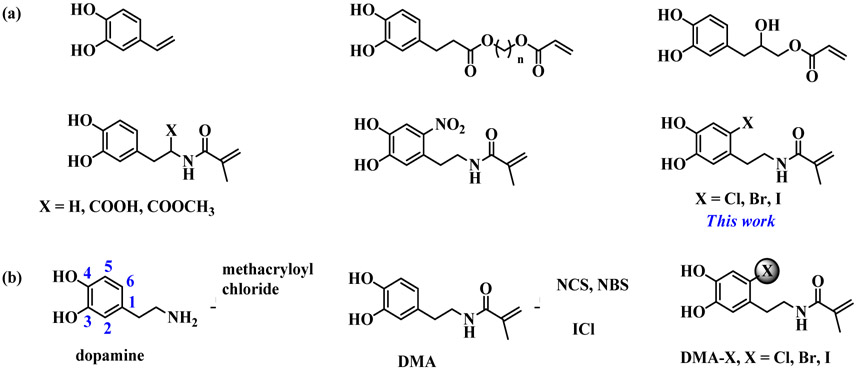
Abstract
Bacterial infection associated with multidrug resistance (MDR) bacteria is increasingly becoming a significant public health risk. Herein, we synthesized a series of halogenated dopamine methacrylamide (DMA), which contains a catechol side chain modified with either chloro-, bromo-, or iodo-functional group. Catechol is a widely used adhesive moiety for designing bioadhesives and coating. However, the intrinsic antimicrobial property of catechol has not been demonstrated before. These halogenated DMA were incorporated into hydrogels, copolymers, and coatings and exhibited more than 99% killing efficiencies against Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli. More importantly, hydrogel containing chlorinated DMA demonstrated broad-spectrum antimicrobial activities towards multiple MDR bacteria, which included methicillin resistant S. aureus (MRSA), vancomycin resistant enterococci (VRE), multi antibiotics resistant Pseudomonas aeruginosa (PAER), multi antibiotics resistant Acinetobacter baumannii (AB) and carbapenem resistant Klebsiella pneumoniae (CRKP). These hydrogels also demonstrated the ability to kill bacteria in a biofilm while exhibiting low cytotoxic. Based on molecular docking and molecular dynamics simulation, Cl-functionalized catechol can potentially inhibit bacterial fatty acid synthesis at the enoyl-acyl carrier protein reductase (FabI) step. The combination of moisture-resistant adhesive property, inherent antimicrobial property, and the versatility of incorporating halogenated DMA into different polymeric materials greatly enhanced the potential for using these monomers for designing multifunctional bioadhesives and coatings.
Keywords: antimicrobial, dopamine methacrylamide, multidrug resistant bacteria, halogenated catechol, mussel adhesive proteins
Graphical Abstract

1. Introduction
Bacterial infections have become a tremendous public health issue [1-3]. Administration of antibiotics is a common practice to control and prevent infections [4]. However, over reliance on antibiotics has led to the development of antibiotic resistance bacteria strains [5]. Multidrug resistant (MDR) bacteria are bacteria that have developed resistance to more than one antimicrobial drug and are a source of hospital-acquired infections especially in immunocompromised and critically ill patients [6]. Over 2 million people are infected with MDR bacteria every year in the United States alone [7] and at least 23,000 people die each year as a direct result of infection from MDR bacteria [8]. According to the World Health Organization (WHO), the most dangerous MDR bacteria that are in need for new antibacterial agents include Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa [9].
Recently, antimicrobial polymers and hydrogels have been developed as alternative antimicrobial agents to combat antibiotic resistant bacteria as these materials possess different antimicrobial mechanisms as those of conventional antibiotics [10-14]. There are several categories of intrinsic antimicrobial polymers, including polymers with quaternary nitrogen atoms [15, 16], guanidine containing polymers [17], polymers that mimic natural antimicrobial peptides [18], halogenated polymers [19] and metal containing polymers [20]. However, few of them exhibited broad-spectrum antimicrobial activities towards MDR bacteria [21, 22]. Another approach is utilizing hydrogels to encapsulate and release antibacterial agents. However, these materials are limited by the rapid depletion of the incorporated antibacterial agents, resulting in short-lasting antimicrobial efficacy when compared to antimicrobial agents that are covalently tethered [23, 24].
Marine mussels secrete catechol-containing adhesive proteins that enable these organisms to adhere to wet surfaces in a turbulent intertidal zone [25-29]. Catechol can participate in reversible interactions such as hydrogen bonding [30], π-π [31] or cation-π interactions [32], complexation with metal ions [33] or boronic acid [34], and oxidation induced crosslinking with nucleophiles [35]. Due to the diverse nature of the catechol chemistry, catechol functionalized polymers are widely used as bioadhesives, antifouling coatings, drug delivery vehicles and even adhesive for batteries [36, 37]. Although there are reports of catechol-containing antimicrobial coatings, catechol served mainly as the adhesive moiety for attaching cationic antimicrobial polymers to a surface [38, 39] or for immobilizing cytotoxic silver ions or nanoparticles [40, 41]. As a departure from these previous usage of catechol, antimicrobial properties of catechol have been recently reported. Our group demonstrated that hydrogen peroxide (H2O2) generated from catechol autoxidation exhibited antimicrobial activities against Staphylococcus epidermidis and Escherichia coli [42, 43]. del Campo and co-workers [44] incorporated chloro-modified dopamine (Cl-dopamine) into polyethylene glycol- (PEG-) based hydrogels and coatings, which reduced fouling of E. coli and biofilm formation. However, these coatings exhibited minimal antimicrobial activities, potentially due to low Cl-dopamine content. Most importantly, these prior works did not demonstrate antimicrobial properties against MDR bacteria.
Triclosan, a polychlorinated aromatic compound, is a synthetic antimicrobial agent widely used in personal care products. Triclosan inhibits the enoyl-acyl carrier protein reductase (FabI), a key enzyme in the biosynthesis of fatty acids, causing bacterial cell death through disintegration of the cellular membrane and the release of intracellular constituents [45, 46]. Most reported approaches relied on the loading and the release of triclosan from a polymeric material [47]. However, the unbound triclosan can be easily released and lead to limited antimicrobial effect. Due to the structural similarity between Cl-catechol and triclosan, we hypothesize that Cl-catechol also possesses intrinsic antimicrobial properties. Because of electron withdrawing effects of chloride, chlorination of catechols lowers the pKa of the phenolic -OH groups and their redox potential, thus, making oxidation to quinone more difficult [48]. Additionally, catechol modified with an electron withdrawing group significantly increases the reactivity and interfacial binding strength of catechol [49-51]. Bromide- and iodide- functionalization will potentially result in similar electron withdrawing effect as Cl-functionalization. However, the intrinsic antimicrobial property of these halogenated catechols have not been previously studied. Due to the unique moisture-resistant adhesive property of catechol, halogenated catechol may be useful in imparting antimicrobial property into designing new adhesive biomaterials with inherent antimicrobial properties. Here we seek to develop a new series of monomers containing halogenated catechol, so that it can be incorporated into different types of antimicrobial polymeric materials.
Various catechol-functionalized vinyl, acrylate, or methacrylate groups have been reported to-date (Scheme 1a) [52-54]. This library of monomers presents an attractive and facile method for creating catechol-containing polymers with varying architectures, compositions, and properties suitable for a wide range of applications [36]. Recently, 6-substituted nitrodopamine methacrylamide (DMA-NO2) was reported and polymerized into hydrogels [55]. However, DMA-NO2 is not stable and decomposes when irradiated with ultraviolet light [56]. To create antimicrobial catechol-based polymer, we reported the synthesis of halogenated DMA, which was 6-substituted with chloride, bromide, and iodide. To demonstrate the versatility of applying these novel monomers, they were copolymerized to form antimicrobial hydrogels, linear polymers, and coatings. The antimicrobial and antibiofilm properties of these polymeric materials against Staphylococcus aureus and E. coli. as well as five strains of MDR bacteria were evaluated. The antimicrobial mechanism of Cl-catechol was investigated by molecular docking and molecular dynamics simulation.
Scheme 1.
(a) Chemical structures of polymerizable catechol-containing monomers. (b) Synthesis of DMA, DMA-Cl, DMA-Br and DMA-I through the modification of DMA with NCS, NBS, and ICI, respectively.
2. Materials and methods
2.1. Materials
Acrylamide (AM), N, N′-methylene bis(acrylamide) (MBAA), azobisisobutyronitrile (AIBN), dopamine hydrochloride, H2O2 (30% stock solution), N-chlorosuccinimide (NCS), N-bromosuccinimide (NBS), methacryloyl chloride, dimethylformamide (DMF), tetrahydrofuran (THF), acetonitrile, hexane, iodine monochloride (ICl) dichloromethane solution, ethyl acetate, di-tert-butyl decarbonate (Boc2O), trifluoroacetic acid (TFA), triethylamine (TEA), dichloromethane, sodium thiosulfate (Na2S2O3), sodium sulfate, chlorotrimethylsilane, 3-[4, 5-dimethylthiazoyl-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT), 3,4-demethoxyl phenyl ethanamine, hydrogen chloride solution (37wt%), sodium chloride and dimethyl sulfoxide (DMSO) were all purchased from Sigma-Aldrich. All the bacteria were purchased from American Type Culture Collection (ATCC). 3T3-E1 cells were purchased from Honsunbio. Ltd. Shanghai China. Lysogeny Broth (LB) agar medium, Mueller Hinton Broth medium (MHB), and phosphate buffer saline (PBS) were purchased from Thermo Fisher Scientific. Other reagents were used as received without further purification.
2.2. Synthesis of dopamine methacrylamide (DMA)
To a cooled solution of dopamine hydrochloride (1.89 g, 10 mmol) and TEA (5.7 mL, 40 mmol) in methanol (50 mL), methacryloyl chloride (1.55 mL, 15 mmol) in THF (10 mL) was slowly added under nitrogen for 30 min. The reaction mixture was then stirred for 2 h at room temperature and the solvent was removed. The residual viscous solid was extracted with ethyl acetate (100 mL) and washed twice with 1 M aqueous hydrochloric acid solution. The organic phase was washed with saturated sodium chloride solution and dried over sodium sulfate. The solution was concentrated under reduced pressure to yield the crude solid, which was further purified by recrystallized in an ethyl acetate and hexane mixture to afford the product (1.54 g, 70%) as a white solid. 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 8.75 (s, 1H, Ph-OH), 8.60 (s, 1H, Ph-OH), 7.89 (t, 1H, -NH), 6.57-6.61 (d, 1H, Ph-H), 6.52-6.55 (d, 1H, Ph-H), 6.32-6.42 (dd, 1H, Ph-H), 5.59 (s, 1H, =CH2), 5.30 (s, 1H, =CH2), 3.18 (m, 2H, -CH2), 2.52 (t, 2H, -CH2), 1.80 (s, 3H, -CH3).
