Abstract
PURPOSE
Sequential drug treatments in metastatic breast cancer (MBC) are disparate. Clinical trial data includes limited reporting of treatment context, primarily including the number of prior therapies. This study evaluates the relationship between prior treatment time, prior lines of treatment, and survival using a novel visualization technique coupled with statistical analyses.
PATIENTS AND METHODS
This retrospective cohort study used a nationwide, de-identified electronic health record–derived database to identify women with hormone receptor–positive, human epidermal growth factor receptor 2–negative MBC diagnosed in 2014 who subsequently received paclitaxel. Images were created, with individual patients represented on the y-axis and time, on the x-axis. Specific treatments were represented by colored bars, with Kaplan-Meier curves overlaying the image. Separate images assessed progression-free survival and overall survival (OS). Hazard ratios (HRs) and 95% CIs from Cox proportional hazards models evaluated the association between prior treatment time and OS.
RESULTS
Of 234 patients, median survival from first paclitaxel administration was 20 months (interquartile range, 8-53 months). An inverse relationship was observed between OS after paclitaxel and timing of administration. In adjusted models, each year on treatment prior to paclitaxel was associated with a 16% increased hazard of death after paclitaxel (HR, 1.16; 95% CI, 1.05 to 1.29).
CONCLUSION
OS after a specific treatment is dependent on when a drug is given in the disease context, highlighting the potential for an overall OS benefit to be observed on the basis of treatment timing. Prior time on treatment should be considered as a stratifying factor in randomized trials and a confounding factor when examining survival in observational data.
INTRODUCTION
The National Comprehensive Cancer Network treatment guidelines provide minimal guidance about optimal sequencing of the > 50 treatment regimens (single drug or combination) listed for women with metastatic breast cancer (MBC).1 As a result, patients with MBC who have similar demographic and clinical characteristics can receive substantially different treatments and highly variable treatment sequences (eg, drug 1 followed by drug 2 followed by drug 3). Prior literature has demonstrated that first-line treatment selection has the potential to affect MBC survival,2-8but limited data are available about treatment efficacy, accounting for treatment timing during the course MBC.
CONTEXT
Key Objective
Using a sample of women with hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer (MBC) treated with paclitaxel, this study evaluates the relationship between prior treatment time, prior lines of treatment, and survival using a novel visualization technique coupled with statistical analyses.
Knowledge Generated
Of 234 women receiving paclitaxel post-MBC diagnosis, the visualization technique displayed an inverse relationship between post-paclitaxel overall survival and timing of paclitaxel administration within the treatment course. In adjusted models, each year on treatment prior to paclitaxel was associated with a 16% increased hazard of death after paclitaxel (hazard ratio, 1.16; 95% CI, 1.05 to 1.29).
Relevance
Overall survival may be dependent on when a specific drug is received in the course of treatment, highlighting potential benefit based on treatment timing. Prior time on treatment should be considered as a stratifying factor in randomized trials and a confounding factor when examining survival in observational data.
In a SEER-linked Medicare evaluation, 56% of 6,639 patients with MBC received a treatment sequence that < 10 other patients received.9 This heterogeneity, in which patients experience variations in duration of prior treatment and specific treatments received, is problematic. Patients who demonstrate chemotherapy resistance are less likely to have future therapeutic response.10 Banerji et al11 reported that the best predictor of response to third-line therapy was response to previous therapies in a small sample of patients with MBC (N = 149). In this study, patients who experienced progression on first- and second-line therapies had a third-line response rate of 20% compared with a third-line response rate of 45% in patients who responded to either prior therapeutic line.11 In a different, larger study of 7,767 patients with MBC, Ray et al12 found that treatment duration decreased with each subsequent therapy, with a mean first-line treatment duration of 163 days compared with a mean fourth-line treatment duration of 130 days. Despite evidence that treatment history influences outcomes, traditional clinical trial data typically include the proportion of patients with 1, 2, or ≥ 3 prior lines of therapy rather than detailed data on type or duration of treatments administered before or after the study drug.13-15 Furthermore, these clinical variables traditionally are not used when analyzing cancer treatment efficacy in either observational studies or clinical trials.
This study seeks to evaluate the timing of administration of paclitaxel, the most common chemotherapy in estrogen receptor (ER)–positive, human epidermal growth factor receptor 2 (HER2)–negative MBC, and the relationship between time on prior treatment and overall survival (OS) after treatment with paclitaxel. We evaluated this relationship in patients with ER-positive/HER2-negative MBC using both a novel visualization approach and traditional statistical techniques. We hypothesized that treatment context, including time on prior treatments, would affect OS in MBC.
PATIENTS AND METHODS
Study Design and Data
This retrospective cohort study used the nationwide, electronic health record–derived Flatiron Health de-identified database, composed of patient-level structured and unstructured data curated via technology-enabled abstraction. The real-world data set includes de-identified data from > 280 cancer clinics (approximately 800 sites of care), representing more than 2.2 million patients with cancer, available for analysis across the United States.16,17 This study evaluated a breast cancer cohort using (1) a novel, qualitative, visualization approach to examine the relationship between treatments prior to paclitaxel and treatment duration and OS; and (2) traditional quantitative statistical methods to assess the relationship between prior treatment and OS. The University of Alabama at Birmingham institutional review board and Copernicus institutional review board approved this study.
