Abstract
Notwithstanding the ongoing coronavirus disease-2019 (Covid-19) pandemic, information on its clinical presentation and prognosis in recipients of a kidney transplant remain scanty. The aim of this registry-based observational study was to explore characteristics and clinical outcomes of recipients of kidney transplants included in the French nationwide Registry of Solid Organ Transplant Recipients with Covid-19. Covid-19 was diagnosed in symptomatic patients who had a positive PCR assay for SARS-CoV-2 or having typical lung lesions on imaging. Clinical and laboratory characteristics, management of immunosuppression, treatment for Covid-19, and clinical outcomes (hospitalization, admission to intensive care unit, mechanical ventilation, or death) were recorded. Risk factors for severe disease or death were determined. Of the 279 patients, 243 were admitted to hospital and 36 were managed at home. The median age of hospitalized patients was 61.6 years; most had comorbidities (hypertension, 90.1%; overweight, 63.8%; diabetes, 41.3%; cardiovascular disease, 36.2%). Fever, cough, dyspnea, and diarrhea were the most common symptoms on admission. Laboratory findings revealed mild inflammation frequently accompanied by lymphopenia. Immunosuppressive drugs were generally withdrawn (calcineurin inhibitors: 28.7%; antimetabolites: 70.8%). Treatment was mainly based on hydroxychloroquine (24.7%), antiviral drugs (7.8%), and tocilizumab (5.3%). Severe Covid-19 occurred in 106 patients (46%). Forty-three hospitalized patients died (30-day mortality 22.8%). Multivariable analysis identified overweight, fever, and dyspnea as independent risk factors for severe disease, whereas age over 60 years, cardiovascular disease, and dyspnea were independently associated with mortality. Thus, Covid-19 in recipients of kidney transplants portends a high mortality rate. Proper management of immunosuppression and tailored treatment of this population remain challenging.
Keywords: COVID-19, immunosuppression, kidney transplantation, mortality, prognosis
Graphical abstract
Editor’s Note.
This is one of several articles we think you will find of interest that are part of our special issue of Kidney International addressing the challenges of dialysis and transplantation during the COVID-19 pandemic. Please also find additional material in our commentaries and letters to the editor sections. We hope these insights will help you in the daily care of your own patients.
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has created an ongoing global pandemic of major concern. Frail patients with comorbidities are at high risk of developing severe disease, as shown by initial reports from China1 , 2 and other countries.3 , 4 Although preexisting kidney disease is a predisposing factor for COVID-19 morbidity and mortality,5 information on its clinical presentation and prognosis in kidney transplant (KT) recipients under immunosuppressive therapy remains scant. Published data are limited to case reports6, 7, 8, 9 and small single-center case series.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
On March 1, 2020, a French nationwide registry of patients with COVID-19 and a history of solid organ transplantation was established under the auspices of the French-Speaking Society of Transplantation. As of April 21, 2020, a total of 598 patients were included in the registry—of whom 426 were KT recipients, 61 heart transplant recipients, 72 liver transplant recipients, and 39 lung transplant recipients. Here, we describe the disease presentation, immunosuppression management, clinical outcomes, and independent prognostic variables in a large sample of 279 KT recipients with COVID-19.
Results
Patient characteristics
Of the 279 KT recipients included in the registry, COVID-19 was diagnosed by reverse transcriptase–polymerase chain reaction in 93% of cases. The diagnosis in the remaining 7% of the study participants was based on clinical presentation and pulmonary computed tomography findings (7%). A total of 243 patients were admitted to the hospital, and 36 were managed at home following assessment by a transplant physician (Table 1 ). In brief, the latter group consisted of younger patients with a lower frequency of dyspnea, fever, and gastrointestinal manifestations. One patient received home treatment with hydroxychloroquine. Antimetabolites and mammalian target of rapamycin (mTOR) inhibitors were stopped in 13 patients (36%). The general characteristics of hospitalized patients are summarized in Table 1. The median age was 61.6 years (interquartile range: 50.8−69.0 years; range: 19−93 years), and two-thirds were men. Most of them were overweight (63.8%), and the most common comorbidities were hypertension (90.1%), cardiovascular disease (36.2%), diabetes (41.3%), and a history of respiratory disease (14.8%). SARS-CoV-2 infection was identified after a median of 74.1 months (interquartile range: 27.6−138.7 months; range: 1−1943 months) from KT. The median delay between the onset of symptoms and hospital admission was 5 days (interquartile range: 3−8 days, range: 0−34 days). The most frequent symptom on admission was fever (80%), followed by cough (63.6%), diarrhea (43.5%), dyspnea (40.3%), and anosmia (14.1%). Median levels of C-reactive protein and procalcitonin were 62 mg/L and 0.20 ng/mL, respectively (Table 2 ). The median lymphocyte count was 0.66 × 109/L, whereas thrombocytopenia was identified in 54 (29%) patients. Lung infiltrates on chest computed tomography images were detected in 87% of cases.
Table 1.
Baseline characteristics of kidney transplant recipients with COVID-19 managed at home versus in-hospital
| Variable | Home management |
In-hospital management |
P | n |
|---|---|---|---|---|
| (n = 36) | (n = 243) | |||
| Baseline characteristics | ||||
| Age, yr | 55.6 [48.0–61.1] | 61.6 [50.8–69.0] | 0.002 | 279 |
| Male | 20 (55.6) | 162 (66.7) | 0.263 | 279 |
| BMI, kg/m2 | 25.0 [23.4–28.9] | 26.1 [23.0–30.7] | 0.608 | 270 |
| BMI >25 kg/m2 | 18 (51.4) | 150 (63.8) | 0.221 | 270 |
| Blood group | 0.691 | 275 | ||
| A | 18 (50.0) | 105 (43.9) | ||
| AB | 1 (2.8) | 12 (5.0) | ||
| B | 6 (16.7) | 29 (12.1) | ||
| O | 11 (30.6) | 93 (38.9) | ||
| Transplanted organ | 0.525 | 279 | ||
| Kidney | 35 (97.2) | 233 (95.9) | ||
| Kidney–heart | 0 (0.0) | 4 (1.6) | ||
| Kidney–liver | 1 (2.8) | 2 (0.8) | ||
| Kidney–pancreas | 0 (0.0) | 4 (1.6) | ||
| Time from Tx to COVID-19 [IQR], mo | 58.9 [25.0–118.9] | 74.1 [27.6–138.7] | 0.626 | 279 |
| Time from Tx to COVID, stratified, mo no. (%): | 0.827 | 279 | ||
| <6 | 3 (8.3) | 20 (8.2) | ||
| 6–11 | 1 (2.8) | 15 (6.2) | ||
| 12–59 | 14 (38.9) | 73 (30.0) | ||
| 60–119 | 9 (25.0) | 60 (24.7) | ||
| ≥120 | 9 (25.0) | 75 (30.9) | ||
| Hypertension | 24 (82.8) | 201 (90.1) | 0.213 | 252 |
| RAS blockers | 15 (55.6) | 97 (44.5) | 0.377 | 245 |
| Cardiovascular disease | 6 (20.0) | 81 (36.2) | 0.122 | 254 |
| Respiratory disease | 5 (16.7) | 33 (14.8) | 0.786 | 253 |
| Diabetes | 12 (40.0) | 92 (41.3) | 1.000 | 253 |
| Cancer | 4 (13.3) | 35 (15.5) | 1.000 | 256 |
| Smoking | 3 (13.0) | 30 (15.5) | 1.000 | 217 |
| Baseline immunosuppression | ||||
| CNIs | 28 (77.8) | 202 (83.1) | 0.581 | 279 |
| Mycophenolate acid | 29 (80.6) | 183 (75.3) | 0.632 | 279 |
| Azathioprine | 1 (2.8) | 11 (4.5) | 1.000 | 279 |
| mTOR inhibitors | 5 (13.9) | 29 (11.9) | 0.784 | 279 |
| Steroids | 25 (69.4) | 177 (72.8) | 0.822 | 279 |
| Belatacept | 1 (2.8) | 15 (6.2) | 0.703 | 279 |
| Clinical presentation | ||||
| Cough | 20 (55.6) | 145 (63.6) | 0.459 | 264 |
| Rhinitis | 6 (16.7) | 20 (9.3) | 0.231 | 251 |
| Dyspnea | 2 (5.6) | 98 (40.3) | <0.001 | 279 |
| Anosmia | 10 (29.4) | 29 (14.1) | 0.046 | 240 |
| Fever | 15 (41.7) | 180 (80.0) | <0.001 | 261 |
| Headache | 11 (30.6) | 39 (17.5) | 0.106 | 259 |
| Diarrhea | 9 (25.0) | 97 (43.5) | 0.056 | 259 |
BMI, body mass index; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; mTOR, mammalian target of rapamycin; RAS, renin–angiotensin system; Ref, reference; Tx, transplantation.