2.3. Synthesis of 6-chlorodopamine methacrylamide (DMA-Cl)
N-Chlorosuccinimide (0.147g, 1.1 mmol) and two drops of concentrated H2SO4 were added to a solution of DMA (0.22g, 1 mmol) in TFA (15 mL). The reaction mixture was stirred for 10 h at room temperature and the solvent was removed. The residual viscous solid was extracted with ethyl acetate (100 mL) and washed twice with 1 M aqueous Na2S2O3 solution. The organic phase was washed with saturated sodium chloride solution and dried with sodium sulfate. The solution was concentrated under reduced pressure to yield the crude solid. The product was purified by silica gel column chromatography using a 70:30 ethyl acetate:hexane mixture (Rf = 0.65) to afford the product (0.11 g, 41%) as a pale yellow solid. 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 9.11 (s, 1H, Ph-OH), 9.00 (s, 1H, Ph-OH), 7.93 (t, 1H, -NH), 6.70 (s, 1H, Ph-H), 6.61 (s, 1H, Ph-H), 5.57 (s, 1H, =cH2), 5.26 (s, 1H, =CH2), 3.20 (m, 2H, -CH2), 2.63 (t, 2H, -CH2), 1.80 (s, 3H, -CH3).
2.4. Synthesis of 6-bromodopamine methacrylamide (DMA-Br)
N-Bromosuccinimide (0.19 g, 1.1 mmol) and two drops of concentrated H2SO4 were added to a solution of DMA (0.22g, 1 mmol) in trifluoroacetate (15 mL). The reaction mixture was stirred for 10 h at room temperature and the solvent was removed. The residual viscous solid was extracted with ethyl acetate (100 mL) and washed twice with 1 M aqueous Na2S2O3 solution. The organic phase was washed with saturated sodium chloride solution and dried with sodium sulfate. The solution was concentrated under reduced pressure to yield the crude solid. The product was purified by silica gel column chromatography using a 70:30 ethyl acetate:hexane mixture (Rf = 0.70) to afford the product (0.15 g, 50%) as a grey solid. 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 9.12 (s, 1H, Ph-OH), 9.02 (s, 1H, Ph-OH), 7.93 (t, 1H, -NH), 6.85 (s, 1H, Ph-H), 6.63 (s, 1H, Ph-H), 5.59 (s, 1H, =CH2), 5.26 (s, 1H, =CH2), 3.18 (m, 2H, -CH2), 2.65 (t, 2H, -CH2), 1.80 (s, 3H, -CH3).
2.5. Synthesis of 6-iododopamine methacrylamide (DMA-I)
1 M ICl in dichloromethane (3 mL, 3 mmol) was added dropwise to a solution of DMA (0.22 g, 1 mmol) in glacial acetic acid (7.5 mL) at room temperature. The reaction mixture was stirred for 8 h and the solvent was removed. The residual viscous solid was extracted with ethyl acetate (100 mL) and washed twice with 1 M aqueous Na2S2O3 solution. The organic phase was washed with saturated sodium chloride solution and dried with sodium sulfate. The solution was concentrated under reduced pressure to yield the crude solid. The product was purified by silica gel column chromatography using a 70:30 ethyl acetate:hexane mixture (Rf = 0.60) to afford the product (0.20 g, 55%) as a light yellow solid. 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 9.11 (b, 2H, Ph-OH), 7.91 (t, 1H, -NH), 6.67 (s, 1H, Ph-H), 6.59 (s, 1H, Ph-H), 5.56 (s, 1H, =CH2), 5.26 (s, 1H, =CH2), 3.16 (m, 2H, -CH2), 2.64 (t, 2H, -CH2), 1.80 (s, 3H, -CH3).
2.6. Synthesis of methyl protected DMA-Cl (MeODMA-Cl)
3,4-dimethoxyl phenyl ethanamine (0.18 g, 1 mmol) and TEA (1 mL) in 10 mL of dichloromethane solution was chilled at 0 °C. Boc2O (0.24 g, 1.1 mmol) was added dropwise and the reaction mixture was stirred for 2 h at room temperature. The solvent was removed. Boc-protected 3,4-dimethoxyl phenyl ethanamine was purified by silica gel column chromatography using 20:80 ethyl acetate:hexane mixture to afford the product (Rf = 0.45, 0.22 g, 80%) as a white solid. NCS (0.15 g, 1.1 mmol) and 1 drop of chlorotrimethylsilane were added into a solution of Boc-protected 3,4-dimethoxyl phenyl ethanamine (0.28 g, 1 mmol) in 10 mL of acetonitrile. The reaction mixture was stirred for 12 h at 60 °C. Boc-protected halogenated catechol amine was purified by silica gel column chromatography using 20:80 ethyl acetate:hexane mixture (Rf = 0.70) to afford the product (0.19 g, 50%) as a brown solid.
Boc-protected halogenated catechol amine (0.38 g, 1mmol) was deprotected with excess TFA (5 mL) for 6 h. After TFA was removed, the vicious crude product was dissolved in 10 mL dichloromethane and 2 mL of TEA was added to the solution. Methacryloyl chloride (0.2 mL, 2mmol) in THF (3 mL) was dropwise added and the reaction mixture was stirred for 1 h at room temperature. MeODMA-Cl was purified by silica gel column chromatography using 50:50 ethyl acetate:hexane mixture (Rf = 0.40) to afford the product (0.14 g, 60%) as a pale yellow solid. 1H NMR (400 MHz, CDCl3): δ (ppm) = 6.98 (s, 1H, Ph-H), 6.70 (s, 1H, Ph-H), 6.04 (dd, 2H, =CH2), 5.71 (d, 1H, -CH), 3.81 (d, 6H, -CH3), 3.60 (m, 2H, -CH2), 2.92 (t, 2H, -CH2).
2.7. Preparation of hydrogels, copolymers, and coatings
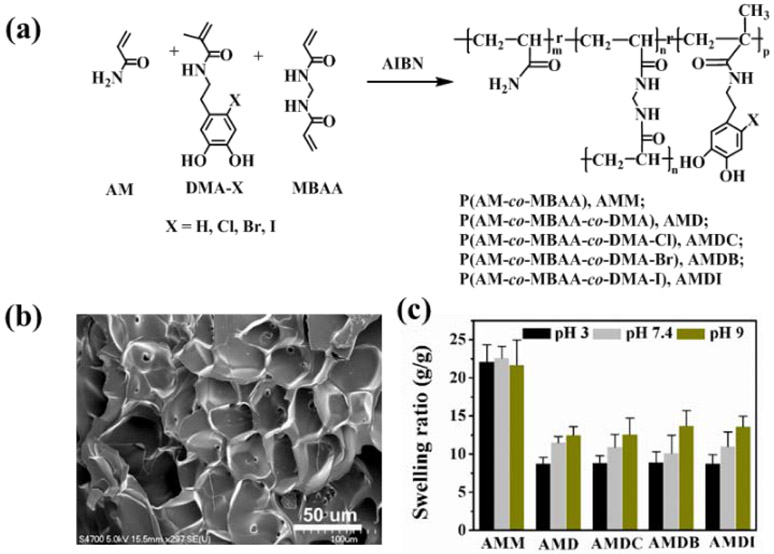
Hydrogels were prepared by curing a precursor solution containing 1 M of AM with up to 10 mol % of the halogenated DMA dissolved in 80% (v/v) DMSO in deionized (DI) water. The bifunctional cross-linker (MBAA) and the thermal initiator (AIBN) were kept at 3 and 1 mol %, respectively, relative to AM. The precursor solutions were degassed three times, added to a mold with a spacer (2 mm thick) and incubated at 80 °C for 3 h. The hydrogels were washed by submerging in an aqueous solution (pH 3) with gentle nutation and frequent medium changes. For antimicrobial testing, the hydrogels were washed with pH 7.4 solution for 12 h before testing. Hydrogels modified with DMA, DMA-Cl, DMA-Br, and DMA-I were abbreviated as AMD, AMDC, AMDB and AMDI, respectively. Control hydrogel without DMA was abbreviated as AMM.
Linear copolymers were prepared by copolymerizing DMA and DMA-Cl with AM. In a typical polymerization process, DMA (0.11 g, 0.5 mmol) and AM (0.71 g, 10 mmol) were dissolved in 2 mL of DMF containing AIBN (5.0 mg). After thoroughly deoxygenating with nitrogen gas, the solution was heated at 75 °C for 3 h. The crude polymer was washed 3 times with ethyl acetate (20 mL) and 1 time with DI water, and dried in vacuum for 48 h. These copolymers were labeled P(AM20-co-DMA1) and P(AM20-co-DMA-Cl1) based on the molar ratio of AM to DMA or DMA-Cl, respectively. Polymers with higher DMA or DMA-Cl contents were prepared by copolymerizing either DMA (1.10 g, 5 mmol) or DMA-Cl (1.28 g, 5 mmol) with AM (0.71 g, 10 mmol) in a similar manner. These copolymers were labeled P(AM2-co-DMA1) and P(AM2-co-DMA-Cl1) based on the molar ratio of AM to DMA or DMA-Cl, respectively. 1H NMR (400 MHz, DMSO-d6) of P(AM-co-DMA): δ (ppm) = 8.66 (Ph-OH), 6.22~7.61 (-NH2, -NH and Ph-H), 3.12 (-CH2 of DMA side chain), 2.05 (-CH of AM), 1.48 (-CH2 of AM and DMA backbone), 0.93 (-CH3 of DMA). 1H NMR (400 MHz, DMSO-d6) of P(AM-co-DMA-Cl): δ (ppm) = 9.16 (Ph-OH), 6.34~7.64 (-NH2, -NH and Ph-H), 3.11 (-CH2 of DMA-Cl side chain), 2.02 (-CH of AM), 1.37 (-CH2 of AM and DMA backbone), 0.94 (-CH3 of DMA-Cl).
