Abstract
The unique repertoire of genes that characterizes the early region 3 (E3) of the different species of human adenovirus (HAdV) likely contributes to their distinct pathogenic traits. The function of many E3 CR1 proteins remains unknown possibly due to unidentified intrinsic properties that make them difficult to express ectopically. This study shows that the species HAdV-B- and HAdV-E-specific E3 CR1 genes can be expressed from vectors carrying the HAdV tripartite leader (TPL) sequence but not from traditional mammalian expression vectors. Insertion of the TPL sequence upstream of the HAdV-B and HAdV-E E3 CR1 open reading frames was sufficient to rescue protein expression from pCI-neo constructs in transfected 293T cells. The detection of higher levels of HAdV-B and HAdV-E E3 CR1 transcripts suggests that the TPL sequence may enhance gene expression at both the transcriptional and translational levels. Our findings will facilitate the characterization of additional AdV E3 proteins.
Keywords: Human adenovirus, Early region 3, CR1 genes, pMT2, Tripartite leader sequence
1. Introduction
Human adenoviruses (HAdVs) are ubiquitous linear double stranded DNA viruses associated with a wide variety of illnesses including respiratory disease of variable severity, conjunctivitis, and gastroenteritis (Berk, 2013). The 51 recognized serotypes and more than 70 genotypes described by bioinformatics analysis of complete genomic sequences are currently classified into 7 different species designated Human mastadenovirus A through G (HAdV-A through HAdV-G) (Hage et al., 2015; Harrach et al., 2012; Robinson et al., 2013).
Early region 3 (E3) is the most divergent coding region of the genome among the different species of HAdVs (Burgert and Blusch, 2000; Davison et al., 2003b). The E3 transcriptional units of HAdVs genomes comprise distinct repertoires of 5–9 open reading frames (ORFs) (Burgert and Blusch, 2000; Wold et al., 1995). E3 is dispensable for virus replication in vitro (Kelly and Lewis, 1973), and encodes several proteins that modulate the host response to infection (Burgert and Blusch, 2000). HAdVs differing only in the genetic content of their E3 regions exhibit different degrees of virulence and host range, highlighting the role of E3 genes in viral pathogenesis (Belak et al., 1986; Linne, 1992). Interestingly, E3 is the only region of the genome that encodes membrane proteins, many of which are HAdV species-specific (Lichtenstein et al., 2004; Windheim et al., 2004). The species-specific ORFs located between the conserved E3–19K and E3–10.4K (also known as RIDα) genes encode proteins that belong to CR1 protein superfamily (CDD: pfam02440 (Blusch et al., 2002; Davison et al., 2003b)). CR1 genes are designated with a Greek letter reflecting their order in the genome, and the corresponding proteins are designated by their predicted molecular weight. The HAdV CR1 proteins exhibit some sequence homology with the RL11 family of proteins encoded by human cytomegalovirus (Davison et al., 2003a). The repertoires of E3 CR1 genes are highly variable —both in number and amino acid sequence— among the different species of HAdV. Furthermore, there is no significant homology between the CR1 gene sequences and any entries in known databases, impeding the prediction of their function based on the sequence alone. To the present only two of the thirteen unique CR1 proteins encoded by the different species of HAdV have been formally assigned a function: Adenovirus Death Protein (ADP), encoded by E3 CR1β of HAdV-C, facilitates efficient viral progeny release at late time points post infection (Tollefson et al., 1996), and E3–49K, encoded by E3 CR1β of HAdV-D, is secreted from infected cells and binds to CD45 on leukocytes inhibiting their effector functions (Windheim et al., 2013). The lack of information regarding the functions for the majority of the E3 CR1 proteins is a major gap in the knowledge. This prevents the full understanding of the molecular bases of the distinct pathobiology of HAdVs classified within different species.
Our laboratory is interested in elucidating the biological function of the HAdV-B- and HAdV-E-specific E3 CR1 proteins. Unexpectedly, constructs generated using commercially available mammalian expression vectors failed to produce adequate levels of our E3 proteins of interest. This led us to the exploration of alternative vectors and to the resurfacing of the pMT2 mammalian expression vector (Kaufman et al., 1989) as a valuable tool. Data from our studies indicate that the presence of the mature adenoviral tripartite leader (TPL) sequence, or certain portions of it, upstream of the ORF is essential for efficient expression of the following E3 CR1 proteins: HAdV-B3 E3 CR1β and E3 CR1γ (also known as E3–20.1K and E3–20.5K, respectively) and HAdV-E4 E3 CR1β and E3 CR1δ (also known as E3–24.8K and E3–29.7K, respectively). These findings will help overcome a major technical obstacle that may have interfered with previous attempts to pursue the functional characterization of many species-specific E3 proteins.
2. Materials and methods
2.1. Cell culture
The 293T cell line (ATCC CRL 3216) was cultured in DMEM/F12 medium (Gibco) supplemented with10% Fetal Bovine Serum (FBS, Atlanta Biologicals), 10 U/ml penicillin and 10 μg/ml streptomycin (Gibco). The HeLa Ohio (HeLa OH) cell line, a generous gift from Dr. Dean Erdman (CDC, Atlanta, GA, USA), was cultured in Minimal Essential Medium (Gibco) supplemented with 10% FBS, 1.5 g/ml Na2CO3 (Amresco), 2 mM l-glutamine, 10 U/ml pencillin, 10 μg/ml streptomycin, 1 mM sodium pyruvate and 25 mM HEPES (Gibco).
2.2. Transfection
The day before transfection 293T cells were seeded in a 6-well dish to be 60–80% confluent. The cells were transfected with 1 μg of mammalian expression construct using Effectene Transfection Reagent (Qiagen) according to manufacturer’s protocol. At 24 h post transfection (hpt), tunicamycin (Abcam) was added at a concentration of 10 μg/ml. At 72 hpt the cells were lysed for Western blot (WB) analysis of protein expression.
2.3. Electroporation
On the day of electroporation, HeLa OH cells at 75% confluency were tripsinized and brought into suspension, counted, and 5×106 cells were spun at 300 g for 10 min. Cells were then resuspended in 200 μl of Gene Pulser Electroporation Buffer (Bio-Rad Laboratories). The resuspended HeLa OH cells and 10 μg of mammalian expression construct DNA were then combined into a 0.2 cm gap electroporation cuvette (USA Scientific) and incubated at room temperature for 2 min. The cells were then electroporated using a Gene Pulser Xcell Electroporation System containing a CE module (Bio-Rad Laboratories) using the manufacturer’s preset protocol for HeLa cells (exponential decay, 500 μF, 160 V). Following electroporation, 200 μl of complete medium was added to the cuvette, and the entire suspension was transferred to 2 ml of prewarmed medium in a single well in a 6 well dish. The cells were washed once with 1x phosphate-buffered saline (PBS) at 24 h post electroporation (hpe) and fresh medium was added. Tunicamycin was added 48 hpe at a concentration of 10 μg/ml. At 72 hpe, the cells were lysed for WB analysis of protein expression.
