Abstract
Purpose
Human epidermal growth factor receptor 2 (HER2) targeting plus endocrine therapy (ET) improved clinical benefit in HER2-positive, hormone receptor (HR)–positive metastatic breast cancer (MBC) versus ET alone. Dual HER2 blockade enhances clinical benefit versus single HER2 blockade. The ALTERNATIVE study evaluated the efficacy and safety of dual HER2 blockade plus aromatase inhibitor (AI) in postmenopausal women with HER2-positive/HR-positive MBC who received prior ET and prior neo(adjuvant)/first-line trastuzumab (TRAS) plus chemotherapy.
Methods
Patients were randomly assigned (1:1:1) to receive lapatinib (LAP) + TRAS + AI, TRAS + AI, or LAP + AI. Patients for whom chemotherapy was intended were excluded. The primary end point was progression-free survival (PFS; investigator assessed) with LAP + TRAS + AI versus TRAS + AI. Secondary end points were PFS (comparison of other arms), overall survival, overall response rate, clinical benefit rate, and safety.
Results
Three hundred fifty-five patients were included in this analysis: LAP + TRAS + AI (n = 120), TRAS + AI (n = 117), and LAP + AI (n = 118). Baseline characteristics were balanced. The study met its primary end point; superior PFS was observed with LAP + TRAS + AI versus TRAS + AI (median PFS, 11 v 5.7 months; hazard ratio, 0.62; 95% CI, 0.45 to 0.88; P = .0064). Consistent PFS benefit was observed in predefined subgroups. Overall response rate, clinical benefit rate, and overall survival also favored LAP + TRAS + AI. The median PFS with LAP + AI versus TRAS + AI was 8.3 versus 5.7 months (hazard ratio, 0.71; 95% CI, 0.51 to 0.98; P = .0361). Common adverse events (AEs; ≥ 15%) with LAP + TRAS + AI, TRAS + AI, and LAP + AI were diarrhea (69%, 9%, and 51%, respectively), rash (36%, 2%, and 28%, respectively), nausea (22%, 9%, and 22%, respectively), and paronychia (30%, 0%, and 15%, respectively), mostly grade 1 or 2. Serious AEs were reported similarly across the three groups, and AEs leading to discontinuation were lower with LAP + TRAS + AI.
Conclusion
Dual HER2 blockade with LAP + TRAS + AI showed superior PFS benefit versus TRAS + AI in patients with HER2-positive/HR-positive MBC. This combination offers an effective and safe chemotherapy-sparing alternative treatment regimen for this patient population.
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2)–positive breast cancer (BC) constitutes approximately 20% to 25% of BC and is associated with poor prognosis and survival outcomes.1-3 Approximately 50% of HER2-positive BCs are hormone receptor (HR)-positive.4,5 Thus HER2-positive/HR-positive represent roughly 10% of all BCs.6,7
Patients with HER2-positive metastatic BC (MBC) are usually treated with chemotherapy-based regimens irrespective of HR status.8 HER2-targeted therapies such as trastuzumab (TRAS), lapatinib (LAP), pertuzumab (PTZ), and trastuzumab-emtansine (T-DM1) have significantly improved treatment outcomes in HER2-positive MBC.9-14 In the first-line setting, dual HER2 blockade with TRAS + PTZ plus chemotherapy (with or without endocrine therapy [ET] in HR-positive BCs) has been shown to improve survival and represents a commonly used standard-of-care therapy for HER2-positive MBC.13,15,16
However, not all patients with HER2-positive/HR-positive MBC may need or tolerate chemotherapy, and these patients could be candidates for anti-HER2 therapies plus ET.16 Preclinical evidence suggests that cross talk between HER2- and HR-signaling pathways contributes to endocrine resistance.5,17,18 Furthermore, in HER2-positive and estrogen receptor (ER)–positive BC cells, either HER2 or ER can function as the major promoter of survival. With effective HER2 inhibition in these cells, ER can become the primary driver of proliferation, resulting in relative resistance to anti-HER2 therapy.19,20 Therefore, cotargeting HER2 and ER simultaneously may be essential for obtaining optimal benefit in patients with HER2-positive/HR-positive MBC. Two previous studies21,22 have demonstrated the benefit of combining single HER2 blockade plus ET, without chemotherapy, in the first-line setting compared with ET alone in HER2-positive/HR-positive MBC. A significant reduction in the risk of progression was observed with the combination of TRAS plus anastrozole (ANA) or LAP plus letrozole (LET) compared with each ET alone (ie, median progression-free survival [mPFS], 4.8 v 2.4 months [TAnDEM; Trastuzumab and Anastrozole Directed Against ER-Positive HER2-Positive Mammary Carcinoma]21 and 8.2 v 3.0 months [EGF30008],22 respectively).
The use of dual anti-HER2 blockade represents a potential strategy to further improve the outcome of these patients.23 Preclinical models of HER2-positive BC have shown that a combination of TRAS + LAP results in a more optimal anticancer effect.24 Dual targeting of HER2-positive tumors with TRAS and LAP is beneficial because of differing mechanisms of action and the well-characterized synergistic interaction between them in HER2 BC models.24-27 In the clinic, dual anti-HER2 blockade has been shown to improve outcomes in both the neoadjuvant and metastatic settings compared with single HER2 blockade.13,25,28,29
Therefore, the present study (EGF114299) was designed to evaluate whether dual HER2 blockade with LAP and TRAS plus aromatase inhibitor (AI), without chemotherapy, improves outcomes compared with TRAS + AI in patients with HER2-positive/HR-positive MBC who experienced disease progression after prior neo(adjuvant)/first-line TRAS plus chemotherapy. The study also included a third arm of LAP + AI to explore if this combination results in different outcomes compared with the other two arms.
METHODS
Study Design and Treatment
In this phase III, open-label study, eligible patients were randomly assigned (1:1:1) to receive either LAP + TRAS or TRAS or LAP, plus an AI, hereafter referred to as the LAP + TRAS + AI, TRAS + AI, and LAP + AI treatment arms, respectively. Random assignment of patients was stratified on the basis of the investigator’s choice of AI (steroidal/nonsteroidal) and prior TRAS usage in the neo(adjuvant)/metastatic setting.
