Abstract
The mitochondrial enzyme succinate dehydrogenase (SDH) acts as a tumor suppressor. Biallelic inactivation of one of the genes encoding for SDH subunits (collectively named SDHx) leads to complete loss of the protein function and the development of diverse group of tumors. Pheochromocytomas-paragangliomas are the prime example of hereditary tumors caused by SDH deficiency. In this review, we discuss the roles of imaging examinations, and illustrate new insights into genotype-imaging phenotype relationships.
Keywords: positron emission tomography, gallium radioisotopes, 6-(18F)fluoro-l-3, 4-dihydroxyphenylalanine, precision medicine, theranostics, succinate dehydrogenase
Origin of PPGL
Paraganglionic cells arise from neural crest and are widely distributed throughout the body. These cells are found in aggregates in specific regions and constitute: 1) the parasympathetic paraganglionic chemoreceptor system that includes the carotid body, aortic bodies, glomus jugulare, and tympanicum; 2) the adrenal medulla; and 3) the sympathetic paraganglia, which under normal circumstances regress via apoptosis or autophagy during the first years of life [1]. In neonates, the organ of Zuckerkandl (OZ) constitutes the largest accumulation of extra-adrenal chromaffin cells. Tumors that derive from either parasympathetic and sympathetic paraganglia are collectively named paragangliomas (PGLs), with the term pheochromocytoma being restricted to adrenal PGL. In up to 70 % of cases, pheochromocytomas and paragangliomas (PPGLs) are associated with germline and somatic mutations in about 15 well-characterized PPGL driver or fusions genes [2, 3].
Molecular-Metabolic Features of SDHx-PPGL
In 2000, Baysal et al. described the first PGL syndrome (PGL1) related to a SDH deficiency, due to a mutation in the SDHD [4]. Later, several familial clusters of PPGLs related to mutations in any gene encoding for subunits of the SDH complex (SDHA-D genes) or its flavination factor (SDHAF2) were described and defined as PGL syndromes PGL1 through PGL5 [5–8]. SDH enzyme (also called mitochondrial complex II) catalyzes the oxidation of succinate to fumarate in the tricarboxylic acid cycle (TCA) and the respiratory chain. Tumorigenesis is related to SDH deficiency that requires a somatic loss of the second allele (bilallelic inactivation of the SDH tumor suppressor gene). Another syndrome named Carney’s triad is in some cases related to epigenetic down-regulation of SDH, which also results in SDH deficiency [9].
SDH deficiency results in a partial TCA blockade with accumulation of enormous concentrations of succinate that can be detected by in vitro and in vivo metabolomic studies. Although SDH deficiency alone does not impair cellular respiration due to compensation by anaplerotic pathways (i. e., glutaminolysis, pyruvate carboxylation), succinate promotes a set of distinct oncogenic features via intra- and extracellular effects. Succinate enhances tumor growth and survival via hypoxia-inducible factors (HIFs) stabilization with increased expression of HIF-target genes despite normal oxygen supply (pseudohypoxia) and hypermethylation profile that is viewed as a contributing factor to both tumor aggressiveness (epithelial to mesenchymal transition) and loss of chromaffin-specific patterns of gene expression. Succinate can also exacerbate inflammation [10] and promote angiogenesis through the HIF-independent signaling pathway [11].
Clinical Phenotypes
Fig. 1 highlights typical clinical characteristics of each syndrome, taking into account that there are some overlaps [12]. Within a European-American registry of patients with PPGLs, the prevalence of underlying SDHx mutations was 10 % among 371 patients with apparently sporadic PPGLs (6 % SDHB, 4 % SDHD) and 28 % among 121 patients with HNPGL (7 % SDHB, 4 % SDHC, and 17 % SDHD) [13].
Fig. 1.
