Abstract
The skin microbiome is an ecosystem comprised of a multitude of microbial species interacting with their surroundings, including other microbes and host epithelial and immune cells. These interactions are the basis of important roles within the skin microbiome that provide benefit to the host, boosting multiple aspects of barrier function, a critical function of this essential organ. However, with reward always comes risk; resident skin microbes function in a context-dependent manner, set on the backdrop of a dynamic host and microbial milieu. Here, we discuss the reward of hosting a microbial ecosystem on the skin, including protection from pathogens and tuning of the skin microenvironment. We also give consideration to how these skin residents, often termed “commensals” can cause disorder, damage, and promote skin disease.
Introduction
The skin is an epithelial barrier to the external environment that also supports diverse microbiota comprised of bacteria, fungi, viruses, and microeukaryotes. The skin microbiota is adapted for life in the unique microhabitats that define the environmental and nutrient conditions of this ecosystem. Interacting together and through mutualistic or commensal interactions with mammalian host cells, the skin microbiota promotes defense and immune responses, inhibits colonization and infection by opportunistic or pathogenic organisms, and promotes tissue repair and barrier functions. In this review, we will discuss the interactions within this ecosystem and the resulting reward of homeostasis, or alternatively, the risk of disorder and disease that might occur as a result of disrupting the skin ecosystem.
The skin offers protective niches and nutrients for microbial survival, competition, and cooperation (Fredricks, 2001; Roth and James, 1988). On the microscopic scale, the pilosebaceous unit is a protective invagination that provides a microaerophilic environment for obligate and facultative anaerobes. On the macroscopic scale, folds such as the umbilicus offer an occluded environment that retains moisture and resists outside perturbation. Sebum, secreted through the sebaceous gland, is a source of lipids that may be utilized as a nutrient source (Figure 1). For example, the skin bacterium Cutibacterium acnes (C. acnes, reclassification of the previous Propionibacterium acnes) produces lipases that break down sebum lipids allowing it to utilize the resulting fatty acids as nutrients. These fatty acids also acidify the skin surface, thereby creating an environment inhibitory of colonization by exogenous microorganisms. Other nutrients available on the skin include salts secreted from sweat (eccrine and apocrine) glands and cellular debris rich in proteins and lipids resulting from desquamation, or sloughing, of the cornified layer of the epidermis through a process of terminal differentiation. Although the skin surface is mostly desiccated with a few notable exceptions, the lipids, salts, and cellular debris provide sufficient nutrients for survival, especially for those microbes that are adapted to the generally inhospitable conditions (Figure 1).
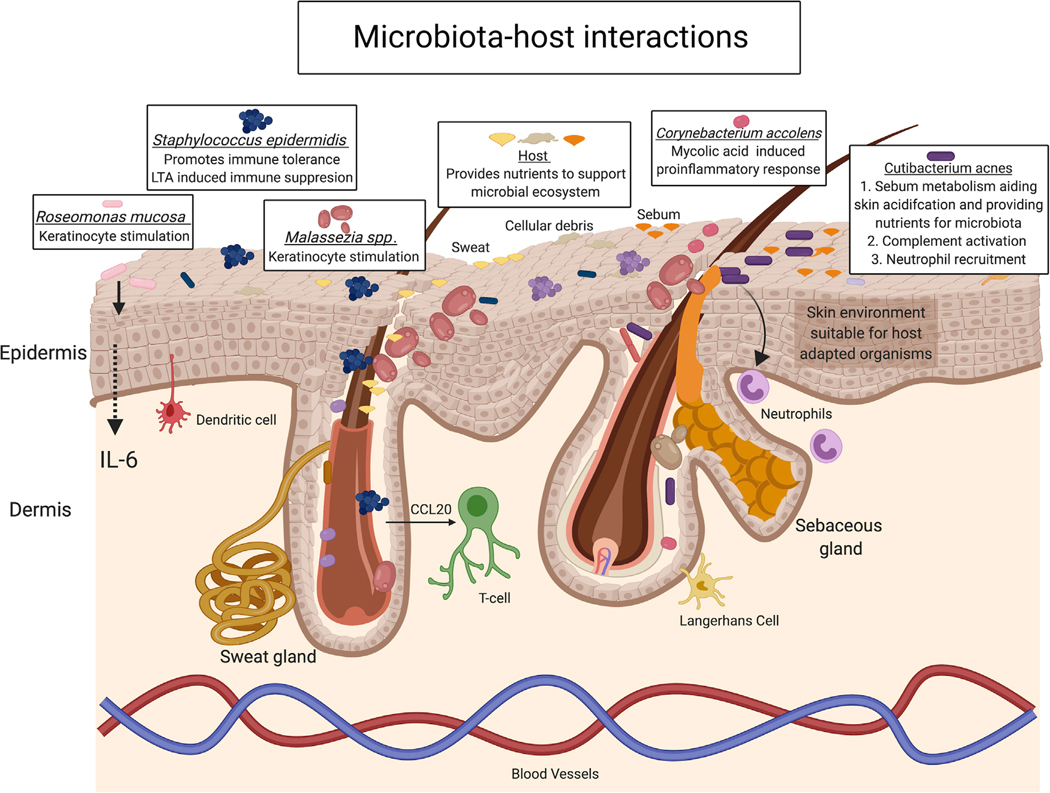
Figure 1. Microbiota-Host Interactions Promote Skin Homeostasis and Immune Response.
Multiple microbial species residing in skin promote immune tolerance, elicit pro-inflammatory response, and aid in skin maintenance; in turn, the host provides nutrients to sustain microbial survival. Staphylococcus epidermidis, a model example of the intimate relationship microbiota has with the host, can both stimulate and suppress inflammation. Such microbial-host interactions contribute to stability of the microbial ecosystem and skin integrity. Other species such as Roseomonas mucosa, Malassezia spp., or Corynebacterium accolens can also tune keratinocyte and host immune responses in a context-dependent manner. Lastly, even the often-problematic skin resident microbe, Cutibacterium acnes, has beneficial interactions with the host as it metabolizes sebum secretions, which in turn aides in maintaining an acidic skin pH, making the skin suitable for select organisms.
DNA sequencing-based methods to identify and survey microbes have illuminated the vast diversity of species within the skin microbiome. Early studies using bacterial 16S ribosomal RNA (rRNA) gene amplicon-based sequencing methods emphasized the topographical diversity of skin microbial communities and the influence of microenvironment. The pivotal study of 20 different anatomical skin sites revealed unique microbial compositional differences that occur across body sites (Grice et al., 2009). Sebaceous sites were enriched in sebum-loving Cutibacterium, while the fastidious and slow growing Corynebacterium localized to moist sites. Overall, Staphylococcal species were prevalent throughout, consistent with culture-based assessments of skin microbiota. Sequencing of fungal ribosomal RNA gene amplicons demonstrated that Malassezia is the overwhelmingly dominant member of the fungal microbiota on the majority of body sites (Findley et al., 2013). The one exception was the feet, which harbored greater diversity of fungal species.
With the advent of shotgun metagenomics, an even more nuanced view of the skin microbiome has emerged. Unlike 16S rRNA gene sequencing which relies on PCR-amplification of a specific gene, in shotgun metagenomics the genomic DNA in a sample is fragmented and deeply sequenced. Bio-informatic methods are then used to assemble the heterogeneous microbial genomes that are recovered and then compared to reference databases to identify species. This type of analysis allows for species- and strain-level interrogation of microbiota, a multi-kingdom analysis that is inclusive of fungi and viruses as well as bacteria, and the analysis of the genetic content and genes and pathways that are enriched (Grogan et al., 2019). These methods revealed the strain-level variation of skin microbiota and its stability over time. Given the frequent perturbations encountered by skin microbes on a daily basis, the microbiota was surprisingly stable (Oh et al., 2014, 2016).
