Abstract
Aim:
This study explored whether inherited variants in genes causing the hereditary neuropathy condition Charcot–Marie–Tooth disease are associated with sensitivity to paclitaxel-induced peripheral neuropathy (PN).
Patients & methods:
Hereditary neuropathy genes previously associated with risk of paclitaxel-induced PN were sequenced in paclitaxel-treated patients. Eight putative genetic predictors in five hereditary neuropathy genes (ARHGEF10, SBF2, FGD4, FZD3 and NXN) were tested for association with PN sensitivity after accounting for systemic exposure and clinical variables.
Results:
FZD3 rs7833751, a proxy for rs7001034, decreased PN sensitivity (additive model, β = -0.41; 95% CI: -0.66 to -0.17; p = 0.0011). None of the other genetic predictors were associated with PN sensitivity.
Conclusion:
Our results support prior evidence that FZD3 rs7001034 is protective of PN and may be useful for individualizing paclitaxel treatment to prevent PN.
Keywords: : ARHGEF10, FZD3, paclitaxel, peripheral neuropathy, pharmacogenomics
Paclitaxel is a critical component of combination chemotherapy treatment for patients with breast cancer [1]. About 25% of patients treated with weekly paclitaxel experience ≥ grade 2 peripheral neuropathy (PN) based on the National Cancer Institute Common Terminology Criteria for Adverse Events grading scale [2]. PN causes symptoms including numbness, tingling, allodynia, hyperalgesia or loss of proprioception in the hands or feet [3,4]. In order to avoid severe PN that can have long-term effects [5], nearly a quarter of patients require dose reductions, delays or discontinuations, which can decrease therapy effectiveness [6–9]. As there are no agents for preventing PN or treating nonpainful PN symptoms [9,10], identification of predictive PN biomarkers could help identify patients likely to experience PN, so their treatment can be adjusted accordingly. Previous research from our group and others have demonstrated that the primary determinant of PN is the patient’s systemic paclitaxel exposure, as estimated by the amount of time (in hours) the patient’s systemic concentration remains above 0.05 μmol/l (‘time above threshold’, Tc>0.05) [7,11]. However, up to 10% of patients treated with individualized paclitaxel doses to achieve a target paclitaxel exposure still experience severe PN [12]. These patients must be inherently PN-sensitive, which may be related to their inherited genetics [13].
Charcot–Marie–Tooth (CMT) disease, the most common inherited PN condition, is caused by more than 1000 inherited variants in 80 genes responsible for various processes in neuronal development and function [14–17]. CMT patients have been reported to be particularly susceptible to paclitaxel-induced PN [18]. Genome-wide association studies (GWAS) conducted in large cohorts of paclitaxel-treated patients have repeatedly identified variants in genes linked to CMT that may affect PN risk [19–22]. Lower PN risk were observed in patients carrying variants in ARHGEF10 [19], FZD3 [21] and NXN [22], which have a role in peripheral nerve myelination [15], neurite growth [17] and neuronal development [23], respectively. Higher PN risk was reported in carriers of variants in EPHA receptors, FGD4 [21] and SBF2 [20], which are involved in synaptogenesis, neuronal regeneration following nerve injury [24,25], and autosomal recessive demyelinating forms of CMT disease (CMT subtype 4) [26,27].
Despite this vast biomarker discovery effort, no genetic predictors of PN have been validated in independent replication attempts [28,29], which is necessary prior to clinical translation. The inability to validate genetic PN biomarkers is likely due to the multifactorial nature of PN and the failure of prior studies to account for variability in systemic paclitaxel exposure, which is the critical determinant of paclitaxel-induced PN [7,11,12,30]. Accounting for interindividual pharmacokinetic variability is the only way to isolate a patient’s true PN-sensitivity, which can then be used as a quantitative end point for pharmacogenetic association testing. We previously used our PN sensitivity model [7], which accounts for cumulative paclitaxel systemic exposure and clinical factors, to demonstrate that EPHA5 rs7349683 increases PN sensitivity [31]. Since PN sensitivity is likely a polygenic trait [32], the objective of this study was to use a similar approach to investigate whether inherited variants in five CMT-linked genes that have been previously associated with PN risk are associated with PN sensitivity after accounting for cumulative systemic paclitaxel exposure.
Materials & methods
Patients, PN & paclitaxel pharmacokinetics
UMCCC 2014.002 (clinicaltrials.gov; NCT0233815) is a previously reported prospective observational clinical study to investigate predictors of PN [7,31]. Participants enrolled in this clinical trial were >18 years old, with stage I–III or oligometastatic breast cancer, without PN or previous exposure to neurotoxic chemotherapy, and scheduled to receive 12 weekly infusions of paclitaxel 80 mg/m2 for curative treatment of breast cancer. Detailed information about patient demographics, cancer treatment, paclitaxel pharmacokinetic sample collection and analysis, neuropathy assessment, germline DNA collection and sequencing of CMT-linked gene have been reported [7,31] and are briefly described below. All participants in this study signed written informed consent. This study was approved by the University of Michigan IRBMed and was conducted in accordance with recognized ethical guidelines.
Patients answered the quality of life questionnaire chemotherapy-induced peripheral neuropathy (CIPN20) from the European Organisation for Research and Treatment of Cancer [33] before their first paclitaxel dose and weekly until the end of treatment. The raw scores from eight sensory items (numbness, tingling and burning/shooting pain, difficulty in standing or walking and difficulty in distinguishing between hot and cold water), excluding the ninth sensory item on ototoxicity, were summed and linearly translated to a 0–100 scale (CIPN8) [7,31] with a higher CIPN8 score indicating greater PN symptoms.
Blood samples were collected 16–26 h after the start of the paclitaxel infusion to measure plasma paclitaxel concentration via liquid chromatography–mass spectrometry (LCMS) by the University of Michigan College of Pharmacy Pharmacokinetics Core (MI, USA). This single measurement was used to estimate time above threshold (Tc>0.05) using a previously published population-pharmacokinetic model [34,35].