Data are expressed as median [interquartile range] or count (%), as appropriate, unless otherwise indicated.
Table 2.
Laboratory data, management of immunosuppression, treatment modalities, and outcomes of kidney transplant recipients hospitalized with COVID-19
| Variable | Value | n |
|---|---|---|
| Laboratory data | ||
| CRP, mg/l | 62 [27–114] | 186 |
| Procalcitonin, ng/ml | 0.20 [0.14–0.48] | 90 |
| Lymphocyte count, ×109/l | 0.66 [0.40–0.96] | 184 |
| Platelet count, ×109/l | 178 [145–238] | 188 |
| Thrombocytopenia <150 × 109/l | 54 (29) | 188 |
| SaO2 | 96 (91–98) | 176 |
| Creatinine, μmol/l | 176 [131–244] | 200 |
| Immunusuppression management | ||
| CNI withdrawal | 58 (28.7) | 202 |
| Antimetabolite withdrawal | 136 (70.8) | 192 |
| mTOR inhibitor withdrawal | 18 (62.1) | 29 |
| Belatacept withdrawal | 7 (46.7) | 15 |
| COVID-19 treatment modalities | ||
| Azithromycin | 71 (29.2) | 243 |
| Other antibiotics | 153 (63.0) | 243 |
| Antifungal drugs | 6 (2.5) | 243 |
| Remdesivir | 2 (0.8) | 243 |
| Lopinavir/ritonavir | 11 (4.5) | 243 |
| Oseltamivir | 6 (2.5) | 243 |
| Hydroxychloroquine | 60 (24.7) | 243 |
| Tocilizumab | 13 (5.3) | 243 |
| Outcome | ||
| Bacterial coinfection | 57 (23.5) | 243 |
| Viral coinfection | 5 (2.1) | 243 |
| Fungal coinfection | 6 (2.5) | 243 |
| Oxygen therapy | 152 (72.4) | 210 |
| Mechanical ventilation | 72 (29.6) | 243 |
| Vasopressor support | 27 (11.1) | 243 |
| Acute kidney injury | 106 (43.6) | 243 |
| Renal replacement therapy | 27 (11.1) | 243 |
CNI, calcineurin inhibitors; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; mTOR, mammalian target of rapamycin; SaO2, arterial oxygen saturation.
Data are expressed as median [interquartile range] or count (%), as appropriate, unless otherwise indicated. Laboratory tests were performed on admission.
Management of immunosuppression
On admission, calcineurin inhibitors (CNIs), antimetabolites, and steroids were being taken by 83.1%, 79.8%, and 72.8% of patients, respectively. Of note, 29 (12%) and 15 (6.2%) patients were on mammalian target of rapamycin inhibitors and belatacept, respectively. During hospitalization (Table 2), antimetabolites, CNIs, and mammalian target of rapamycin inhibitors were withdrawn in 70.8% (136 of 192), 28.7% (58 of 202), and 62.1% (18 of 29) of patients, respectively. Moreover, belatacept administration was postponed in 7 of the 15 participants taking this drug. Of note, changes in immunosuppressive drugs other than those withdrawn were not recorded.