Oral LAP of 1,000 mg/day was self-administered by patients in the LAP + TRAS + AI arm and 1,500 mg/day in the LAP + AI arm. TRAS was administered intravenously at a loading dose of 8 mg/kg, followed by the maintenance dose of 6 mg/kg intravenously every 3 weeks in both TRAS arms. Investigator’s choice of oral AIs included LET (2.5 mg/day) or ANA (1 mg/day) or exemestane (EXE; 25 mg/day) as shown in the Appendix (online only). Prophylactic antidiarrheal agents were not mandated. It was highly recommended for patients receiving LAP to initiate a prescription of loperamide treatment at the onset of diarrhea.
The study was conducted in accordance with the Good Clinical Practice guidelines and Declaration of Helsinki and all applicable country-specific regulatory requirements. The study-related protocol was approved by the institutional review board or independent ethics committee of each study site. Written informed consent was obtained from all patients before performing any study-related procedures. This trial was registered with the European Clinical Trials database (EUDRACT ID: 2010-019577-16) and ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT01160211).
Patients
The study enrolled postmenopausal women ≥ 18 years of age with histologically or cytologically confirmed ER-positive and/or progesterone receptor–positive (HR-positive), HER2-positive MBC as determined in a local laboratory. Prior treatment with ET and disease progression during or after a prior regimen containing TRAS plus chemotherapy in the neo(adjuvant) setting and/or in the first-line metastatic setting was required (maximum one prior regimen in the metastatic setting). Patients with either measurable or nonmeasurable disease per Response Evaluation Criteria in Solid Tumors (RECIST version 1.1), Eastern Cooperative Oncology Group performance status 0 or 1, adequate baseline organ function (hematologic, hepatic, and renal), and no active or history of cardiac disease were included. Patients for whom chemotherapy was intended per investigator’s judgement were excluded.
Study Objectives
The primary objective was to evaluate the superiority of PFS (radiologic progression only per investigator assessment or death) with LAP + TRAS + AI versus TRAS + AI using RECIST version 1.1. Nonradiologic response was on the basis of clinical signs and symptoms. Of note, PFS and response rate were only on the basis of radiologic assessment. Secondary objectives were PFS with TRAS + AI versus LAP + AI and LAP + TRAS + AI versus LAP + AI arms, PFS in defined subgroups, overall response rate (ORR) and clinical benefit rate (CBR; either complete response or partial response or stable disease for at least 6 months), duration of response, overall survival (OS), safety, and change in quality of life status from baseline (Appendix, online only).
Procedures
Tumor samples were collected (Covance Laboratory) and submitted to a central laboratory (University of Southern California) for retrospective confirmation of HER2 and HR status according to American Society of Clinical Oncology-College of American Pathologists clinical practice guidelines criteria for fluorescent in situ hybridization (FISH; Abbott Molecular) and immunohistochemistry (IHC; HercepTest, Dako). Tumor assessments were performed at baseline, within 28 days of randomization, and thereafter every 12 weeks until disease progression or discontinuation of the study treatment and at the end of the study. Efficacy assessments were performed locally using radiologic scans, including computed tomography or magnetic resonance imaging. Safety was monitored throughout the study. The severity of adverse events (AEs) was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Left ventricular ejection fraction (LVEF) was evaluated every 12 weeks using echocardiogram and/or Multi Gated Acquisition Scan. Patient-reported health-related quality of life outcomes were evaluated using the Functional Assessment of Cancer Therapy-Breast questionnaire (Appendix Table A1, online only).
Statistical Analysis
At the time of amendment implementation (to change the primary end point from OS to PFS), a minimum of 121 PFS events in the LAP + TRAS + AI and TRAS + AI treatment arms were required to achieve 80% power (two-sided alpha = 0.05) to detect a 67% increase (hazard ratio, 0.60) in mPFS (from an estimated 7 months in the TRAS + AI arm to 11.7 months in the LAP + TRAS + AI arm). The intent-to-treat population, comprising all randomly assigned patients, was used for efficacy analyses.
PFS and OS across treatment arms was estimated using the Kaplan-Meier method as shown in the Appendix. Comparison of PFS for the primary end point was performed using a two-sided stratified log-rank test (using baseline stratification factors). The Pike estimator was used to estimate the hazard ratio, along with the 95% CI. Two sensitivity analyses for PFS were performed, in which nonradiologic progressions and patients who received a subsequent anticancer therapy, respectively, were treated as events. The effect of prespecified baseline prognostic factors (including measurable disease, type of AI, and prior TRAS usage) on PFS was determined using a predefined stepwise Cox regression model. A stratified Fisher’s exact test was used for ORR comparison between treatment arms.
RESULTS
Baseline Characteristics and Study Treatment
At the data cutoff defined by the required number of PFS events (March 11, 2016), 355 patients were enrolled across 112 sites in 29 countries (Fig 1). Baseline demographic characteristics were well balanced across the three treatment arms. Two-thirds of the patients received a prior TRAS chemotherapy–containing regimen in the neo(adjuvant) setting only and one-third in the metastatic setting (with or without prior TRAS in the neo[adjuvant] setting; Table 1). Prior ET included tamoxifen (55%), ANA (29%), LET (28%), and EXE (8%). In the LAP + TRAS + AI, TRAS + AI, and LAP + AI arms, 68%, 57%, and 65% of patients received study treatment in the first-line metastatic setting and 32%, 41%, and 35% in the second-line setting (or later), respectively. EXE (47%) was the most common AI received by patients in the study, followed by LET (42%) and ANA (11%). Prior therapy in the metastatic setting included fulvestrant (n = 2), PTZ (n = 17), and T-DM1 (n = 1).
Fig 1.