2.4. Generation of mammalian expression constructs
The pMT2-PL mammalian expression vector used in this study was derived from pMT2 (Kaufman et al., 1989) by the incorporation of an expanded polylinker (PL) to provide better options for sub-cloning. A portion of the polylinker from the pCI-neo mammalian expression vector (Promega, Madison, WI) was PCR-amplified using iProof High Fidelity DNA Polymerase (Bio-Rad Laboratories) using the Fw (5′ GCT AGC CTC GAG AAT TCA CGC GTG GTA CCT CTA G 3′) and Rv (5′ GCT CGA AGC ATT AAC CCT CAC TAA AGG GAA GCG 3′) primers. The amplified 83 bp fragment containing restriction sites for 5′ EcoRI, XhoI, SmaI, NotI, ClaI, and EcoRI 3′ was inserted into the EcoRI site in the pMT2 plasmid. A map of pMT2-PL is shown in Fig. 1A. The complete sequence for this plasmid was deposited in GenBank under accession number KX757040.
Fig. 1.
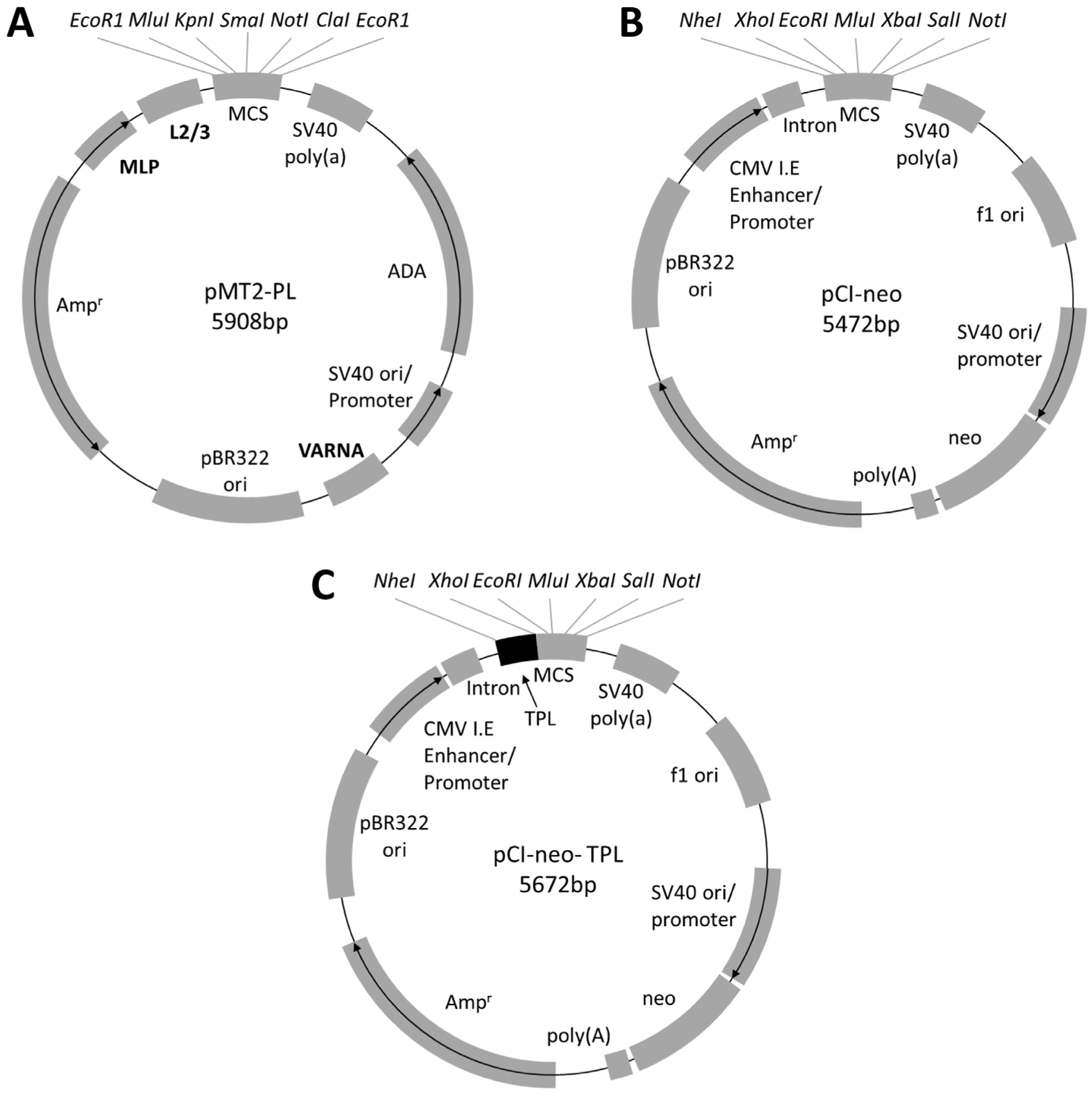
Map of mammalian expression vector used in this study. (A) pMT2-PL was derived from pMT2 by inserting a polylinker (obtained from pCI-neo vector) containing 5′ EcoRI, MluI, KpnI, SmaI, NotI, ClaI and EcoRI 3′ sites. The key features of this vector include: HAdV-C2 major late promoter (MLP) driving the expression of the gene cloned into the MCS, HAdV-C2 tripartite leader sequences 2 and 3 (L2/3), SV40 poly(a) signal, human adenosine deaminase (ADA), SV40 ori/promoter, HAdV-C2 VA RNA, pBR322 ori, and ampicillin resistance marker. (B) pCI-neo’s (Promega) key features include: the cytomegalovirus (CMV) immediate early (I.E.) enhancer/promoter, a chimeric intron, multiple cloning site (MCS), SV40 poly(a) signal, the origin of replication from the filamentous phage f1, the SV40 origin of replication (ori)/promoter which drives expression of the neomycin selectable marker along with a synthetic polyadenylation signal, ampicillin resistance marker (Ampr), and the pBR322 ori. (C) pCI-neo-TPL was derived from pCI-neo by insertion of the spliced complete HAdV-E4 tripartite leader sequence (L1/L2/L3) immediately upstream of the MCS.
Portions of the E3 regions including the HAdV-B3 E3–20.1K and E3–20.5K and HAdV-E4 E3–24.8K and E3–29.7K ORFs were PCR-amplified from genomic clones pKBS2 Ad3 (Sirena et al., 2005) and Ad4 pVQ Wt #11183 (ABL, Inc. through an MTA with NIH), respectively, using iProof High Fidelity DNA Polymerase and cloned into the pCR-BluntII Topo vector using the Zero Blunt Topo PCR Cloning kit (Invitrogen) according to the manufacturer’s protocol. Small epitope tags (VSV-G (YTDIEMNRLGK), HA (YPYDVPDYA), myc (EQKLISEEDL), and V5 (GKPIPNPLLGLDST)) were inserted into the 5′ end of the coding sequence for E3–20.1K, E3–20.5K, E3–24.8K, and E3–29.7K, respectively, immediately downstream of the corresponding predicted signal sequences (Signal IP 4.1, http://www.cbs.dtu.dk/services/SignalP) by site-directed mutagenesis using the Quikchange II XL kit (Agilent) following the manufacturer’s directions. These tagged coding sequences were then PCR-amplified using iProof High Fidelity DNA Polymerase and cloned into the pCI-neo and pMT2-PL vectors. As a control, enhanced green fluorescent protein (eGFP) was PCR-amplified from the pEGFP-N1 vector (Clontech) and cloned into the pCI-neo and pMT2-PL vectors.