Study Sample
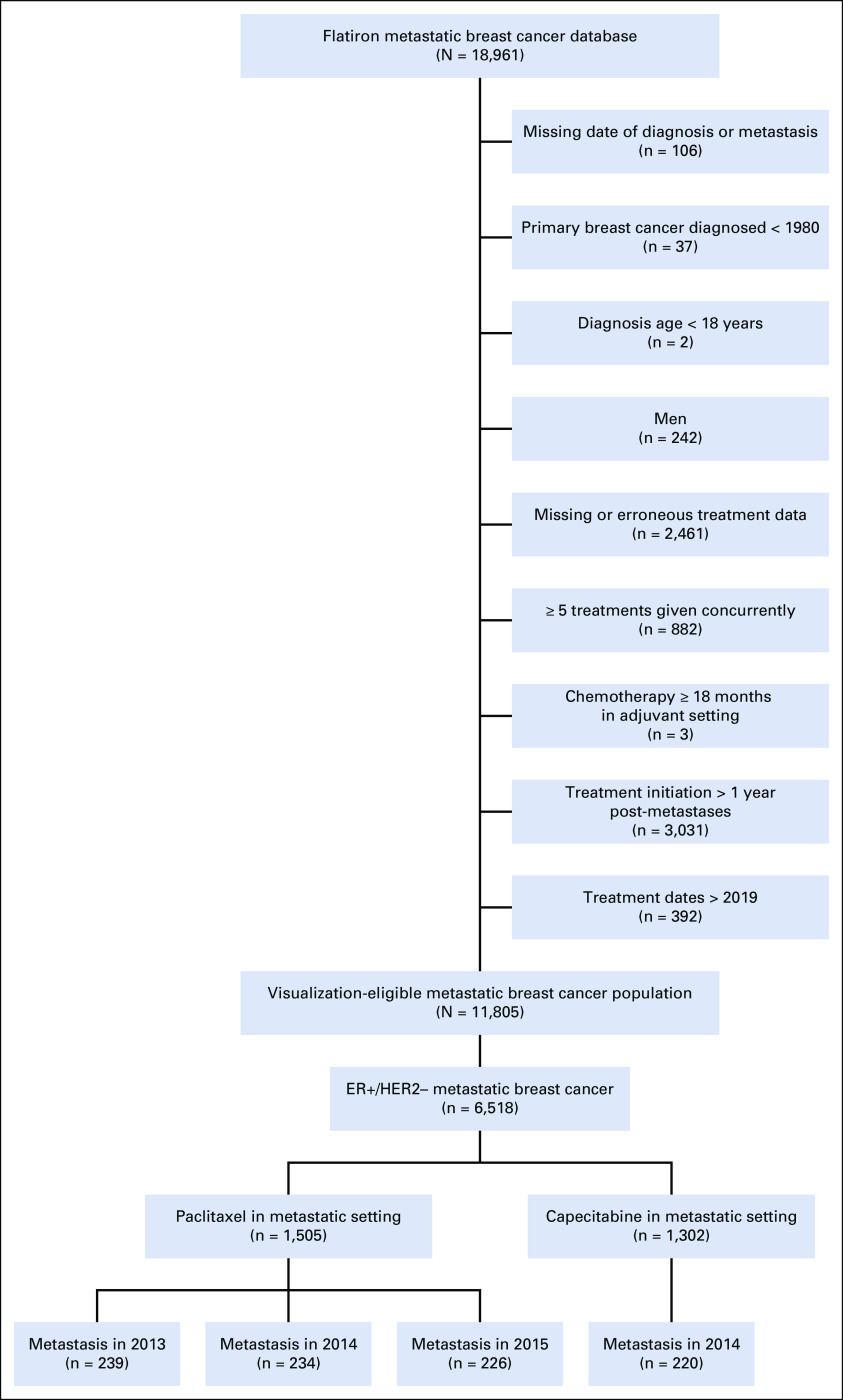
Our study population included women with MBC whose primary breast cancer was diagnosed in 1980 or later. Women with ER-positive/HER2-negative MBC with distant (not breast or axillary lymph node) metastasis in 2014 and subsequent treatment with paclitaxel were included. The year 2014 was selected to ensure that all patients would have consistent guideline-based treatment options. The selection of a single year also ensured the availability of 5-year follow-up and minimized the variation in available therapies over time. ER and HER2 statuses were identified using the biomarker data set. Consistent ER and HER2 diagnoses across all patient records were required to ensure diagnostic accuracy. The cohort excluded patients who were male, were age < 18 years, had primary breast cancer diagnosed before 1980, and had missing or suspected erroneous data for diagnosis or treatments (eg, no treatment data, treatments not typically used for patients with breast cancer). Additional details about the algorithm for inclusion and exclusion criteria are described in Appendix Figure A1.
Outcome Measures
The primary outcome was OS, defined as time from initiation of drug of interest in the metastatic setting to death as a result of any cause. The secondary outcomes were treatment duration and real-world progression-free survival (PFS).18-20 Patients were followed for a maximum of 70 months, to censoring or end of study (last available data date).
Treatment Characterization
All treatments after primary breast cancer diagnosis were identified using generic drug names from the medications administered (infusion/oral therapies) or ordered (oral therapies only) real-world data sets. Treatment duration was calculated on the basis of the initial administered/ordered date until death or 30 days after the final administered/ordered date for patients whose data were censored. Instances in which a gap of > 6 months existed between administered/ordered dates for a single drug resulted in the categorization of the drug as a separate treatment. This rule did not apply to endocrine therapies because of the ability to prescribe 90-day supplies with refills. Real-world PFS was measured from the initial administered/ordered date until the date of progression reported within the database. Real-world PFS was based upon clinician documentation of disease burden, as previously described.18-20
Visualization and Qualitative Analysis
A composite treatment graphic for all patients was created and displayed using a novel visualization technique. Individual patients were represented on the y-axis, and treatment time, on the x-axis. Specific treatments were represented by a color-coded treatment bar, with paclitaxel after metastasis in black, endocrine therapy in shades of red, chemotherapy in shades of blue, HER2-targeted therapy in shades of green, and other targeted therapies in shades of orange. Treatment gaps and time between treatment termination and death/censoring were represented by white space. For each patient, treatments administered concurrently were represented by up to 4 different colors stacked within a single treatment bar. A Kaplan-Meier curve was generated as a function of time from paclitaxel to death or censoring and was overlaid on the visualization graphic. To qualitatively assess both timing of paclitaxel within the course of MBC treatment and OS, time zero was defined as the initial diagnosis of breast cancer for both recurrent and de novo MBC (Fig 1). Gaps between time 0 and the initial drug therapy could include surgery or other nondrug treatments. Subsequently, to qualitatively to determine the association between survival and paclitaxel treatment patterns, time zero was defined as the initiation of paclitaxel in the metastatic setting, with previous treatments on the left side of the y-axis and treatments given after paclitaxel initiation on the right side of the y-axis. Two primary visualizations were generated. The first visualization was sorted by OS (Fig 2), and the second was sorted by time on treatment (Fig 3). To assess data validity across time and chemotherapy type, the visualization approach was repeated for patients receiving paclitaxel in 2013 and 2015 as well as for patients receiving capecitabine in 2014 (Appendix Figs A2-A4).
FIG 1.
