Abstract
Background
Covid-19 spread through blood transfusion has not yet been reported. Despite the prevailing pandemic, there are no recommendations available as yet for testing SARS-CoV-2 antibodies as part of blood screening.
Objective
To determine the seroprevalence of SAR-CoV-2 antibodies, its clinical significance and to identify if total antibodies(IgA, IgM, IgG) should be tested or just the specific IgG antibodies only.
Method
Consecutive blood donors donated were screened for standard serological panel of HbsAg, Anti-HCV, Anti-HIV and Syphilis using Cobas-411 analyser and Malaria. All seronegative donors were then screened for COVID serology using the same instrument. These results were compared with the blood donors’ seroprevalence checked in a cohort in the first week of June 2020. Pre-COVID-19 period (October 2019) blood donors’ archived samples were also compared. Donors who were positive on ECLIA were then tested for specific antibodies (IgM or IgG) by ELISA.
Results
A total of 380 healthy blood donors were included. All were males with the mean age being 30.6 ± 6.3 years. Ten pre-pandemic samples did not show COVID-19 antibodies, whereas out of 70 samples in the 3rd week of June, only 15 (21.4 %) were positive. However, in July out of the 300 blood donors, 113 (37.7 %) were found to be reactive. To reconfirm our findings, these 113 donors were then tested on ELISA for presence of IgG specifically. Out of these 128 samples, 81 were IgG positive, 23 were borderline positive and 24 were negative.
Conclusion
Almost 40 % of blood donors are now seroconverted for COVID-19. This is a reflection of widespread seroprevalence in the adult male population.
Keywords: Anti-SARS-Cov-2 antibodies, Blood donors, Transfusion transmitted infections, Seroprevalence
1. Introduction
Corona virus first emerged in Wuhan, China in December 2019and now due to its rapid transmission throughout the world, it is regarded a pandemic [1]. SARS-CoV-2, is a Beta Corona virus, which is an enveloped, positive sense-single stranded RNA virus containing four major structural proteins: Spike(S), membrane (M), envelope (E) and the nucleocaspid (N) [2]. Virus invades the host cells by interaction of its Spike protein (S) with specific receptor on host’s cell membrane (ACE-2 receptor) and then gains entry into cell via the process of endocytosis and uses its own RNA and host machinery for replication [3]. Coronavirus has a range of presentation varying from no or mild symptoms like fever, cough cold, body aches, abdominal pain, diarrhea to severe acute respiratory symptoms including Acute Respiratory Distress Syndrome that could lead to death [[4], [5], [6]].
More than 20 million COVID-19 cases are recorded throughout the world causing around 0.7 million deaths and around 14 million recoveries [7]. In Pakistan, the first 2 cases of COVID-19 were reported in February 2020 [8] in individuals who travelled from Iran, with all the appropriate measures immediately taken to prevent its spread. Pakistan has reported around 0.28 million cases till date, with more than 6000 deaths [9]. This data revealed better control of Covid-19 in our population and decreased mortality as compared to some other countries; however, these are the cases that had been symptomatic or tested because of contact tracing and were C-OVID-19 PCR positive. The testing rate of Pakistan is much lower compare to the rest of the world and South East Asia due to many reasons. One is the fear of community to the COVID-19 infection, the other and very important being the limited socioeconomic resources.
As COVID-19 has become a global threat, it was really important to force the health care systems around the world to take necessary steps in early recognition and prevention of spread of the virus. Nucleic acid testing for SARS-CoV-2 by RT-PCR (real time polymerase chain reaction) is the gold standard and helps in early recognition of confirmed COVID cases [10]. However RT-PCR sensitivity can be influenced by many factors like biological sampling, inadequate sample collection, time between sample collection and onset of symptoms and fluctuation in viral load, giving false negative results [11]. Moreover, because of a major proportion of patients being asymptomatic, an easy, sensitive and inexpensive investigation is required to know the actual frequency and seroprevalence of the virus. This could be achieved by performing specific antibody screening by validated serological assays in order to promptly identify the individuals who have been infected with COVID 19in order to facilitate the control of transmission of the disease and ensure timely public health management [12].
Evaluating the prevalence of COVID-19 infection among healthy blood donors is important. WHO has currently provided no recommendations about screening the donors for SARS-CoV-2 by RT-PCR or immunoassays, however, it recommends temporary deferral for 28 days if any symptoms (cough, fever, flu) are present, or if they are exposed to a confirmed COVID-19 patient or have travelled to an epidemic area. WHO also recommends that the potential donors also have to inform the blood bank if they develop symptoms within 28 days of donation [13]. However, COVID-19 virus does not transmit through blood donations and is not a blood borne disease but identification of seroprevalence among the blood donors can give an estimate of circulation of the virus among healthy individuals, providing actual disease burden and real case fatality rate in a population.
