Abstract
Hydroxychloroquine (HCQ) has been largely used and investigated as therapy for COVID-19 across various settings at a total dose usually ranging from 2400 mg to 9600 mg. In Belgium, off-label use of low-dose HCQ (total 2400 mg over 5 days) was recommended for hospitalised patients with COVID-19. We conducted a retrospective analysis of in-hospital mortality in the Belgian national COVID-19 hospital surveillance data. Patients treated either with HCQ monotherapy and supportive care (HCQ group) were compared with patients treated with supportive care only (no-HCQ group) using a competing risks proportional hazards regression with discharge alive as competing risk, adjusted for demographic and clinical features with robust standard errors. Of 8075 patients with complete discharge data on 24 May 2020 and diagnosed before 1 May 2020, 4542 received HCQ in monotherapy and 3533 were in the no-HCQ group. Death was reported in 804/4542 (17.7%) and 957/3533 (27.1%), respectively. In the multivariable analysis, mortality was lower in the HCQ group compared with the no-HCQ group [adjusted hazard ratio (aHR) = 0.684, 95% confidence interval (CI) 0.617–0.758]. Compared with the no-HCQ group, mortality in the HCQ group was reduced both in patients diagnosed ≤5 days (n = 3975) and >5 days (n = 3487) after symptom onset [aHR = 0.701 (95% CI 0.617–0.796) and aHR = 0.647 (95% CI 0.525–0.797), respectively]. Compared with supportive care only, low-dose HCQ monotherapy was independently associated with lower mortality in hospitalised patients with COVID-19 diagnosed and treated early or later after symptom onset.
Keywords: Hydroxychloroquine, COVID-19, SARS-CoV-2, Mortality, Observational study
1. Introduction
There is currently no robust antiviral or immunomodulatory treatment for infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19). Chloroquine (CQ), an antimalarial drug, has been shown to have in vitro antiviral properties both against SARS-CoV and SARS-CoV-2 by different mechanisms [1], [2], [3], [4]. It has also been hypothesised that CQ could have a positive impact on COVID-19 outcome through immunomodulatory properties [5,6]. Hydroxychloroquine (HCQ), a derivative of CQ, has a long clinical track record as a treatment for malaria and inflammatory diseases such as systemic lupus erythematous and rheumatoid arthritis, with a favourable safety profile in acute and chronic use [7]. Both CQ and HCQ were selected by the World Health Organization (WHO) for potential repurposing for COVID-19. Early during the amplification phase of the epidemic in Belgium, and pending results of clinical trials, off-label administration of a ‘low-dose’ regimen of HCQ sulphate in monotherapy (400 mg twice on Day 1, followed by 200 mg twice a day from Days 2 to 5, i.e. a total dose of 2400 mg) was recommended as an acceptable immediate treatment option for hospitalised COVID-19 patients [8]. This guidance, officially released on 13 March 2020, was based on the following considerations: (i) HCQ was the only drug with demonstrated in vitro effect against SARS-CoV-2 available in Belgium at that time; (ii) HCQ exhibited a superior in vitro antiviral effect in comparison with CQ, likely explained by the higher accumulated intracellular drug concentrations [9]; (iii) limited pharmacokinetic data suggested that the selected dosage should have sufficient antiviral activity [10]; (iv) chronic administration of HCQ for rheumatological disorders has not been associated with major safety signals over decades of use; (v) restricting HCQ use to well-selected COVID-19 patients monitored at hospitals appeared as a reasonable risk/benefit compromise considering the well-known dose-dependent cardiotoxicity of the drug; and (vi) it was advised to Belgian hospitals to administer this off-label regimen whenever possible within clinical studies. Of note, azithromycin (AZM) and systemic use of corticosteroids were not recommended in the guidance [8]. Simultaneously, Sciensano, the Belgian Scientific Institute of Public Health, initiated a national surveillance of COVID-19 hospitalised patients that included treatments and outcomes among its variables, enabling the clinical surveillance of drug use and outcome.
So far, the impact of HCQ on the outcome of SARS-CoV-2 infection in humans remains undetermined. An increasing number of single-centre and multicentre retrospective studies using various HCQ dosages are being published with conflicting results [11], [12], [13], [14], [15], [16], [17], [18], [19]. Recently, the UK-based RECOVERY and WHO-led SOLIDARITY trials communicated that HCQ at the study dosage of 9200–9600 mg over 10 days provided no benefit in hospitalised patients with COVID-19 [20].
In the present study, we retrospectively assessed the association between HCQ monotherapy and in-hospital mortality in a nationwide registry of 8075 COVID-19 patients. Next, the impact of HCQ treatment on mortality was investigated according to the time between symptom onset and COVID-19 diagnosis.
2. Methods
2.1. Data collection
Sciensano's data collection of patients hospitalised with confirmed COVID-19 was initiated on 14 March 2020, 2 weeks after the first symptomatic case was reported in Belgium, and systematic registering was strongly encouraged by health authorities. Two independent online secured questionnaires in LimeSurvey (LimeSurvey GmbH, Hamburg, Germany) were made available: one with information after admission and the second after discharge. Information collected at admission included sociodemographic characteristics, clinical presentation, co-morbidities, chronic treatment with renin–angiotensin–aldosterone system inhibitors and diagnostic workup. Data collected at discharge included COVID-19 treatment details (antiviral and immunomodulatory drugs, including date of initiation and termination, mode of administration), clinical and laboratory markers of disease severity during hospital stay, admission to the intensive care unit (ICU) and final outcome at hospital discharge (dead or discharged alive).