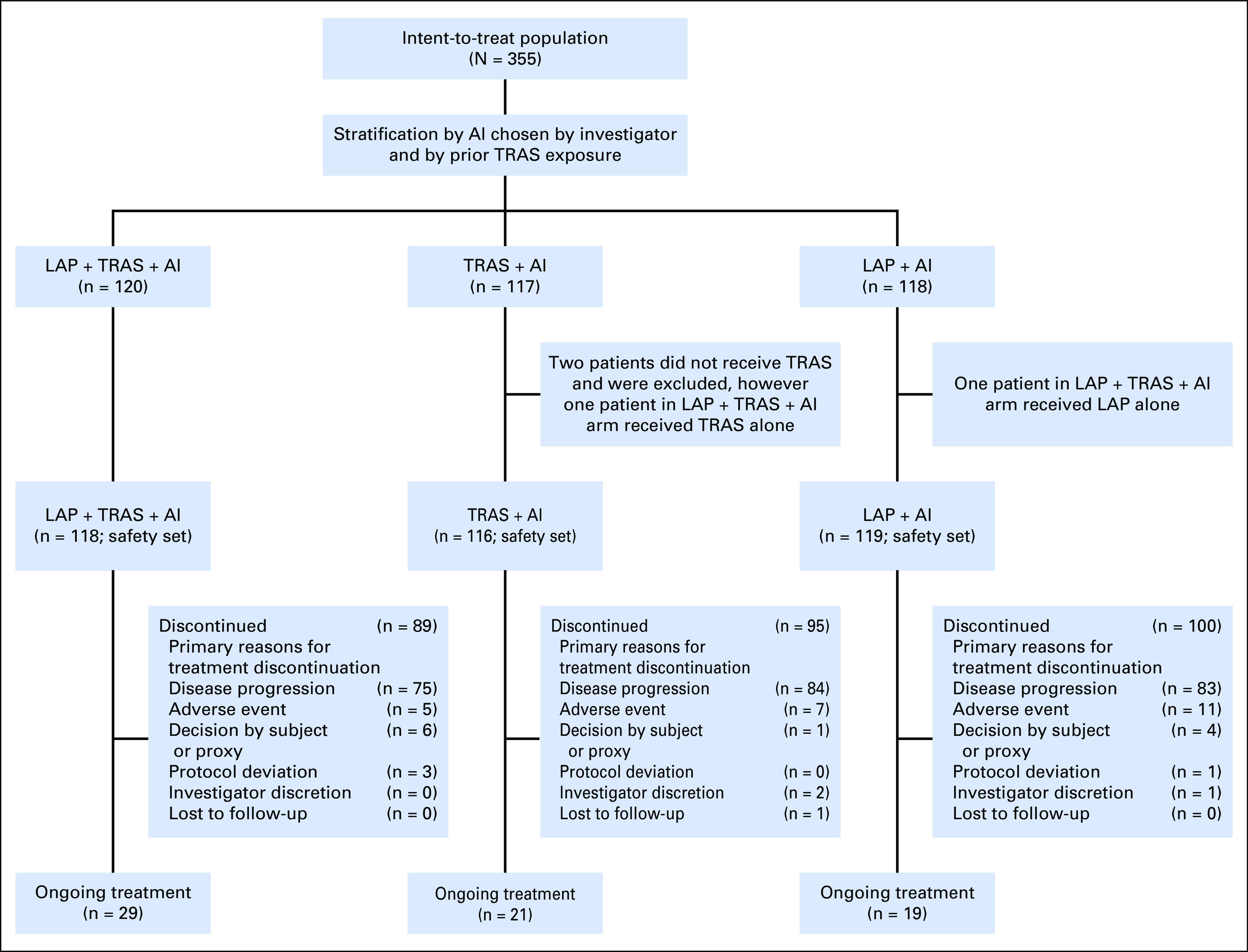
CONSORT diagram: Study design. AI, aromatase inhibitor; LAP, lapatinib; TRAS, trastuzumab.
Table 1.
Baseline Characteristic, Other Demographic Data, and Study Treatment* (intent to treat, n = 355)
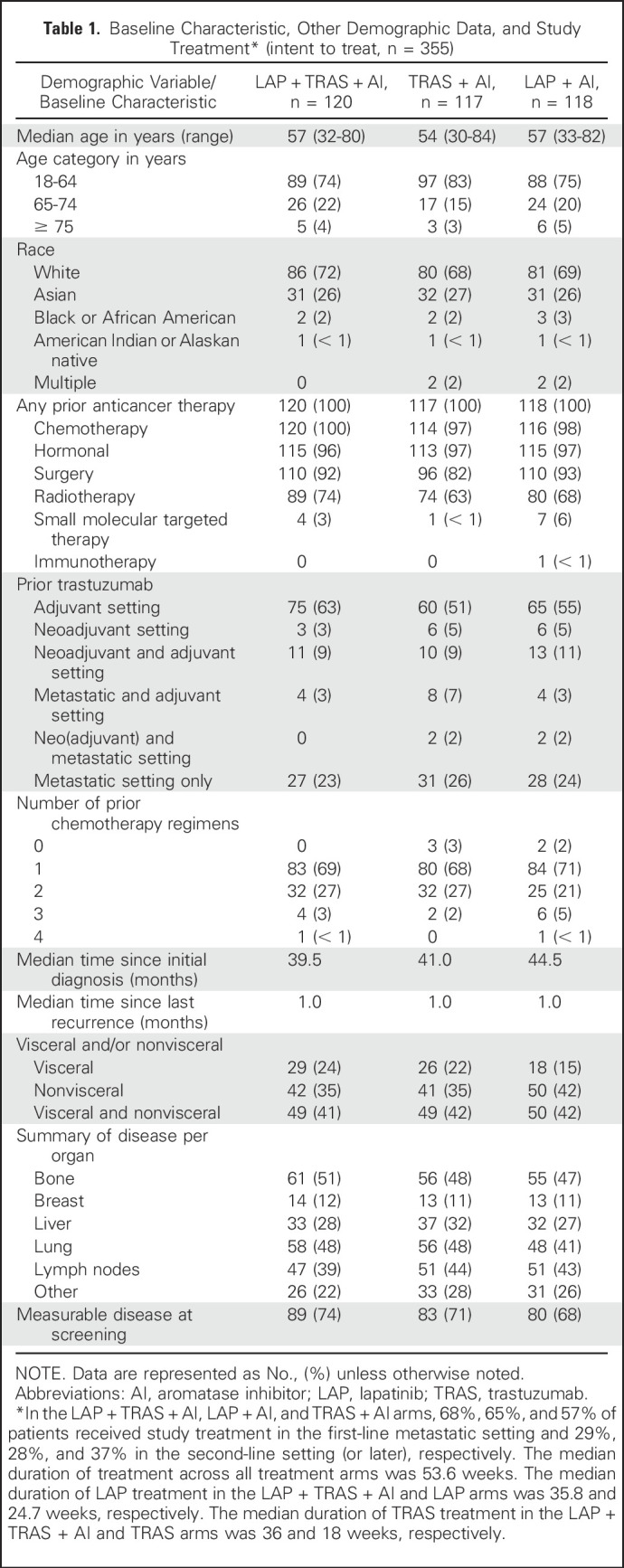
Patients were HER2-positive and HR-positive by local assessment and received prior HER2-targeted therapy and prior ET. Two hundred ninety-one tumor samples were available for central testing. HER2 assessment by FISH was evaluable in 220 samples (173 [78.6%] were HER2 positive). In addition to these 173 HER2-positive tumors (by FISH), 17 additional samples, in which FISH was not evaluable, were assessed as HER2-positive by IHC (total, 190 HER2-positive). HR assessment confirmed HR-positive status in 241 of 285 evaluable samples (84.5%) by IHC. Of 190 HER2-positive tumors, 154 were confirmed as HR-positive by central testing.