Treatment and clinical course
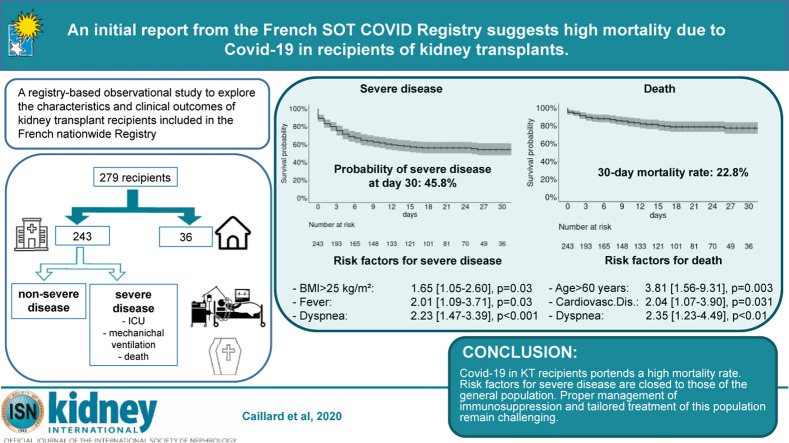
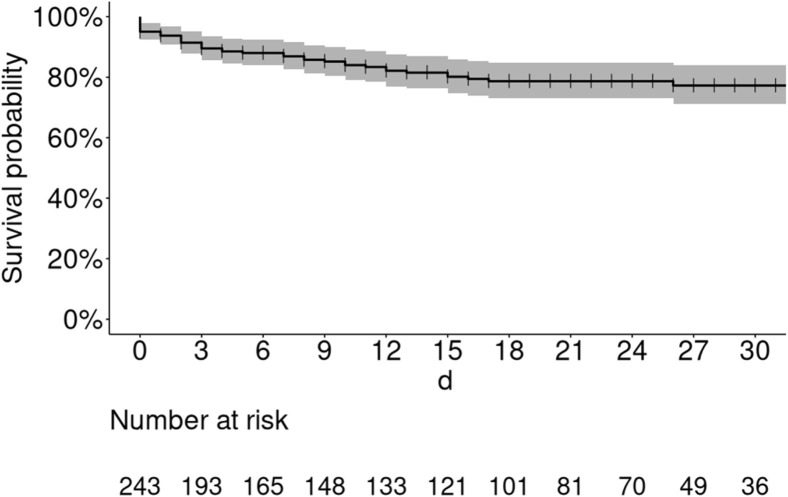
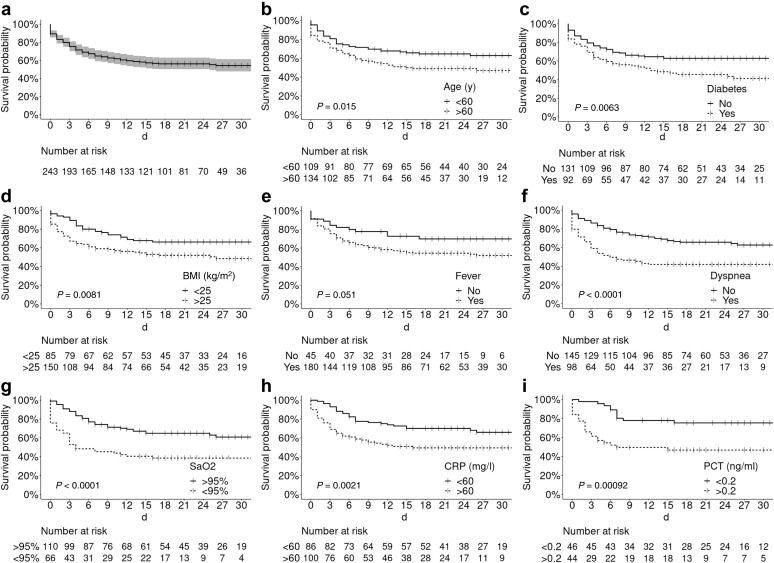
Most patients received nasal oxygen therapy (72.4%) and antibiotics other than azithromycin (63%). Hydroxychloroquine and azithromycin were given to 60 (24.7%) and 71 (29.2%) patients, respectively (Table 2). CNIs were stopped in 7 of the 11 patients treated with lopinavir/ritonavir. Tocilizumab was administered to 13 (5.3%) cases. Bacterial coinfections were identified in 57 (23.5%) participants. Mechanical ventilation was required for approximately 30% of cases. Acute kidney injury occurred in 43.6% of patients, with renal replacement therapy being necessary in 11.1% of cases. A total of 88 patients (36%) required intensive care unit (ICU) care either on admission (n = 25) or during hospitalization (n = 63). In the latter subgroup, the median interval between hospitalization and transfer to the ICU was 4 days (range: 1−25 days). The 30-day mortality rate of hospitalized patients was 22.8% (Figure 1 ). Nine patients lost their graft during hospitalization, 4 of whom died. The composite endpoint of severe COVID-19 within 30 days of hospital admission was reached by 46% of the study patients (Figure 2 a).
Figure 1.
Kaplan–Meier plot of survival in kidney transplant recipients who were hospitalized with coronavirus 2019. The 30-day mortality rate after admission was 22.8% (16.1%–28.9%).
Figure 2.
Probability of reaching the composite endpoint of severe disease. (a) The 30-day severe disease–free survival in the entire study cohort was 54.2% (48%–61.4%). Kaplan–Meier plots stratified according to (b) age (<60 years vs. >60 years), (c) diabetes (yes vs. no), (d) body mass index (BMI; <25 kg/m2 vs. >25 kg/m2), (e) fever on admission (yes vs. no), (f) dyspnea on admission (yes vs. no), (g) arterial oxygen saturation (SaO2) on admission (>95% vs. <95%), (h) C-reactive protein (CRP) level on admission (<60 mg/l vs. >60 mg/l), and (i) procalcitonin level on admission (<0.2 ng/ml vs. >0.2 ng/ml). PCT, procalcitonin.
Risk factors for severe COVID-19
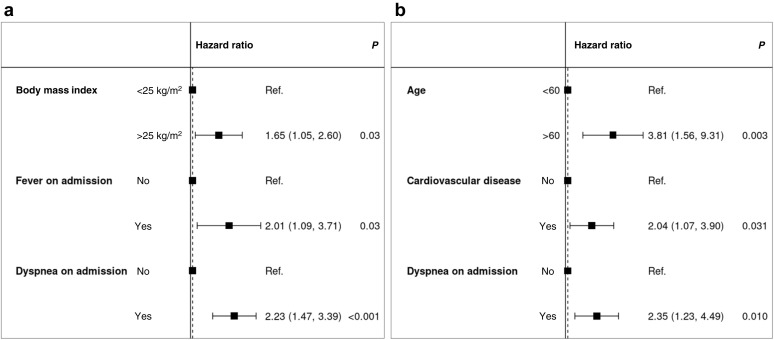
Table 3 compares the general characteristics of hospitalized patients who developed severe COVID-19 (n = 109) versus those who did not (n = 137). Patients aged >60 years who were overweight or had diabetes were significantly overrepresented in the former group. Fever and dyspnea on admission—but not cough—were associated with severe disease. However, the time elapsed between symptom onset and hospitalization was similar in the 2 groups (5 days). C-reactive protein levels >60 mg/L, procalcitonin concentrations >0.2 g/L, and a partial pressure of oxygen <95% on admission were significantly associated with severe COVID-19. No similar associations were observed with lymphocyte count, platelet count, or creatinine levels. Treatment modalities and management of immunosuppression (Table 4 ) were slightly different in the 2 study groups in relation to disease presentation and the clinical evolution over time. These differences were especially evident with respect to CNI withdrawal (52% and 11% in patients with severe and nonsevere disease, respectively, P < 0.001). Kaplan–Meier plots of severe COVID-19–free survival according to different risk factors are provided in Figure 2b–i. Multivariable analysis identified overweight, fever, and dyspnea as independent risk factors for severe disease (Figure 3 a).
Table 3.
