Abstract
COVID-19 is a new pandemic, caused by Severe Acute Respiratory Syndrome-CoronaVirus-2 (SARS-Cov2) infection and characterized by a broad spectrum of clinical manifestations.
Inflammation and the innate immune system have been recently recognized as pivotal players in the most severe forms, characterized by significantly elevated levels of pro-inflammatory cytokines. In this setting, several studies have also reported the presence of abnormalities in coagulation parameters and platelets count, possibly identifying a subgroup of patients with poor prognosis. Some reports of full-blown thromboembolic events are emerging.
Among the possible mechanisms underlying coagulation dysfunction, the so-called "cytokine storm" seems to play a pivotal role. Other candidate factors include virus-specific mechanisms, related to the virus interaction with renin angiotensin system (RAS) and the fibrinolytic pathway, but also comorbidities affecting these patients.
Coagulation dysfunction is therefore a candidate risk factor for adverse outcomes in COVID-19 and should be carefully addressed in clinical practice.
Keywords: COVID-19, SARS-CoV2, Coagulation, Thrombosis, Inflammation
1. Introduction
Severe Acute Respiratory Syndrome-CoronaVirus-2 (SARS-CoV2) infection disease (COVID-19) is a novel pandemic, initially identified in a cluster of pneumonia in the Wuhan province in China.
According to the report of the World Health Organization (WHO), published on 6th July 2020, 11,327,790 COVID-19 cases were confirmed worldwide, with 532,340 deaths. Currently, Europe is the second most involved region, with Italy representing the fourth affected country with 241,611 cases, which are clustered in the Northern region Lombardy (94,580 cases), according to the Italian Ministry of Health daily report. During the pandemic peak, the mean percentage of patients in an Intensive Care Unit (ICU) reported in Italy between March 1st and March 11st, 2020, has been reported between 9% and 11% of patients actively infected [1].
A wide range of clinical outcomes has been observed in patients with COVID-19, from mild and moderate disease to severe and rapidly progressive pneumonia evolving in acute respiratory distress syndrome (ARDS) and multi-organ failure [2]. Several evidences suggest that patients with the worst prognosis might have a condition characterized by a cytokine storm, hyperinflammation and possibly a secondary viral-induced hemophagocytic lymphohistiocytosis [3].The correlation between the immune system, especially the innate immune system, the inflammation, and the coagulations system is well-known and has been addressed in different contexts [4].
Here we reviewed the main findings regarding coagulation dysfunction during COVID-19.
2. Laboratory abnormalities in coagulation parameters and platelet levels in COVID-19 infection
In patients with COVID-19 abnormalities of mechanisms of primary haemostasis, coagulation and fibrinolytic parameters have been reported and related with severe illness, ARDS development and death [[5], [6], [7], [8]].
In one of the first studies reporting the complete coagulation parameters of 183 consecutive patients with COVID-19 pneumonia [6], the non-survivors as compared to survivors, revealed significantly higher levels of fibrin-related markers (D-dimer and fibrinogen degradation product (FDP)) and longer prothrombin time (PT) at admission, while lower fibrinogen levels and antithrombin (AT) activity at the end of the hospitalization. Moreover, 71.4% of the non-survivors as compared to 0.6% of the survivors matched the criteria for disseminated intravascular coagulation (DIC) in the later stages of pneumonia, according to the International Society on Thrombosis and Haemostasis (ISTH). Increasing clinical evidences have underlined the importance of coagulation markers monitoring in all COVID-19 cases, leading to ISTH release of guidelines for the management of coagulopathy in COVID-19 [9].
Thrombocytopenia (platelet count<150 × 109/L) is quite infrequent in COVID-19 [10], with a lower frequency than that reported in SARS-infection (45% in one of the largest cohorts [11]) and seems to represent a marker of disease severity. In fact, a meta-analysis of 9 studies with 1779 COVID-19 patients [12], including 22.4% with severe disease, revealed that the platelet count was significantly lower in those with more severe infection, with even lower count in non-survivors as compared to survivors. In particular, the Weighted Mean Difference (WMD) of platelets count was -31 × 109/L (95% CI -35 to −29 × 109/L) for more severe COVID-19 cases. The non-survivors, as compared to survivors, had the lowest platelets counts with WMD -48 × 109/L (95% CI -57 to −39 × 109/L). The prevalence of thrombocytopenia was reported in 4 of the 9 studies (1427 patients) and widely ranged from 7% to 41.7%.
At the same time, thrombocytosis was also recorded in the moderately severe cases with prolonged hospitalization [13].
It was reported that in 94 patients with COVID-19 the levels of D-dimer and FDP were significantly higher than in 40 healthy controls during the same period and were also higher in severe forms as compared to milder forms [7]. Elevated levels of D-dimer at the time of admission were also identified as risk factor for mortality in a retrospective cohort of 191 Chinese patients [14]. Higher levels of D-dimer also emerged as one of the risk factors for ARDS and death in a series of 201 patients with COVID-19 pneumonia in China [8]. At the time of admission, 23.3% of them had D-dimer above the reference, while activated partial thromboplastin time (APTT) and PT were increased in 9.7% and 2.1% respectively, and platelets levels were reduced in 18.8%.