Metagenomic techniques have also enhanced our understanding of viral communities associated with the skin. Unlike bacteria and fungi, there is not a common “marker gene” that can be broadly amplified and sequenced to infer viral identify. In the healthy skin virome bacteriophages dominate, which like their hosts, are associated with skin microenvironment (Hannigan et al., 2015). Eukaryotic viruses, especially human papillomaviruses (HPVs) seem to be controlled to some degree by immune surveillance, as HPVs expand and dominate the skin virome in patients with primary immunodeficiencies (Tirosh et al., 2018; Pastrana et al., 2018).
A picture of a diverse resident microbiota has emerged, but key questions remain regarding the function of the skin microbiome and which members are beneficial, neutral, or harmful. Similarly, how do changes in the composition of the skin microbiota relate to disease, and are these changes causal? Here, we focus on members of the healthy human skin microbiota, which we consider as those taxa that stably reside (intransient) on the normal skin in the majority of humans. We examine roles for skin microbes within this ecosystem and how these roles present risk as well as reward in the context of the host. Finally, we consider how changes in the normal skin microbiota can predispose to or exacerbate skin disease and infection.
Cutibacterium acnes: Friend in Health, Foe in Acne, or Both?
Cutibacterium acnes is one of the most abundant bacterial species in the adult skin microbiome (Fitz-Gibbon et al., 2013). Formerly known as Propionibacterium acnes, C. acnes is an aerotolerant anaerobe that utilizes lipid-rich sebum as its nutrient source (Brown and Shalita, 1998). Comprising minimal abundance in pre-pubertal skin, C. acnes begins to appear in the human skin microbiota concomitant with maturation of the sebaceous gland and sebum secretion during puberty (Oh et al., 2012). In its role as a commensal component of the skin microbiota, C. acnes produces propionic acid, which helps to maintain the acidic pH of healthy skin, thus inhibiting colonization by more pathogenic microbes (Youn et al., 2013). Adapted to the nutrient conditions of the human pilosebaceous unit, C. acnes secretes an extracellular lipase that cleaves sebum triglycerides, thus freeing glycerol as a growth substrate and free fatty acids that further acidify the skin (Brown and Shalita, 1998) (Figure 1).
However, C. acnes may be more notorious for its role in the inflammatory skin disorder acne vulgaris. Acne affects the pilosebaceous unit, which consists of small hair follicles associated with a sebaceous gland and is localized to the face, chest, and back (Brown and Shalita, 1998). Here, C. acnes has the potential to directly and indirectly cause inflammation and tissue damage (Figure 2). Free fatty acids freed from sebum triglycerides by C. acnes are also pro-inflammatory. C. acnes are also capable of producing a variety of pro-inflammatory virulence factors including proteases and factors that activate complement and recruit neutrophils (Burkhart et al., 1999).
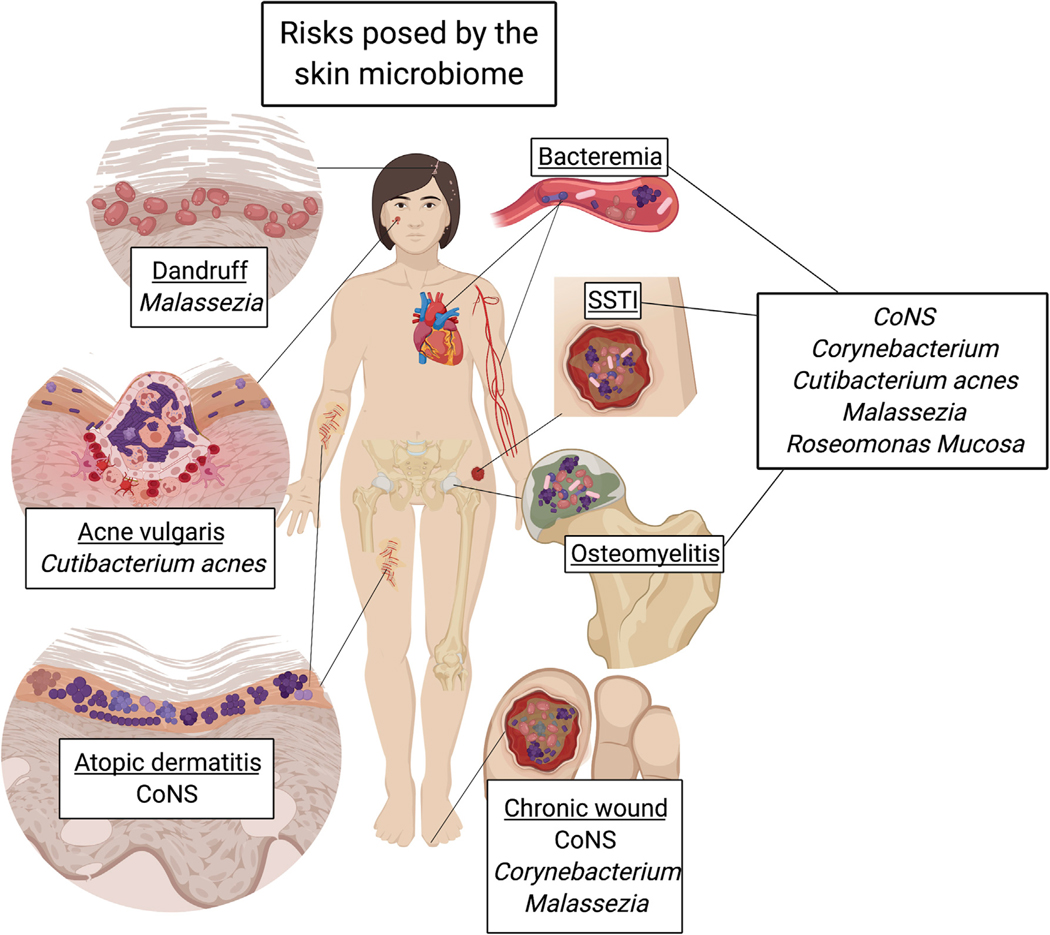
Figure 2. Risk: Diseases with Causative Agents from or Associated with Skin Microbiota.
Microbiota that are most abundant and commonly found in skin microbiome datasets have been identified as the causative agents of the skin disorders and diseases shown here. The species contributing to disease are often body site dependent, a reflection of microbial niche specificity. For instance, both Malassezia spp. and Cutibacterium acnes prefer sebum rich environments, thus high abundance of these species at sebum rich sites, such as the scalp or the face, can lead to host disease. Unfortunately, many species can cause serious systemic disease if given the opportunity to transit to deeper tissue. This occurs most often in immunocompromised host in the form of bacteremia, surgical site soft tissue infections (SSTI), osteomyelitis, and/or chronic wounds.
Comparative genomic analysis of C. acnes isolates cultured from acne and normal skin has shed some light on the elusive nature of this bacterium’s relationship with the skin. Fitz-Gibbon et al. showed that C. acnes genomes recovered from acne skin were different than C. acnes genomes from healthy controls (Fitz-Gibbon et al., 2013). C. acnes from acne patients harbored unique genomic elements encoding virulence factors that were rarely present in C. acnes genomes from healthy controls, suggesting that genomic diversification may drive pathogenicity of the organism. Strain-specific differences in promoting immune responses have also been observed, where C. acnes isolated from acne skin induced higher production of the pro-inflammatory cytokines interferon (IFN)-γ and interleukin 17 (IL-17) from peripheral blood mononuclear cells, whereas strains from healthy skin were more robust in inducing the anti-inflammatory cytokine IL-10 (Yu et al., 2016). Comparative proteomic analysis of bacterial secreted factors provided mechanistic clues, as acne strains were found to produce higher levels of an adhesin and a cell wall hydrolase compared with C. acnes strains isolated from healthy individuals.
Porphyrin production by C. acnes may be an important virulence mechanism that promotes skin inflammation. C. acnes strains isolated from acne skin have been observed to produce higher levels of the pro-inflammatory porphyrin molecule than strains from healthy subjects (Johnson et al., 2016). Linking genomic variation to molecular mechanism, these phenotypic changes could be explained by loss of a repressor gene in the porphyrin biosynthetic pathway. Porphyrin production also appears to be positively regulated by vitamin B12, driving increased porphyrin in acne-associated strains, but with no effect in healthy strains (Barnard et al., 2020). These findings are consistent with metatranscriptomic profiling of acne skin, which was depleted in expression of vitamin B12 biosynthesis genes compared with normal skin (Kang et al., 2015). Oral supplementation of healthy humans with vitamin B12 over a course of two weeks similarly resulted in decreased expression of C. acnes vitamin B12 biosynthesis genes in their skin microbiota. These findings together provide evidence for a microbially mediated link connecting acne pathogenesis with porphyrin and vitamin B12 supplementation (Kang et al., 2015).