Baseline characteristics of kidney transplant recipients with severe versus nonsevere COVID-19
| Characteristics | Nonsevere |
Severe |
HR [95% CI] | P | n |
|---|---|---|---|---|---|
| (n =137) | (n = 106) | ||||
| Baseline | |||||
| Age, yr | 59.5 [48.7–67.8] | 63.5 [54.7–69.6] | 1.02 [1.00–1.04] | 0.013 | 243 |
| Age >60 yr | 67 (48.9) | 67 (63.2) | 1.63 [1.10–2.43] | 0.015 | 243 |
| Male | 90 (65.7) | 72 (67.9) | 1.07 [0.71–1.61] | 0.740 | 243 |
| BMI > 25 kg/m2 | 78 (57.8) | 72 (72.0) | 1.80 [1.16–2.79] | 0.008 | 235 |
| Blood group | 239 | ||||
| A | 65 (48.5) | 40 (38.1) | Ref | Ref | |
| AB | 6 (4.48) | 6 (5.71) | 1.52 [0.64–3.59] | 0.340 | |
| B | 16 (11.9) | 13 (12.4) | 1.27 [0.68–2.38] | 0.449 | |
| O | 47 (35.1) | 46 (43.8) | 1.32 [0.86–2.02] | 0.198 | |
| Transplanted organ | 243 | ||||
| Kidney | 129 (94.2) | 104 (98.1) | Ref | Ref | |
| Kidney–heart | 2 (1.46) | 2 (1.89) | 1.36 [0.34–5.51] | 0.668 | |
| Kidney–liver | 2 (1.46) | 0 (0.00) | 0.00 [–] | 0.997 | |
| Kidney–pancreas | 4 (2.92) | 0 (0.00) | 0.00 [–] | 0.996 | |
| Time from Tx to COVID-19, mo | 73.4 [30.9–151] | 77.8 [25.4–131] | 1.00 [1.00–1.00] | 0.660 | 243 |
| Tx within 1 yr | 19 (13.9) | 16 (15.1) | 0.97 [0.57–1.65] | 0.912 | 243 |
| Hypertension | 112 (89.6) | 89 (90.8) | 1.14 [0.57–2.25] | 0.717 | 223 |
| RAS blockers | 58 (47.2) | 39 (41.1) | 0.83 [0.55–1.25] | 0.377 | 218 |
| Cardiovascular disease | 41 (32.5) | 40 (40.8) | 1.32 [0.88–1.98] | 0.176 | 224 |
| Respiratory disease | 19 (15.2) | 14 (14.3) | 0.96 [0.54–1.69] | 0.885 | 223 |
| Diabetes | 42 (33.6) | 50 (51.0) | 1.73 [1.16–2.57] | 0.007 | 223 |
| Cancer | 17 (13.4) | 18 (18.2) | 1.33 [0.80–2.21] | 0.276 | 226 |
| Smoking | 16 (14.8) | 14 (16.3) | 0.99 [0.56–1.76] | 0.977 | 194 |
| CNIs | 115 (83.9) | 87 (82.1) | 0.96 [0.58–1.58] | 0.868 | 243 |
| Mycophenolate acid | 102 (74.5) | 81 (76.4) | 1.08 [0.69–1.69] | 0.743 | 243 |
| Azathioprine | 5 (3.65) | 6 (5.66) | 1.32 [0.58–3.01] | 0.509 | 243 |
| mTOR inhibitors | 15 (10.9) | 14 (13.2) | 1.08 [0.62–1.90] | 0.785 | 243 |
| Steroids | 96 (70.1) | 81 (76.4) | 1.24 [0.79–1.94] | 0.347 | 243 |
| Belatacept | 8 (5.84) | 7 (6.60) | 1.08 [0.50–2.33] | 0.844 | 243 |
| On admission | |||||
| Cough | 81 (62.3) | 64 (65.3) | 1.20 [0.79–1.82] | 0.390 | 228 |
| Rhinitis | 12 (9.76) | 8 (8.70) | 0.82 [0.40–1.69] | 0.592 | 215 |
| Dyspnea | 42 (30.7) | 56 (52.8) | 2.28 [1.55–3.34] | <0.001 | 243 |
| Anosmia | 19 (16.1) | 10 (11.4) | 0.71 [0.37–1.38] | 0.315 | 206 |
| Fever | 98 (75.4) | 82 (86.3) | 1.77 [0.99–3.19] | 0.055 | 225 |
| Headache | 25 (19.5) | 14 (14.7) | 0.75 [0.43–1.32] | 0.322 | 223 |
| Diarrhea | 59 (46.1) | 38 (40.0) | 0.86 [0.57–1.30] | 0.486 | 223 |
| Time from symptom onset to admission, d | 5.00 [3.00–9.00] | 5.00 [3.00–7.00] | 1.00 [0.96–1.04] | 0.873 | 219 |
| C-reactive protein >60 mg/l | 51 (46.4) | 49 (64.5) | 2.07 [1.29–3.31] | 0.003 | 186 |
| Procalcitonin > 0.2 ng/ml | 21 (37.5) | 23 (67.6) | 3.19 [1.55–6.57] | 0.002 | 90 |
| Lymphocyte count, ×109/l | 0.70 [0.40–0.95] | 0.60 [0.40–0.96] | 1.10 [0.74–1.64] | 0.627 | 184 |
| Platelet count, ×109/l | 178 [146–229] | 178 [145–247] | 1.00 [1.00–1.00] | 0.742 | 188 |
| Thrombocytopenia < 150 × 109/l | 31 (28.7) | 23 (28.7) | 0.98 [0.60–1.58] | 0.923 | 188 |
| SaO2 < 95% | 26 (26.8) | 40 (50.6) | 2.47 [1.59–3.84] | <0.001 | 176 |
| Creatinine, μmol/l | 173 [126–230] | 182 [132–251] | 1.00 [1.00–1.00] | 0.378 | 200 |
BMI, body mass index; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; HR, hazard ratio; mTOR, mammalian target of rapamycin; RAS, renin–angiotensin system; Ref, reference; Tx, transplantation.
Data are expressed as median [interquartile range] or count (%), as appropriate, unless otherwise indicated.
Table 4.
Treatment modalities and immunosuppression management in kidney transplant recipients hospitalized for COVID-19 according to the presence of severe versus nonsevere disease
| Therapy | Nonsevere |
Severe |
P | n |
|---|---|---|---|---|
| n = 137 | n = 106 | |||
| COVID-19 treatment | ||||
| Azithromycin | 38 (27.7) | 33 (31.1) | 0.790 | 243 |
| Other antibiotics | 81 (59.1) | 72 (67.9) | 0.190 | 243 |
| Antifungal drugs | 1 (0.7) | 5 (4.7) | 0.060 | 243 |
| Remdesivir | 0 (0.0) | 2 (1.9) | 0.035 | 243 |
| Lopinavir/ritonavir | 2 (1.5) | 9 (8.5) | 0.002 | 243 |
| Oseltamivir | 3 (2.2) | 3 (2.8) | 0.708 | 243 |
| Hydoxychloroquine | 28 (20.4) | 32 (30.2) | 0.168 | 243 |
| Tocilizumab | 4 (2.9) | 9 (8.5) | 0.077 | 243 |
| Immunosuppression management | ||||
| CNI withdrawal | 13 (11.3) | 45 (51.7) | <0.001 | 202 |
| Antimetabolite withdrawal | 73 (68.2) | 63 (74.1) | 0.376 | 192 |
| mTOR inhibitor withdrawal | 8 (53.3) | 10 (71.4) | 0.187 | 29 |
| Belatacept withdrawal | 4 (50.0) | 3 (42.9) | 0.549 | 15 |
COVID-19, coronavirus disease 2019; CNI, calcineurin inhibitor; mTOR, mammalian target of rapamycin.
Values are n (%), unless otherwise indicated.
Figure 3.
Multivariable analysis of risk factors for (a) severe disease (intensive care unit admission/need for mechanical ventilation/mortality) and (b) mortality. Concordance for the severe disease model: 0.66; concordance for the mortality model: 0.76. Ref., reference.