Noteworthy, elevated D-dimer levels have also been described and related to worst outcomes in patients with other acute lung diseases, such as community acquired pneumonia [15], acute lung injury [16] and ARDS [17].
Altogether, these findings indicate that elevated levels of D-dimer and FDP, prolonged PT and APTT could mark severe forms and possibly later stages of COVID-19 infection, characterized by poor prognosis.
On the contrary, the majority of patients, possibly those with milder forms or in earlier stages of the disease, seems to have normal platelet levels and coagulation parameters; a minority can also show reactive thrombocytosis, possibly related to the acute inflammatory status, and reduced PT and APTT [18].
Therefore, coagulation dysfunction not only could reflect the severity of the disease among different patients, but in the same patient could vary according to the stage of the disease, suggesting a careful monitoring of these parameters during hospitalization [19].
The prognostic role of coagulation parameters was also assessed in a clinical series with 449 COVID-19 patients, by demonstrating the role of heparin use >7 days during hospitalization in reducing the 28-days mortality [20]. This outcome was positively associated with D-dimer, PT and age, and negatively correlated with platelet count in the multivariate analysis.
The greatest benefit in terms of highest 28-days survival among the 99 heparin users was demonstrated for those with D-dimer levels >6 fold of upper limit of normal and “sepsis induced coagulopathy” (SIC) score ≥ 4. SIC is a novel definition provided by ISTH to define patients in earlier phases of sepsis associated DIC, who could experience the highest benefit from anticoagulant treatment.
To date, no experimental investigations on the coagulation cascade are available apart from data coming from clinical practice. Considering the similarities between this new pandemic and SARS infection, an upregulation of genes associated with the coagulation pathway could be hypothesized. In fact, the overexpression of procoagulant genes such as those of factors II, III and X, has been demonstrated in an in vitro model containing SARS-infected peripheral blood mononuclear cells [21].
An impairment in fibrinolysis also seems to play a central role in COVID-19. A ‘fibrinolytic shutdown’ was demonstrated to be associated with thrombotic events in a clinical series of 44 patients, as revealed by thromboelastography measurements [22]. Moreover, another study taking advantage of thromboelastometry and considering 40 patients admitted to ICU was not able to find any sign of secondary hyperfibrinolysis [23].
3. Clinical aspects: increased rate of thromboembolic events in COVID-19
As early as coagulation abnormalities were reported in many Chinese cohorts from Wuhan, clinical reports of thromboembolic events became increasingly reported.
Initially, some Chinese Authors have suggested an increased rate of thrombotic and thromboembolic events in these patients [19], supported by their clinical practice and by similarities with the SARS infection, in which the rate of thrombotic events was estimated to be 20.5% for deep venous thrombosis (DVT) and 11.4% for pulmonary embolism (PE) in a report from Singapore [24]. One study also reported 5 SARS patients who developed large artery ischaemic strokes, but 3 of them were treated with high-doses Intravenous Immunoglobulins (IVIg), that could have enhanced the pro-coagulant status [25].
One of the first studies from Wuhan, in which 1008 patients with COVID-19 were hospitalized between January and February 2020, retrospectively identified 25 patients who also underwent to computed tomography pulmonary angiography (CTPA) on the basis of clinical suspicion [26]. Among them, 10 had acute pulmonary embolism (APE), that was dominantly located in small branches of the pulmonary artery. Interestingly, all 25 patients had increased D-dimer levels, but the 10 with APE had significantly higher levels as compared to the 15 without APE.
Subsequently, a large multicentre retrospective study showed an incidence of thrombotic complications in ICU patients around 30%, reinforcing the recommendations to consider an appropriate thrombotic prophylaxis in all COVID-19 ICU patients [27]. At the same time, in a single-centre retrospective study, the incidence of venous thromboembolism of 25% was reported by the same group of patients [28].
Importantly, different studies reported the necessity of changing the usual schedule of thrombo-prophylaxis (including dose and duration), because of the excess of risk in COVID-19 cohort as compared to other patients with acute lung injury [29,30].
Moreover, from a clinical point of view, different studies have underlined the importance to consider demographic characteristics and comorbidities of each patient to correctly estimate the thrombotic risk of COVID-19 patients. The role of concomitant risk factors should also be considered.
