Abstract
Research in the last five years has made great strides toward mechanistic explanations of how the brain enables memory. This progress builds upon decades of research from two complementary strands: a Levels of Analysis approach and a Levels of Organization approach. We review how research in cognitive psychology and cognitive neuroscience under these two approaches has recently converged on mechanistic, brain-based theories, couched at the optimal level for explaining cognitive phenomena – the intermediate level. Furthermore, novel empirical and data analysis techniques are now providing ways to test these theories’ predictions, a crucial step in unraveling the mechanisms of memory.
Introduction: Levels of Analysis versus Levels of Organization
The goal of life sciences is usually to provide mechanistic explanations of phenomena [1, 2]. Bechtel and Abrahamsen [3] define a mechanism as “a structure performing a function in virtue of its component parts, component operations, and their organization. The orchestrated functioning of the mechanism is responsible for one or more phenomena”. In cognitive neuroscience, such a phenomenon might be “sequential recall effects in human memory”. One challenge in providing such explanations is choosing the appropriate level of analysis and level of biological organization (or scale) at which to construct the theory.
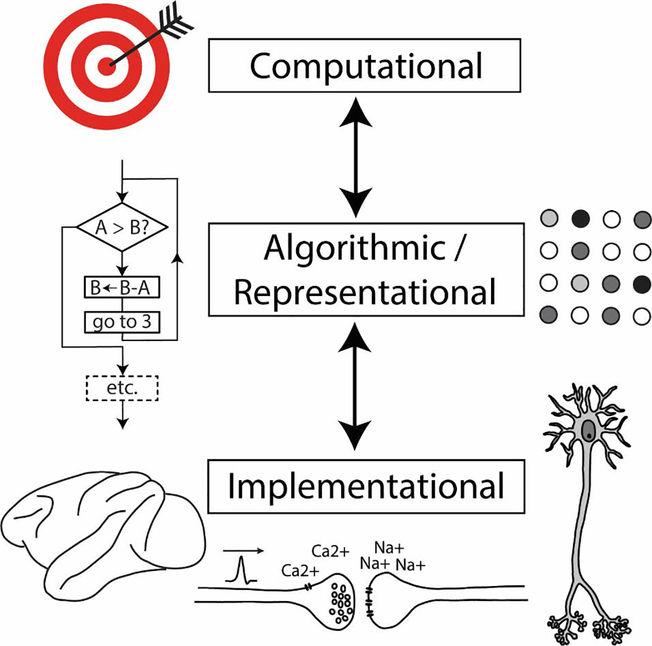
David Marr [4] identified three levels of analysis for understanding information-processing tasks such as vision (Figure 1). The highest, computational level defines the task goals; the middle, representational or algorithmic level, supplies the mechanistic steps by which the task is achieved; and the lowest, implementational level describes the physical realization of the task in biological tissue. The three levels are almost independent: a computational goal may be achieved by multiple algorithms and an algorithm can be implemented in many physical hardwares. According to Marr, an information-processing task should be analyzed by first considering the behavioral goals of the system, because the manner in which a system solves a task will depend more on nature of the problem to be solved than on the hardware that solves it. Thus, a levels of analysis approach is inherently top-down, allowing the researcher to freely invent algorithms and representations to explain behavioral phenomena (in an analogy from physics, the hypothesized representation for the phenomenon of galaxy rotation is dark matter, even though the subatomic particles of dark matter are completely unspecified).
Figure 1.
Marr’s Levels of Analysis. There are three levels as which the explanation of a task can reside. The highest level, termed computational, defines the overall goals of the task. The middle level, called representational or algorithmic, supplies the “how” in an explanation of a visual or cognitive phenomenon – the critical, mechanistic steps by which the task is carried out. The lowest level, the implementational level, describes the physical realization of the task in the biological tissue of the nervous system. The three levels are only loosely connected – they may influence but do not tightly constrain each other. In particular, understanding the algorithmic/representational level can proceed without specifying the implementational level.