2.2. Hydroxychloroquine treatment
On 13 March 2020, a task force (ND, SVI and EB, affiliated to the national reference institutions for emerging infections), co-ordinated by Sciensano, published a guidance for the management of patients hospitalised with COVID-19. Based on the above-described rationale, the ‘low-dose’ HCQ regimen (2400 mg in total over 5 days) was recommended as a reasonable emergency therapeutic option for hospitalised patients and was centrally provided for free [8]. A set of warnings were provided on its use, including corrected QT (QTc) determination in all admitted patients and close cardiac monitoring in case of baseline QTc exceeding 450 ms and in all conditions that could favour arrhythmia (underlying cardiopathy, congenital or acquired QTc prolongation, electrolytic disturbances, or use of other drugs prolonging the QTc interval) [8]. Treatment initiation was advised as soon as a diagnosis was made, with information to the patient about the off-label use. The final treatment decision was, however, left to the discretion of the treating physician.
2.3. Inclusion and exclusion criteria
We analysed all COVID-19 cases for whom both admission and discharge questionnaires were reported up to 24 May 2020. The analysis was restricted to those confirmed before 1 May 2020 by reverse transcription PCR (RT-PCR) and/or rapid antigen test on respiratory samples, with exclusion of those diagnosed by pulmonary computed tomography (CT) scan only. The SARS-CoV-2 rapid antigen test used in Belgium has a specificity of 99.5% compared with RT-PCR in respiratory samples [21]. Children aged <16 years, pregnant and post-partum women as well as patients who were discharged (either alive or dead) within 24 h after hospital admission or before diagnosis confirmation were excluded. In addition, we removed from this analysis all patients having started any COVID-19-related treatment before symptom onset, including for other clinical indications, as well as those having a missing date of diagnosis. To compare patients treated with HCQ monotherapy and supportive care with those receiving only supportive care, any patients treated with another COVID-19-related treatment (macrolides, tocilizumab, lopinavir/ritonavir, remdesivir, atazanavir or anakinra), whether prescribed with or without HCQ, were also excluded.
2.4. Statistical analyses
Participants meeting the inclusion criteria were divided into two groups: (i) COVID-19 patients treated with HCQ monotherapy in addition to supportive care (HCQ group); and (ii) those receiving supportive care alone (no-HCQ group). Demographic characteristics, pre-existing conditions, laboratory parameters, clinical features and outcome were described first by discharge status (survivors versus non-survivors) and second by treatment group (HCQ versus no-HCQ). The χ2 test for categorical variables and the Wilcoxon test for continuous variables were used to assess differences between groups. We considered a P-value of <0.05 to be statistically significant.
Missing data among important prognostic baseline covariates were assumed to be missing at random, i.e. independent of the underlying missing values given the observed data. This was handled by ten-fold multiple imputation performed in R software through the MICE package v.3.8.0 [22]. A competing risks proportional hazards regression with robust standard errors allowing for clustering within hospitals (R package SURVIVAL v.3.1-12) was then used to analyse in-hospital death competing with alive discharge from hospital. Hazards of this in-hospital death thus dropped to zero post discharge alive. Cause-specific hazards of treatment effect were adjusted for the baseline covariates age, sex, co-morbidities (cardiovascular disease, arterial hypertension, diabetes mellitus, chronic renal, liver and lung diseases, neurological and cognitive disorders, immunosuppressive conditions, malignancies, obesity and smoking status), clinical features [pneumonia diagnosis, acute respiratory distress syndrome (ARDS), admission to ICU within the 24 h following admission and time from symptom onset to diagnosis] and baseline laboratory parameters of disease severity consisting of lactate dehydrogenase (LDH) ≥ 350 IU/L, C-reactive protein (CRP) ≥ 150 mg/L and partial pressure of oxygen (paO2) < 60 mmHg. As HCQ prescription decreased over time, the calendar time of diagnosis was also included in the model.
The propensity of HCQ treatment was estimated from those same baseline covariates (R package IPW v.1.0-11[23]). An inverse propensity-weighted standardised cumulative incidence of in-hospital death for each treatment was derived using R package RISCA v.0.8.2 [24]. The competing risks analysis was repeated for patients treated within or beyond 5 days of onset of symptoms. Sensitivity analyses were performed (Supplementary material): they considered additional adjustments in the model, missing data impact and possible immortal time bias associated with delayed treatment receipt. Analyses were performed in SAS Enterprise Guide 7.1 and in R 3.6.3.
2.5. Ethical and privacy considerations
The hospital data collection is being performed by Sciensano, the Belgian Scientific Institute of Public Health, legally entitled for surveillance of infectious diseases in Belgium (Royal Decree of 21/03/2018). This COVID-19 hospital surveillance was authorised by an independent administrative authority protecting privacy and personal data and was approved by the ethical committee of Ghent University Hospital.
3. Results
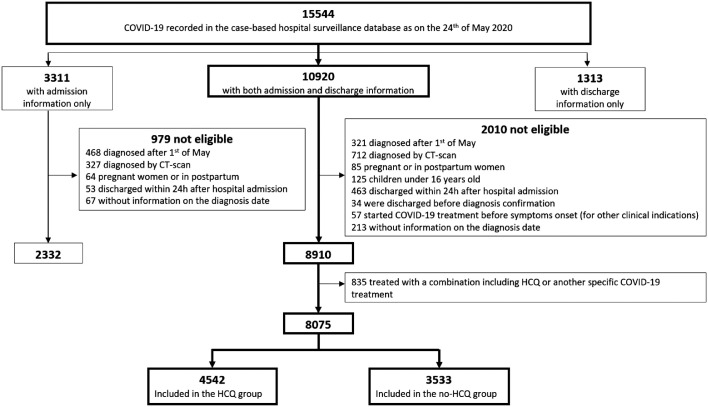
As recorded on 24 May 2020, the Sciensano database contained a total of 15 544 case records of COVID-19 patients (Fig. 1 ), originating from 109 Belgian hospitals. Among those, both admission and discharge report forms were received for 10 920 patients [3311 (21.3%) discharge forms were missing for patients with admission data and 1313 (8.4%) admission forms were missing for patients with discharge data]. After having excluding patients not meeting the inclusion criteria (Fig. 1), 8910 cases were included for the descriptive analysis.