CMT gene sequencing & identification of CMT genes of interest
A whole blood sample was collected prior to the first infusion for isolation of germline DNA. Targeted exonic sequencing of genes known to cause CMT was conducted followed by alignment to a reference genome (grch37), as previously described [19,29,31]. Although exonic regions were targeted, some nonexonic regions were sequenced as a byproduct [36]. Identified SNV were ranked by variant quality score recalibration according to the variant quality log-odds, and only SNV that had a specificity of >99.9% and sensitivity of >90% were included [19,31]. The annotations included are based on Ensemble GRCh37.75.
From the CMT genes sequenced, genes and SNVs of interest were selected based on a literature review of previously published pharmacogenetic studies of paclitaxel-induced PN. For ARHGEF10 two, individual SNVs (rs9657362 and rs17683288) and the overall SNV gene burden were previously reported to decrease PN risk [19,29]. For SBF2, five individual SNVs (rs149501654, rs117957652, rs141368249, rs146987383 and rs7102464), and SNV gene burden have been reported to increase PN risk in African–American patients [20]. A GWAS reported rs10771973 in FGD4 and rs7001034 in FZD3 were associated with increased and decreased PN, respectively [21]. Finally, rs910920 in NXN was reported to be protective of PN [22]. These ten SNVs in five CMT-linked genes that were previously reported to be associated with PN occurrence were selected as candidate genetic predictors for this analysis.
Genetic data cleaning & selection of genetic predictors
The following describes the process for selecting which potential genetic predictors of chemotherapy-induced neuropathy to include in the analysis. Starting with the five CMT-linked genes described above, many potential genetic predictors, either individual SNV or gene-burden tests, were considered. Most of these potential genetic predictors were rejected prior to analysis based on a requirement that any tested predictor has at least ten patients in each genotype group. The ten-patient threshold was used to ensure that each group had sufficient patients for meaningful association testing but was not based on a formal power analysis. After this filtering process, pharmacogenetic association testing was only conducted for the remaining eight genetic predictors described in this manuscript.
Each SNV analysis was conducted either based on the presence of a single variant or the total number of variant alleles carried by the patient, based partially on the previously reported genetic effect and ensuring an adequate number of patients for analysis (>10). Six candidate SNVs of interest that were previously associated with PN risk (rs149501654, rs141368249, rs146987383, rs10771973, rs7001034 and rs910920) were not detected by our sequencing. HaploReg was used to identify proxy variants in linkage disequilibrium (LD) >0.8 in the American population to be analyzed as tagging SNVs [37], where possible.
Similarly, for gene-based analyses, patients were classified by the presence of any missense variant, any functional variant, or by the total number of functional variant alleles the patient carried. Functional variants refer to genetic variants that are predicted to affect protein activity, including by affecting protein expression. Whether a variant has functional consequences was determined by three predictive bioinformatics tools: combined annotation-dependent depletion (CADD) [38,39], GWAVA [40] and PROVEAN [41], similar to our previous analysis of EPHA genes [31]. Coding variants were functional if they had CADD PHRED-like scaled C-score rankings ≥15 and PROVEAN scores <-2.5. Noncoding variants were functional if their CADD rankings ≥15 and GWAVA transcription start site scores ≥0.5. Functional noncoding variants that were located upstream or downstream of the candidate gene were only included, if they were an expression quantitative trait loci (eQTL) (p < 0.005) of their target gene in the GTex database [42]. Analyses of the total number of functional variants include both coding and noncoding functional variants. HaploReg was used to ensure each SNV included within any analysis was independent (LD <0.8), to prevent double counting.
Since ARHGEF10 SNV rs9657362 and rs17683288 have been replicated as protective for PN [19,29], our a priori defined primary hypothesis was that patients carrying an rs9657362 or rs17683288 variant have decreased PN sensitivity (#1 in Table 3). After genetic data cleaning, seven additional genetic predictors were selected for secondary analyses, each with a prespecified direction of effect on PN sensitivity: carrying any ARHGEF10 missense SNV (2), carrying SBF2 rs117957652 or rs7102464 (3), the number of functional SBF2 SNV alleles a patient carried (4), carrying FGD4 rs10844253 (tag SNV for rs10771973) (5), carrying any functional FGD4 SNV (6), the number of FZD3 rs7833751 alleles a patient carried (tag SNV for rs7001034) (7) and carrying any functional NXN SNV (8).
Table 3. . Genetic associations with peripheral neuropathy sensitivity.
| Entry | Genetic predictor | Genetic predictor distribution | Expected effect on PN Sensitivity | Beta† | 95% CI | p-value |
|---|---|---|---|---|---|---|
| 1 | ARHGEF10: carrying rs9657362 or rs17683288 | Yes: 21/58 = 36.2% | Lower | -0.27 | -0.68–0.14 | 0.20 |
| 2 | ARHGEF10: carrying any missense SNV | Yes: 30/58 = 51.7% | Lower | 0.23 | -0.16–0.63 | 0.25 |
| 3 | SBF2: carrying rs117957652 or rs7102464 | Yes: 18/58 = 31.0% | Higher | -0.32 | -0.75–0.10 | 0.14 |
| 4 | SBF2: carrying more functional SNV alleles | 0: 21/58 = 36.2% 1: 26/58 = 44.8% 2: 11/58 = 19.0% |
Higher | 0.12 | 0.15–0.40 | 0.39 |
| 5 | FGD4: carrying rs10844253‡ | Yes: 36/58 = 62.1% | Higher | 0.31 | -0.09–0.71 | 0.13 |
| 6 | FGD4: carrying any functional SNV | Yes: 17/58 = 29.3% | Higher | -0.07 | -0.51–0.36 | 0.75 |
| 7 | FZD3: carrying more rs7833751‡ alleles | 0: 13/58 = 22.4% 1: 20/58 = 34.5% 2: 25/58 = 43.1% |
Lower | -0.41§ | -0.66 to -0.17§ | 0.0011§ |
| 8 | NXN: carrying any functional SNV | Yes: 42/58 = 72.4% | Lower | -0.23 | -0.66–0.20 | 0.29 |
Positive β-coefficient indicates higher PN sensitivity, negative indicates lower PN sensitivity. Bold indicates statistical significance (p < 0.05).
These alleles are tagging SNV of the variant of interest (FGD4: rs10771973 and FZD3: rs7001034).