DMA and DMA-Cl containing polymers were coated on to glass slides. Glass slides (length × height ÷ thickness = 1cm × 1cm × 0.1cm) were first treated with piranha solution for 1 min, washed with DI water for several times, and dried at 50 °C for overnight. Glass slides were immersed in a solution containing 1 % w/w of the polymer dissolved in DMF for 30 min and washed with ethanol and DI water several times.
2.8. Material characterization
Flourier transformed infrared spectroscopy (FTIR) was performed using a Perkin Elmer Spectrum One spectrometer. To image the network morphology, hydrogels were freeze-dried, coated with platinum, and imaged using a field emission scanning electron microscope (FE-SEM, HITACHI S-4700). 1H NMR spectra of monomers and polymers were collected on a Varian Unity Inova 400 MHz instrument using DMSO-d6 and CDCl3 as the solvents. UV spectra of halogenated DMA (0.6 mM) or copolymer (0.2 g/L) solutions was examined using a UV-vis spectrometer (LAMBDA35, PerkinElmer, MA). Lyophilized hydrogels were incubated in pH 3, pH 7.4 and pH 9 at 25 °C for up to 48 h. The mass of hydrogels in the swollen (Ws) and dried (Wd) states were used to determine the swelling ratio (SR) as calculated by: SR = (Ws-Wd)/Wd ~ 100%. The molecular weights and molecular weight distributions of copolymers were determined by gel permeation chromatography (GPC) performed with a Shimadzu LC-20A Series GPC system equipped with a pump, a BC-PL gel mixed column, and a RID-10A refractive index detector using DMF with 0.02 M lithium bromide as the eluent and polystyrene standards as reference.
X-ray photoelectron spectroscopy (XPS) was performed to characterize polymer coated glass slide surface using a PHI 5800 instrument. A Mg anode was used to collect the XPS data, which was charge corrected with respect to aliphatic carbon at 284.6 eV. Johnson Kendall Roberts (JKR) contact mechanics test was performed using a custom-built setup comprising a 10 g load cell (Transducer Techniques; Temecula, CA) and a miniature linear stage stepper motor (MFA-PPD, Newport; Irvine, CA). Substrates were affixed to an indenter stem (ALS-06, Transducer Techniques; Temecula, CA) using Super Glue (Adhesive Systems MG 100) and compressed at a rate of 1 μm/s against a buffer-wetted quartz surface until a fixed maximum preload of 20 mN was reached. The substrates were then retracted at the same speed. One contact cycle composed of bringing the substrates into contact with the hydrogels (diameter = 0.5 cm, thickness = 0.2 cm) at a constant speed until the fixed preload was reached and then retracting it at the same speed. Hydrogels were equilibrated at pH 3 for 24 h, and then pH 7.4 for 1 h prior to testing. A borosilicate glass surface (Pearl microscope slides, cat. no. 7101) was used as test substrate, and it was wetted with 25 μL of pH 7.4 buffer medium. The force (F) and the work of adhesion (Wadh) were calculated according to our previous report [34]. The rheological properties of the hydrogels were examined using a Malvern Kinexus pro rheometer. A cone-and-plate geometry with a 20 mm diameter plate, and 3 mm gap was used for all experiments on hydrogels. Hydrogels (diameter = 2 cm, thickness = 0.5 cm) were equilibrated in pH 7.4 with nutation for 48 h. The storage (G’) and loss (G”) modulus were determined at an oscillatory strain range of 10% to 10000% at a constant frequency (f) of 1 Hz.
2.9. H2O2 generation using FOX assay
The concentration of H2O2 released from the hydrogels (diameter = 0.5 cm, thickness = 0.2 cm) after 24 h incubation was quantified using a Pierce Quantitative Peroxide Assay Kit (FOX Assay). A standard curve was prepared by performing serial dilutions with a 30% H2O2 stock solution, which created 10 standards with concentrations from 1-1000 μM H2O2. In a microplate, 20 μL of sample and 200 μL of FOX Assay working reagent were added to each well, incubated for 15 min, and characterized using a plate reader at an absorbance wavelength of 595 nm. The peroxide concentration was calculated in each sample through referencing to an absorbance from the standard curve. The results were reported as mean concentration +/− standard deviation (n = 3).
2.10. Minimum inhibitory concentrations (MIC) of the copolymers against bacteria
Overnight bacteria cultures were prepared in MHB at 37 °C, and sub-cultured to mid-log phase for further use. A series of sample solutions were prepared by two-fold dilution of 2000 mg/mL polymer stock in MHB to obtain final concentrations ranging from 1000 to 1 mg/mL. Then 100 μL of each diluted sample from the serial dilutions was added to a 96-well plate and 100 μL of bacterial suspension in MHB (1×106 colony forming units (CFU)mL) was added. The 96-well plate was mixed on the plate shaker for 20 min and subsequently incubated statically at 37 °C for 16 h. The killing percentage was calculated by determining its optical density (OD) value using a microplate spectrophotometer (SpectraMax® i3, USA) at a wavelength of 600 nm, compared with positive control (bacteria without sample treatment) and negative control (medium only).
2.11. Antimicrobial testing
The antimicrobial property of samples against S. aureus, E. coli and MDR bacteria were quantitatively evaluated using viable cell count method. Overnight bacteria cultures (106-108 CFU/mL) were prepared in LB at 37 °C, and sub-cultured to mid-log phase for further use. 10 μL of bacterial suspension was spread evenly onto the surface of samples, which were further incubated at 37 °C for 1 h to inoculate the bacteria. Samples were transferred to vessels containing 1 mL PBS and vortexed to obtain bacterial suspensions. The suspension was diluted and plated on LB agar. The agar plates were incubated at 37 °C for 24 h. The CFU number was counted and expressed in killing efficiency: [(cell count of control - survivor count on sample)/cell count of control] × 100.
2.12. Antibiofilm activities of hydrogels
The ability of the hydrogels to kill bacteria in preformed biofilms was characterized by FE-SEM (Zeiss Sigma 500) and LIVE/DEAD assay. Bacteria suspensions (106-108 CFU/mL) were seeded onto glass dishes to simulate the formation of thick and dense bacterial biofilms after 24 h of inoculation [21, 57]. Hydrogels (diameter = 0.5 cm, thickness = 0.2 cm) were placed over the glass dishes seeded with bacteria and further incubated at 37 °C for up to 24 h. After removing the hydrogel, the surface was immersed in 2 mL of 2.5 wt% glutaraldehyde in PBS and refrigerated at 4 °C for overnight. Bacteria were dehydrated serially in an ethanol and water mixture with increasing ethanol concentration (25%, 50%, 75%, and 100%). Dehydrated samples were further dried under nitrogen before coating with platinum for imaging. The morphology of bacteria after treatment with hydrogels were observed using FE-SEM (Zeiss Sigma 500). Hydrogel-treated glass dishes were further stained using L13152 LIVE/DEAD® Bac Light TM Bacterial Viability Kit following manufacturer’s protocol. 40 μL of the stain solution (the final concentration of each dye would be 6 μM SYTO 9 stain and 30 μM propidium iodide) was added and spread over the hydrogel-covered zone and incubated in the dark for 15 min before examination under a fluorescence microscope (Zeiss LSM 710 microscope). Fluorescence imaging was performed with 480/500 nm filter for SYTO 9 stain and 490/635 nm filter for propidium iodide in the microscope optical path.
2.13. Cytotoxicity study
The cytotoxicity of the hydrogels was performed by exposing 3T3-E1 fibroblast to hydrogel extracts as well as directly exposing the cells through direct contact. To prepare the hydrogel extracts, sterilized hydrogel samples (diameter = 0.5 cm, thickness = 0.2 cm) were immersed in 5 mL of PBS for 24 h at room temperature. Cells were seeded at a density of 105 cells/cm2 in a 96-well cell culture plate for 24 h at 37 °C in 5% CO2. 100 μL of the hydrogel extract was then added to each well containing 100 μL of Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum and 1% penicillin-streptomycin. After 24 h incubation, 20 μL of MTT (5 mg/mL) was added to each well followed by another 4 h of incubation. The supernatant was discarded, and 150 μL of DMSO was added to each well and shook for 15 min. OD values were measured at 570 nm using Infinite F50 TECAN plate reader (TECAN, Swiss). Cell viability was calculated based on the following equation: Cell viability (%) = ODhydrogel well/ODcontrol well × 100%. To visualize the morphology of 3T3-E1 after exposure to hydrogel extracts, cells were stained with 2 mM calcein-acetoxymethyl and 1mM propidium iodide following published protocol [58] and visualized using a polarizing optical microscope (Nikon, ECLIPSE E200).
For the direct contact cytotoxicity test, 3T3-E1 cells were first seeded at 105 cells/cm2 in a 24-well cell culture plate for 24 h at 37 °C in 5% CO2. Sterilized hydrogel (diameter = 0.5 cm, thickness = 0.2 cm) along with 500 μL of DMEM were added over the cells and incubated for another 24 h. Hydrogels were then removed and MTT assay and relative cell viability were determined as described above. 500 μL of PBS was used as the control instead of hydrogels.
2.14. Molecular model and docking
The crystal structure of FabI in complex with oxidized nicotinamide adenine dinucleotide (NAD+) was extracted from the RCSB Protein data bank (protein data bank ID: 1C14). The polar hydrogen atom of FabI/NAD+ binary complex was added, and the partial charges of atoms were calculated by AutoDockTools-1.5.6. N-(2-chloro-4,5-dihydroxyphenethyl)-2-propylpentanamide (CDPPA) with a 6-C alkyl chain and Cl-catechol was chosen to imitate polymer-tethered DMA-Cl. CDPPA and DMA-Cl were optimized by using the Molecular Mechanics 2 force field. AutoDock 4 was used for the docking process, in which Lamarckian genetic algorithm was used for the conformational search [59]. The grid map was constructed to be large enough to include the active site of the protein by autogrid4. The grid centers were defined by the center (X = 3.51, Y = 20.246, Z = 136.478) of DMA-Cl/FabI/NAD+ and CDPPA/FabI/NAD+ ternary complexes. The number of grid points in each Cartesian direction was 60.
