Abstract
Cannabis use initiated during adolescence might precipitate negative consequences in adulthood. Thus, predicting adolescent cannabis use prior to any exposure will inform the aetiology of substance abuse by disentangling predictors from consequences of use. In this prediction study, data were drawn from the IMAGEN sample, a longitudinal study of adolescence. All selected participants (n = 1,581) were cannabis-naïve at age 14. Those reporting any cannabis use (out of six ordinal use levels) by age 16 were included in the outcome group (N = 365, males n = 207). Cannabis-naïve participants at age 14 and 16 were included in the comparison group (N = 1,216, males n = 538). Psychosocial, brain and genetic features were measured at age 14 prior to any exposure. Cross-validated regularized logistic regressions for each use level by sex were used to perform feature selection and obtain prediction error statistics on independent observations. Predictors were probed for sex- and drug-specificity using post-hoc logistic regressions. Models reliably predicted use as indicated by satisfactory prediction error statistics, and contained psychosocial features common to both sexes. However, males and females exhibited distinct brain predictors that failed to predict use in the opposite sex or predict binge drinking in independent samples of same-sex participants. Collapsed across sex, genetic variation on catecholamine and opioid receptors marginally predicted use. Using machine learning techniques applied to a large multimodal dataset, we identified a risk profile containing psychosocial and sex-specific brain prognostic markers, which were likely to precede and influence cannabis initiation.
Keywords: marijuana, neuroimaging, prediction, specificity
Introduction
Cannabis use in adolescence is associated with a range of adversity in adulthood including cannabis dependence (DSM-IV; Hall & Degenhardt, 2009; Moss, Chen, & Yi, 2014), poly-drug use (Secades-Villa, Garcia-Rodríguez, Jin, Wang, & Blanco, 2015), cognitive deficits (Meier et al., 2012; Schuster, Hoeppner, Evins, & Gilman, 2016), compromised physical (Kalant, 2004) and mental health (Degenhardt et al., 2013; Kedzior & Laeber, 2014; Malone, Hill, & Rubino, 2010), and diminished life attainment goals (e.g., socioeconomic factors; Fergusson & Boden, 2008). These findings are supported by animal models linking adolescent cannabis exposure with detrimental outcomes in adulthood (O’Shea, 2004; Quinn et al., 2008). However, in humans, it is difficult to assert a causal role for cannabis in subsequent outcomes as any negative outcomes arising from use could be related to a number of factors confounded with the choice to initiate use (Jackson et al., 2016).
Results from the 2013 National Survey on Drug Use and Health indicated that nearly 25% of 10th graders reported ever trying cannabis (NSDUH, 2014). From 2005 to 2010 rates of cannabis-related emergency room visits increased 54% in males and 42% in females aged 15–17 years (NSDUH, 2014). Moreover, beliefs concerning the risk of use are declining (Johnston, O’Malley, Bachman, & Schulenberg, 2011) despite the increase in drug potency relative to previous decades (ElSohly et al., 2016). These trends are a source of concern as in vitro models indicate that delta-9-tetrahydrocannabinol (THC), a psychoactive compound in cannabis, could be more toxic in adolescent than in adult tissue (Pope et al., 2003; Quinn et al., 2008; Renard et al., 2016; Rubino et al., 2015; Schneider, 2008), and human studies suggest early, compared to adult, initiation of cannabis is associated with worse outcomes (Brook, Lee, Brown, Finch, & Brook, 2011; Coffey & Patton, 2016).
Global studies suggest cannabis use is typically initiated prior to age 18 (Degenhardt et al., 2008). Thus, adolescence might be a developmental period during which initiation can be best predicted. Investigations of the risk factors associated with cannabis initiation commonly report features like temperament (Creemers et al., 2010), delinquent behaviours (van den Bree & Pickworth, 2005), alcohol and tobacco use (von Sydow, Lieb, Pfister, Höfler, & Wittchen, 2002) and parental (Day, Goldschmidt, & Thomas, 2006) and peer influences (Ellickson, Tucker, Klein, & Saner, 2004), while rarely considering any neurobiological or genetic contributions. Incorporating these domains may uncover biobehavioural processes that are specific to the initiation of cannabis use. Therefore, we sought to uncover a comprehensive risk profile of adolescent cannabis use by predicting the initiation of use via a large multimodal biobheavioural dataset.
Prior studies have stressed the importance of attending to sex differences in substance abuse research. Indeed, males and females differ in their biological response to cannabis, such that females produce more psychoactive THC metabolites (Narimatsu, Watanabe, Yamamoto, & Yoshimura, 1991) and exhibit elevated gene expression levels of both CB1 and CB2 cannabinoid receptors (Onaivi et al., 1999) relative to males. Behaviourally, female cannabis users endorse more positive subjective ratings associated with abuse liability to smoked cannabis (vs. placebo; (Cooper & Haney, 2014). Moreover, converging evidence using animal (Fattore et al., 2007) and human studies (Hernandez-Avila, Rounsaville, & Kranzler, 2004; Schepis et al., 2011) indicates the transition from cannabis use initiation to regular use is accelerated in females. Hence, the identification of a predictive profile may identify sex-specific aetiological mechanisms while also informing sex-specific interventions to attenuate the risk of ever becoming a user.
While prediction analyses can illuminate the nature of drug initiation, these studies are rare as they necessitate large, longitudinal samples, especially when feature-rich domains are considered (Whelan & Garavan, 2014). Large samples are also needed for cross-validation schemes to ensure predictive models are tested on independent samples. Hence, we modelled our analytic approach on a related study using the IMAGEN dataset in which Whelan et al. (2014) developed predictive models which identified multidomain features at age 14 that predicted binge drinking at age 16. Given this work, we hypothesized cannabis use could be predicted in a similar fashion using multidomain data from the IMAGEN sample. We extend the methods of Whelan et al. by identifying multidomain risk profiles for each sex while considering a range of subsequent cannabis use levels. In doing so, we identify predictive features that are both common and unique between the sexes, and between future cannabis use and binge drinking. While we anticipate replicating many psychosocial predictors and uncovering a sparse set of brain and genetic predictors, these exploratory analyses are data driven. In an era where large multisite neuroimaging projects and big datasets are becoming more prevalent, we leverage machine learning techniques to uncover a sparse set of predictors of cannabis use from a large multidomain set of variables that generalize to predict use in independent samples.
Methods and materials
Full details of the multisite IMAGEN study (Schumann et al., 2010) are available in the online Standard Operating Procedures (https://imagen-europe.com/). Imaging acquisition parameters and quality assurance procedures were standardized across site to ensure comparable data (see Schumann et al., 2010 for standardization of procedures across sites). The IMAGEN study conformed to the ethical standards outlined by Declaration of Helsinki and was approved by ethics committees at each site including King’s College, London; Central Institute of Mental Health, Mannheim; Charite, Universitatsmedizin Berlin; University Medical Center Hamburg-Eppendorf; University of Nottingham; Trinity College Dublin; Institut National de la Sante et de la Recherche Medicale, Orsay. After description of the IMAGEN study to the participants and their parents, written informed consent was obtained. Individuals who provided assent were studied at age 14 and 16.
Participants
Inclusion was determined by a self-report drug use questionnaire (using the “ESPAD”, described below). Participants from the baseline sample (age 14) who provided ESPAD data and were cannabis-naïve were eligible for inclusion (n = 2,018). At age 16, n = 1,581 participants (78% of the cannabis-naïve sample) provided usable data (see Supporting Information Table S1 for evaluation of participants unavailable for follow-up) and were thus included in the analysis. Participants reporting any level of cannabis use by age 16 were assigned to the outcome groups (n = 365). Participants who remained cannabis-naïve at age 14 and 16 were assigned to the comparison group (n = 1,216).
The European School Survey Project on Alcohol and Drugs (ESPAD; Hibell et al., 1997) was administered at age 14 and 16 using Psytools (London, UK). Lifetime usage was measured on an ordinal scale: 0, 1 = 1–2x, 2 = 3–5x, 3 = 6–9x, 4 = 10–19x, 5 = 20–39x, 6 = 40x+. See Table 1 for sample demographics and drug use levels.
Table 1.