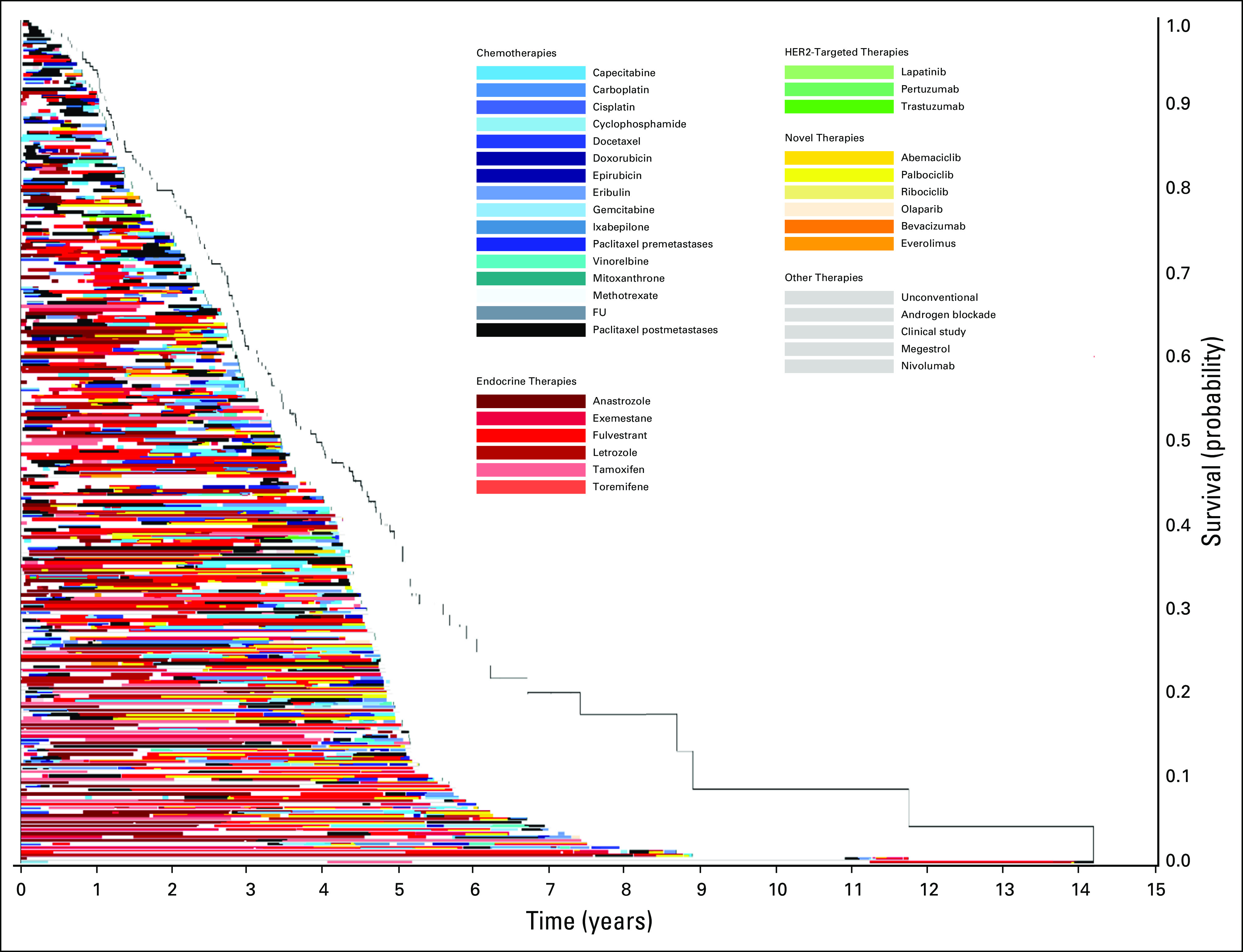
Treatment of patients who received paclitaxel after diagnosis of metastatic disease in 2014. The x-axis includes time in years with initial treatment of any stage breast cancer at time zero. The y-axis includes individual patients. Image is sorted according to overall survival from initial breast cancer diagnosis, with longest survival on the bottom. FU, fluorouracil; HER2, human epidermal growth factor receptor 2.
FIG 2.
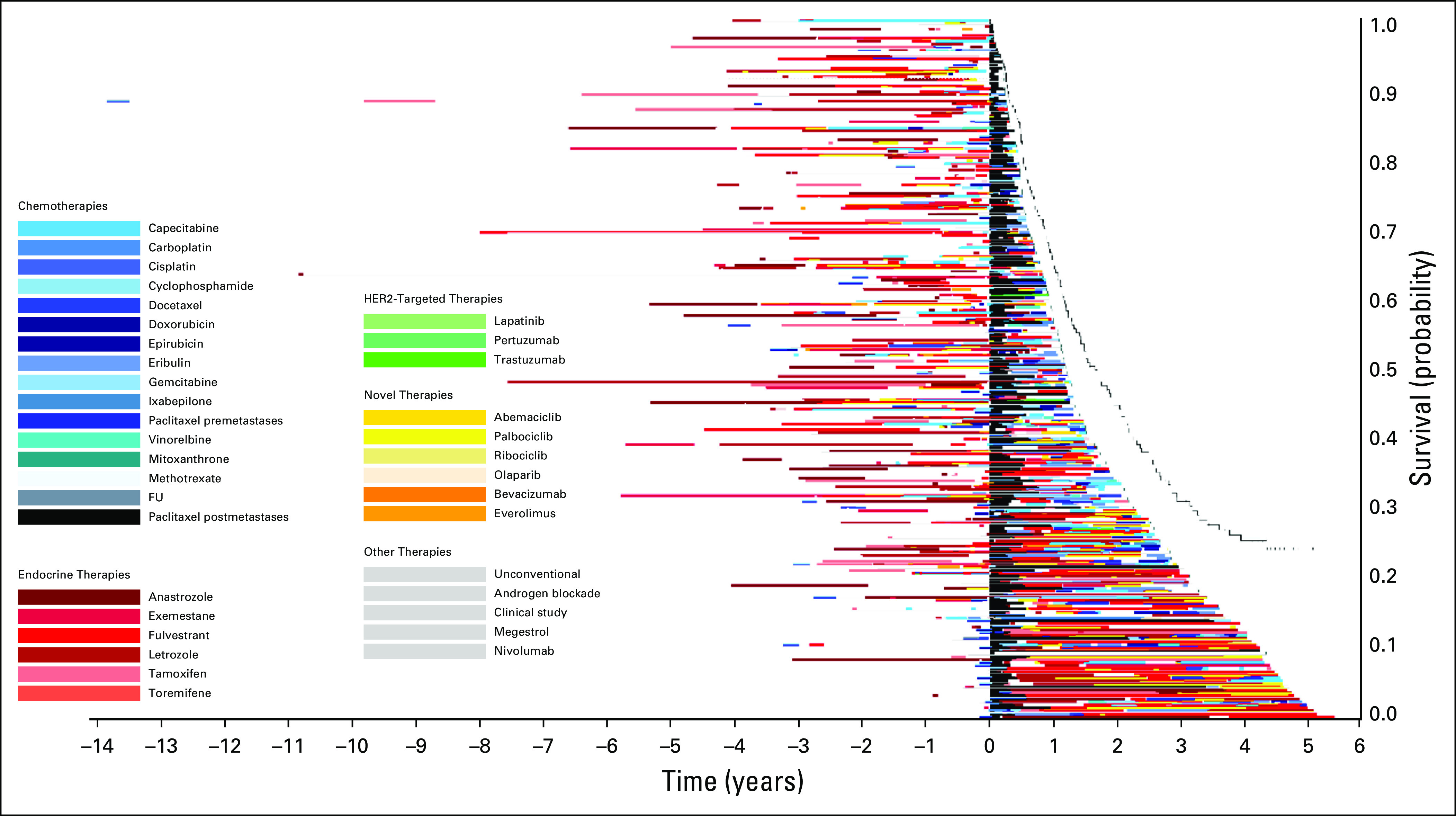
Treatment of patients who received paclitaxel after diagnosis of metastatic disease in 2014. The x-axis includes time in years with initiation of paclitaxel at time zero. The y-axis includes individual patients. Image is sorted according to overall survival from initiation of paclitaxel, with longest survival on the bottom. FU, fluorouracil; HER2, human epidermal growth factor receptor 2.
FIG 3.