2.15. Molecular dynamics simulation
Molecular dynamics simulation was performed under periodic boundary using the GROMACS 2018 and water molecules were performed by SPCE model. DMA-Cl/FabI/NAD+ and CDPPA/FabI/NAD+ ternary complexes were set in a cubic box (side length = 7 nm) with a minimal distance of 1 nm between the complex and box. Four sodium ions were added to make sure the system is neutral. Energy minimization and steepest descent methods were used to optimize the system, so that the whole system was in the lowest energy state. Molecular dynamics simulations were used the periodic boundary conditions with constant temperature (300 K) and pressure (1 atm). Temperature and pressure were controlled by V-rescale and Parrinello-Rahman method, respectively. The coupling constants of temperature and pressure were 0.1 and 0.5 ps. The time of molecular dynamics simulations is 50 ns. The binding free energy was calculated based on the molecular mechanics/Poisson-Bolzmann surface area (MM/PBSA) method [60].
3. Results and discussions
Synthesis of DMA derivatives.
Halogenated DMA derivatives were prepared using a simple, two-step process (Scheme 1b). Polymerizable DMA was first synthesized by reacting dopamine with methacryloyl chloride with a high yield (80%) and purity (Figure S1). The halogenated DMA was prepared by subsequently reacting DMA with either NCS, NBS, or ICl to yield DMA-Cl, DMA-Br, or DMA-I, respectively. The chemical structures of various DMA derivatives were confirmed by 1H NMR spectra (Figures S2-S4). For halogenated DMA, two sharp single peaks at around 6.65 and 6.57 ppm were assigned to the two aromatic protons instead of three aromatic protons found in the spectra of DMA. The maximum absorption wavelength (λmax) of DMA occurred at 282 nm based on UV-vis spectroscopy. Functionalizing DMA with halogen atoms red shifted the λmax to 284-289 nm due to the electronic withdrawing halogen atoms (Figure S5).
Preparation of DMA-containing hydrogels.
DMA derivatives were incorporated into AM-based hydrogels using thermal initiated free-radical polymerization (Figure 1a). FTIR spectra of the hydrogels confirmed the characteristics peaks for AM (-NH2 3450–3050 cm−1, C=O 1650 cm−1) and catechol (-OH, 3450–3050 cm−1 and benzene rings 1600–1500 cm−1) (Figure S6). The lyophilized gels exhibited typical porous structures of a hydrogel network (Figures 1b and S7). Swelling behavior of the hydrogels were measured at pH 3, pH 7.4 and pH 9 (Figure 1c). The swelling ratio of DMA-free control hydrogel was over 22 and was independent of pH. In contrast, hydrogels containing DMA derivatives exhibited significantly lower swelling ratios (8-13), potentially due to the hydrophobic nature of catechol and its ability to form intermolecular interactions (e.g., H-bonding, π-π interaction) [35]. The swelling ratios of catechol-containing hydrogels increased with increasing pH, potentially due to the increased formation of negatively charged semiquinone [61]. The color of DMA-containing hydrogels also gradually changed from colorless to pink and then red due to autoxidation of catechol moiety at pH 9, while DMA-free hydrogel remained colorless (Figure S8). These observations collectively confirmed the incorporation of DMA derivatives into AM-based hydrogels.
Figure 1.
(a) Thermal initiated polymerization of halogenated DMA with AM and MBAA using AIBN to fabricate hydrogels. (b) FE-SEM of lyophilized AMDC. (c) Swelling ratios of hydrogels (g swollen hydrogel/g dried hydrogel) at pH 3, pH 7.4 and pH 9 after 48 h.
Oscillatory rheometry was performed to characterize the mechanical property of the cured hydrogels. All the hydrogels exhibited linear elasticity, where G’ value was independent of applied strain between 10 to 1000% (Figure S9). Additionally, G’ values were higher than those of G” values, indicating that the hydrogels were chemically crosslinked [62]. Catechol-containing hydrogels (AMD, AMDC, AMDB, AMDI) exhibited higher G’ values (260-460 Pa) in the linear elastic regions when compared to that of the DMA-free hydrogel AMM (80 Pa). The increased stiffness is attributed to catechol’s ability to form intermolecular interactions such as H-bonding and π-π interaction) [35].
Antimicrobial activities of hydrogels.
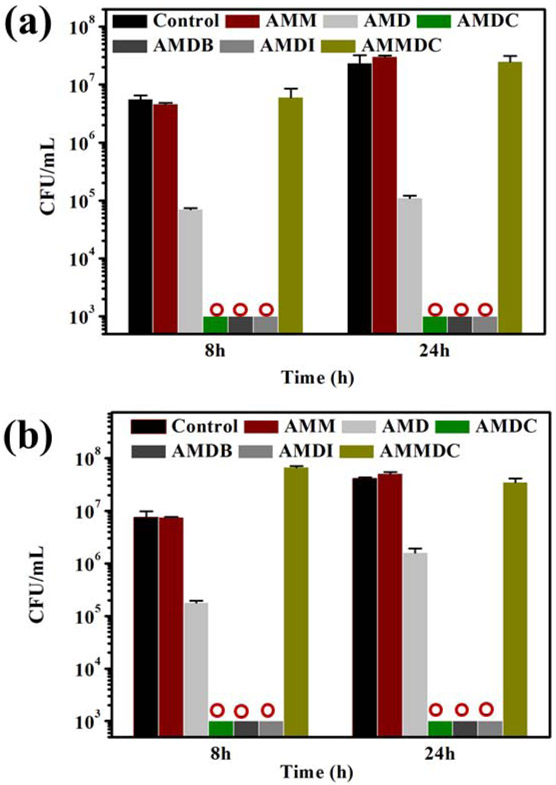
Antimicrobial property of hydrogels was firstly evaluated by using contact killing protocol against Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria (Figure 2). DMA-free control exhibited no antibacterial activity and CFU counts were almost the same as the untreated bacteria control, where CFU counts increased by nearly an order of magnitude with increasing incubation time. In contrast, DMA-containing hydrogel exhibited 98.7 and 95.3% killing efficiency against S. aureus and 97.1 and 96.1% killing efficiency against E. coli after 8 h and 24 h, respectively. Hydrogels incorporated with the halogenated DMA derivatives (DMA-Cl, DMA-Br and DMA-I) completely suppressed bacterial growth and killed the bacteria (a 7 log reduction in CFU). Interestingly, when the two hydroxyl groups on DMA-Cl were protected with methyl groups (Scheme S1 and Figure S10; AMMDC), the protected DMA-Cl lost its antimicrobial activity (Figures 2a and 2b). Chemical protection renders the halogenated DMA non-adhesive (Figure S11), even though AMDC exhibited work of adhesion value that was twice as high as that of AMD due to the electron-withdrawing chloro-functional group. This suggests that the ability for catechol to adhere to the bacteria is potentially critical for the contact killing induced by membrane disruption.
Figure 2.
CFU/mL for (a) S. aureus and (b) E. coli treated with hydrogels containing halogenated DMA and protected DMA-Cl after 8 h and 24 h (red circles indicate zero CFU/mL).
The antimicrobial activity of the hydrogel containing the unmodified DMA is likely associated with the generation of H2O2 during catechol autoxidation [42]. However, DMA-Cl generated a lower amount of H2O2 when compared to that of DMA over 24 h (Figure S12). This is potentially due to the electron withdrawing chloro-functional group that reduces the rate of catechol oxidation [50]. Due to large difference between the antimicrobial efficacy between AMD and hydrogels containing halogenated catechol (2 orders of magnitude difference in the CFU count, Figure 2), H2O2 is not the main contributor to the strong antimicrobial activity exhibited by halogenated DMA. Additionally, we previously reported that H2O2-releasing microgel was only able to disinfect bacteria up to 105 CFU/mL [42], whereas the halogenated DMA-containing hydrogels completely killed bacteria with a starting concentration of 107 CFU/mL in this report. Bacteria such as Staphylococcus secretes catalase, an antioxidant enzyme that decomposes H2O2 [63], which reduces the antimicrobial efficacy of H2O2.
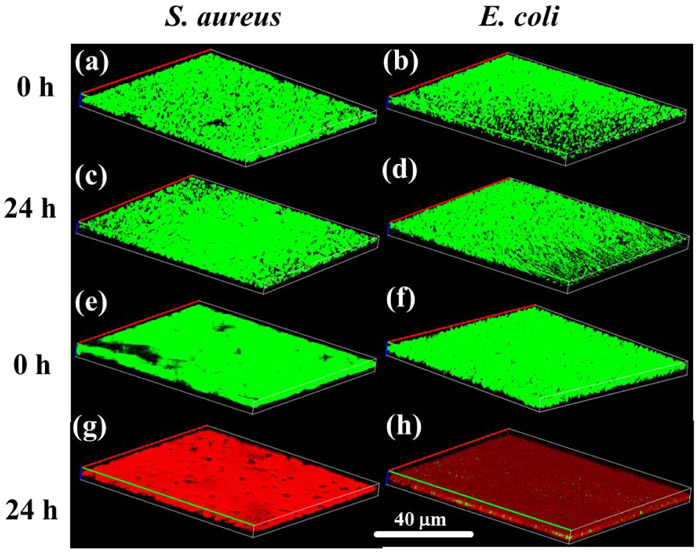
The antibiofilm activities of hydrogel were evaluated by bring it in contact with a surface with an established culture of bacteria to simulate biofilm formation [57]. FE-SEM images of S. aureus and E. coli seeded on glass dishes appeared spherical and rod-like, respectively, as expected (Figures 3a and 3d). Both bacteria assembled to form thick and dense bacterial biofilms after 24 h of inoculation [21]. After treated with AMDC, the density of bacteria decreased sharply (Figures 3b and 3e). Additionally, these treated cells appeared deformed with increased surface roughness. Moreover, debris and lysed cells were observed for both bacteria (Figures 3c and 3f). These results indicate that DMA-Cl induced deformation of the bacterial cell wall after contact, leading to the leakage of intracellular components and cell death. This antimicrobial mechanism resembles those of halogenated phenol such as triclosan and hexachlorophene [64]. The viability of bacterial biofilms after treatment with AMM and AMDC was further evaluated using the LIVE/DEAD assay (Figure 4). AMM-treated bacteria formed dense and thick biofilms after 24 h incubation and the bacteria remained alive (stained green) (Figures 4c and 4d). In contrast, bacteria treated with AMDC were mostly dead (stained red) (Figures 4g and 4h).
Figure 3.