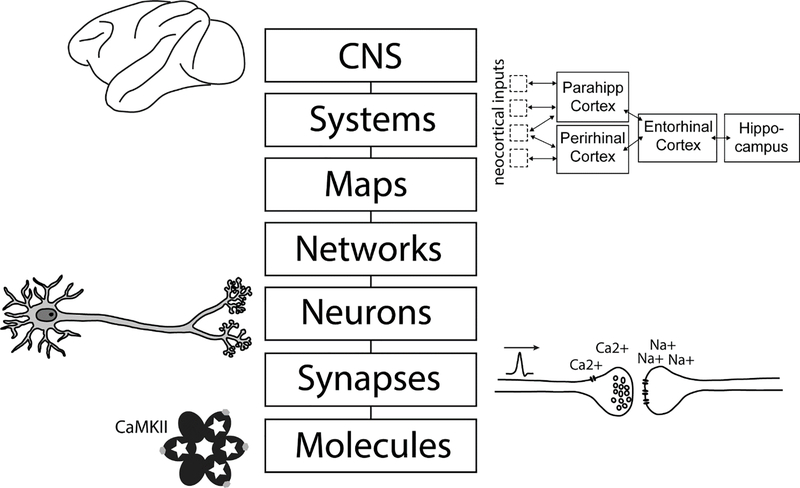
Other researchers have operated under a “levels of organization” approach (Figure 2), in which the problem space for theorizing is defined in terms of levels of biological scale (e.g., synapses, neural assemblies or anatomical systems). In contrast to Marr’s levels of analysis, these levels are tightly coupled; indeed, the goal of theories under this approach is often to define the relation between structures and phenomena at vertically adjacent levels [5, 6]. This approach is at least partly reductionist: a phenomenon at one level is explained in terms of structures at the level below (e.g., the behavior of a neural assembly depends on neural coupling via synapses). Although researchers can look both “up and down” to higher and lower levels for inspiration [6, 7], they are always constrained by implementational details. Under this approach, a significant challenge is to determine the appropriate “intermediate” sized components: if too small, interactions between component parts cannot account for the phenomenon of interest (e.g., it is difficult to explain the subjective experience of recollection in terms of interacting molecules), and if too large, the component operations become a restatement of the target phenomenon (e.g., a sub-region of prefrontal cortex is “responsible for retrieval”) [6].
Figure 2.
Levels of Neurobiological Organization (after Churchland and Sejnowski, 1988). Theories of memory that pay heed to Marr’s implementational level can be specified at one or more level of structural organization, corresponding to a particular range of biological scales. Such theories are reductionist in that it must always be possible, in principle, to construct the structures postulated at any given level from components residing at a lower level. However, these theories can be influenced and inspired by levels both above and below the level at which their explanation primarily resides.
Searching for the representations and algorithms of memory
Memory research in cognitive psychology has largely proceeded under a levels of analysis approach, seeking abstract explanations at the computational/algorithmic levels without heed to implementation. Many influential memory theories in this tradition have been formalized with mathematics. One of the earliest theories of learning is Estes’ stimulus sampling theory (SST) [8]. SST hypothesizes a large set of possible stimulus-response “elements”, which are statistically sampled in an all-or-none manner. Following on the heels of behaviorism, which disavowed unobservable theoretical constructs, this theory signaled a new approach by postulating both the form of mnemonic representations and the operations that act upon them.
As in SST, Atkinson and Shiffrin [9] appealed to the statistical sampling of latent memory traces, but their theory laid out how a memory moves from sensory memory, to short-term memory, and then into or out of long-term memory (via encoding and retrieval). These different “modes” of memory were defined in terms of content (e.g., visual, verbal, semantic), duration (e.g., less than a second, up to 30 seconds, a lifetime), and capacity (e.g., a whole visual display, approximately 7 chunks, unlimited). Subsequent work with the Search of Associative Memory (SAM) model specified the mathematical algorithms of statistical sampling and recovery in memory recall [10], and global matching in recognition [11]. Concurrent with the algorithmic developments in the SAM model, other memory researchers formalized memory representations by assuming that every event is specified as a vector of features, with vectors concatenated in a giant repository [12] or convolved with the existing memory structure to form a composite distributed representation [e.g., 13].
Further important progress was made in defining the representations and algorithms of temporal context. Early work with the SAM model [14] proposed that context randomly fluctuates over time, explaining interference and forgetting effects. To explain recall dynamics (e.g., how participants use each recalled item to cue subsequent recall attempts), Howard and Kahana [15] proposed the Temporal Context Model (TCM), in which context fluctuations are not random, but strongly influenced by the current item. This was a radical departure from Atkinson and Shiffrin’s modal model, explaining short-term memory phenomena without reference to a separate short-term memory system. The TCM model was subsequently given feature representations and learning rules compatible with neural network theory, producing the Context Maintenance and Retrieval (CMR) model [16]. Rather than assuming arbitrary features, CMR uses real-world representations extracted using Latent Semantic Analysis (LSA) [17] – a statistical corpus-based analysis of the co-occurrences of words within paragraphs. Attesting to the applicability of this genre of latent representations, such representations are the driving force behind Google and other text-based search engines.