C. acnes may also serve as an accomplice to skin pathogenic species, namely S. aureus. Coproporphyrin III (CIII), the most abundant extracellular porphyrin molecule produced by C. acnes, was shown to induce aggregation of S. aureus and biofilm attachment to abiotic (plasma free) surfaces (Wollenberg et al., 2014). Other studies have shown that C. acnes enhances S. aureus hemolytic and cytolytic activity; these effects translated to heightened lesion severity in a murine model of co-infection, and were dependent on pore-forming toxins from these bacteria, notably C. acnes CAMP (Christie, Atkins, Munch Peterson) factor and S. aureus β-hemolysin (Lo et al., 2011). These studies highlight how microbes regarded as skin commensals may cooperate with traditional pathogens to enhance their virulence and are yet another example of the context-dependent relationships among skin microbes and their effects on the host.
Coagulase-Negative Staphylococci: Bacterial Warfare on the Skin
To ensure survival, skin resident bacteria must compete to maintain colonization of their niche and access to nutrients. As in any ecosystem, competitive interactions are continuously occurring between microorganisms. In the skin, resident microbiota protect the skin from invading, pathogenic, or opportunistic microbes through the process of colonization resistance (Figures 3 and 4). One manner in which bacteria can eliminate competition is through direct killing of competing microbes. The full extent of these interactions is unknown, but the most studied are those where coagulase-negative Staphylococcus (CoNS) species inhibit their close but pathogenic relative, S. aureus (O’Sullivan et al., 2019). CoNS species are highly abundant across the skin microbiome. These Gram-positive, facultative anaerobes are distinguished from S. aureus, which is coagulase positive, or capable of coagulating blood. On human skin, the CoNS species largely comprise S. epidermidis, S. capitis, S. caprae, S. hominis, S. lugdunensis, and S. haemolyticus.
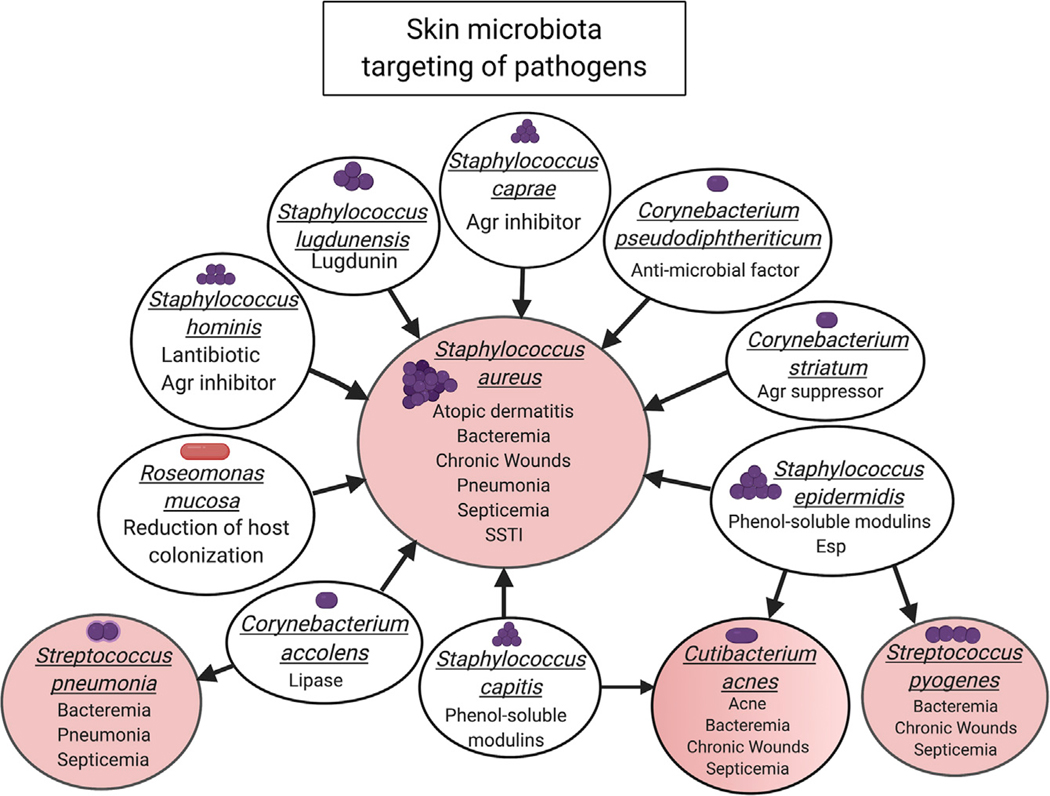
Figure 3. Reward: Microbiota Targeting of Pathogens Associated with Skin Disorders or Disease.
Microbial-microbial interactions between the skin microbiome (white circles) and pathogens (pink circles) demonstrate the powerful benefit the skin microbiome can have for the host. CoNs, Corynebacterium spp, and Roseomonas mucosa target (arrows point from the source to the target) Staphylococcus aureus. These bacteria can either directly kill through the secretion of antimicrobial factors or limit the virulence of Staphylococcus aureus through the secretion of an inhibitor or suppressor of a major S. aureus virulence regulator. Other skin microbial species can target and limit Cutibacterium acnes, Streptococcus pyogenes, and Streptococcus pneumoniae.
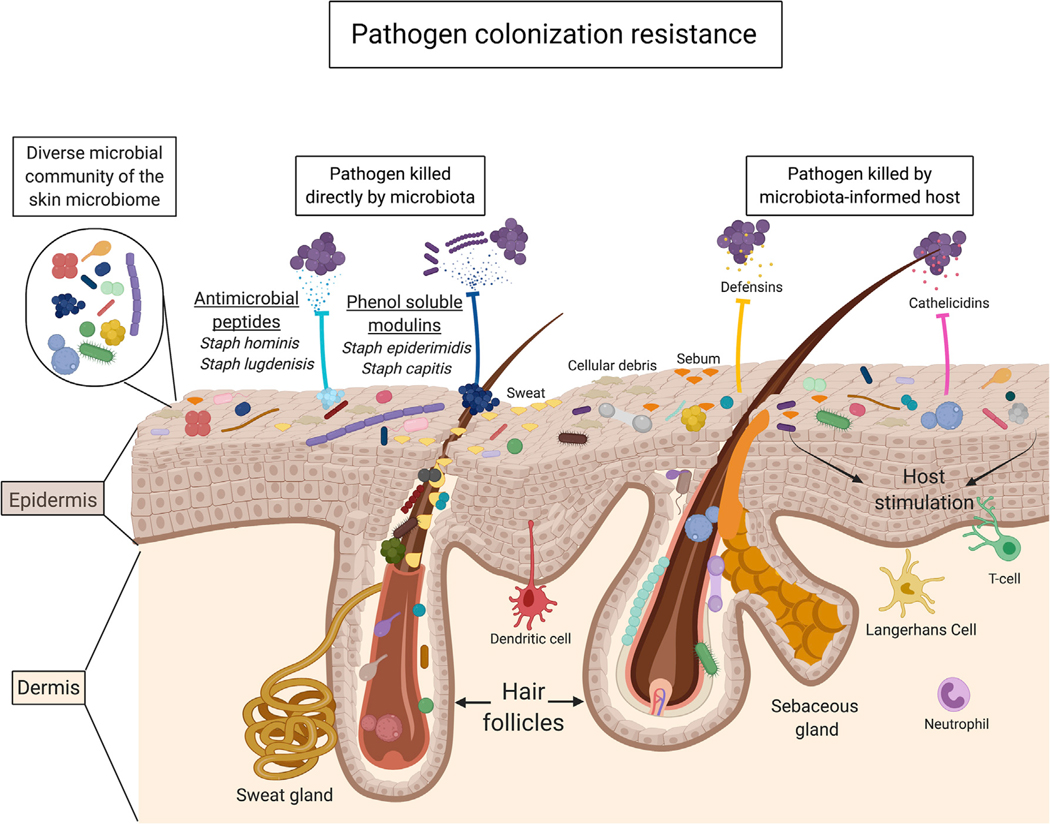
Figure 4. Colonization Resistance, a Collective Contribution of the Skin Microbiome.