Statistical significance p < 0.05.
PN: Peripheral neuropathy.
Statistical analysis
A previously developed PN sensitivity prediction model [7] was used to analyze the contribution of our eight genetic predictors with PN severity, as defined by the square root of CIPN8. This PN sensitivity model includes baseline CIPN8 (0–100), cumulative dose (mg/m2, actual-weight body surface area adjusted), relative dose intensity (proportion of cumulative planned dose received to expected cumulative dose, to account for delays and decreases), measured systemic paclitaxel exposure (Tc>0.05) and an interaction term with Tc>0.05 and cumulative dose. Each putative genetic predictor was introduced into the model independently to determine whether it has a significant contribution to PN sensitivity, using an uncorrected significance threshold (α = 0.05). Significant associations were then tested in the model including the EPHA5 SNV rs7349683 to investigate whether these were independent genetic predictors of PN sensitivity [31]. All analyses were conducted in SAS v.9.4.
Results
Patient demographics & clinical data
Detailed information about the 58 patients enrolled in this prospective cohort study that are included in this secondary pharmacogenetic analysis (Figure 1) has been previously reported [7,31]. Patients included in this analysis had a mean age of 52.5 years (range: 28–71), mean body surface area of 1.83 m2 (standard deviation [SD]: 0.21) and 93.1% were Caucasian (Table 1). The average Tc>0.05 was 10.72 h (SD: 2.73). As previously reported, CIPN8 was low at baseline (mean = 1.29 ± 3.04) and increased throughout treatment (mean maximum CIPN8 = 13.26 ± 1.76).
Figure 1. . Patient flow from observational study into this analysis.
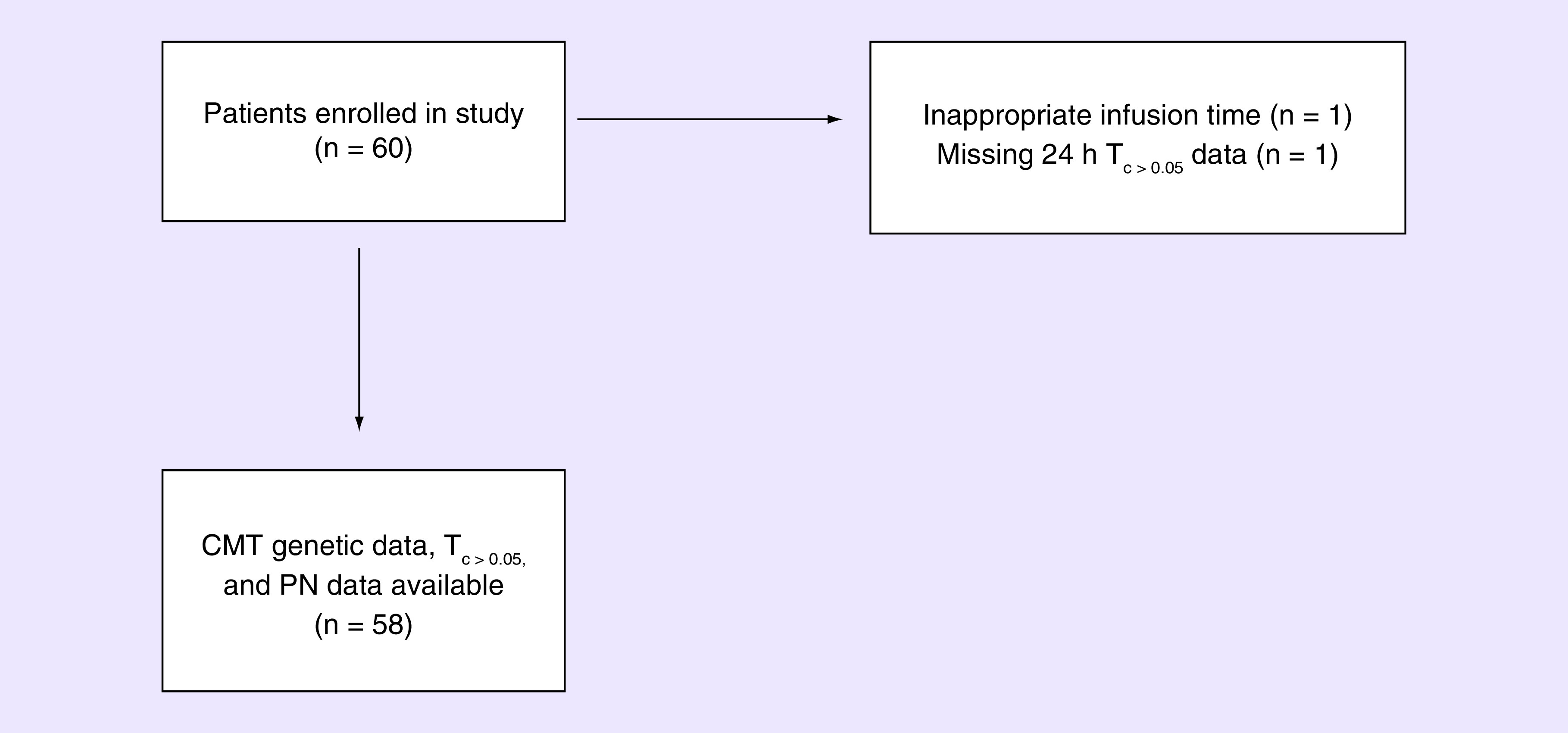
CMT: Charcot–Marie–Tooth; PN: Peripheral neuropathy.
Table 1. . Demographic and treatment information (n = 58).
| Patient demographics | n or mean (% or SD) |
|---|---|
| Age (years) | 52.52 (10.31) |
| BSA (m2) | 1.83 (0.21) |
| Race (Caucasian) | 54 (93.1%) |
| Tc>0.05 (h) | 10.72 (2.73) |
| Baseline CIPN8 (range: 0–100) | 1.29 (3.04) |
| Cumulative dose (mg/m2) | 883.95 (163.82) |
| Relative dose intensity | 0.95 (0.01) |
BSA: Body surface area; SD: Standard deviation.