Thus, cognitive models of memory under a levels of analysis approach have arrived at increasingly realistic representations and algorithms. However, they have largely avoided the question of compatibility with biological brains.
Searching for the structures of memory
In contrast to the abstract models of cognitive psychology, cognitive neuroscience research in the same period attempted to identify the brain structures of memory. This more data-driven, levels of organization approach was kick-started by the case of patient H.M. [18, 19]. H.M.’s deficits following brain surgery suggested that the medial temporal lobe (MTL) performs a highly selective function – the formation of new, long-term, declarative memories. Moreover, the preservation of H.M.’s other skills implied that the MTL makes no contribution to other functions (e.g., perceptual, linguistic, motoric). Subsequent work demonstrated that declarative memory is further divisible into sub-types such as episodic and semantic [20, 21], inspiring the “multiple memory systems” framework in which different forms of memory are independent and rely upon distinct brain regions [20, 22–24].
Although the multiple memory systems account provides a decomposition of memory by carving it into sub-types based on phenomenology, and mapping each sub-type onto intermediate-level structures in the brain, it skirts the question of how each sub-type arises. That is, it fails to describe the mechanisms that produce the observed memory phenomena [6, 25, 26, 27**]. This exemplifies Bechtel’s claim [6] that the search for cognitive mechanisms has been hindered by a failure to correctly identify the intermediate level: multiple memory systems accounts identify the structural parts but not the intermediate-level operations that act upon them.
Later, memory systems accounts were challenged (or complemented) by components of processing accounts, which decompose memory according to the type of information processing required, such as top-down (conceptual) versus bottom-up (perceptual) processing [28–31]. Bechtel [6] argued that components of processing accounts better dissect the mechanisms of memory, because rather than providing only a descriptive, phenomenal decomposition, they emphasize constituent operations, which are needed to define a working mechanism.
However, even components of processing accounts have provided scant details regarding the specific operations that underpin memory [6, 27]. Furthermore, the utility of a component process account is limited by how appropriately it decomposes a phenomenon [27]. For example, one theory decomposes retrieval into recollection and familiarity processes, and maps them onto hippocampal versus neocortical systems [32–35]. We have argued [27, 36] that recollection and familiarity are inadequate as components of a mechanistic theory because to identify these processes as memory operations is to make what Gilbert Ryle identified as a “category mistake” [37]: they are sub-types of memory retrieval, not sub-components. Recollection and familiarity are the high-level phenomena to be explained, rather than intermediate-level operations of memory, so applying them to component structures falls short of a mechanistic explanation. In our view, while 20th century cognitive neuroscience enjoyed success in identifying the brain structures of memory, it failed to identify the operations of memory.
Mechanism at an intermediate level: structures, operations and representations
As briefly summarized above, the levels of analysis approach elucidated mnemonic representations and operations in the abstract but did not tie them to the brain, whereas the levels of organization approach delineated the brain structures underpinning memory but did not specify its neural operations, nor its representations. The convergence of these approaches was inevitable, and fruitful.
McClelland, McNaughton and O’Reilly’s Complementary Learning Systems (CLS) account [38] was an early example of convergence. Pairing neuropsychological facts (e.g., that damage to the hippocampus affects some but not all kinds of memory) with information-processing principles of learning and representation, this work and subsequent extensions [39] supplied a mechanism for many long-term declarative memory phenomena. It proposed candidate operations for encoding and retrieval – e.g., pattern separation, pattern completion, “sharpening” of stimulus representations – and tied them to neuroarchitectural properties. Moreover, specifying the operations required explication of the representations: in pattern completion, the activity pattern representing each stimulus must be sparse so that a partial input does not trigger completion of an incorrect representation owing to excessive overlap [40–42]. In fact, it is difficult to say whether the operations of CLS are simply the best means by which to arrive at representations with the desired properties (i.e., representations are the central explanatory factor), or rather the properties of CLS representations are an inevitable consequence of the operations (i.e., operations are the crux of the account). The point is that we need both operations and representations for a mechanistic explanation.
Recognizing the importance of representations led to another promising approach. Echoing earlier accounts that assigned “memory systems” or “processes” to brain regions, this new framework instead assigns “representations”. This approach analyzes vision and memory in information processing terms, asking what representations are needed for each task (a levels of analysis philosophy), but exploiting the fact that neurons can be characterized in terms of their receptive fields, i.e., what they represent (a data-driven premise). For example, the Representational-Hierarchical account assumes that successive regions in the ventral visual stream and MTL capture increasingly complex conjunctions of features, culminating in objects in perirhinal cortex and associations of items with context, time and space in the hippocampus. Critically, the contribution of a brain region to cognition is said to be determined by what information it represents rather than what process it computes [36, 43–45, see also 46]. This view is now well evidenced [47*, 48–52]. Although this will surely prove an imperfect account of memory, especially for other levels of biological scale (e.g., within sub-regions of the hippocampus, functional distinctions may be best characterized by operations), it improves on multiple memory systems and process-based accounts because the representations that it postulates are intermediate-level components.