Participant demographics
| Groups | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||||||||||
| Measure | Cannabis use by age 16 (n = 207) | Comparison group (n = 538) | p | Cannabis use by age 16 (n = 158) | Comparison group (n = 678) | p | ||||||||||
| Age (M, SD) | 14.50, 0.47 | 14.52, 0.39 | 0.54 | 14.51, 0.53 | 14.54, 0.42 | 0.33 | ||||||||||
| Handedness (L, R) | 25, 182 | 66, 472 | 0.94 | 18, 140 | 60, 618 | 0.32 | ||||||||||
| PDS (M, SD) | 2.65, 0.49 | 2.54, 0.55 | 0.01 | 3.22, 0.39 | 3.17, 0.44 | 0.13 | ||||||||||
| Perceptual IQ (M, SD) | 108.11, 13.55 | 108.18, 14.56 | 0.95 | 109.40, 13.49 | 107.77, 13.23 | 0.17 | ||||||||||
| Verbal IQ (M, SD) | 114.19, 13.267 | 112.07, 13.14 | 0.05 | 112.93, 12.29 | 109.22, 13.80 | 0.002 | ||||||||||
| SES (M, SD) | 18.52, 3.97 | 17.88, 3.82 | 0.05 | 18.26, 3.94 | 17.88, 3.68 | 0.26 | ||||||||||
| Cannabis use levels | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | ||||
| (N) | 62 | 35 | 26 | 24 | 23 | 37 | 56 | 39 | 19 | 20 | 9 | 15 | ||||
Note. PDS: Puberty Development Scale (Carskadon & Acebo, 1993); SES: Socioeconomic status.
Cannabis use levels from the ESPAD and measured on an ordinal scale (1 = 1–2x, 2 = 3–5x, 3 = 6–9x, 4 = 10–19x, 5 = 20–39x, 6 = 40x+). All measures (with the exception of cannabis use) were obtained at age 14. All demographics measures were also included as predictors in feature selection analyses.
Data
Participants were extensively characterized at age 14 using psychosocial (of parent and child), neuroimaging, and genetic assessments (see Supporting Information Data S1). Psychosocial data were largely self-reported and included demographics, summary scores for personality dimensions (Cloninger, 1999; Costa & McCrae, 1995; Woicik, Stewart, Pihl, & Conrod, 2009), frequency of candidate life events (Newcomb, Huba, & Bentler, 1981), cognitive (Robbins et al., 1994) and intelligence (Wechsler, 2003) assessments and drug use levels of the parent and child (additional features described in Supporting Information Data S1). Genetic data included 108 candidate single nucleotide polymorphisms (SNPs) on genes coding for neurotransmitter receptors (cannabinoid, opioid and catecholamines), related enzymes (FAAH), eight SNPs previously associated with cannabis dependence (Hartman et al., 2009; Hopfer et al., 2006; Hurd, Michaelides, Miller, & Jutras-Aswad, 2014) and one genetic risk-score based on the summation of those eight risk alleles (Cornelis, 2009). Brain data included three fMRI tasks designed to engage cognitive processes associated with substance abuse (reward processing, motor response inhibition, and social affective (face) processing; see Supporting Information Data S1 for task specifics) and one structural MRI scan. Whole-brain fMRI contrast maps (generated using a standard GLM) and grey matter volume maps (GMV; generated using voxel-based morphometry) were each parcellated into 278 regions of interest (ROIs) (Shen, Tokoglu, Papademetris, & Constable, 2013). All data (except the cannabis use outcome) were collected at age 14 and used to predict cannabis use by age 16, and all predictors (n variables = 2,413; see Supporting Information Table S2 for summary) from each domain were considered during predictive model estimation.
Statistical analyses
The overall analytic procedure was designed to accomplish three goals: (a) perform feature selection to identify the predictors of light to heavy use in males and females separately; the selected features then informed post hoc analyses to (b) probe the identified predictors for sex- and drug-specificity, and (c) assess the relative contribution of each data domain to the prediction of cannabis use initiation.
Feature selection
Six prediction analyses were conducted for each sex to predict each level of use via the ESPAD scale (use levels of 1 and above (Males n = 207; Females n = 158), levels 2 and above (Males n = 172; Females n = 120), and so on up to level 6). Predictive models were estimated using elastic-net regularization (Zou & Hastie, 2005) with logistic regression to perform feature selection (from n variables = 2,413) and reduce model overfit. The elastic-net minimizes both the sum of the squared and absolute values of the regression coefficients, effectively setting some coefficients to zero, thereby performing feature selection during model estimation. Elastic-net parameters (see Supporting Information Data S1) were tuned on independent samples (via nested k-fold cross-validation), and then final models were tested on an independent internal validation set. These analyses were implemented using the “glmnet” function in MATLAB (v. R2014a, Natick, MA, USA).
k(10)-fold cross-validation was used during model estimation to evaluate predictive models on independent observations. Partitioning a completely external validation set would have reduced an already small group of interest. Therefore, internal validation using k-fold cross-validation was used as a proxy for external validation. During k-fold cross-validation, the full sample of data is partitioned into subsamples of data, where k equals the number of partitions (or “folds”) of the original starting sample. k-fold cross-validation then becomes an iterative process whereby a single fold is set aside as the test sample “test fold”, and a “training model” is estimated on the observations in the remaining k-1 folds “training folds”. The training model is then used to predict the observations in the set aside test fold, thereby ensuring the independence of the test fold sample. This procedure returns k final models.
Each of the six sex-specific prediction analyses was run 100 times to account for the subtle differences in results incurred due to the random assignment of participants to folds. Results were thresholded to identify only the predictors that were present in at least six final models (from k = 10) across all 100 runs within a use level analysis. Predictors passing this threshold were selected for use in post hoc analyses. See Supporting Information Figure S1 for a schematic of the analytic method.
The area under the curve (AUC) of the receiver-operating characteristic (ROC) was calculated based on the model’s ability to predict cannabis use in the independent samples segregated during cross-validation. In a wide fashion, the ROC AUC represents the probability that a randomly selected individual from the outcome group will be predicted as a future user (Fawcett, 2006). Null-hypothesis significance testing on the AUC was conducted using a Mann-Whitney U-test (Mason & Graham, 2002) (significance set using a Bonferroni corrected p < 0.008 [p < 0.05/6 models]) to test the hypothesis that models predicted independent samples better than chance.
Features selected from each use level analysis were then used in post hoc analyses described below. Correlations between each identified feature and cannabis use were also analysed using Pearson’s point-biserial correlation to predict any level of future use in a binary fashion.
Specificity analyses
Sex-specificity was assessed by including the selected features of male cannabis use as the independent variables of a logistic regression model estimated on the female sample (and vice versa). Drug-specificity was assessed by including the selected features of male cannabis use as the independent variables of a logistic regression model estimated on an independent sample of binge drinking males (and likewise for females). The binge drinking sample contained new individuals (n = 400) who were naïve to binge drinking at age 14 (with a maximum of two lifetime drinks), but endorsed binge drinking episodes (i.e., being drunk from alcoholic beverages) by age 16 (see Supporting Information Table S3 for binge drinking sample demographics).
Domain contribution analyses
The selected features for each sex were also modelled in a hierarchical fashion to measure the relative change in model fit after the inclusion of each domain-specific set of predictors. Model fit for all post hoc regressions were determined using a chi-square goodness of fit statistic and the delta Akaike information criterion of model selection (ΔAIC; Akaike, 1974).
Results
Feature selection analyses predicting each use level returned a range of ROC AUC values (Males: AUC=0.65–0.74, p = 1.4 × 10−8-5.3 × 10−10; Females: AUC = 0.74–0.82, p = 1.8 × 10−16-5.5 × 10−13), indicating high accuracy in predicting independent samples for each use level (Figure 1). Best performance was achieved predicting ≥20 uses for males (AUC = 0.74, p = 5.3 × 10−10) and ≥10 uses for females (AUC = 0.82, p = 5.5 × 10−13). For context, in a study using only psychosocial features to predict the initiation of cannabis use, authors reported a final predictive logistic regression model returning a ROC AUC = 0.78 (von Sydow et al., 2002). In addition, Whelan et al. (2014) reported a cross-validated ROC AUC = 0.75 in their study of brain, psychosocial, and genetic predictors of binge drinking. Hence, the AUCs reported here are in line with previous research, while the AUCs from the female models reflect an even higher degree of cross-validated prediction than what has been previously reported..
Fig. 1.
