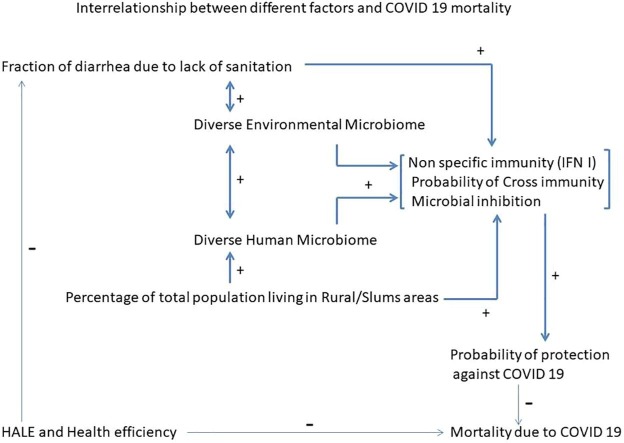
Graphical abstract
Keywords: COVID19, Mortality, Immunity, Microbiome, TLR4, Interferon I
Abstract
There is a significant difference between COVID 19 associated mortality between different countries. Generally the number of deaths per million population are higher in the developed countries despite better health care efficiency, drinking water quality and expected healthy life span (HALE) at the time of birth. Developing and underdeveloped countries on the other hand have lower mortality even with higher rural and slum populations along with incidence of diarrhea because of lack of sanitation. We analyzed data from 122 countries out of which 80 were high or upper middle income and 42 were low or low middle income countries. There was statistically significant positive correlation between COVID 19 deaths /million population and water current score, health efficiency, and HALE. Statistically significant negative correlation was observed with % rural population and fraction of diarrhea because of inadequate sanitation for all ages. Moreover analysis of 51 countries showed that there is significant negative correlation between COVID 19 deaths /million population and proportion of total population living in slums. We propose that high microbial exposure particularly gram negative bacteria can possibly induce interferon type I which might have a protective effect against COVID 19 since the countries with less mortality also tend to have lack of sanitation and high incidence of attendant diseases. So, far none of the predictive models have taken into account immune status of populations engendered by environmental microbial exposure or microbiome. There might be a need to look at dynamics of COVID 19 pandemic using immune perspective. The approach can potentially inform better policies including interventions.
Introduction
There are remarkable global disparities between COVID 19 associated disease burden. Some western European countries and USA have seen explosive rate of mortalities whereas many of the Asian and African countries have remained relatively unscathed [1]. Broad patterns can be discerned and it appears that countries with better health care, clean environment, clean food and water have higher COVID associated mortality, whereas developing and underdeveloped countries have lower mortality in terms of deaths per million population. Predictive models have acknowledged varied rate of spread in different countries among other factors that might affect the disease burden. However the immune status of any population is notably absent in all of these models [2] [3].
It might turn out to be a significant oversight since any infection and the consequences of the same ought not to be considered without taking into context the immunity, whether specific or innate. The measures for specific immunity are considered to be either prior infection or vaccination and both can be quantified by measuring relevant antibodies in the serum.Measuring innate immunity is much more challenging though and generally involves quantifying levels of interferons and interleukins [4], [5]]. It has been suggested that BCG vaccination induced beneficial effects on innate immunity might explain relatively lower COVID 19 disease burden in certain populations. That BCG vaccination has been shown to be associated with decreased viral load in certain infections strongly supports the suggestion [6]. BCG vaccination is administered within one year of age and it appears to be doubtful whether single vaccination would lead to sustained higher levels of protective effects. It is possible that in BCG covered countries, frequent subclinical tuberculosis infections could sustain the protective effects, if any.
We would like to bring to notice somewhat similar association between infections by gram negative bacteria and raised levels of Interferon Type I which could have beneficial effect in the context of present pandemic. Bacteria like E coli have been used as a marker for faecal contamination of water [7]. It is interesting to note that countries with lower water quality index and generally higher population exposure to microbial load have lower COVID 19 associated mortality.
Hypothesis
We hypothesize that COVID19 associated death per million population will continue to be lower in countries with higher population exposure to microbial diversity particularly gram negative bacteria.
