks reported recently (15).
The current COVID-19 pandemic demonstrates performing a rapid diagnosis in pregnant women presenting with fever allow for appropriate care, including hospitalization vs. outpatient management and whether or not to prescribe antibiotics.
Our study demonstrates that fever during pregnancy is associated with a high rate of adverse events. The main strength of this study is the inclusion of all patients consulting for fever, and therefore including those where diagnosis was not certain. There is no answer available for patients who reported fever only at home, even though clinicians are regularly face to this situation. The analysis of this population in our studies is an original approach. These patients are mostly excluded from other studies, although etiology or inflammation associated with fever may have consequences for them as well [16,17]. In this subgroup, we found a significant rate of complications (14 %), one half of the total complications, even if complications are probably most often the result of the etiology or inflammation, and not fever per se. Because of this result, not shown until now, it seems necessary to conduct further prospective studies evaluating the rate of complication in case of fever, even if in cases where diagnosis is unclear. The complication rate was lower when fever was not confirmed, possibly related to less severe infections and in an unknown proportion an overestimation of the temperature when taken at home. Management of pregnant women with fever at home but not confirmed at presentation has not been described previously.
Management of fever during pregnancy, particularly the rate of antibiotic prescription, has never been evaluated, unlike in other populations (emergency department for adult or pediatric population), although administration of an antibiotic is much higher in pregnant women than in these populations. Beyond the allergic risk, cost-efficacy, and the issue of antibiotic resistance, recent data suggests that antibiotic therapy during pregnancy could influence the microbiota of newborn and influence risk of diabetes and obesity [7,[18], [19], [20]]. Most patients (58 %) have an uncertain diagnosis at the end of the consultation, and listeriosis is considered, given the high risk of complications including fetal loss. This may lead to excessive prescription of antibiotics despite the rarity of the diagnosis (about 30 cases per year in France among 800 000 births). In the study conducted by Charlier et al. out of 107 cases of fetal infection with Listeria monocytogenes, only 5% of patients had an isolated fever. [21] We believe that developing more reliable diagnostic and prognostic strategies would reduce unnecessary hospitalizations, and unnecessary antibiotic prescriptions and thus economic costs, while being more vigilant when there is a significant risk of complications.
The limitations of this study are the size of our population and its retrospective observational nature. There are no confirmed criteria to predict a complication when a pregnant woman presents with fever, except according to specific diagnoses such as intrauterine infection. The low size of our population does not allow us to make this type of prediction. As our series was retrospective, some data may be lacking. All women with a temperature of 38 °C or more at presentation were identified. However, fever before presentation may be under-reported. Also, while all drug prescriptions were recorded, actual antibiotic consumption, especially concerning duration of the treatment, may have been variable depending on patients’ compliance. Classifications of etiologies was done by several observers, but in some cases symptoms was unusual and not systematically confirm with microbiological or histologic investigations. As example, only the late miscarriage had placental analysis, which confirm histologic intra uterine infection. Unfortunately, for other patients, placental analysis was not available.
5. Conclusion
Fever during pregnancy was associated with a high rate of complications, 22 % overall. Further, prospective studies on larger cohorts, should be conducted to clarify the medical and public health consequences, including in the longer term, identify prognostic factors and allow for the study of new strategies.
References
- 1.Charlier C., Perrodeau E., Levallois C., Cachina T., Dommergues M., Salomon L.J. Causes of fever in pregnant women with acute undifferentiated fever: a prospective multicentric study. Eur J Clin Microbiol Infect Dis. 2020;(January):18. doi: 10.1007/s10096-019-03809-3. [DOI] [PubMed] [Google Scholar]
- 2.Surgers L., Valin N., Carbonne B., Bingen E., Lalande V., Pacanowski J. Evolving microbiological epidemiology and high fetal mortality in 135 cases of bacteremia during pregnancy and postpartum. Eur J Clin Microbiol Infect Dis. 2013;32(1):107–113. doi: 10.1007/s10096-012-1724-5. [DOI] [PubMed] [Google Scholar]