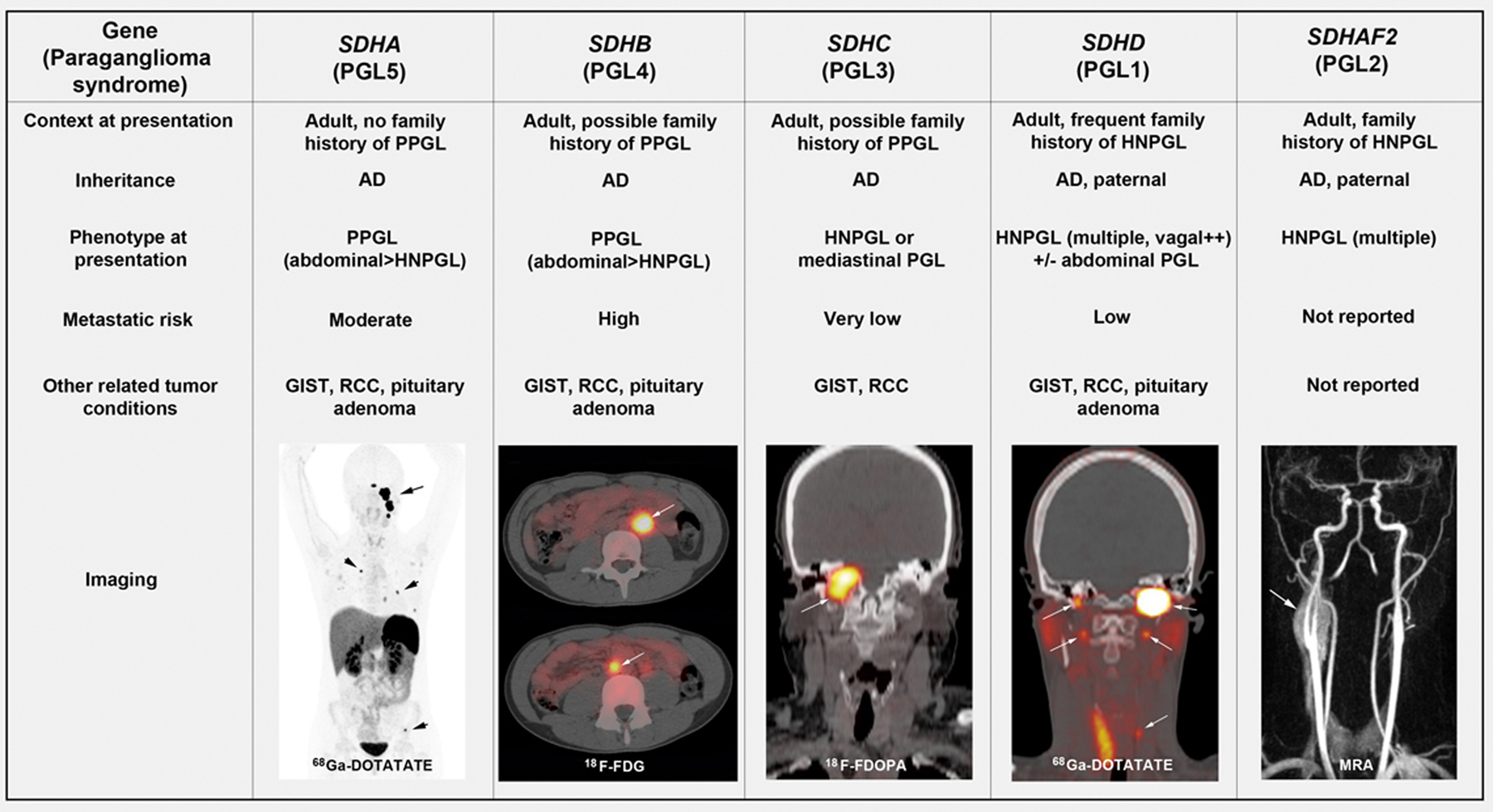
Clinical characteristics of PGL syndromes with typical findings on PET/CT at initial presentation. AD: Autosomal dominant; MRA: Magnetic Resonance Angiography. High ~ 30 %, Moderate ~10 %, Low ~ 5 %, very low ~ 0 %.
Beyond PPGLs, these mutations can also predispose a patient to renal cell carcinoma, gastrointestinal stromal tumors, pituitary adenomas, and rarely neuroblastomas and carcinoids. Carney’s triad is usually a sporadic association of at least 2 of the 5 following lesions: stomach (gastric GIST), lungs (pulmonary chondroma), paraganglionic system (extra-adrenal PGL), adrenal cortex (adenoma), and esophagus (leiomyoma).