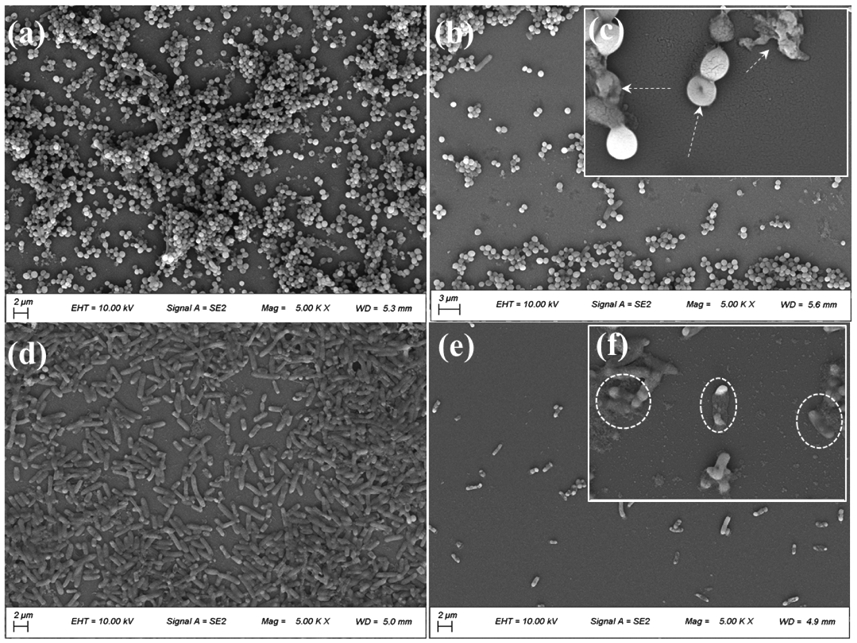
FE-SEM images of (a) S. aureus biofilm grown for 24 h and (b) S. aureus biofilm treated with AMDC for 24 h. (c) Inset showing magnified morphology of deformed S. aureus and bacterial debris (white arrows). FE-SEM images of (d) E. coli biofilm grown for 24 h and (e) E. coli biofilm treated with AMDC for 24 h. (f) Inset showing magnified morphology of bacterial debris (white dashed circles).
Figure 4.
Fluorescence images of LIVE/DEAD bacterial staining assay of S. aureus (a and c) with AMM and (e and g) AMDC after 0 h and 24 h. Fluorescence images of LIVE/DEAD bacterial staining assay of E. coli (b and d) with AMM and (f and h) AMDC after 0 h and 24 h.
Given that hydrogels modified with all three versions of the halogenated DMA (AMDC, DMDB, and AMDI) completely killed both S. aureus and E. coli, AMDC was chosen as a representative for further investigation for its killing efficiencies against five MDR bacteria that are of great concern worldwide were further determined (Figure 5). These bacteria strains are Gram-positive methicillin resistant S. aureus (MRSA) and vancomycin resistant enterococci (VRE), and Gram-negative Pseudomonas aeruginosa (PAER), multi antibiotics resistant Acinetobacter baumannii (AB) and carbapenem resistant Klebsiella pneumoniae (CRKP). For all the MDR bacteria, DMA-Cl-containing hydrogel exhibited greater than 8 log reduction in CFU count, reaching the detection limit of 10 CFU/mL after 24 h incubation (Figure 5a). To the best of our knowledge, such an excellent antimicrobial effect against multiple MDR bacteria has not been reported in other type of hydrogels bearing catechol moieties. The antibiofilm activities of AMDC against PAER and MRSA were also investigated by FE-SEM and LIVE/DEAD assay. The densities of PAER and MRSA treated with AMDC were greatly reduced and these bacteria were mostly stained red (dead) after 24 h treatment (Figure S13). Moreover, bacterial debris and deformation were also observed (Figures 5c and 5e).
Figure 5.
(a) CFU/mL of MDR bacteria treated with AMM and AMDC after 24 h (red circles indicate zero CFU/mL). Morphology of PAER (b and c) and MRSA (d and e) biofilms visualized using FE-SEM after the biofilms were treated with AMM (b and d) and AMDC (c and e) for 24 h (White dashed circles indicate bacterial debris and deformation).
Cytotoxicity of hydrogels.
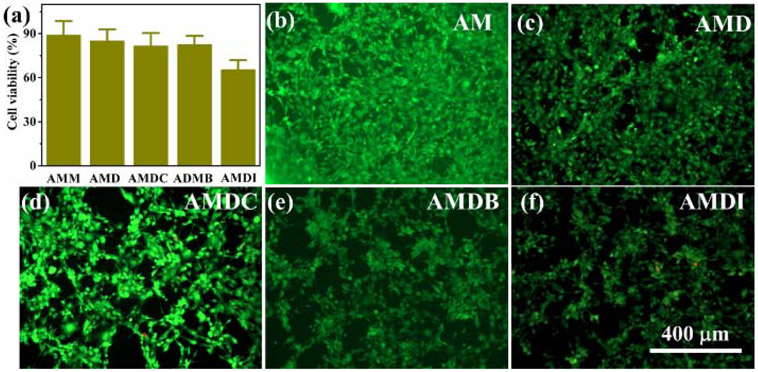
The cytotoxicity of hydrogels was evaluated using MTT assay. The relative cell viability of 3T3-E1 fibroblast remained above 80% when these cells were exposed with the hydrogel extracts of AMM, AMD, AMDC and AMDB, indicating these hydrogels exhibited good biocompatibility (Figure 6a). Only DMA-I containing hydrogel exhibited a slightly lower relative cell viability of 66%. The fluorescence images also showed live 3T3-E1 cells spreading on the surfaces of these hydrogels, exhibiting spindle-like morphology typical of fibroblast (Figures 6b-6f). However, when 3T3-E1 fibroblasts were brought into direct contact with the hydrogels, cell viability reduced by around 5-10% (Figure S14). Future work may be required to optimize the hydrogel formulation to improve its biocompatibility while maintaining superior antimicrobial property. Nevertheless, hydrogels modified with halogenated DMA demonstrated a combined strong killing efficiency against multiple strains of bacteria and low cytotoxicity.
Figure 6.
(a) Cell viability of 3T3-E1 incubated with hydrogels for 24 h with extraction MTT assay. (b-f) Fluorescence images of LIVE/DEAD staining of 3T3-E1 cells seeded on hydrogels for 24 h.
Antimicrobial activities of DMA-Cl-containing polymer and coating.
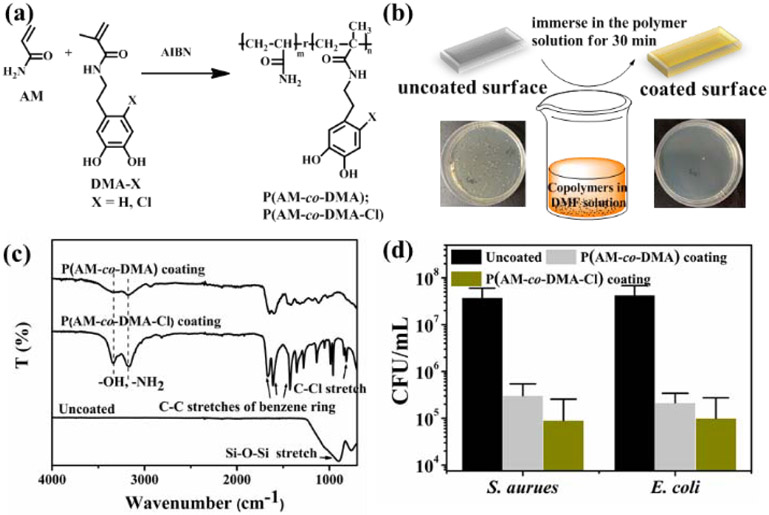
Linear copolymer of containing either DMA or DMA-Cl (P(AM20-co-DMA1) or P(AM 20-co-DMA-Cl1), respectively) was prepared through thermal-initiated free-radical polymerization using a feed molar ratio of AM:DMA or DMA-Cl of 20:1 (Figure 7a). The measured contents of DMA and DMA-Cl in the copolymers were 9.2% and 9.7%, respectively, as determined by 1H NMR (Table S1). Minimum inhibitory concentration (MIC90 μg/mL) is the lowest sample concentration that can inhibit bacteria growth by over 90%. MIC90 for these copolymers against S. aureus and E. coli were found to be 256 μg/mL, which were similar with those of chitosan [65]. The relatively low DMA and DMA-Cl contents in these copolymers were likely responsible for this underwhelming antimicrobial activity. However, due to the hydrophobic nature of the catechol side chain, copolymers with a higher content of DMA became insoluble in PBS, the medium used in MIC tests.
Figure 7.
(a) Synthesis of copolymers P(AM-co-DMA) and P(AM-co-DMA-Cl). (b) Dip-coating the copolymers on the glass slide to render the surface antimicrobial. (c) FTIR spectra of uncoated glass slide and glass slides coated with P(AM2-co-DMA1) and P(AM2-co-DMA-Cl1). (d) CFU/mL of S. aureus and E. coli seeded on uncoated, P(AM2-co-DMA1) and P(AM2-co-DMA-Cl1) coated glass slides after 24 h.
To evaluate the antimicrobial property of DMA- and DMA-Cl-containing polymers with higher DMA and DMA-Cl contents, P(AM2-co-DMA1) and P(AM2-co-DMA-Cl1) were synthesized with a molar feed ratio of AM to DMA or DMA-Cl of 2:1 (Figures S15-S17). Copolymers were coated onto glass slides surface using a simple dip-coating method (Figure 7b). XPS wide scans of three coatings showed peaks at 153.2 and 102.6 eV corresponding to Si2s and Si2p, respectively. The C1s core-level spectra of P(AM2-co-DMA1) coating and P(AM2-co-DMA-Cl1) coating showed peaks at 284.6, 285.2, and 287.9 eV attributed to C–C/C–H, C–O and C=O, respectively. The Cl2p core level of P(AM2-co-DMA-Cl1) coating showed a new peak at 199.4 eV corresponding to C-Cl of DMA-Cl (Figure S18). In the FTIR spectra of P(AM2-co-DMA-Cl1) coating, a new peak appeared at 811 cm−1, which is attributed to the stretching of C-Cl group from DMA-Cl (Figure 7c). The antimicrobial property of the coated surface was evaluated by applying 107-108 CFU/mL of bacteria to the polymer-coated surfaces. P(AM2-co-DMA1) coated surfaces demonstrated 92.1 and 95.5% contact killing efficiencies against S. aureus and E. coli, respectively, after 24 h of incubation and P(AM2-co-DMA-Cl1) coating exhibited a 99% contact killing efficiencies against both bacteria (Figure 7d). However, the antibacterial efficiencies of the coatings were not as effective when compared to hydrogels containing halogenated DMA (Figure 2).