Elderly (70 years) patients are prone to develop the most complicated infections as confirmed in a recent meta-analysis collecting the results of 147 studies on 20,662 Chinese patients in which the mean patient age was 49 years and 53% of patients were male [31]. A significant prevalence of cardiovascular comorbidities was also described in this large Chinese cohort, including systemic hypertension (21%), diabetes mellitus (12%), cardiovascular (9%) and cerebrovascular diseases (6%) [31]. In another large report of 1099 patients with confirmed COVID-19, systemic arterial hypertension and diabetes mellitus were recorded in 23.7% and 16.2% respectively [32]. In a report from the Italian Institute of Health, 68% of 3032 patients who died for COVID-19 had hypertension, 30% diabetes, 28% ischemic heart disease and 11% obesity [33]. Interestingly, acute cardiac injury determined by elevated high-sensitivity troponin levels is frequently reported in critically ill patients and is strongly associated with mortality. Different potential mechanisms for acute effects on the cardiovascular system during the infection have been proposed, including a direct infection of myocardial cells causing myocarditis, but also side effects of anti-viral treatments [34,35]. Cardiovascular diseases, such as hypertension, diabetes and obesity are often associated with Angiotensin/Angiotensin I-converting enzyme 2 (ACE2) dysregulation, which aggravates the imbalance caused by the infection, suggesting a role of these comorbidities as risk factors for a poor prognosis in COVID-19 [36]. Additionally, in the setting of ICU admission that concern critically ill patients, respiratory failure and prolonged mechanical ventilation further increase the risk of thrombotic events [37]. It is important to consider that patients with comorbidities take a concomitant variety of drugs. Alarm has emerged for ACE inhibitors and angiotensin-receptor blockers (ARBs), which are commonly used for hypertension. In some reports it was reported an increase of risk for SARS-CoV-2 infection because of the potential increasing of expression of the entry receptor of the virus (ACE2) [38]. On the contrary, other studies demonstrated that ARBs' treatment might reduce lung injury modulating the angiotensin type 1 receptors [39]. Globally, the latest data support the continued use of these drugs during the pandemic.
Table 1 summarizes the principal studies recently published on the occurrence of thromboembolic events during COVID-19.
Table 1.
Features of patients with thromboembolic events in COVID-19 at the beginning of observation.
| Study | Enrolled subject (n) | Study population | Age (mean of years); Male sex (n) | Comorbidities | TE event (n) | Arterial thrombotic event (n) | Prophylaxis for possible events |
|---|---|---|---|---|---|---|---|
| Tang et al. [20] | 449 | Hospitalized patients | 65; 268 | 177 hypertension 93 diabetes 41 heart disease |
na | na | 94 patients received LMWH (40–60 mg/day) |
| Klok et al. [27] | 184 | ICU patients | 64; 139 | 5 cancer | 25 APE; 3 DVT |
3 ischemic strokes | Standard dose |
| Helms et al. [30] | 150 | ICU patients | 63 (median); 122 | 9 cancer 72 CV disease 30 diabetes |
8 | 7 ischemic strokes | 105 Standard dose, 43 Therapeutic dose |
| Cui et al. [28] | 81 | ICU patients | 60; 37 | 20 hypertension 8 diabetes 10 coronary disease |
20 DVT | na | None |
n = number; TE = thromboembolic event; LMWH = low molecular weight heparin; DVT = deep venous thrombosis; APE = pulmonary embolism; ICU = intensive care unit; CV = cardiovascular; na = not available.
4. Mechanisms of coagulation dysfunction in COVID-19
Different mechanisms are possibly implicated in the pathogenesis of coagulation dysfunction in patients with SARS-CoV2 infection. They could be summarized in 2 main pathways:
-
•
Inflammation and cytokines storm;
-
•
Specific-virus mechanisms.
4.1. Inflammation and cytokines storm
During SARS-CoV2 infection elevated levels of different pro-inflammatory cytokines induced by the innate immunity activation have been described and the so-called “cytokine release syndrome” seems to be responsible of the most severe manifestations of the disease [3]. Among these cytokines, a prominent role of interleukin-6 (IL-6) has emerged and is currently addressed in clinical trials with anti-IL-6 agents to treat severe forms [40].
In vitro, most pro-inflammatory cytokines have been demonstrated to activate the coagulation system; in vivo, high levels of tumor necrosis factor (TNF), IL-6 and IL-1 are detectable in patients with acute inflammatory conditions (such as sepsis) together with a hyper-coagulable status, sometimes evolving in DIC [41]. Results from clinical trials in sepsis with drugs targeting these pathways, showed that IL-6 rather than TNF seems to be the most important mediator for cytokine-induced coagulation activation. The role of IL-1 is uncertain, as its levels in sepsis become detectable significantly after the appearance of coagulation abnormalities [41].
IL-6 could also stimulate megakaryopoiesis [42] and has a prominent role in the induction of tissue factor (TF) expression in inflamed tissues. Therefore, TF expression could be enhanced in the lungs of patients affected by COVID-19, both via direct exposure due to tissue damage and inflammation, and via increased expression induced by IL-6 [43].