The pCI-neo-TPL constructs were derived from the corresponding pCI-neo constructs described above by insertion of the HAdV-E4 mature TPL sequence upstream of the gene of interest. The TPL sequence was amplified from cDNA reverse-transcribed from total RNA extracted from A549 cells infected with HAdV-E4 vaccine strain using the primers Fw (5′ TAC CTG CTA GCA CTG TCT TCC GGA TCG CTG TC 3′) and Rv (5′ TTC ACT CGA GCT TGC GAC TGC GAC TGG C 3′). The amplified TPL sequence was cloned into the NheI and XhoI sites upstream of the E3 CR1 genes or eGFP in the pCI-neo constructs. Maps of the pCI-neo and pCI-neo TPL vectors are shown in Figs. 1B and C, respectively.
All mammalian expression constructs were controlled by DNA sequencing. No unintended start codons before or premature stop codons after the initiating ATG were identified. Sequences representative of all pMT2-PL, pCIneo and pCI-neo-TPL constructs generated for this study are shown in Supplemental Fig. 1.
2.5. RT-PCR
Expression levels for the four E3 CR1 genes of interest were examined in 293T cells transfected with pCI-neo, pCI-neo-TPL and pMT2-PL constructs. Total RNA was extracted from transfected cells using the RNAqueous kit (Ambion) following the manufacturer’s recommendations. Residual DNA contamination was eliminated from 20 μg of total RNA using Turbo DNA-free kit (Invitrogen). To ensure the elimination of contaminating DNA, the conserved ribosomal subunit Rig/S15 gene was amplified using the Fw (5′ TTC CGC AAG TTC ACC TAC C 3′) and Rv (5′ CGG GCC GGC CAT GCT TTA CG 3′) primers and goTaq Flexi DNA Polymerase (Promega) following the general manufacturer’s recommendations. No amplicon was detected. One μg of DNAse-treated RNA was then reverse-transcribed using Oligo(dT) primers and the Retroscript Reverse Transcription kit (Invitrogen) following the manufacture’s recommendations. One μl of cDNA was used as a template and amplified using goTaq Flexi DNA Polymerase with primers specific for HAdV-B3 E3–20.1K (Fw 5′ CCA TAT TAC CTT AGG ACA TAA TCA CAC 3′ and Rv 5′ GTG CCA TTC CC AAT G 3′), and HAdV-B3 E3–20.5K (Fw 5′ CCT TGC AGC TGT AAC TTA TGG 3′ and Rv 5′ AAT CCC ACT ACC ACG GC 3′), HAdV-E4 E3–24.8K (Fw 5′ CAC ACA CCT TTT TCA GAC CTA 3′ and Rv 5′ GTC CAG CTT GTT GTT CAG 3′), and HAdV-E4 E3–29.7K (Fw 5′ ACA GTT TAT ACA TGC GAG GGA G 3′ and Rv 5′ ACA GTT TAT ACA TGC GAG GGA G 3′). The constitutively expressed Rig/S15 was amplified using the primers described above as a housekeeping gene for semi-quantitative analysis of gene expression. The cycle number for all CR1 genes and Rig/S15 was optimized empirically and set at 20 to fit within the linear range of amplification. The amplification products were analyzed by electrophoresis on agarose gels. Densitometric analysis of EtBr-stained gel bands was performed using the open source Fiji platform (https://imagej.net/Fiji; Schindelin et al., 2012). The E3 CR1 mRNA levels were normalized to those of Rig/S15 in the corresponding samples and expressed in arbitrary units (AU).
2.6. Cell lysates and WB
At 72 hpt the transfected 293T cells were lysed with lysis buffer (for E3–20.1K and E3–20.5K: 0.14 M NaCl, 10 mM Tris pH 8.5, 0.5% Igepal CA-630, 1 mM MgCl2; for E3–24.8K and E3–29.7K: 150 mM NaCl, 50 mM Tris pH 7.5, 1% Igepal CA-630, 1% sodium deoxycholate) containing 1X complete mini EDTA free protease inhibitor cocktail (Roche Diagnostics Corporation, Indianapolis, IN). WB analysis was performed as described by Kotha and colleagues (Kotha et al., 2015). Briefly, cells were scraped in the lysis buffer and sonicated with five pulses for 30 s each. Protein concentration was determined with the Bio-Rad protein assay dye (Bio-Rad Laboratories) according to manufacturer’s protocol. Equal amounts of protein were mixed with SDS-PAGE sample buffer, heated at 85 °C for 10 min and resolved on a 4–12% SDS polyacrylamide gel (Invitrogen). Gels were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore), blocked with 5% BSA and probed with primary antibodies (anti-VSV-G tag (RVV-45A-Z, 1:1000, Immunology Consultant Laboratories, Inc.), anti-HA tag (16B12, 1:1000, Covance, Inc.), anti-myc tag (9B11, 1:1000, Cell Signaling Technology), and anti-V5 tag (D3H8Q, 1:1000, Cell Signaling Technology) overnight at 4 °C. The blots were then washed 3 times with tris-buffered saline and tween-20 (TBS-T) and incubated with HRP-conjugated secondary antibodies (Invitrogen). Protein bands were detected with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific) and imaged using Chemi Doc MP Imaging system (Bio-Rad Laboratories).
2.7. Fluorescence microscopy
293T cell monolayers were transfected with the pCI-neo, pCI-neo-TPL or pMT2-PL vectors encoding eGFP or HAdV-B3 E3–20.1K in a 6-well dish. At 48 hpt, the cells were put into suspension by pipetting up and down gently, then plated onto an 8 mm coverslip in a 12-well dish at a density resulting in 50% confluency on the day of processing for immunofluorescence. At 72 hpt, coverslips were fixed with 4% paraformaldehyde, quenched with 0.1 M glycine, permeabilized with 0.2% saponin, and blocked with 2% BSA in SuperBlock (PBS) blocking buffer (Thermo Scientific). VSV-G E3–20.1K was visualized with anti VSV-G tag primary antibody (Immunology Consultants Laboratory, Inc.) followed by FITC conjugated secondary antibody (Jackson ImmunoResearch Laboratories). 293T cells transfected with constructs expressing eGFP were fixed with 4% paraformaldehyde, quenched with 0.1 M glycine and blocked with 2% BSA in SuperBlock (PBS) blocking buffer. After washing with 1X PBS, coverslips were mounted on microscope slides using ProLong Diamond Antifade Mountant with DAPI (Molecular Probes). Slides were then imaged using a Zeiss Axioskop epifluorescence microscope equipped with a Hamamatsu digital camera controlled with SlideBook image analysis software version 6.0 (Intelligent Imagining Innovations) at 40X magnification.