Fig. 1.
Data flow for coronavirus disease 2019 (COVID-19) patient selection for the observational cohort study. CT, computed tomography; HCQ, hydroxychloroquine.
Approximately 60% of the hospitalised COVID-19 patients were aged ≥65 years (Table 1 ). In general, participants were severely ill with more than 80% having radiological pneumonia, large proportions presenting with laboratory parameters of severity, including pronounced hypoxaemia, and 5.5% requiring immediate admission to the ICU. The median time from symptom onset to COVID-19 diagnosis was 5 days. Patients with incomplete discharge data (n = 2332) were similar to the study population in terms of age and sex distribution as well as the frequency of pre-existing conditions, except for the proportion of active smokers (Supplementary Table S1). In the univariate analysis, compared with survivors, non-survivors were older and were more likely to be male and to suffer from pre-existing conditions (Table 1). In addition, non-survivors presented more often with laboratory markers of disease severity such as high levels of LDH (≥350 IU/L) and CRP (≥150 mg/L) and severe hypoxaemia (paO2 < 60 mmHg). Time from symptom onset to diagnosis was shorter in non-survivors (median 3 days vs. 6 days in survivors; P < 0.0001). Length of hospital stay was similar in both groups.
Table 1.
Characteristics of coronavirus disease 2019 (COVID-19) patients by survival or non-survival status during hospitalisation
| Characteristic | No./total no. (%) | P-value | ||
|---|---|---|---|---|
| Total (n = 8910) | Survivors (n = 6981) | Non-survivors (n = 1929) | ||
| Demographic characteristics | ||||
| Age (years) | ||||
| 16–30 | 149/8906 (1.7) | 149/6979 (2.1) | 0/1927 (0.0) | <0.0001* |
| 31–44 | 607/8906 (6.8) | 596/6979 (8.5) | 11/1927 (0.6) | |
| 45–64 | 2685/8906 (30.2) | 2503/6979 (35.9) | 182/1927 (9.4) | |
| 65–79 | 2655/8906 (29.8) | 2017/6979 (28.9) | 638/1927 (33.1) | |
| ≥80 | 2810/8906 (31.6) | 1714/6979 (24.6) | 1096/1927 (56.9) | |
| Median (IQR) age (years) | 71 (57–82) | 66 (54–79) | 82 (73–87) | |
| Male sex | 4807/8819 (54.5) | 3711/6919 (53.6) | 1096/1900 (57.7) | 0.0017± |
| Pre-existing conditions | ||||
| Cardiovascular disease | 3084/8910 (34.6) | 2093/6981 (30.0) | 991/1929 (51.4) | <0.0001± |
| Arterial hypertension | 3622/8910 (40.7) | 2641/6981 (37.8) | 981/1929 (50.9) | <0.0001± |
| Diabetes mellitus | 1985/8910 (22.3) | 1442/6981 (20.7) | 543/1929 (28.1) | <0.0001± |
| Chronic renal disease | 1166/8910 (13.1) | 733/6981 (10.5) | 433/1929 (22.4) | <0.0001± |
| Chronic liver disease | 237/8910 (2.7) | 160/6981 (2.3) | 77/1929 (4.0) | <0.0001± |
| Chronic lung disease | 1353/8910 (15.2) | 976/6981 (14.0) | 377/1929 (19.5) | <0.0001± |
| Neurological disorders | 832/8910 (9.3) | 555/6981 (8.0) | 277/1929 (14.4) | <0.0001± |
| Cognitive disorders a | 1001/8338 (12.0) | 627/6539 (9.6) | 374/1799 (20.8) | <0.0001± |
| Immunosuppressive conditions | 248/8910 (2.7) | 191/6981 (2.7) | 57/1929 (3.0) | 0.6049± |
| Malignancy | ||||
| Solid | 730/8910 (8.2) | 507/6981 (7.3) | 223/1929 (11.6) | <0.0001± |
| Haematological | 174 /8910 (2.0) | 118/6981 (1.7) | 56/1929 (2.9) | 0.0007± |
| Obesity a | 545/5457 (10.0) | 450/4313 (10.4) | 95/1144 (8.3) | 0.0327± |
| Current smoker | 407/4757 (8.6) | 312/3793 (8.2) | 95/964 (9.9) | 0.1064± |
| Medications | ||||
| ACE inhibitor | 1368/8907 (15.3) | 1030/6979 (14.8) | 338/1928 (17.5) | 0.0028± |
| Angiotensin receptor blocker | 806/8907 (9.0) | 604/6979 (8.7) | 202/1928 (10.5) | 0.0135± |
| COVID-19 treatments | ||||
| Supportive care only | 3533/8910 (39.6) | 2576/6981 (36.9) | 957/1929 (49.6) | <0.0001± |
| HCQ | 4542/8910 (51.0) | 3738/6981 (53.5) | 804/1929 (41.7) | <0.0001± |
| HCQ + macrolides | 761/8910 (8.5) | 617/6981 (8.5) | 144/1929 (7.5) | 0.0561± |