IL-6 promotes also the synthesis of other coagulation factors such as fibrinogen and factor VIII [44,45] and, acting on endothelial cells, induces vascular permeability by stimulating VEGF secretion [46]. It was reported that soluble IL-6 receptor (IL-6R) is abnormally elevated in the plasma of COVID-19 patients as consequence of enhanced cleavage from the cells surface during the infection. Afterwards, the circulating complexes of soluble IL-6 and IL-6R can activate directly most cells, including endothelial cells [47]. It's possible that the infection-related continuous coagulopathy may contribute also to the thrombocytopenia and, at the same time, the cytokine storm could stimulate the proliferation of megakaryocytes with consequent thrombocytosis [13]. The opposite platelets' alterations could reflect different phases of this disease, but their underlying specific mechanisms have to be elucidated [9].
4.2. Virus-specific mechanisms
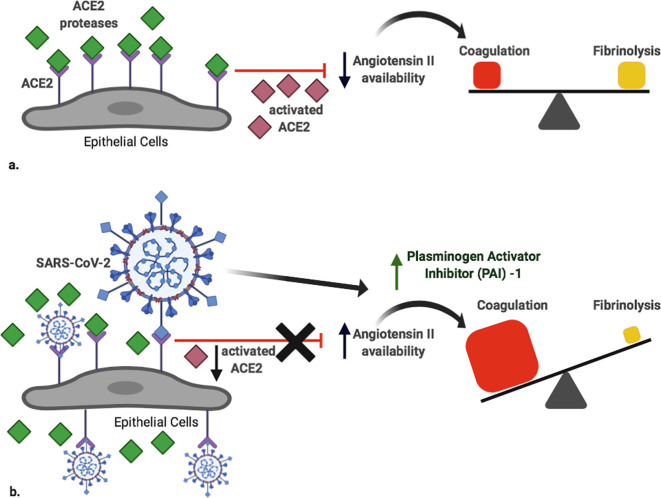
SARS-CoV-2 belongs to beta-coronavirus genus, enveloped and positive-stranded RNA viruses. Spike surface glycoprotein of the virus engages ACE2, which is an integral membrane receptor expressed in many cells, including the lung, heart, kidney and intestine. The physiological function of ACE2 is to counter-regulate ACE activity by reducing angiotensin II availability [48]. The virus-mediated engagement of ACE2 decreases its expression and activates the renin-angiotensin system (RAS), promoting platelet adhesion and aggregation [49] (Fig. 1 ). Moreover, RAS is known to exert substantial control over the fibrinolytic system, with strong evidences that it represents a mediator in the association between reduced fibrinolytic activity and ischemic clinical events in patients with systemic hypertension [50]. In fact, pharmacologic interventions that reduce the activity of angiotensin II also have positive effects on fibrinolytic balance and frequency of cardiovascular events.
Fig. 1.
Simplified scheme of the potential virus-specific effects in the imbalance between coagulation and fibrinolysis.
a. Physiologically, ACE2 counterbalances the effects of renin-angiotensin system by degrading Ang II, with no effects on the balance between coagulation and fibrinolysis.
b. During SARS-CoV-2 infection, the availability of ACE2 decreases because scavenged by the virus, enhancing Ang II availability. This favours the activation of a systemic procoagulant environment, together with the virus-mediated enhancement of PAI-1 production.
ACE2: Angiotensin Converting Enzyme II; Ang II: Angiotensin II; PAI-1: Plasminogen Activator Inhibitor-1.
Created with BioRender (academic license).
In particular, high levels of Plasminogen Activator Inhibitor-1 (PAI-1), the principal inhibitor of fibrinolysis interfering with tissue plasminogen activator (tPA) and urokinase, have been related with increased risk of thromboembolic events [50].
Interestingly, ACE2 was recognized to play a crucial role as receptor also for in vivo infection by SARS-CoV [51]. In a report including 16 patients with SARS-CoV infection, the blood levels of PAI-1 were significantly higher as compared to 20 healthy controls, but also to 19 patients with infectious pneumonia of other etiology [52]. These findings were consistent with another study, in which the PAI-1 mRNA and protein levels in the human hepatoma cell line Huh7 infected with SARS-coronavirus were much higher as compared to infection with human Coronavirus 229E (associated with the common cold), as evidenced by transcriptome experiments, qRT-PCR and ELISA [53], thereby explaining a possible direct effect of infection on the production of anti-coagulant factors. Furthermore, by infecting Serpine1-knockout mice, some Authors showed that the urokinase pathway had a significant effect also on lung pathology in SARS-CoV [54]. Plasminogen also contributes to inflammation caused by influenza virus through fibrinolysis [55], confirming the strictly connection between virus-induced lung injuries and abnormalities in the coagulation system.
During SARS-CoV pneumonia the expression of platelet derived vitronectin (VN), a mediator of platelet adhesion, was also dramatically increased but it was uncertain whether it originated from increased expression by the liver or from lung damage [56].
It was demonstrated that SARS-CoV2 can infect primary endothelial cells in vitro [57] and there is some evidence of the infection of endothelial cells in severe cases of COVID-19 [58]. The replication within endothelial cells induces cell death causing the activation of pro-coagulant reactions [59].
Another possible virus-specific mechanism could be related to the induction of autoimmunity, that was also described in SARS patients, as a consequence of molecular mimicry mechanisms [60].