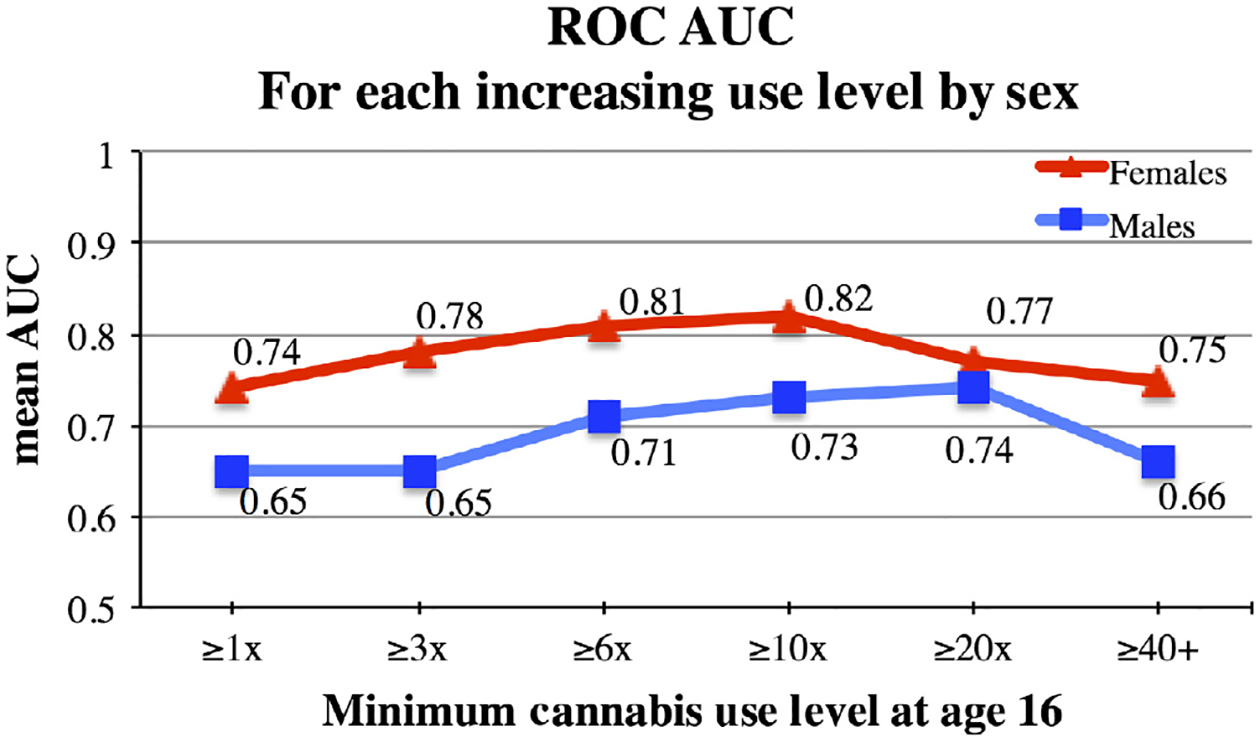
Mean Receiver-operating characteristic (ROC) AUC For Each Use Level by Sex. Mean ROC AUC indicates the performance of the predictive models on independent samples across 100 runs for each use level by sex.
Selected psychosocial predictors
Six psychosocial predictors were found to be common to both sexes, including greater lifetime alcohol and cigarette use, parental lifetime cannabis use, novelty-seeking personality and the disorderliness personality subscale (Cloninger, 1999), and less-negative feelings towards deviant behaviours (Newcomb et al., 1981). Post hoc regressions indicated these predictors returned strong model fit for the full sample (males and females) for all levels of cannabis use (, p = 4.7 × 10−37; ΔAIC = 175.02) and also predicted binge drinking (, p = 1.8 × 10−5; ΔAIC = 19.58) in an independent sample. See Figure 3 for a summary of all identified predictors and their point-biserial correlation with use initiation.
Fig. 3.
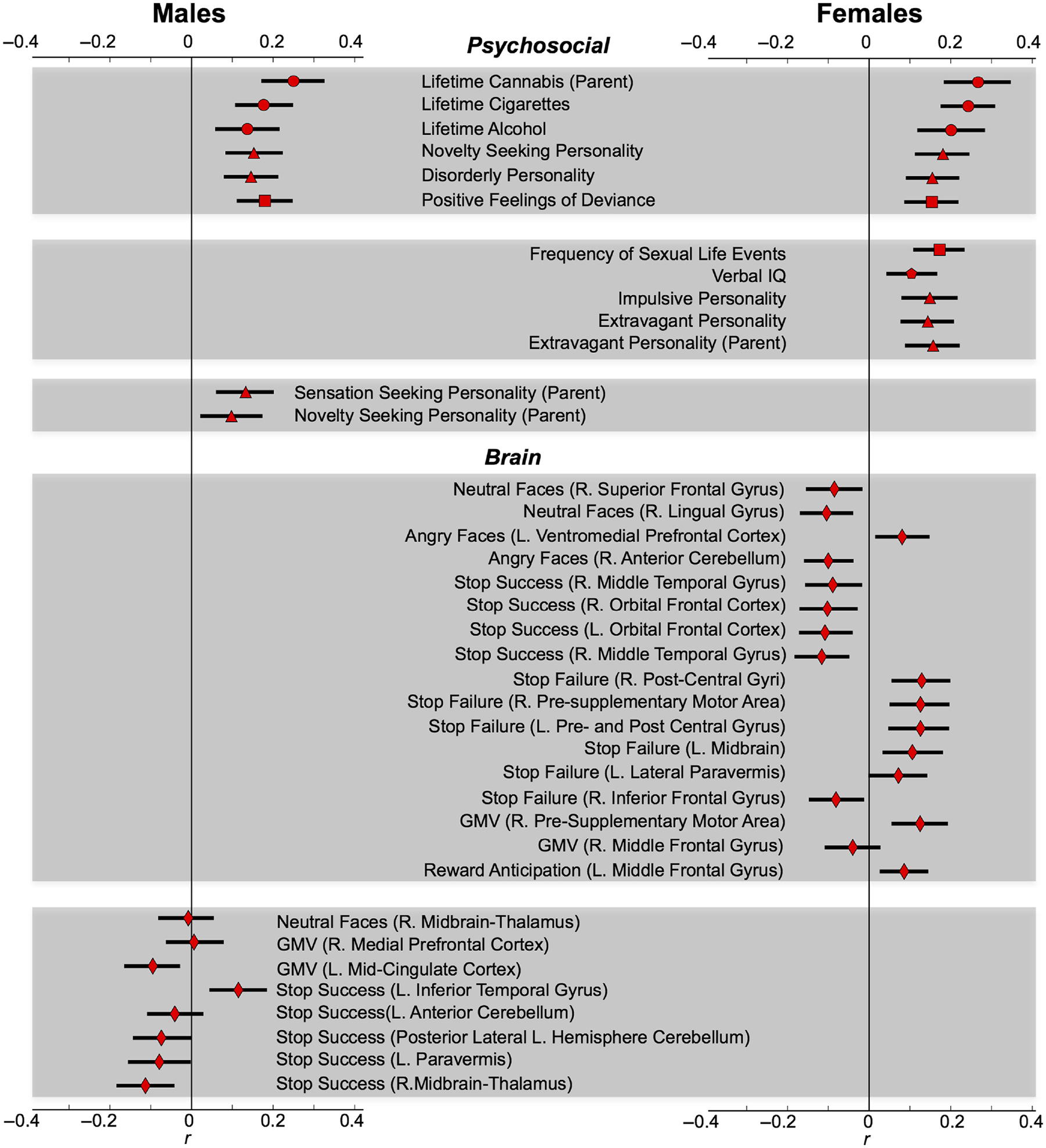
Correlations Between Identified Predictors and Outcome Measure by Sex. Pearson’s point-biserial correlation (r) between predictor and outcome. Error bars represent 95% confidence intervals generated from 5,000 bootstrap samples. Circles = drug use (ESPAD). Triangles = personality (from TCI and SURPS). Squares = life event (from LEQ). Pentagon = verbal IQ. Diamonds = neuroimaging data.
Male-specific predictors included greater parental novelty-seeking (Cloninger, 1999) and sensation seeking personality. While these parental personality traits measure similar constructs, partial correlations indicated parent sensation seeking predicted use (r739=0.10, p = 0.005) after accounting for parent novelty-seeking personality (r740=0.10, p = 0.007). Furthermore, although personality traits are heritable, partial correlations also indicated child novelty-seeking personality predicted use (r739 = 0.14, p = 2.1 × 10−4) after accounting for parent novelty-seeking personality, r740 = 0.10, p = 0.007).
