Abstract
TRPC channels are Ca2+-permeable nonselective cation channels activated downstream from phospholipase C (PLC). Although most TRPC channels can be activated by stimulating Gq/11-coupled receptors, TRPC4 requires simultaneous stimulation of Gi/o-coupled receptors, making it a perfect detector of coincident Gi/o and Gq/11 signaling. Evidence shows that activated Gαi/o proteins work together with PLCδ1 to induce robust TRPC4 activation and the process is accelerated by stimulation of other PLC isozymes, such as PLCβ through Gq/11 proteins. Mechanistically, Gq/11-PLCβ activation produces triggering proton and calcium signals to initiate self-propagating PLCδ1 activity, crucial for Gi/o-mediated TRPC4 function. Thus, TRPC4-containing channels are activated under conditions not only when coincident Gi/o and Gq/11 stimulation occurs, but also when Gi/o stimulation coincides with proton and Ca2+ signals. The resulting cytosolic Ca2+ rise and membrane depolarization switch the inhibitory Gi/o response to excitation. The conditions and implications of Gi/o-mediated TRPC4 activation in physiology and pathophysiology warrant further investigation.
Keywords: heterotrimeric G proteins, GPCR, phospholipase C, TRP channels, acidosis, calcium signaling
1. Introduction
TRPC4 is a member of the canonical subfamily of Transient Receptor Potential (TRP) channels. TRP superfamily contains 28 mammalian members of related proteins that share homology with a Drosophila protein named as TRP due to the transient receptor potential phenotype found in the loss-of-function mutations that affected phototransduction of fruit flies 1,2. All TRPs function as cation channels with variable selectivities and Ca2+ permeabilities and thus affecting membrane potential and Ca2+ signaling in different ways to contribute to physiology and pathophysiology.
Structurally, they share a common tetrameric assembly with each subunit containing 6 transmembrane (TM) segments (S1-S6) and the intervening pore loop between the S5 and S6 segments, as well as the cytoplasmically localized amino-termius and carboxyl-terminus (Fig. 1A). The S5-pore-S6 domains from the four subunits form the ion conducting pore, encircled by the S1-S4 TM regulatory domains. This architecture resembles that of classical voltage-gated K+, Na+, and Ca2+ channels, to which TRP channels display identical membrane topology and some sequence homology 3, except for the more extended lengths and functional motifs found in the cytoplasmic termini of TRP channels. Based on sequence homology, the mammalian TRPs are divided into six subfamilies, TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPP (polycystin), and TRPML (mucolipin), among which the seven TRPC members are closest to the original Drosophila TRP protein, sharing 30-35% sequence identity at the protein level 2.
Figure 1. Structural organization of TRPC4 channel.
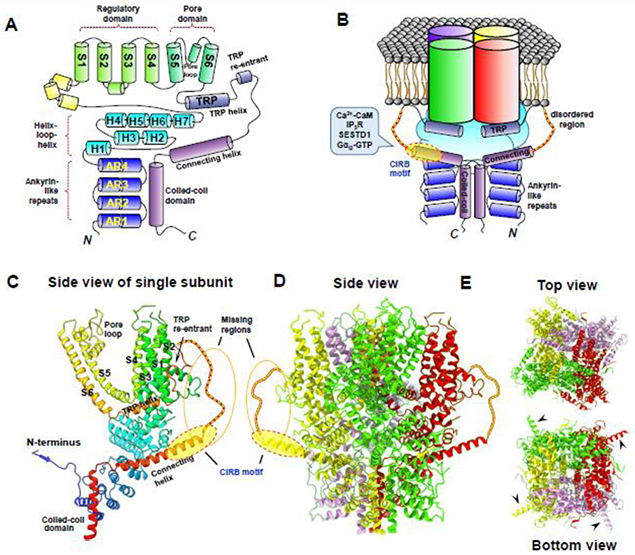
A. Diagram of main structural features of TRPC4 protein single subunit. Cylinders highlight α-helices in the transmembrane domain (S1-S6), amino (N) and carboxyl (C) terminal domains. AR1-AR4 indicate ankyrin-like repeats and H1-H7 indicate helix-loop-helix domains in the N-terminus. TRP helix or TRP domain (TRP) is present in most TRP channels. The regulatory domain formed by S1-S4 and pore domain formed by S5-pore loop-S6 are common for all TRP channels and most voltage-gated cation channels 3. B. Assembly of the TRPC4 channel with four subunits. For the cytoplasmic area, only two subunits are shown. Note the crossover of the C-terminal coiled-coil domain downstream from the connecting helix. The disordered region between TRP re-entrant (labeled in A) and the connecting helix is highlighted by the red chain. The calmodulin and IP3 receptor binding (CIRB) motif that encompasses parts of the connecting helix and the disordered region is indicated by the oval shade. The CIRB motif has been implicated to be critical for TRPC4 interaction with and/or regulation by calcium calmodulin (Ca2+-CaM), IP3 receptors (IP3R), SEC14 domain and spectrin repeat-containing protein 1 (SESTD1), and Gαi2-GTP. C-E. Ribbon diagrams of closed mouse TRPC4 channel structure based on single particle cryogenic electron microscopy 32, with side views for a single subunit (C) and all four protomers (D), as well as the top and bottom views of the whole channel (E). Adapted from ref. 28 with modifications. Key structural regions are labeled in (C). CIRB motif and the disordered region (missing in the resolved structures 32,33) are indicated as yellow ovals and red chains in (C) and (D). Arrowheads in (E) indicate that the CIRB motif is largely exposed in the periphery of the cytoplasmic area of the channel.