Why this hypothesis is different from current thinking
So far it has been suggested that the difference is because of higher proportion of younger population in developing countries which seems plausible since COVID associated mortality is higher in older age group. One of the recent publications has projected COVID associated mortality in African continent somewhere near 150,000 using Markov Chain Model and four contributory factors viz. tendency of people to cluster together, weather factor, distribution factor i.e. the ease of people to move around spread the infection and sanitation/hygiene practices[2], [3]]. However one of the important factors is missing in the analysis which is potential immune attributes of a given population.
How has this idea evolved?
Significantly higher death rate has been observed in industrialized countries like USA, UK, Italy, France and Spain. Whereas many of the developing and underdeveloped countries including south and south east Asian along with African and Latin American countries have not shown as high mortality [1]. It was feared and also predicted that crowded slums in these countries in particular would be a fertile ground for massive explosion of COVID associated disease burden and death but that has not happened so far.
To our mind the only way to explain comparative lower mortality in these regions with relative lack of sanitation and resultant higher incidence of diarrhoea coupled with low health efficiency, low expected healthy life span, higher percentage of people living in slums or rural area is the possible role of immunity.
The immunity can either be innate or non-specific or it can be adaptive immune response in response to prior infection or vaccination. None of the countries have any history of prior exposure to COVID19 and therefore the immune protection has to be either non specific or cross immunity owing to infection by related microbial species already existing in some areas.
With the starting point of production of interferon I, one of the key molecules providing protection against viral infections including coronavirus, in response to gram negative bacterial infections the hypothesis was built to explain disparity in global COVID19 associated mortality.[8], [9], [10], [11]]
Microbial inhibition is another important concept that ought to be taken into consideration especially in pandemic scenario. Said inhibition is mediated by commensal microbial population in the body at sites such as intestine, oral and nasal cavity and skin referred to as human microbiome. Diverse human microbiome has been shown to provide better immunity against external infection.
Human microbiomic diversity is in turn dictated by environmental microbiome which is sum total of microbes in the surroundings and socio economic status [12], [13], [14]].
Therefore it was expected that the regions with higher microbial diversity including zoonotic infections resulting in potential cross immunity ought to have lower COVID associated mortality.
Evaluation of hypothesis
Significant COVID 19 associated disparity between most of the developing and developed countries cannot be ignored.The reasons for such difference can potentially dictate the interventional measures and policy decisions.
The disease burden following infection is determined by multiple factors namely principle among which is immune status. Immunity in context of infection is mostly implied as adaptive specific immunity following either prior infection or vaccination. Value of cross immunity owing to infection by related microbes is also acknowledged even as potential role of innate immunity or any kind of host resistance and/or tolerance to new infection that is not because of prior infection or vaccination goes unexplored. Possible role of widespread BCG vaccination has been proposed to explain lower COVID 19 disease burden on the grounds that the said vaccine is known to induce nonspecific immune response, in other words innate immunity [6].
In all of the countries, populations have neither had prior exposure to COVID 19 nor vaccination. Therefore, excluding the possibility of cross immunity owing to native microbes, all the countries should be at par and rate of infection along with mortality ought to be similar which does not appear to be the case thus far.
We propose that population exposed to more microbial load, in particular gram negative bacteria or in other words the population with less sanitation and access to safe drinking water are likely to have less COVID associated disease burden.
The relative protection against COVID 19 might be the combined result of host immunity, resistance, tolerance and microbial interference.
Interferon I and bacterial infections
Interferon Type I, is known to directly inhibit replication of most viruses. Interferon, via different pathways stimulates both dendritic cells and CD8 + T cells which are instrumental in killing virus infected cells. They also effect B cell activation and antibody production. Interferons are produced in response to viral infections and are considered to be a bridge between innate and adaptive immune response owing to its immunomodulatory actions [15] In addition, interferons are also produced in response to bacterial infections such as gram negative E coli. The link between interferon I production and microbes is family of Toll like receptors [TLRs) [16]. TLR 4 is known to be associated with interferon I production [17]. TLR 4 appears to be of interest in the present context since it recognises lipopolysaccharide component of gram negative bacteria in addition to viral proteins and help produce type I interferon[18], [8], [9], [10]].In mice models interferon has been demonstrated to be essential to prevent viral replication and prevent mortality [11]. Previous SARS epidemic had actuated multiple studies to explore role of interferon in preventing replication of coronavirus and it was found to be so in vitro [19], [20]].