Female-specific predictors included greater extravagant personality subscale (Cloninger, 1999) in both the parent and daughter. The extravagant subscale assesses overspending behaviours and diminished planning, and conveys a tendency to approach reward cues. Similar to males, greater extravagance of both the parent and daughter made separate contributions to the prediction (post hoc partial correlation between the outcome measure and child extravagance r823 = 0.12, p = 3.6 × 10−4, after accounting for parent extravagance r824 = 0.16, p = 6.0 × 10−6). In addition, greater impulsive personality subscale (Cloninger, 1999), frequent sexual experiences and higher verbal IQ predicted female use.
Selected brain predictors
For males, six functional and two structural brain features predicted cannabis use. For females, fifteen functional and two structural brain features predicted use with no overlap with the predictors for males. Post hoc point-biserial correlations indicated that five regions for males, and sixteen regions for females, significantly predicted any level of use across each sample. See Figures 2 and 3 for visualization of all brain features and direction of effects.
Fig. 2.
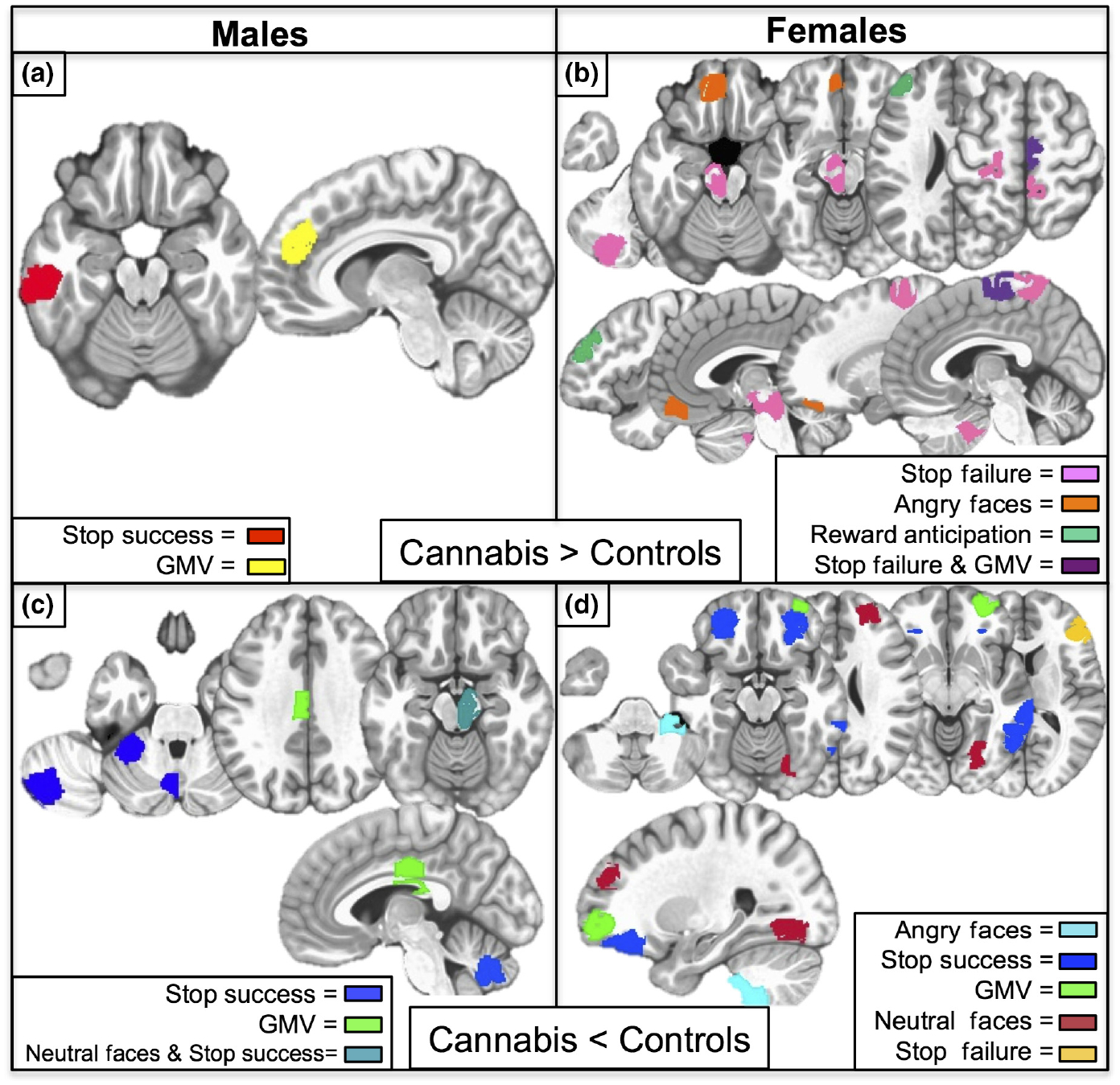
Sex-Specific Brain Predictors of Adolescent Cannabis Use. Panels a and b: Brain regions where age 16 cannabis users displayed higher average group-level activation or grey matter volume relative to their nonusing peers. Panel a: Male-Specific Predictive ROIs. Stop success refers to successful inhibition trials minus implicit baseline during the stop signal task; ROI (red) in left inferior temporal gyrus. GMV ROI (yellow) in right medial prefrontal cortex. Panel b: Female-Specific Predictive ROIs. Stop Failure refers to failed inhibition trials minus implicit baseline during the stop signal task; ROIs (pink) in left lateral paravermis, left midbrain, left pre- and postcentral gyrus, right postcentral gyrus. Angry faces refer to passive viewing of angry faces minus control images; ROI (orange) in left ventromedial prefrontal cortex. Reward anticipation refers to the processing of monetary reward cues; ROI (dark green) in left middle frontal gyrus. Stop failure and GMV overlapping ROI (purple) in right presupplementary motor area. Panels c and d: Brain regions where age 16 cannabis users displayed lower average group-level activation or grey matter volume relative to their nonusing peers. Panel c: Male-Specific ROIs. Stop success ROIs (dark blue) in left cerebellum include the anterior cerebellum, paravermis and posterior-lateral portion of the left hemisphere. GMV ROI (bright green) in left middle cingulate. Neutral Faces (passive viewing of neutral faces minus control images) and GMV overlapping ROI (teal) in right midbrain with extent into thalamus. Panel d: Female-Specific ROIs. Angry faces ROI (light blue) in right cerebellar tonsil. Stop success ROIs (dark blue) in bilateral orbitofrontal cortex and two contiguous regions in the right middle temporal gyrus. GMV ROI (bright green) in right middle frontal gyrus. Neutral faces ROIs (maroon) in right superior frontal gyrus and lingual gyrus. Stop failure ROI (dark yellow) in right inferior frontal gyrus.
Sex- and drug-specificity
Post hoc regressions confirmed that male-specific brain predictors of use returned strong model fits when estimated on the male sample (, p = 0.002; ΔAIC=8.3), as did the female-specific brain predictors estimated on the female sample (, p = 4.3 × 10−14 ΔAIC = 67.7). The male-specific brain predictors failed to predict use in females (, p = 0.272; model with predictors ΔAIC = 6.1 relative to the base rate model) and failed to predict binge drinking in males (, p = 0.405; model with predictors ΔAIC = 7.6 relative to the base rate model). Likewise, the female-specific brain predictors failed to predict use in males (, p = 0.341; model with predictors ΔAIC=15.2 relative to the base rate model) and failed to predict binge drinking in females (, p = 0.482; model with predictors ΔAIC = 17.4 relative to the base rate model). See Supporting Information Table S4 for all sex- and drug-specific post-hoc regression summaries.
Genetic predictors
Sex-specific feature selection analyses did not identify any SNPs, therefore, as a post hoc exploratory analysis, we collapsed across sex and reran the analyses with only the genetic predictors (plus nuisance covariates). This analysis returned an ROC AUC range = 0.54–0.61; p = 0.01–1.4 × 10−6 (Supporting Information Figure S2). We note that given the relatively small p-values, these models do not pass a Bonferroni correction, and as the highest use level analysis (use level 6) yielded a nonsignificant prediction (AUC = 0.53, p = 0.23), only results from the uncorrected significant models (use levels 1–5) were probed further. Moreover, the genetic multidimensional scaling factors plus demographic covariates inflated model performance. With that in consideration, two SNPs on genes coding for the β2-adrenergic receptor, one SNP on a gene coding for the α1b-adrenergic receptor, two SNPs on genes coding for the DRD1 receptor and five SNPs on genes coding for the μ1-opioid receptor, predicted cannabis use. Post hoc analyses suggested three SNPs were significantly related to cannabis use for the male sample (β2-adrenergic: rs1042711, rs1801704; and DRD1: rs1174661), whereas none of the SNPs were significant for the female sample (see Supporting Information Table S7 and Figure S8 for SNP statistics, including their correlation with the outcome measure across the entire sample).