2.8. Statistical analysis
Statistical analysis was carried out using the SPSS statistics suite version 24 (IBM). Five random fields of 293T cells transfected with constructs pCI-Neo-eGFP, pMT2-PL-eGFP, or pCI-Neo-TPL-eGFP were imaged. The percentage of cells expressing eGFP was calculated for each condition and the means were compared using one-way ANOVA analysis. Levene’s test was performed to verify the assumed equality of variances among experimental groups.
3. Results
3.1. Species-specific HAdV-B and HAdV-E E3 CR1 genes cannot be expressed at detectable levels in vitro from traditional mammalian expression vectors
With the goal of ectopically expressing HAdV-B-specific CR1 genes E3–20.1K and E3–20.5K and HAdV-E-specific CR1 genes E3–24.8K and E3–29.7K we sub-cloned the corresponding ORFs into pCI-neo, a traditional mammalian expression vector. In this vector, expression is driven by the cytomegalovirus (CMV) immediate-early promoter. The pCI-neo vector also contains the SV40 origin of replication that allows the propagation of the plasmid in mammalian cells constitutively expressing the SV40 T antigen, such as 293T cells (Mahon, 2011). To express N-terminally tagged E3 CR1 polypeptides, the small epitope tags VSV-G, HA, myc, and V5 were inserted into the 5′ end of HAdV-B3 E3–20.1K and E3–20.5K and HAdV-E4 E3–24.8K and E3–29.7K genes, respectively, bypassing their corresponding signal sequence predicted using Signal IP 4.1 (http://www.cbs.dtu.dk/services/SignalP/). Monolayers of 293T cells were transfected with the pCI-neo constructs encoding the tagged E3 genes of interest and examined for E3 protein expression by WB analysis with anti-tag antibodies. Because the four E3 proteins of interest are N-glycosylated and exhibit a diffused migration pattern on SDS PAGE gels (Hawkins and Wold, 1995b; Li and Wold, 2000), transfected 293T cells were treated with tunicamycin, an N-glycosylation inhibitor. To our surprise, we failed to detect protein expression for all four E3 genes tested (Fig. 2A, B, C, and D). Additionally, no protein expression was detectable for any of the E3 genes tested when we used an alternative mammalian expression vector, pCEP4 that also carries the CMV promoter (Data not shown).
Fig. 2.
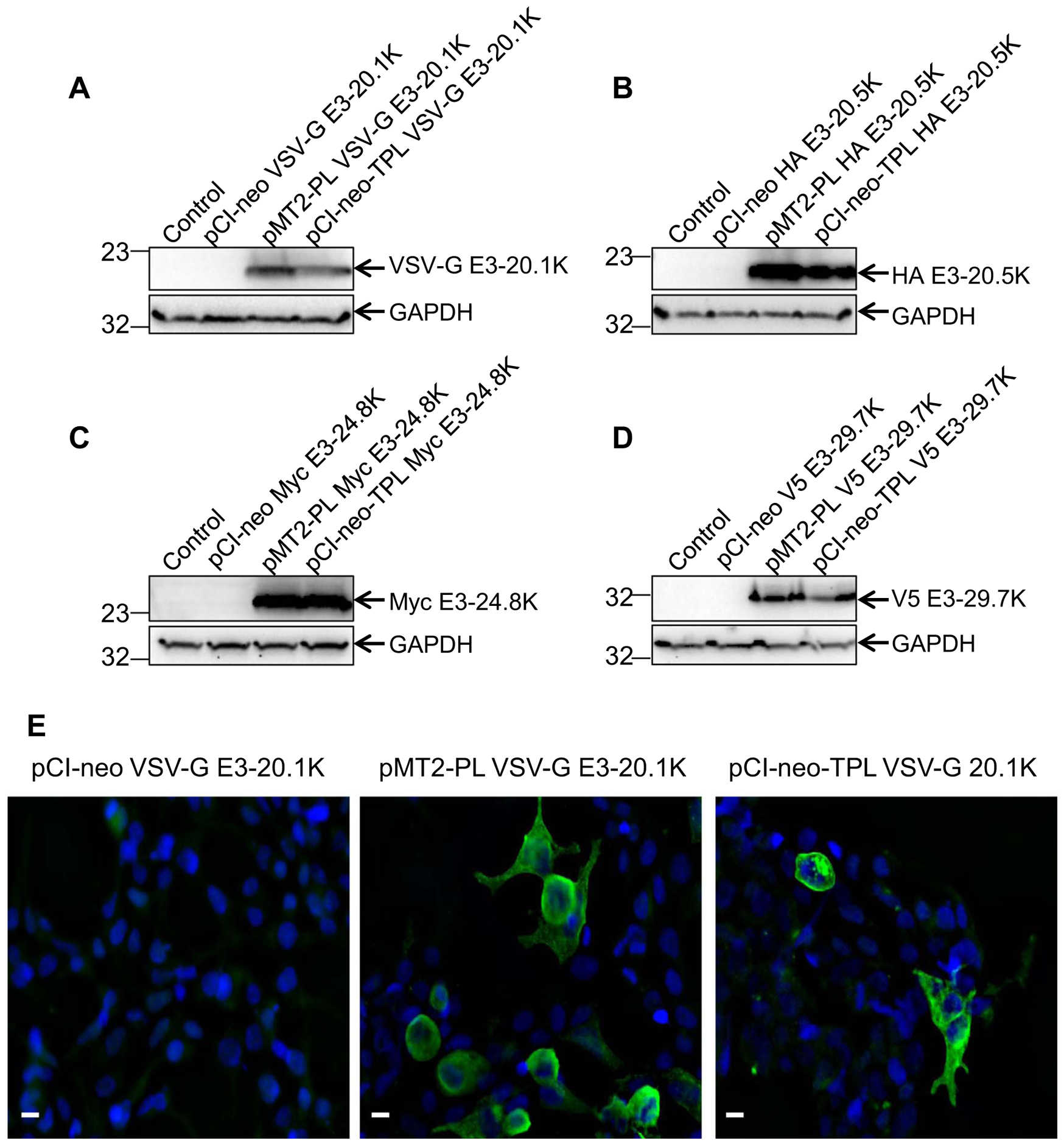
Insertion of the TPL sequence into the pCI-neo vector rescues the protein expression of HAdV-B and HAdV-E-specific E3 CR1 genes. 293T cells were either untransfected (Control) or transfected with the pCI-Neo, pMT2-PL or pCI-Neo-TPL constructs encoding N-terminally tagged (A) HAdV-B3 VSV-G E3–20.1K, (B) HAdV-B3 HA E3–20.5K, (C) HAdV-E4 Myc E3–24.8K, or (D) HAdV-E4 V5 E3–29.7K. To inhibit N-glycoslysation, cells were treated with 10 μg/ml of tunicamycin at 24 hpt. At 72 hpt, the cells were lysed and analyzed by WB using the primary antibody specific to the corresponding tag. E) 293T cells were transfected with the pCI-Neo, pMT2-PL, or pCI-Neo-TPL constructs encoding HAdV-B3 VSV-G E3–20.1K. At 72 hpt, immunofluorescence was performed with a VSV-G tag-specific antibody (green) and imaged at 40X magnification using Zeiss Axioskop epifluorescence microscope. DAPI staining of the nucleus is shown in blue. Scale bar =10 μm.