| Lopinavir/ritonavir | 12/8910 (0.1) | 7/6981 (0.1) | 5/1929 (0.3) | 0.2358± |
| HCQ + lopinavir/ritonavir | 18/8910 (0.2) | 10/6981 (0.1) | 8 /1929 (0.4) | 0.0504± |
| HCQ + tocilizumab | 17/8910 (0.2) | 12/6981 (0.2) | 5/1929 (0.3) | 0.4367± |
| HCQ + tocilizumab + macrolides | 7/8910 (0.1) | 5/6981 (0.1) | 2/1929 (0.1) | 0.6565± |
| HCQ + remdesivir | 4/8910 (0.0) | 2/6981 (0.0) | 2/1929 (0.1) | 0.1685± |
| Others | 16/8910 (0.2) | 14/6981 (0.2) | 2/1929 (0.1) | 0.3738± |
| Laboratory parameters | ||||
| LDH (IU/L) (median (IQR) [no.]) | 343 (258–477) [7385] | 329 (251–459) [5909] | 394 (288–548) [1476] | <0.0001* |
| LDH ≥ 350 IU/L | 3563/7385 (48.2) | 2663/5909 (45.1) | 900/1476 (61.0) | <0.0001± |
| CRP (mg/L) (median (IQR) [no.]) | 62 (26–118) [8624] | 55.9 (21.8–108.2) [6802] | 91.2 (44.4–162) [1822] | <0.0001* |
| CRP ≥ 150 mg/L | 1487/8624 (17.2) | 973/6802 (14.3) | 514/1822 (28.2) | <0.0001± |
| paO2 (mmHg) (median (IQR) [no.]) | 66 (57–76) [6013] | 67 (70–77) [4713] | 61 (52–73) [1300] | <0.0001* |
| paO2 < 60 mmHg | 1834/6013 (30.5) | 1221/4713 (25.9) | 613/1300 (47.2) | <0.0001± |
| Clinical features | ||||
| Pneumonia b | 7184/8567 (83.9) | 5545/6710 (82.6) | 1639/1857 (88.2) | <0.0001± |
| ARDS | 1197/8423 (14.2) | 601/6710 (9.0) | 596/1713 (34.8) | <0.0001± |
| Invasive ventilation support | 736/8691 (8.5) | 367/6810 (5.4) | 369/1881 (19.6) | <0.0001± |
| Admission to ICU within 24 h after admission | 488/8900 (5.5) | 298/6974 (4.3) | 190/1926 (9.9) | <0.0001± |
| Time from symptom onset to diagnosis (days) (median (IQR) [no.]) | 5 (2–9) [8097] | 6 (2–9) [6393] | 3 (1–7) [1704] | <0.0001* |
| Length of hospital stay (days) (median (IQR) [no.]) | 9 (5–15) [8894] | 9 (5–15) [6970] | 9 (5–16) [1924] | 0.9320* |
IQR, interquartile range; ACE, angiotensin-converting enzyme; HCQ, hydroxychloroquine; LDH, lactate dehydrogenase; CRP, C-reactive protein; paO2, partial pressure of oxygen; ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
NOTE: All of the pre-existing conditions and COVID-19 features were reported as assessed by the clinician.
Missingness is due to later onset of data collection.
Diagnosis by imaging [chest radiography and/or computed tomography (CT) scan].
Wilcoxon test.
χ2 test.
After further exclusion of patients who received alternative COVID-19 treatments either with (n = 818; including macrolides, n = 761) or without HCQ (n = 17), the comparative analysis was restricted to 8075 subjects: 4542 in the HCQ group and 3533 in the no-HCQ group (Fig. 1). Of the HCQ-treated patients, 78.2% initiated the treatment within 24 h after diagnosis.
As shown in Table 2 , COVID-19 patients in the HCQ group were younger and male sex was predominant. Several co-morbidities were significantly less frequent in the HCQ group, including cardiovascular diseases, arterial hypertension, chronic renal disease, neurological and cognitive disorders, solid cancer and obesity, as well as the proportion of active smokers. On the other hand, at admission, patients in the HCQ group appeared to be sicker as reflected by a higher frequency of radiological pneumonia, ARDS, ICU transfer within the 24 h after admission and invasive ventilation support as well as a higher frequency of elevated LDH and CRP levels. The case fatality rate of the study population was 21.8% (1761 deaths/8075 patients) but was lower in the HCQ group (804/4542; 17.7%) than in the no-HCQ group (957/3533; 27.1%) (P < 0.001). Incidental use of steroids was very low in both groups, although it was slightly higher in the HCQ group (8.1% vs. 5.9%). On a side note, mortality in the 761 participants who received HCQ and AZM was 18.9%.
Table 2.
Characteristics of coronavirus disease 2019 (COVID-19) patients (n = 8075) by treatment group.