The combined antibacterial results indicated that both the concentration of the halogenated DMA and how it is presented in a polymeric material played a vital role in the observed antibacterial response. When DMA-Cl content in the copolymer is low (i.e., P(AM20-co-DMA-Cl1)), the antibacterial activity was not significant. In comparison, DMA-Cl-containing hydrogel (AMDC) and surface coated with P(AM2-co-DMA-Cl1) with 2- and 6-fold increase in DMA-Cl content, respectively, exhibited significantly better antibacterial property. However, P(AM2-co-DMA-Cl1)-coated surface demonstrated poorer antimicrobial property when compared to that of DMA-Cl-containing hydrogel, despite having a significantly higher DMA-Cl content. It is possible that DMA-Cl participated in surface binding, which reduced the amount of the halogenated catechol for antimicrobial activities. Additionally, AMDC network was highly swollen (Figure 1c), which enabled the halogenated DMA to have a better interaction with bacteria cells [66].
Molecular docking and molecular dynamics studies on Cl-catechol binding to FabI.
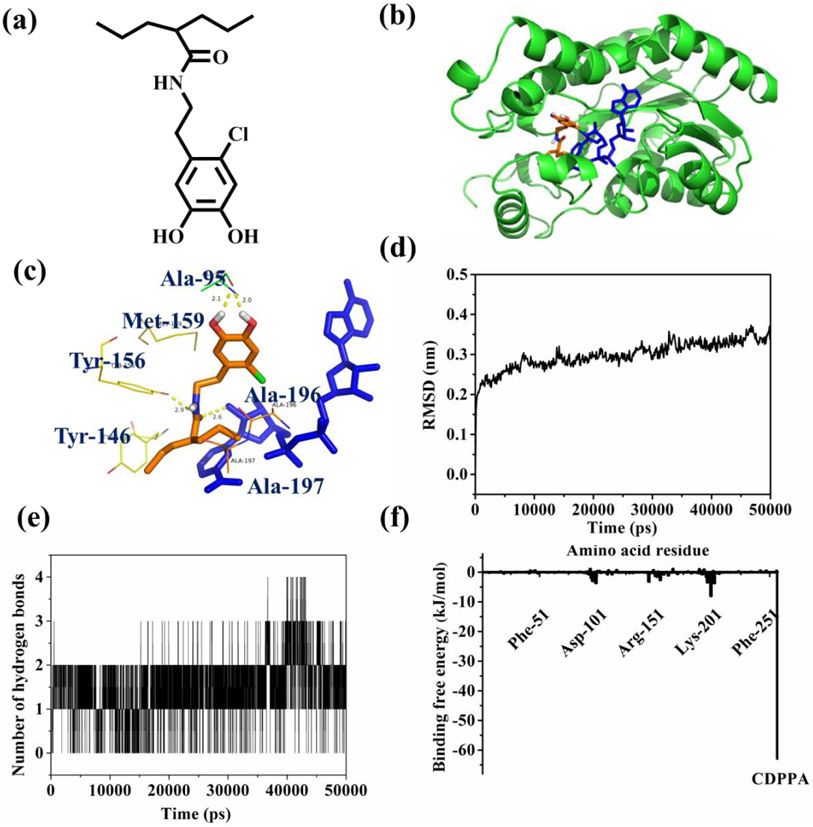
To gain a better understanding of the antimicrobial mechanism of Cl-catechol, molecular docking and molecular dynamics simulations were performed to determine the interactions between the ligand modified with Cl-catechol and FabI of E. coli. CDPPA (Figure 8a) with a 6-C alkyl chain was chosen to imitate a polymer chain grafted with DMA-Cl. FabI is the rate-limiting enzyme in the reduction step of the type II fatty acid synthase pathway [67, 68]. This is an important regulatory step in the pathway, playing a vital role in completing each round of fatty acyl carbon chain elongation. Targeting the FabI enzyme and inhibiting bacterial fatty acid biosynthesis have been demonstrated to be essential for antimicrobial agents [46]. Previously, it was demonstrated that triclosan can tightly bind to FabI/NAD+ complex by the hydrogen bond formation and π-π stacking interaction, leading to the inhibition of the bacterial fatty acid biosynthesis and broad-spectrum antimicrobial property [69, 70]. Autodock redocked CDPPA into the binding area of FabI/NAD+ binary complex to form the CDPPA/FabI/NAD+ ternary complex (Figure 8b). CDPPA was bound to the amino acids (Ala-95, Ala-196, Ala-197, Tyr-156, Met-159 and Try-146) at the active site of the FabI/NAD+ complex by hydrophobic interactions and van der Waals forces (Figure 8c). Additionally, the two -OH groups of the catechol and -NH group of amide formed hydrogen bonds with Ala-95 and NAD+, respectively (Figure 8c). These H-bonds can potentially enhance the ability of CDPPA to bind to the FabI/NAD+ binary complex.
Figure 8.
(a) The chemical structure of CDPPA. (b) The crystal structure of CDPPA /FabI/NAD+ ternary complex (FabI is colored green; CDPPA is colored orange; NAD+ is colored blue). (c) Binding models of CDPPA (orange) with the amino acids found at the active site of FabI and NAD+ (purple). (d) RMSD of the CDPPA/FabI/NAD+ ternary complex. (e) Number of hydrogen bonds formed in the CDPPA/FabI/NAD+ ternary complex. (f) Binding free energy contributions by amino acid residues and CDPPA.
50 ns molecular dynamics simulation was performed to verify the stability and secondary structures of CDPPA/FabI/NAD+ ternary complex, hydrogen bonds between CDPPA and FabI/NAD+ binary complex, and binding energy of protein. Root mean square deviations (RMSD) of the ternary complex was used to confirm its stability and RMSD of the complex reached equilibrium after 20 ns (Figure 8d). Moreover, RMSD values deviated less than 0.2 nm, indicating that the conformation of the ternary complex was stable during the molecular dynamics simulations [60]. Hydrogen bonding was the main force for maintaining interactions of CDPPA and FabI/NAD+ complex. The number of hydrogen bonds formed in the CDPPA/FabI/NAD+ ternary complex was around 2 in the 50 ns molecular dynamics simulation (Figure 8e), which was likely coming from two hydroxyl groups of catechol, ensuring the binding interaction between Cl-catechol and FabI/NAD+ complex. Among the different calculated energies, van der Waals energy formed between hydrophobic amino acid residues (Arg-151, Lys-201, Asp-101, Phe-51 and Phe-251) and CDPPA made the most contribution (−62.892 kJ/mol, Figure 8f). Similarly, van der Waals energy and the unpolymerized form of the DMA-Cl monomer also played the most role in the DMA-Cl/FabI/NAD+ ternary complex (Table S2). The calculated binding free energy between CDPPA and the FabI/NAD+ complex was −99.609 kJ/mol which was higher in magnitude when compared to that of DMA-Cl (−67.0303 kJ/mol). Although this calculated energy was lower when compared to that of triclosan (−172.050 kJ/mol) [60], the interaction of both CDPPA and DMA-Cl with the binary complex was significantly more negative than 0 kJ/mol, which indicated strong interaction. These results indicated that Cl-catechol can potentially inhibit bacterial fatty acid synthesis at the FabI step by forming a strong ternary complex, similar to the antimicrobial mechanism of triclosan. The results from molecular modeling is in agreement with the antimicrobial experiment, where the content and availability of halogenated catechol plays a critical role in the antimicrobial activity.
Taken together, halogenated DMA were incorporated into hydrogels, linear polymers and coatings to impart these materials with antimicrobial property. Previously, antimicrobial catechol-containing polymers or hydrogels were reported [39-42]. However, the antimicrobial activities of these materials were derived from the incorporation of antimicrobial monomers or metal ions (e.g., silver ion) rather than the intrinsic antimicrobial property of the catechol itself. Similarly, other antimicrobial hydrogels have been prepared through the incorporation of antimicrobial metal ions or nanoparticles, antimicrobial drugs, cationic polymers, or antimicrobial peptides [14]. Halogenated DMA are chemically linked to a polymer network and its antimicrobial effects are localized, which will limit systemic complications associated with the administration of drug eluting hydrogels [71]. The antimicrobial property of cationic polymers and hydrogels is partially derived from their abilities to interact with the negatively charged bacterial cell wall through electrostatic interactions [16]. However, this results in bacteria adhering to the cationic polymers or surfaces, and subsequently retarding the antimicrobial property [72]. Moreover, some cationic polymers are cytotoxic and can cause hemolysis [10]. Halogenated DMA are electrical neutral and oxidizes to form negatively charged semiquinone, which can prevent the formation of biofilm in comparison to ordinary cationic antimicrobial hydrogels. Most importantly, hydrogels bearing halogenated catechol moieties demonstrated excellent bactericidal efficiencies against multiple MDR bacteria, with the ability of killing bacteria in a biofilm. Except for selected cationic antimicrobial hydrogels, few hydrogels have demonstrated antimicrobial activities against a broad-spectrum of MDR bacteria [19].
Our collective antimicrobial experiments and simulation results indicated that the inherent antimicrobial property of halogenated catechol stem from its availability for contacting the bacteria. In the previous work, Cl-dopamine-modified PEG hydrogels demonstrated low antimicrobial efficacy potentially due to low Cl-dopamine contents and the inaccessibility of the catechol after oxidative crosslinking [44]. In contrast, our hydrogels were crosslinked with MBAA and did not involve catechol-catechol crosslinking, which preserved it for antimicrobial activity. Additionally, the reported halogenated DMA can be easily incorporated into polymeric materials of different chemical structures with the ability to manipulate the catechol concentrations in these materials. The combination of moisture-resistant adhesive property, inherent antimicrobial property, and the versatility of these halogenated DMA greatly enhanced the potential for using these monomers for designing multifunctional bioadhesives. Specifically, halogenated DMA reported here can potentially be incorporated into coatings for implantable medical devices to preventing infection, antimicrobial wound dressings, or a materials for disinfecting contaminated surfaces.