Poor drinking water quality in general and particularly in low and middle income countries reflect high coliform content which in turn is usually results from faecal contamination both human and cattle [21], [22]]. Diarrhoea owing to lack of sanitation is one of the key outcomes. One of the key Abovementioned molecular mechanisms can possibly explain the results of our analysis of correlation between COVID associated mortality and water quality of different countries.
Microbiome mediated protection
Bacterial exposure of a given population is not only confined to drinking water or food but it extends to the whole environment. Environmental microbiome is defined as sum total of microbes in a given ecosystem. Human gastrointestinal, oropharyngeal, cutaneous and urogenital microbiomes , to a significant extent are dependent upon individual diet, socioeconomic status, medicine intake and environment. Human gut microbiota composed of diverse bacteria, fungi, viruses and bacteriophages, has been the subject of intensive investigations compared to other sites [23]. It has been demonstrated that gut microbiota modulates host immunity which in turn confers protection against external microbes either via immunity or competitive exclusion [24], [25]]. Mechanisms of conferring resistance are diverse. Commensals are known to induce interleukins in the gut that are protective against infections [12]. Metabolites and nutrients essential for the external microbes have limited availability owing to stiff competition for utilization of the same metabolites by commensal microbes [26], [27]]. The effects of commensals are not only local but may be systemic. It has been demonstrated that reduction of gut commensals owing to antibiotics is associated with blunted T and B cell response against intranasal influenza [28]. Rectal administration of TLR agonists resulting in production of IL 1 beta and IL 18 restores the protective immunity against intranasal influenza [29]. What is true for gut microbiome is context of protection against external microbes is also true for other microbiomes, including nasal [13].
Importance of microbiome is underscored by the observation that rural population from underdeveloped or developing countries harbour more diverse gut microflora compared to urban populations from industrialized countries and considered to be protective against infections.[14]
The flip side of diverse environmental microbiome include environmental pathogens like gram negative bacilli which coupled with lack of sanitation do cause significant diarrhoea but paradoxically the same can possibly provide protection via mechanisms detailed above.
Therefore it can be concluded with a degree of confidence that individuals from developing and underdeveloped regions with diverse microbiome are likely to offer more resistance against external pathogen than an individual with relatively diminished microbiome from developed countries. It should be noted that most of the regions covered by BCG vaccination are also likely to have less sanitation and lower access to clean water along with likely diverse physiological microbiomes.
Importance of environmental microbiome is further underscored by the fact that repeated exposure to microbes in people working with animals can result in reduced incidence of zoonotic clinical illness due to acquired immunity and therefore these individuals though asymptomatic and relatively immune are likely to spread the infection to unexposed population. Total of 18 species of Cornonavirus have been found in 47 species of bats in addition to ferrets, mice, pigs, rats and birds in different regions of China including Hong Kong, Taiwan and Macau. Bat coronaviruses have been also been found in Myanmar , Lao PDR and Combodia, Vietnam, Indonesia and USA [30], [31], [32], [33], [34], [35]]
Excluding USA and China, all other countries have shown remarkable limited COVID 19 associated disease burden. Such scenario in part could be, in addition to other factors mentioned in this article, due to possible cross immunity as a consequence of select populations exposed to zoonotic infections. In context of 2002–03 SARS epidemic mortality it is notable that while the origin of epidemic was china the fatality percentage rate was 6.6% in the country, whereas the mortality was more than double in other countries/provinces like Hong Kong, Taiwan, Canada and Singapore [36]. Even COVID 19 associated mortality shows similar pattern with higher mortality in industrialized nations even as the origin was China.
The phenomenon illustrates a paradoxical advantage. The populations living in areas with higher microbial load and exposure, while at risk of infectious diseases are also likely to be protected from the same for a given inoculum or dose. That up to 70% of the visitors from industrialized countries to developing or underdeveloped regions suffers from traveller’s diarrhoea attests to the importance of exposure to microbial dose that is pathogenic for travellers [37].On the other hand there are geographical pockets where native populations are resistant to very high doses of bacterial contaminants in environment including drinking water [38].