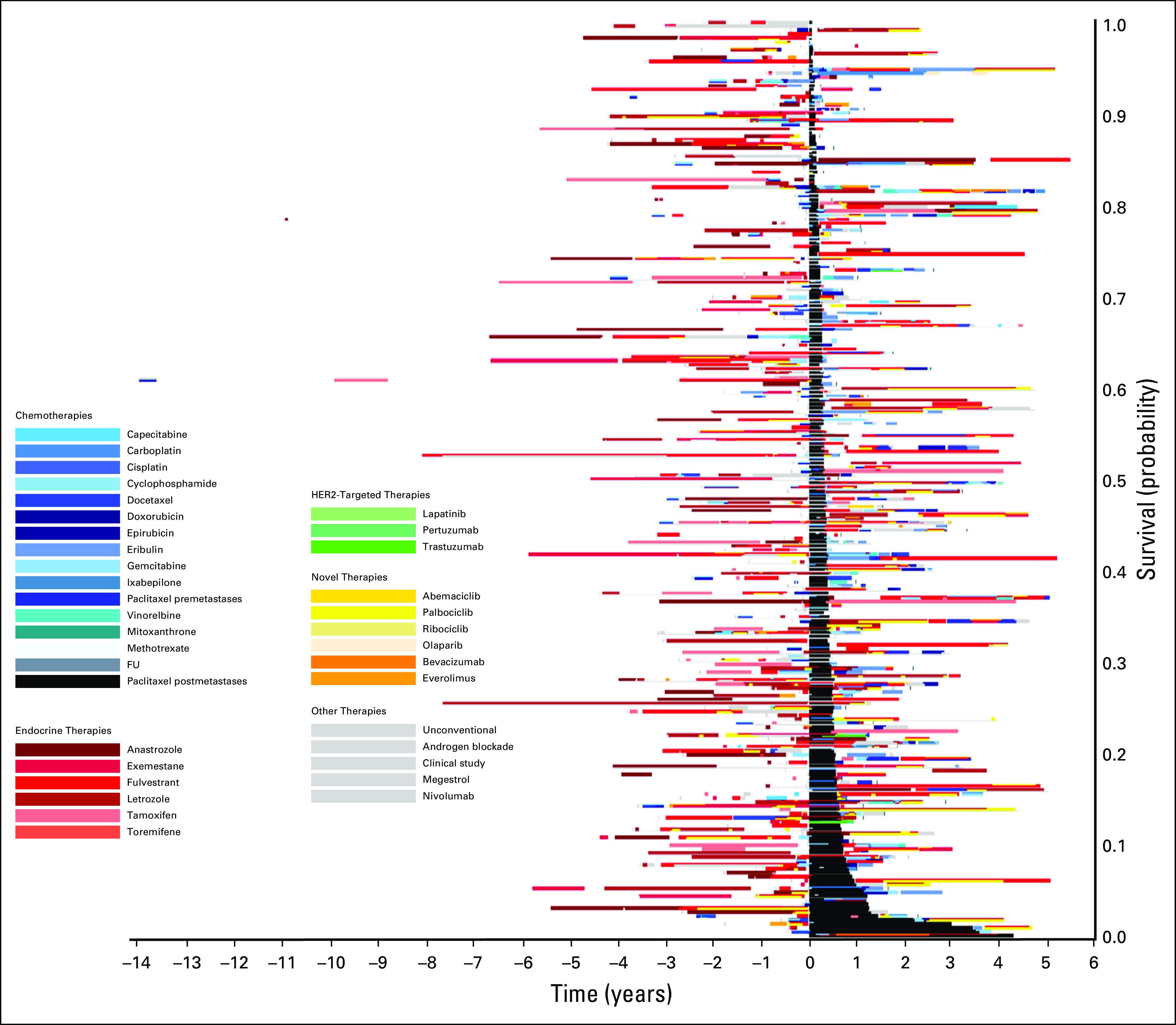
Treatment of patients who received paclitaxel after diagnosis of metastatic disease in 2014. The x-axis includes time in years with initiation of paclitaxel at time zero. The y-axis includes individual patients. Image is sorted according to time on treatment from initiation of paclitaxel, with longest time on treatment on the bottom. FU, fluorouracil; HER2, human epidermal growth factor receptor 2.
Statistical Analysis
Descriptive statistics were calculated using medians and interquartile ranges (IQRs) or frequencies. The median OS from paclitaxel initiation after metastatic diagnosis was calculated using the Kaplan-Meier estimator. Medians and IQRs of paclitaxel treatment duration were calculated. Associations between treatment duration and number of treatments prior to paclitaxel and OS were evaluated using hazard ratios (HRs) and 95% CIs from Cox proportional hazards models. Unadjusted models and models adjusting for site of metastasis, ethnicity, and age at paclitaxel initiation were quantified. Sensitivity analyses examining the same outcomes were performed using (1) patients with MBC receiving paclitaxel in 2013 and 2015, and (2) patients receiving capecitabine in the metastatic setting after a MBC diagnosis in 2014. A subset analysis was conducted to examine patients with documented disease progression while on paclitaxel. Median (IQR) treatment duration, PFS, and the association between treatment duration and real-world progression after paclitaxel initiation were examined.18,19 All data preparation and analysis were accomplished using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Sample Demographics
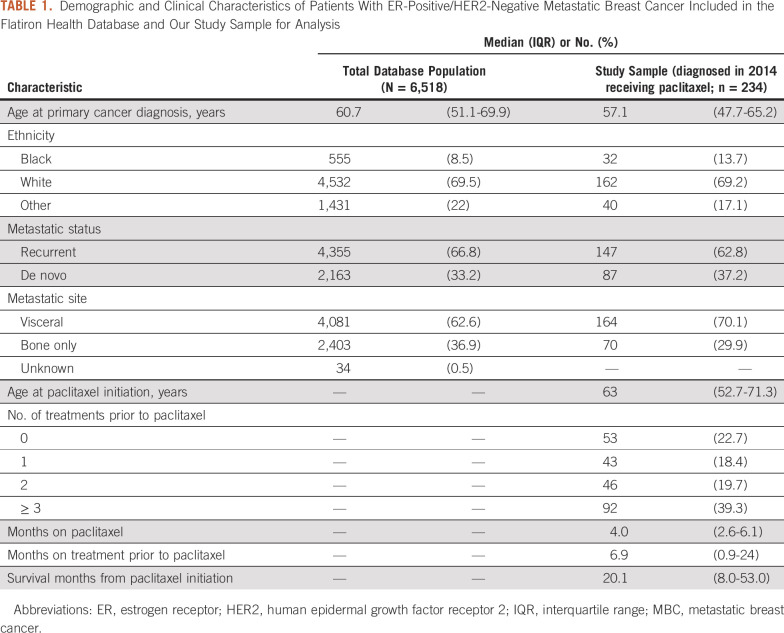
We identified 234 women receiving paclitaxel after diagnosis of ER-positive/HER2-negative MBC in 2014. Population and sample demographic and clinical characteristics are listed in Table 1. Our sample of patients who received paclitaxel reflected the entire population of patients with ER-positive/HER2-negative MBC included in the Flatiron Health database. The median age at primary breast cancer diagnosis was 57 years (IQR, 48-66 years). The sample was predominantly white (69%), and most had recurrent MBC (63%).
TABLE 1.