| Characteristic | No./total No. (%) | P-value | |
|---|---|---|---|
| HCQ (n = 4542) | No-HCQ (n = 3533) | ||
| Demographic characteristics | |||
| Age (years) | |||
| 16–30 | 62/4541 (1.4) | 72/3530 (2.0) | <0.0001* |
| 31–44 | 372/4541 (8.2) | 175/3530 (5.0) | |
| 45–64 | 1656/4541 (36.5) | 695/3530 (19.7) | |
| 65–79 | 1395/4541 (30.7) | 1015/3530 (28.8) | |
| ≥80 | 1056/4541 (23.3) | 1573/3530 (44.6) | |
| Median (IQR) age (years) | 66 (54–78) | 77 (63–85) | |
| Male sex | 2646/4494 (58.9) | 1671/3492 (47.8) | <0.0001± |
| Pre-existing conditions | |||
| Cardiovascular disease | 1392/4542 (30.7) | 1444/3533 (40.9) | <0.0001± |
| Arterial hypertension | 1757/4542 (38.7) | 1513/3533 (42.8) | 0.0002± |
| Diabetes mellitus | 998/4542 (22.0) | 796/3533 (22.5) | 0.5498± |
| Chronic renal disease | 508/4542 (11.2) | 585/3533 (16.6) | <0.0001± |
| Chronic liver disease | 122/4542 (2.7) | 99/3533 (2.8) | 0.7511± |
| Chronic lung disease | 698/4542 (15.4) | 517/3533 (14.6) | 0.3599± |
| Neurological disorders | 330/4542 (7.3) | 450/3533 (12.7) | <0.0001± |
| Cognitive disorders a | 331/4260 (7.8) | 582/3266 (17.8) | <0.0001± |
| Immunosuppressive conditions | 159/4542 (3.5) | 78/3533 (2.2) | 0.0006± |
| Malignancy | |||
| Solid | 314/4542 (6.9) | 345/3533 (9.8) | <0.0001± |
| Haematological | 90/4542 (2.0) | 70/3533 (2.0) | 0.9995± |
| Obesity a | 297/2643 (11.2) | 186/2284 (8.1) | 0.0003± |
| Current smoker | 183/2390 (7.7) | 196/1916 (10.2) | 0.0031± |
| Medications | |||
| ACE inhibitor | 669/4541 (14.7) | 569/3531 (16.1) | 0.0874± |
| Angiotensin receptor blocker | 388/4541 (8.5) | 318/3531 (9.0) | 0.4665± |
| Laboratory parameters at admission | |||
| LDH (U/L) (median (IQR) [no.]) | 359 (270–497) [3890] | 314 (239–442) [2764] | <0.0001* |
| LDH ≥ 350 U/L | 2036/3890 (52.3) | 1146/2764 (41.5) | <0.0001± |
| CRP (mg/L) (median (IQR) [no.]) | 68.9 (32.1–125.0) [4461] | 50.6 (16.0–105.2) [3340] | <0.0001* |
| CRP ≥150 mg/L | 835/4461 (18.7) | 471/3340 (14.1) | <0.0001± |
| paO2 (mmHg) (median (IQR) [no.]) | 66 (57–75) [3442] | 68 (58–80) [1967] | 0.0033* |
| paO2 < 60 mmHg | 1046/3442 (30.4) | 557/1967 (28.3) | 0.1084± |
| Clinical features | |||
| Pneumonia b | 4055/4423 (91.7) | 2329/3313 (70.3) | <0.0001± |
| ARDS | 720/4306 (16.7) | 299/3320 (9.0) | <0.0001± |
| Invasive ventilation support | 503/4407 (11.4) | 114/3457 (3.3) | <0.0001± |
| Admission to ICU within 24 h after admission | 313/4539 (6.9) | 96/3529 (2.7) | <0.0001± |
| Time from symptom onset to diagnosis (days) (median (IQR) [no.]) | 6 (3–9) [4542] | 4 (1–8) [3049] | <0.0001* |
| Length of hospital stay (days) (median (IQR) [no.]) | 9 (6–15) [3324] | 9 (4–17) [3526] | 0.2061* |
| Outcome | |||
| Time from diagnosis to death (days) (median (IQR) [no.]) | 8 (5–13) [4542] | 6 (4–12) [3533] | <0.0001* |
| Death | 804/4542 (17.7) | 957/3533 (27.1) | <0.0001± |
IQR, interquartile range; ACE, angiotensin-converting enzyme; LDH, lactate dehydrogenase; CRP, C-reactive protein; paO2, partial pressure of O2; ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
NOTE: All of the pre-existing conditions and COVID-19 features were reported as assessed by the clinician.
Missingness is due to later onset of data collection.
Diagnosis by imaging [chest radiography and/or computed tomography (CT) scan].
Wilcoxon test.
χ2 test.
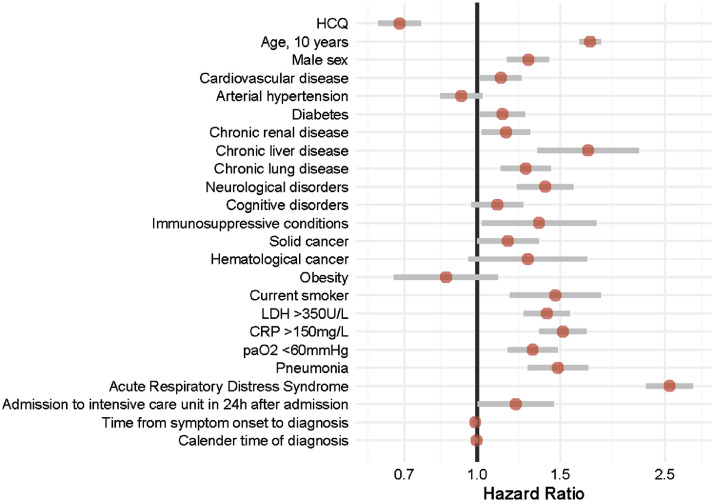
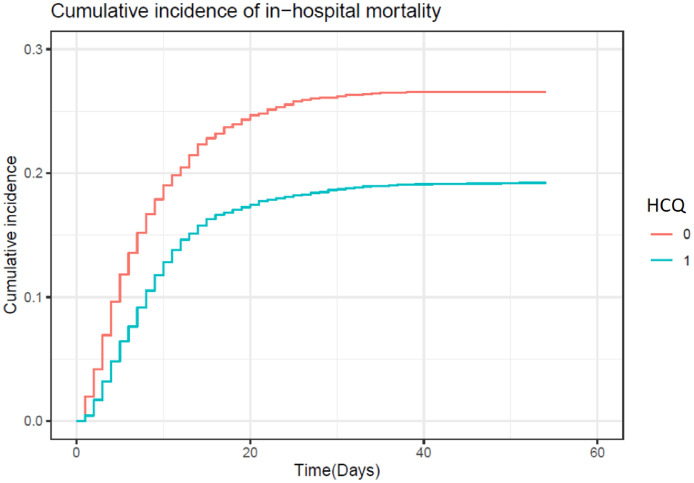
Independent predictors of in-hospital mortality are shown in Fig. 2 . Age, male sex, cardiovascular disease, diabetes mellitus, chronic renal, liver and lung diseases, neurological disorders, immunosuppressive conditions, smoking status as well as radiological pneumonia, ARDS and immediate admission to the ICU were all associated with a higher risk of in-hospital death. It was also the case for the measured laboratory parameters of disease severity at baseline. Treatment with HCQ alone was in contrast independently associated with a decreased risk of in-hospital mortality [adjusted hazard ratio (aHR) = 0.684, 95% confidence interval (CI) 0.617–0.758] compared with the no-HCQ group. Fig. 3 shows the inverse propensity-weighted standardised cumulative incidence of in-hospital death for each treatment with multiple imputations. From this model, estimated direct-adjusted mortality at 40 days was 19.1% with HCQ alone and 26.5% with supportive care only.