Nuclear Imaging Phenotypes of SDHx-PPGL
SDHx-related PPGLs exhibit a highly elevated 18F-FDG uptake compared to their sporadic or other hereditary counterparts. The connecting hub between this imaging phenotype and genotype is not completely elucidated but there are two main hypotheses, both related to the effect of succinate [14]. The first one is related to increased glucose uptake and/or glycolysis of tumor cells. The second one is related to a stromal cell-succinate interaction. In SDHx deficient cells, succinate accumulates and inhibits VHL-mediated prolyl hydroxylation of HIF by competitively inhibiting HIF prolyl hydroxylases (PHDs). HIF accumulates and increases the transcription of VEGF and transporters/enzymes involved in glucose metabolism. Although this hypothesis is attractive, it does not fully explain all situations and does not match with all experimental data. More recently, a second hypothesis involving the stroma has emerged. Succinate can migrate through the mitochondrial and plasma membrane via different transport systems and act as an extracellular ligand. It has been shown that intratumoral injection of succinate could increase 18F-FDG uptake and this effect was related to an effect on endothelial cells [15]. Taking into account that PPGL are highly vascularized tumors, stromal cell-succinate interaction could be a major determinant of 18F-FDG phenotype.
SDHx-PPGLs are also characterized by a loss a differentiated features that probably contribute to a lower 18F-FDOPA uptake pattern. Interestingly, there have been false negative results on
18F-FDOPA, mainly in abdominal PPGLs (chromaffin-derived tumors) [16]. Since a decrease in expression of LAT transporters are rarely observed in negative PPGLs, it was hypothesized that this phenotype could be related to a depletion of intracellular amino acids that are required for 18F-FDOPA entry in an obligatory exchange system [17].
More recently, unlike PPGLs related to somatic EPAS1 mutations [18], SDHx-PPGLs overexpress somatostatin receptors (SSTRs) and are therefore targetable with somatostatin analogs (SSAs) labeled with diagnostic radionuclides [19]. This phenotype is currently largely unexplained.
Role of Imaging in The Management of SDHx-PPGLs
Diagnosis of PPGL
The diagnosis of PPGL relies on the presence of elevated plasma or urinary metanephrines. A dopaminergic phenotype is characterized by significant elevation of either dopamine or its metabolite methoxytyramine (MTY), or both. Usually, dopamine or MTY elevation is associated with an increase in norepinephrine or normetanephrine, which is commonly seen in patients with SDHx mutations.
In the presence of a non-secreting tumor mass (normal plasma free metanephrines and MTY), the diagnosis of PPGL can be made by the following criteria: typical anatomical location, imaging features on CT and/or MRI, and uptake using specific radiopharmaceuticals.
On contrast-enhanced CT, PPGL demonstrates avid enhancement with frequent cystic, necrotic, or degenerated regions within the lesion. On MR, they typically demonstrate avid contrast enhancement. The classic imaging appearance of a PHEO is “light-bulb” bright on T2-weighted imaging, which is seen in two-third of cases.
Identification of tumor multiplicity and/or metastases
For detecting multifocality or metastases, functional imaging is superior to anatomical imaging. This is due to the high signal-to-noise ratio obtained with PET radiopharmaceuticals which guarantee a high lesion detectability with a high interobserver agreement. Recent studies have shown that SDHx-PPGL are better visualized by 68Ga-DOTA-SSAs than 18F-FDOPA PET/CT or even 18F-FDG PET/CT, especially those located in head and neck or metastatic ones [19–21]. 18F-FDA PET/CT appeared very specific but lacks sensitivity in the setting of SDHx-PPGL, even those associated with the sympathetic nervous system. Furthermore, it is only available in very few centers worldwide. In absence of 68Ga-DOTA-SSAs, the combination of 18F-FDOPA and 18F-FDG PET/CT is the second choice to fully delineate the disease. For HNPGLs, 4D gadolinium MR angiography were also found to be particularly sensitive for detecting small PGL. Three-dimensional (3D) time-of-flight sequences are also very sensitive but much more time consuming with a limited field of exploration and therefore could be centered over uptake foci on PET imaging [22–26]. For the detection of retroperitoneal PPGLs, contrasted enhanced CT is more sensitive than whole-body diffusion-weighted MRI.
Assessment of locoregional extension
Anatomic imaging is superior to functional imaging in the locoregional staging of PPGL. For HNPGL, CT and MR provide complementary information. CT enables better evaluation of the temporal bone extension of jugular PGL (JP), tympanic PGL (TP), and vagal PGL (VP) extending to the skull base. MRI provides better soft-tissue contrast than CT and thus offers unique information for tumor delineation. MRI is also preferred for radiotherapy treatment planning. Several classifications (i. e., Fisch and Mattox’s or Glasscock and Jackson’s for TP and JP, Netterville’s for VP, and Shamblin’s for CBP) help predict surgical outcome and should be used in the evaluation of these patients. Fusion images between 3D-volumetric interpolated breath-hold examination, fat-saturated T1-weighted, and 4D-MR angiography are particularly informative to delineating tumor spread. For retroperitoneal PGL, contrast-enhanced CT also provides excellent staging specificity [26, 27].