The overall median duration of treatment across all treatment arms was 53.6 weeks. The median duration of LAP treatment in the LAP + TRAS + AI and LAP + AI arms was 35.8 weeks and 24.7 weeks, respectively. The median duration of TRAS treatment was 36 weeks in LAP + TRAS + AI and 18 weeks in TRAS + AI arms.
Efficacy
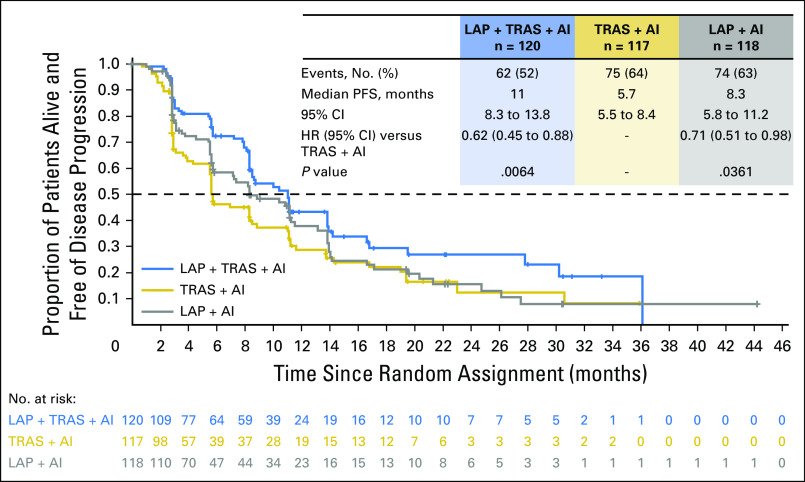
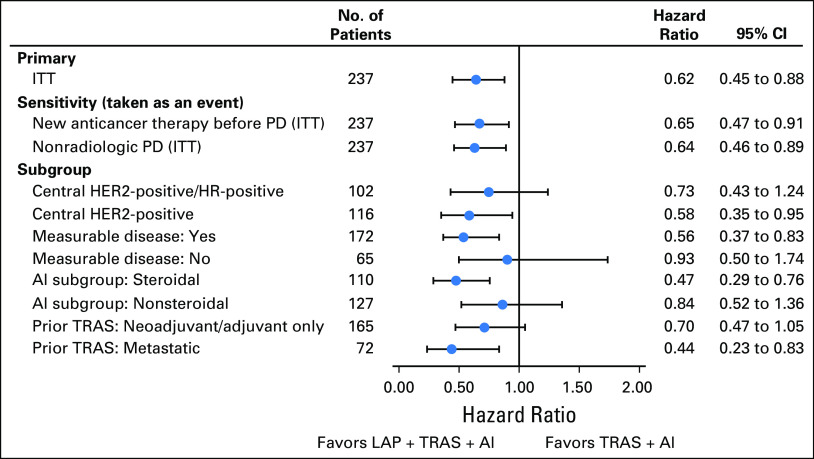
The PFS analysis (intent-to-treat population) was performed after 137 PFS events were reported in the LAP + TRAS + AI and TRAS + AI arms. The trial met its primary end point; superior PFS was observed with LAP + TRAS + AI versus TRAS + AI (mPFS, 11 v 5.7 months; hazard ratio, 0.62; 95% CI, 0.45 to 0.88; P = .0064; Fig 2). A similar magnitude of benefit favoring the LAP + TRAS + AI arm was observed in the following two sensitivity analyses of PFS: (1) not censoring patients receiving alternative anticancer therapies before a PFS event (hazard ratio, 0.65; 95% CI, 0.47 to 0.91), and (2) inclusion of nonradiologic progressions (three and two additional events, respectively) as events (hazard ratio, 0.64; 95% CI, 0.46 to 0.89; Fig 3). The mPFS with LAP + AI versus TRAS + AI was 8.3 versus 5.7 months, respectively (hazard ratio, 0.71; 95% CI, 0.51 to 0.98; P = .0361; Fig 2). A consistent PFS benefit was observed in predefined subgroups: patients with measurable disease, patients treated with AIs (steroidal/nonsteroidal), and patients receiving prior TRAS in a neo(adjuvant) or metastatic setting (Fig 3). Cox regression analysis including all factors that had a significant effect on PFS in addition to treatment showed that the PFS benefit with LAP + TRAS + AI was consistent with the primary analysis (hazard ratio, 0.56; 95% CI, 0.394 to 0.794). A consistent PFS benefit with LAP + TRAS + AI was observed in the centrally confirmed HER2-positive subpopulation (by FISH or IHC: hazard ratio, 0.62; 95% CI, 0.39 to 0.99 and by FISH only: hazard ratio, 0.58; 95% CI, 0.35 to 0.95). Similarly, in the centrally confirmed HER2-positive/HR-positive subpopulation, the benefit of LAP + TRAS + AI was confirmed (hazard ratio, 0.73; 95% CI, 0.43 to 1.24).
Fig 2.
Kaplan-Meier graph of progression-free survival in all treatment arms (intent to treat). AI, aromatase inhibitor; HR, hazard ratio; LAP, lapatinib; PFS, progression-free survival; TRAS, trastuzumab.