Moreover, antiphospholipid antibodies (aPL), recognized as risk factors for arterial and venous thrombosis, have been associated with different viral infections, such as parvovirus B19, several herpes viruses, hepatitis viruses and human immunodeficiency virus. Whether these autoantibodies contribute to clinically important thrombotic events during infections remains controversial [60]. Some evidences that lupus anticoagulant activity (LA) and anti-beta2 glycoprotein I antibodies were induced after immunization with viral peptides were found [61]. On the other hand, in some reports aPL were transient and not predictive of thrombotic complications [62]. Interestingly, post-infectious aPL were reported to have distinct immunochemical characteristics; for example, anti-cardiolipin antibodies after viral infections were described to be non–beta2 glycoprotein I-dependent, differently from those detectable in autoimmune diseases [63].
Furthermore, in some patients the presence of infection-related aPL was also associated with the clinical manifestations of antiphospholipid syndrome, especially when other risk factors for thrombosis (such as inherited thrombophilia) were present [64].
A first case-report of COVID-19 patient with aPL and arterial ischemia was described at the end of April 2020 by Chinese Authors [65]. Moreover, a high rate of Lupus Anticoagulant positivity, but not of the other aPL tests (anti-cardiolipin and anti-Beta2 glycoprotein I) was reported [66]. A larger multicentric cohort systematically assessed classic aPL tests together with ‘non-criteria’ aPL tests demonstrated a low rate of aPL positivity, as defined by classification criteria, and supported the idea that aPL found in COVID-19 patients are different from aPL found in antiphospholipid syndrome [67]. Further studies are necessary to clarify this issue.
5. Anti-coagulant and anti-platelet agents in COVID-19: more than thrombo-prophylaxis?
5.1. Heparin
Heparin and its derivatives are an under-exploited antiviral drug class, despite possessing broad-spectrum activity against a multitude of distinct viruses, including SARS-associated Coronaviridae [68].
Even though the ACE2 protein is required for SARS-CoV2 entry, it was hypothesized that it was not the primary binding site on the target cell surface [68]. In fact, binding of the virus with heparan sulphates is probably required in the earlier phase of the interaction with human cells, as demonstrated for other human Coronaviridae [68,69].
Heparin is a well-known anticoagulant drug and is extensively used in clinical practice for its anticoagulant function in binding and activating antitrombin. Over the treatment of venous thromboembolism, it is widely prescribed in a prophylactic setting in general medical inpatients, as well as in COVID-19 patients, according to the current guidelines for the clinical management [9].
Since both heparin and heparan sulphate are complex, linear and acidic polysaccharides, belonging to the glycosaminoglycan (GAG) family, a possible competition in coronavirus binding was supposed.
A recent study demonstrated that SARS-CoV-2 surface protein S1 receptor binding domain binds to heparin and afterward induce a significant structural change [68]. Further proofs of the inhibitory potential of heparin against coronavirus infection come from in vitro studies, in which the incubation of susceptible epithelial cells (Vero cells) with heparin 30 min before SARS-CoV injection curtailed infection by 50% [70].
An increasing number of cytokines and interleukins, such as IL-6, are now known to bind to GAGs of the heparin and heparan sulphate family. This binding allows the retaining of cytokines close to their site of release by the tissues in a mechanism of local regulation, thus enhancing their paracrine functions [71]. Therefore, this could be considered as another potential mechanism by which heparin could interfere with the systemic cytokine storm detected in COVID-19.
Currently, eleven clinical trials are ongoing (ClinicalTrials.gov) on Enoxaparin in different subsets of COVID-19 patients with the aim to establish the most effective dose in primary or secondary prophylaxis in outpatient or hospitalized patients. Furthermore, a clinical trial is ongoing in United States with the aim to determine if therapeutic dose of anticoagulation improves mortality in comparison with intermediate dose prophylaxis. The drugs in study are Enoxaparin and Unfractionated Heparin, but also Fondaparinux and Argatroban.
5.2. Dypiridamole
Dypiridamole (DIP) is an anti-platelet agent and acts as a phosphodiesterase (PDE) inhibitor that increases intracellular cAMP/cGMP levels [72]. In the past, it was demonstrated that DIP was efficacious in inhibiting the positive-stranded RNA viruses' replication [73], in suppressing inflammation [74] and in preventing acute injury and progressive fibrosis of the lung, heart, liver, and kidney [75]. A recent study demonstrated an additional therapeutic benefit for COVID-19 patients [76]. In fact, DIP suppressed SARS-CoV-2 replication in vitro, detected through the determination of viral titres in Vero cells. In the same study, the Authors reported that 12 COVID-19 patients treated with prophylactic anti-coagulation and DIP had significantly increased platelet and lymphocyte counts and decreased D-dimer levels in comparison to control patients. Larger scale clinical trials of DIP are now needed to validate these prophylactic effects.