The skin microbiome is composed of a diverse microbial community; as also defined in Figure 1, this diversity spans through domains, from kingdom to strain. A recently recognized essential function of the skin microbiome is to provide protection against pathogens, termed colonization resistance. The skin microbiome achieves this through the capability to directly kill or alter the virulence of pathogens through the secretion of antimicrobial peptides and other molecules. Additionally, as many species are capable of stimulating keratinocytes, the microbiome can signal to the host to mount a protective immune response to invading pathogens. Importantly, the combined microbial-microbial and microbial-host interactions play a critical role in maintaining host health.
Some species of CoNS directly kill their S. aureus competitors by secreting antimicrobial peptides (summarized in Figure 3). For example, some strains of S. hominis secrete lantibiotics that were shown to be protective against S. aureus colonization in murine models and human subjects with the inflammatory skin condition atopic dermatitis (Nakatsuji et al., 2017). Nasal strains of S. lugdunensis were found to produce lugdunin, a novel thiazolidine-containing cyclic peptide that also has potent anti-S. aureus activity (Zipperer et al., 2016). Phenol-soluble modulins (PSM) are produced by several different CoNS species including S. epidermidis, S. capitis, and promote killing of Streptococcus pyogenes and S. aureus, and C. acnes, respectively (Cogen et al., 2010; O’Neill et al., 2020).
Bacterially produced AMPs may enhance or synergize with host immune responses, in addition to their direct killing activity. S. epidermidis PSMs work in concert with the host derived antimicrobial peptide LL-37 to enhance host immune defense (Cogen et al., 2010). Similarly, combining S. hominis lantibiotics with LL-37 enhanced the antimicrobial activity against S. aureus (Nakatsuji et al., 2017).
Another mechanism of bacterial warfare on the skin involves interfering with cell-to-cell communications of the pathogenic S. aureus, referred to as quorum sensing. All staphylococci communicate through quorum sensing, specifically through the accessory gene regulator (agr) system, and this system is necessary for S. aureus skin infection and damage (Wright et al., 2005). This system also provides a target for commensal bacteria to inhibit the growth and virulence of their competition. For example, S. caprae inhibited methicillin-resistant S. aureus agr activity in an intradermal skin infection model, thereby improving infection outcome (Paharik et al., 2017). Such mechanisms of cross talk have also been identified in atopic dermatitis skin, where CoNS such as S. hominis interfere with S. aureus quorum sensing to prevent S. aureus toxin production and associated tissue destruction and inflammation (Williams et al., 2019).
Production of proteases is another potential mechanism whereby CoNS species interfere with S. aureus virulence. S. epidermidis is capable of producing a serine protease, Esp, that inhibits biofilm formation and disrupts intact biofilm of S. aureus (Iwase et al., 2010). In addition to epidemiological evidence suggesting that colonization with Esp producing strains is protective, introduction of Esp producing strains into the nasal cavity of S. aureus carriers effectively eliminated S. aureus, while the isogenic Esp mutant did not.
The above described competitive interactions highlight the role of microbial competition as a force in shaping the skin microbiome. Among the staphylococci, genomic diversification though mobile genetic transfer may also shape the skin microbiome and its risk versus reward. The diverse species in the skin microbiome represent a reservoir of genetic material, in particular pathogenicity islands, transposons, plasmids, and other mobile elements that are transferrable among staphylococci (Otto, 2013). In fact, the chromosomal cassette carrying methicillin resistance and other virulence factors likely originated from S. epidermidis and potentially other CoNS species. Diversification through horizontal gene transfer represents a potential mechanism whereby resident skin commensal bacteria could become more virulent or invasive.
Corynebacterium
The Corynebacterium are also prevalent and abundant members of the skin microbiota, in particular in moist and occluded areas of the body. Corynebacterium, of the phylum Actinobacteria and family Actinomycetales, are Gram-positive, aerobic, “club-shaped” microorganisms. Corynebacterium species have been implicated in producing odorous volatile compounds characteristic of axillary sweat (Kligman and Strauss, 1956), though emerging evidence suggests that CoNS species S. lugdunenesis, S. hominis, and S. haemolyticus are more efficient at sweat biotransformation to the malodourous free thioalchohol (Bawdon et al., 2015). Because they grow slower than other skin commensal species and have specialized nutritional requirements, Corynebacterium can be difficult to isolate and cultivate under artificial growth conditions (Forbes et al., 1998).
Corynebacterium species and their role in competitive interactions with the skin pathogen S. aureus have been studied most thoroughly in the nasal cavity. Many of the microbial species residing in the nasal cavity are also found on the skin and vice versa (Human Microbiome Project, 2012; Grice et al., 2009), including Corynebacterium species. This is despite the nasal cavity being occluded and comprised non-cornified stratified epithelium. The nasal cavity is also a reservoir of S. aureus carriage, and nasal carriage is a well-established risk factor for infection (Kluytmans and Wertheim, 2005). Studies defining the human nasal microbiota have led to the identification of microbial community determinants influencing the competitive exclusion of S. aureus in the nasal cavity (Yan et al., 2013). One such species, C. pseudodiphtheriticum, was identified from culture-independent sequencing and random forest-supervised learning models as a predictor that was strongly negatively correlated with S. aureus carriage. In vitro co-culture confirmed the predictive model, as cultured isolates of C. pseudodiphtheriticum demonstrated strong inhibition of S. aureus. More recent studies have built on these findings to show a novel direct killing mechanism of C. pseudodiphtheriticum that was dependent on S. aureus virulence components (Hardy et al., 2019).
Skin commensal Corynebacterium species may also limit the virulence of S. aureus through modulating the agr quorum sensing system and expression of agr-inducible virulence genes (Figure 3). In vitro co-culture of C. striatum or C. striatum cell-free conditioned media with S. aureus resulted in decreased expression of S. aureus genes regulated by agr, reduced hemolytic activity, and increased epithelial adhesion, characteristic of virulence attenuation. In a dermal murine abscess infection model, co-infection of S. aureus with C. striatum reduced S. aureus microbial burden compared with infection with S. aureus alone (Ramsey et al., 2016). While it is unknown how C. striatum elicits such interference, these findings demonstrate how mechanisms of microbial interference have the potential to limit S. aureus colonization and virulence.
Corynebacterium species have also been shown to inhibit streptococcal pathogens. The observation that children with high relative abundances of nasal Corynebacterium were free of Streptococcus pneumoniae (pneumococcus) informed further mechanistic investigations of microbial antagonism (Bomar et al., 2016). C. accolens, commonly found in adult and human nasal cavity, depends on a lipase for its inhibitory activity that cleaves triacylglycerols that are found in skin and sebum. In hydrolyzing these triacylglycerols, free fatty acids are released that inhibit S. pneumoniae (Bomar et al., 2016). Biological observations, such as these regarding microbial-microbial interactions from nasal isolates are likely to be relevant to the skin, considering the overlap in microbiome species composition, and demonstrate the role of microbial-microbial interactions in sculpting the overall composition of the microbiome.