Genetic variants included in each genetic predictor
Each SNV included in any analysis is listed in Table 2. The primary analysis included two ARHGEF10 SNV (rs9657362 and rs17683288). The secondary analysis of carrying any ARHGEF10 missense SNV allele included seven independent missense variants. Six SBF2 SNVs were considered functionally consequential. In the analysis of SBF2, patients were classified as to whether they carried SBF2 rs117957652 or rs7102464 or by the number of functional SBF2 SNV alleles. The analyses of FGD4 were conducted on the basis of carrying FGD4 rs10844253 (tag SNV for rs10771973, r2 = 0.92), or carrying any functional FGD4 SNV. Two functional FGD4 SNVs were identified: rs11539445 and rs10844308, but due to LD only rs11539445 was considered a functional SNV in the analysis. In the analysis of FZD3, patients were classified by the number of FZD3 rs7833751 alleles (tag SNV for rs7001034, r2 = 0.98). In the NXN analysis, rs11247571 was the only functionally consequential SNV identified and patients were classified by whether they carried this functional SNV.
Table 2. . All variants included in genetic analysis.
| Gene | rs ID | Chromosomal position | Reference allele | Variant type | Reason for variant inclusion (candidate SNV, tag SNV, missense or functional) | Corresponding genetic predictor† |
|---|---|---|---|---|---|---|
| ARHGEF10 | rs9657362 | 8:1833801 | G | Missense | Candidate, missense | 1, 2 |
| rs17683288 | 8:1877480 | T | Missense | Candidate, missense | 1, 2 | |
| rs141069028 | 8:1851564 | C | Missense | Missense | 2 | |
| rs2294039 | 8:1857591 | G | Missense | Missense | 2 | |
| rs201516531 | 8:1905361 | C | Missense | Missense | 2 | |
| rs887797448 | 8:1900880 | G | Missense | Missense | 2 | |
| rs139515492 | 8:1905048 | C | Missense | Missense | 2 | |
| SBF2 | rs117957652 | 11:9861208 | C | Missense | Candidate | 3 |
| rs7102464 | 11:9879838 | T | Missense | Candidate | 3 | |
| rs59613534 | 11:9800552 | C | 3 prime UTR | Functional (CADD: 19.22, GWAVA: 0.71) | 4 | |
| rs60154961 | 11:9800566 | G | 3 prime UTR | Functional (CADD: 18.12, GWAVA: 0.67) | 4 | |
| rs360126 | 11:9800346 | G | 3 prime UTR | Functional (CADD: 17.02, GWAVA: 0.68) | 4 | |
| rs360125 | 11:9800650 | G | 3 prime UTR | Functional (CADD: 17.01, GWAVA: 0.63) | 4 | |
| rs1045634 | 11:9800450 | T | 3 prime UTR | Functional (CADD: 15.01, GWAVA: 0.70) | 4 | |
| rs146366305 | 11:9989990 | A | Missense | Functional (CADD: 25.3, PROVEAN: -2.53 | 4 | |
| FGD4 | rs10844253 | 12:32764184 | A | Synonymous | Tag SNV of rs10771973 (r2 = 0.92) | 5 |
| rs11539445 | 12:32908237 | A | Regulatory | Functional (CADD: 24.2, GWAVA: 0.50, GTEx p = 0.00012) | 6 | |
| rs10844308 | 12:32854366 | C | Regulatory | Functional (CADD: 17.03, GWAVA: 0.54, GTEx p = 0.00012) | Excluded due to LD‡ | |
| FZD3 | rs7833751 | 8:28362792 | G | Intron | Tag SNV of rs7001034 (r2 = 0.98) | 7 |
| NXN | rs11247571 | 17:908502 | G | Regulatory | Functional (CADD: 17.79, GWAVA: 0.51, GTEx p = 0.0000049) | 8 |
Corresponding genetic predictor: the genetic predictor (Table 3) in which each SNV was included.
Variant excluded from analysis due to LD with rs11539445 (r2 = 0.93).
CADD: Combined annotation-dependent depletion; LD: Linkage disequilibrium.
Genetic associations with PN sensitivity
Table 3 lists each genetic predictor analyzed, the distribution of that genetic predictor in the cohort, the expected direction of effect on PN sensitivity, and the association for that genetic predictor when introduced in the PN sensitivity model that accounts for cumulative treatment, systemic paclitaxel exposure, and clinical factors. In the primary analysis, carrying either rs9657362 or rs17683288 in ARHGEF10 was not associated with lower PN sensitivity (β-coefficient: -0.27, 95% CI: -0.68–0.14; p = 0.20; Table 3 & Figure 2). In a secondary analysis, each additional FZD3 rs7833751 variant allele a patient carried decreased her PN sensitivity (additive β-coefficient = -0.41; 95% CI: -0.66 to -0.17; p = 0.0011; Figure 3), which is consistent with the expected direction of effect. Although the exploratory secondary analyses were not corrected for multiple comparisons, this association does retain significance after strict Bonferonni multiple comparisons testing correction (α = 0.05/8 = 0.00625). The PN sensitivity model parameter estimates for all clinical covariates with FZD3 rs7833751 alone, or including EPHA5 rs7349683, are reported in Table 4. The final model indicates that both variants were independently associated with PN sensitivity, though with opposing direction of effect. None of the other six genetic predictors tested in secondary analyses was associated with PN sensitivity.
Figure 2. . CIPN8 score by cumulative exposure stratified by whether a patient carried either ARHGEF10 SNV rs9657362 or rs17683288 (green line) or not (red line).