TRPCs form Ca2+-permeable nonselective cation channels activated downstream of phospholipase C (PLC) signaling 4,5. Of the many mechanisms that activate PLC, the Gq/11-PLCβ and receptor tyrosine kinase (RTK)-PLCγ pathways are the most commonly known, and both have been shown to induce robust TRPC channel activation, resulting in membrane depolarization and cytosolic Ca2+ concentration ([Ca2+]c) elevation. Therefore, TRPC channels have been referred to as receptor-operated channels and because of their pronounced effects on [Ca2+]c, they are also frequently studied in the context of receptor-operated Ca2+ entry and/or store-operated Ca2+ entry. The latter designation implies a mechanism of activation by Ca2+ depletion from the endoplasmic reticulum (ER) store. Whether or not TRPC channels are directly activated in response to ER Ca2+ store depletion in the absence of receptor activation is still a subject of debate. Evidence exists for TRPC channels or their mediation of Ca2+ influx to respond to store depletion, perhaps through interaction with STIM1 6-8, an ER membrane protein that transmits store depletion signal to plasma membrane channels 9,10. However, the TRPC currents exhibit distinct Ca2+ selectivity, unitary conductance, and voltage dependence from the most commonly known store-operated current, namely Ca2+-release activated Ca2+ current 11. Here, we focus on receptor-operated TRPC activation, giving specific attention to channels formed by TRPC4 because of its unique requirement on pertussis toxin (PTX)-sensitive Gi/o proteins.
2. PLC dependence of TRPC channel activation
Nearly all constituents of PLC signaling have been implicated in the activation of TRPC channels5. These include diacylglycerols (DAGs), inositol 1,4,5-trisphosphate (IP3) or IP3 receptors, Ca2+, and phosphatidylinositol 4,5-bisphosphate (PIP2). DAGs are thought to act as direct ligands of TRPC channels; however, the binding site on the channel remains undefined 12, although recent evidence suggests that DAGs may gain access to TRPC3 channels through a subunit-joining fenestration behind the selectivity filter 13. Besides DAG, IP3 receptors can bind to a conserved C-terminal motif, known as Calmodulin IP3 Receptor Binding (CIRB) site (Fig. 1B), found in all TRPC proteins; whereas the binding by IP3 receptors promoted, that by Ca2+-calmodulin inhibited the channel activation 14. Moreover, Ca2+ and PIP2 exert both positive and negative effects on TRPC activities 5. The dependence on PIP2 may reflect the needs for the PIP2 hydrolysis products, e.g. DAGs and protons, to support TRPC activation 15-17, and in the case of TRPC4, it may also reflect the need for plasma membrane targeting of PLCδ1, an isozyme specifically required for the activation of TRPC4 channels 18 (see later). On the other hand, phosphoinositides, including PIP2, may exert a tonic inhibition on TRPC channels 5,16,18. The PIP2 concentrations optimal for the activation and inhibition of different TRPC channels, including both homomeric and heteromeric ones 19, and under different stimulation conditions still remain to be elucidated. Generally, TRPC channel activation is regulated by multiple factors associated with PLC activation. Additionally, there are other modulatory factors, such as nitric oxide, reactive oxygen species and thioredoxin 20-22, indicating complex mechanisms of regulation.
Most studies have relied on overexpression of TRPC proteins in heterologous cells, which enhances the chance of spontaneous channel activation likely due to high protein abundance and/or uncontrolled endogenous factors. As a result, it is not easy to distinguish modulation from true activation mechanisms. Examining TRPC4 activation through Gq/11-PLCβ signaling, Thakur et al18 discovered that while the muscarinic receptor agonist, carbachol, caused robust [Ca2+]c rise in parental HEK293 cells, the same treatment only elicited very weak, if any, TRPC4 current, unless a Gq/11-coupled muscarinic receptor, M1, M3, or M5, was also overexpressed in these cells. By contrast, there was no difficulty in inducing TRPC3, C5, C6, and C7 currents via carbachol stimulation of the endogenously expressed muscarinic receptors in the HEK293 cells 18,23,24. Since overexpressed receptors can promiscuously couple to multiple G protein subtypes 25, PTX was tested and shown to be able to abolish the carbachol-evoked TRPC4 currents no matter if M1, M3, or M5 receptors were overexpressed. Therefore, unlike other TRPCs, the receptor-operated activation of TRPC4 shows an absolute dependence on PTX-sensitive Gi/o proteins. This is in line with the findings that stimulating Gi/o-coupled receptors, such as M2 and M4 muscarinic receptors, μ opioid receptors (μOR), and receptors for sphingosine-1-phosphate (S1P) or oxidized phospholipids, also activate TRPC4 or TRPC5 channels 18,26-29. Notably, although TRPC5 is closely related to and shares many common features and regulatory mechanisms with TRPC4, PTX only inhibited ~50% of TRPC5 currents elicited by either endogenous or overexpressed M3 receptors, indicative of partial dependence on Gi/o signaling 18.
