Abstract
COVID-19 is associated with acute and lethal pneumonia, causing the severe acute respiratory syndrome (SARS), which is not confined to the respiratory tract, as demonstrated by clinical evidence of the involvement of multiple organs, including the central nervous system (CNS). In this context, we hypothesized that both oligosymptomatic and symptomatic patients present an imbalance in the microbiota-gut (immune system) and nervous system axis, worsening the clinical picture. The brain constantly receives a direct and indirect influence from the intestine, more specifically from the immune system and intestinal microbiota. The presence of SARS-CoV-2 in the intestine and CNS, can contribute to both neurological disorders and gut immune system imbalance, events potentialized by an intestinal microbiota dysbiosis, aggravating the patient’s condition and causing more prolonged harmful effects.
Introduction
The novel Coronavirus 2019 (COVID-19) pandemic, which had the first diagnosed case in Wuhan (China), has different clinical manifestations, ranging from asymptomatic to severe or critical conditions [1]. Worldwide data confirms a higher incidence in elderly patients. For these patients, severe cases cause acute and lethal pneumonia, developing the severe acute respiratory syndrome (SARS). This later is not confined to the respiratory tract, as demonstrated by clinical evidence of the involvement of multiple organs [2]. Based on experimental research, in vitro studies, laboratory analyses from patient’s samples, comparative analyses between the SARS and the Middle East respiratory syndrome (MERS) infection, and computational modeling studies, the course of the disease is still being understood and there are gaps regarding the understanding concerning the evolution, pathogenesis and, mainly, therapeutic strategies [3].
One of these gaps concerns the relationship between SARS-CoV-2 and the nervous system, especially with the brain-gut-microbiota axis. It is now known that a neuro-invasive and neurovirulence profile was demonstrated, in a great number of case reports, in other Coronavirus belonging to the same genetic grouping (Beta Coronavirus), such as the SARS-CoV-1 and the MERS-CoV [4], [5], [6]. During the SARS pandemic of 2002–2003, patients presented neurological complications and in brain tissue specimens’ autopsies, SARS-CoV-1 was detected mainly in the cytoplasm of neurons and associated tissue inflammation [7]. It is probable that brain invasion by SARS-CoV-1 occurs via an olfactory route, and is associated with neuronal death as demonstrated in experimental models using mice transgenic for human ACE2 (Angiotensin-converting enzyme). Unlike SARS-CoV (type 1 and 2), MERS-CoV uses a distinct receptor in the cell surface to get access to cytoplasm, named dipeptidyl peptidase 4 receptor (DPP-4), broadly expressed throughout the body on the epithelia, vascular endothelia, and on the brain [7]. It is important to note that in many patients with MERS-CoV infection, was related to neurological complications were observed, ranging from mild to severe clinical manifestations [8], [9]. Since they share genetic, structural, and clinical aspects of the disease, it is likely that many similar mechanisms may apply to SARS-CoV-2. In this respect, an increase in scientific publications suggesting the possibility that the SARS-CoV-2 may affect the central nervous system (CNS) has been observed, with symptoms starting after hospitalization, including headache, disturbed consciousness, and paresthesia as the most frequent of them [10], [11], [12]. It is not known by which pathway the virus accesses the CNS, and it is speculated that it is through retrograde transport through the olfactory nerve and blood pathways infecting endothelial cells or leukocytes [5], [13].
In addition to the possible direct impacts of the tropism of the virus to the CNS, the homeostatic relationship on the microbiota-intestine and nervous system axis, due to the presence of SARS-CoV-2 in the intestine, can contribute to both neurological disorders and gut immune system imbalance. Since the brain-gut microbiota-immune axis presents bidirectional communication, and microbiota is important for the maintenance of immunological homeostasis and Th17/Treg dynamic balance, which suppress pro-inflammatory responses throughout the body [14], an intestinal dysbiosis induced by SARS-CoV-2 may favor a cerebral CNS vulnerability to different injury-causing agents, including the coronavirus itself.