Fig 3.
Progression-free survival across predefined subgroups. AI, aromatase inhibitor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; ITT, intent to treat; LAP, lapatinib; PD, progressive disease; TRAS, trastuzumab.
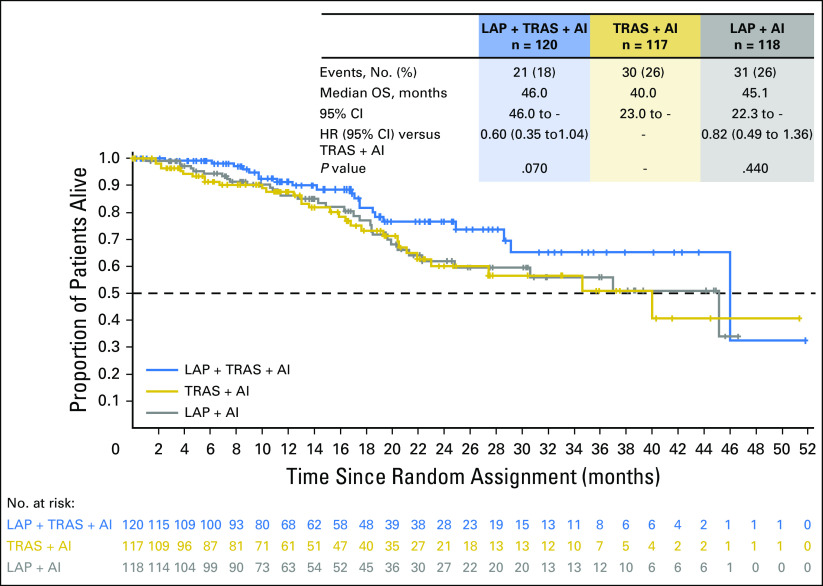
The ORR with LAP + TRAS + AI, TRAS + AI, and LAP + AI was 31.7%, 13.7%, and 18.6%, respectively. The median duration of response was 13.9 months, 8.3 months, and 11.1 months, respectively. The CBR was 41%, 31%, and 33%, respectively (Table 2). OS data were immature at the time of the present analysis but trended in favor of the LAP + TRAS + AI versus the TRAS + AI arm, in line with the primary PFS analysis (median OS, 46.0 v 40.0 months; hazard ratio, 0.60; 95% CI, 0.35 to 1.04; Fig 4). The median OS was 45.1 and 40.0 months with LAP + AI and TRAS + AI, respectively (hazard ratio, 0.82).
Table 2.
Best Overall Response (intent to treat)
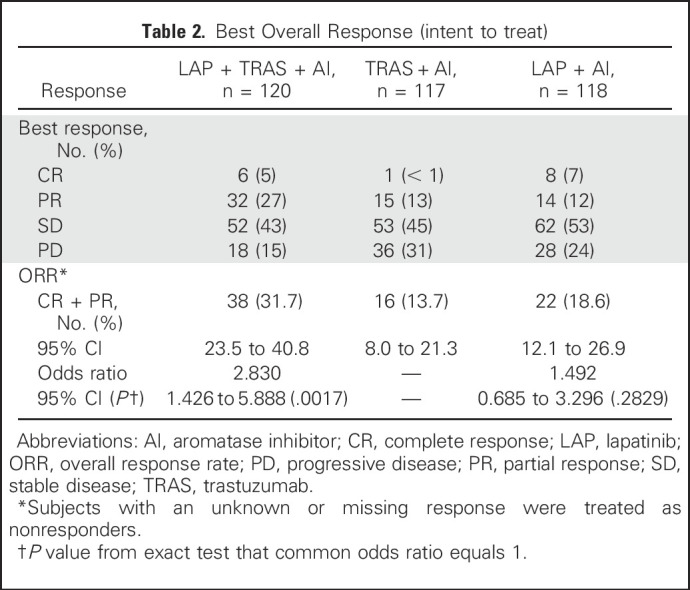
Fig 4.
Kaplan-Meier graph of overall survival in all treatment arms (intent to treat). AI, aromatase inhibitor; HR, hazard ratio; LAP, lapatinib; OS, overall survival; TRAS, trastuzumab.
Safety
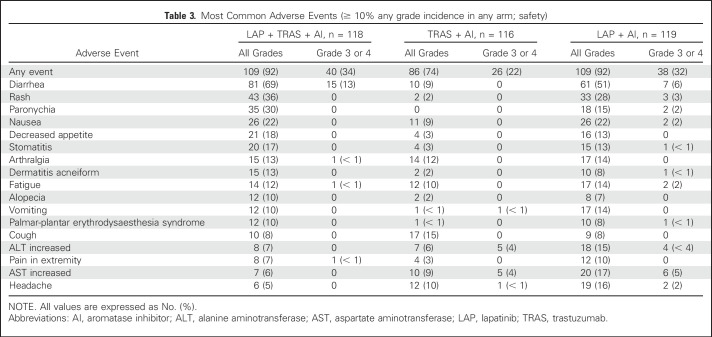
The safety population included 353 patients; two patients did not receive study treatment and were excluded. The most common AEs (any grade) with LAP + TRAS + AI, TRAS + AI, and LAP + AI (≥ 10% in any arm) were diarrhea (69%, 9%, and 51%, respectively), rash (36%, 2%, and 28%, respectively), nausea (22%, 9%, and 22%, respectively), and paronychia (30%, 0%, and 15% respectively; Table 3). Most events were grade 1 or 2. The frequency of grade 3 or 4 AEs was low in the three treatment arms (< 5%), with the exception of 13% and 6% of grade 3 diarrhea in the LAP + TRAS + AI and LAP + AI arms, respectively. There were no grade 4 diarrhea events. The incidence of serious AEs (SAEs) irrespective of causality and treatment-related SAEs in the LAP + TRAS + AI, TRAS + AI, and LAP + AI arms was 14%, 10%, and 17%, and 5%, 2%, and 4%, respectively. On-treatment deaths were reported in three (3%), five (4%), and six patients (5%), respectively. All deaths were due to disease progression, except for one death each due to cardiogenic shock and organ failure in the LAP + AI arm and one death due to cardiopulmonary arrest in the TRAS + AI arm. Cardiac safety-related events were noted in 7%, 3%, and 2%, respectively. Any decrease in the LVEF was observed in 59%, 65%, and 65%, respectively, but was > 20% in 3%, 2%, and 0%, and less than the lower limit of normal in 5%, < 1%, and 4%, respectively. One, three, and two patients in the LAP + TRAS + AI, TRAS + AI, and LAP + AI arms, respectively, discontinued treatment as the result of LVEF decrease/cardiogenic shock.