3. Gi/o-mediated TRPC4 activation and its dependence on PLCδ1
Receptor-mediated activation of Gi/o proteins produces two transducers, the activated Gαi/o-GTP and free Gβγ dimer, between which the latter could directly activate certain PLCβ subtypes 30 and thereby induce TRPC activation similarly as Gq/11. However, this mechanism does not seem to work in the HEK293 cells used, as stimulating the heterologously expressed μOR barely elicited any Ca2+ transients; nor it evoked detectable responses of the co-expressed TRPC3, C6, or C7 18. Thus, at least in HEK293 cells, Gi/o stimulation is not efficiently coupled to PLC signaling. On the other hand, in experiments where different G protein subunits were coexpressed with TRPC4 or TRPC5, the constitutively active Gαi2, Gαi3, and Gαo, but not Gαi1 or various combinations of Gβγ, were found to support TRPC4/C5 activation in the absence of any stimulus 31. Also, the activated Gαi2 physically interacts with TRPC4 carboxyl terminus and the CIRB motif of TRPC4 is important for Gαi2-mediated channel activation 31.
Interestingly, the CIRB motif is located in an exposed region at the end of a long α-helix that extends all the way to the center core of the cytoplasmic assembly in recently reported high resolution TRPC4 cryogenic electron microscopy structures 32,33. A long stretch of unresolved structure of ~40 residues, including a short amino-terminal portion (~6 residues) of the CIRB motif, exists upstream from CIRB, outlining the widest area of the channel complex (Fig. 1B-E). This suggests flexibility of the pre-CIRB region for binding to different protein partners, whereby variable conformations may be adopted for specific functional outcomes by pulling or pushing the upstream TRP re-entrant loop connected to the TRP helix (Fig. 1A-C). The long α-helix initiated from the CIRB motif is also perfectly situated to transmit the protein-protein interaction signals allosterically to channel gating through its central access and perhaps interactions with TRP helix, S4-S5 linker, and/or the C-terminal end of the S6 TM segment (Fig. 1A-C).
Although the stimulation of Gi/o-coupled μOR did not cause obvious PLC activation in the absence of TRPC4, μOR-mediated TRPC4 activation was strongly dependent on PLC, as it was completely prevented by the PLC inhibitor, U73122. Through a number of complementary approaches, RNA interference, dominant negative suppression and inhibition by RhoA, it was determined that a specific PLC isozyme, PLCδ1, underlies this absolute dependence on PLC 18. PLCδ1 is likely the simplest and most ancient form of all thirteen mammalian PLC isoforms known to date 34. Unlike other PLC isoforms that have developed sensing mechanisms for various extra- and intracellular signals, it is only known to be activated by Ca2+, with PIP2 also required as both the substrate and membrane anchor. Therefore, PLCδ1 may partially explain the Ca2+ and PIP2 dependence of TRPC4 activation. Consistent with the functional coupling, the levels of both TRPC4 and PLCδ1 were increased in surgical specimens of epilepsy patients with either cortical lesions of focal cortical dysplasia II or tuberous sclerosis complex as compared to controls 35.
Notably, although PLCβs are not absolutely required for Gi/o-mediated TRPC4 activation, costimulating a Gq/11-coupled receptor or an RTK (e.g. EGF receptors) facilitated TRPC4 current development. Because of the Ca2+ dependence of PLCδ1, it was thought that Gq/11-PLCβ or RTK-PLCγ signaling probably provided the initial [Ca2+]c elevation that was propagated to PLCδ1, which then further amplified the signal to induce robust channel activation. However, substituting the receptor agonist with the Ca2+ ionophore, ionomycin, only increased the probability but not the rate of Gi/o-mediated TRPC4 activation 18. More recently, this missing component of Gq/11-PLCβ pathway is found to be protons 17, a by-product of PIP2 hydrolysis 16. Indeed, intracellular acidification resulting from PIP2 hydrolysis had been shown to facilitate activation of TRP and TRP-like channels in Drosophila photoreceptors 16. It was shown that intracellular protons act at PLCδ1, rather than TRPC4, to accelerate Ca2+-dependent PLCδ1 activation via a positive-feedback mechanism 17. Such a self-propagating capability of PLCδ1, supported by H+ and Ca2+ signals generated from its own action on PIP2 hydrolysis, likely also underpins its function in amplifying signaling initiated by Gq/11-PLCβ, RTK-PLCγ, and other PLC isozymes. Interestingly, for Drosophila TRP, intracellular protons also affected the channel through an indirect effect36. Thus, although Gi/o signaling is crucial for receptor-operated TRPC4 activation, it requires coincident PLCδ1 activity, which can be triggered through ligand stimulation of Gq/11-PLCβ or RTK-PLCγ, or intracellular acidification and [Ca2+]c rise (Figs. 2, 3A).
Figure 2. Mechanism of TRPC4 channel activation by coincident Gi/o and Gq/11 signaling.