When including these ten SNPs in a post hoc hierarchical logistic regression predicting cannabis use, the model exhibited strong fit to the full sample after first modelling the nuisance covariates (, p = 0.002; ΔAIC=7.7). However, these SNPs returned poor model fits to the full sample of binge drinkers after first modelling the nuisance covariates (, p = 0.435; ΔAIC=9 relative to the model with nuisance covariates only).
Domain contribution effects
The psychosocial predictors were entered first and significantly improved model fit relative to the base rate model for the male sample (, p = 5.5 × 10−17; ΔAIC = 78.53) and the female sample (, p = 2.5 × 10−23; ΔAIC = 112.13). Next, the brain predictors were added and significantly improved model fit for the male sample (, p = 0.027; ΔAIC = 1.3) and the female sample (, p = 5.8 × 10−14; ΔAIC = 67.1). At last, the 10 SNPs were added and significantly improved model fit for the male sample (, p = 0.007; ΔAIC = 6.2) but not the female sample (, p = 0.689; psychosocial and brain model ΔAIC = 11.5). These findings held irrespective of the order in which each domain was entered. Thus, while psychosocial data alone can be used to significantly predict use, models containing both psychosocial and sex-specific brain features return superior fits, highlighting the utility of capturing individual neurobiological differences in predicting adolescent cannabis use.
Discussion
Psychosocial findings
The six shared psychosocial predictors replicate previous findings establishing alcohol and tobacco as predictors of cannabis use (Hall & Pacula, 2003; Siegel et al., 2014), as are novelty-seeking and disorderliness personality traits (Hale, Whiteman, Muehl, & Faynberg, 2003; Sher & Trull, 1994), and parental transmission of drug use (Brook et al., 2001; Kandel, Kessler, & Margulies, 1978; Kosty et al., 2015). As these features also predicted binge drinking, they may be considered general risk factors for adolescent drug use. In considering the parental influence, parents with behaviourally disinhibited personality traits, coupled with a history of cannabis use, were found to increase risk for use in their children, mirroring previously published studies (Day et al., 2006; Kerr, Tiberio, & Capaldi, 2015). Moreover, less-negative feelings towards deviant behaviours may signal a predisposition towards conduct disorder, which previous literature has linked to cannabis use (Crowley, Mikulich, MacDonald, Young, & Zerbe, 1998). Risk of use was also identified for females exhibiting higher verbal IQ, which has been implicated in cannabis experimentation (Fried, Watkinson, James, & Gray, 2002). In addition, higher impulsivity, extravagance and sexual experiences are consistent with the novelty-seeking phenotype of individuals most likely to initiate substance use.
Brain findings
For males, the brain predictors were largely related to cerebellar activation differences during response inhibition. Animal models suggest the lateral cerebellum is involved in motor preparation and inhibition via projections to cortical motor and inhibitory regions through the thalamus (Middleton & Strick, 2001). In addition, the cerebellar regions identified have also been implicated in a network underlying motor inhibitory control (Stevens, Kiehl, Pearlson, & Calhoun, 2007). Thus, hypoactivity in all three cerebellar regions may suggest a compromised motor inhibitory control system constitutes a neurobiological vulnerability that influences the initiation of cannabis consuming behaviours. Moreover, larger GMV in the right medial prefrontal cortex (PFC) might indicate a neurodevelopmental delayed maturation in regions supporting executive functioning. This finding is supported by studies reporting an adolescent male-specific increase in PFC volume with alcohol use disorder (Medina et al., 2008) and conduct use disorder (Brito et al., 2009).
In females, a structural-functional finding in the right presupplemental motor area (pre-SMA) predicted cannabis use. As myelination proliferates during adolescence, especially in motor areas requiring expedited signal propagation (Paus, 1999), higher GMV and activity during failed inhibitions observed in the right pre-SMA suggests a functional consequence of delayed cortical maturation. This structural finding is notable for the female sample as cortical maturation (thinning) occurs earlier in females compared to their male peers (Giedd, 2004).
In addition, lower activity compared to nonusers in the right inferior frontal gyrus (IFG) during failed inhibitions was predictive of cannabis use in females. As the right IFG is a key region implicated in the stop task (Garavan, Ross, & Stein, 1999), lower activity is notable as hypoactivity here is also associated with cigarette use (Spechler et al., 2016). As our test for drug-specificity was restricted to binge drinking, some brain predictors might generalize to other drugs of abuse not tested here. In the orbitofrontal cortex (OFC), females also displayed lower bilateral activations during successful inhibitions and lower right-sided GMV. The volumetric finding is concordant with Cheetham et al. (2012) who reported lower OFC GMV at age 12 predicts use at age 16, with only the right OFC remaining significant after accounting for poly-drug use, thus under-scoring the right OFC specificity to cannabis initiation. Furthermore, as other studies have correlated OFC hypoactivity with adolescent substance use (Whelan et al., 2012), the anterior prefrontal cortex might be especially valuable for inquiry relating female-specific neurobiological pathways with substance abuse.
For females, more predictors related to face processing were identified. In a specific way, lower processing of neutral faces in the right superior frontal and lingual gyri. Previous studies suggest neutral faces can be misperceived as threatening, especially in individuals with social anxiety disorder (Cooney, Atlas, Joormann, Eugène, & Gotlib, 2006; Yoon & Zinbarg, 2008). Given the higher prevalence of social anxiety in females (Schneier, 1992) and the correlation between social anxiety and prevalence of cannabis use in females (Buckner, Bonn-Miller, Zvolensky, & Schmidt, 2007; Buckner, Mallott, Schmidt, & Taylor, 2006) these results suggest a female-specific pathway towards cannabis use. In addition, higher female-specific activation to angry faces in the ventromedial prefrontal cortex is notable given this region’s involvement in emotion regulation (Urry et al., 2006).
Genetic findings
The number of predictive μ1-opioid receptor SNPs highlights the importance of the opioid system in substance abuse. Opioid and cannabinoid systems co-localize in the striatum (Rodriguez, Mackie, & Pickel, 2001) and exhibit reciprocal signalling (Robledo, Berrendero, Ozaita, & Maldonado, 2008). However, the biobehavioural effects orchestrated by these systems remain unclear in humans. Animal models suggest the μ1-opioid receptor is specifically involved in reinforcement as μ1-opioid receptor knockout mice failed to exhibit THC-induced conditioned place preference compared to δ1-knockout and wild-type mice (Ghozland et al., 2002). Hence, our findings that cannabis users had a greater number of risk alleles for both DRD1 SNPs and three μ1-receptor SNPs suggest alterations in their neurobiological processing of rewards. As these findings were uncovered from exploratory models that were not as robust to predict use as the multidomain models, larger GWAS studies or candidate SNP analyses are needed to reinforce these results.
Conclusions
In this large longitudinal study, we offer evidence that psychosocial and sex-specific neurobiological predictors of cannabis use preceded, and likely influenced, teenage cannabis consuming behaviours. Hence, these analyses identified individual differences at age 14 that predict later cannabis use and thus have potential for guiding proactive interventions. Despite having thousands of multidomain variables per individual, prediction with high generalizability was achieved with a sparse set of sex-specific brain and psychosocial features, and six shared psychosocial features. And while the psychosocial data alone were found to predict both cannabis and binge drinking, the addition of the brain features improved cannabis prediction and augmented the sex-specificity of the findings.
The superior prediction of the female sample suggests they exhibit a more distinct predictive profile at age 14, despite having lower levels of subsequent use. These findings are clinically meaningful given the female-specific vulnerability towards accelerated dependency. Moreover, the fMRI findings highlight the sex-specific psychological processes potentially driving the initiation of cannabis use in adolescence. Thus, our findings underscore the importance of attending to sex differences in addiction research and fulfils the recent NIH policy for investigators to examine sex differences in biobheavioural research (Clayton & Collins, 2014).
Limitations of this study include the absence of measures of peer influences. The addition of these variables, as well as interactions between features, might yield a higher AUC, as the reported AUCs indicate a departure from perfect prediction. Future analyses to identify how psychosocial, brain and genetic feature interact to influence the likelihood of cannabis use are needed. In addition, the convenient community sampling of predominantly white Europeans may impact generalizability to other populations.