3.2. Species-specific HAdV-B and HAdV-E E3 CR1 transcripts require the 5′ TPL sequence for efficient protein translation in vitro
All E3 CR1 genes that have been examined to date, including HAdV-B3 E3–20.1K and E3–20.5K, and HAdV-E4 E3–24.8K and E3–29.7K, are expressed at late times post infection ((Hawkins and Wold, 1995a; Li and Wold, 2000; Tollefson et al., 1992; Windheim and Burgert, 2002; Frietze et al., 2010) Kajon laboratory, unpublished data). The late phase E3 CR1 transcripts carry the TPL sequence upstream of the ORF ((Chow et al., 1979; Frietze et al., 2010; Robinson et al., 2011); Kajon laboratory unpublished data). We, therefore, sub-cloned all four N-terminally tagged E3 genes of interest into pMT2-PL (Fig. 1). The four pMT2-PL constructs were transfected into 293T cells and E3 protein expression was evaluated by WB analysis. Abundant expression of both HAdV-B3- and HAdV-E4-specific E3 proteins was detected in transfected 293T cells with protein bands migrating at the molecular weight predicted for the corresponding unglycosylated polypeptides (Fig. 2A, B, C, and D).
We next evaluated the contribution of the TPL sequence to the observed dramatic change in expression of E3 proteins. We sub-cloned the mature HAdV-E4 complete TPL sequence into the pCI-neo constructs immediately upstream of the HAdV-B3 and HAdV-E4 E3 CR1 ORFs. Strikingly, E3 protein expression levels comparable to those obtained using pMT2-PL constructs were detected in 293T cells transfected with the new pCI-neo-TPL constructs (Fig. 2A, B, C, and D). The results from the WB analysis are in agreement with the immunofluorescence assay showing detectable protein expression of HAdV-B3 E3–20.1K in 293T cells transfected with the pMT2-PL and pCI-neo-TPL constructs but not in cells transfected with the pCI-neo constructs (Fig. 2E).
To evaluate the possibility that differences in transfection efficiencies between the pCI-neo, pCI-neo-TPL, and pMT2-PL vectors may have contributed to the observed differences in protein expression levels, we compared the transfection efficiencies of these three vectors. Monolayers of 293T cells were transfected individually with pCI-neo, pCI-neo-TPL, and pMT2-PL vectors encoding eGFP. At 72 hpt the proportion of cells expressing eGFP relative to cells not expressing eGFP was calculated for each of the conditions and compared. We observed no significant difference in the percentage of fluorescent cells between monolayers transfected with the pCI-neo, pCI-neo-TPL, and pMT2-PL constructs, F(2,12) =3.03, p > 0.05 (Fig. 3).
Fig. 3.

The transfection efficiencies of the pCI-Neo, pCI-Neo-TPL, and pMT2-PL vectors are similar in 293T cells. 293T cells plated in a 6-well dish were transfected individually with (A) pCI-neo, (B) pCI-neo-TPL, and (C) pMT2-PL mammalian expression vectors encoding eGFP. At 72 hpt, the cells were examined for green fluorescence and imaged at 40X magnification using an Zeiss Axioskop epifluorescence microscope. DAPI staining of the nucleus is shown in blue. (D) The transfection efficiency was evaluated by determining the percentage of fluorescent cells and is shown in a graphical representation. Error bars represent the standard error of the mean of 5 fields. No significant difference was detected by one-way ANOVA analysis, F(2,12) =3.03, p > 0.05. Scale bar =10 μm, NS = Not significant.
Similar results were obtained in HeLa OH cells electroporated with the expression constructs encoding tagged HAdV-B3 E3–20.1K or HAdV-E4 E3–24.8K polypeptides. No detectable protein expression was observed for the pCI-neo constructs by WB analysis while protein expression was evident for the pMT2-PL and pCI-Neo TPL constructs (Supplemental Fig 2).
Taken together, the results of these experiments indicate that the incorporation of the TPL sequence into the pCI-neo vector rescued— and is necessary for—the efficient expression of species-specific HAdV-B and HAdV-E E3 CR1 genes.
3.3. The presence of the TPL sequence increases the mRNA transcript levels of species-specific HAdV-B and HAdV-E E3 CR1 genes
To further understand the mechanism by which the TPL sequence rescues E3 CR1 protein expression, we examined the transcript levels of each of the E3 genes expressed from the corresponding pCI-neo, pCI-neo-TPL, and pMT2-PL constructs. Monolayers of 293T cells were transfected with the pCI-neo, pCI-neo-TPL, and pMT2-PL vectors encoding N-terminus tagged HAdV-B3 E3–20.1K and E3–20.5K, and HAdV-E4 E3–24.8K and E3–29.7K. At 72 hpt, total RNA was extracted and mRNA was reverse-transcribed to cDNA. PCR analysis was performed using primers designed to specifically amplify sequences within the respective E3 ORF. As shown in Fig. 4, after reverse transcription and 20 cycles of PCR amplification a readily apparent increase in the levels of E3 CR1 transcripts was detectable in cells transfected with pCI-neo-TPL and pMT2-PL constructs relative to the levels detected in cells transfected with the corresponding pCI-neo constructs for all four genes examined.
Fig. 4.

The TPL sequence increases the levels HAdV-B- and HAdV-E-specific E3 CR1 mRNA. 293T cells were either untransfected (Control) or transfected individually with the pCI-Neo, pMT2-PL, and pCI-Neo-TPL mammalian expression vectors encoding (A) HAdV-B3 VSV-G E3–20.1K (B) HAdV-B3 HA E3–20.5K, (C) HAdV-E4 Myc E3–24.8K, and (D) HAdV-E4 V5 E3–29.7K. At 72 hpt, total RNA was extracted, mRNA was reverse-transcribed to cDNA, and PCR was performed for a total of 20 cycles with primer pairs designed to specifically amplify E3–20.1K, E3–20.5K, E3–24.8K, and E3–29.7K cDNA. The constitutively expressed Rig/S15 gene was amplified as a housekeeping control. The RT-PCR products were quantified by densitometry analysis of EtBr-stained gel bands using the Fiji platform (https://imagej.net/Fiji). The densitometric values for the target E3 CR1 mRNA were normalized to those of Rig/S15 in the same samples and the resulting values plotted using arbitrary units (AU). The densitometry data are shown for each E3 CR1 gene below the corresponding gel image.