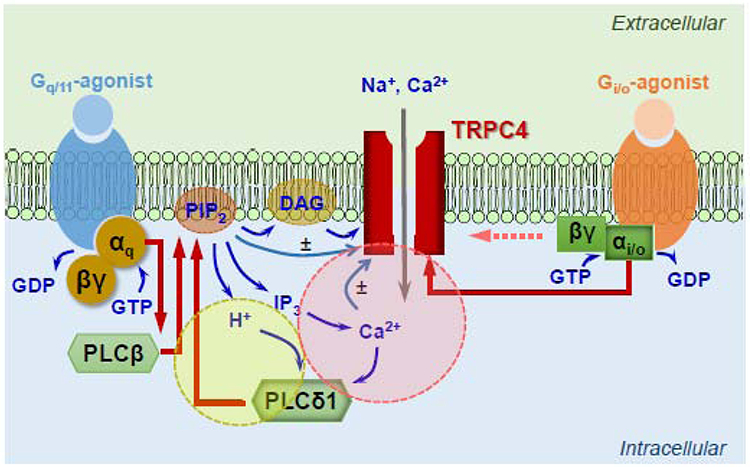
Schematic drawing of factors critical for receptor-operated TRPC4 channel activation in response to agonists that simultaneously activate both Gi/o-coupled and Gq/11-coupled receptors. The light yellow and light pink circles highlight the propagating H+ and Ca2+ signals, respectively, that support continued PLCδ1 activation via positive feedback mechanisms, which can be initiated by stimulating Gq/11-PLCβ with a Gq/11-coupled receptor agonist (Gq/11-agonist). The Gq/11-PLCβ requirement may be bypassed by intracellular acidification in combination with an increase in cytosolic Ca2+ level. The propagating PLCδ1 activation needs simultaneous Gi/o activation, most likely Gαi/o-GTP although the contribution of Gβγ cannot be completely ruled out at this point (pink dashed arrow), in order to support robust TRPC4 activation. Red arrows, protein-based regulation; blue arrows, regulation by ions and small diffusible messengers. ± indicate that the effects (of PIP2 and Ca2+) on TRPC4 activation can be both positive and negative. Abbreviations are given in the text.
Figure 3. Physiological and pathophysiological functions mediated by TRPC4 in response to receptor-operated activation of Gq/11 and/or Gi/o proteins. A & B.
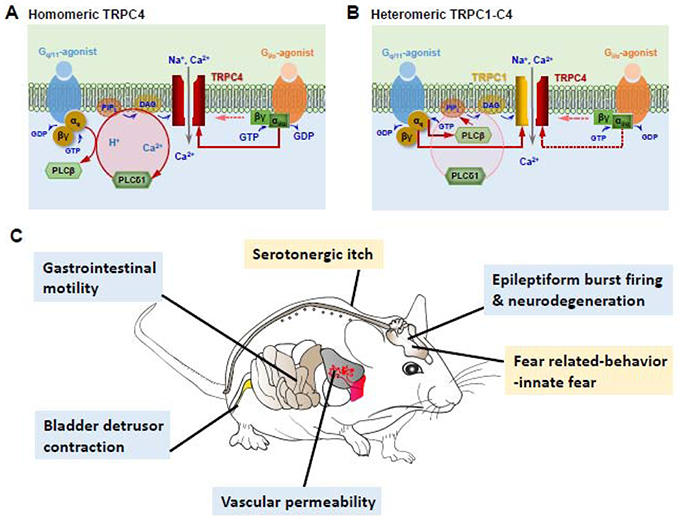
Possible differences in the mechanisms of activation of homotetrameric TRPC4 channel (A) from TRPC1-TRPC4 heterotetrameric channels (B) in response to coincident Gq/11 and Gi/o signaling. For homomeric TRPC4, the Gq/11-PLCβ pathway mainly serves to initiate the self-propagating and H+- and Ca2+-dependent positive feedback activation of PLCδ1, and the channel opening requires coincident Gi/o input (A). For heteromeric TRPC1-C4 channels, Gαq/11-GTP might exert a direct stimulatory effect through TRPC1 48, which might obliviate the requirement for PLCδ1 and/or Gi/o (B). However, these possibilities have not been directly tested. C. Physiological and pathophysiological functions demonstrated to require TRPC4 based on studies using Trpc4 knockout mice. Among them, gastrointestinal motility and bladder detrusor contraction regulations are cholinergic and require M2 (Gi/o-coupled) and M3 (Gq/11-coupled) muscarinic receptors 39,41,42. Lung vascular permeability regulation 46 likely requires both Gq/11 and Gi/o when it is induced via thrombin receptors. The depolarizing plateau that underlies epileptiform burst firing and neurodegeneration of lateral septal neurons43, is triggered by stimulating group 1 metabotropic glutamate receptors 43,47, but the response is inhibited by pertussis toxin (unpublished). For fear related-behavior and serotonergic itch, only Gq/11 signaling has been suggested 44,45; however, no report has ruled out the involvement of Gi/o signaling at this point.