Risk factors for mortality
Table 5 compares the general characteristics of hospitalized patients who died (n = 43) versus those who did not (n = 200). Patients aged >60 years, who had cardiovascular disease, were receiving immunosuppressive drugs different from CNIs, and who presented with dyspnea or a partial pressure of oxygen <95% on admission, were significantly overrepresented in the former group. Multivariable analysis identified age >60 years, cardiovascular disease, and dyspnea as independent risk factors for death in hospitalized patients (Figure 3b).
Table 5.
Baseline characteristics of kidney transplant recipients with COVID-19 who died versus those who did not
| Characteristics | Alive |
Dead |
HR [95% CI] | P | n |
|---|---|---|---|---|---|
| (n = 200) | (n = 43) | ||||
| Baseline | |||||
| Age, yr | 59.8 [49.8–67.5] | 68.9 [61.7–75.1] | 1.07 [1.04–1.10] | <0.001 | 243 |
| Age >60 yr | 99 (49.5) | 35 (81.4) | 3.98 [1.85–8.59] | <0.001 | 243 |
| Male | 137 (68.5) | 25 (58.1) | 0.68 [0.37–1.25] | 0.215 | 243 |
| BMI >25 kg/m2 | 122 (61.9) | 28 (73.7) | 1.65 [0.80–3.39] | 0.177 | 235 |
| Blood group | 239 | ||||
| A | 89 (45.2) | 16 (38.1) | Ref. | Ref. | |
| AB | 11 (5.58) | 1 (2.38) | 0.58 [0.08–4.40] | 0.601 | |
| B | 23 (11.7) | 6 (14.3) | 1.36 [0.53–3.48] | 0.521 | |
| O | 74 (37.6) | 19 (45.2) | 1.42 [0.73–2.77] | 0.299 | |
| Transplanted organ | 243 | ||||
| Kidney | 190 (95.0) | 43 (100) | Ref. | Ref. | |
| Kidney–heart | 4 (2.00) | 0 (0.00) | 0.00 [0.00] | 0.997 | |
| Kidney–liver | 2 (1.00) | 0 (0.00) | 0.00 [0.00] | 0.998 | |
| Kidney–pancreas | 4 (2.00) | 0 (0.00) | 0.00 [0.00] | 0.997 | |
| Time from Tx to COVID-19, mo | 72.5 [27.7–147] | 83.7 [25.7–116] | 1.00 [1.00–1.00] | 0.933 | 243 |
| Tx within 1 yr | 29 (14.5) | 6 (14.0) | 0.95 [0.40–2.26] | 0.914 | 243 |
| Hypertension | 165 (89.7) | 36 (92.3) | 1.39 [0.43–4.53] | 0.580 | 223 |
| RAS blockers | 80 (44.4) | 17 (44.7) | 1.07 [0.56–2.03] | 0.836 | 218 |
| Cardiovascular disease | 59 (31.9) | 22 (56.4) | 2.74 [1.45–5.17] | 0.002 | 224 |
| Respiratory disease | 28 (15.2) | 5 (12.8) | 0.77 [0.30–1.96] | 0.577 | 223 |
| Diabetes | 69 (37.5) | 23 (59.0) | 2.27 [1.20–4.29] | 0.012 | 223 |
| Cancer | 28 (15.0) | 7 (17.9) | 1.17 [0.52–2.65] | 0.708 | 226 |
| Smoking | 25 (15.5) | 5 (15.2) | 0.97 [0.38–2.52] | 0.953 | 194 |
| CNIs | 172 (86.0) | 30 (69.8) | 0.46 [0.24–0.88] | 0.019 | 243 |
| Mycophenolate acid | 152 (76.0) | 31 (72.1) | 0.83 [0.43–1.62] | 0.586 | 243 |
| Azathioprine | 8 (4.00) | 3 (6.98) | 1.41 [0.43–4.55] | 0.569 | 243 |
| mTOR inhibitors | 22 (11.0) | 7 (16.3) | 1.38 [0.61–3.10] | 0.439 | 243 |
| Steroids | 147 (73.5) | 30 (69.8) | 0.81 [0.42–1.56] | 0.533 | 243 |
| Belatacept | 12 (6.00) | 3 (6.98) | 1.15 [0.36–3.71] | 0.817 | 243 |
| On admission | |||||
| Cough | 123 (64.4) | 22 (59.5) | 0.81 [0.42–1.56] | 0.521 | 228 |
| Rhinitis | 16 (8.89) | 4 (11.4) | 1.24 [0.44–3.51] | 0.687 | 215 |
| Dyspnea | 74 (37.0) | 24 (55.8) | 1.99 [1.09–3.63] | 0.025 | 243 |
| Anosmia | 28 (16.0) | 1 (3.23) | 0.20 [0.03–1.45] | 0.110 | 206 |
| Fever | 151 (79.9) | 29 (80.6) | 1.05 [0.46–2.41] | 0.901 | 225 |
| Headache | 35 (18.6) | 4 (11.4) | 0.59 [0.21–1.68] | 0.323 | 223 |
| Diarrhea | 84 (44.7) | 13 (37.1) | 0.75 [0.38–1.48] | 0.401 | 223 |
| Time from symptom onset to admission, d | 6.00 [3.00–9.00] | 4.00 [2.75–6.00] | 0.94 [0.88–1.02] | 0.138 | 219 |
| CRP >60 mg/l | 82 (51.9) | 18 (64.3) | 1.69 [0.78–3.66] | 0.185 | 186 |
| Procalcitonin > 0.2 ng/ml | 34 (44.7) | 10 (71.4) | 2.79 [0.87–8.89] | 0.083 | 90 |
| Lymphocyte count, ×109/l | 0.70 [0.40–0.97] | 0.60 [0.44–0.96] | 0.80 [0.38–1.65] | 0.538 | 184 |
| Platelet count, ×109/l | 178 [144–232] | 178 [155–257] | 1.00 [1.00–1.00] | 0.894 | 188 |
| Thrombocytopenia <150 ×109/L | 48 (30.8) | 6 (18.8) | 0.54 [0.22–1.32] | 0.176 | 188 |
| SaO2 <95% | 47 (32.2) | 19 (63.3) | 3.39 [1.61–7.14] | 0.001 | 176 |
| Creatinine level, μmol/l | 176 [131–249] | 184 [131–230] | 1.00 [1.00–1.00] | 0.864 | 200 |
BMI, body mass index; CI, confidence interval; CNI, calcineurin inhibitor; COVID-19, coronavirus 2019; CRP, C-reactive protein; HR, hazard ratio; IQR, interquartile range; mTOR, mammalian target of rapamycin; RAS, renin-angiotensin system; Ref, reference; SaO2, arterial oxygen saturation; Tx, transplantation.
Data are expressed as median [interquartile range] or count (%), as appropriate, unless otherwise indicated.
Subgroup analyses conducted in patients who tested negative on reverse transcriptase-polymerase chain reaction (7%) yielded similar results both in terms of severe disease and mortality (data not shown). The median follow-up time was 22 days; a total of 66 patients were still in the ICU at the time the manuscript was written.