Table 3.
Most Common Adverse Events (≥ 10% any grade incidence in any arm; safety)
Overall, the incidence of AEs leading to discontinuation of study treatment was low, and it was lower in the LAP + TRAS + AI arm (3%; n = 4), than in the TRAS + AI (6%; n = 7) and LAP + AI (9%; n = 11) arms. The most frequent AE leading to discontinuation was transaminases increase (n = 8), which occurred in the LAP + AI arm where the dose of LAP was the highest. LAP dose reduction was observed in 20% (LAP + TRAS + AI) and 17% (LAP + AI) of patients; the primary reasons for reduction were AEs (71% and 37%, respectively) and patient noncompliance (25% and 63%, respectively). Dose interruptions of LAP in the LAP + TRAS + AI and LAP + AI arms were required in 38% and 35% of patients, respectively, mainly due to AEs (51% and 50%, respectively). In the LAP + TRAS + AI and TRAS + AI arms, dose delays of TRAS were recorded in 19% and 9%; the main reasons for the delays were AEs (38% and 15%, respectively) and noncompliance (28% and 15%, respectively). Treatment discontinuation was reported in 75%, 82%, and 84% of patients in the LAP + TRAS + AI, TRAS + AI, and LAP + AI arms, respectively. The most common reasons for discontinuation in the LAP + TRAS + AI, TRAS + AI, and LAP + AI arms were disease progression (64%, 72%, and 70%, respectively) and AEs (4%, 6%, and 9%, respectively).
DISCUSSION
The ALTERNATIVE trial evaluated if dual HER2 blockade with LAP + TRAS + AI was superior to TRAS + AI in patients with HER2-positive/HR-positive MBC. To our knowledge, this is the first large, randomized clinical trial to exclusively evaluate this chemotherapy-sparing regimen in this specific HER2-positive/HR-positive MBC population of patients who had already received prior ET and prior TRAS plus chemotherapy in the neo(adjuvant) and/or first-line metastatic setting. Our study demonstrated that the combination of LAP + TRAS + AI provided a clinically meaningful prolongation of PFS compared with TRAS + AI (mPFS, 11.0 v 5.7 months, respectively), representing a statistically significant 38% reduction in the risk of disease progression (hazard ratio, 0.62). The PFS benefit was consistently observed in various subgroups of patients. Furthermore, the ORR (31.7% v 13.7%) and CBR (41% v 31%) favored the LAP + TRAS + AI combination. Although survival data were immature, there was also a trend in favor of the dual-blockade treatment (median, 46.0 v 40.0 months).
Although cross-trial comparisons need to be interpreted with caution because of differences in patient populations, the PFS outcome observed with LAP + TRAS + AI in ALTERNATIVE is in line with various chemotherapy plus single HER2 blockade regimens in patients with HER2-positive MBC (including both HR-positive and HR-negative patients) previously treated with TRAS and ET. Trials specifically designed for patients with HER2-positive/HR-positive MBC evaluating chemotherapy plus single blockade are limited. The PERTAIN30 trial showed the benefit of dual HER2 blockade (hazard ratio, 0.65) with a mPFS of 18.9 months with PTZ + TRAS + ET versus 15.80 months with TRAS + ET. However, this trial was conducted only in the first-line metastatic setting, and approximately 77% of patients were TRAS naïve and 55% of patients had received induction chemotherapy.
The meaningful clinical benefit with dual HER2 blockade observed in ALTERNATIVE and in other MBC trials is in contrast with the lack of benefit observed in the adjuvant setting in ALTTO31 (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization; with LAP) or the rather modest benefit in APHINITY32 (with PTZ). This discrepancy may be the result, at least in part, of the excellent outcome with adjuvant single HER2 blockade with TRAS, making the demonstration of additional benefit with dual blockade harder. Dual HER2 blockade may benefit only a small subset of high-risk patients.
ALTERNATIVE included a third arm investigating LAP + AI, which was a mandated study design after the EGF3000822 study and the approval of LAP + LET. Although ALTERNATIVE was not powered for comparison between LAP + AI versus TRAS + AI arms, the results showed that LAP + AI (mPFS, 8.3 months) was superior to TRAS + AI (mPFS, 5.7 months) in patients with HER2-positive/HR-positive MBC previously treated with TRAS and ET. This finding is in contrast with other randomized trials in BC comparing LAP- and TRAS-containing regimens where no difference or better outcome with TRAS was reported. However, these trials were conducted in different patient populations (both HR-positive and HR-negative tumors) and in a different clinical setting (neo[adjuvant]), included chemotherapy-containing regimens, and/or had different requirements for prior TRAS treatment. The better outcome in ALTERNATIVE with LAP + AI may suggest that in HER2-positive/HR-positive MBC that has progressed after prior TRAS, LAP may be the preferred anti-HER2 treatment in combination with ET. This hypothesis needs to be confirmed in a trial designed with that purpose, although the feasibility of such a trial is questionable considering that dual HER2 blockade plus ET would be the preferred option.
The safety profile of the three treatment arms was consistent with the known safety profile of LAP and TRAS. The frequency of AEs, mostly grade 1 or 2, was higher in the two LAP arms (92% each) than in the TRAS arm (74%). Diarrhea, rash, paronychia, and nausea were among the most frequent AEs. However, the frequency of grade 3 or 4 AEs and SAEs was similar in the three arms, with the exception of grade 3 diarrhea, which was higher in the LAP + TRAS + AI arm (13%).
In conclusion, the PFS benefit obtained with LAP + TRAS + AI in patients with HER2-positive, HR-positive MBC who had been previously treated with TRAS and ET is clinically meaningful and robust. The ALTERNATIVE trial showed relevant clinical benefit with a relatively good tolerability, supporting that patients with HER2-positive/HR-positive MBC who are not candidates for chemotherapy can be adequately treated with dual HER2 blockade (LAP + TRAS) plus an AI. This combination can potentially offer an effective and well-tolerated, chemotherapy-sparing alternative treatment regimen for patients for whom chemotherapy is not intended.