4. Outstanding questions on Gi/o-mediated TRPC4 activation
Mechanism of TRPC4 activation by Gi/o remains undefined. Experimental evidence supporting the involvement of Gαi/o but not Gβγ includes a) coexpression of constitutively active Gαi2, Gαi3, or Gαo, but not Gαi1, Gαq or Gβγ supported spontaneous TRPC4 activation 31, b) selectively inhibiting Gαi/o-GTP while enhancing free Gβγ production through GoLoco domain proteins suppressed TRPC4 activation 37, and c) intracellular pipette dialysis of antibodies for Gαo, but not that for Gβ, suppressed the development of muscarinic cation current (m/CAT) 38, an endogenous current found in gastrointestinal smooth muscle cells mediated mainly by TRPC4 39. However, unequivocal evidence ruling out the involvement of Gβγ has not been obtained. Secondly, although a direct interaction between TRPC4 carboxyl-terminus (residues 621-890) and Gαi2 has been demonstrated by glutathione S-transferase pull-down using purified proteins, other experimental support of the interaction had come from co-immunoprecipitation of cell lysates and Forster resonance energy transfer (FRET) in intact cells 31,40. It remains to be elucidated whether Gαi/o or other Gα or Gβγ also binds to TRPC4 and where exactly they bind or whether other proteins are involved in facilitating the interaction. More importantly, solving structures of TRPC4 in complex with Gi/o subunit(s) will reveal molecular details on how Gi/o proteins gate this channel.
Physiological relevance of Gi/o-mediated TRPC4 activation is another important question. The best example is m/CAT being mostly mediated by TRPC4 and responsible for neurogenic cholinergic contraction of gastrointestinal smooth muscles 39. m/CAT is dependent on both M2 and M3 muscarinic receptors 41, and is suppressed by PTX treatment42, suggesting a codependence on Gi/o and Gq/11 pathways. However, in other systems where TRPC4 function has been implicated, the studies have only considered Gq/11-PLCβ signaling (Fig. 3C)43-45. It would be important to examine the unintended involvement of Gi/o proteins in these functions. In some cases, for instance thrombin-induced endothelial permeation 46, this would be obvious as thrombin receptors are coupled to both Gq/11 and Gi/o. In other cases, the coregulation might involve a different transmitter or receptor subtype, which would require more careful examinations. Indeed, we found that PTX treatment completely eliminated the large depolarizing plateau in lateral septal neurons induced by (S)-3,5-dihydroxyphenylglycine (DHPG) (unpublished), a group 1 metabotropic glutamate receptor agonist that induces epileptiform burst firing through TRPC4-containing channels 43,47. Alternatively, the Gi/o requirement might be abrogated by including TRPC1 in the heteromeric TRPC1-C4 channels (Fig. 3A-B)43,48,49. However, since neither the native nor heterologously expressed TRPC1-C4 channels had been tested for PTX sensitivity, the involvement of Gi/o proteins cannot be completely rule out. The likelihood of TRPC4 to function as a detector of coincident Gi/o and Gq/11 signaling in various physiological settings should not be overlooked. Given that in the nervous system, the activation of Gq/11 is typically associated with excitation whereas that of Gi/o is accompanied with inhibition 50, the consequence and functional significance of Gi/o and Gq/11 costimulation, which likely happens often and with Gi/o and Gq/11 inputs having different strengths, warrant further investigation.
Although stimulation of Gi/o-coupled receptors evoked TRPC4 currents in heterologous systems 18,31, no similar response has been demonstrated in native systems. Therefore, the physiological activation of TRPC4 most likely requires coincident inputs of Gi/o and Gq/11, with the latter probably substitutable by RTK or other means that triggers PLC activation. The latest finding, however, suggests that tissue acidosis, which occurs commonly under pathological conditions and lowers not only extracellular but also intracellular pH, can facilitate Gi/o-dependent TRPC4 activation through sensitizing PLCδ1 17. This could underlie the mechanism by which TRPC4 contributes to neurodegeneration under pathological conditions 43
In summary, as a nonselective cation channel codependent on Gi/o and PLCδ1 for activation, TRPC4 is perfectly suited in integrating metabotropic signaling through the Gi/o and Gq/11 pathways. The Gq/11 pathway may be substituted by stimulating other PLC isozymes or intracellular acidification to give greater versatility of this coincident sensor for signaling. Although the physiological and pathological implications remain to be fully elucidated, the detailed regulatory mechanisms revealed thus far have tremendously enriched our knowledge on receptor-operated channels and their functional significance.
Acknowledgments
Fundings
The research in the authors’ lab was supported by NIH grants R01 NS092377 and R01 NS102452.