Discussion
Despite the growing literature focusing on the clinical manifestations and prognosis of COVID-19, data on certain selected clinical populations that merit special consideration—including immunocompromised patients with a history of solid organ transplantation—remain scant. To address this knowledge gap, herein we report the general characteristics and the main risk factors for adverse outcomes—including severe disease and mortality—of a large nationwide French cohort consisting of 279 KT recipients with COVID-19.
First, we demonstrate that the clinical presentation of COVID-19 in KT recipients is similar to that reported in the general population—with fever and cough being the 2 more common symptoms. These findings are in line with those from initial large reports showing fever in 77%−94% and cough in 68%−79% of cases, respectively.1, 2, 3 However, the occurrence of gastrointestinal symptoms (mainly diarrhea) was as high as 42% in our patients (i.e., significantly more frequent than that previously reported in general population studies conducted in both China [3%−5%]1 , 2 and the United States [24%]).3 Patients with a history of solid organ transplantation are at high risk of gastrointestinal disorders—which may be exacerbated by immunosuppressive drugs. Importantly, anosmia was present in 14% of our patients, and in accordance with previous findings obtained in the general population,22 tended to be associated with more favorable survival figures. We also demonstrate that some immunocompromised patients with COVID-19 were manageable at home with a favorable outcome, as described in an Italian cohort from Brescia.23 This decision was made on a case basis and was chiefly implemented for young patients without dyspnea and high fever. This patient subgroup was offered daily teleconsultation surveillance until disease resolution, a strategy that has been successfully implemented in a recent report from the United States.21 The laboratory findings of our patients on admission are also in line with previous studies. In general, there was evidence of mild inflammation—with lymphopenia being present in most patients, and thrombocytopenia in approximately one third. Notably, high procalcitonin levels were identified in 16% of our study participants—a markedly lower prevalence compared with that previously reported in KT recipients (42%).13
The initially reported mortality rate for COVID-19 in the general population of Wuhan, China, was 1.4%.1 Higher mortality figures have been published for hospitalized patients in New York (10%),3 and for Italian patients admitted to the ICU (26%).4 Previous data obtained in small-sized series of transplanted patients indicated a death rate similar to that observed in our cohort.24 Here, the 30-day mortality rate of our hospitalized KT recipients with COVID-19 was 22.8%, a value similar to that reported for Italian patients admitted to the ICU.4 The high mortality rate observed in these patients may reflect the frailty of KT recipients and/or a high burden of comorbidities. Mechanical ventilation and ICU transfer were required in 36% of our patients—a slightly higher percentage than that reported for immunocompetent subjects (16%−33%).2 , 3
Male sex has been previously linked to severe COVID-19.25 However, no significant association between male sex and severe disease or mortality was observed in our cohort— possibly because of the high burden of comorbidities. Conversely, overweight, fever, and dyspnea were independent risk factors for severe disease in our cohort. The association between overweight/obesity and severe COVID-19—which has been shown here for the first time in transplant recipients—is in accordance with previous data obtained in the general population.3 In our study, age, cardiovascular disease, and dyspnea were independent risk factors for mortality. Age2 , 25 and comorbidities have been reported to have an adverse prognostic significance in previous general population studies. The lack of prognostic significance of hypertension in our sample may be explained by its high prevalence (90%). In accordance with previous studies,2 , 3 severe inflammation on admission was found to have an adverse prognostic significance. Procalcitonin and C-reactive protein levels were higher in patients in the United States requiring mechanical ventilation,3 whereas procalcitonin levels were an unfavorable predictor of mortality in Chinese patients.2 However, in contrast to previous studies,2 , 26 lymphopenia did not predict severe COVID-19 or mortality in our sample. A potential explanation may lie in the fact that lymphopenia occurs commonly in KT patients and thus might not be invariably linked to SARS-CoV-2 infection.
The debate on the management of immunosuppression in transplant recipients following SARS-CoV-2 infection remains unresolved.27 Published case reports and small-size series of KT recipients diagnosed with COVID-19 have consistently documented a reduction in maintenance immunosuppression,6, 7, 8, 9, 10, 11, 12, 13, 14 and this approach is currently being recommended by guidelines.28 However, precise guidance on the management of CNIs, antimetabolites, and steroids is still lacking. In our registry, CNIs and antimetabolites were withdrawn in 28.7% and 70.8% of the study patients, respectively. Similar figures have been reported in United States case series.11 , 13 These management strategies have been chiefly informed by alterations in T-cell responses induced by SARS-CoV-2. Although CNIs may exert an inhibitory effect against the replication of coronaviruses in vitro,29 , 30 whether or not this effect can have clinical implications is arguable. In our study, patients who were free from CNIs on admission had a lower risk of mortality in univariable but not multivariable analysis (probably because of their older age; data not shown). No firm conclusions can therefore be drawn on the potential beneficial or detrimental effects of CNIs in KT recipients with COVID-19.
A minority of our patients received specific antiviral drugs. The lopinavir/ritonavir combination has strong pharmacological interactions with CNIs and mammalian target of rapamycin inhibitors, which have been related to the onset of acute renal failure in solid organ transplant recipients.9 , 10 , 12 Only 25% of our patients received hydroxychloroquine. The lower usage of this drug compared with the usage level in other cohorts10 , 13 may be explained by low-quality evidence on its effectiveness31 , 32 and the potential risk of severe adverse events in KT recipients. The potential benefits of interleukin-6 inhibition merit comment. A hyperinflammatory state characterized by the release of massive amounts of cytokines (cytokine storm) has been reported in patients with severe or catastrophic forms of COVID-19.33 Because interleukin-6 plays a central role in the cytokine storm, interleukin-6–targeting therapies have been proposed to tackle its occurrence.34 , 35 Trials of tocilizumab have been already attempted in nontransplanted36 and transplanted patients,10, 11, 12, 13 and this drug was given to 13 patients included in our registry. Of them, 11 had favorable outcomes despite severe COVID-19. Although no firm conclusions can be drawn because of the retrospective, nonrandomized nature of our study, our results are in line with those by Alberici et al. 10 who demonstrated a 50% reduction in the oxygen therapy requirement and a significant improvement in imaging features of pulmonary lesions upon tocilizumab administration.
Our findings need to be interpreted in the context of several limitations. First, we acknowledge that some baseline clinical, laboratory, and imaging data were missing. Similarly, information on the exact management of immunosuppression (i.e., dose reduction) and changes in laboratory parameters over time is lacking. Second, we are aware that the follow-up time is limited, and 88 patients were still being hospitalized at the time of analysis. We cannot exclude the possibility that some of these cases will ultimately develop severe disease and eventually die. We also acknowledge that some patients with severe disease did not qualify for admission to the intensive care unit. Third, we are aware that representativeness can affect the generalizability of our registry data and that our findings need external validation. However, efforts to address potential sources of bias in our registry included the prospective data collection and the controlling for potential confounders in multivariable analysis. Notwithstanding the potential caveats, this study is by far the largest so far to provide a comprehensive description of KT recipients with COVID-19.