At last, despite predicting high levels of use (e.g., ≥40 uses by age 16), it is unknown if these individuals will meet DSM-V diagnostic criteria for cannabis use disorder later in life. However, by design of the analysis, all participants were early initiators of cannabis, with the heavy users always present in the prediction models. Therefore, these predictors may signify risk for higher use. Still, the heavy users only encompassed a small proportion of the sample, therefore even larger studies are needed. And while our predictive models generalized to independent observations via internal cross-validation, a completely set aside external validation set was not possible due to the limited sample sizes. As such, the gold standard remains a completely independent external validation set. Studies assessing the degree by which cross-validated prediction metrics may differ by cross-validation scheme are also needed (although Whelan et al., 2014 reports similar AUCs for internal and external validation). Taken together, our findings supply new hypotheses to be tested using additional time points from the ongoing IMAGEN and larger ABCD (www.ABCDstudy.org) studies.
Supplementary Material
Acknowledgements
This work received support from the following sources: National Institutes of Health Center of Biomedical Research Excellence award P20GM103644 from the National Institute of General Medical Sciences; the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the FP7 projects IMAGEMEND (602450; IMAging GEnetics for MENtal Disorders) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), a Medical Research Council Programme Grant “Developmental pathways into adolescent substance abuse” (93558), the Swedish funding agency FORMAS, the Medical Research Council and the Wellcome Trust (Behavioural and Clinical Neuroscience Institute, University of Cambridge), the National Institute for Health Research (NIHR) Biomedical Research Centre at SouthLondon and Maudsley NHS Foundation Trust and King’s College London, the Bundesministerium fur Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc 01ZX1311A; Forschungsnetz AERIAL), the Deutsche Forschungsgemeinschaft (DFG): Reinhart-Koselleck Award SP 383/5-1 and grants SM 80/7-1, SFB 940/1, FOR 1617), the French MILDT (Mission Interministe rielle de Lutte contre la Drogue et la Toxicomanie), the CENIR (Centre de NeuroImagerie de Recherche, Pr. S. Lehe ricy) within the ICM institute and the National Institute of Mental Health (MH082116). HG was supported, in part, by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centers of Excellence.
Members of the IMAGEN Consortium not listed as individual authors include: Karl Mann, Maren Struve, Marcella Rietschel, Rainer Spanagel, Mira Fauth-Bühler, Sabina Millenet, and Yvonne Grimmer at the Central Institute of Mental Health, University of Heidelberg; Nikolay Ivanov, Nicole Strache, Michael Rapp, Andreas Ströhle, and Jan Reuter at Charité, Universitätsmedizin Berlin; Alexis Barbot, Benjamin Thyreau, Yannick Schwartz, and Christophe Lalanne at the Comissariat à l’Energie Atomique; Jean-Luc Martinot, Zuleima Bricaud, Fanny Gollier Briand, Hervé Lemaitre, Jessica Massicotte, Helene Vulser, Jani Pentillä, and André Galinowski at the Institut national de la santé et de la recherche médicale; Tianye Jia, Helen Werts, Lauren Topper, Laurence Reed, Chris Andrew, Catherine Mallik, Barbara Ruggeri, Charlotte Nymberg, Lindsay Smith, Eva Loth, Stephanie Havatzias, Kerstin Stueber, and Argyris Stringaris at the Institute of Psychiatry, King’s College London; Patrick Constant at PERTIMM (Asnières-Sur-Seine); Ruediger Brühl, Albrecht Ihlenfeld, and Bernadeta Walaszek at the Physikalisch-Technische Bundesanstalt; Thomas Hübner, Kathrin Müller, Stephan Ripke, Sarah Rodehacke, Eva Mennigen, Dirk Schmidt, Nora Vetter, and Veronika Ziesch at the Technische Universität Dresden; Jennifer Jones at the University College Dublin; Jean-Baptiste Poline at the University of California, Berkeley; Tahmine Fadai, Juliana Yacubian, and Sophia Schneider at the University of Hamburg; Claire Lawrence, Craig Newman, Kay Head, and Nadja Heym at the University of Nottingham; and Zdenka Pausova, and Amir Tahmasebi at the University of Toronto.
Conflict of interests
Dr. Banaschewski has served as an advisor or consultant to Bristol-Myers Squibb, Desitin Arzneimittel, Eli Lilly, Medice, Novartis, Pfizer, Shire, UCB and Vifor Pharma; he has received conference attendance support, conference support or speaking fees from Eli Lilly, Janssen McNeil, Medice, Novartis, Shire and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis and Shire; the present work is unrelated to these relationships. Dr. Gallinat has received research funding from the German Federal Ministry of Education and Research, AstraZeneca, Eli Lilly, Janssen-Cilag and Bristol-Myers Squibb; he has received speaking fees from AstraZeneca, Janssen-Cilag and Bristol-Myers Squibb; the present work is unrelated to these relationships. The other authors report no biomedical financial interests or potential conflict of interests.
Abbreviations
- AIC
Akaike information criterion
- AUC
Area under the curve
- CB1
Primary cannabinoid receptor
- CB2
Secondary cannabinoid receptor
- DRD1
Dopamine receptor subtype 1
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders-4th Edition
- ESPAD
European School Survey Project on Alcohol and Drugs
- FAAH
Fatty acid amide hydrolase
- fMRI
Functional magnetic resonance imaging
- GLM
General linear model
- GMV
Grey matter volume
- GWAS
Genomewide association study
- IFG
Inferior frontal gyrus
- IQ
Intelligence quotient
- LEQ
Life events questionnaire
- NIH
National Institutes of Health
- OFC
Orbitofrontal cortex
- PDS
Pubertal development scale
- PFC
Prefrontal cortex
- pre-SMA
pre-supplemental motor area
- ROC
Receiver-operating characteristic
- ROI
Region of interest; SES, Socioeconomic status
- SNPs
Single nucleotide polymorphisms
- SURPS
Substance use risk profile scale
- TCI
Temperament and character inventory
- THC
delta-9-tetrahydrocannabinol
Footnotes
Supporting Information
Additional supporting information can be found in the online version of this article:
Data S1. Materials and methods.
Figure S1. Schematic of analytic method.
Figure S2. Receiver-operating characteristic (ROC) mean AUC for Gene-specific Analysis.
Figure S3. Correlations between identified SNPs and outcome measure by sex.
Table S1. Comparison of age 16 dropouts vs. retained sample.
Table S2. Summary of data used as independent variables in predictive modeling.
Table S3. Binge drinking sample demographics.
Table S4. Post-hoc regression model summaries.
Table S5. Statistics and frequencies for cannabis predictive SNPs.
Table S6. Frequency of selected male features.
Table S7. Frequency of selected female features.
Table S8. Analysis of head motion.
Data accessibility
Access to the IMAGEN dataset can be obtained by following a formal request procedure. This procedure involves writing a study proposal that outlines and defends the aims of a study. The proposal is then submitted to the IMAGEN consortium for approval. Full details on the IMAGEN study data access policy and proposal materials can be found on the IMAGEN website (www.imagen-europe.com).