Footnotes
Conflict of Interest statement
The authors claim no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cosens DJ & Manning A (1969). Abnormal electroretinogram from a Drosophila mutant. Nature, 224, 285–287. doi: 10.1038/224285a0 [DOI] [PubMed] [Google Scholar]
- 2.Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, & Zhu MX (2002). A unified nomenclature for the superfamily of TRP cation channels. Mol Cell, 9, 229–231. doi: 10.1016/s1097-2765(02)00448-3 [DOI] [PubMed] [Google Scholar]
- 3.Yu FH, Yarov-Yarovoy V, Gutman GA, & Catterall WA (2005) Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 57, 387–395. doi: 10.1124/pr.57.4.13 [DOI] [PubMed] [Google Scholar]
- 4.Trebak M, Vazquez G, Bird GSJ, & Putney JW Jr (2003). The TRPC3/6/7 subfamily of cation channels. Cell Calcium, 33, 451–461. doi: 10.1016/s0143-4160(03)00056-3 [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Cheng X, Tian J, Xiao Y, Tian T, Xu F, Hong X, & Zhu MX (2020) TRPC channels: Structure, function, regulation and recent advances in small molecular probes. Pharmacol Ther. 209, 107497. doi: 10.1016/j.pharmthera.2020.107497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, & Muallem S (2008) STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell, 32, 439–448. doi: 10.1016/j.molcel.2008.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asanov A, Sampieri A, Moreno C, Pacheco J, Salgado A, Sherry R, & Vaca L (2015). Combined single channel and single molecule detection identifies subunit composition of STIM1-activated transient receptor potential canonical (TRPC) channels. Cell Calcium, 57, 1–13. doi: 10.1016/j.ceca.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 8.Cheng KT, Ong HL, Liu X, & Ambudkar IS (2013). Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr Top Membr., 71, 149–179. doi: 10.1016/B978-0-12-407870-3.00007-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr., & Meyer T (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol., 15, 1235–1241. doi: 10.1016/j.cub.2005.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, & Cahalan MD (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature, 437, 902–905. doi: 10.1038/nature04147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putney JW, Steinckwich-Besançon N, Numaga-Tomita T, Davis FM, Desai PN, D'Agostin DM, Wu S, & Bird GS (2017) The functions of store-operated calcium channels. Biochim Biophys Acta Mol Cell Res, 1864, 900–906. doi: 10.1016/j.bbamcr.2016.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Q, Guo W, Zheng L, Wu JX, Liu M, Zhou X, Zhang X, & Chen L (2018) Structure of the receptor-activated human TRPC6 and TRPC3 ion channels. Cell Res, 28, 746–755. doi: 10.1038/s41422-018-0038-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenegger M, Tiapko O, Svobodova B, Stockner T, Glasnov TN, Schreibmayer W, Platzer D, de la Cruz GG, Krenn S, Schober R, Shrestha N, Schindl R, Romanin C, & Groschner K (2018) An optically controlled probe identifies lipid-gating fenestrations within the TRPC3 channel. Nat Chem Biol., 14, 396–404. doi: 10.1038/s41589-018-0015-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, & Zhu MX (2001) Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J Biol Chem., 276, 21303–21310. doi: 10.1074/jbc.M102316200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai Y, Itsuki K, Okamura Y, Inoue R, & Mori MX (2012) A self-limiting regulation of vasoconstrictor-activated TRPC3/C6/C7 channels coupled to PI(4,5)P2-diacylglycerol signalling. J Physiol., 590, 1101–1119. doi: 10.1113/jphysiol.2011.221358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, & Hardie RC (2010) Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol., 20, 189–197. doi: 10.1016/j.cub.2009 [DOI] [PubMed] [Google Scholar]
- 17.Thakur DP, Wang Q, Jeon J, Tian JB, & Zhu MX (2020) Intracellular Acidification facilitates receptor-operated TRPC4 activation through PLCδ1 in a Ca2+ dependent manner. J Physiol., 598, 2651–2667. doi: 10.1113/JP279658.**The study reveals that the accelerating effect of Gq/11-PLCβ on Gi/o-dependent TRPC4 activation is mainly due to protons generated from PIP2 hydrolysis. This local drop in intercellular pH sensitizes PLCδ1 for Ca2+. The activation of PLCδ1 in turn produces more H+ and Ca2+, creating a positive feedback loop to support self-propagating PLCδ1 activity, which not only facilitates Gi/o-mediated TRPC4 activation but also the loss of KCNQ2/3 channel function in the absence of Gi/o and TRPC4.