Identification of non-paraganglionic tumors
As previously detailed, SDH mutations predispose to other tumor types with some malignancies, such as RCC and GIST, that may significantly impact the prognosis of the disease. SDH deficient-GIST occur in the setting of Carney-Stratakis syndrome (PGL-GIST dyad caused in most cases by germline SDHx mutations) or Carney triad. In both cases, GIST commonly occur in young adult (mostly in female for Carney triad), and are almost always confined to the stomach and multifocal. The screening of non-paraganglionic tumors relies on endoscopy and anatomical imaging.
Diagnosis of recurrences
Identification of tumor remnants or recurrences can be difficult after treatment, mainly due to postoperative or radiation-induced morphological changes (e. g., fibrosis, edema, necrosis, or presence of surgical material). Functional imaging using specific radiopharmaceuticals is only slightly influenced by the post-treatment sequelae and therefore, enables accurate diagnosis of tumor recurrences that could be missed by anatomical imaging [28].
Screening of SDHx-mutation carriers
The optimal follow-up algorithm has not yet been validated in SDHx-PPGL but most likely requires a more frequent and complete imaging work-up than for their sporadic counterparts. The aim is to detect tumors at early stages of development, thereby minimizing tumor extension, facilitating curative treatment, and potentially reducing the occurrence of metastases, especially in more aggressive genotypes. The reduction of radiation exposure as a dogma from CT and/or radiopharmaceuticals can be counterproductive [29]. In our opinion, at initial staging, the use of 68Ga-DOTA-SSAs should provide an adequate sensitivity and specificity at whole body scale with limited radiation exposure and very low, if any, risks (2–2.5 mSv for radiopharmaceutical, 1–3 mSv for CT). In absence of PPGL, follow-up should include annual biochemical screening, and MRI at regular time intervals [30].
Future Directions
The future of precision medicine in PPGLs will depend on the identification of new targets for imaging and therapy, which is heavily dependent on a better understanding of the disease and its relation with stroma and immunity. The identification of succinate as a major player in tumorigenesis and imaging phenotype has opened new perspectives for diagnosis and treatment monitoring of PPGL via PET/MR spectroscopy [31, 32]. Excellent results obtained with 68Ga-SSAs have also considerably simplified the imaging approach to the PPGL patient and led to the implementation of Peptide Receptor Radionuclide Therapy (PRRT) with SSAs labeled with therapeutic radionuclides. PRRT could also be viewed as an efficient synergistic combination to immunotherapy via dose delivery and subsequent induction of de novo antitumor immune responses.
Funding
This work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health in Bethesda, Maryland.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- [1].Unsicker K, Huber K, Schober A, Kalcheim C. Resolved and open issues in chromaffin cell development. Mech Dev 2013; 130: 324–329 [DOI] [PubMed] [Google Scholar]
- [2].Crona J, Taieb D, Pacak K. New perspectives on pheochromocytoma and paraganglioma: Towards a molecular classification. Endocr Rev 2017; 38: 489–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fishbein L, Leshchiner I, Walter V, Danilova L, Robertson AG, Johnson AR, Lichtenberg TM, Murray BA, Ghayee HK, Else T, Ling S, Jefferys SR, de Cubas AA, Wenz B, Korpershoek E, Amelio AL, Makowski L, Rathmell WK, Gimenez-Roqueplo AP, Giordano TJ, Asa SL, Tischler AS, Cancer Genome Atlas Research N.Pacak K, Nathanson KL, Wilkerson MD. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell 2017; 31: 181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW 3rd, Cornelisse CJ, Devilee P, Devlin B. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000; 287: 848–851 [DOI] [PubMed] [Google Scholar]
- [5].Niemann S, Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet 2000; 26: 268–270 [DOI] [PubMed] [Google Scholar]