While widely regarded as a commensal bacterium, as usual, this relationship is context dependent. Multi-drug resistance has been noted among Corynebacterium species, but especially C. striatum, which is considered an emerging pathogen that causes various device and skin and soft tissue infections in immunocompromised, hospitalized, and chronically ill hosts (Figure 2) (Patel et al., 2016; Wang et al., 2019). Almost all species of Corynebacterium contain a mycolic acid layer, long-chain fatty acids (usually C22 to C36 in Corynebacterium; Collins et al., 1982) with key roles in resisting stress, such as detergents, antimicrobials, and even lysozyme, allowing colonization and survival across various conditions (Burkovski, 2018; Tauch and Burkovski, 2015). Mycolic acid also can influence host responses, but this is also contextual. In a murine model of epicutaneous colonization, it was found that C. accolens only promoted inflammation in an IL-23-dependent manner when animals were fed a high-fat diet (Ridaura et al., 2018). This effect was dependent on mycolic acid in the cell envelope and demonstrates how the metabolic state of the host can influence its relationship with components of the skin microbiota.
Staphylococcus epidermidis: A Model Commensal to Probe Homeostatic Immune Responses
The majority of studies that probe immune responses to commensal bacteria have utilized Staphylococcus epidermidis as a model skin commensal. Study designs in murine models typically use topical association procedures to colonize the skin. Liquid cultures of the bacterium, usually 107–109 CFU, are concentrated and swabbed onto the skin multiple times over a time-course of several days to several weeks. This design is typically used when colonizing murine skin with bacterial strains isolated from human skin. Due to differences in nutrient availability, pH, and other microenvironment parameters, isolates specialized to life on human skin may be recalcitrant to colonization of murine skin. Thus, optimization and experimental confirmation of colonization are critical to robust experimental designs.
In neonatal life, colonization is of particular importance during a critical window of hair follicle morphogenesis. Scharschmidt et al. observed that S. epidermidis colonization of murine neonatal skin provided protection, or immune tolerance, later in life when challenged (Scharschmidt et al., 2015). This effect was dependent on a migratory wave of activated regulatory T cells (Tregs) into neonatal skin, which was concomitant with hair follicle morphogenesis (Scharschmidt et al., 2017). The mechanism underlying this selective wave of Treg infiltration is dependent on S. epidermidis stimulation of the hair follicle-derived chemokine Ccl20 and its receptor Ccr6, which is expressed on Tregs (Scharschmidt et al., 2017). This work demonstrates how skin morphogenic processes coordinate with the immune system to establish tolerance to our commensal microbiota (Figure 1).
In adult murine skin, microbial colonization has been shown to control the local inflammatory milieu and T cell function (Naik et al., 2015, 2012). For example, infection of germ-free mice with the parasitic pathogen leishmania resulted in uncontrolled parasite growth and blunted protective immune response, despite lesser infection pathology compared to conventionally raised control mice (Naik et al., 2012). Notably, these responses could be rescued in germ-free mice by colonization with S. epidermidis (Naik et al., 2012). Thus, S. epidermidis not only promotes tolerance to the commensal microbiota but also tunes protective immune responses appropriately when a pathogen is encountered.
S. epidermidis also has demonstrated roles in modulating innate immune responses during tissue repair and wound healing of the skin. Like other Gram-positive bacteria, S. epidermidis cell membrane/wall contains lipoteichoic acid (LTA), an adhesion molecule that is released upon bacteriolysis (Ginsburg, 2002). LTA from Staphylococcal species suppressed inflammation during tissue injury through a TLR2-dependent mechanism to prevent excessive damage (Lai et al., 2009). Staphylococcal LTA may also have applications for the treatment of inflammatory disease. In an acne model of P. acnes induced skin inflammation, staphylococcal LTA application abrogated inflammatory effects via induction of a microRNA, miR-143, which destabilizes the TLR2 mRNA and decreases protein production (Xia et al., 2016).
S. epidermidis interactions with its host exist against the backdrop of a complex microbial milieu that may not always behave in the interest of the host. The mechanisms that S. epidermidis use to compete in the skin microbial community may double as virulence mechanisms that promote the adherence, survival, and dissemination in certain situations. For example, the S. epidermidis protease Esp may prevent S. aureus biofilm formation, but it also degrades human complement component C5 and fibrinogen, critical proteins for the complement and coagulation cascade which forms a pillar of the innate immune response (Moon et al., 2001). S. epidermidis PSMs have potent anti-bacterial activity but are also essential for structuring and dissemination of biofilm in S. epidermidis indwelling device infection (Le et al., 2019). S. epidermidis also produces proteases that promote its adherence to plastic (EcpA), degrade host AMPs, and evade elimination by immune cells (SepA, APS) (Cheung et al., 2010; Martínez-García et al., 2018). These mechanisms highlight potential reasons why S. epidermidis is prone to cause device-associated infections and is the most common microbe associated with such (Zheng et al., 2018). Recently, the S. epidermidis protease EcpA was identified as a factor contributing to tissue destruction and inflammation in the monogenetic skin disorder Netherton syndrome (Williams et al., 2020), highlighting how host-dependent interactions with the resident skin microbiota can impact disease severity.
Malassezia
Malassezia are the most abundant fungi found on human skin (Findley et al., 2013). In a similar fashion as other resident skin microbes, Malassezia spp. are highly evolved for life on the skin and exhibit niche specificity where nutrients necessary for survival are readily available. Malassezia sp. thrive in lipid-rich environments; thus, they are commonly found in skin sites enriched with sebaceous glands such as the face, scalp, and outer ears (Kaneko et al., 2010). The human-associated species of Malassezia include M. dermatis, M. furfur, M. globosa, M. restricta, and M. sympodialis. The role of this species in the skin microbiome is quite nuanced because they frequently dominate diseased skin with varying levels of disease severity (Figure 2).
Despite being an integral part of the resident skin microbiota, Malassezia species have a clear connection to skin disease. Dandruff, a frequent scalp issue, and seborrheic dermatitis are most commonly associated with M. globose, M. furfur, and M. restricta (Han et al., 2019; Johansson et al., 2018). In this case, Malassezia spp. metabolize sebum to different fatty acids, which then act as irritants, causing flaking and irritation (DeAngelis et al., 2005). Malassezia spp. have also been tied to atopic dermatitis, and recent studies have shed light on how orchestration of anti-fungal immunity may go awry to promote IL-17/IL-23-dependent inflammation under conditions of barrier impairment (Sparber et al., 2019). Furthermore, unchecked colonization by M. globosa and M. restricta can result in pityriasis, skin that is considered to be infected and characterized by rash and inflammatory signals, such as skin redness and thickening (Pradhan et al., 2018). Finally, M. furfur is an emerging culprit in invasive fungal infections in infants (Chen et al., 2020).
Indeed, Malassezia spp. can be the causative agents in disease and are often framed as such, but there is abundant evidence of positive impact on the skin microbiome and the host. Many Malassezia spp. secrete extracellular vesicles that signal keratinocytes to secrete pro-inflammatory cytokines (Vallhov et al., 2020; Watanabe et al., 2001; Zhang et al., 2019). Like other skin resident microbes, Malassezia spp. are able to inhibit and compete with the pathogen S. aureus. The dominant secreted protease of M. globosa (MgSAP1) not only cleaves the S. aureus protein A, a major virulence factor, but also is able to dissociate biofilms (Li et al., 2018). These studies suggest that despite the risk, there is potential for Malassezia in promoting skin health.
The Microbiome and Skin Disease
Changes in the skin microbiota are often investigated in cutaneous disease and disorders. It is possible that these searches will identify a novel and/or previously unsuspected species in association with disease. More often, they reveal “dysbiosis” or an imbalance of microbial inhabitants on the skin. This terminology is imprecise and inaccurate for multiple reasons, as fully elaborated by Oleson et al. (Olesen and Alm, 2016); namely, dysbiosis suggests that (1) a “balanced” skin microbiome has been defined; (2) a “balanced” skin microbiome is equivalent to skin health; and (3) an “imbalance” is causal of disease. Demonstrating causality of dysbiotic states presents challenges as appropriate model systems may not be readily available and/or there is difficulty isolating microbes in culture. Furthermore, the relevance of “Who is there?” is less relevant than “What are they doing?,” as highlighted by our discussion thus far of the context-dependent risk versus reward of microbial colonization. Thus, while associations are abundant in the literature, the relevance of such changes is often ambiguous.