- 18.Thakur DP, Tian J.-b., Jeon J, Xiong J, Huang Y, Flockerzi V, & Zhu MX (2016). Critical roles of Gi/o proteins and phospholipase C-δ1 in the activation of receptor-operated TRPC4 channels. Proc Natl Acad Sci U S A., 113, 1092–1097. doi: 10.1073/pnas.1522294113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko J, Myeong J, Shin YC, & So I (2019) Differential PI(4,5)P2 Sensitivities of TRPC4, C5 Homomeric and TRPC1/4, C1/5 Heteromeric Channels. Sci Rep. 2019 February 12;9(1):1849. doi: 10.1038/s41598-018-38443-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, & Mori Y (2004) Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol., 2, 596–607. doi: 10.1038/nchembio821 [DOI] [PubMed] [Google Scholar]
- 21.Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, & Beech DJ (2008) TRPC channel activation by extracellular thioredoxin. Nature, 451, 69–72. doi: 10.1038/nature06414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa N, Kurokawa T, & Mori Y (2016) Sensing of redox status by TRP channels. Cell Calcium, 60, 115–122. doi: 10.1016/j.ceca.2016.02.009. doi: 10.1016/j.ceca.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 23.Qu C, Ding M, Zhu Y, Lu Y, Du J, Miller M, Tian J, Zhu J, Xu J, Wen M, Er-Bu A, Wang J, Xiao Y, Wu M, McManus OB, Li M, Wu J, Luo HR, Cao Z, Shen B, Wang H, Zhu MX, & Hong X (2017) Pyrazolopyrimidines as potent stimulators for transient receptor potential canonical 3/6/7 channels. J Med Chem., 60, 4680–4692. doi: 10.1021/acs.jmedchem.7b00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding M, Wang H, Qu C, Xu F, Zhu Y, Lv G, Lu Y, Zhou Q, Zhou H, Zeng X, Zhang J, Yan C, Lin J, Luo HR, Deng Z, Xiao Y, Tian J, Zhu MX, & Hong X (2018) Pyrazolo[1,5-a]pyrimidine TRPC6 antagonists for the treatment of gastric cancer. Cancer Lett., 432, 47–55. doi: 10.1016/j.canlet.2018.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuho I, Ostrovskaya O, Kramer GM, Jones CD, Xie K, & Martemyanov KA (2015) Distinct profiles of functional discrimination among G proteins determine the actions of G protein-coupled receptors. Sci Signal., 8, ra123. doi: 10.1126/scisignal.aab4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, & Beech DJ (2006). A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res., 98, 1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otsuguro K, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, Ito S, Flockerzi V, Zhu MX, & Zholos AV (2008). Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J Biol Chem., 283, 10026–10036. doi: 10.1074/jbc.M707306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Shawaf E, Naylor J, Taylor H, Riches K, Milligan CJ, O'Regan D, Porter KE, Li J, & Beech DJ (2010). Short-term stimulation of calcium-permeable transient receptor potential canonical 5-containing channels by oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol, 30, 1453–1459. doi: 10.1161/ATVBAHA.110.205666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller M, Shi J, Zhu Y, Kustov M, Tian JB, Stevens A, Wu M, Xu J, Long S, Yang P, Zholos AV, Salovich JM, Weaver CD, Hopkins CR, Lindsley CW, McManus O, Li M, & Zhu MX (2011). Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J Biol Chem., 286, 33436–33446. doi: 10.1074/jbc.M111.274167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bristol JA & Rhee SG (1994) Regulation of phospholipase C-beta isozymes by G-proteins. Trends Endocrinol Metab., 5, 402–406. doi: 10.1016/1043-2760(95)92522-k [DOI] [PubMed] [Google Scholar]
- 31.Jeon JP, Hong C, Park EJ, Jeon JH, Cho NH, Kim IG, Choe H, Muallem S, Kim HJ, & So I (2012). Selective Gαi subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels. J Biol Chem., 287, 17029–17039. doi: 10.1074/jbc.M111.326553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan J, Li J, Zeng B, Chen GL, Peng X, Zhang Y, Wang J, Clapham DE, Li Z, & Zhang J (2018) Structure of the mouse TRPC4 ion channel. Nat Commun., 9, 3102. doi: 10.1038/s41467-018-05247-9** This single particle cryogenic electron microscopy work provides the first high resolution structure of mammalian TRPC4 in the closed state. Many interesting structural features of the TRPC4 channel are revealed.
- 33.Vinayagam D, Mager T, Apelbaum A, Bothe A, Merino F, Hofnagel O, Gatsogiannis C, & Raunser S (2018) Electron cryo-microscopy structure of the canonical TRPC4 ion channel. Elife, 7, e36615. doi: 10.7554/eLife.36615** The single particle cryogenic electron microscopy structure of zebrafish TRPC4 was published about the same time as mouse TRPC4 (ref 32). This provides an independent validation of the resolved structures and some different details of certain structural features.
- 34.Gresset A, Sondek J, & Harden TK (2012) The phospholipase C isozymes and their regulation. Subcell Biochem., 58, 61–94. doi: 10.1007/978-94-007-3012-0_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang LK, Chen X, Zhang CQ, Liang C, Wei YJ, Yue J, Liu SY, & Yang H (2017) Elevated Expression of TRPC4 in Cortical Lesions of Focal Cortical Dysplasia II and Tuberous Sclerosis Complex. J Mol Neurosci. 62, 222–231. doi: 10.1007/s12031-017-0923-z* Using surgical specimens from epilepsy patients with Cortical Lesions of Focal Cortical Dysplasia II (n = 24) and Tuberous Sclerosis Complex (n = 11), the researchers found upregulated expression of both TRPC4 and PLCδ1 proteins, as compared to age-matched autopsy control samples (n = 12). The results provide clinical relavence to the findings in ref 43 that TRPC4 plays a critical role in seizure-induced neurodegeneration and in ref 18 that PLCδ1 and TRPC4 are functionally coupled.