- 3.Knowles S.J., O’sullivan N.P., Meenan A.M., Hanniffy R., Robson M. Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study. Bjog Int J Obstet Gynaecol. 2015;122(5):663–671. doi: 10.1111/1471-0528.12892. [DOI] [PubMed] [Google Scholar]
- 4.Mertz D., CK-F Lo, Lytvyn L., Ortiz J.R., Loeb M. Pregnancy as a risk factor for severe influenza infection: an individual participant data meta-analysis. BMC Infect Dis. 2019;19(1):683. doi: 10.1186/s12879-019-4318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wing D.A., Fassett M.J., Getahun D. Acute pyelonephritis in pregnancy: an 18-year retrospective analysis. Am J Obstet Gynecol. 2014;210(March (3)):219. doi: 10.1016/j.ajog.2013.10.006. e1-6. [DOI] [PubMed] [Google Scholar]
- 6.Towers C.V., Yates A., Zite N., Smith C., Chernicky L., Howard B. Incidence of fever in labor and risk of neonatal sepsis. Am J Obstet Gynecol. 2017;216(6):596. doi: 10.1016/j.ajog.2017.02.022. e1-596.e5. [DOI] [PubMed] [Google Scholar]
- 7.Laxminarayan R., Matsoso P., Pant S., Brower C., Røttingen J.-A., Klugman K. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(January (10014)):168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 8.Broberg E.K., Waris M., Johansen K., Snacken R., Penttinen P., European Influenza Surveillance Network Seasonality and geographical spread of respiratory syncytial virus epidemics in 15 European countries, 2010 to 2016. Euro Surveill. 2018;23(5) doi: 10.2807/1560-7917.ES.2018.23.5.17-00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beucher G., Charlier C., Cazanave C. Diagnosis and management of intra-uterine infection: CNGOF preterm premature rupture of membranes guidelines. Gynecol Obstet Fertil Senol. 2018;46(12):1054–1067. doi: 10.1016/j.gofs.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Pilorgé L., Chartier M., Méritet J.-F., Cervantes M., Tsatsaris V., Launay O. Rhinoviruses as an underestimated cause of influenza-like illness in pregnancy during the 2009-2010 influenza pandemic. J Med Virol. 2013;85(August (8)):1473–1477. doi: 10.1002/jmv.23614. [DOI] [PubMed] [Google Scholar]
- 11.Paño-Pardo J.R., Martínez-Sánchez N., Martín-Quirós A., Romero-Gómez M.P., Muñoz-Muñiz M., Sánchez-Pastor M. Influenza-like illness in pregnant women during summertime: clinical, epidemiological and microbiological features. Eur J Clin Microbiol Infect Dis. 2011;30(December (12)):1497–1502. doi: 10.1007/s10096-011-1248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallières E., Renaud C. Clinical and economical impact of multiplex respiratory virus assays. Diagn Microbiol Infect Dis. 2013;76(July (3)):255–261. doi: 10.1016/j.diagmicrobio.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anselem O., Baraud C., L’Honneur A.-S., Gobeaux C., Rozenberg F., Goffinet F. Improving care for pregnant women with suspected influenza: a retrospective study before and after introduction of a rapid molecular assay. PLoS One. 2019;14(June (6)) doi: 10.1371/journal.pone.0217651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahajan P., Kuppermann N., Mejias A., Suarez N., Chaussabel D., Casper T.C. Association of RNA biosignatures with bacterial infections in febrile infants aged 60 days or younger. JAMA. 2016;316(August (8)):846–857. doi: 10.1001/jama.2016.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Méreaux J., Picone O., Vauloup-Fellous C., Khediri Z., Benachi A., Mandelbrot L. [Enterovirus infection during pregnancy: underestimated cause of fetal and neonatal complications?] Gynecol Obstet Fertil Senol. 2017;45(April (4)):231–237. doi: 10.1016/j.gofs.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Kalagiri R.R., Carder T., Choudhury S., Vora N., Ballard A.R., Govande V. Inflammation in complicated pregnancy and its outcome. Am J Perinatol. 2016;33(14):1337–1356. doi: 10.1055/s-0036-1582397. [DOI] [PubMed] [Google Scholar]
- 17.Ashwal E., Salman L., Tzur Y., Aviram A., Ben-Mayor Bashi T., Yogev Y. Intrapartum fever and the risk for perinatal complications - the effect of fever duration and positive cultures. J Matern Fetal Neonatal Med. 2018;31(June (11)):1418–1425. doi: 10.1080/14767058.2017.1317740. [DOI] [PubMed] [Google Scholar]
- 18.Stoll B.J., Hansen N., Fanaroff A.A., Wright L.L., Carlo W.A., Ehrenkranz R.A. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347(July (4)):240–247. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 19.Jauréguy F., Carton M., Panel P., Foucaud P., Butel M.-J., Doucet-Populaire F. Effects of intrapartum penicillin prophylaxis on intestinal bacterial colonization in infants. J Clin Microbiol. 2004;42(November (11)):5184–5188. doi: 10.1128/JCM.42.11.5184-5188.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuperman A.A., Koren O. Antibiotic use during pregnancy: how bad is it? BMC Med. 2016;14(1):91. doi: 10.1186/s12916-016-0636-0. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlier C., Perrodeau É, Leclercq A., Cazenave B., Pilmis B., Henry B. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis. 2017;17(5):510–519. doi: 10.1016/S1473-3099(16)30521-7. [DOI] [PubMed] [Google Scholar]