A species that repeatedly appears and dominates the microbiota in association with skin disorders is S. aureus, often found where there is a breach in the skin barrier, such as atopic dermatitis or wounds. While the current discussion is focused on resident skin microbiota, this pathogen deserves mention with regard to skin disease as it is also the leading cause of skin and soft tissue and surgical site infection and a major antimicrobial resistance threat (Tong et al., 2015). Yet, S. aureus is sometimes characterized as having a commensal relationship in the skin microbiome (Krismer et al., 2017). Indeed, S. aureus can asymptomatically co-exist as part of the human microbiota and in the nares, about 30% of healthy individuals are asymptomatically colonized; however, colonization is a significant risk factor for subsequent infection (von Eiff et al., 2001). In an example of how skin commensals cooperate with pathogens, Micrococcus luteus enhanced S. aureus pathogenesis in a murine sepsis model while M. luteus itself was cleared (Boldock et al., 2018). Thus, while the risks are evident, it is less clear what “rewards” S. aureus has to offer in the human skin microbiota.
At the genomic level, specialization and diversification of S. aureus is also a significant factor that can drive skin disease and clinical variation and outcomes. In pediatric atopic dermatitis flares, shotgun metagenomic profiling approaches have allowed strain-level analysis of S. aureus and S. epidermidis populations (Byrd et al., 2017). In this cohort, S. aureus strains were clonal within each patient and highly personal, suggesting strain variation in driving different disease outcomes. In a murine model of cutaneous colonization, S. aureus isolates from more severe AD flares elicited increased epidermal thickening and promoted T cell infiltration in the skin, compared with strains from less severe disease and/or healthy individuals (Byrd et al., 2017). Similarly, in chronic wounds, shotgun metagenomics in a prospective cohort study of diabetic foot ulcers revealed that S. aureus relative abundance was significantly increased in non-healing wounds, and that some S. aureus strains were exclusively linked with non-healing outcomes (Kalan et al., 2019). Unlike atopic dermatitis, “generalist” strains of S. aureus were also present across both healing and non-healing outcomes. These findings suggested that S. aureus strain-level variation was associated with poor healing outcomes. Using a murine model of diabetic wound healing coupled with matched patient wound isolates, these associations were shown to be biologically relevant and suggested mechanisms whereby the same species can cause differential disease outcomes (Kalan et al., 2019).
One of the thrilling aspects of the skin microbiome is the abundance of microbes still to be identified. Excitingly, now with insights from DNA-based microbiome analysis, targeted culturing has yielded the successful isolation and classification of new species within genera—Roseomonas is a prime example (Rihs et al., 1993). Guided by genomic analyses of the skin microbiota, targeted culturing approaches uncovered Roseomonas mucosa as inhibitory to S. aureus. Roseomonas are pink Gram-negative bacteria found on the skin, the teeth, and in aquatic environments; dominant strains identified on human skin include: R. gilardii, R. cervicalis, and R. mucosa (Han et al., 2003). Similar to other skin microbiota discussed in this review, they have the potential to be pathogenic and have previously been identified as the causative agent in bacteremia and wound infections (Figure 2) (Romano-Bertrand et al., 2016; Shao et al., 2019). Yet the reward with regard to atopic dermatitis is promising. In a murine model of topical colonization, R. mucosa controlled growth of S. aureus and in human skin promoted production of IL-6 (Myles et al., 2016). Furthermore, transplanting R. mucosa on the skin of human atopic dermatitis patients resulted in reduced S. aureus burden and significant reduction in clinical disease (Myles et al., 2018). These studies demonstrate the potential for discovery in the skin microbiome, while highlighting how microbiota-directed therapy could ameliorate dermatological disease.
Conclusions
The skin microbiome is home to thousands of microbial species that create a rich ecosystem providing many benefits to the host as we have highlighted here. These benefits are derived from their interactions with different components of the skin ecosystem. Furthermore, strain-specific attributes are critical in the outcome of host-microbial interactions. Such interactions have the potential to provide novel, translatable targets for the prevention and treatment of skin disease and disorders. Thus, one must consider the potential risks along with the rewards, including how changing one component of a highly dynamic, intertwined consortium will affect other interactions.
Here, we have highlighted ways in which the most abundant and stable (“resident”) skin microbial species may be detrimental to the host. Thus, we ponder the question, as others have posed, is it fair and accurate to refer to these microbes as “commensals”? Given the opportunity or if forced into a survival state, any commensal may become pathogenic. However, this “switch” in lifestyle can be true of many organisms in eco-systems beyond the microbiome. Therefore, we surmise that classification of a given microbial species as a commensal is dependent on its interaction state with the host. Furthermore, both strain and host specificity are highly relevant as has been demonstrated by CoNS and S. aureus studies.
Importantly, the wealth of data generated from DNA-based techniques combined with culturing techniques has set the groundwork for asking the persistent but fundamental questions, “What microbial species compose a healthy skin microbiome? How is a healthy skin microbiome maintained”? In order to answer these questions, future studies examining understudied species and their molecular and biochemical mechanisms of interacting in their ecosystem are needed. At the same time, it is critical to consider that these mechanisms exist against a backdrop of the entire ecosystem and thus are one of many, many “conversations” that are taking place at the same time. A challenge to the field will be to demonstrate that these mechanisms have biological and clinical relevance, when considered outside of model systems and in the complex ecosystem of the human skin microbiome.
ACKNOWLEDGMENTS
We thank the members of the Grice lab for their underlying contributions and the funding sources that make our work possible. Support to E.A.G. includes grants from the National Institutes of Health (R01NR015639, R01AI143790, and R01AR066663), the Linda Pechenik Montague Investigator Award, and awards from the Burroughs Wellcome Fund (PATH Award) and the Dermatology Foundation (Sun Pharma Research Award). L.F. was supported by a NIH/NIAMS training grant (T32AR007465).
All figures were created with BioRender.com.