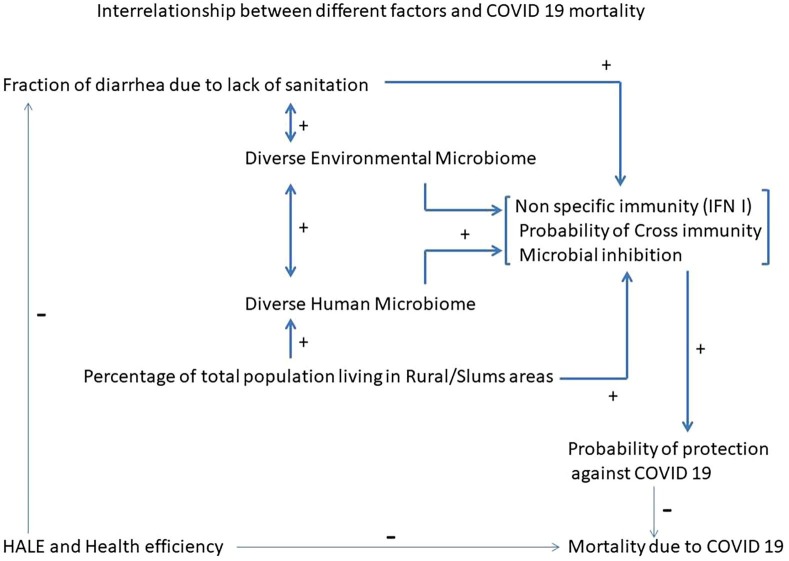
Interactions between different factors and their relationship to COVID19 mortality is depicted in Fig. 1 (Graphical Abstract).
Fig. 1.
Interrelationship between different factors and COVID 19 mortality
Possible protective immunity acquired by different populations because of exposure to different environmental microbial profile and load so far has not been factored into explaining COVID19 associated disease burden. This oversight might have serious consequences since the purpose of different vaccines is to provide similar protective immunity.
Empirical data
To support our hypothesis total of 122 countries were included in this analysis. Out of 122 countries 80 were high or upper middle income and 42 were low or low middle income countries. Countries were selected based on availability of relevant data pertinent to the study. Dependent variable was the number of deaths due to corona virus disease 19 (COVID 19) per million of population. Data pertaining to number of deaths in each country was obtained from John Hopkins University centre for systems science and engineering (CSSE) [1] Explanatory variables included were water current score [39], health efficiency[40], percent rural population [41],fraction of diarrhoea because of inadequate sanitation in all ages [42] and healthy life expectancy (HALE) at birth [43]. Raw data shown in Table 01 Supplementary
Correlations of COVID 19 deaths/million population with slum population percentage was done for 51 countries as per the availability of data [44].
Raw data is shown in Supplementary Tables 01 and 02.
Statistical analysis
Pearson’s Correlation coefficients were calculated for each of the explanatory variables with number of deaths per million of population due to COVID 19. Finally step wise multiple linear regression was performed on all variables with significant correlation coefficient. P value of < 0.05 was considered statistically significant. All the variables were checked for multicollinearity and variables with variance inflation factor (VIF) of 5 or more were dropped from final model. Data was analyzed using SPSS version 22.
Also calculated was correlation between different variables separately for group of 122 and 51 countries.
Results:
Mean COVID 19 deaths/million of population was 6.56 with standard deviation of 13.95. Minimum number of deaths was 0.01 for Angola and maximum number of COVID 19 deaths was 21.6/million of population for Equador. Maximum water current score of 100 was for 8 high income countries and minimum score of 0.32 was for Chad.
There was statistically significant positive correlation between COVID 19 deaths /million population and water current score, health efficiency, and HALE at birth. Countries with better water current score, better health systems and better HALE at birth were more likely to have higher COVID 19 deaths. Statistically significant negative correlation as observed between COVID 19 deaths/million population and % rural population and fraction of diarrhea because of inadequate sanitation all ages (Table 1 ).
Table 1.