4. Discussion
HAdV types classified within species HAdV-B and HAdV-E cause acute respiratory disease of variable severity in infants and adults (Barraza et al., 1999; Chen et al., 2015; Lin et al., 2015; Siminovich and Murtagh, 2011; Tullo and Higgins, 1980). Despite their clinical importance, little is known about the molecular biology and pathobiology of these viruses. Historically, most studies have focused on species HAdV-C, hindering the understanding of the molecular determinants of pathogenicity that are unique to other HAdV species. At the genomic level, the most striking differences between HAdV-C and other species map to the E3 region that encodes non-structural proteins. The functions of only a few E3 proteins are known to the present. The majority of these proteins are modulators of the host immune response to infection (Deryckere and Burgert, 1996; Hilgendorf et al., 2003; Lichtenstein et al., 2004; Windheim et al., 2013). Strikingly, the functions of most of the species-specific E3 CR1 proteins including HAdV-B3 E3–20.1K and E3–20.5K, and HAdV-E4 E3–24.8K and E3–29.7K remain unknown.
Based on the difficulties we have encountered in expressing the full-length HAdV-B and HAdV-E-specific E3 proteins it is reasonable to speculate that the gap in the knowledge regarding the function of species-specific repertoires of E3 CR1 genes may be in part the result of some intrinsic properties of these genes that hamper their expression at adequate levels using traditional mammalian expression vectors.
The use of expression vectors carrying the CMV promoter, such as pCI-neo, is common practice for studies addressing the functional characterization of viral proteins (Lindwasser et al., 2008; Lu and Zhou, 2013). Therefore, we initially sub-cloned our E3 CR1 genes of interest into pCI-neo with the goal of expressing the encoded proteins ectopically for studies of sub-cellular localization and protein-protein interaction. Intriguingly, we were unable to detect any of these proteins by WB analysis. Similar attempts using pCEP4 constructs also failed.
Based on the fact that the E3 CR1 protein expression is barely detectable at early times post infection, and that late E3 transcripts carry the TPL sequence, we hypothesized that the TPL sequence is necessary for the efficient expression of E3 CR1 genes.
The TPL sequence is a 200 nucleotide non-coding sequence made up of three exons (leaders 1 through 3, L1, L2 and L3) that is spliced onto the 5′ end (upstream of the ORF) of most late viral mRNAs (Chow and Broker, 1978; Berget et al., 1977; Beveren et al., 1981; Zain et al., 1979). The presence of the TPL sequence at the 5′ end of the late viral mRNAs eliminates the requirement of the cap-binding protein eIF4F. eIF4F is otherwise essential for initiation of protein translation from mRNAs that possess 5′ 7-methyl guanosine cap (7mG) (Dolph et al., 1990, 1988). The TPL sequence consists of unstructured 25–44 nucleotides at the 5′ region followed by stable hairpin structures. While the unstructured region of the TPL sequence facilitates binding to ribosomes, the stable hairpins are essential for ribosomal shunting (Xi et al., 2004; Zhang et al., 1989). During the late phase of infection, the TPL sequence in conjunction with other viral proteins orchestrates the preferential translation of viral proteins by ribosome shunting (Xi et al., 2004, 2005; Yueh and Schneider, 2000).
A number of studies have shown that the TPL sequence influences protein expression by 1) having a direct effect on protein translation (Berkner and Sharp, 1985), 2) regulating the transcription of the gene (Alonso-Caplen et al., 1988; Davis et al., 1985), and 3) facilitating efficient export of mRNA out of the nucleus into the cytoplasm (Huang and Flint, 1998). Taking advantage of these features, several eukaryotic expression vectors developed during the 1980’s and 1990’s incorporated either portions of or the full length TPL sequence upstream of the cloned ORF (Kaufman, 1985; Kaufman et al., 1991; Sheay et al., 1993). Our detection of increased levels of both translated protein and mRNA transcripts in the presence of TPL sequence in two different cell lines (Fig. 2, Fig. 4 and Supp. Fig. 2) corroborate previous observations.
Interestingly, the ectopic expression of E3 CR1 genes using pMT2 was reported by the Wold laboratory for HAdV-E4 E3–29.7K in 2000 (Li and Wold, 2000) and later for HAdV-C ADP (Ying and Wold, 2003) although the publications did not specifically attribute any major significance to the accomplishment, or mentioned any particular difficulties encountered for the expression of these genes. Our success expressing HAdV-B3 E3–20.1K and E3–20.5K, and HAdV-E4 E3–24.8K and E3–29.7K from similar constructs support the identification of the TPL sequence as a regulatory element required for the efficient translation of CR1 transcripts. Interestingly, we were able to detect E3 CR1 protein expression using constructs encoding either a partial (L2 and L3; pMT2-PL) or the full length TPL sequence (L1/L2/L3; pCI-neo-TPL), indicating that the latter two leaders are sufficient for E3 CR1 protein expression.
Like the parental vector pMT2, pMT2-PL carries the HAdV-C2 VA RNA gene encoding VA RNAI. VA RNAI contributes to the selective translation of viral protein late during adenovirus infection and also can stimulate the protein expression of transfected genes (Ma and Mathews, 1996; Schneider et al., 1984; Svensson and Akusjarvi, 1985). Additionally, VA RNAI has been shown to increase the translation of some genes from mammalian expression vectors (Kaufman et al., 1989). Although the contribution of VA RNAI to the successful expression of the E3 CR1 genes from the pMT2-PL constructs cannot be ruled out, our data from transfections with pCI-neo-TPL constructs suggest that only the presence of the TPL sequence upstream of the E3 CR1 ORFs is required for ectopic protein expression in vitro (Fig. 2, Fig. 4, and Supp. Fig. 2).
Why TPL is necessary for the expression of the HAdV-B3 E3–20.1K and E3–20.5K and HAdV-E4 E3–24.8K and E3–29.7K in vitro remains unclear and warrants future research efforts. Interestingly, E3 CR1 genes encoding larger proteins such as HAdV-D E3–49K can be expressed from the pSG5 expression vector which carries the SV40 promoter (Windheim and Burgert, 2002). Other studies have shown that E3 CR1 genes fused to sequences encoding large tags such as GFP or human IgG1 can be ectopically expressed in the absence of the TPL sequence (Frietze et al., 2010; Martinez-Martin et al., 2016).
Our study resurfaces the TPL sequence and the mammalian expression vectors that include it as valuable molecular tools that may be useful for studying proteins that are difficult to express in vitro. The recent examination of the extracellular interactome of various HAdV E3 CR1 protein N-terminal ectodomains using a human extracellular protein microarray (Martinez-Martin et al., 2016) identified a putative role for many of these proteins in immunomodulation. Our findings may enable/facilitate the expression of the corresponding full-length proteins for a more physiologically relevant approach to investigate their function and thus encourage new research initiatives addressing the characterization of E3 CR1 proteins, and the study of the mechanisms used by adenoviruses to fine-tune their levels of expression at late times post infection.