Demographic and Clinical Characteristics of Patients With ER-Positive/HER2-Negative Metastatic Breast Cancer Included in the Flatiron Health Database and Our Study Sample for Analysis
Qualitative Results
Figure 1 displays the timing of paclitaxel use within the overall course of treatment of MBC. Within this image, paclitaxel (denoted by the black bars) is displayed at different times through the treatment course, highlighting the variability in timing of delivery. Patients with longer survival from initial diagnosis commonly had prolonged duration of endocrine therapy prior to paclitaxel and other chemotherapies. Figure 2 qualitatively demonstrates that patients who received treatments for longer durations prior to paclitaxel initiation in 2014 had less remaining survival time compared with those who received paclitaxel earlier in their treatment course (ie, shorter time on treatment prior to receiving paclitaxel). This is reflected by the group of patients on the top of the image (shorter post-paclitaxel survival) having longer pretreatment than those on the bottom of the image (longer post-paclitaxel survival). Often, patients at the top of the image had overall length of time on any treatment similar to that of patients at the bottom of the image, who appeared to have better post-paclitaxel survival. Within this image, the time on paclitaxel does not increase for patients at the bottom of the image. Rather, many of those with the greatest time on paclitaxel are within the top half of the image. The relationships between time on paclitaxel and either prior treatment or OS are less visually apparent using Figure 3. In this image, the pre-paclitaxel and post-paclitaxel treatments are variable for all paclitaxel time on treatment, without a clear pattern from the top to the bottom of the image. Similar relationships were observed for patients receiving paclitaxel in 2013 (n = 239; Appendix Fig A2) and 2015 (n = 226; Appendix Fig A3). For patients receiving capecitabine in 2014, the relationship between prior treatment time and survival was less apparent (n = 220; Appendix Fig A4).
Quantitative Results
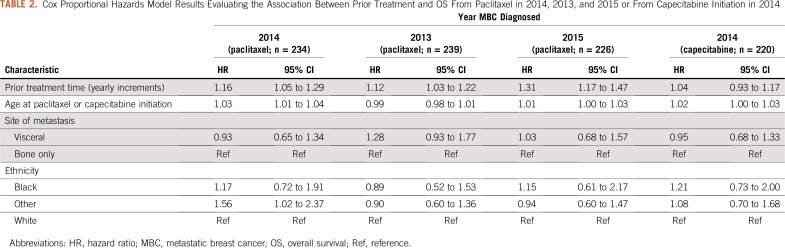
The median OS after paclitaxel initiation for all patients with ER-positive/HER2-negative MBC was 20 months (IQR, 8-53 months; Table 1). Cox proportional hazards model results are listed in Table 2. For every year increase on treatments given prior to paclitaxel, the hazard of death after paclitaxel initiation increased 16% (HR, 1.16; 95% CI, 1.05 to 1.29). Results were comparable when assessing the relationship between OS and number of prior treatment lines. An increased hazard of death was observed for patients with 1 prior treatment (HR, 1.71; 95% CI, 1.03 to 2.84), 2 prior treatments (HR, 1.30; 95% CI, 0.77 to 2.21), and ≥ 3 treatments (HR, 2.23; 95% CI, 1.42 to 3.53) compared with those who had no prior treatment. Similar relationships between prior treatment duration and OS were observed among those receiving paclitaxel who experienced metastasis in 2013 (HR, 1.12; 95% CI, 1.03 to 1.22) and in 2015 (HR, 1.30; 95% CI, 1.16 to 1.47). Though estimates showed the same directionality, no significant relationship was found between prior treatment time and OS in patients receiving capecitabine who experienced metastasis in 2014 (HR, 1.04; 95% CI, 0.93 to 1.17).
TABLE 2.
Cox Proportional Hazards Model Results Evaluating the Association Between Prior Treatment and OS From Paclitaxel in 2014, 2013, and 2015 or From Capecitabine Initiation in 2014
The majority of patients (71%) had a documented progression on paclitaxel. The median duration of paclitaxel treatment and the median real-world PFS in this subset (n = 165) were 4 months (IQR, 3-6 months) and 6 months (IQR, 3-11 months), respectively. A relationship between prior treatment and real-world progression also was observed (HR, 1.13; 95% CI, 1.03 to 1.24).
DISCUSSION
Edward Tufte, a pioneer in data visualization, wrote, “Graphics reveal data. Indeed, graphics can be more precise and revealing than conventional statistical computations.”21(p13) This visualization approach displays the big picture of cancer treatment rather than a single treatment line or time point. Survival after paclitaxel in this study, for all patients receiving paclitaxel, was 20 months. This compares with 22 months reported for weekly paclitaxel in patients with ER-positive/HER2-negative MBC who received at least one prior line of hormone therapy and no prior chemotherapy.22 In other MBC studies evaluating patients with both ER-positive and ER-negative cancers, post-paclitaxel survival ranged from 15-16 months.23,24 These studies had different population enrolled and did not include specific details of prior treatments, which may have confounded results, given that our study demonstrated that patients with longer post-paclitaxel OS had a much briefer clinical course leading up to receipt of paclitaxel. This relationship was less prominent for capecitabine, which may have been secondary to the relatively few patients who received capecitabine very early in the course of their disease. Although the relationship between prior treatment and survival is intuitive for clinicians, who expect less robust responses to treatment later in the disease course, current reporting of clinical trial outcomes uses a standard Kaplan-Meier curve without consideration of prior number of treatments or time on those treatments. Although large phase III trial randomization can balance prestudy treatment, sufficient sample size is needed to ensure similar baseline characteristics.25 Therefore, interpretation of small randomized trials and nonrandomized, single-arm studies may be limited when type and duration of prior treatment are not incorporated. Given that the hazard of death after paclitaxel initiation increased 16% per year in this study, omitting pretreatment contextual details has the potential to confound results. This approach may result in observed survival benefits merely reflecting early versus late receipt of treatment. Thus, we advocate for randomization schemes that account for time on previous treatment and statistical models that account for confounding by prior treatment duration.
Even with balanced treatment arms, contextual data are valuable for clinicians when considering patient similarity to the study population in terms of previous treatment type and duration. Current clinical trials are not designed to capture the entire disease course and thus do not often report on details of prior or subsequent therapies. Therefore, this information is not often integrated into trial interpretation—for instance, to examine the heterogeneity of effects. However, future clinical trials should capture these rich data elements for the invaluable contextual information they provide. This is especially important for clinicians during clinical encounters, when they interpret clinical trial results for application to individual patients.
The visualization of paclitaxel use in metastatic disease from initial diagnosis shown in Figure 1 highlights the heterogeneity of specific drug use, which may contribute to previously reported challenges of using PFS as a surrogate endpoint for long-term survival in MBC.26 This imperfect relationship is supported by Figure 3 in this analysis, in which patients are sorted by time on treatment as a surrogate for PFS. In a meta-analysis of 51 oncology drug trials, nearly half showed benefit in PFS without a corresponding benefit in OS.27 In ER-positive/HER2-negative MBC, > 90% of randomized controlled trials showed no significant improvements in both PFS and OS.28 Further evaluation of this relationship is warranted, with additional attention to the pre- and poststudy treatments received by patients.
This visualization methodology can be deployed to evaluate patterns of care for patients with cancer receiving sequential treatments. For example, this approach could be used to assess adoption of specific novel therapies over time, using images with patients receiving treatment in different years, to evaluate the proportion of patients with specific first-line therapies (eg, endocrine therapy first) or to ascertain which chemotherapeutic agents have the longest duration of treatment. Visualization, when used in conjunction with traditional statistical analysis, has the potential to enhance our understanding of these complex treatment questions.