References
- Akaike H (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19, 716–723. 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- van den Bree MBM, & Pickworth WB (2005). Risk factors predicting changes in marijuana involvement in teenagers. Archives of General Psychiatry, 62, 311–319. 10.1001/archpsyc.62.3.311 [DOI] [PubMed] [Google Scholar]
- Brito DAS, Mechelli A, Wilke M, Laurens KR, Jones AP, … Viding E (2009). Size matters: Increased grey matter in boys with conduct problems and callous-unemotional traits. Brain, 132, 843–852. 10.1093/brain/awp011 [DOI] [PubMed] [Google Scholar]
- Brook JS, Brook DW, De La Rosa M, Whiteman M, Johnson E, & Montoya I (2001). Adolescent illegal drug use: The impact of personality, family, and environmental factors. Journal of Behavioral Medicine, 24, 183–203. 10.1023/A:1010714715534 [DOI] [PubMed] [Google Scholar]
- Brook JS, Lee JY, Brown EN, Finch SJ, & Brook DW (2011). Developmental trajectories of marijuana use from adolescence to adulthood: Personality and social role outcomes. Psychological Reports, 108, 339–357. 10.2466/10.18.PR0.108.2.339-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Bonn-Miller MO, Zvolensky MJ, & Schmidt NB (2007). Marijuana use motives and social anxiety among marijuana-using young adults. Addictive Behaviors, 32, 2238–2252. 10.1016/j.addbeh.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Mallott MA, Schmidt NB, & Taylor J (2006). Peer influence and gender differences in problematic cannabis use among individuals with social anxiety. Journal of Anxiety Disorders, 20, 1087–1102. 10.1016/j.janxdis.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Carskadon MA, & Acebo C (1993). A self-administered rating scale for pubertal development. Journal of Adolescent Health, 14, 190–195. 10.1016/1054-139X(93)90004-9 [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yücel M, & Lubman DI (2012). Orbitofrontal volumes in early adolescence predict initiation of cannabis use: A 4-year longitudinal and prospective study. Biological Psychiatry, 71, 684–692. 10.1016/j.biopsych.2011.10.029 [DOI] [PubMed] [Google Scholar]
- Clayton JA, & Collins FS (2014). Policy: NIH to balance sex in cell and animal studies. Nature, 509, 282–283. 10.1038/509282a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR (1999). The temperament and character inventory-revised. St Louis MO: Cent. Psychobiol. Personal., Wash. Univ. [Google Scholar]
- Coffey C, & Patton GC (2016). Cannabis use in adolescence and young adulthood: A review of findings from the victorian adolescent health cohort study. Canadian Journal of Psychiatry, 61, 318–327. 10.1177/0706743716645289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugène F, & Gotlib IH (2006). Amygdala activation in the processing of neutral faces in social anxiety disorder: Is neutral really neutral?. Psychiatry Research Neuroimaging, 148, 55–59. 10.1016/j.pscychresns.2006.05.003 [DOI] [PubMed] [Google Scholar]
- Cooper ZD, & Haney M (2014). Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug and Alcohol Dependence, 136, 85–91. 10.1016/j.drugalcdep.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis MC (2009). Joint effects of common genetic variants on the risk for type 2 diabetes in U.S. men and women of European ancestry. Annals of Internal Medicine, 150, 541 10.7326/0003-4819-150-8-200904210-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT Jr, & McCrae RR (1995). Domains and facets: Hierarchical personality assessment using the revised NEO personality inventory. Journal of Personality Assessment, 64, 21–50. 10.1207/s15327752jpa6401_2 [DOI] [PubMed] [Google Scholar]
- Creemers HE, Dijkstra JK, Vollebergh WAM, Ormel J, Verhulst FC, & Huizink AC (2010). Predicting life-time and regular cannabis use during adolescence; the roles of temperament and peer substance use: The TRAILS study. Addiction, 105, 699–708. 10.1111/j.1360-0443.2009.02819.x [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Mikulich SK, MacDonald M, Young SE, & Zerbe GO (1998). Substance-dependent, conduct-disordered adolescent males: Severity of diagnosis predicts 2-year outcome1. Drug and Alcohol Dependence, 49, 225–237. 10.1016/S0376-8716(98)00016-7 [DOI] [PubMed] [Google Scholar]
- Day NL, Goldschmidt L, & Thomas CA (2006). Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction, 101, 1313–1322. 10.1111/j.1360-0443.2006.01523.x [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, … Wells JE (2008). Toward a global view of alcohol, tobacco, cannabis, and cocaine use: Findings from the WHO world mental health surveys. PLoS Medicine, 5, e141 10.1371/journal.pmed.0050141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Coffey C, Romaniuk H, Swift W, Carlin JB, Hall WD, & Patton GC (2013). The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood: Adolescent cannabis use and mental health. Addiction, 108, 124–133. 10.1111/j.1360-0443.2012.04015.x [DOI] [PubMed] [Google Scholar]
- Ellickson PL, Tucker JS, Klein DJ, & Saner H (2004). Antecedents and outcomes of marijuana use initiation during adolescence. Preventive Medicine, 39, 976–984. 10.1016/j.ypmed.2004.04.013 [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, & Church JC (2016). Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biological Psychiatry, 79, 613–619. 10.1016/j.biopsych.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, & Fratta W (2007). Cannabinoid self-administration in rats: Sex differences and the influence of ovarian function. British Journal of Pharmacology, 152, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett T (2006). An introduction to ROC analysis. Pattern Recognition Letters, 27, 861–874. 10.1016/j.patrec.2005.10.010 [DOI] [Google Scholar]
- Fergusson DM, & Boden JM (2008). Cannabis use and later life outcomes. Addiction, 103, 969–976. 10.1111/j.1360-0443.2008.02221.x [DOI] [PubMed] [Google Scholar]
- Fried P, Watkinson B, James D, & Gray R (2002). Current and former marijuana use: Preliminary findings of a longitudinal study of effects on IQ in young adults. Canadian Medical Association Journal, 166, 887–891. [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, & Stein EA (1999). Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proceedings of the National Academy of Sciences, 96, 8301–8306. 10.1073/pnas.96.14.8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghozland S, Matthes HWD, Simonin F, Filliol D, Kieffer BL, & Maldonado R (2002). Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. Journal of Neuroscience, 22, 1146–1154. 10.1523/JNEUROSCI.22-03-01146.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN (2004). Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences, 1021, 77–85. 10.1196/annals.1308.009 [DOI] [PubMed] [Google Scholar]
- Hale RL, Whiteman S, Muehl K, & Faynberg E (2003). Tridimensional personality traits of college student marijuana users. Psychological Reports, 92, 661–666. 10.2466/pr0.2003.92.2.661 [DOI] [PubMed] [Google Scholar]
- Hall W, & Degenhardt L (2009). Adverse health effects of non-medical cannabis use. The Lancet, 374, 1383–1391. 10.1016/S0140-6736(09)61037-0 [DOI] [PubMed] [Google Scholar]
- Hall W, & Pacula RL (2003). Cannabis use and dependence: Public health and public policy. Cambridge, UK: Cambridge University Press; 10.1017/CBO9780511470219 [DOI] [Google Scholar]
- Hartman CA, Hopfer CJ, Haberstick B, Rhee SH, Crowley TJ, Corley RP, … Ehringer MA (2009). The association between cannabinoid receptor 1 gene (CNR1) and cannabis dependence symptoms in adolescents and young adults. Drug and Alcohol Dependence, 104, 11–16. 10.1016/j.drugalcdep.2009.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, & Kranzler HR (2004). Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug and Alcohol Dependence, 74, 265–272. 10.1016/j.drugalcdep.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Hibell B, Andersson B, Bjarnason T, Kokkevi A, Morgan M, & Narusk A (1997). The 1995 ESPAD report. Alcohol Drug Use Stud. In., 26,. [Google Scholar]
- Hopfer CJ, Young SE, Purcell S, Crowley TJ, Stallings MC, Corley RP, … Ehringer MA (2006). Cannabis receptor haplotype associated with fewer cannabis dependence symptoms in adolescents. American Journal of Medical Genetics, 141, 895–901. 10.1002/(ISSN)1552-485X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Michaelides M, Miller ML, & Jutras-Aswad D (2014). Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology, 76, 416–424. 10.1016/j.neuropharm.2013.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson NJ, Isen JD, Khoddam R, Irons D, Tuvblad C, Iacono WG, … Baker LA (2016). Impact of adolescent marijuana use on intelligence: Results from two longitudinal twin studies. Proceedings of the National Academy of Sciences, 113, E500–E508. 10.1073/pnas.1516648113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, & Schulenberg JE (2011). Monitoring the Future National Survey Results on Drug Use, 1975–2010 Volume I, Secondary School Students; Inst. Soc. Res [Google Scholar]
- Kalant H (2004). Adverse effects of cannabis on health: An update of the literature since 1996. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 28, 849–863. 10.1016/j.pnpbp.2004.05.027 [DOI] [PubMed] [Google Scholar]
- Kandel DB, Kessler RC, & Margulies RZ (1978). Antecedents of adolescent initiation into stages of drug use: A developmental analysis. Journal of Youth and Adolescence, 7, 13–40. 10.1007/BF01538684 [DOI] [PubMed] [Google Scholar]