- 36.Liu W, Wen W, Wei Z, Yu J, Ye F, Liu CH, Hardie RC, & Zhang M (2011). The INAD scaffold is a dynamic, redox-regulated modulator of signaling in the Drosophila eye. Cell, 145, 1088–1101. doi: 10.1016/j.cell.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 37.Jeon JP, Thakur DP, Tian JB, So I, & Zhu MX (2016). Regulator of G-protein signalling and GoLoco proteins suppress TRPC4 channel function via acting at Galphai/o. Biochem J., 473, 1379–1390. doi: 10.1042/BCJ20160214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan HD, Okamoto H, Unno T, Tsytsyura YD, Prestwich SA, Komori S, Zholos AV, & Bolton TB (2003) Effects of G-protein-specific antibodies and G beta gamma subunits on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. Br J Pharmacol., 139, 605–615. doi: 10.1038/sj.bjp.0705289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, & Flockerzi V (2009) Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology, 137, 1415–1424. doi: 10.1053/j.gastro.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myeong J, Kwak M, Jeon JP, Hong C, Jeon JH, & So I (2015) Close spatio-association of the transient receptor potential canonical 4 (TRPC4) channel with Gαi in TRPC4 activation process. Am J Physiol Cell Physiol., 308, C879–C889. doi: 10.1152/ajpcell.00374.2014. [DOI] [PubMed] [Google Scholar]
- 41.Zholos AV & Bolton TB (1997) Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br J Pharmacol., 122, 885–893. doi: 10.1038/sj.bjp.0701438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pucovský V, Zholos AV, & Bolton TB (1998) Muscarinic cation current and suppression of Ca2+ current in guinea pig ileal smooth muscle cells. Eur J Pharmacol., 346, 323–330. doi: 10.1016/s0014-2999(98)00059-4 [DOI] [PubMed] [Google Scholar]
- 43.Phelan KD, Mock MM, Kretz O, Shwe UT, Kozhemyakin M, Greenfield LJ, Dietrich A, Birnbaumer L, Freichel M, Flockerzi V, & Zheng F (2012) Heteromeric canonical transient receptor potential 1 and 4 channels play a critical role in epileptiform burst firing and seizure-induced neurodegeneration. Mol Pharmacol. 81, 384–392. doi: 10.1124/mol.111.075341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riccio A, Yan L, Tsvetkov E, Gapon S, Gui LY, Smith KS, Engin E, Rudolph U, Bolshakov VY, & Clapham DE (2014). Decreased anxiety-like behavior and Gαq/11-dependent responses in the amygdala of mice lacking TRPC4 channels. J Neurosci., 34, 3653–3667. doi: 10.1523/JNEUROSCI.2274-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SH, Cho PS, Tonello R, Lee HK, Jang JH, Park GY, Hwang SW, Park CK, Jung SJ, & Berta T (2018) Peripheral serotonin receptor 2B and transient receptor potential channel 4 mediate pruritus to serotonergic antidepressants in mice. J Allergy Clin Immunol. 142, 1349–1352.e16. doi: 10.1016/j.jaci.2018.05.031* This work shows that TRPC4 is uniquely involved in itch induced by serotonergic antidepressants such as sertraline through activation of Gq/11-coupled serotonin receptor, 5-HT2B, in primary afferents of dorsal root ganglion neurons. Although the response requires TRPV1 positive neurons, it does not require TRPV1, TRPA1, or TRPV4. The main pathway clearly involves 5-HT2B-Gq/11-PLCβ, but Gi/o should not be ruled out since PTX was not examined.
- 46.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, & Malik AB (2002) Impairment of store-operated Ca2+ entry in TRPC4−/− mice interferes with increase in lung microvascular permeability. Circ Res., 91, 70–76. doi: 10.1161/01.res.0000023391.40106.a8 [DOI] [PubMed] [Google Scholar]
- 47.Tian J, Thakur DP, Lu Y, Zhu Y, Freichel M, Flockerzi V, & Zhu MX (2014) Dual depolarization responses generated within the same lateral septal neurons by TRPC4-containing channels. Pflugers Arch., 466, 1301–1316. doi: 10.1007/S00424-013-1362-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Myeong J, Ko J, Kwak M, Kim J, Woo J, Ha K, Hong C, Yang D, Kim HJ, Jeon JH, & So I (2018) Dual action of the Gαq-PLCβ-PI(4,5)P2 pathway on TRPC1/4 and TRPC1/5 heterotetramers. Sci Rep., 8, 12117. doi: 10.1038/S41598-018-30625-0* This study shows that TRPC1-C4 and TRPC1-C5 heteromeric channels are both inhibited by Gq-PLCβ pathway through hydrolysis of PIP2 and activated by Gαq involving direct protein-protein interaction. The interaction was tested by FRET using Gαq-YFP and TRPC4β-CFP or CFP-TRPC5 in the presence of TRPC1. However, it was not shown whether TRPC1 was absolutely required for the Gq-mediated responses and the sensitivity to PTX was not examined.
- 49.Bröker-Lai J, Kollewe A, Schindeldecker B, Pohle J, Nguyen Chi V, Mathar I, Guzman R, Schwarz Y, Lai A, Weiβgerber P, Schwegler H, Dietrich A, Both M, Sprengel R, Draguhn A, Köhr G, Fakler B, Flockerzi V, Bruns D, & Freichel M (2017) Heteromeric channels formed by TRPC1, TRPC4 and TRPC5 define hippocampal synaptic transmission and working memory. EMBO J., 36, 2770–2789. doi: 10.15252/embj.201696369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth BL (2016) DREADDs for Neuroscientists. Neuron, 89, 683–694. doi: 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]