- [6].Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, Skoldberg F, Husebye ES, Eng C, Maher ER. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet 2001; 69: 49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burnichon N, Briere JJ, Libe R, Vescovo L, Riviere J, Tissier F, Jouanno E, Jeunemaitre X, Benit P, Tzagoloff A, Rustin P, Bertherat J, Favier J, Gimenez-Roqueplo AP. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet 2010; 19: 3011–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, Kunst H, Devilee P, Cremers CW, Schiffman JD, Bentz BG, Gygi SP, Winge DR, Kremer H, Rutter J. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science 2009; 325: 1139–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Haller F, Moskalev EA, Faucz FR, Barthelmess S, Wiemann S, Bieg M, Assie G, Bertherat J, Schaefer IM, Otto C, Rattenberry E, Maher ER, Strobel P, Werner M, Carney JA, Hartmann A, Stratakis CA, Agaimy A. Aberrant DNA hypermethylation of SDHC: A novel mechanism of tumor development in Carney triad. Endocr Relat Cancer 2014; 21: 567–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGet-trick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 2013; 496: 238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mu X, Zhao T, Xu C, Shi W, Geng B, Shen J, Zhang C, Pan J, Yang J, Hu S, Lv Y, Wen H, You Q. Oncometabolite succinate promotes angiogenesis by upregulating VEGF expression through GPR91-mediated STAT3 and ERK activation. Oncotarget 2017; 8: 13174–13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Benn DE, Robinson BG, Clifton-Bligh RJ. 15 YEARS OF PARAGANGLIOMA: Clinical manifestations of paraganglioma syndromes types 1–5. Endocr Relat Cancer 2015; 22: T91–T103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schiavi F, Boedeker CC, Bausch B, Peczkowska M, Gomez CF, Strassburg T, Pawlu C, Buchta M, Salzmann M, Hoffmann MM, Berlis A, Brink I, Cybulla M, Muresan M, Walter MA, Forrer F, Valimaki M, Kawecki A, Szutkowski Z, Schipper J, Walz MK, Pigny P, Bauters C, Willet-Brozick JE, Baysal BE, Januszewicz A, Eng C, Opocher G, Neumann HP. European-American Paraganglioma Study G. Predictors and prevalence of paraganglioma syndrome associated with mutations of the SDHC gene. JAMA 2005; 294: 2057–2063 [DOI] [PubMed] [Google Scholar]
- [14].Taieb D, Pacak K. New insights into the nuclear imaging phenotypes of cluster 1 pheochromocytoma and paraganglioma. Trends Endocrinol Metab 2017; 28: 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Garrigue P, Bodin-Hullin A, Balasse L, Fernandez S, Essamet W, Dignat-George F, Pacak K, Guillet B, Taieb D. The evolving role of succinate in tumor metabolism: An (18)F-FDG-based study. J Nucl Med 2017; 58: 1749–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gabriel S, Blanchet EM, Sebag F, Chen CC, Fakhry N, Deveze A, Barlier A, Morange I, Pacak K, Taieb D. Functional characterization of nonmetastatic paraganglioma and pheochromocytoma by (18) F-FDOPA PET: Focus on missed lesions. Clin Endocrinol 2013; 79: 170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Feral CC, Tissot FS, Tosello L, Fakhry N, Sebag F, Pacak K, Taieb D. 18 F-fluorodihydroxyphenylalanine PET/CT in pheochromocytoma and paraganglioma: Relation to genotype and amino acid transport system L. Eur J Nucl Med Mol Imaging 2017; 44: 812–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Darr R, Nambuba J, Del Rivero J, Janssen I, Merino M, Todorovic M, Balint B, Jochmanova I, Prchal JT, Lechan RM, Tischler AS, Popovic V, Miljic D, Adams KT, Prall FR, Ling A, Golomb MR, Ferguson M, Nilubol N, Chen CC, Chew E, Taieb D, Stratakis CA, Fojo T, Yang C, Kebebew E, Zhuang Z, Pacak K. Novel insights into the polycythemia-paraganglioma-somatostatinoma syndrome. Endocr Relat Cancer 2016; 23: 899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Janssen I, Blanchet EM, Adams K, Chen CC, Millo CM, Herscovitch P, Taieb D, Kebebew E, Lehnert H, Fojo AT, Pacak K. Superiority of [68Ga]-DOTATATE PET/CT to Other functional imaging modalities in the localization of SDHB-associated metastatic pheochromocytoma and paraganglioma. Clin Cancer Res 2015; 21: 3888–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Archier A, Varoquaux A, Garrigue P, Montava M, Guerin C, Gabriel S, Beschmout E, Morange I, Fakhry N, Castinetti F, Sebag F, Barlier A, Loundou A, Guillet B, Pacak K, Taieb D. Prospective comparison of (68) Ga-DOTATATE and (18)F-FDOPA PET/CT in patients with various pheochromocytomas and paragangliomas with emphasis on sporadic cases. Eur J Nucl Med Mol Imaging 2016; 43: 1248–1257 [DOI] [PubMed] [Google Scholar]