- Kedzior KK, & Laeber LT (2014). A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population- a meta-analysis of 31 studies. BMC Psychiatry, 14, 136 10.1186/1471-244X-14-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DCR, Tiberio SS, & Capaldi DM (2015). Contextual risks linking parents’ adolescent marijuana use to offspring onset. Drug and Alcohol Dependence, 154, 222–228. 10.1016/j.drugalcdep.2015.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosty DB, Farmer RF, Seeley JR, Gau JM, Duncan SC, & Lewinsohn PM (2015). Parental transmission of risk for cannabis use disorders to offspring: Parent transmission of cannabis use disorder. Addiction, 110, 1110–1117. 10.1111/add.12914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DT, Hill MN, & Rubino T (2010). Adolescent cannabis use and psychosis: Epidemiology and neurodevelopmental models. British Journal of Pharmacology, 160, 511–522. 10.1111/j.1476-5381.2010.00721.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SJ, & Graham NE (2002). Areas beneath the relative operating characteristics (ROC) and relative operating levels (ROL) curves: Statistical significance and interpretation. Quarterly Journal Royal Meteorological Society, 128, 2145–2166. 10.1256/003590002320603584 [DOI] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, & Tapert SF (2008). Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcoholism, Clinical and Experimental Research, 32, 386–394. 10.1111/j.1530-0277.2007.00602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, … Moffitt TE (2012). Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences, 109, E2657–E2664. 10.1073/pnas.1206820109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, & Strick PL (2001). Cerebellar projections to the prefrontal cortex of the primate. Journal of Neuroscience, 21, 700–712. 10.1523/JNEUROSCI.21-02-00700.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, & Yi H (2014). Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug and Alcohol Dependence, 136, 51–62. 10.1016/j.drugalcdep.2013.12.011 [DOI] [PubMed] [Google Scholar]
- Narimatsu S, Watanabe K, Yamamoto I, & Yoshimura H (1991). Sex difference in the oxidative metabolism of D9-tetrahydrocannabinol in the rat. Biochemical Pharmacology, 41, 1187–1194. 10.1016/0006-2952(91)90657-Q [DOI] [PubMed] [Google Scholar]
- Newcomb MD, Huba GJ, & Bentler PM (1981). A Multidimensional assessment of stressful life events among adolescents: Derivation and correlates. Journal of Health and Social Behavior, 22, 400 10.2307/2136681 [DOI] [Google Scholar]
- NSDUH (2014). The NSDUH report: Substance use and mental health estimates from the 2013 national survey on drug use and health: Overview of findings. [PubMed]
- Onaivi ES, Chaudhuri G, Abaci AS, Parker M, Manier DH, Martin PR, & Hubbard JR (1999). Expression of cannabinoid receptors and their gene transcripts in human blood cells. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 23, 1063–1077. 10.1016/S0278-5846(99)00052-4 [DOI] [PubMed] [Google Scholar]
- O’Shea M (2004). Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. Journal of Psychopharmacology, 18, 502–508. 10.1177/0269881104047277 [DOI] [PubMed] [Google Scholar]
- Paus T (1999). Structural maturation of neural pathways in children and adolescents. In vivo study. Science, 283, 1908–1911. 10.1126/science.283.5409.1908 [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, & Yurgelun-Todd D (2003). Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug and Alcohol Dependence, 69, 303–310. 10.1016/S0376-8716(02)00334-4 [DOI] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, … McGregor IS (2008). Adolescent rats find repeated D9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology, 33, 1113–1126. 10.1038/sj.npp.1301475 [DOI] [PubMed] [Google Scholar]
- Renard J, Rosen LG, Loureiro M, De Oliveira C, Schmid S, Rushlow WJ, & Laviolette SR (2016). Adolescent cannabinoid exposure induces a persistent sub-cortical hyper-dopaminergic state and associated molecular adaptations in the prefrontal cortex. Cerebral Cortex, 27, 1297–1310. 10.1093/cercor/bhv335 [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, & Rabbitt P (1994). Cambridge neuropsychological test automated battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia and Geriatric Cognitive Disorders, 5, 266–281. 10.1159/000106735 [DOI] [PubMed] [Google Scholar]
- Robledo P, Berrendero F, Ozaita A, & Maldonado R (2008). Advances in the field of cannabinoid-opioid cross-talk. Addiction Biology, 13, 213–224. 10.1111/j.1369-1600.2008.00107.x [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, & Pickel VM (2001). Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. Journal of Neuroscience, 21, 823–833. 10.1523/JNEUROSCI.21-03-00823.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, … Parolaro D (2015). Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiology of Diseases, 73, 60–69. 10.1016/j.nbd.2014.09.015 [DOI] [PubMed] [Google Scholar]
- Schepis TS, Desai RA, Cavallo DA, Smith AE, McFetridge A, Liss TB, … Krishnan-Sarin S (2011). Gender differences in adolescent marijuana use and associated psychosocial characteristics. Journal of Addiction Medicine, 5, 65–73. 10.1097/ADM.0b013e3181d8dc62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M (2008). Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure: Consequences of cannabis exposure dsuring puberty. Addiction Biology, 13, 253–263. 10.1111/j.1369-1600.2008.00110.x [DOI] [PubMed] [Google Scholar]
- Schneier FR (1992). Social Phobia: Comorbidity and morbidity in an epidemiologic sample. Archives of General Psychiatry, 49, 282 10.1001/archpsyc.1992.01820040034004 [DOI] [PubMed] [Google Scholar]
- Schumann G, the IMAGEN consortium, Loth E, Banaschewski T, Barbot A, Barker G, … Struve M (2010). The IMAGEN study: Reinforcement-related behaviour in normal brain function and psychopathology. Molecular Psychiatry, 15, 1128–1139. 10.1038/mp.2010.4 [DOI] [PubMed] [Google Scholar]
- Schuster RM, Hoeppner SS, Evins AE, & Gilman JM (2016). Early onset marijuana use is associated with learning inefficiencies. Neuropsychology, 30, 405–415. 10.1037/neu0000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secades-Villa R, Garcia-Rodríguez O, Jin CJ, Wang S, & Blanco C (2015). Probability and predictors of the cannabis gateway effect: A national study. International Journal of Drug Policy, 26, 135–142. 10.1016/j.drugpo.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Tokoglu F, Papademetris X, & Constable RT (2013). Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage, 82, 403–415. 10.1016/j.neuroimage.2013.05.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, & Trull TJ (1994). Personality and disinhibitory psychopathology: Alcoholism and antisocial personality disorder. Journal of Abnormal Psychology, 103, 92–102. 10.1037/0021-843X.103.1.92 [DOI] [PubMed] [Google Scholar]
- Siegel JT, Crano WD, Alvaro EM, Lac A, Hackett JD, & Hohman ZP (2014). Differentiating common predictors and outcomes of marijuana initiation: A retrospective longitudinal analysis. Substance Use and Misuse, 49, 30–40. 10.3109/10826084.2013.817427 [DOI] [PubMed] [Google Scholar]
- Spechler PA, Chaarani B, Hudson KE, Potter A, Foxe JJ, & Garavan H (2016). Response inhibition and addiction medicine: From use to abstinence. Progress in Brain Research, 223, 143–164. 10.1016/bs.pbr.2015.07.024 [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, & Calhoun VD (2007). Functional neural networks underlying response inhibition in adolescents and adults. Behavioral Brain Research, 181, 12–22. 10.1016/j.bbr.2007.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Sydow K, Lieb R, Pfister H, Höfler M, & Wittchen H-U (2002). What predicts incident use of cannabis and progression to abuse and dependence? A 4-year prospective examination of risk factors in a community sample of adolescents and young adults. Drug and Alcohol Dependence, 68, 49–64. 10.1016/S0376-8716(02)00102-3 [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, … Davidson RJ (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience, 26, 4415–4425. 10.1523/JNEUROSCI.3215-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler intelligence scale for children-Fourth Edition (WISC-IV). San Antonio, TX: Psychol. Corp. [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, … the IMAGEN Consortium (2012). Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience, 15, 920–925. 10.1038/nn.3092 [DOI] [PubMed] [Google Scholar]
- Whelan R, & Garavan H (2014). When optimism hurts: Inflated predictions in psychiatric neuroimaging. Biological Psychiatry, 75, 746–748. 10.1016/j.biopsych.2013.05.014 [DOI] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, … the IMAGEN Consortium (2014). Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature, 512, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woicik PA, Stewart SH, Pihl RO, & Conrod PJ (2009). The substance use risk profile scale: A scale measuring traits linked to reinforcement-specific substance use profiles. Addictive Behaviors, 34, 1042–1055. 10.1016/j.addbeh.2009.07.001 [DOI] [PubMed] [Google Scholar]
- Yoon KL, & Zinbarg RE (2008). Interpreting neutral faces as threatening is a default mode for socially anxious individuals. Journal of Abnormal Psychology, 117, 680–685. 10.1037/0021-843X.117.3.680 [DOI] [PubMed] [Google Scholar]
- Zou H, & Hastie T (2005). Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 67, 301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