Hypothesis
Since SARS-CoV-2 has been observed in feces of patients, adding to the fact that there is evidence of intestinal disorders in 30% of the infected [15], we hypothesize that both oligosymptomatic and symptomatic patients present an imbalance in the microbiota-gut (immune system) and nervous system axis. This results in immediate consequences for symptomatic patients, mainly with regards to the regulation of the inflammatory phase of COVID-19, as well as in oligosymptomatic ones. These changes may have a late repercussion on immunological phenomena, which is crucial to the homeostasis of the body.
Rationale and discussion
Microorganisms in the gut influence immune system function and health brain
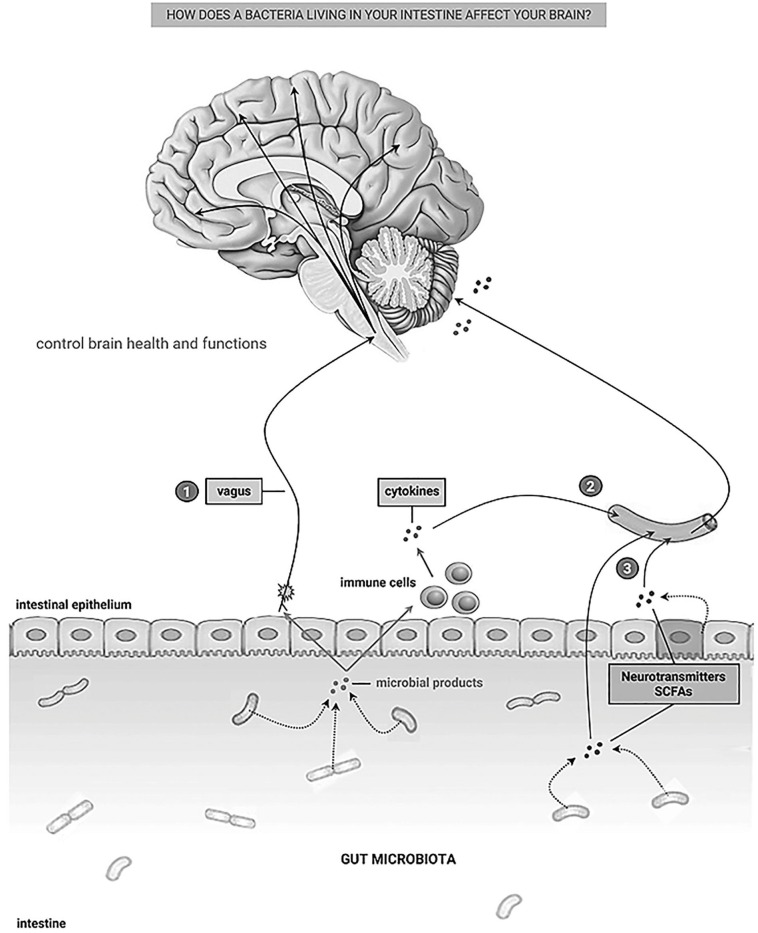
Over 95% of the body's entire microbiome (about 100 trillion) resides in the intestine. In turn, the intestine is the largest human immune organ, in continuous contact with the diversity of bacterial products. This microbiome is crucially fundamental for the maturation and maintenance of immunophysiology. The intestinal microbiota is responsible for aiding digestion, synthesizing vitamins, degrading toxins, and forms the microbiome-gut axis. It provides two-way communication (Fig. 1 ) through cytokine, immunological, hormonal, and neuronal signals [16], [17], [18]. Several studies show that the brain constantly receives a direct and indirect influence from the intestine, more specifically from the intestinal microbiota, which modulates neural functions, regulates its health, and reverses disease processes [19], [20], [21]. Bravo and coauthors [19] showed that the administration of L. rhamnosus was able to reduce stress and anxiety/depression behaviors in rodents, whereas in vagotomized animals these benefic effects were not observed. Demyelinating diseases could be modulated by the action of anti-inflammatory cytokines produced by regulatory T lymphocytes (Treg) induced by microbial products of B. fragilis [20], [22]. Antigen-presenting cells (APCs) in Peyer’s patches in the intestine, present a distinct phenotype and play a crucial role in the generation of Treg cells, contributing to maintaining the immune tolerance in the intestine, and several studies show the contribution of the gut microbiota and its products in regulating the development of APCs [23]. Currently, there are no studies that establish the relationship between the presence of SARS-CoV-2 and alterations in immune tolerance mediated by local APCs and Treg cells.