Fig. 2.
Independent predictors of in-hospital mortality among 8075 patients with coronavirus disease 2019 (COVID-19). Competing risks proportional hazards regression with robust standard errors analysing in-hospital death competing with alive discharge from hospital. HCQ, hydroxychloroquine; LDH, lactate dehydrogenase; CRP, C-reactive protein; paO2, partial pressure of oxygen.
Fig. 3.
Cumulative incidence of in-hospital mortality. Inverse propensity-weighted standardised cumulative incidence of in-hospital death according to treatment received: hydroxychloroquine (HCQ) (blue line) versus no-HCQ (red line).
Next we compared the association between HCQ treatment and in-hospital mortality in patients diagnosed and treated within 5 days after onset of symptoms (early diagnosis, n = 3975) with those diagnosed >5 days later (late diagnosis, n = 3487). Compared with the no-HCQ group, administration of HCQ appears to be associated to a lower risk of death both in the early diagnosis group (aHR = 0.701, 95% CI 0.617–0.796) and the late diagnosis group (aHR = 0.647, 95% CI 0.525–0.797).
4. Discussion
In this large analysis of patients admitted for COVID-19 in Belgium, HCQ monotherapy administered at a dosage of 2400 mg over 5 days was independently associated with a lower in-hospital mortality rate compared with patients treated with supportive care only, even after adjustment for age, major co-morbidities and disease severity at admission. Importantly, mortality was reduced regardless of the time from symptom onset to diagnosis and HCQ treatment initiation.
Our study has several limitations and strengths. It is an observational study of data collected using standardised report forms during the most critical phase of the epidemic in Belgium. The cohort was established within an ongoing surveillance that aims at monitoring the epidemic and identifying risk factors for severe COVID-19 and unfavourable outcome. The evaluation of HCQ efficacy in this population was therefore not the primary objective of the data collection itself. Also, the actual HCQ dosage was not systematically checked, but qualitative surveys pointed out that the ‘low-dose’ recommendation was very well adhered to, since the risk of dose-dependent cardiotoxicity and the necessary precautions for use in patients at risk were particularly stressed in the treatment guidance [8]. Not surprisingly, HCQ has been less administered in several groups of patients with pre-existing conditions or co-medications that correspond to contra-indications of its use (cardiac and renal diseases). The implementation of this surveillance during the initial phase of the epidemic when hospitals were under pressure and its non-mandatory nature resulted in missing admission or discharge report forms for a sizeable proportion of patients. The absence of difference in baseline characteristics and in outcome for subgroups with missing data and the study population is somehow reassuring, although some hidden sources of bias cannot be fully excluded. Strengths of this study include the very large sample size obtained in a timely manner, the strict comparison between groups exposed to HCQ in monotherapy and to supportive care only (with no other COVID-19 treatments as confounders), the multicentric design covering the vast majority of Belgian hospitals, and the real-life representativeness of the data. Indeed, this hospital-based surveillance captured complete admission and discharge information for 64.0% (10 920/17 052) of all aggregated COVID-19 patients admitted across the country until 24 May 2020 [25]. Finally, rigorous sensitivity analysis taking into account censored data and immortal bias all confirmed the positive impact of HCQ on in-hospital mortality.
This nationwide observational study provides a robust description of the COVID-19 patients admitted in Belgian hospitals. The demographic and clinical characteristics were similar to hospital cohorts reported in other countries, with a large proportion of patients with well-established risk factors for COVID-19 complications. The observed in-hospital mortality rate (~21.8%) was in line with that of previous observational studies and ongoing trials [26], [27], [28]. Risk factors for death were comparable with previous clinical experience. Notably, we found that biological markers previously related to disease severity and mortality in univariate analysis (increased CRP and LDH) [29] were independently associated with mortality in our study. Other well-established predictors of mortality such as lymphopenia and increased levels of D-dimer were not recorded in our data set. High D-dimer levels have been meanwhile associated with increased risk of thrombotic events, a major cause of death in hospitalised COVID-19 patients [30,31].
Interestingly, we observed that patients who died in hospital had on average a shorter duration of symptoms before admission, suggesting that abrupt clinical deterioration is associated with a worse prognosis. More research is required to clarify the phenotype of this subgroup of patients, to assess whether patients who develop signs of severity very soon after the onset of symptoms require more intensive care earlier, and to investigate whether very early predictors of subsequent severity and targeted (pre-hospital) interventions could prevent admission due to acute complications.
In the context of the COVID-19 pandemic, HCQ therapy has been in the centre of debates, between hype and bashing, within and beyond the scientific community. Uncertainty about treatment efficacy relies mainly on the observational nature of the published studies so far and the major risks of bias and confounders. Many small single-centre retrospective studies did not find any impact of HCQ treatment on outcome in hospitalised COVID-19 patients [11,[13], [14], [15], [16]], but were not powered to explore associations with mortality endpoint through robust multivariate analysis. Recently, larger observational studies found that the use of HCQ alone or in combination with AZM was independently associated with lower in-hospital mortality [17], [18], [19], in line with our results. Some therapeutic differences, however, have to be highlighted that impede full comparison of these studies with our data. In the study by Lagier et al. [17], the HCQ dosage was 6000 mg in total over 10 days and the vast majority of participants were given AZM concomitantly. In the study by Mikami et al. [18], the HCQ dosage was not reported, but most hospitalised patients were also exposed to a combination of HCQ and AZM. Finally, Arshad et al. used the same HCQ dosage as in our study and reported specifically on the subgroup treated with HCQ, but a large proportion of participants were also given steroids, which might have been beneficial in severe COVID-19 cases [19,32]. Of note, in our data set, corticosteroid uptake was low in both groups. In Belgium, AZM was not recommended owing to lack of robust information on viral efficacy at the time of writing the guidance. It has, however, been administered in combination with HCQ in 761 patients in our real-life database (but excluded from this analysis). The combination treatment of AZM+HCQ was associated with a decrease in mortality similar to that of HCQ alone (data not shown).