Characteristics of independent variables and Pearson’s correlation coefficients among dependent and independent variables.
| Variables | Mean | Standard deviation | Maximum | Minimum | Pearson’s Correlation Coefficient |
|---|---|---|---|---|---|
| Water current score | 54 | 30.9 | 100 | 32 | 0.470* |
| Health efficiency | 0.67 | 0.21 | 0.994 | 0 | 0.381* |
| % Rural population | 35.5 | 21.32 | 83.57 | 0 | −0.327* |
| Fraction of diarrhoea because of inadequate sanitation all age | 0.34 | 0.22 | 0.72 | 0.08 | −0.392* |
| HALE at birth | 64.5 | 6.8 | 76.2 | 47.2 | 0.401* |
*Statistically significant at 1%.
Multiple regression was performed on all the variables with significant Pearson’s coefficients. HALE at birth was removed due to high VIF. After multiple regression only significant variable was water current score (Beta = 0.470, p value = 0.001) (Table 2 ).
Table 2.
Linear regression model for COVID 19 deaths per million population.
| Variable | Beta Coefficients | P value |
|---|---|---|
| Water current score | 0.470 | 0.001 |
| Health efficiency | −0.69 | 0.655 |
| % Rural population | 0.008 | 0.945 |
| Fraction of diarrhoea because of inadequate sanitation All age | 0.119 | 0.498 |
R2 = 0.221; F = 34.08; P value = <0.001.
Correlation coefficient between COVID 19 deaths /million population and % slum population was calculated for 51 countries. The same was found to be statistically significant and negative with a value of -0.295 with p value of 0.036.
Correlations between different variables for 122 and 51 countries were found to be significant. Only exception was COVID mortality and health efficiency for 51 countries, all of which were low income regions.
The hypothesized protective effects of gram negative bacilli induced interferon I are demonstrated by the fact that twenty countries with lowest water score ranging from 0.32 to 12.9 reflecting high levels of bacterial contamination have COVID 19 deaths per million population ranging from 0.01 to 0.6 whereas the corresponding figures for twenty countries with highest water score ranging between 94.6 and 100 and 0.7 to 84.3 respectively.
Therefore it might not be surprising to note that COVID19 mortality in countries with more than sixty percent diarrhoea cases attributed to lack of sanitation ranges from 0.02 to 1.61 whereas countries with less than one percent of diarrhoea due to inadequate sanitation show mortality ranging from 0.06 to 61.98.
Negative correlation between percentage of total population living in slums with attendant higher probability of overcrowding and lack of sanitation and COVID19 mortality points towards possible underlying mechanism similar to the one mentioned above.
Negative correlation between COVID19 mortality and percentage of total population living in rural areas can be attributed to diverse microbiome resulting in protective immunity. Countries with more than 60% of the total population living in rural areas have COVID 19 mortality ranging from 0.02 to 2.44 whereas mortality for countries with <10% rural population ranges from 0.09 to 84.3.
Expected healthy life expectancy (HALE) is a function of not only spectrum of morbidities in a given population but also the quality of health care available. Once again contrary to expectations countries with lower HALE have lower COVID 19 mortality. It must be noted that countries have different health care efficiencies reflecting not only access but also the quality of care. Our analysis of 122 countries found health efficiency significantly related to COVID 19 mortality in a negative manner. However the same was not true for 51 lower income countries. It appears that adverse HALE and health efficiency are compensated by microbial exposure.
Conclusions
It is inherently illogical and unscientific to consider populations belonging to different regions but of similar age group to be equally susceptible to COVID 19. Populations of developing and underdeveloped countries are likely to have more resistance to COVID19 owing to high microbial load exposure and resulting microbial interference and/or immunity.
That there appears to be some protective effect of high microbial load exposure, it should not be interpreted as advocacy against sanitation drives. Diarrhea and other diseases owing to lack of sanitation results in much more morbidity and mortality over time compared to global COVID 19.
That diverse microbiome appears to have protective effect against external infection including COVID 19, unnecessary use to antibiotics resulting in diminished human microbiome must be avoided.
That there are at present countries with very low COVID19 mortality, it should act as an impetus to explore population immunity along with regional microbiome in order to gain newer insights into potential protective effects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110209.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.https://coronavirus.jhu.edu/data/mortality Accessed on 15/6/20.