6. Summary and future directions
COVID-19 is a novel pandemic, caused by a new Coronavirus named as SARS-CoV2. A wide spectrum of severity of pulmonary involvement has been described, together with different kinds of systemic manifestations. Coagulation abnormalities have been frequently reported in patients with the most severe forms, especially markedly increased levels of D-dimer, but also prolonged APTT and/or PT and different degrees of thrombocytopenia in a smaller proportion of patients. Concomitantly, reports of both arterial and venous thromboembolic events are emerging.
This observation provides many insights into the pathogenetic mechanisms of COVID-19, starting from hyper-inflammation, that can represent the link between the infection and the hyper-coagulable state. IL-6 seems to have a pivotal role in the cytokine storm which is characteristic of the most severe forms and could induce a hyper-coagulable status, both at systemic and local levels. This could be enhanced by SARS-CoV2-specific mechanisms, by acting on the RAS and inhibiting the fibrinolysis. Autoimmunity could be another potential player, for example by inducing antiphospholipid (aPL) antibody production as observed in other viral infections.
According to the current WHO indications for COVID-19 hospitalized patients, heparin should be prescribed as primary prophylaxis for thrombosis. In fact, besides hyper-inflammation, the most critical patients are elderly patients with reduced mobility and cardiovascular comorbidities (systemic hypertension, diabetes). In addition to its anticoagulant effect, potential anti-inflammatory and anti-viral actions have been described for heparin in vitro. Similar evidences have been provided for Dypiridamole, an anti-platelet agent with PDE-inhibition activity.
From a clinical point of view, evaluation of coagulation parameters seems to be crucial to identify the patients affected by the most severe forms of COVID-19 and should also be monitored in patients during the disease course. Prophylactic low molecular weight heparin should be virtually prescribed to all COVID-19 hospitalized patients, except otherwise contraindicated. Increased heparin dosage might be considered in critically ill patients, especially those with the highest levels of D-dimer and pro-inflammatory markers or with other concomitant risk factors. The analysis of the results of the ongoing clinical trials are urgently needed to evaluate the most appropriate prophylactic strategy, to prevent thromboembolic events that dramatically increase the morbidity and the mortality of these patients, but also to assess its ancillary anti-inflammatory and anti-viral effects.
Regarding research proposals, SARS-CoV2 potential of inducing coagulation dysfunction could provide insights into the pathogenetic mechanisms of the virus, potentially addressed by specifically targeted treatment strategies.
Practice points
-
•
Coagulation dysfunction is not infrequent in COVID-19 patients, especially those with the most severe forms, yielding to lower survival rates.
-
•
Increased levels of D-dimer is the most frequently reported coagulation abnormality; a minority of patients also have APTT and/or PT prolongation and variable degrees of thrombocytopenia.
-
•
The increased rate of thrombotic and thromboembolic events is now well recognized in COVID-19 series.
-
•
In the cytokine storm, that characterize the most severe forms of COVID-19, IL-6 seems to play a pivotal role also in inducing a hyper-coagulable status both at systemic and local levels.
-
•
SARS-CoV2 specific mechanisms seems to play an additional role by inhibiting the fibrinolysis via the renin-angiotensin system (RAS).
-
•
Heparin prophylaxis should be virtually prescribed to all COVID-19 hospitalized patients, in the light of hyper-coagulation provided by the inflammation and the virus, but also of the frequent concomitant cardiovascular risk factors.
Research agenda
-
•
Coagulation dysfunction is a novel aspect which should be elucidated in COVID-19, from both a pathogenetic and clinical points of view.
-
•
The role of different players, including inflammation, virus-specific mechanisms and autoimmunity, should be clarified.
-
•
Results from clinical trials on anticoagulant and anti-platelet agents in COVID-19 are needed to assess the optimal protocol to be adopted in clinical practice and to explore in vivo anti-viral and anti-inflammatory properties of these compounds.