REFERENCES
- Barnard E, Johnson T, Ngo T, Arora U, Leuterio G, McDowell A, and Li H. (2020). Porphyrin production and regulation in cutaneous propionibacteria. mSphere 5, e00793–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawdon D, Cox DS, Ashford D, James AG, and Thomas GH (2015). Identification of axillary Staphylococcus sp. involved in the production of the malodorous thioalcohol 3-methyl-3-sufanylhexan-1-ol. FEMS Microbiol. Lett. 362, fnv111. [DOI] [PubMed] [Google Scholar]
- Boldock E, Surewaard BGJ, Shamarina D, Na M, Fei Y, Ali A, Williams A, Pollitt EJG, Szkuta P, Morris P, et al. (2018). Human skin commensals augment Staphylococcus aureus pathogenesis. Nat. Microbiol. 3, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomar L, Brugger SD, Yost BH, Davies SS, and Lemon KP (2016). Corynebacterium accolens Releases antipneumococcal free fatty acids from human nostril and skin surface triacylglycerols. mBio 7, e01725–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SK, and Shalita AR (1998). Acne vulgaris. Lancet 351, 1871–1876. [DOI] [PubMed] [Google Scholar]
- Burkhart CG, Burkhart CN, and Lehmann PF (1999). Acne: a review of immunologic and microbiologic factors. Postgrad. Med. J. 75, 328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkovski A. (2018). The role of corynomycolic acids in Corynebacterium-host interaction. Antonie Leeuwenhoek 111, 717–725. [DOI] [PubMed] [Google Scholar]
- Byrd AL, Deming C, Cassidy SKB, Harrison OJ, Ng WI, Conlan S, NISC Comparative Sequencing Program, Belkaid Y, Segre JA, and Kong HH (2017). Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 9, eaal4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IT, Chen CC, Huang HC, and Kuo KC (2020). Malassezia furfur Emergence and candidemia trends in a neonatal intensive care unit during 10 years: the experience of fluconazole prophylaxis in a single hospital. Adv. Neonat. Care 20, E3–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR, Whitney AR, Teintze M, DeLeo FR, and Otto M. (2010). Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog. 6, e1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, Torpey JW, Otto M, Nizet V, Kim JE, and Gallo RL (2010). Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J. Invest. Dermatol. 130, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MD, Goodfellow M, and Minnikin DE (1982). A survey of the structures of mycolic acids in Corynebacterium and related taxa. J. Gen. Microbiol. 128, 129–149. [DOI] [PubMed] [Google Scholar]
- DeAngelis YM, Gemmer CM, Kaczvinsky JR, Kenneally DC, Schwartz JR, and Dawson TL Jr. (2005). Three etiologic facets of dandruff and seborrheic dermatitis: Malassezia fungi, sebaceous lipids, and individual sensitivity. J. Investig. Dermatol. Symp. Proc. 10, 295–297. [DOI] [PubMed] [Google Scholar]
- Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M, NIH Intramural Sequencing Center Comparative Sequencing Program, et al. (2013). Topographic diversity of fungal and bacterial communities in human skin. Nature 498, 367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, Elashoff D, Erfe MC, Loncaric A, Kim J, et al. (2013). Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Invest. Dermatol. 133, 2152–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes BA, Sahm DF, and Weissfeld AS (1998). Bailey & Scott’s Diagnostic Microbiology (Elsevier). [Google Scholar]
- Fredricks DN (2001). Microbial ecology of human skin in health and disease. J. Investig. Dermatol. Symp. Proc. 6, 167–169. [DOI] [PubMed] [Google Scholar]
- Ginsburg I. (2002). Role of lipoteichoic acid in infection and inflammation. Lancet Infect. Dis. 2, 171–179. [DOI] [PubMed] [Google Scholar]
- Grice EA, and Dawson TL Jr. (2017). Host-microbe interactions: malassezia and human skin. Curr. Opin. Microbiol. 40, 81–87. [DOI] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, NISC Comparative Sequencing Program, Bouffard GG, Blakesley RW, Murray PR, et al. (2009). Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan MD, Bartow-McKenney C, Flowers L, Knight SAB, Uberoi A, and Grice EA (2019). Research techniques made simple: profiling the skin microbiota. J. Invest. Dermatol. 139, 747–752.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han XY, Pham AS, Tarrand JJ, Rolston KV, Helsel LO, and Levett PN (2003). Bacteriologic characterization of 36 strains of Roseomonas species and proposal of Roseomonas mucosa sp nov and Roseomonas gilardii subsp rosea subsp nov. Am. J. Clin. Pathol. 120, 256–264. [DOI] [PubMed] [Google Scholar]
- Han Y, Zhang YJ, Wang HX, Sun YZ, Yang Y, Li ZX, Qi RQ, and Gao XH (2019). Malassezia furfur promoting growth of Staphylococcus epidermidis by increasing pH when cultured in a lipid-free environment. Chin. Med. J. 132, 873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan GD, Meisel JS, Tyldsley AS, Zheng Q, Hodkinson BP, San-Miguel AJ, Minot S, Bushman FD, and Grice EA (2015). The human skin double-stranded DNA virome: topographical and temporal diversity, genetic enrichment, and dynamic associations with the host microbiome. mBio 6, e01578–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy BL, Dickey SW, Plaut RD, Riggins DP, Stibitz S, Otto M, and Merrell DS (2019). Corynebacterium pseudodiphtheriticum exploits Staphylococcus aureus virulence components in a novel polymicrobial defense strategy. mBio 10, e02491–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, and Mizunoe Y. (2010). Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465, 346–349. [DOI] [PubMed] [Google Scholar]
- Johansson HJ, Vallhov H, Holm T, Gehrmann U, Andersson A, Johansson C, Blom H, Carroni M, Lehtiö J, and Scheynius A. (2018). Extracellular nanovesicles released from the commensal yeast Malassezia sympodialis are enriched in allergens and interact with cells in human skin. Sci. Rep. 8, 9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T, Kang D, Barnard E, and Li H. (2016). Strain-level differences in porphyrin production and regulation in Propionibacterium acnes elucidate disease associations. mSphere 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalan LR, Meisel JS, Loesche MA, Horwinski J, Soaita I, Chen X, Uberoi A, Gardner SE, and Grice EA (2019). Strain- and species-level variation in the microbiome of diabetic wounds is associated with clinical outcomes and therapeutic efficacy. Cell Host Microbe 25, 641–655.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Shiota R, Shibuya S, Watanabe S, Umeda Y, Takeshita K, Yamamoto M, Nishioka K, and Makimura K. (2010). Human external ear canal as the specific reservoir of malassezia slooffiae. Med. Mycol. 48, 824–827. [DOI] [PubMed] [Google Scholar]
- Kang D, Shi B, Erfe MC, Craft N, and Li H. (2015). Vitamin B12 modulates the transcriptome of the skin microbiota in acne pathogenesis. Sci. Transl. Med. 7, 293ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman AM, and Strauss JS (1956). The bacteria responsible for apocrine odor. J. Invest. Dermatol. 27, 67–71. [DOI] [PubMed] [Google Scholar]
- Kluytmans JA, and Wertheim HF (2005). Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 33, 3–8. [DOI] [PubMed] [Google Scholar]
- Krismer B, Weidenmaier C, Zipperer A, and Peschel A. (2017). The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat. Rev. Microbiol. 15, 675–687. [DOI] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu ZR, Hooper LV, Schmidt RR, von Aulock S, et al. (2009). Commensal bacteria regulate toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 15, 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le KY, Villaruz AE, Zheng Y, He L, Fisher EL, Nguyen TH, Ho TV, Yeh AJ, Joo HS, Cheung GYC, and Otto M. (2019). Role of phenol-soluble modulins in Staphylococcus epidermidis biofilm formation and infection of indwelling medical devices. J. Mol. Biol. 431, 3015–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Goh BN, Teh WK, Jiang Z, Goh JPZ, Goh A, Wu G, Hoon SS, Raida M, Camattari A, et al. (2018). Skin commensal Malassezia globosa secreted protease attenuates Staphylococcus aureus biofilm formation. J. Invest. Dermatol. 138, 1137–1145. [DOI] [PubMed] [Google Scholar]
- Lo CW, Lai YK, Liu YT, Gallo RL, and Huang CM (2011). Staphylococcus aureus hijacks a skin commensal to intensify its virulence: immunization targeting beta-hemolysin and CAMP factor. J. Invest. Dermatol. 131, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marples MJ (1965). The Ecology of the Human Skin (Charles C Thomas, Bannerhouse). [Google Scholar]
- Martínez-García S, Rodríguez-Martínez S, Cancino-Diaz ME, and Cancino-Diaz JC (2018). Extracellular proteases of Staphylococcus epidermidis: roles as virulence factors and their participation in biofilm. APMIS 126, 177–185. [DOI] [PubMed] [Google Scholar]
- Moon JL, Banbula A, Oleksy A, Mayo JA, and Travis J. (2001). Isolation and characterization of a highly specific serine endopeptidase from an oral strain of Staphylococcus epidermidis. Biol. Chem. 382, 1095–1099. [DOI] [PubMed] [Google Scholar]