Information regarding antibody response against SARS-CoV-2 in asymptomatic individuals is lacking in our part of the subcontinent. In our study, we conducted specific serological testing (total antibodies) to identify prevalence of SARS-2-CoV antibodies among the healthy blood donors who visited Blood Bank at our Institute. Their results were compared with specific serologic results of blood donors that came before the onset of pandemic (October 2019). With this aim, we elucidate the seroprevalence of blood donors and association of any blood groups with the seroconversion along with association with the certain age group.
2. Materials and Methods
2.1. Study design and participants
A cohort of voluntary/exchange blood donors who came at the National institute of blood diseases and bone marrow transplantation, Karachi, during the pandemic in May, June and July were enrolled in the study. Retrospectively, healthy blood donors who visited our blood bank in October 2019 were also tested by recovering their previous samples. Blood donors meeting the AABB 18th edition donor acceptance and deferral criteria were included or excluded accordingly. Moreover, history was thoroughly taken for presence of fever and any respiratory symptoms for at least 28 days; donors, who had a history of COVID-19 infection, were excluded from the study. Any donors having close contact with other COVID-19 patients were also excluded. Furthermore, donors having positive screening for hepatitis B, hepatitis C, HIV, syphilis or malaria were also excluded. Demographic data of donors including age and gender was noted.
2.2. Methodology
Electro-Chemiluminescence immunoassay (ECLIA): Total antibody against SARS-CoV-2(including IgG, IgM and IgA) detected by using double-antigen sandwich assay on Coba e-411Immunoassay analyzer (Roche diagnostics International Ltd at Rotkreuz Switzerland).The assay used a recombinant protein representing the nucleocapsid (N) antigen. Result reported as Reactive = if Cut of Index (COI)>1.0 and Non-Reactive = COI<1.0.
Enzyme linked immunosorbent assay (ELISA): Specific antibodies (IgG,IgM) against nucleocapsid (NP) protein of corona virus were detected quantitatively. Kits of AESKULISA® SARS-CoV-2 made by AESKU, Diagnostics GmbH & Co. KG, Wendelsheim Germany were used. The result Interpreted as negative if value is < 8 U/mL, borderline if value is 8–12 U/mL and positive if value is ≥ 12 U/mL.
Blood groups: As per protocol, blood groups of all the donors were performed by tube method using commercially prepared anti sera (BIO RAD).
3. Statistical analysis
For categorical variables, frequency with percentage was calculated whereas mean and standard deviation was calculated for quantitative variables. Anti-SARS-CoV-2(Total) is qualitative assay; mean was calculated by using numerical cut of index (COI) value. t-Test was used to compare the mean and p value of less than 0.05 was taken as statistically significant. All analysis was done on statistical package for social science SPSS (Version 23).
4. Results
A total of 380 healthy blood donors were included in the study. All were males and their mean age was 30.6 ± 6.3years. Ten samples from October 2019 were checked for anti-SARS-CoV antibodies by ECLIA, and none of them was found to be positive. In 3rd week of June, 70 donors were tested for presence or absence of anti SARS-CoV antibodies and 15 were tested positive (21.4 %). In July 2020, we tested 300 healthy blood donors, 113 donors (37.7 %) were found to be reactive for anti-SARS-CoV-2antibodies.
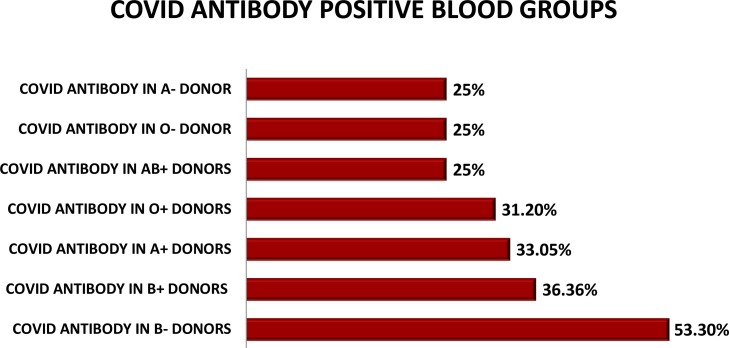
To reconfirm our findings, these 128 donors were then tested on ELISA for presence of IgG specifically. Out of these 128 samples, 81 were IgG positive, 24 were negative and 23 were found to be borderline positive. To further assess our findings, 24 negative IgG samples were tested for IgM ELISA and out of these 24, 22 were found to be negative for IgM whereas 2 were borderline positive as shown in Fig. 1 .