Fig. 1.
Communication pathways between the gut - brain. (1) through vagal afferent neurons, (2) through cytokines of the immune system, (3) through microbial products themselves and intestinal epithelial cells. SCFAs (Short-chain fatty acids). Figure is modified from Cryan, JF and Dinan, TG. Nat Rev Neurosci. 2012;13(10):701‐712.
In all likelihood, the intestine acts as a secondary site for novel coronavirus tropism and infection through the communication among mucosal tissues. The entrance in the respiratory mucosa and the lack of control in this environment could favor the virus spread to other mucous tissues. However, it is also possible that the intestine represents a primary site of infection through the mouth. The presence of SARS-CoV-2 in the intestinal lumen and its entry into the body through the intestinal epithelial cells which express ACE2 entrance receptor [24], [25], may affect the homeostasis of the microbiota-mucosal immune system’s relationship, with repercussions to the CNS, once neuroinflammatory and functional consequence after brain injury are affected by the gut microbiota homeostasis [26]. The real impact of SARS-CoV-2 on the gut microbiota with consequent dysbiosis remains to be defined, but it has become evident that COVID-19 patients with gastrointestinal symptoms such as diarrhea, nausea, vomiting, and abdominal pain had more severe disease. Still, some of them presented microbial dysbiosis with decreased levels of Lactobacillus and Bifidobacterium [27]. After invading the gut, the virus can reach the CNS through the circulatory pathway or the vagal nerve [28]. Neurological consequences may initially be subjective, such as the above-mentioned headache, disturbed consciousness, and paresthesia [10], but there may be more severe symptoms (such as depressed level of consciousness, seizure, and stroke) with the course of infection [29] and the inflammatory process, as glial cells and neurons have been reported to express ACE-2 receptors, which intermediates the entry of the virus into cells.
Another key point is related to immunological phenomena that occurs in the intestine. This contributes to the generation of fundamental regulatory activities for the modulation of local and systemic inflammatory immune responses. It is known that the generation and maintenance of this mucosal immunity are stimulated and maintained by the acquisition of a complex microbiota, with which symbiotic relationships are established [30].
A breakdown of this dynamic relationship results in chronic inflammatory disorders, including autoimmunity, allergies, and metabolic syndromes and compromises the control of exacerbated immune responses in many diseases [30]. It is known that COVID-19 presents a biphasic course, initially viremic and then uncontrolled inflammation. Therefore, we hypothesize that greater severity of pathogenesis is related to an immune dysregulation added to SARS-CoV-2 gut infection. Therefore, a mucosa proinflammatory response instead of an immune regulation, associated with microbial dysbiosis, may affect the CNS.
Conclusion
In conclusion, it is reasonable to consider this worsening of the clinical picture when the new coronavirus reaches the intestine. This may also cause more severe neurological impairment. Furthermore, the control of the inflammatory process will be even more difficult due to changes in the immune regulation in the intestinal mucosa microenvironment. To the best of our knowledge, there is no publication specifically exploring this hypothesis, but there are data and independent results that support this theory.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Minas Gerais State Research Foundation (FAPEMIG, Minas Gerais, Brazil), the National Council for Scientific and Technological Development (CNPq, Brazil), and the Coordination of Training of Higher Education Graduate Foundation (CAPES, Brasilia, Brazil).