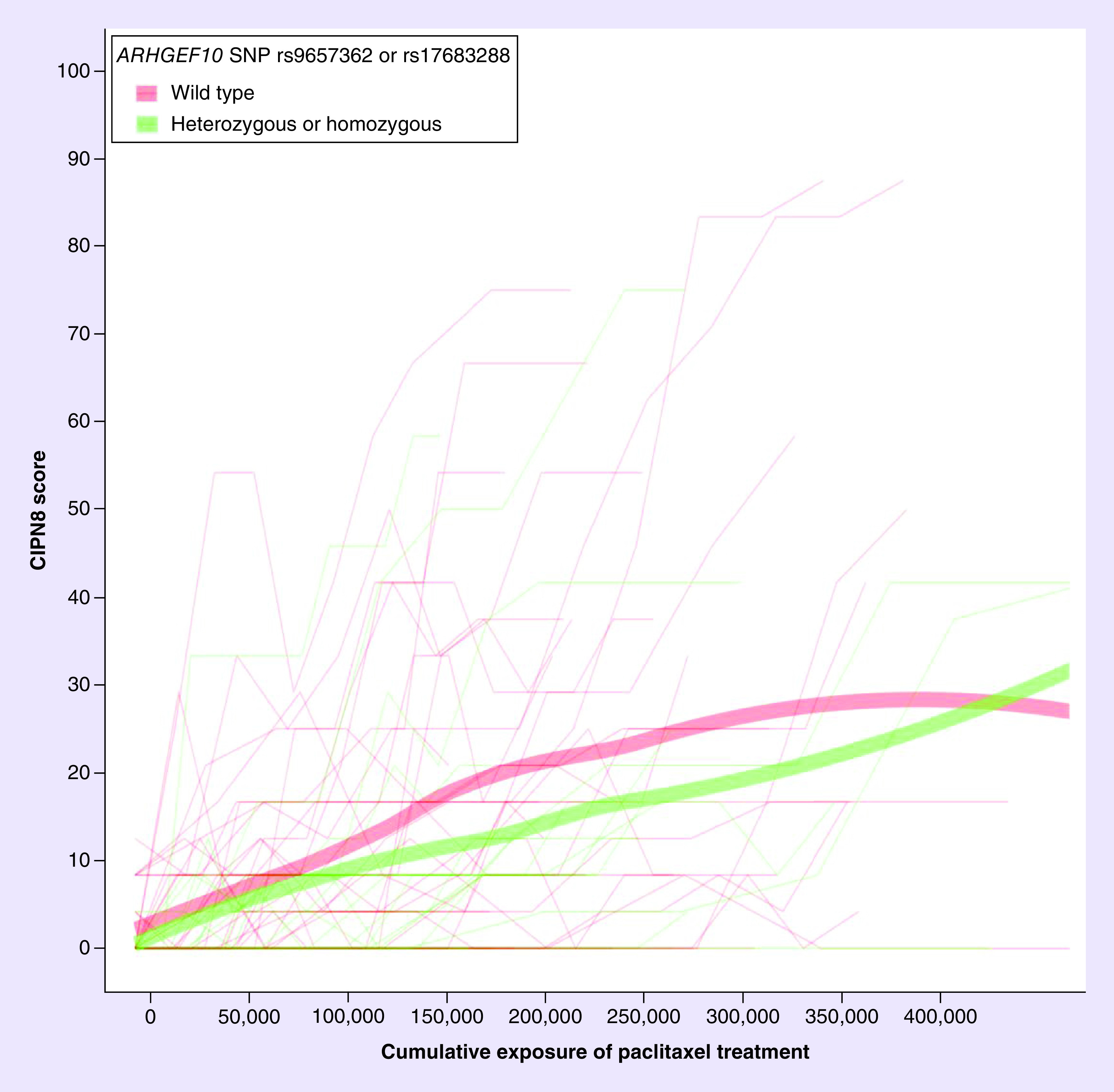
Carrying ARHGEF10 rs9657362 or rs17683288 was not associated with peripheral neuropathy sensitivity. Wider lines represent lines of best fit.
Figure 3. . CIPN8 score by cumulative dose (cumulative dose * Tc >0.05) stratified by whether a patient carried 0 (red line), 1 (green line) or 2 (blue line) FZD3 tag SNV rs7833751 alleles.
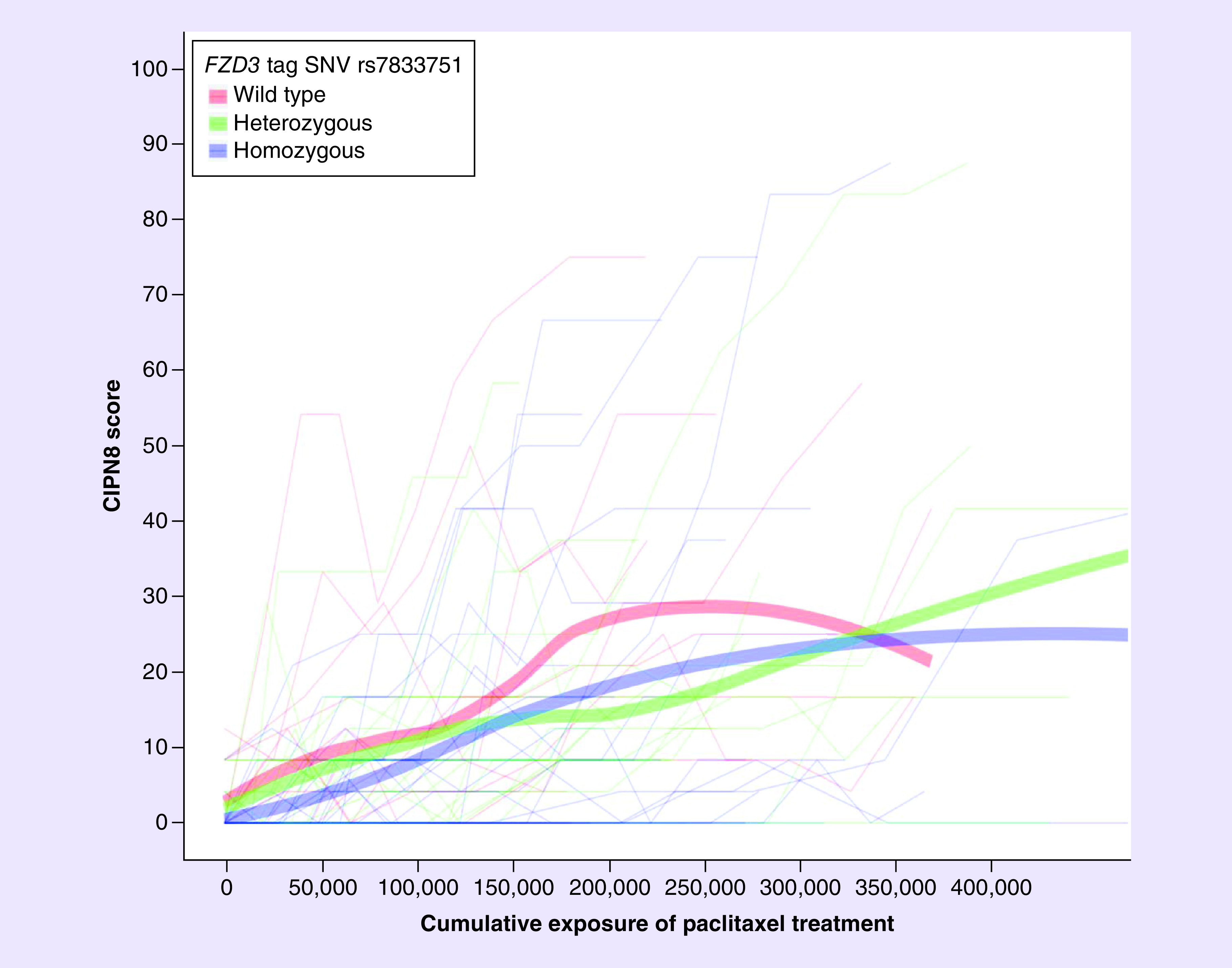
Peripheral neuropathy sensitivity was decreased for each rs7833751 variant allele a patient carried (β-coefficient = -0.41; 95% CI: -0.66 to -0.17; p = 0.0011). Thick lines represent lines of best fit.