4. Conclusions
In conclusion, we reported the synthesis of polymerizable DMA derivatives with electron withdrawing halogen substituents at the 6-position. Halogenated DMA can be easily incorporated into hydrogels, polymers, and coatings. Halogenated DMA were copolymerized to form antimicrobial hydrogels, which exhibited strong antimicrobial and antibiofilm activities against both S. aureus and E. coli, as well as five strains of MDR bacteria. DMA-Cl was also copolymerized into a polymer that was used to prepare antimicrobial coating through a simple dip coating approach. Coating functionalized with DMA-Cl imparted glass surfaces with antimicrobial property and demonstrated 99% bacterial killing efficiencies. Molecular docking and molecular dynamics simulation demonstrated that DMA-Cl inhibited bacterial fatty acid synthesis at the FabI step potentially by binding to the FabI/NAD+ complex.
Supplementary Material
Highlights.
Polymerizable monomers containing halogenated catechol were used to prepare antimicrobial hydrogels, polymers, and coatings.
Halogenated catechol demonstrated intrinsic antimicrobial property toward five strains of multidrug resistant bacteria.
Halogenated catechol potentially inhibits bacterial fatty acid synthesis based on molecular docking and dynamics simulation.
Acknowledgment
This work was supported by the National Institutes of Health [grant numbers R15GM104846 (B.P.L.) and R15GM135875 (B.P.L.)]; the Office of Naval Research [grant numbers N00014-16-1-2463 (B.P.L.) and N00014-20-1-2230 (B.P.L.)]; and the Office of the Assistant Secretary of Defense for Health Affairs through the Defense Medical Research and Development Program [grant number W81XWH181061 (B.P.L.)].
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sugden R, Kelly R, Davies S, Combatting antimicrobial resistance globally, Nat. Microbiol 1 (2016) 2. [DOI] [PubMed] [Google Scholar]
- [2].Morales E, Cots F, Sala M, Comas M, Belvis F, Riu M, Salvado M, Grau S, Horcajada JP, Montero MM, Castells X, Hospital costs of nosocomial multi-drug resistant pseudomonas aeruginosa acquisition, BMC Health Serv. Res 12 (2012) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nathwani D, Raman G, Sulham K, Gavaghan M, Menon V, Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant pseudomonas aeruginosa Infections: a systematic review and meta-analysis, Antimicrob. Resist. In 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tan SY, Tatsumura Y, Alexander fleming (1881–1955): discoverer of penicillin, Singap. Med. J 56 (2015) 366–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stewart PS, Costerton JW, Antibiotic resistance of bacteria in biofilms, Lancet 358 (2001) 135–138. [DOI] [PubMed] [Google Scholar]
- [6].Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL, Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance, Clin. Microbiol. Infect 18 (2012) 268–281. [DOI] [PubMed] [Google Scholar]
- [7].Darouiche RO, Current concepts - treatment of infections associated with surgical implants, N. Engl. J. Med 350 (2004) 1422–1429. [DOI] [PubMed] [Google Scholar]
- [8].Barriere SL, Clinical, economic and societal impact of antibiotic resistance, Expert Opin. Pharmacother 16 (2015) 151–153. [DOI] [PubMed] [Google Scholar]
- [9].Ahmad F, Ahmad L, Aqil F, Khan MS, Hayat S, Diversity and potential of nonsymbiotic diazotrophic bacteria in promoting plant growth, in: Ahmad I, Pichtel J, Hayat S (Eds.) Plant-Bacteria Interactions, John Wiley & Sons Inc, New Jersey, 2008, pp. 81–109. [Google Scholar]
- [10].Munoz-Bonilla A, Fernandez-Garcia M, Polymeric materials with antimicrobial activity. Prog. Polym. Sci 37 (2012) 281–339. [Google Scholar]
- [11].Kratochvil MJ, Yang T, Blackwell HE, Lynn DM, Nonwoven polymer nanofiber coatings that inhibit quorum sensing in Staphylococcus aureus: toward new nonbactericidal approaches to infection control, ACS Infect. Dis 3 (2017) 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guo JS, Kim GB, Shan DY, Kim JP, Hu JQ, Wang W, Hamad FG, Qian G, Rizk EB, Yang J, Click chemistry improved wet adhesion strength of mussel-inspired citrate-based antimicrobial bioadhesives. Biomaterials 112 (2017) 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ren W, Cheng WR, Wang G, Liu Y, Developments in antimicrobial polymers, J. Polym. Sci. Pol. Chem 55 (2017) 632–639. [Google Scholar]
- [14].Li SQ, Dong SJ, Xu WG, Tu SC, Yan LS, Zhao CW, Ding JX, Chen XS, Antibacterial hydrogels, Adv. Sci 5 (2018) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen Y, Li J, Li Q, Shen Y, Ge Z, Zhang W, Chen S. Enhanced water-solubility, antibacterial activity and biocompatibility upon introducing sulfobetaine and quaternary ammonium to chitosan, Carbohydr. Polym 143 (2016) 246–253. [DOI] [PubMed] [Google Scholar]
- [16].Liang J, Li J, Zhou C, Jia W, Song H, Zhang L, Zhao F, Lee BP, Liu B, In situ synthesis of biocompatible imidazolium salt hydrogels with antimicrobial activity, Acta Biomater. 99 (2019) 133–140. [DOI] [PubMed] [Google Scholar]
- [17].Yu H, Liu L, Yang H, Zhou R, Che C, Li X, Li C, Luan S, Yin J. Shi H, Water-insoluble polymeric guanidine derivative and application in the preparation of antibacterial coating of catheter, ACS Appl. Mater. Interfaces 10 (2018) 39257–39267. [DOI] [PubMed] [Google Scholar]
- [18].Takahashi H, Caputo GA, Vemparala S, Kuroda K, Synthetic random copolymers as a molecular platform To mimic host-defense antimicrobial peptides. Bioconjugate Chem. 28 (2017) 1340–1350. [DOI] [PubMed] [Google Scholar]
- [19].Dong A, Wang Y-J, Gao Y, Gao T, Gao G, Chemical insights into antibacterial N-halamines, Chem. Rev 117 (2017) 4806–4862. [DOI] [PubMed] [Google Scholar]
- [20].Yan Y, Zhang JY, Ren LX, Tang CB, Metal-containing and related polymers for biomedical applications, Chem. Soc. Rev 45 (2016) 5232–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li Y, Fukushima K, Coady DJ, Engler AC, Liu S, Huang Y, Cho JS, Guo Y, Miller LS, Tan JPK, Ee PLR, Fan W, Yang YY, Hedrick JL, Broad-spectrum antimicrobial and biofilm-disrupting hydrogels: stereocomplex-driven supramolecular assemblies, Angew. Chem., Int. Ed 52 (2013)674–678. [DOI] [PubMed] [Google Scholar]
- [22].Chin W, Zhong G, Pu Q, Yang C, Lou W, De Sessions PF, Periaswamy B, Lee A, Liang ZC, Ding X, Gao S, Chu CW, Bianco S, Bao C, Tong YW, Fan W, Wu M, Hedrick JL, Yang YY, A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset, Nat. Commun 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hoque J, Bhattacharjee B, Prakash RG, Paramanandham K, Haldar J, Dual function injectable hydrogel for controlled release of antibiotic and local antibacterial therapy. Biomacromolecules 19 (2018) 267–278. [DOI] [PubMed] [Google Scholar]
- [24].Lu Y, Wu Y, Liang J, Libera MR, Sukhishvili SA, Self-defensive antibacterial layer-by-layer hydrogel coatings with pH-triggered hydrophobicity. Biomaterials 45 (2015) 64–71. [DOI] [PubMed] [Google Scholar]
- [25].Ahn BK, Perspectives on mussel-inspired wet adhesion, J. Am. Chem. Soc 139 (2017) 10166–10171. [DOI] [PubMed] [Google Scholar]
- [26].Mogal V, Papper V, Chaurasia A, Feng G, Marks R, Steele T, Novel on-demand bioadhesion to soft tissue in wet environments, Macromol. Biosci 14 (2014) 478–484. [DOI] [PubMed] [Google Scholar]
- [27].Forooshani PK, Lee BP, Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein, J. Polym. Sci. Pol. Chem 55 (2017) 9–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Siebert HM, Wilker JJ, Deriving commercial level adhesive performance from a bio-based mussel mimetic polymer, ACS Sustainable Chem. Eng 7 (2019) 13315–13323. [Google Scholar]
- [29].Lee BP, Messersmith PB, Israelachvili JN, Waite JH, Mussel-inspired adhesives and coatings, in: Clarke DR, Fratzl P (Eds.) Annual Review of Materials Research, Vol 41, Annual Reviews, Palo Alto, 2011, pp. 99–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schnurrer J, Lehr C-M, Mucoadhesive properties of the mussel adhesive protein, Int. J. Pharm 141 (1996) 251–256. [Google Scholar]
- [31].Baty AM, Leavitt PK, Siedlecki CA, Tyler BJ, Suci PA, Marchant RE, Geesey GG, Adsorption of adhesive proteins from the marine mussel, mytilus edulis, on polymer films in the hydrated state using angle dependent X-ray photoelectron spectroscopy and atomic force microscopy, Langmuir 13 (1997) 5702–5710. [Google Scholar]
- [32].Lu Q, Oh DX, Lee Y, Jho Y, Hwang DS, Zeng H, Nanomechanics of cation–π interactions in aqueous solution, Angew. Chem., Int. Ed 52 (2013) 3944–3948. [DOI] [PubMed] [Google Scholar]
- [33].Lee BP, Konst S, Novel hydrogel actuator inspired by reversible mussel adhesive protein chemistry, Adv. Mater 26 (2014) 3415–3419. [DOI] [PubMed] [Google Scholar]
- [34].Narkar AR, Barker B, Clisch M, Jiang J, Lee BP, pH responsive and oxidation resistant wet adhesive based on reversible catechol-boronate complexation, Chem. Mater 28 (2016) 5432–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lee H, Dellatore SM, Miller WM, Messersmith PB, Mussel-inspired surface chemistry for multifunctional coatings. Science 318 (2007) 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Patil N, Jerome C, Detrembleur C, Recent advances in the synthesis of catechol-derived (bio)polymers for applications in energy storage and environment. Prog. Polym. Sci 82 (2018) 34–91. [Google Scholar]