Although observational studies, even of large scale, do not provide final conclusions on treatment efficacy, their results are important to consider in order to guide clinical trials. Well-designed prospective studies combined with large, randomised control trials should provide definitive evidence about the clinical impact of HCQ in severe hospitalised and in mild ambulatory COVID-19 patients. Meanwhile, in a preprint publication at the time of writing, the results of the RECOVERY trial did not show any clinical benefit (death or discharge) in the high-dose HCQ arm (9200 mg in total over 10 days) compared with usual care in hospitalised COVID-19 patients (median number of days from symptom onset to randomisation, 9 days) [20]. Of note, the dose administered during the first 24 h (2400 mg) is equivalent to the dose administered over 5 days in Belgium. The pharmacokinetic rationale for the high dosage remains poorly described. Moreover, 10% of the patients included in the HCQ arm had negative SARS-CoV-2 test and mortality was high (25%) in both groups, indicating advanced disease.
Whilst both CQ and HCQ have in vitro antiviral activity against SARS-CoV-2 [3,4,9], concerns about a real antiviral activity in vivo have emerged early in the pandemic based on previous experience in other viral infections [33]. Antiviral efficacy of HCQ in humans has been poorly studied so far with adequate methods. Questions have also been raised about whether safe HCQ dosages are sufficient to reach antiviral activity in target pulmonary cells [34]. Translating in vitro data into in vivo drug concentration in tissue appears particularly challenging for HCQ, as plasma concentrations do not appear to be a reliable surrogate [35]. Preprint studies in animal models (non-humans primates and Syrian hamsters) also suggest that HCQ has no antiviral efficacy [36,37]. Clinical efficacy might, however, be mediated through immunomodulatory mechanisms [7], preventing the progression toward severe disease with over-inflammatory responses by dampening the cytokine storm [38]. HCQ has indeed been shown to decrease the production of pro-inflammatory cytokines, both ex vivo and in lung explant model [5,39,40]. In the same line, use of low-dose dexamethasone (one of the RECOVERY arms) was recently reported to significantly decrease mortality in COVID-19 patients requiring oxygen [28]. Also, HCQ has been suggested to have some anticoagulant properties that may be beneficial in preventing thrombotic events in complement to low-molecular-weight heparin [41].
The potential detrimental effect of HCQ, mainly due to dose-dependent cardiotoxicity, has become a major clinical concern, especially following the publication of an article that reported an association between HCQ therapy and increased in-hospital mortality, but that was retracted shortly after [42]. A randomised controlled trial evaluating high-dosage (12 000 mg over 10 days) and low-dosage CQ was halted prematurely due to serious toxicity in the high-dose group [43]. However, many studies have meanwhile reported on the safety of a short-term/low-dosage course of HCQ monotherapy [16,44]. Our study provides further support to the claim that this regimen is not associated with increased short-term risk of cardiotoxicity and mortality in the hospital setting and in well-selected COVID-19 patients. Accordingly, as of 17 June 2020, the Federal Agency for Medicines and Health Products had registered in total eight reports of adverse reactions suspected to be associated with HCQ use for the treatment of COVID-19 in Belgium, among which were three cases of cardiac toxicity (all having received concomitant medication), and no reported deaths [45].
In conclusion, in this large nationwide observational study of patients hospitalised with COVID-19, HCQ monotherapy administered at a dosage of 2400 mg over 5 days was independently associated with a significant decrease in mortality compared with patients not treated with HCQ. This impact was observed both in the early and late treatment groups, suggesting that this benefit might be mediated by immunomodulatory properties, a hypothesis worth addressing as evidence of an antiviral activity of HCQ on SARS-CoV-2 appears increasingly inconsistent. Considering the availability and cheapness of HCQ, it seems worth further investigating the clinical effect of an optimised dosage of HCQ and designing add-on studies in ongoing trials to monitor, beyond viral shedding and infectiousness, a relevant set of inflammatory markers during the course of SARS-CoV-2 infection.
Acknowledgments
The authors are very grateful to all of the clinicians and hospital directions which allowed this systematic and timely data collection and reporting to Sciensano, in very stretched circumstances. ND is a Post-Doctorate Clinical Master Specialist of the FRS-FNRS.
Funding: None.
Competing interests: None declared.
Ethical approval: This study was approved by the Ethical committee of Ghent University Hospital [BC-07507].
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2020.106144.
Contributor Information
Belgian Collaborative Group on COVID-19 Hospital Surveillance:
Kristof Bafort, Leïla Belkhir, Nathalie Bossuyt, Philippe Caprasse, Vincent Colombie, Paul De Munter, Jessika Deblonde, Didier Delmarcelle, Mélanie Delvallee, Rémy Demeester, Thierry Dugernier, Xavier Holemans, Benjamin Kerzmann, Pierre Yves Machurot, Philippe Minette, Jean-Marc Minon, Saphia Mokrane, Catherine Nachtergal, Séverine Noirhomme, Denis Piérard, Camelia Rossi, Carole Schirvel, Erica Sermijn, Frank Staelens, Filip Triest, Nina Van Goethem, Jens Van Praet, Anke Vanhoenacker, Roeland Verstraete, and Elise Willems
Appendix. Supplementary materials
References
- 1.Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grassin-Delyle S, Salvator H, Brollo M, Catherinot E, Sage E, Couderc L-J, et al. Chloroquine inhibits the release of inflammatory cytokines by human lung explants. Clin Infect Dis. 2020 May 8 doi: 10.1093/cid/ciaa546. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 8.Sciensano Interim clinical guidance for adults with suspected or confirmed COVID-19 in Belgium. Sciensano; 2020. https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_InterimGuidelines_Treatment_ENG.pdf.