References
- 1.Pascarella G., Strumia A., Piliego C., Bruno F., Buono R., Costa F. COVID-19 diagnosis and management: a comprehensive review [published online ahead of print, 2020 Apr 29] J Intern Med. 2020 doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendoza-Pinto C., Escárcega R.O., García-Carrasco M., Bailey D.J.O., Gálvez-Romero J.L., Cervera R. Viral Infections and their relationship with catastrophic antiphospholipid syndrome: a possible pathogenic mechanism of severe COVID-19 thrombotic complications [published online ahead of print, 2020 Jun 7] J Intern Med. 2020 doi: 10.1111/joim.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao N., Zhou Z.L., Wu L., Zhang X.D., Han S.B., Bao H.J. An update on the status of COVID-19: a comprehensive review. Eur Rev Med Pharmacol Sci. 2020;24(8):4597–4606. doi: 10.26355/eurrev_202004_21046. [DOI] [PubMed] [Google Scholar]
- 4.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. Epub 2020 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng Kee Kwong K.C., Mehta P.R., Shukla G., Mehta A.R. COVID-19, SARS and MERS: a neurological perspective. J Clin Neurosci. 2020;77:13–16. doi: 10.1016/j.jocn.2020.04.124. Epub 2020 May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Q., Yang Y., Gao J. Infectivity of human coronavirus in the brain. EBioMedicine. 2020;56 doi: 10.1016/j.ebiom.2020.102799. Epub 2020 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease: a review. JAMA Neurol. 2019 doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saad M., Omrani A.S., Baig K., Bahloul H., Elzein F., Matin M.A. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43(4):495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liguori C., Pierantozzi M., Spanetta M., Sarmati L., Cesta N., Iannetta M. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.05.037. S0889-1591(20)30876-X. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan. China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. [published online ahead of print, 2020 Apr 6] Eur Arch Otorhinolaryngol. 2020:1–11. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J., Zheng M., Tang X., Chen Y., Tong A., Zhou L. Potential of SARS-CoV-2 to Cause CNS infection: biologic fundamental and clinical experience. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omenetti S., Pizarro T.T. The Treg/Th17 Axis: a dynamic balance regulated by the gut microbiome. Front Immunol. 2015;17(6) doi: 10.3389/fimmu.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neurath M.F. COVID-19 and immunomodulation in IBD. Gut. 2020;69(7):1335–1342. doi: 10.1136/gutjnl-2020-321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol. 2018;6(2):133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sochocka M., Donskow-Łysoniewska K., Diniz B.S., Kurpas D., Brzozowska E., Leszek J. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer's disease-a critical review. Mol Neurobiol. 2019;56(3):1841–1851. doi: 10.1007/s12035-018-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochoa-Repáraz J., Mielcarz D.W., Wang Y., Begum-Haque S., Dasgupta S., Kasper D.L. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3(5):487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 21.Kirby T.O., Ochoa-Repáraz J. The gut microbiome in multiple sclerosis: a potential therapeutic avenue. Med Sci (Basel) 2018;6(3):69. doi: 10.3390/medsci6030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochoa-Repáraz J., Kirby T.O., Kasper L.H. The gut microbiome and multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(6) doi: 10.1101/cshperspect.a029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H.J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3(1):4–14. doi: 10.4161/gmic.19320. Epub 2012 Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgueño J.F., Reich A., Hazime H., Quintero M.A., Fernandez I., Fritsch J. Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis. 2020;26(6):797–808. doi: 10.1093/ibd/izaa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, et al. SARS-CoV-2 productively infects human gut enterocytes. Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes [published online ahead of print, 2020 May 1]. Science 2020; eabc1669. Advance online publication. https://doi.org/10.1126/science.abc1669. [DOI] [PMC free article] [PubMed]
- 26.Singh V., Roth S., Llovera G., Sadler R., Garzetti D., Stecher B. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016;36(28):7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He L.H., Ren L.F., Li J.F., Wu Y.N., Li X., Zhang L. Intestinal flora as a potential strategy to Fight SARS-CoV-2 infection. Front Microbiol. 2020;9(11):1388. doi: 10.3389/fmicb.2020.01388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bostancıklıoğlu M. Temporal correlation between neurological and gastrointestinal symptoms of SARS-CoV-2. Inflamm Bowel Dis. 2020;26(8):e89–e91. doi: 10.1093/ibd/izaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bostanciklioglu M. Severe acute respiratory syndrome coronavirus 2 is penetrating to dementia research [published online ahead of print, 2020 May 22] Curr Neurovasc Res. 2020 doi: 10.2174/1567202617666200522220509. [DOI] [PubMed] [Google Scholar]
- 30.Belkaid Y., Harrison O.J. Homeostatic Immunity and the Microbiota. Immunity. 2017;46(4):562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]