Fig. 1.
DONORS BLOOD GROUP HAVING COVID ANTIBODIES. (n = 128).
Analyzing the blood groups of these healthy donors, we found out that 109 blood donors were O Rh D positive, 103 were B Rh D positive whereas 118 were A Rh D positive while the remaining were AB RhD positive and A,B,O and AB Rh D negative. The 31.1 % O RhD positive donors were found to have anti-SARS-CoV-2 antibodies whereas 36.4 % of B positive donors had COVID antibodies.33.05 % of A Rh D positive donors were tested positive for antibodies. No significant association was noted with either of blood groups in regard to SARS CoV antibodies (Table 1 ).
Table 1.
Laboratory outcomes of Covid positive blood donors.
| Techniques | Positive | Negative | Borderline |
|---|---|---|---|
| ECLIA (n = 380) | 128(33.6 %) | 252(66.3 %) | 0 |
| ELISA-IgG(n = 128) | 81(63.2 %) | 24(18.7 %) | 23(17.9 %) |
| ELISA-IgM(n = 24) | 0 | 22(91.6 %) | 2(8.3 %) |
5. Discussion
In this study, 380 healthy blood donors were investigated for SARS COV-2 antibodies by ECLIA (total antibodies) at three different time points. Though samples of donors of October 2019 tested were less, it helped us to assess the specificity of testing kit used (no false positive results were seen). The samples tested in mid of June showed 21.4 % positivity in random blood donors, supporting the evidence of increase in infection spread along with substantiating the idea of asymptomatic COVID-19 carriers. The results of July showed drastic increase (37.8 %) within a month’s time that equates with the increasing number of COVID-19 symptomatic and PCR confirmed cases at that time. The use of specific ELISA kits for IgM and IgG further complemented that these donors were infected in the past as majority (63.2 %) were positive for IgG. Of all the negative IgG ELISA samples, IgM was performed and none of the samples was found to be positive (2 were reported to be borderline positive).
These findings elaborated upon some important concerns. The first being the actual number of COVID cases in Pakistan because only symptomatic individuals were tested for COVID by PCR. Secondly, the increasing number of SARS COV-2 antibodies in healthy population is raising herd immunity. Lastly, the samples that were positive for SARS COV-2 antibodies by ECLIA but were negative for ELISA IgG and IgM, either that were false positive or some other category of antibody was present in those donors. We rechecked the samples of ECLIA and the result was found to be same. Considering the high specificity of ECLIA, we preclude the idea of false positivity. Hypothetically, we can assume those antibodies to be IgA but we could not confirm due to the lack of testing availability.
A study in China was published regarding antibodies in healthy blood donors. They demonstrated overall COVID-19 antibody prevalence as 2.29 %, 0.029 % and 0.0074 % in Wuhan, Shenzhen and Shijiazhuang respectively from January 2020 to April 2020 [1]. These results contradict our findings probably because of the strict social restrictions followed in China as compared to our country. In another study in China, association of gender was also demonstrated with a Gender ratio was 0.99(male/female) [14]. As all of our blood donors were males, we could not make any association with the gender. Again, since the majority of our blood donors were young adults, any association with age could not be established. However, compared to rest of the world, Pakistan has a higher ratio of young adults who were COVID-19 positive; although the mortality ratio was lower among them. It could be due to the fact that our country has a much lower average age as compared to the rest of the world.
Association of blood group with COVID-19 infection has also been described in recent studies. Individuals with blood group A have been found to be more at risk as compared to those with blood group O [15,16]. The exact reason for this is unknown, but partly may be due to the protective mechanism of circulating anti-A antibodies which inhibit the interaction of virus to its specific host ACE2 receptor [17]. However, in our study we did not observe any significant difference between different blood groups, which might be because none of the donors were symptomatic during disease period.
Different studies throughout the world were conducted in the general population for checking Covid-19 antibodies status; one such study in Netherlands was reported in April 2020 which showed 2.7 % of population being reactive for Covid antibodies [18], however they did not exclude previously symptomatic cases. Another study in Northern France reported 25.8 % of population positive for COVID-19 antibody [19] but they also did not exclude previously symptomatic cases. In April 2020, Italy reported around 9.4 % of healthy blood donors to be seropositive for COVID-19 [20]. Our study showed the largest number of seroconversion partly because of the time period selected for testing and because of implementation of social restrictions stringently in other parts of the world.