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
References
- 1.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M., van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38:S26–S34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han H., Yang L., Liu R., Liu F., Wu K.-l., Li J., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 8.Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 12.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu R., Ling Y., Zhang Y.H., Wei L.Y., Chen X., Li X.M., et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020 doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Querol-Ribelles J.M., Tenias J.M., Grau E., Querol-Borras J.M., Climent J.L., Gomez E., et al. Plasma d-dimer levels correlate with outcomes in patients with community-acquired pneumonia. Chest. 2004;126:1087–1092. doi: 10.1378/chest.126.4.1087. [DOI] [PubMed] [Google Scholar]
- 16.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31(S213):S20. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 17.Ware L.B., Matthay M.A., Parsons P.E., Thompson B.T., Januzzi J.L., Eisner M.D. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35:1821. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mei H., Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19. Zhonghua xueyexue zazhi. 2020;41:185–191. doi: 10.3760/cma.j.issn.0253-2727.2020.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng L.F., Hibberd M.L., Ooi E.-E., Tang K.-F., Neo S.-Y., Tan J., et al. A human in vitro model system for investigating genome-wide host responses to SARS coronavirus infection. BMC Infect Dis. 2004;4:34. doi: 10.1186/1471-2334-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright F.L., Vogler TO, Moore E.E., Moore H.B., Wohlauer M.V., Urban S., et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;S1072–7515(20):30400–30402. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavoni V., Gianesello L., Pazzi M., Stera C., Meconi T., Frigieri F.C. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020:1–6. doi: 10.1007/s11239-020-02130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong P.Y., Chui P., Ling A.E., Franks T.J., Tai D.Y., Leo Y.S., et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004;128:195–204. doi: 10.1043/1543-2165(2004)128<195:AODDTS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Umapathi T., Kor A.C., Venketasubramanian N., Lim C.T., Pang B.C., Yeo T.T., et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J Neurol. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J., Wang X., Zhang S., Liu B., Wu X., Wang Y., et al. 2020. Findings of acute pulmonary embolism in COVID-19 patients. available at SSRN 3548771. [DOI] [Google Scholar]
- 27.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T., Chen R., Liu C., Liang W., Guan W., Tang R., et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;4:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Chong, Zhang Keshi, Wang Wenlong, Pei Zheng, Liu Zheng, Yuan Ping, Guan Zhenpeng, Gu Jin. Clinical characteristics of 20,662 patients with COVID-19 in mainland China: A systemic review and meta-analysis. medRxiv preprint. 2020 doi: 10.1101/2020.04.18.20070565. [DOI] [Google Scholar]
- 32.W-j Guan, Z-y Ni, Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmieri L., Vanacore N., Donfrancesco C., Lo Noce C., Canevelli M., Punzo O., et al. Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. J Gerontol A Biol Sci Med Sci. 2020 doi: 10.1093/gerona/glaa146. glaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 35.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labò N., Ohnuki H., Tosato G. Vasculopathy and coagulopathy associated with SARS-CoV-2 infection. Cells. 2020;9:E1583. doi: 10.3390/cells9071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibrahim E.H., Iregui M., Prentice D., Sherman G., Kollef M.H., Shannon W. Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis. Crit Care Med. 2002;30:771–774. doi: 10.1097/00003246-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 38.South A.M., Tomlinson L., Edmonston D., Hiremath S., Sparks M.A. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020;16:305–307. doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. Crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levi M., van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Folman C.C., Linthorst G.E., van Mourik J., van Willigen G., de Jonge E., Levi M., et al. Platelets release thrombopoietin (Tpo) upon activation: another regulatory loop in thrombocytopoiesis? Thromb Haemost. 2000;83:923–930. [PubMed] [Google Scholar]
- 43.Levi M., van der Poll T., Schultz M. Systemic versus localized coagulation activation contributing to organ failure in critically ill patients. Semin Immunopathol. 2012;34:167–179. doi: 10.1007/s00281-011-0283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stouthard J.M., Levi M., Hack C.E., Veenhof C.H., Romijn H.A., Sauerwein H.P., et al. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost. 1996;76:738–742. [PubMed] [Google Scholar]
- 45.Stirling D., Hannant W.A., Ludlam C.A. Transcriptional activation of the factor VIII gene in liver cell lines by interleukin-6. Thromb Haemost. 1998;79:74–78. [PubMed] [Google Scholar]
- 46.Cohen T., Nahari D., Cerem L.W., Neufeld G., Levi B.Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 47.Zunke F., Rose-John S. The shedding protease ADAM17: physiology and pathophysiology. Biochim Biophys Acta Mol Cell Res. 1864;2017:2059–2070. doi: 10.1016/j.bbamcr.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Guang C., Phillips R.D., Jiang B., Milani F. Three key proteases–angiotensin-I-converting enzyme (ACE), ACE2 and renin–within and beyond the renin-angiotensin system. Arch Cardiovasc Dis. 2012;105:373–385. doi: 10.1016/j.acvd.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall R.P. The pulmonary renin-angiotensin system. Curr Pharm Des. 2003;9:715–722. doi: 10.2174/1381612033455431. [DOI] [PubMed] [Google Scholar]