Supplementary Material
Acknowledgements
We thank William Wold and Ann Tollefson from Saint Louis University for the generous gift of the pMT2 vector, and Daryl Lamson and Kirsten St. George from NY State Department of Health, Wadsworth Center, for the sequencing of pMT2-PL. This work was supported by intramural funding provided by Lovelace Respiratory Research Institute. C.R.B. is supported by University of New Mexico Infectious Diseases and Inflammation NIH Training Grant T32-AI007538.
Footnotes
Conflict of interest statement
No conflicts declared.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.virol.2017.02.021.
References
- Alonso-Caplen FV, Katze MG, Krug RM, 1988. Efficient transcription, not translation, is dependent on adenovirus tripartite leader sequences at late times of infection. J. Virol 62, 1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraza EM, Ludwig SL, Gaydos JC, Brundage JF, 1999. Reemergence of adenovirus type 4 acute respiratory disease in military trainees: report of an outbreak during a lapse in vaccination. J. Infect. Dis 179, 1531–1533. [DOI] [PubMed] [Google Scholar]
- Belak S, Virtanen A, Zabielski J, Rusvai M, Berencsi G, Pettersson U, 1986. Subtypes of bovine adenovirus type 2 exhibit major differences in region E3. Virology 153, 262–271. [DOI] [PubMed] [Google Scholar]
- Beveren C, Maat J, Dekker B, van Ormondt H, 1981. The nucleotide sequence of th egene for protein IVa2 and of the 5′ leader segment of the major late mRNAs of adenovirus type 5. Gene 16 (1–3), 179–189. [DOI] [PubMed] [Google Scholar]
- Berget SM, Moore C, Sharp PA, 1977. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Rev. Med Virol 10, 356–362. [PubMed] [Google Scholar]
- Berk AJ, 2013. Adenoviridae In: Knipe DM, Howley PM (Eds.), Fields Virology6 ed. Lippincott Williams & Wilkins, Philadelphia, 1704–1731. [Google Scholar]
- Berkner KL, Sharp PA, 1985. Effect of the tripartite leader on synthesis of a non-viral protein in an adenovirus 5 recombinant. Nucleic Acids Res. 13, 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusch JH, Deryckere F, Windheim M, Ruzsics Z, Arnberg N, Adrian T, Burgert HG, 2002. The novel early region 3 protein E3/49K is specifically expressed by adenoviruses of subgenus D: implications for epidemic keratoconjunctivitis and adenovirus evolution. Virology 296, 94–106. [DOI] [PubMed] [Google Scholar]
- Burgert HG, Blusch JH, 2000. Immunomodulatory functions encoded by the E3 transcription unit of adenoviruses. Virus Genes 21, 13–25. [PubMed] [Google Scholar]
- Chen M, Zhu Z, Huang F, Liu D, Zhang T, Ying D, Wu J, Xu W, 2015. Adenoviruses associated with acute respiratory diseases reported in Beijing from 2011 to 2013. PLoS One 10, e0121375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L, Broker T, 1978. The spliced structures of adenovirus 2 fiber message and other late mRNAs. Cell 15, 497–510. [DOI] [PubMed] [Google Scholar]
- Chow LT, Broker TR, Lewis JB, 1979. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J. Mol. Biol 134, 265–303. [DOI] [PubMed] [Google Scholar]
- Davis AR, Kostek B, Mason BB, Hsiao CL, Morin J, Dheer SK, Hung PP, 1985. Expression of hepatitis B surface antigen with a recombinant adenovirus. Proc. Natl. Acad. Sci. USA 82, 7560–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AJ, Akter P, Cunningham C, Dolan A, Addison C, Dargan DJ, Hassan-Walker AF, Emery VC, Griffiths PD, Wilkinson GW, 2003a. Homology between the human cytomegalovirus RL11 gene family and human adenovirus E3 genes. J. Gen. Virol 84, 657–663. [DOI] [PubMed] [Google Scholar]
- Davison AJ, Benko M, Harrach B, 2003b. Genetic content and evolution of adenoviruses. J. Gen. Virol 84, 2895–2908. [DOI] [PubMed] [Google Scholar]
- Deryckere F, Burgert HG, 1996. Early region 3 of adenovirus type 19 (subgroup D) encodes an HLA-binding protein distinct from that of subgroups B and C. J. Virol 70, 2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph PJ, Huang JT, Schneider RJ, 1990. Translation by the adenovirus tripartite leader: elements which determine independence from cap-binding protein complex. J. Virol 64, 2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph PJ, Racaniello V, Villamarin A, Palladino F, Schneider RJ, 1988. The adenovirus tripartite leader may eliminate the requirement for cap-binding protein complex during translation initiation. J. Virol 62, 2059–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frietze KM, Campos SK, Kajon AE, 2010. Open reading frame E3–10.9K of subspecies B1 human adenoviruses encodes a family of late orthologous proteins that vary in their predicted structural features and subcellular localization. J. Virol 84, 11310–11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage E, Gerd Liebert U, Bergs S, Ganzenmueller T, Heim A, 2015. Human mastadenovirus type 70: a novel, multiple recombinant species D mastadenovirus isolated from diarrhoeal faeces of a haematopoietic stem cell transplantation recipient. J. Gen. Virol 96, 2734–2742. [DOI] [PubMed] [Google Scholar]
- Harrach BBM, Both GW, Brown M, Davison AJ, Echavarría M, Hess M, Jones MS, Kajon A, Lehmkuhl HD, Mautner V, Mittal SK, Wadell G, 2012. Adenoviridae In: King AM, Adams MJ, Carstens EB, Lefkowitz EJ (Eds.), Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses.. Elsevier Academic Press, San Diego, 125–141. [Google Scholar]
- Hawkins LK, Wold WS, 1995a. A 20,500-Dalton protein is coded by region E3 of subgroup B but not subgroup C human adenoviruses. Virology 208, 226–233. [DOI] [PubMed] [Google Scholar]
- Hawkins LK, Wold WS, 1995b. The E3–20.5K membrane protein of subgroup B human adenoviruses contains O-linked and complex N-linked oligosaccharides. Virology 210, 335–344. [DOI] [PubMed] [Google Scholar]
- Hilgendorf A, Lindberg J, Ruzsics Z, Honing S, Elsing A, Lofqvist M, Engelmann H, Burgert HG, 2003. Two distinct transport motifs in the adenovirus E3/10.4–14.5 proteins act in concert to down-modulate apoptosis receptors and the epidermal growth factor receptor. J. Biol. Chem 278, 51872–51884. [DOI] [PubMed] [Google Scholar]
- Huang W, Flint SJ, 1998. The tripartite leader sequence of subgroup C adenovirus major late mRNAs can increase the efficiency of mRNA export. J. Virol 72, 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ, 1985. Identification of the components necessary for adenovirus translational control and their utilization in cDNA expression vectors. Proc. Natl. Acad. Sci. USA 82, 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ, Davies MV, Pathak VK, Hershey JW, 1989. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol. Cell Biol 9, 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ, Davies MV, Wasley LC, Michnick D, 1991. Improved vectors for stable expression of foreign genes in mammalian cells by use of the untranslated leader sequence from EMC virus. Nucleic Acids Res. 19, 4485–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TJ Jr., Lewis AM Jr., 1973. Use of nondefective adenovirus-simian virus 40 hybrids for mapping the simian virus 40 genome. J. Virol 12, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotha PL, Sharma P, Kolawole AO, Yan R, Alghamri MS, Brockman TL, Gomez-Cambronero J, Excoffon KJ, 2015. Adenovirus entry from the apical surface of polarized epithelia is facilitated by the host innate immune response. PLoS Pathog. 11, e1004696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wold WS, 2000. Identification and characterization of a 30K protein (Ad4E3–30 K) encoded by the E3 region of human adenovirus type 4. Virology 273, 127–138. [DOI] [PubMed] [Google Scholar]