A study has demonstrated that if around 60 % of population [21] develops antibodies, the prevention of disease can be strengthened. However, whether these antibody responses are long lasting or not needs to be studied and investigated. As RNA viruses have a tendency to mutate, it is still not clear that if the virus mutates, will these antibodies be protective against the disease or not.
Antibody testing of general population can help in detecting the actual number of asymptomatic carriers; however, it cannot be used for the diagnosis and PCR remains the choice of investigation for diagnosis. Talking about limitations, this is a single centre study but the first local study of its type to our knowledge to elucidate the seroprevalence of blood donors, its association with any blood groups and age group. We need more local studies to authenticate these findings. Moreover, there were limited pre-covid samples available to study from.
6. Conclusion
To conclude, seroprevalence of SARS-COV-2 antibodies has increased in Pakistan over a period of time and could help in recognizing the actual number of COVID-19 cases.
Ethical Review Board
The study was approved by the Ethical Review Committee of National Institute of blood disease and bone marrow transplantation.
Author’s contribution
AY and SW wrote the manuscript. SWK and MI did data collection and analysis. MB, TSS conceived main idea andcritically evaluated the paper.
Declaration of Competing Interest
None.
Acknowledgement
We thank all the staffs and technologists of the blood bank, laboratory and donor centre for providing endless support during the pandemic.
References
- 1.Chang L., Hou W., Zhao L., Zhang Y., Wang Y., Wu L. The prevalence of antibodies to SARS-CoV-2 among blood donors in China. medRxiv. 2020;(January (1)) doi: 10.1038/s41467-021-21503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik Y.A. Properties of coronavirus and SARS-CoV-2. Malays J Pathol. 2020;42(1):3–11. Apr 1. [PubMed] [Google Scholar]
- 3.Gaze David C. 2020. ACE2: the molecule that helps coronavirus invade your cells. May. [Google Scholar]
- 4.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization, COVID 19 Pandemic Emergency Information.
- 8.Noreen N., Dil S., Niazi S., Naveed I., Khan N., Khan F. COVID 19 pandemic & Pakistan; limitations and gaps. Global Biosecurity. 2020;1(4) May 21. [Google Scholar]
- 9.Covid.gov.pk. Government of Pakistan, COVID-19 updates.
- 10.Wang P., Anderson N., Pan Y., Poon L., Charlton C., Zelyas N. The SARS-CoV-2 outbreak: diagnosis, infection prevention, and public perception. Clin Chem. 2020;66(5):644–651. doi: 10.1093/clinchem/hvaa080. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theel E.S., Slev P., Wheeler S., Couturier M.R., Wong S.J., Kadkhoda K. The role of antibody testing for SARS-CoV-2: is there one? J Clin Microbiol. 2020;(April (29)) doi: 10.1128/JCM.00797-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Maintaining a safe and adequate blood supply during the pandemic outbreak of coronavirus disease (COVID-19) Interim guidance. 2020;(March (20)) [Google Scholar]
- 14.Novel C.P. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghualiuxingbingxuezazhi= Zhonghualiuxingbingxuezazhi. 2020;41(2):145. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. Feb 17. [DOI] [PubMed] [Google Scholar]
- 15.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J., Yang Y., Huang H.P., Li D., Gu D.F., Lu X.F. Relationship between the ABO blood group and the COVID-19 susceptibility. medRxiv. 2020;(January (1)) [Google Scholar]
- 17.Li J., Wang X., Chen J., Cai Y., Deng A., Yang M. Association between ABO blood groups and risk of SARS‐CoV‐2 pneumonia. Br J Haematol. 2020;(May (7)) doi: 10.1111/bjh.16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slot Ed, Boris M. Hogema, Reusken C.B.E.M. Research Square; 2020. Herd immunity is not a realistic exit strategy during a COVID-19 outbreak. published online April 29. [Google Scholar]
- 19.Fontanet A., Tondeur L., Madec Y. Cluster of COVID-19 in northern France: a retrospective closed cohort study. MedRxiv. 2020 published online April 23. [Google Scholar]
- 20.Valenti L., Bergna A., Pelusi S. SARS-CoV-2 seroprevalence trends in healthy blood donors during the COVID-19 Milan outbreak. MedRxiv. 2020 doi: 10.2450/2021.0324-20. published online May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altmann D.M., Douek D.C., Boyton R.J. What policy makers need to know about COVID-19 protective immunity. Lancet. 2020;395(10236):1527–1529. doi: 10.1016/S0140-6736(20)30985-5. May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]