- Myles IA, Earland NJ, Anderson ED, Moore IN, Kieh MD, Williams KW, Saleem A, Fontecilla NM, Welch PA, Darnell DA, et al. (2018). First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 3, e120608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles IA, Williams KW, Reckhow JD, Jammeh ML, Pincus NB, Sastalla I, Saleem D, Stone KD, and Datta SK (2016). Transplantation of human skin microbiota in models of atopic dermatitis. JCI Insight 1, e86955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. (2015). Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. (2012). Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, et al. (2017). Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 9, eaah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Deming C, Conlan S, NISC Comparative Sequencing Program, Kong HH, and Segre JA (2014). Biogeography and individuality shape function in the human skin metagenome. Nature 514, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Park M, NISC Comparative Sequencing Program, Kong HH, and Segre JA (2016). Temporal stability of the human skin microbiome. Cell 165, 854–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Conlan S, Polley EC, Segre JA, and Kong HH (2012). Shifts in human skin and nares microbiota of healthy children and adults. Genome Med. 4, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen SW, and Alm EJ (2016). Dysbiosis is not an answer. Nat. Microbiol. 1, 16228. [DOI] [PubMed] [Google Scholar]
- O’Neill AM, Nakatsuji T, Hayachi A, Williams MR, Mills RH, Gonzalez DJ, and Gallo RL (2020). Identification of a human skin commensal bacterium that selectively kills Cutibacterium acnes. J. Invest. Dermatol. 10.1016/j.jid.2019.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan JN, Rea MC, O’Connor PM, Hill C, and Ross RP (2019). Human skin microbiota is a rich source of bacteriocin-producing staphylococci that kill human pathogens. FEMS Microbiol. Ecol. 95, fiy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. (2013). Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection: staphylococcal commensal species such as Staphylococcus epidermidis are being recognized as important sources of genes promoting MRSA colonization and virulence. BioEssays 35, 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paharik AE, Parlet CP, Chung N, Todd DA, Rodriguez EI, Van Dyke MJ, Cech NB, and Horswill AR (2017). Coagulase-negative staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe 22, 746–756.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana DV, Peretti A, Welch NL, Borgogna C, Olivero C, Badolato R, Notarangelo LD, Gariglio M, FitzGerald PC, McIntosh CE, et al. (2018). Metagenomic discovery of 83 new human papillomavirus types in patients with immunodeficiency. mSphere 3, e00645–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Iacovella J, and Cornell RS (2016). Corynebacterium striatum: a concerning pathogen of osteomyelitis in the diabetic patient. J. Am. Podiatr. Med. Assoc. 106, 9. [Google Scholar]
- Pradhan S, Ran X, Zhang C, Li X, and Ran Y. (2018). Cover image: naevus sebaceus affected by overgrowth of Malassezia globosa. Br. J. Dermatol. 179, 1432–1433. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Freire MO, Gabrilska RA, Rumbaugh KP, and Lemon KP (2016). Staphylococcus aureus shifts toward commensalism in response to Corynebacterium species. Front. Microbiol. 7, 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura VK, Bouladoux N, Claesen J, Chen YE, Byrd AL, Constantinides MG, Merrill ED, Tamoutounour S, Fischbach MA, and Belkaid Y. (2018). Contextual control of skin immunity and inflammation by Corynebacterium. J. Exp. Med. 215, 785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs JD, Brenner DJ, Weaver RE, Steigerwalt AG, Hollis DG, and Yu VL (1993). Roseomonas, a new genus associated with bacteremia and other human infections. J. Clin. Microbiol. 31, 3275–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano-Bertrand S, Bourdier A, Aujoulat F, Michon AL, Masnou A, Parer S, Marchandin H, and Jumas-Bilak E. (2016). Skin microbiota is the main reservoir of Roseomonas mucosa, an emerging opportunistic pathogen so far assumed to be environmental. Clin. Microbiol. Infect. 22, 737.e1–7. [DOI] [PubMed] [Google Scholar]
- Roth RR, and James WD (1988). Microbial ecology of the skin. Annu. Rev. Microbiol. 42, 441–464. [DOI] [PubMed] [Google Scholar]
- Scharschmidt TC, Vasquez KS, Pauli ML, Leitner EG, Chu K, Truong HA, Lowe MM, Sanchez Rodriguez R, Ali N, Laszik ZG, et al. (2017). Commensal microbes and hair follicle morphogenesis coordinately drive Treg migration into neonatal skin. Cell Host Microbe 21, 467–477.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt TC, Vasquez KS, Truong HA, Gearty SV, Pauli ML, Nosbaum A, Gratz IK, Otto M, Moon JJ, Liese J, et al. (2015). A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 43, 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Guo X, Guo P, Cui Y, and Chen Y. (2019). Roseomonas mucosa infective endocarditis in patient with systemic lupus erythematosus: case report and review of literature. BMC Infect. Dis. 19, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparber F, De Gregorio C, Steckholzer S, Ferreira FM, Dolowschiak T, Ruchti F, Kirchner FR, Mertens S, Prinz I, Joller N, et al. (2019). The skin commensal yeast Malassezia triggers a type 17 response that coordinates anti-fungal immunity and exacerbates skin inflammation. Cell Host Microbe 25, 389–403.e6. [DOI] [PubMed] [Google Scholar]
- Tauch A, and Burkovski A. (2015). Molecular armory or niche factors: virulence determinants of Corynebacterium species. FEMS Microbiol. Lett. 362, fnv185. [DOI] [PubMed] [Google Scholar]
- Tirosh O, Conlan S, Deming C, Lee-Lin SQ, Huang X, NISC Comparative Sequencing Program, Su HC, Freeman AF, Segre JA, and Kong HH (2018). Expanded skin virome in DOCK8-deficient patients. Nat. Med. 24, 1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong SY, Davis JS, Eichenberger E, Holland TL, and Fowler VG Jr. (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallhov H, Johansson C, Veerman RE, and Scheynius A. (2020). Extracellular vesicles released From the skin commensal yeast Malassezia sympodialis activate human primary keratinocytes. Front. Cell. Infect. Microbiol. 10, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eiff C, Becker K, Machka K, Stammer H, and Peters G. (2001). Nasal carriage as a source of Staphylococcus aureus bacteremia. Study group. N. Engl. J. Med. 344, 11–16. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhou H, Chen D, Du P, Lan R, Qiu X, Hou X, Liu Z, Sun L, Xu S, et al. (2019). Whole-genome sequencing reveals a prolonged and persistent intrahospital transmission of Corynebacterium striatum, an emerging multidrug-resistant pathogen. J. Clin. Microbiol. 57, e00683–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Kano R, Sato H, Nakamura Y, and Hasegawa A. (2001). The effects of Malassezia yeasts on cytokine production by human keratinocytes. J. Invest. Dermatol. 116, 769–773. [DOI] [PubMed] [Google Scholar]
- Williams MR, Cau L, Wang Y, Kaul D, Sanford JA, Zaramela LS, Khalil S, Butcher AM, Zengler K, Horswill AR, et al. (2020). Interplay of staphylococcal and host proteases promotes skin barrier disruption in netherton syndrome. Cell Rep. 30, 2923–2933.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Costa SK, Zaramela LS, Khalil S, Todd DA, Winter HL, Sanford JA, O’Neill AM, Liggins MC, Nakatsuji T, et al. (2019). Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci. Transl. Med. 11, eaat8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg MS, Claesen J, Escapa IF, Aldridge KL, Fischbach MA, and Lemon KP (2014). Propionibacterium-produced coproporphyrin III induces Staphylococcus aureus aggregation and biofilm formation. mBio 5, e01286–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JS 3rd, Jin R, and Novick RP (2005). Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc. Natl. Acad. Sci. USA 102, 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Li Z, Liu K, Wu Y, Jiang D, and Lai Y. (2016). Staphylococcal LTA-induced miR-143 inhibits Propionibacterium acnes-mediated inflammatory response in skin. J. Invest. Dermatol. 136, 621–630. [DOI] [PubMed] [Google Scholar]
- Yan M, Pamp SJ, Fukuyama J, Hwang PH, Cho DY, Holmes S, and Relman DA (2013). Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe 14, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn SH, Choi CW, Choi JW, and Youn SW (2013). The skin surface pH and its different influence on the development of acne lesion according to gender and age. Skin Res. Technol. 19, 131–136. [DOI] [PubMed] [Google Scholar]
- Yu Y, Champer J, Agak GW, Kao S, Modlin RL, and Kim J. (2016). Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J. Invest. Dermatol. 136, 2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Han Y, Sun YZ, Jiang HH, Liu M, Qi RQ, and Gao XH (2019). Extracellular vesicles derived from Malassezia furfur stimulate IL-6 production in keratinocytes as demonstrated in in vitro and in vivo models. J. Dermatol. Sci. 93, 168–175. [DOI] [PubMed] [Google Scholar]
- Zheng Y, He L, Asiamah TK, and Otto M. (2018). Colonization of medical devices by staphylococci. Environ. Microbiol. 20, 3141–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, Burian M, Schilling NA, Slavetinsky C, Marschal M, et al. (2016). Human commensals producing a novel antibiotic impair pathogen colonization. Nature 535, 511–516. [DOI] [PubMed] [Google Scholar]