This study has several limitations. No validated method of deriving comorbidities from electronic health record data are available, so comorbidities were not accounted for in our model. This may overestimate the relationship between OS after paclitaxel initiation and prior treatment. Qualitatively, treatment duration was used in this study as a proxy for PFS, which may not fully capture the relationship between prior treatment duration, PFS, and OS within the visualizations. However, relatively few patients (29%) changed treatments for reasons other than progression. This visualization approach does not account for other biologic variables contributing to PFS and OS, like genetic tumor alterations. Shortened survival after late delivery of paclitaxel may be secondary to additional mutation development that renders the disease refractory to chemotherapy. Additional evaluation of the biologic factors contributing to findings observed in this study is needed, as is future work to evaluate treatment and survival patterns for tumors with differing mutation profiles. The analysis on prior lines of therapy did not differentiate whether the patient had chemotherapy or hormone therapy prior to paclitaxel, which may differ in the effect on post-paclitaxel survival. This lack of differentiation may contribute to the similar results found for the impact of 1 or 2 prior lines of therapy compared with no prior therapy on post-paclitaxel survival. The evaluation of the sequence of specific medications is inherently complex, and more research is needed on how individual sequences affect OS. Finally, this analysis was conducted before the introduction of cyclin-dependent kinase 4/6 inhibitors, which are now considered part of standard care for ER-positive/HER2-negative MBC. Phase III trials have demonstrated benefits of cyclin-dependent kinase 4/6 inhibitors as first-line treatments,4,5 and in later lines of therapy.6-8 The order of and use of specific agents remain controversial, making these ideal topics for further evaluation using visualization techniques. Although this study was limited to ER-positive/HER2-negative MBC, our visualization could be applied to other cancers with treatment duration or sequencing variability to better understand treatment context.
In summary, visualizing paclitaxel in the context of the disease course demonstrates an association between time on prior therapy and both PFS and OS. This result highlights the potential for an OS benefit to be observed merely on the basis of early versus late receipt of treatment if treatment arms are not balanced. Future trials should consider stratifying according to prior time on treatment and displaying the treatment context to aid in interpretation of results. This visualization approach can enhance our capacity to harness the power of patient-level data to better understand treatment patterns and their influence on survival outcomes. Considerations of treatment sequencing when studying treatment repercussions will be increasingly important as new US Food and Drug Administration–approved medications are incorporated into existing treatment paradigms.
APPENDIX
FIG A1.
Study sample exclusion cascade. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
FIG A2.
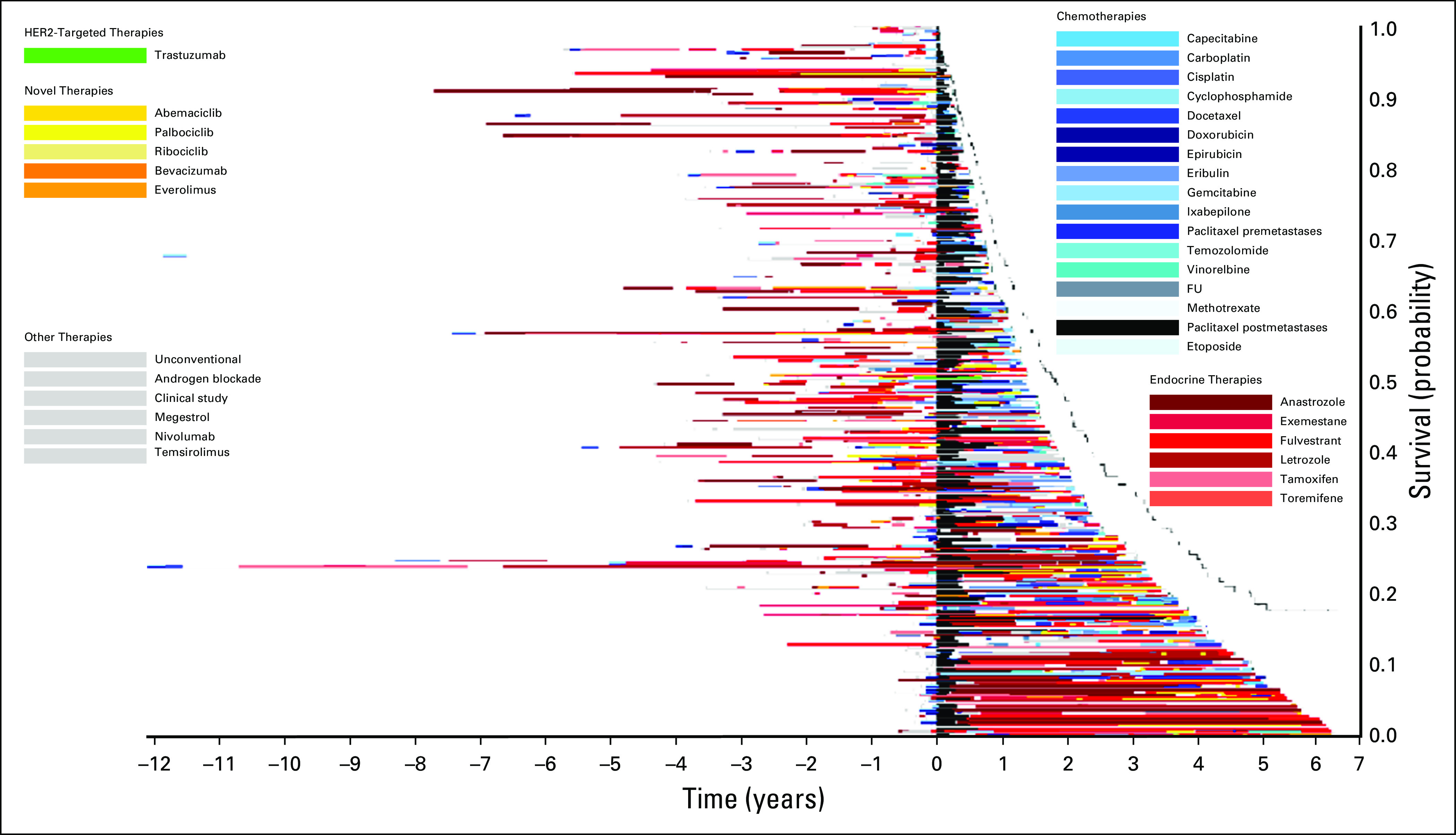
Treatment of patients who received paclitaxel after diagnosis of metastatic disease in 2013. The x-axis includes time in years with initiation of paclitaxel at time zero. The y-axis includes individual patients. Image is sorted according to overall survival from initiation of paclitaxel, with longest survival on the bottom. FU, fluorouracil; HER2, human epidermal growth factor receptor 2.
FIG A3.