- 9.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perinel S, Launay M, É Botelho-Nevers, É Diconne, Louf-Durier A, Lachand R, et al. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. Clin Infect Dis. 2020 Apr 7 doi: 10.1093/cid/ciaa394. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautret P, Lagier J, Parola P, Hoang V, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Mahévas M, Tran V-T, Roumier M, Chabrol A, Paule R, Guillaud C, et al. Clinical efficacy of hydroxychloroquine in patients with COVID-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paccoud O, Tubach F, Baptiste A, Bleibtreu A, Hajage D, Monsel G, et al. Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe COVID-19 in a French university hospital. Clin Infect Dis. 2020;323:2493–2502. doi: 10.1093/cid/ciaa791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sbidian E, Josse J, Lemaitre G, Mayer I, Bernaux M, Gramfort A, et al. Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France. medRxiv. 2020 Jun 19 doi: 10.1101/2020.06.16.20132597. [DOI] [Google Scholar]
- 17.Lagier J-C, Million M, Gautret P, Colson P, Cortaredona S, Giraud-Gatineau A, et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Travel Med Infect Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikami T, Miyashita H, Yamada T, Harrington M, Steinberg D, Dunn A, et al. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med. 2020 Jun 30 doi: 10.1007/s11606-020-05983-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020 Jul 15 doi: 10.1101/2020.07.15.20151852. [DOI] [Google Scholar]
- 21.Mertens P, De Vos N, Martiny D, Jassoy C, Mirazimi A, Cuypers L, et al. Development and potential usefulness of the COVID-19 Ag Respi-Strip diagnostic assay in a pandemic context. Front Med. 2020;7:225. doi: 10.3389/fmed.2020.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 23.van der Wal WM, Geskus RB. ipw : an R package for inverse probability weighting. J Stat Softw. 2011;43 doi: 10.18637/jss.v043.i13. [DOI] [Google Scholar]
- 24.Le Borgne F, Giraudeau B, Querard AH, Giral M, Foucher Y. Comparisons of the performance of different statistical tests for time-to-event analysis with confounding factors: practical illustrations in kidney transplantation. Stat Med. 2016;35:1103–1116. doi: 10.1002/sim.6777. [DOI] [PubMed] [Google Scholar]
- 25.Sciensano . 2020. COVID-19 — Bulletin épidémiologique du 25 mai 2020. [Google Scholar]
- 26.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with COVID-19—preliminary report. N Engl J Med. 2020 Jul 17 doi: 10.1056/NEJMoa2021436. [Epub ahead of print] [DOI] [Google Scholar]
- 29.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee TC, MacKenzie LJ, McDonald EG, Tong SYC. An observational cohort study of hydroxychloroquine and azithromycin for COVID-19: (can't get no) satisfaction. Int J Infect Dis. 2020;98:216–217. doi: 10.1016/j.ijid.2020.06.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020;177 doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan J, Zhang X, Liu J, Yang Y, Zheng N, Liu Q, et al. Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients. Clin Infect Dis. 2020 May 21 doi: 10.1093/cid/ciaa623. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin-Blondel G, Ruiz S, Murris M, Faguer S, Duhalde V, Eyvrard F, et al. Hydroxychloroquine in COVID-19 patients: what still needs to be known about the kinetics. Clin Infect Dis. 2020 May 11 doi: 10.1093/cid/ciaa558. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaptein SJ, Jacobs S, Langendries L, Seldeslachts L, ter Horst S, Liesenborghs L, et al. Antiviral treatment of SARS-CoV-2-infected hamsters reveals a weak effect of favipiravir and a complete lack of effect for hydroxychloroquine. bioRxiv. 2020 Jun 19 doi: 10.1101/2020.06.19.159053. [DOI] [Google Scholar]
- 37.Maisonnasse P, Guedj J, Contreras V, Behillil S, Solas C, Marlin R, et al. Hydroxychloroquine in the treatment and prophylaxis of SARS-CoV-2 infection in non-human primates. Nature. 2020 Jul 22 doi: 10.1038/s41586-020-2558-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang C-H, Choi J-H, Byun M-S, Jue D-M. Chloroquine inhibits production of TNF-α, IL-1β and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology. 2006;45:703–710. doi: 10.1093/rheumatology/kei282. [DOI] [PubMed] [Google Scholar]
- 40.Silva J, Mariz H, Jr Rocha L, Oliveira P, Dantas A, Duarte A, et al. Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics (Sao Paulo) 2013;68:766–771. doi: 10.6061/clinics/2013(06)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oscanoa TJ, Romero-Ortuno R, Carvajal A, Savarino A. A pharmacological perspective of chloroquine in SARS-CoV-2 infection. Int J Antimicrob Agents. 2020 Jul 4 doi: 10.1016/j.ijantimicag.2020.106078. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ledford H, Noorden RV. High-profile coronavirus retractions raise concerns about data oversight. Nature. 2020;582:160. doi: 10.1038/d41586-020-01695-w. [DOI] [PubMed] [Google Scholar]
- 43.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lane JCE, Weaver J, Kostka K, Duarte-Salles T, Abrahao MTF, Alghoul H, et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. medRxiv. 2020 May 31 doi: 10.1101/2020.04.08.20054551. [DOI] [Google Scholar]
- 45.Federal Agency for Medicines and Health Products (FAMHP) FAMHP; Brussels, Belgium: 2020. Summary adverse drug reactions in corona virus infection. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.