- [37].Ye Q, Zhou F, Liu WM, Bioinspired catecholic chemistry for surface modification, Chem. Soc. Rev 40 (2011)4244–4258. [DOI] [PubMed] [Google Scholar]
- [38].Faure E, Lecomte P, Lenoir S, Vreuls C, Van De Weerdt C, Archambeau C, Martial J, Jerome C, Duwez AS, Detrembleur C, Sustainable and bio-inspired chemistry for robust antibacterial activity of stainless steel, J. Mater. Chem 21 (2011) 7901–7904. [Google Scholar]
- [39].Charlot A, Sciannamea V, Lenoir S, Faure E, Jerome R, Jerome C, Van De Weerdt C, Martial J, Archambeau C, Willet N, Duwez AS, Fustin CA, Detrembleur C, All-in-one strategy for the fabrication of antimicrobial biomimetic films on stainless steel, J. Mater. Chem 19 (2009) 4117–4125. [Google Scholar]
- [40].Vatankhah-Varnoosfaderani M, Ina M, Adelnia H, Li Q, Zhushma AP, Hall LJ, Sheiko SS, Well-defined zwitterionic microgels: synthesis and application as acid-resistant microreactors. Macromolecules 49 (2016) 7204–7210. [Google Scholar]
- [41].Faure E, Falentin-Daudre C, Lanero TS, Vreuls C, Zocchi G, Van De Weerdt C, Martial J, Jerome C, Duwez A-S, Detrembleur C, Functional nanogels as platforms for imparting antibacterial, antibiofilm, and antiadhesion activities to stainless steel, Adv. Funct. Mater 22 (2012) 5271–5282. [Google Scholar]
- [42].Meng H, Forooshani PK, Joshi PU, Osborne J, Mi X, Meingast C, Pinnaratip R, Kelley J, Narkar A, He W, Frost MC, Heldt CL, Lee BP, Biomimetic recyclable microgels for on-demand generation of hydrogen peroxide and antipathogenic application, Acta Biomater. 83 (2019) 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Forooshani PK, Polega E, Thomson K, Bhuiyan MSA, Pinnaratip R, Trought M, Kendrick C, Gao Y, Perrine KA, Pan L, Lee BP, Antibacterial properties of mussel-inspired polydopamine coatings prepared by a simple two-step shaking-assisted method. Front. Chem 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Garcia-Fernandez L, Cui J, Serrano C, Shafiq Z, Gropeanu RA, San Miguel V, Ramos JI, Wang M, Auernhammer GK, Ritz S, Golriz AA, Berger R, Wagner M, del Campo A, Antibacterial strategies from the sea: polymer-bound Cl-catechols for prevention of biofilm formation, Adv. Mater 25 (2013) 529–533. [DOI] [PubMed] [Google Scholar]
- [45].Park E-S, Moon W-S, Song M-J, Kim M-N, Chung K-H, Yoon J-S, Antimicrobial activity of phenol and benzoic acid derivatives, Int. Biodeterior. Biodegrad 47 (2001) 209–214. [Google Scholar]
- [46].Heath RJ, Yu YT, Shapiro MA, Olson E, Rock CO, Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis, J. Biol. Chem 273 (1998) 30316–30320. [DOI] [PubMed] [Google Scholar]
- [47].Hu Y, Ren G, Deng L, Zhang J, Liu H, Mu S, Wu T, Degradable UV-crosslinked hydrogel for the controlled release of triclosan with reduced cytotoxicity. Mater. Sci. Eng., C 67 (2016) 151–158. [DOI] [PubMed] [Google Scholar]
- [48].Proudfoot G, Ritchie I, A cyclic voltammetric study of some 4-substituted Benzene-1,2-diols, Aust. J. Chem 36 (1983) 885–894. [Google Scholar]
- [49].Amstad E, Gehring AU, Fischer H, Nagaiyanallur VV, Hähner G, Textor M, Reimhult E, Influence of electronegative substituents on the binding affinity of catechol-derived anchors to Fe3O4 nanoparticles, J Phys Chem C 115 (2011) 683–691. [Google Scholar]
- [50].Ding X, Vegesna GK, Meng H, Winter A, Lee BP, Nitro-group functionalization of dopamine and its contribution to the viscoelastic properties of catechol-containing nanocomposite hydrogels, Macromol. Chem. Phys 216 (2015) 1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shafiq Z, Cui J, Pastor-Pérez L, San Miguel V, Gropeanu RA, Serrano C, del Campo A, Bioinspired Underwater Bonding and Debonding on Demand, Angew. Chem., Int. Ed 51 (2012) 4332–4335. [DOI] [PubMed] [Google Scholar]
- [52].Nakahata M, Mori S, Takashima Y, Hashidzume A, Yamaguchi H, Harada A, pH- and sugar-responsive gel assemblies based on boronate-catechol interactions, ACS Macro Lett. 3 (2014) 337–340. [DOI] [PubMed] [Google Scholar]
- [53].Seo S, Lee DW, Ahn JS, Cunha K, Filippidi E, Ju SW, Shin E, Kim B-S, Levine ZA, Lins RD, Israelachvili JN, Waite JH, Valentine MT, Shea JE, Ahn BK, Significant performance enhancement of polymer resins by bioinspired dynamic bonding, Adv. Mater 29 (2017) 1703026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yang J, Keijsers J, van Heek M, Stuiver A, Stuart MAC, Kamperman M, The effect of molecular composition and crosslinking on adhesion of a bio-inspired adhesive, Polym. Chem 6 (2015) 3121–3130. [Google Scholar]
- [55].Zhao P, Wei K, Feng Q, Chen H, Wong DSH, Chen X, Wu C-C, Bian L, Mussel-mimetic hydrogels with defined cross-linkers achieved via controlled catechol dimerization exhibiting tough adhesion for wet biological tissues, Chem. Commun 53 (2017) 12000–12003. [DOI] [PubMed] [Google Scholar]
- [56].Shafiq Z, Cui J, Pastor-Perez L, San Miguel V, Gropeanu RA, Serrano C, del Campo A, Bioinspired underwater bonding and debonding on demand, Angew. Chem., Int. Ed 51 (2012) 4332–4335. [DOI] [PubMed] [Google Scholar]
- [57].Zhou C, Song H, Loh JLC, She J, Deng L, Liu B, Grafting antibiofilm polymer hydrogel film onto catheter by SARA SI-ATRP, J. Biomat. Sci-Polym. E 29 (2018) 2106–2123. [DOI] [PubMed] [Google Scholar]
- [58].She J, Li Y, Zhou C, Chen S, Li J, Zhang Y-F, Zhang F, Liu B, Unusual allyl diazoacetate/acrolein copolymer-based hydrogels as promising antimicrobial agents for effective bacteria therapy, Chem. Eng. J 388 (2020) 124114. [Google Scholar]
- [59].Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ, Automated docking using a lamarckian genetic algorithm and an empirical binding free energy function, J. Comput. Chem 19 (1998) 1639–1662. [Google Scholar]
- [60].Yang X, Lu J, Ying M, Mu J, Li P, Liu Y, Docking and molecular dynamics studies on triclosan derivatives binding to fabI, J. Mol. Model 23 (2017) 25. [DOI] [PubMed] [Google Scholar]
- [61].Kalyanaraman B, Felix CC, Sealy RC, Semiquinone anion radicals of catechol(amine)s, catechol estrogens, and their metal ion complexes. Environ. Health Perspect 64 (1985) 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Liu Y, Meng H, Qian Z, Fan N, Choi W, Zhao F, Lee BP, A Moldable Nanocomposite Hydrogel Composed of a Mussel-Inspired Polymer and a Nanosilicate as a Fit-to-Shape Tissue Sealant, Angew. Chem., Int. Ed 56 (2017) 4224–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mustafa HSI, Staphylococcus aureus can produce catalase enzyme when adding to human WBCs as a source of H2O2 productions in human plasma or serum in the laboratory. Open J. Med. Microbiol 04 (2014) 249–251. [Google Scholar]
- [64].Lucchini JJ, Corre J, Cremieux A, Antibacterial activity of phenolic compounds and aromatic alcohols. Res. Micobiol 141 (1990) 499–510. [DOI] [PubMed] [Google Scholar]
- [65].Holappa J, Hjalmarsdottir M, Masson M, Runarsson O, Asplund T, Soininen P, Nevalainen T, Jarvinen T, Antimicrobial activity of chitosan N-betainates, Carbohydr. Polym 65 (2006) 114–118. [Google Scholar]
- [66].Mi G, Shi D, Wang M, Webster TJ, Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces, Adv. Healthcare Mater 7 (2018) 1800103. [DOI] [PubMed] [Google Scholar]
- [67].Heath RJ, Rubin JR, Holland DR, Zhang EL, Snow ME, Rock CO, Mechanism of triclosan inhibition of bacterial fatty acid synthesis, J. Biol. Chem 274 (1999) 11110–11114. [DOI] [PubMed] [Google Scholar]
- [68].Yu M, Kumar TRS, Nkrumah LJ, Coppi A, Retzlaff S, Li CD, Kelly BJ, Moura PA, Lakshmanan V, Freundlich JS, Valderramos J-C, Vilcheze C, Siedner M, Tsai JHC, Falkard B, Sidhu ABS, Purcell LA, Gratraud P, Kremer L, Waters AP, Schiehser G, Jacobus DP, Janse CJ, Ager A, Jacobs WR Jr., Sacchettini JC, Heussler V, Sinnis P, Fidock DA, The fatty acid biosynthesis enzyme fabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe 4 (2008) 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sivaraman S, Sullivan TJ, Johnson F, Novichenok P, Cui G, Simmerling C, Tonge PJ, Inhibition of the bacterial enoyl reductase fabI by triclosan: a structure–reactivity analysis of fabI inhibition by triclosan analogues, J. Med. Chem 47 (2004) 509–518. [DOI] [PubMed] [Google Scholar]
- [70].Rafi SB, Cui G, Song K, Cheng X, Tonge PJ, Simmerling C, Insight through molecular mechanics poisson–boltzmann surface area calculations into the binding affinity of triclosan and three analogues for fabI, the E. coli enoyl reductase, J. Med. Chem 49 (2006) 4574–4580. [DOI] [PubMed] [Google Scholar]
- [71].Han H, Wu J, Avery CW, Mizutani M, Jiang X, Kamigaito M, Chen Z, Xi C, Kuroda K, Immobilization of amphiphilic polycations by catechol functionality for antimicrobial coatings, Langmuir 27 (2011) 4010–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ding X, Duan S, Ding X, Liu R, Xu F-J, Versatile antibacterial materials: an emerging arsenal for combatting bacterial pathogens, Adv. Funct. Mater 28 (2018) 1802140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.