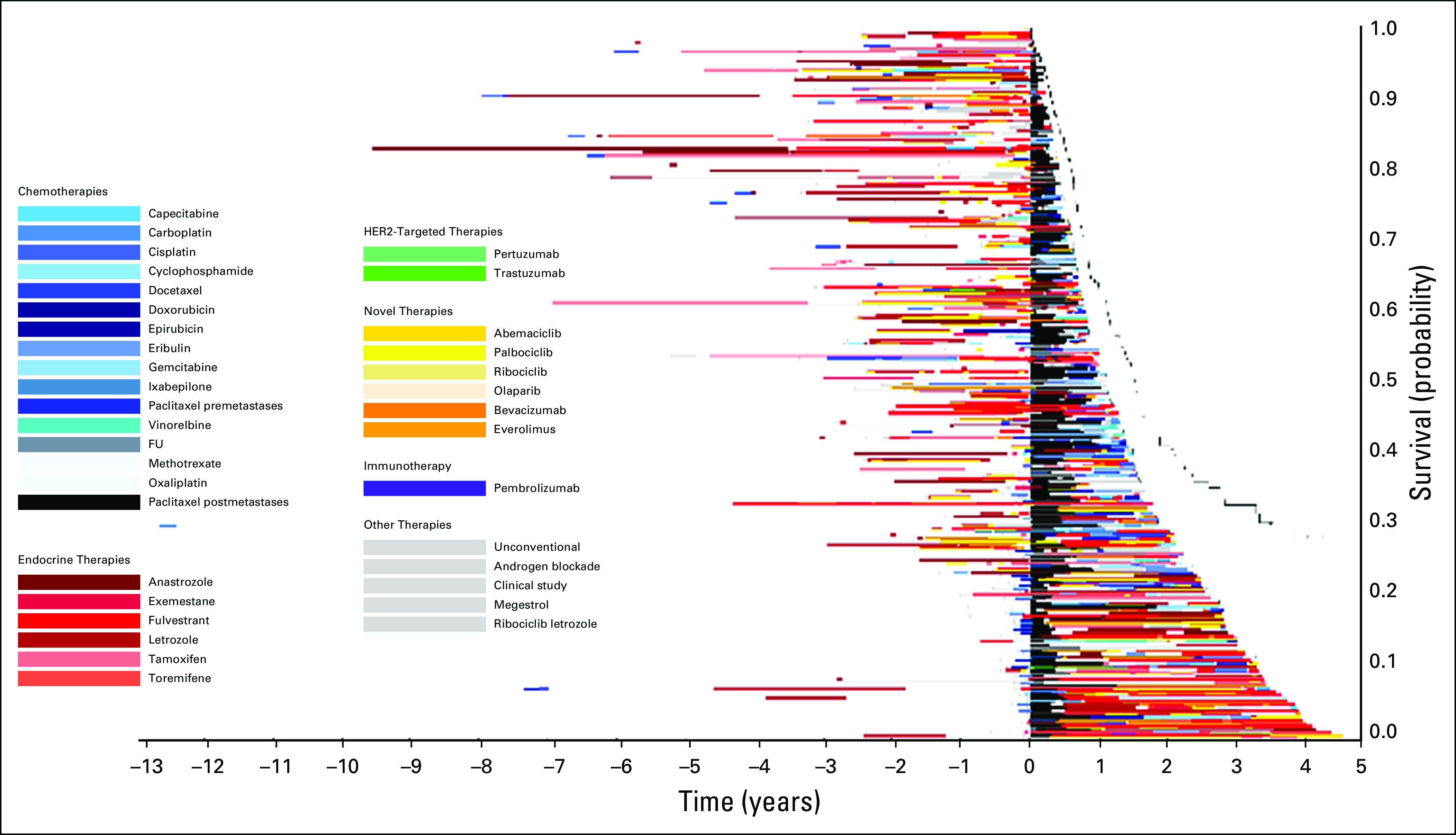
Treatment of patients who received paclitaxel after diagnosis of metastatic disease in 2015. The x-axis includes time in years with initiation of paclitaxel at time zero. The y-axis includes individual patients. Image is sorted according to overall survival from initiation of paclitaxel, with longest survival on the bottom. FU, fluorouracil; HER2, human epidermal growth factor receptor 2.
FIG A4.
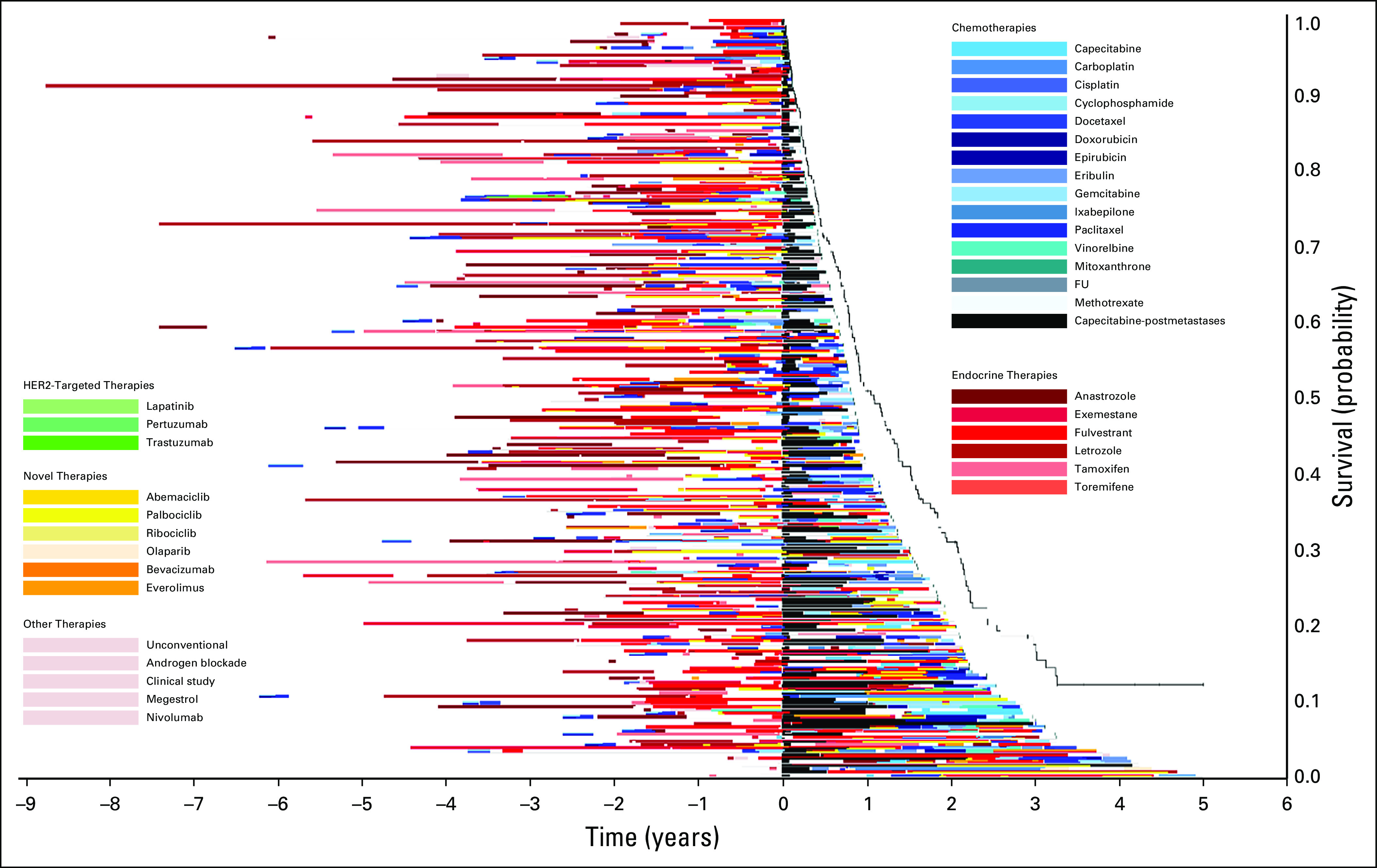
Treatment of patients who received capecitabine after diagnosis of metastatic disease in 2014. The x-axis includes time in years with initiation of capecitabine at time zero. The y-axis includes individual patients. Image is sorted according to overall survival from initiation of capecitabine, with longest survival on the bottom. FU, fluorouracil; HER2, human epidermal growth factor receptor 2.