- [21].Kroiss A, Putzer D, Frech A, Decristoforo C, Uprimny C, Gasser RW, Shulkin BL, Url C, Widmann G, Prommegger R, Sprinzl GM, Fraedrich G, Virgolini IJ. A retrospective comparison between 68Ga-DOTA-TOC PET/CT and 18 F-DOPA PET/CT in patients with extra-adrenal paraganglioma. Eur J Nuc Med Mol Imaging 2013; 40: 1800–1808 [DOI] [PubMed] [Google Scholar]
- [22].van den Berg R, Schepers A, de Bruine FT, Liauw L, Mertens BJ, van der Mey AG, van Buchem MA. The value of MR angiography techniques in the detection of head and neck paragangliomas. Eur J Radiol 2004; 52: 240–245 [DOI] [PubMed] [Google Scholar]
- [23].van den Berg R, Wasser MN, van Gils AP, van der Mey AG, Hermans J, van Buchem MA. Vascularization of head and neck paragangliomas: Comparison of three MR angiographic techniques with digital subtraction angiography. AJNR Am J Neuroradiol 2000; 21: 162–170 [PMC free article] [PubMed] [Google Scholar]
- [24].Ferre JC, Brunet JF, Carsin-Nicol B, Larralde A, Godey B, Gauvrit JY. Optimized time-resolved 3D contrast-enhanced MRA at 3 T: Appreciating the feasibility of assessing cervical paragangliomas. J Neuroradiol 2010; 37: 104–108 [DOI] [PubMed] [Google Scholar]
- [25].Neves F, Huwart L, Jourdan G, Reizine D, Herman P, Vicaut E, Guichard JP. Head and neck paragangliomas: Value of contrast-enhanced 3D MR angiography. AJNR Am J Neuroradiol 2008; 29: 883–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Taieb D, Varoquaux A, Chen CC, Pacak K. Current and future trends in the anatomical and functional imaging of head and neck paragangliomas. Semin Nucl Med 2013; 43: 462–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Taieb D, Kaliski A, Boedeker CC, Martucci V, Fojo T, Adler JR Jr., Pacak K. Current approaches and recent developments in the management of head and neck paragangliomas. Endocr Rev 2014; 35: 795–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Heimburger C, Veillon F, Taieb D, Goichot B, Riehm S, Petit-Thomas J, Averous G, Cavalcanti M, Hubele F, Chabrier G, Namer IJ, Charpiot A, Imperiale A. Head-to-head comparison between 18 F-FDOPA PET/CT and MR/CT angiography in clinically recurrent head and neck paragangliomas. Eur J Nucl Med Mol Imaging 2017; 44: 979–987 [DOI] [PubMed] [Google Scholar]
- [29].Siegel JA, Sacks B, Pennington CW, Welsh JS. Dose Optimization to Minimize Radiation Risk for Children Undergoing CT and Nuclear Medicine Imaging Is Misguided and Detrimental. J Nucl Med 2017; 58: 865–868 [DOI] [PubMed] [Google Scholar]
- [30].Daniel E, Jones R, Bull M, Newell-Price J. Rapid-sequence MRI for long-term surveillance for paraganglioma and phaeochromocytoma in patients with succinate dehydrogenase mutations. Eur J Endocrinol 2016; 175: 561–570 [DOI] [PubMed] [Google Scholar]
- [31].Lussey-Lepoutre C, Bellucci A, Morin A, Buffet A, Amar L, Janin M, Ottolenghi C, Zinzindohoue F, Autret G, Burnichon N, Robidel E, Banting B, Fontaine S, Cuenod CA, Benit P, Rustin P, Halimi P, Fournier L, Gimenez-Roqueplo AP, Favier J, Tavitian B. In vivo detection of succinate by magnetic resonance spectroscopy as a hallmark of SDHx mutations in paraganglioma. Clin Cancer Res 2016; 22: 1120–1129 [DOI] [PubMed] [Google Scholar]
- [32].Varoquaux A, le Fur Y, Imperiale A, Reyre A, Montava M, Fakhry N, Namer IJ, Moulin G, Pacak K, Guye M, Taieb D. Magnetic resonance spectroscopy of paragangliomas: New insights into in vivo metabolomics. Endocr Relat Cancer 2015; 22: M1–M8 [DOI] [PMC free article] [PubMed] [Google Scholar]