- Lichtenstein DL, Toth K, Doronin K, Tollefson AE, Wold WS, 2004. Functions and mechanisms of action of the adenovirus E3 proteins. Int. Rev. Immunol 23, 75–111. [DOI] [PubMed] [Google Scholar]
- Lin YC, Lu PL, Lin KH, Chu PY, Wang CF, Lin JH, Liu HF, 2015. Molecular epidemiology and phylogenetic analysis of human adenovirus caused an outbreak in Taiwan during 2011. PLoS One 10, e0127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwasser OW, Smith WJ, Chaudhuri R, Yang P, Hurley JH, Bonifacino JS, 2008. A diacidic motif in human immunodeficiency virus type 1 Nef is a novel determinant of binding to AP-2. J. Virol 82, 1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linne T, 1992. Differences in the E3 regions of the canine adenovirus type 1 and type 2. Virus Res. 23, 119–133. [DOI] [PubMed] [Google Scholar]
- Lu HZ, Zhou JH, 2013. Hepatitis B virus X protein up-regulates tumor necrosis factor-alpha expression in cultured mesangial cells via ERKs and NF-kappab pathways. Asian Pac. J. Trop. Biomed 3, 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Mathews MB, 1996. Structure, function, and evolution of adenovirus-associated RNA: a phylogenetic approach. J. Virol 70, 5083–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon MJ, 2011. Vectors bicistronically linking a gene of interest to the SV40 large T antigen in combination with the SV40 origin of replication enhance transient protein expression and luciferase reporter activity. Biotechniques 51, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Martin N, Ramani SR, Hackney JA, Tom I, Wranik BJ, Chan M, Wu J, Paluch MT, Takeda K, Hass PE, Clark H, Gonzalez LC, 2016. The extracellular interactome of the human adenovirus family reveals diverse strategies for immunomodulation. Nat. Commun 7, 11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Rajaiya J, Zhou X, Singh G, Dyer DW, Chodosh J, 2011. The E3 CR1-gamma gene in human adenoviruses associated with epidemic keratoconjunctivitis. Virus Res 160, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Singh G, Lee JY, Dehghan S, Rajaiya J, Liu EB, Yousuf MA, Betensky RA, Jones MS, Dyer DW, Seto D, Chodosh J, 2013. Molecular evolution of human adenoviruses. Sci. Rep 3, 1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RJ, Weinberger C, Shenk T, 1984. Adenovirus VAI RNA facilitates the initiation of translation in virus-infected cells. Cell 37, 291–298. [DOI] [PubMed] [Google Scholar]
- Sheay W, Nelson S, Martinez I, Chu TH, Bhatia S, Dornburg R, 1993. Downstream insertion of the adenovirus tripartite leader sequence enhances expression in universal eukaryotic vectors. Biotechniques 15, 856–862. [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovich M, Murtagh P, 2011. Acute lower respiratory tract infections by adenovirus in children: histopathologic findings in 18 fatal cases. Pedia. Dev. Pathol 14, 214–217. [DOI] [PubMed] [Google Scholar]
- Sirena D, Ruzsics Z, Schaffner W, Greber UF, Hemmi S, 2005. The nucleotide sequence and a first generation gene transfer vector of species B human adenovirus serotype 3. Virology 343, 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C, Akusjarvi G, 1985. Adenovirus VA RNAI mediates a translational stimulation which is not restricted to the viral mRNAs. EMBO J. 4, 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ, Wold WS, 1996. The adenovirus death protein (E3–11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J. Virol 70, 2296–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson AE, Scaria A, Saha SK, Wold WS, 1992. The 11,600-MW protein encoded by region E3 of adenovirus is expressed early but is greatly amplified at late stages of infection. J. Virol 66, 3633–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullo AB, Higgins PG, 1980. An outbreak of adenovirus type 4 conjunctivitis. Br. J. Ophthalmol 64, 489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windheim M, Burgert HG, 2002. Characterization of E3/49K, a novel, highly glycosylated E3 protein of the epidemic keratoconjunctivitis-causing adenovirus type 19a. J. Virol 76, 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windheim M, Hilgendorf A, Burgert HG, 2004. Immune evasion by adenovirus E3 proteins: exploitation of intracellular trafficking pathways. Curr. Top. Microbiol Immunol 273, 29–85. [DOI] [PubMed] [Google Scholar]
- Windheim M, Southcombe JH, Kremmer E, Chaplin L, Urlaub D, Falk CS, Claus M, Mihm J, Braithwaite M, Dennehy K, Renz H, Sester M, Watzl C, Burgert HG, 2013. A unique secreted adenovirus E3 protein binds to the leukocyte common antigen CD45 and modulates leukocyte functions. Proc. Natl. Acad. Sci. USA 110, E4884–E4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold WS, Tollefson AE, Hermiston TW, 1995. E3 transcription unit of adenovirus.Curr. Top. Microbiol Immunol 199 (Pt 1), 237–274. [DOI] [PubMed] [Google Scholar]
- Xi Q, Cuesta R, Schneider RJ, 2004. Tethering of eIF4G to adenoviral mRNAs by viral 100k protein drives ribosome shunting. Genes Dev. 18, 1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Cuesta R, Schneider RJ, 2005. Regulation of translation by ribosome shunting through phosphotyrosine-dependent coupling of adenovirus protein 100k to viral mRNAs. J. Virol 79, 5676–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying B, Wold WS, 2003. Adenovirus ADP protein (E3–11.6 K), which is required for efficient cell lysis and virus release, interacts with human MAD2B. Virology 313, 224–234. [DOI] [PubMed] [Google Scholar]
- Yueh A, Schneider RJ, 2000. Translation by ribosome shunting on adenovirus and hsp70 mRNAs facilitated by complementarity to 18S rRNA. Genes Dev. 14, 414–421. [PMC free article] [PubMed] [Google Scholar]
- Zain S, Sambrook J, Roberts RJ, Keller W, Fried M, Dunn AR, 1979. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell 16, 851–861. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dolph PJ, Schneider RJ, 1989. Secondary structure analysis of adenovirus tripartite leader. J. Biol. Chem 264, 10679–10684. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