Conclusion
COVID-19 in KT recipients portends a high risk of mortality. Proper management of immunosuppression and tailored treatment of this fragile population remain challenging. Overweight, fever, and dyspnea were independent risk factors for severe COVID-19 in this patient group, whereas age >60 years, cardiovascular disease, and dyspnea were independently associated with mortality.
Patients and Methods
Patients
Data from all French patients with COVID-19 and a history of KT included in a nationwide registry—termed French Solid Organ Transplant (SOT) COVID—between March 4 and April 21, 2020, were retrieved. Inclusion criteria were age >18 years at the diagnosis of COVID-19 and presence of a functioning kidney graft. Patients who received double solid organ transplantation (kidney with pancreas, liver, or heart transplantation) were deemed eligible. The diagnostic criteria for COVID-19 were as follows: (i) evidence of SARS-CoV-2 infection on reverse transcriptase–polymerase chain reaction testing performed on nasopharyngeal swab specimens; or (ii) presence of typical respiratory symptoms accompanied by evocative pulmonary lesions on low-dose chest computed tomography even when reverse transcriptase–polymerase chain reaction yielded negative results. Clinical and laboratory variables were extracted from medical records. In case of hospitalization, data on presentation and other clinical and biological variables (including ongoing immunosuppressive therapy) were collected on admission. Changes in immunosuppression during the course of hospitalization were thoroughly recorded. Patients were divided into 2 groups according to their need for hospitalization (admitted to hospital vs. managed at home). Severe COVID-19 was defined as admission (or transfer) to an intensive care unit (ICU), need for mechanical ventilation, or death. All other patients were considered nonsevere cases. Acute kidney injury was defined according to the Kidney Disease Improving Global Outcomes guidelines as an increase in serum creatinine of >50%. The creation of the French SOT COVID Registry was approved by the Institutional Review Board of Strasbourg University (approval number 02.26) and registered at clinicaltrials.gov (NCT04360707). The need for informed consent was waived. However, all patients were informed about their inclusion in the registry.
Statistical analysis
Categorical data are presented as counts and percentages. Continuous variables are expressed as medians and interquartile ranges upon verification of their skewed distribution with the Shapiro-Wilk test. Two time-dependent variables served as the outcome measures. The first was a composite endpoint of severe COVID-19 (including admission/transfer to an ICU, need for mechanical ventilation, or death), whereas the second was a hard endpoint consisting of death only. Survival curves were plotted with the Kaplan-Meier method and compared with the log-rank test. Cox proportional hazard univariable and multivariable models were constructed to identify predictors of the study endpoints. All variables showing an association with a P < 0.1 in univariable analysis were included as covariates in the multivariable model using a backward conditional selection procedure. The optimal model was selected according to the highest concordance value. Results are expressed as hazard ratios with their 95% confidence intervals. All analyses were conducted in the R environment (R Foundation for Statistical Computing, Vienna, Austria), and 2-tailed P values <0.05 were considered statistically significant.
Footnotes
see commentary on page 1404
Contributor Information
French SOT COVID Registry:
Sophie Caillard, Bruno Moulin, Samira Fafi-Kremer, Marc Hazzan, Dany Anglicheau, Alexandre Hertig, Jérôme Tourret, Benoit Barrou, Emmanuel Morelon, Olivier Thaunat, Lionel Couzi, Pierre Merville, Valérie Moal, Tristan Legris, Pierre-François Westeel, Maïté Jaureguy, Luc Frimat, Didier Ducloux, Jamal Bamoulid, Dominique Bertrand, Michel Tsimaratos, Florentine Garaix-Gilardo, Jérôme Dumortier, Sacha Mussot, Antoine Roux, Laurent Sebbag, Yannick Le Meur, Gilles Blancho, Christophe Masset, Nassim Kamar, Hélène Francois, Eric Rondeau, Nicolas Bouvier, Christiane Mousson, Matthias Buchler, Philippe Gatault, Jean-François Augusto, Agnès Duveau, Cécile Vigneau, Marie-Christine Morin, Jonathan Chemouny, Leonard Golbin, Philippe Grimbert, Marie Matignon, Antoine Durrbach, Clarisse Greze, Renaud Snanoudj, Charlotte Colosio, Betoul Schvartz, Paolo Malvezzi, Christophe Mariat, Antoine Thierry, Moglie Le Quintrec, Antoine Sicard, Jean Philippe Rerolle, Anne-Élisabeth Heng, Cyril Garrouste, Henri Vacher Coponat, Éric Epailly, Olivier Brugiere, Sébastien Dharancy, Éphrem Salame, and Faouzi Saliba
Appendix
French SOT COVID Registry
Sophie Caillard and Bruno Moulin, Service de Néphrologie et Transplantation, Hôpitaux Universitaires de Strasbourg, Strasbourg; Samira Fafi-Kremer, Laboratoire de Virologie, Hôpitaux Universitaires de Strasbourg, Strasbourg; Marc Hazzan, Service de Néphrologie, Hôpital Huriez, Lille; Dany Anglicheau, Service de Néphrologie et Transplantation Adultes, AP-HP, Hôpital Necker, Paris; Alexandre Hertig, Jérôme Tourret, and Benoit Barrou, Service de Néphrologie, AP-HP, Hôpital La Pitié Salpétrière, Paris; Emmanuel Morelon and Olivier Thaunat, Service de Néphrologie, Hôpital Edouard Herriot, Lyon; Lionel Couzi and Pierre Merville, Service de Néphrologie–Transplantation–Dialyse, Hôpital Pellegrin, Bordeaux; Valérie Moal and Tristan Legris, Service de Néphrologie et Transplantation, AP-HM, Hôpital de la Conception, Marseille; Pierre-François Westeel and Maïté Jaureguy, Service de Néphrologie, CHU Amiens Picardie, Amiens; Luc Frimat, Service de Néphrologie, CHRU Nancy, Vandoeuvre; Didier Ducloux and Jamal Bamoulid, Service de Néphrologie, Hôpital Jean-Minjoz, Besancon; Dominique Bertrand, Service de Néphrologie, CHU de Rouen, Rouen; Michel Tsimaratos and Florentine Garaix-Gilardo, Service de Pédiatrie Multidisciplinaire, Hôpital La Timone, Marseille; Jérôme Dumortier, Service d’Hépato-Gastroentérologie, Hôpital Edouard Herriot, Lyon; Sacha Mussot and Antoine Roux, Centre Chirurgical Marie Lannelongue, Le Plessis Robinson; Laurent Sebbag, Service d’insuffisance Cardiaque, Hôpital Louis Pradel, Bron; Yannick Le Meur, Service de Néphrologie, Hôpital de la Cavale Blanche, Brest; Gilles Blancho and Christophe Masset, Service de Néphrologie–Transplantation, Hôtel Dieu, Nantes; Nassim Kamar, Service de Néphrologie et Transplantation, Hôpital Rangueil, Toulouse; Hélène Francois and Eric Rondeau, Service de Néphrologie, Dialyse et Transplantation, AP-HP, Hôpital Tenon, Paris; Nicolas Bouvier, Service de Néphrologie, Dialyse, Transplantation Rénale, CHU, Caen; Christiane Mousson, Service de Néphrologie, Dijon; Matthias Buchler and Philippe Gatault, Service de Néphrologie, Tours; Jean-François Augusto and Agnès Duveau, Service de Néphrologie, Dialyse, Transplantation, CHU Angers, Angers; Cécile Vigneau, Marie-Christine Morin, Jonathan Chemouny, Leonard Golbin, Service de Néphrologie, CHU de Rennes, Rennes; Philippe Grimbert, Marie