ACKNOWLEDGMENT
Supported by the NIHR Biomedical Research Centre at The Royal Marsden NHS Foundation Trust (S.R.D.J.). The authors acknowledge Ivonne Villalobos, Angela Santiago, Roberta Guzman, Marianne Banzuelo, and Monica Estrada for their technical support in the central laboratory. The authors thank all of the Steering Committee members of this study: William J. Gradishar (chair), Stephen R.D. Johnston (co-chair), Fatima Cardoso, Sergio Simon, Hiroji Iwata, Zsuzsanna Kahán, Sergei Tjulandin, Kristina Lübbe, Zora Nešković-Konstantinović, Janice Tsang, Damir Vrbanec, and Constanta Timcheva. The authors also thank the patients who participated in the trial, the investigators, study nurses, and clinical research associates from the individual trial centers who provided ongoing support, as well as Bhavani Yamsani (Novartis Healthcare Pvt. Ltd) for providing medical editorial assistance with this manuscript.
Appendix
METHODS
Study design and treatment.
Oral lapatinib (LAP) 1,000 mg/day was self-administered by patients in the LAP plus trastuzumab (TRAS) plus aromatase inhibitor (AI) arm and 1,500 mg/day in the LAP + AI arm. TRAS was administered intravenously at a loading dose of 8 mg/kg, followed by the maintenance dose of 6 mg/kg intravenously every 3 weeks in both TRAS arms. Investigator’s choice of oral AIs included letrozole (2.5 mg/day) or anastrozole (1 mg/day) or exemestane (25 mg/day). Treatment continued until disease progression, unacceptable toxicity or death, withdrawal of consent, or investigator decision.
The study was initially designed to evaluate the overall survival (OS) benefit with LAP + TRAS + AI versus TRAS + AI. However, since the trial was initiated, newly available treatment options for this patient population have resulted in a substantial prolongation of OS outcomes, making the initial protocol assumptions obsolete and significantly extending the time required to achieve the required number of OS events.13,14 In addition, next-line treatments would influence survival assessments, causing OS to be a less relevant end point. Accordingly, in agreement with the regulatory authorities, a non–data-driven protocol amendment to change the primary end point to progression-free survival was issued on March 18, 2016.
Statistical analysis.
Progression-free survival (primary end point) was defined as time from randomization until the earlier of a radiologically assessed progression event or death as the result of any cause. Patients without such an event or who initiated another anticancer therapy were censored.
RESULTS
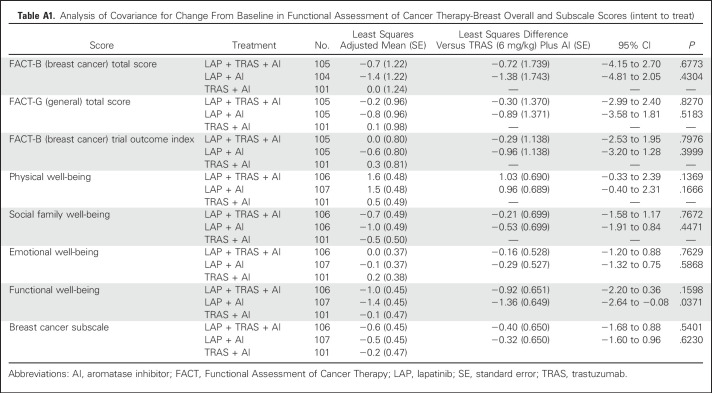
Fourteen additional patients were enrolled after the cutoff; data on these patients will be included in the final report of the trial. Patient-reported health-related quality of life outcomes were evaluated using the Functional Assessment of Cancer Therapy-Breast questionnaire. The analysis of covariance performed on the Functional Assessment of Cancer Therapy-Breast data overall showed no relevant changes over time and no differences between the treatment arms for health-related quality of life (Appendix Table A1).
Table A1.
Analysis of Covariance for Change From Baseline in Functional Assessment of Cancer Therapy-Breast Overall and Subscale Scores (intent to treat)
Footnotes
Original funding for the ALTERNATIVE study was provided by GlaxoSmithKline; as of March 2, 2015, lapatinib is an asset of Novartis Pharmaceutical Corporation, the current sponsor of this study.
Clinical trial information: EUDRACT ID: 2010-019577-16 NCT01160211.