Table 4. . Final peripheral neuropathy sensitivity model including clinical and genetic predictors.
| Predictor | PN sensitivity model with FZD3 rs7833751 | PN sensitivity model with FZD3 rs7833751 and EPHA5 rs7349683 | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Beta† | 95% CI | p-value | Beta† | 95% CI | p-value | ||
| Each additional FZD3 rs7833751 variant allele a patient carried | -0.41 | -0.66 to -0.17 | 0.0011 | -0.47 | -0.72 to -0.23 | <0.001 | |
| Baseline CIPN8 | 0.19 | 0.12–0.25 | <0.001 | 0.18 | 0.12–0.24 | <0.0001 | |
| Cumulative dose | -0.13 | -0.55–0.29 | 0.55 | -0.14 | -0.56–0.28 | 0.52 | |
| Relative dose intensity | -1.53 | -3.01 to -0.04 | 0.04 | -1.44 | -2.91–0.03 | 0.06 | |
| Tc>0.05 | -0.23 | -0.45 to -0.02 | 0.03 | -0.31 | -0.53 to -0.10 | <0.01 | |
| Cumulative dose Tc>0.05 interaction† | 0.14 | 0.04–0.25 | <0.01 | 0.14 | 0.04–0.25 | <0.01 | |
| Each additional EPHA5 rs7349683 variant allele a patient carried |
– | – | – | 0.47 | 0.19–0.75 | <0.01 | [31] |
Positive beta-coefficient indicates higher PN sensitivity, negative indicates lower PN sensitivity.
PN: Peripheral neuropathy.
Discussion
PN is a common, debilitating, sometimes irreversible side effect of paclitaxel treatment [5,8,43]. PN is primarily determined by cumulative systemic paclitaxel exposure [7,11], but there also seems to be an inherent sensitivity to PN that may be determined by patient genetics [12,31]. Predictive PN sensitivity biomarkers could be used to individualize paclitaxel dosing or select non-neuropathic alternative regimens, to prevent PN and improve treatment outcomes. Using a previously published PN sensitivity model that accounts for measured cumulative paclitaxel systemic exposure, we were unable to confirm that patients carrying ARHGEF10 rs9657362 or rs17683288, who have been previously reported to have lower risk of developing PN [19,29], are less PN-sensitive. In a statistically uncorrected secondary analysis, each additional variant allele of FZD3 rs7833751, a tag SNV for rs7001034, a patient carried was associated with lower PN sensitivity.
Our results that FZD3 rs7833751, a proxy for rs7001034, decreases PN sensitivity is consistent with an observation from a previously published GWAS that European patients carrying FZD3 rs7001034 had lower risk of PN [21]. Our study had to use a proxy SNV of rs7001034 due to lack of intronic coverage on our sequencing panel. Though previous replication studies failed to support the PN protective effect of rs7001034, perhaps due to insufficient study power [28], the consistency of our findings with the original publication warrant additional replication attempts to confirm that rs7001034 decreases PN sensitivity and is protective of PN. FZD3 encodes a G-protein-coupled receptor involved in Wnt signaling that is important for neurite outgrowth [17] and development of the neural crest [44]. Further biological experiments should be conducted to confirm this variant’s functional impact on FZD3 activity and its possible contribution to PN sensitivity. Our results indicate that each rs7001034 variant allele a patient carries could increase their optimal systemic paclitaxel exposure (i.e., Tc >0.05) [7]. This finding is similar to our previous finding that each EPHA rs7349683 variant a patient carriers decreases their optimal exposure by approximately 1 h [31]. Our final model suggests that these two SNVs act independent of each other, and with opposite directions of effect on optimal exposure, and both would need to be considered when selecting an optimal exposure target for a patient. Upon validation of these PN sensitivity biomarkers, and determination of whether they act independently or whether there are gene-by-gene interactions between them, genotype-specific optimal exposure targets would need to be tested in prospective clinical trials to demonstrate the clinical benefit of individualized paclitaxel dosing.
Since PN sensitivity is likely to be a complex polygenic trait, genotype-guided paclitaxel dosing may require consideration of multiple genetic predictors. We investigated four other genes previously reported to be associated with PN risk, however, none of these genes were associated with PN sensitivity in this analysis. Rs9657362 and rs17683288 in ARHGEF10 were included in the primary analysis because they have been successfully replicated to have protective effects on PN susceptibility [19,29]. FGD4 SNV rs10771973 was included as a candidate SNV since it was originally reported to be associated with earlier-onset of paclitaxel-induced sensory PN and subsequently replicated to increase PN risk or risk of paclitaxel dose reduction in multiple independent patient cohorts [21,45]. SBF2 and NXN variants were reported to be associated with occurrence of severe PN in individual studies [20,22] but have not been successfully replicated to our knowledge. Our study was not able to detect an association with PN sensitivity for any of these genes, again perhaps due to limited analytical power.