- 50.Vaughan D.E. Angiotensin, fibrinolysis, and vascular homeostasis. Am J Cardiol. 2001;87:18–24. doi: 10.1016/s0002-9149(01)01509-0. [DOI] [PubMed] [Google Scholar]
- 51.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y.P., Wei R., Liu Z.H., Chen B., Lisman T., Ren D.L., et al. Analysis of thrombotic factors in severe acute respiratory syndrome (SARS) patients. Thromb Haemost. 2006;96:100–101. doi: 10.1160/TH05-12-0827. [DOI] [PubMed] [Google Scholar]
- 53.Tang B.S., K-h Chan, Cheng V.C., Woo P.C., Lau S.K., Lam C.C., et al. Comparative host gene transcription by microarray analysis early after infection of the Huh7 cell line by severe acute respiratory syndrome coronavirus and human coronavirus 229E. J Virol. 2005;79:6180–6193. doi: 10.1128/JVI.79.10.6180-6193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gralinski L.E., Bankhead A., Jeng S., Menachery V.D., Proll S., Belisle S.E., et al. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. MBio. 2013;4(e00271):13. doi: 10.1128/mBio.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berri F., Rimmelzwaan G.F., Hanss M., Albina E., Foucault-Grunenwald M.-L., Le V.B., et al. Plasminogen controls inflammation and pathogenesis of influenza virus infections via fibrinolysis. PLoS Pathog. 2013;9:e1003229. doi: 10.1371/journal.ppat.1003229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reheman A., Gross P., Yang H., Chen P., Allen D., Leytin V., et al. Vitronectin stabilizes thrombi and vessel occlusion but plays a dual role in platelet aggregation. J Thromb Haemost. 2005;3:875–883. doi: 10.1111/j.1538-7836.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 57.Monteil V., Kwon H., Prado P., Hagelkruys A., Wimmer R.A., Stahl M., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stern D., Nawroth P., Handley D., Kisiel W. An endothelial cell-dependent pathway of coagulation. Proc Natl Acad Sci U S A. 1985;82:2523–2527. doi: 10.1073/pnas.82.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goeijenbier M., Van Wissen M., Van De Weg C., Jong E., Gerdes V., Meijers J., et al. Viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84:1680–1696. doi: 10.1002/jmv.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gharavi A.E., Pierangeli S.S., Espinola R.G., Liu X., Colden-Stanfield M., Harris E.N. Antiphospholipid antibodies induced in mice by immunization with a cytomegalovirus-derived peptide cause thrombosis and activation of endothelial cells in vivo. Arthritis Rheum. 2002;46:545–552. doi: 10.1002/art.10130. [DOI] [PubMed] [Google Scholar]
- 62.Josephson C., Nuss R., Jacobson L., Hacker M.R., Murphy J., Weinberg A., et al. The varicella-autoantibody syndrome. Pediatr Res. 2001;50:345–352. doi: 10.1203/00006450-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 63.McNally T., Purdy G., Mackie I., Machin S., Isenberg D. The use of an anti-β2-glycoprotein-I assay for discrimination between anticardiolipin antibodies associated with infection and increased risk of thrombosis. Br J Haematol. 1995;91:471–473. doi: 10.1111/j.1365-2141.1995.tb05324.x. [DOI] [PubMed] [Google Scholar]
- 64.Consigny P., Cauquelin B., Agnamey P., Comby E., Brasseur P., Ballet J., et al. High prevalence of co-factor independent anticardiolipin antibodies in malaria exposed individuals. Clin Exp Immunol. 2002;127:158–164. doi: 10.1046/j.1365-2249.2002.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borghi M.O., Beltagy A., Garrafa E., Curreli D., Cecchini G., Bodio C., et al. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. medRxiv and bioRxiv. 2020 doi: 10.1101/2020.06.17.20134114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mycroft-West C., Su D., Elli S., Guimond S., Miller G., Turnbull J., et al. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 receptor binding domain undergoes conformational change upon heparin binding. BioRxiv. 2020 doi: 10.1101/2020.02.29.971093. [DOI] [Google Scholar]
- 69.Milewska A., Zarebski M., Nowak P., Stozek K., Potempa J., Pyrc K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J Virol. 2014;88:13221–13230. doi: 10.1128/JVI.02078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vicenzi E., Canducci F., Pinna D., Mancini N., Carletti S., Lazzarin A., et al. Coronaviridae and SARS-associated coronavirus strain HSR1. Emerg Infect Dis. 2004;10:413. doi: 10.3201/eid1003.030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mummery R.S., Rider C.C. Characterization of the heparin-binding properties of IL-6. J Immunol. 2000;165:5671–5679. doi: 10.4049/jimmunol.165.10.5671. [DOI] [PubMed] [Google Scholar]
- 72.Gresele P., Momi S., Falcinelli E. Anti-platelet therapy: phosphodiesterase inhibitors. Br J Clin Pharmacol. 2011;72:634–646. doi: 10.1111/j.1365-2125.2011.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fata-Hartley C.L., Palmenberg A.C. Dipyridamole reversibly inhibits mengovirus RNA replication. J Virol. 2005;79:11062–11070. doi: 10.1128/JVI.79.17.11062-11070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang B., Chen Z., Geng L., Wang J., Liang H., Cao Y., et al. Mucosal profiling of pediatric-onset colitis and IBD reveals common pathogenics and therapeutic pathways. Cell. 2019;179(e24):1160–1176. doi: 10.1016/j.cell.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 75.Insel P.A., Murray F., Yokoyama U., Romano S., Yun H., Brown L., et al. cAMP and Epac in the regulation of tissue fibrosis. Br J Pharmacol. 2012;166:447–456. doi: 10.1111/j.1476-5381.2012.01847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu X., Li Z., Liu S., Chen Z., Zhao Z., Huang Y.-Y., et al. Therapeutic effects of dipyridamole on COVID-19 patients with coagulation dysfunction. MedRxiv. 2020 doi: 10.1101/2020.02.27.20027557. [DOI] [Google Scholar]