- 2.Petropoulos F, Makridakis S. Forecasting the novel coronavirus COVID-19. PLoS One. 2020;15(3):e0231236. Published 2020 Mar 31. doi:10.1371/journal.pone.0231236. [DOI] [PMC free article] [PubMed]
- 3.Kim S., Seo Y.B., Jung E. Prediction of COVID-19 transmission dynamics using a mathematical model considering behavior changes in Korea. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Measuring Innate Immune Responses to Bacterial Viability.Moretti J, Vabret N, Blander JM.Methods Mol Biol. 2018;1714:167-190. [DOI] [PubMed]
- 5.Sander LE, Davis MJ, Boekschoten MV, Amsen D, Dascher CC, Ryffel B, Swanson JA, Müller M, Blander JM. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature. 2011 May 22;474(7351):385-9. [DOI] [PMC free article] [PubMed]
- 6.Gursel M., Gursel I. Is Global BCG Vaccination Coverage Relevant To The Progression Of SARS-CoV-2 Pandemic? [published online ahead of print, 2020 Apr 6] Med Hypotheses. 2020;109707 doi: 10.1016/j.mehy.2020.109707. [DOI] [Google Scholar]
- 7.KImani-Murage, EW., Ngindu. AM. Quality of water the slum dwellers use: the case of a Kenyan slum. J Urban Health 2007 Nov; 84(6):829-38. Epub 200Jun 6. [DOI] [PMC free article] [PubMed]
- 8.Kurt-Jones E.A., Popova L., Kwinn L., Haynes L.M., Jones L.P., Tripp R.A. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1(5):398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 9.Quilliam R.S., Cross P., Williams A.P., Edwards-Jones G., Salmon R.L., Rigby D. Subclinical infections and asymptomatic carriage of gastrointestinal zoonoses: occupational exposure, environmental pathways, and anonymous spread of the disease. Epidemiol Infect. 2013;141(10):2011–2021. doi: 10.1017/S0950268813001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monroe K.M., McWhirter S.M., Vance R.E. Induction of type I interferons by bacteria. Cell Microbiol. 2010;12(7):881–890. doi: 10.1111/j.1462-5822.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cervantes-Barragan L., Zust R., Weber F., Spiegel M., Lang K.S., Akira S. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franchi L., Kamada N., Nakamura Y., Burberry A., Kuffa P., Suzuki S. NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat Immunol. 2012;13(5):449–456. doi: 10.1038/ni.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, Turroni S, Biagi E, Peano C, Severgnini M, Fiori J, Gotti R, De Bellis G, Luiselli D, Brigidi P, Mabulla A, Marlowe F, Henry AG, Crittenden AN. Gut microbiome of the Hadza hunter-gatherers.Nat Commun 2014; 5:3654. [DOI] [PMC free article] [PubMed]
- 14.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teijaro J.R. Type I interferons in viral control and immune regulation. Curr Opin Virol. 2016;16:31–40. doi: 10.1016/j.coviro.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uematsu S., Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282(21):15319–15323. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- 17.Richez C., Yasuda K., Watkins A.A., Akira S., Lafyatis R., Seventer J.V. Toll-like Receptor 4 Ligands Induce IFN-α Production By Mouse Conventional Dendritic Cells and Human Monocytes After IFN-β Priming. J Immunol. 2009;182(2):820–828. doi: 10.4049/jimmunol.182.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes L.M., Moore D.D., Kurt-Jones E.A., Finberg R.W., Anderson L.J., Tripp R.A. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75(22):10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahl H, Linde A, Strannegård, Scand O. In vitro inhibition of SARS virus replication by human interferons. J Infect Dis 2004;36(11-12):829-31. [DOI] [PubMed]
- 20.Hensley LE, Fritz LE, Jahrling PB, Karp CL, Huggins JW, Geisbert TW, Interferon-beta 1a and SARS coronavirus replication. Emerg Infect Dis. 10, (2) 2004 Feb 317-319. [DOI] [PMC free article] [PubMed]