Strengths of this study include the use of a PN sensitivity model that accounted for cumulative systemic paclitaxel exposure to explore genetic PN predisposition, the inclusion of gene-based genetic predictors, and the use of a reliable and valid patient-reported questionnaire for PN assessment [46–48]. There are also some limitations in this study. First, the small sample size limited the statistical powers to detect association with PN sensitivity for several genes that were previously reported to be associated with PN risk, including our primary hypothesis that SNVs in ARHGEF10 decrease PN sensitivity [19,29], and precludes meaningful analysis of gene-by-gene interactions. Second, our genetic dataset was derived from targeted exonic sequencing, which precluded direct analysis of several previously SNVs previously reported to be associated with PN risk. Although we attempted to include proxy SNVs with high LD, these surrogates may not perfectly represent the previously reported SNVs. Finally, our gene-based hypotheses assume all variants have similar functional consequences, which is unlikely to be true at the level of protein expression or function.
Conclusion
This study supports prior findings that FZD3 SNV rs7001034 decreases PN risk and indicates that the causal mechanism is by decreasing patients’ PN sensitivity. Additional validation studies in larger patient cohorts that account for cumulative paclitaxel exposure are necessary to confirm this predictive PN sensitivity biomarker, followed by prospective clinical trials testing individualized treatment strategies based on the patient’s PN sensitivity. This work could enable personalized treatment to prevent PN and improve therapeutic outcomes in patients with cancer.
Summary points.
Genetic variation in genes linked to hereditary neuropathy, specifically Charcot–Marie–Tooth (CMT) disease, have been reported to effect the risk of paclitaxel-induced peripheral neuropathy (PN).
Since PN is primarily determined by cumulative systemic paclitaxel exposure, analyses accounting for exposure can isolate a patient’s sensitivity to PN for use as an end point in pharmacogenetic analyses.
A total of 58 paclitaxel-treated patients were sequenced for a panel of genes linked to CMT. Their PN were measured using the eight sensory items of the patient-reported European Organisation for Research and Treatment of Cancer-Quality of Life Questionnaire (EORTC-QLQ) CIPN20 subscale.
Eight putative genetic predictors in five CMT genes (ARHGEF10, SBF2, FGD4, FZD3 and NXN) with prespecified expected direction of effect were analyzed.
Consistent with previous genome-wide association studies findings, each additional variant allele of FZD3 rs7833751 (a tagging variant of FZD3 rs7001034) a patient carried decreased her PN sensitivity.
This study did not find evidence that carrying ARHGEF10 rs9657362 or rs17683288 was associated with lower PN sensitivity.
Future biological studies and larger validation studies of rs7001034 and prospective trials that verify the clinical benefit of rs7001034-guided paclitaxel dosing could enable personalized treatment to prevent PN and improve therapeutic outcomes in patients with cancer.
Consistent replication in independent patient cohorts is necessary prior to clinical translation of pharmacogenetic biomarkers.
Footnotes
Financial & competing interests disclosure
D Hertz is funded by the National Center for Advancing Translational Sciences under award nos. KL2TR000434 and 2UL1TR000433. K Kidwell is funded by the National Cancer Institutes of Health under award no. P30CA046592. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided by the Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale (DF Hayes) and the Breast Cancer Research Foundation (BCRF) (N003173 to J.M. Rae). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study was approved by the University of Michigan IRBMed and was conducted in accordance with recognized ethical guidelines. All participants in this study signed written informed consent.
Data sharing statement
Data is available upon reasonable request to the corresponding author.
References
- 1.Perez EA. Paclitaxel in breast cancer. Oncologist 3(6), 373–389 (1998). [PubMed] [Google Scholar]
- 2.Sparano JA, Wang M, Martino S. et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med. 358(16), 1663–1671 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur. J. Cancer 44(11), 1507–1515 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Mielke S, Sparreboom A, Mross K. Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. Eur. J. Cancer 42(1), 24–30 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Bandos H, Melnikow J, Rivera DR. et al. Long-term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG oncology/NSABP B-30. J. Natl Cancer Inst. 110(2), djx162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speck RM, Sammel MD, Farrar JT. et al. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J. Oncol. Pract. 9(5), e234–e240 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Hertz DL, Kidwell KM, Vangipuram K. et al. Paclitaxel plasma concentration after the first infusion predicts treatment-limiting peripheral neuropathy. Clin. Cancer Res. 24(15), 3602–3610 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy‐induced peripheral neuropathy: a current review. Ann. Neurol. 81(6), 772–781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: current status and progress. Gynecol. Oncol. 140(1), 176–183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hershman DL, Lacchetti C, Dworkin RH. et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 32(18), 1941–1967 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Mielke S, Sparreboom A, Steinberg SM. et al. Association of paclitaxel pharmacokinetics with the development of peripheral neuropathy in patients with advanced cancer. Clin. Cancer Res. 11(13), 4843–4850 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Joerger M, von Pawel J, Kraff S. et al. Open-label, randomized study of individualized, pharmacokinetically (PK)-guided dosing of paclitaxel combined with carboplatin or cisplatin in patients with advanced non-small-cell lung cancer (NSCLC). Ann. Oncol. 27(10), 1895–1902 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Hertz DL, McLeod HL. Using pharmacogene polymorphism panels to detect germline pharmacodynamic markers in oncology. Clin. Cancer Res. 20(10), 2530–2540 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Timmerman V, Strickland AV, Züchner S. Genetics of Charcot–Marie–Tooth (CMT) disease within the frame of the human genome project success. Genes 5(1), 13–32 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaya T, Shibata S, Tokuhara Y. et al. Identification of a negative regulatory region for the exchange activity and characterization of T332I mutant of Rho guanine nucleotide exchange factor 10 (ARHGEF10). J. Biol. Chem. 286(34), 29511–29520 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stendel C, Roos A, Deconinck T. et al. Peripheral nerve demyelination caused by a mutant Rho GTPase guanine nucleotide exchange factor, frabin/FGD4. Am. J. Hum. Genet. 