PRIOR PRESENTATION
Presented at the 2019 San Antonio Breast Cancer Research Symposium, San Antonio, TX, December 10-14, 2019.
SUPPORT
Supported by grant from the Breast Cancer Research Foundation of Alabama and by an American Cancer Society Mentored Research Scholar Grant (No. MRSG-17-051-01-PCSM to G.B.R.).
AUTHOR CONTRIBUTIONS
Conception and design: Gabrielle B. Rocque, Aidan Gilbert, Arie Nakhmani, Smita Bhatia, Mark E. Burkard
Administrative support: Gabrielle B. Rocque
Financial support: Gabrielle B. Rocque
Collection and assembly of data: Gabrielle B. Rocque, Aidan Gilbert
Data analysis and interpretation: Gabrielle B. Rocque, Aidan Gilbert, Courtney P. Williams, Kelly M. Kenzik, Arie Nakhmani, Pravinkumar G. Kandhare, Andres Azuero
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Gabrielle B. Rocque
Consulting or Advisory Role: Pfizer (Inst), Genentech
Research Funding: Carevive Systems (Inst), Genentech (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Genentech, Carevive Systems (I)
Mark E. Burkard
Consulting or Advisory Role: Pointcare Genomics, Strata Oncology, Novartis
Research Funding: AbbVie (Inst), Strata Oncology (Inst), Puma Biotechnology (Inst), Loxo (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: I have patent for implantable/localized drug delivery device that can sample the tumor microenvironment and deliver drug; I have a patent for a method to detect recombination events with CRISPR-mediated editing.
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Comprehensive Cancer Network https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Invasive breast cancer, 2019.
- 2.Rocque GB, Williams CP, Jackson BE, et al. Impact of nonconcordance with NCCN guidelines on resource utilization, cost, and mortality in de novo metastatic breast cancer. J Natl Compr Canc Netw. 2018;16:1084–1091. doi: 10.6004/jnccn.2018.7036. [DOI] [PubMed] [Google Scholar]
- 3.Rocque GB, Williams CP, Kenzik KM, et al. Concordance with NCCN treatment guidelines: Relations with health care utilization, cost, and mortality in breast cancer patients with secondary metastasis. Cancer. 2018;124:4231–4240. doi: 10.1002/cncr.31694. [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 5.Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 6.Cristofanilli M, DeMichele A, Giorgetti C, et al. Predictors of prolonged benefit from palbociclib plus fulvestrant in women with endocrine-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer in PALOMA-3. Eur J Cancer. 2018;104:21–31. doi: 10.1016/j.ejca.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Sledge GW, Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy: MONARCH 2—A randomized clinical trial. JAMA Oncol. doi: 10.1001/jamaoncol.2019.4782. [epub ahead of print on September 19, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 9.Rocque GB, Kandhare PG, Williams CP, et al. Visualization of sequential treatments in metastatic breast cancer. JCO Clin Cancer Inform. 2019;3:1–8. doi: 10.1200/CCI.18.00095. [DOI] [PubMed] [Google Scholar]
- 10.Swallow E, Zhang J, Thomason D, et al. Real-world patterns of endocrine therapy for metastatic hormone-receptor-positive (HR+)/human epidermal growth factor receptor-2-negative (HER2-) breast cancer patients in the United States: 2002-2012. Curr Med Res Opin. 2014;30:1537–1545. doi: 10.1185/03007995.2014.908829. [DOI] [PubMed] [Google Scholar]
- 11.Banerji U, Kuciejewska A, Ashley S, et al. Factors determining outcome after third line chemotherapy for metastatic breast cancer. Breast. 2007;16:359–366. doi: 10.1016/j.breast.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Ray S, Bonthapally V, McMorrow D, et al. Patterns of treatment, healthcare utilization and costs by lines of therapy in metastatic breast cancer in a large insured US population. J Comp Eff Res. 2013;2:195–206. doi: 10.2217/cer.13.1. [DOI] [PubMed] [Google Scholar]
- 13.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 14.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 15.André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 16.Curtis MD, Griffith SD, Tucker M, et al. Development and validation of a high-quality composite real-world mortality endpoint. Health Serv Res. 2018;53:4460–4476. doi: 10.1111/1475-6773.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger ML, Curtis MD, Smith G, et al. Opportunities and challenges in leveraging electronic health record data in oncology. Future Oncol. 2016;12:1261–1274. doi: 10.2217/fon-2015-0043. [DOI] [PubMed] [Google Scholar]
- 18.Griffith SD, Tucker M, Bowser B, et al. Generating real-world tumor burden endpoints from electronic health record data: Comparison of RECIST, radiology-anchored, and clinician-anchored approaches for abstracting real-world progression in non–small-cell lung cancer. Adv Ther. 2019;36:2122–2136. doi: 10.1007/s12325-019-00970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khozin S, Miksad RA, Adami J, et al. Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non–small-cell lung cancer. Cancer. 2019;125:4019–4032. doi: 10.1002/cncr.32383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith SD, Miksad RA, Calkins G, et al. Characterizing the feasibility and performance of real-world tumor progression end points and their association with overall survival in a large advanced non–small-cell lung cancer data set. JCO Clin Cancer Inform. 2019;3:1–13. doi: 10.1200/CCI.19.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tufte ER. The Visual Display of Quantitative Information. 1983. Cheshire, CT, Graphics Press, [DOI] [PubMed] [Google Scholar]
- 22.Aapro M, Ruiz-Borrego M, Hegg R, et al. Randomized phase II study evaluating weekly oral vinorelbine versus weekly paclitaxel in estrogen receptor-positive, HER2-negative patients with advanced breast cancer (NorBreast-231 trial) Breast. 2019;45:7–14. doi: 10.1016/j.breast.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542–5551. doi: 10.1200/JCO.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Paridaens R, Biganzoli L, Bruning P, et al. Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: A European Organization for Research and Treatment of Cancer randomized study with cross-over. J Clin Oncol. 2000;18:724–733. doi: 10.1200/JCO.2000.18.4.724. [DOI] [PubMed] [Google Scholar]
- 25.Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2–21. doi: 10.1016/j.socscimed.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26:1987–1992. doi: 10.1200/JCO.2007.10.8407. [DOI] [PubMed] [Google Scholar]
- 27.Tan A, Porcher R, Crequit P, et al. Differences in treatment effect size between overall survival and progression-free survival in immunotherapy trials: A meta-epidemiologic study of trials with results posted at ClinicalTrials.gov. J Clin Oncol. 2017;35:1686–1694. doi: 10.1200/JCO.2016.71.2109. [DOI] [PubMed] [Google Scholar]
- 28.Kaklamani VG. Clinical implications of the progression-free survival endpoint for treatment of hormone receptor-positive advanced breast cancer. Oncologist. 2016;21:922–930. doi: 10.1634/theoncologist.2015-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]