- 21.Onyango AE, Okoth MW, Kunyanga CN, Aliwa BO. Microbiological Quality and Contamination Level of Water Sources in Isiolo County in Kenya. J Environ Public Health 2018; 2018: 2139867. Published 2018 Jul 9. doi:10.1155/2018/2139867. [DOI] [PMC free article] [PubMed]
- 22.Wardrop N.A., Hill A.G., Dzodzomenyo M., Aryeetey G., Wright J.A. Livestock ownership and microbial contamination of drinking-water: Evidence from nationally representative household surveys in Ghana, Nepal and Bangladesh. Int J Hyg Environ Health. 2018;221(1):33–40. doi: 10.1016/j.ijheh.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manrique P, Bolduc B, Walk ST, van der Oost J, de Vos WM, Young MJ. Healthy human gut phageome .Proc Natl Acad Sci U S A. 2016 Sep 13; 113(37):10400-5. [DOI] [PMC free article] [PubMed]
- 24.Cerf-Bensussan N., Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10(10):735–744. doi: 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- 25.Kamada N., Chen G.Y., Inohara N., Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14(7):685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota.Nat Immunol. 2013 Jul; 14(7):685-90. [DOI] [PMC free article] [PubMed]
- 27.Pacheco A.R., Curtis M.M., Ritchie J.M., Munera D., Waldor M.K., Moreira C.G. Fucose sensing regulates bacterial intestinal colonization. Nature. 2012;492(7427):113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichinohe T., Pang I.K., Kumamoto Y., Peaper D.R., Ho J.H., Murray T.S. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brugger S.D., Bomar L., Lemon K.P. Commensal-Pathogen Interactions along the Human Nasal Passages. PLoSPathog. 2016;12(7) doi: 10.1371/journal.ppat.1005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan Y., Zhao K., Shi Z., Zhou P. Bat Coronaviruses in China. Viruses. 2019;11(3):210. doi: 10.3390/v11030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valitutto M.T., Aung O., Tun K.Y.N., Vodzak M.E., Zimmerman D., Yu J.H. Detection of novel coronaviruses in bats in Myanmar. PLoS ONE. 2020;15(4) doi: 10.1371/journal.pone.0230802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Infect Genet Evol 2017 Mar;48:10-18. doi: 10.1016/j.meegid.2016.11.029. Epub 2016 Dec 6.
- 33.Virus Evol 2018 Dec 15;4(2):vey035. doi: 10.1093/ve/vey035. eCollection 2018 Jul. [DOI] [PMC free article] [PubMed]
- 34.Arch Virol 2015 Apr;160(4):1113-8. doi: 10.1007/s00705-015-2342-1. Epub 2015 Feb 4. [DOI] [PMC free article] [PubMed]
- 35.Dominguez S.R., O'Shea T.J., Oko L.M., Holmes K.V. Detection of group 1 coronaviruses in bats in North America. Emerg Infect Dis. 2007;13(9):1295–1300. doi: 10.3201/eid1309.070491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.“Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003”.World Health Organization. 21 April 2004.
- 37.Porter C.K., Olson S., Hall A., Riddle M.S. Travelers’ Diarrhea: An Update on the Incidence, Etiology, and Risk in Military Deployments and Similar Travel Populations. Mil Med. 2017;182:9/10:4. doi: 10.7205/MILMED-D-17-00064. [DOI] [PubMed] [Google Scholar]
- 38.Moe C.L., Sobsey M.D., Samsa G.P., Mesolo V. Bacterial indicators of risk of diarrhoeal disease from drinking-water in the Philippines. Bull World Health Organ. 1991;69(3):305–317. [PMC free article] [PubMed] [Google Scholar]
- 39.https://epi.envirocenter.yale.edu/epi-indicator-report/H2O Accessed on 10/6/20.
- 40.https://www.who.int/healthinfo/paper29.pdf accessed on 15/5/20 Accessed on 10/6/20.
- 41.https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS?view=chart Accessed on 10/6/20.
- 42.https://www.who.int/data/gho/data/indicators/indicator-details/GHO/attributable-fraction-of-diarrhoea-to-inadequate-hygiene Accessed on 10/6/20.
- 43.https://www.who.int/data/gho/data/indicators/indicator-details/GHO/healthy-life-expectancy-(hale)-at-birth-(years) Accessed on 10/6/20.
- 44.https://data.worldbank.org/indicator/EN.POP.SLUM.UR.ZS?view=chart Accessed on 10/6/20.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.