81(1), 158–164 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endo Y, Beauchamp E, Woods D. et al. Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3-and c-Jun N-terminal kinase-dependent mechanism. Mol. Cell. Biol. 28(7), 2368–2379 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martino MA, Miller E, Grendys Jr EC. The administration of chemotherapy in a patient with Charcot–Marie–Tooth and ovarian cancer. Gynecol. Oncol. 97(2), 710–712 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Beutler AS, Kulkarni AA, Kanwar R. et al. Sequencing of Charcot–Marie–Tooth disease genes in a toxic polyneuropathy. Ann. Neurol. 76(5), 727–737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider BP, Lai D, Shen F. et al. Charcot–Marie–Tooth gene, SBF2, associated with taxane-induced peripheral neuropathy in African–Americans. Oncotarget 7(50), 82244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldwin RM, Owzar K, Zembutsu H. et al. A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin. Cancer Res. 18(18), 5099–5109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sucheston-Campbell LE, Clay-Gilmour AI, Barlow WE. et al. Genome-wide meta-analyses identifies novel taxane-induced peripheral neuropathy associated loci. Pharmacogenet. Genomics 28(2), 49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urbainsky C, Nölker R, Imber M. et al. Nucleoredoxin-dependent targets and processes in neuronal cells. Oxid. Med. Cell. Longev. 2018, 4829872 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akaneya Y, Sohya K, Kitamura A. et al. Ephrin-A5 and EphA5 interaction induces synaptogenesis during early hippocampal development. PLoS ONE 5(8), e12486 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrette B, Calvo E, Vallieres N, Lacroix S. Transcriptional profiling of the injured sciatic nerve of mice carrying the wld(S) mutant gene: identification of genes involved in neuroprotection, neuroinflammation, and nerve regeneration. Brain Behav. Immun. 24(8), 1254–1267 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Bird TD. Charcot–Marie–Tooth neuropathy Type 4 – archived chapter, for historical reference only. : GeneReviews®. Adam MP, Ardinger HH, Pagon RAet al.. et al. (). University of Washington, WA, USA: (1993). [Google Scholar]
- 27.Delague V, Jacquier A, Hamadouche T. et al. Mutations in FGD4 encoding the Rho GDP/GTP exchange factor FRABIN cause autosomal recessive Charcot–Marie–Tooth Type 4H. Am. J. Hum. Genet. 81(1), 1–16 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boora GK, Kanwar R, Kulkarni AA. et al. Testing of candidate single nucleotide variants associated with paclitaxel neuropathy in the trial NCCTG N08C1 (Alliance). Cancer Med. 5(4), 631–639 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boora GK, Kulkarni AA, Kanwar R. et al. Association of the Charcot–Marie–Tooth disease gene ARHGEF10 with paclitaxel induced peripheral neuropathy in NCCTG N08CA (Alliance). J. Neurol. Sci. 357(1–2), 35–40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan A, Hertz DL, Morales M. et al. Biological predictors of chemotherapy-induced peripheral neuropathy (CIPN): MASCC neurological complications working group overview. Support Care Cancer. 27(10), 3729–3737 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcath LA, Kidwell KM, Vangipuram K. et al. Genetic variation in EPHA contributes to sensitivity to paclitaxel‐induced peripheral neuropathy. Br. J. Clin. Pharmacol. 86(5), 880–890 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chhibber A, Mefford J, Stahl EA. et al. Polygenic inheritance of paclitaxel-induced sensory peripheral neuropathy driven by axon outgrowth gene sets in CALGB 40101 (Alliance). Pharmacogenomics J. 14(4), 336–342 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fayers P, Aaronson NK, Bjordal K, Sullivan M. EORTC QLQ–C30 scoring manual. European Organisation for Research and Treatment of Cancer, Brussels, Belgium: (1995). https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf [Google Scholar]
- 34.Joerger M, Huitema AD, van den Bongard DH, Schellens JH, Beijnen JH. Quantitative effect of gender, age, liver function, and body size on the population pharmacokinetics of paclitaxel in patients with solid tumors. Clin. Cancer Res. 12(7), 2150–2157 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Kraff S, Lindauer A, Joerger M, Salamone SJ, Jaehde U. Excel-based tool for pharmacokinetically guided dose adjustment of paclitaxel. Ther. Drug Monit. 37(6), 725–732 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Long J, He J. et al. Exome sequencing generates high quality data in non-target regions. BMC Genomics 13(1), 194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 40(D1), D930–D934 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 46(3), 310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 47(D1), D886–D894 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritchie GR, Dunham I, Zeggini E, Flicek P. Functional annotation of noncoding sequence variants. Nat. Methods 11(3), 294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the functional effect of amino acid substitutions and indels. PLoS ONE 7(10), e46688 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lonsdale J, Thomas J, Salvatore M. et al. The genotype-tissue expression (GTEx) project. Nat. Genet. 45(6), 580–585 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivera E, Cianfrocca M. Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother. Pharmacol. 75(4), 659–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pantavou KG, Braliou GG, Kontou PI, Dimou NL, Bagos PG. A meta-analysis of FZD3 gene polymorphisms and their association with schizophrenia. Psychiatr. Genet. 26(6), 272–280 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Lam SW, Frederiks CN, Van Der Straaten T, Honkoop AH, Guchelaar H-J, Boven E. Genotypes of CYP2C8 and FGD4 and their association with peripheral neuropathy or early dose reduction in paclitaxel-treated breast cancer patients. Br. J. Cancer 115(11), 1335–1342 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cleeland CS, Farrar JT, Hausheer FH. Assessment of cancer-related neuropathy and neuropathic pain. Oncologist 15(Suppl 2), 13–8 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Kuroi K, Shimozuma K. Neurotoxicity of taxanes: symptoms and quality of life assessment. Breast Cancer 11(1), 92 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Postma T, Heimans J. Grading of chemotherapy-induced peripheral neuropathy. Ann. Oncol. 11(5), 509–513 (2000). [DOI] [PubMed] [Google Scholar]