Matignon, and Antoine Durrbach, Service de Néphrologie, Hôpital Henri-Mondor, Creteil; Clarisse Greze, Service de Néphrologie, AP-HP, Hôpital Bichat Claude Bernard, Paris; Renaud Snanoudj, Service de Néphrologie, Hôpital Foch, Service de Néphrologie et Transplantation Hôpital du Kremlin Bicêtre, Le Kremlin Bicetre; Charlotte Colosio, Betoul Schvartz, Service de Néphrologie, Hôpital Maison Blanche, Reims; Paolo Malvezzi, Service de Néphrologie, Hémodialyse, Transplantation Rénale, Hôpital La Tronche, Grenoble; Christophe Mariat, Service de Néphrologie, CHU de Saint Etienne, Saint Etienne; Antoine Thierry, Service de Néphrologie, Hémodialyse et Transplantation Rénale, Hôpital Jean Bernard, Poitiers ; Moglie Le Quintrec, Service de Néphrologie-Transplantation-Dialyse, CHU Lapeyronie, Montpellier; Antoine Sicard, Service de Néphrologie, Hôpital Pasteur, Nice; Jean Philippe Rerolle, Service de Néphrologie, CHU Dupuytren, Limoges; Anne-Élisabeth Heng and Cyril Garrouste, Service de Néphrologie, CHU Gabriel Montpied, Clermont-Ferrand; Henri Vacher Coponat, Service de Néphrologie, CHU de La Réunion, Saint Denis; Éric Epailly, Service de Cardiologie, Hôpitaux Universitaires de Strasbourg, Strasbourg; Olivier Brugiere, Service d’hépatologie, Hôpital Foch, Suresnes; Sébastien Dharancy, Service d’hépatologie, Hôpital Huriez, Lille; Éphrem Salame, Service de Chirurgie Hépatique, Hôpital Universitaire de Tours, Tours; and Faouzi Saliba, Service d’Hépatologie, Centre Hépato-Biliaire Paul Brousse, Villejuif
Disclosure
All the authors declared no competing interests.
Author Contributions
SC and MH had full access to all the data of the cohort study and take responsibility for the integrity of the data and the accuracy of the analyses. Conception and design were completed by SC, MH, and YL. Acquisition, analysis, and interpretation of data were conducted by SC, DA, MM, ADur, CG, LF, OT, TL, VM, PFW, NK, PGa, RS, AS, DB, CC, LC, JC, CM, GB, JB, ADuv, NB, NC, PGr, BM, YL, and MH. Drafting of the manuscript was completed by SC and MH. Statistical analysis was conducted by MH. Critical revision of the manuscript for important intellectual content was performed by SC, DA, MM, ADur, CG, LF, OT, TL, VM, PFW, NK, PGa, RS, AS, DB, CC, LC, JC, CM, GB, JB, ADuv, NB, NC, PGr, BM, YL, and MH.
References
- 1.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal P., Choi J.J., Pihneiro L.C. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillen E., Pineiro G.J., Revuelta I. Case report of COVID-19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20:1875–1878. doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L., Xu X., Ma K. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20:1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong Z., Zhang Q., Xia H. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20:1916–1921. doi: 10.1111/ajt.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandolfini I., Delsante M., Fiaccadori E. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1941–1943. doi: 10.1111/ajt.15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberici F., Delbarba E., Manenti C. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia Kidney. Int. 2020;97:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Columbia University Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31:1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez Ruiz M., Andres A., Lopez-Medrano F., San Juan R. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akalin E., Azzi Y., Bartash R. COVID-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira M.R., Mohan S., Cohen D.J. COVID-19 in solid transplant organ recipients: initial report of the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee D., Popoola J., Shah S. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi S.G., Rogers A.W., Saharia A. Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center. Transplantation. 2020;104:2208–2214. doi: 10.1097/TP.0000000000003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair V., Jandovitz N., Hirsch J.S. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L., Gong N., Liu B. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77:748–754. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen T.Y., Farghaly S., Cham S. COVID-19 pneumonia in kidney transplant recipients: focus on immunosuppression management. Transpl Infect Dis. 2020;23:e13378. doi: 10.1111/tid.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoek R.A.S., Manintveld O.C., Betjes M.G.H. COVID-19 in solid organ transplant recipients: a single-center experience. Transpl Int. 2020;33:1099–1105. doi: 10.1111/tri.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Husain S.A., Dube G., Morris H. Early outcomes of outpatient management of kidney transplant recipients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15:1174–1178. doi: 10.2215/CJN.05170420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim G.U., Kim M.J., Ra S.H. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020;26:948.e1–948.e3. doi: 10.1016/j.cmi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bossini N., Alberici F., Delbarba E. Kidney transplant recipients with SARS-CoV-2 infection: The Brescia Renal Covid task force experience. Am J Transplant. 2020;20:3019–3029. doi: 10.1111/ajt.16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maggiore U., Abramowicz D., Crespo M. How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion. Nephrol Dial Transplant. 2020;35:899–904. doi: 10.1093/ndt/gfaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y., Yu X., Zhao H. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial [epub ahead of print]. MedRvix. doi.org/10.1101/2020.03.22.20040758. Accessed May 15, 2020.
- 27.Willicombe M., Thomas D., McAdoo S. COVID-19 and calcineurin inhibitors: Should they get left out in the storm? J Am Soc Nephrol. 2020;31:1145–1146. doi: 10.1681/ASN.2020030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.French guidelines for COVID-19 management in solid organ transplant recipients A collegial statement endorsed by the «Société Francophone de Transplantation» (SFT), the «Société Francophone de Néphrologie, Dialyse et Transplantation» (SFNDT) the «Groupe Infection et Immunodépression», and the «Société de pathologie infectieuse de langue française» (SPILF) https://www.transplantation-francophone.org/images/public/COVID19_et_transplantees_d_organes_solides_Guide_pratiquev1_SFT_SFNDT_SP.pdf Available at: Accessed May 15, 2020.
- 29.De Wilde A.H., Zevenhoven-Dobbe J.C., Van der Meer Y. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol. 2011;92:2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carbajo-Lozoya J., Müller M.A., Kallies S. Replication of human coronaviruses SARS-CoV, HCoVNL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165:112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang W., Cao Z., Han M. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Giambenedetto S., Ciccullo A., Borghetti A. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J Med Virol. 2020;92:1787–1788. doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]