See accompanying article on page 808
AUTHOR CONTRIBUTIONS
Conception and design: Stephen R.D. Johnston, Seock-Ah Im, Sergei Tjulandin, Hiroji Iwata, Sergio D. Simon, Lisa S. Williams, William J. Gradishar
Provision of study materials or patients: Stephen R.D. Johnston, Roberto Hegg, Seock-Ah Im, In Hae Park, Olga Burdaeva, Galina Kurteva, Michael F. Press, Sergei Tjulandin, Hiroji Iwata, Sergio D. Simon, William J. Gradishar
Collection and assembly of data: All authors
Data analysis and interpretation: Stephen R.D. Johnston, Seock-Ah Im, Galina Kurteva, Michael F. Press, Sergei Tjulandin, Hiroji Iwata, Sarah Kenny, Severine Sarp, Miguel A. Izquierdo, Lisa S. Williams, William J. Gradishar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III, Randomized Study of Dual Human Epidermal Growth Factor Receptor 2 (HER2) Blockade With Lapatinib Plus Trastuzumab in Combination With an Aromatase Inhibitor in Postmenopausal Women With HER2-Positive, Hormone Receptor–Positive Metastatic Breast Cancer: ALTERNATIVE
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Stephen R.D. Johnston
Consulting or Advisory Role: Eli Lilly, AstraZeneca, Novartis, Pfizer, OBI Pharma
Speakers’ Bureau: OBI Pharma, Puma Biotechnology
Research Funding: Pfizer (Inst)
Roberto Hegg
No relationship to disclose
Seock-Ah Im
Consulting or Advisory Role: Spectrum Pharmaceuticals, AstraZeneca, Novartis, Genentech, Hanmi
Research Funding: AstraZeneca
In Hae Park
No relationship to disclose
Olga Burdaeva
No relationship to disclose
Galina Kurteva
No relationship to disclose
Michael F. Press
Honoraria: Cepheid, Karyopharm Therapeutics, Eli Lilly, Puma Biotechnology, Halozyme Therapeutics, Biocartis, ADC Therapeutics, Scripps Health
Consulting or Advisory Role: Cepheid, Karyopharm Therapeutics, Eli Lilly, Puma Biotechnology, Halozyme Therapeutics, Biocartis, ADC Therapeutics
Research Funding: Cepheid
Sergei Tjulandin
Speakers’ Bureau: AstraZeneca, Sanofi, Eli Lilly
Research Funding: AstraZeneca (Inst)
Hiroji Iwata
Honoraria: Novartis, Chugai Pharma, Pfizer, AstraZeneca, Eisai, Daiichi Sankyo
Research Funding: MSD, Kyowa Hakko Kirin, GlaxoSmithKline, Daiichi Sankyo, Chugai Pharma, Eli Lilly, Novartis, Bayer, Pfizer
Sergio D. Simon
Consulting or Advisory Role: Roche, AstraZeneca, MSD Oncology, Pfizer
Speakers’ Bureau: AstraZeneca, Novartis, Roche
Travel, Accommodations, Expenses: Roche, AstraZeneca, Pfizer
Sarah Kenny
Employment: Novartis
Stock or Other Ownership: Novartis
Severine Sarp
Employment: Novartis
Stock or Other Ownership: Novartis
Miguel A. Izquierdo
Employment: Novartis
Stock or Other Ownership: Novartis
Lisa S. Williams
Employment: Novartis
Stock or Other Ownership: Novartis, GlaxoSmithKline
William J. Gradishar
Consulting or Advisory Role: Genentech, Puma Biotechnology, AstraZeneca/MedImmune, Pfizer, MacroGenics
REFERENCES
- 1.Ross JS, Slodkowska EA, Symmans WF, et al. : The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 14:320-368, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, et al. : Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177-182, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Godolphin W, Jones LA, et al. : Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707-712, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Quénel N, Wafflart J, Bonichon F, et al. : The prognostic value of c-erbB2 in primary breast carcinomas: A study on 942 cases. Breast Cancer Res Treat 35:283-291, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Schettini F, Buono G, Cardalesi C, et al. : Hormone receptor/human epidermal growth factor receptor 2-positive breast cancer: Where we are now and where we are going. Cancer Treat Rev 46:20-26, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Advani P, Cornell L, Chumsri S, et al. : Dual HER2 blockade in the neoadjuvant and adjuvant treatment of HER2-positive breast cancer. Breast Cancer (Dove Med Press) 7:321-335, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howlader N, Altekruse SF, Li CI, et al. : US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106:dju055, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montemurro F, Di Cosimo S, Arpino G: Human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-positive breast cancer: New insights into molecular interactions and clinical implications. Ann Oncol 24:2715-2724, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Geyer CE, Forster J, Lindquist D, et al. : Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733-2743, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Ma B, Ma Q, Wang H, et al. : Clinical efficacy and safety of T-DM1 for patients with HER2-positive breast cancer. Onco Targets Ther 9:959-976, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marty M, Cognetti F, Maraninchi D, et al. : Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol 23:4265-4274, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Leyland-Jones B, Shak S, et al. : Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Swain SM, Baselga J, Kim SB, et al. : Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372:724-734, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma S, Miles D, Gianni L, et al. : Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783-1791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. NCCN Evidence Blocks (Version 2.2017) https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf.
- 16.Santa-Maria CA, Nye L, Mutonga MB, et al. : Management of metastatic HER2-positive breast cancer: Where are we and where do we go from here? Oncology (Williston Park) 30:148-155, 2016 [PubMed] [Google Scholar]
- 17.Tripathy D, Kaufman PA, Brufsky AM, et al. : First-line treatment patterns and clinical outcomes in patients with HER2-positive and hormone receptor-positive metastatic breast cancer from registHER. Oncologist 18:501-510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Sun T, Wan D, et al. : Hormone receptor status predicts the clinical outcome of human epidermal growth factor 2-positive metastatic breast cancer patients receiving trastuzumab therapy: A multicenter retrospective study. Onco Targets Ther 8:3337-3348, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YC, Morrison G, Gillihan R, et al. : Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers—Role of estrogen receptor and HER2 reactivation. Breast Cancer Res 13:R121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia W, Bacus S, Hegde P, et al. : A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA 103:7795-7800, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman B, Mackey JR, Clemens MR, et al. : Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: Results from the randomized phase III TAnDEM study. J Clin Oncol 27:5529-5537, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Johnston S, Pippen J, Jr, Pivot X, et al. : Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 27:5538-5546, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Abramovitz M, Williams C, Loibl S, et al. : Dual blockade of HER-2 provides a greater magnitude of benefit in patients with hormone-negative versus hormone-positive breast cancer. Clin Breast Cancer 16:444-455, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Alvarez RH, Hortobagyi GN: Dual human epidermal growth factor receptor 2 blockade for the treatment of HER2-positive breast cancer. Breast Cancer 20:103-110, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackwell KL, Burstein HJ, Storniolo AM, et al. : Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28:1124-1130, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Konecny GE, Pegram MD, Venkatesan N, et al. : Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res 66:1630-1639, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Xia W, Gerard CM, Liu L, et al. : Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene 24:6213-6221, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Baselga J, Bradbury I, Eidtmann H, et al. : Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet 379:633-640, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gianni L, Pienkowski T, Im YH, et al. : Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25-32, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Rimawi M, Ferrero JM, Haba-Rodriguez J, et al. : Primary analysis of PERTAIN: A randomized, two-arm, open-label, multicenter phase II trial assessing the efficacy and safety of pertuzumab given in combination with trastuzumab plus an aromatase inhibitor in first-line patients with HER2-positive and hormone receptor-positive metastatic or locally advanced breast cancer. Presented at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 6-10, 2016 [Google Scholar]
- 31.Piccart-Gebhart M, Holmes E, Baselga J, et al. : Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: Results from the randomized phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization trial. J Clin Oncol 34:1034-1042, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Minckwitz G, Procter M, de Azambuja E, et al. : Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 377:122-131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]