What have we learned about the intermediate-level mechanisms of memory?
Thus, cognitive psychology and cognitive neuroscience have converged on a detailed sketch of the brain structures, operations and representations of memory. Recent technical advances have given us new methods for probing neural representations and functional networks. Armed with these methods, what advances in mechanistic understanding have been built on that sketch?
Many researchers have risen to Marr’s challenge of determining the representations underlying cognition by exploiting new tools such as multi-variate pattern analysis (MVPA; [53]) and representational similarity analysis [54]. That is, memory theories developed by cognitive psychologists at an abstract level – with representational structures that were freely invented – are now being tested in the brain. For example, fMRI studies have supported and refined the temporal context theory [15], by revealing information-bearing states that reflect temporal context (e.g., [55–58]). In another example, hierarchical schemas in memory, well-established in the abstract [59, 60], have been fleshed out in neural terms by electrophysiological studies in rodents employing novel analysis techniques [61]. Another abstract construct proposed to be important for the structure and segmentation of episodic memory is the event boundary [62]. Modern brain imaging has confirmed this proposal and helped unpack its mechanisms, for example by demonstrating that the stability of hippocampal representations across events is related to memory for temporal order [63, 64]. Brunec et al. [65] recently suggested that boundary segmentation mechanisms are shared across spatial and episodic domains, integrating two hippocampal functions that have often been studied separately.
A second, more data-driven approach within neuroscience has fine-tuned the intermediate-level structures and operations of memory. For example, recent work has uncovered functional networks rather than focal, contiguous brain regions as the structural components of memory [66–68]. Using new connectivity analyses, previously unknown structural components such as the Default Mode Network (DMN) have been revealed [69] and the DMN has been linked to episodic memory via possible roles in memory consolidation [70] and autobiographical reminiscence [71]. Further, the operations of memory are undergoing significant revision. By applying classifiers to intracranial electrophysiological data collected in humans performing an episodic recall task, Weidemann et al. [72] arrived at a novel proposition: that semantic and episodic information interact primarily through retrieval operations, not through the similarity of representations laid down at encoding.
Other breakthroughs have stemmed from combining theory-driven and data-driven approaches. Zhang et al. [73] used RSA to identify stimulus-specific activity patterns in humans performing a memory task, then tracked the replay of these representations during sleep. Replay trigged by hippocampal “ripples” during non-REM sleep predicted later memory. Thus, guided by theory (that consolidation depends on replay) and exploiting modern tools for mining complex data (RSA), this study shed light on the representations and operations of memory consolidation. In a similarly top-down/bottom-up approach, Howard Eichenbaum, Mark Howard and colleagues have combined new empirical evidence for “time cells” in the hippocampus [74, 75*] with existing theories of hippocampal function, to generate new theories that integrate temporal, spatial and mnemonic processing in the hippocampus [76–78].
In light of these advances, some are advocating a new approach to cognition more broadly [27, 47, 79**, 80, 81]. Under this approach, research into brain function puts the computational and representational capabilities of a brain region (or network) first, and cognitive function second. Rather than carving cognition into folk-psychologically intuitive functions and mapping these onto brain structures, this approach first asks what a brain structure computes or represents, then considers how it can contribute to cognition [27, 36, 79, 82]. A mechanism in one brain region for performing some function can be recapitulated in another region to serve another function (e.g., “pattern completion” may underpin recollection in hippocampus and priming in visual cortex [27, 47]). Conversely, a brain region may serve more than one cognitive function: the perirhinal cortex contributes to recognition memory and visual discrimination [48, 83] and the hippocampus is functionally versatile because its associative representations are suited to many cognitive purposes and its computations are flexible [79, 84*, 85].
Conclusions
Twentieth century memory research delineated key memory phenomena, sketched their representations and algorithms in the abstract, and identified the brain structures that support them. Subsequent convergence of abstract models with neuropsychological accounts has produced mechanistic, brain-based explanations. These gains were achieved by choosing intermediate-level components while adopting a levels of analysis philosophy that asks how a brain region’s representations and operations might serve the goals of a cognitive task. The intermediate level is optimal for explanations in cognitive neuroscience because its components – structures like the hippocampus, representational properties such as “associative/relational”, and operations like pattern completion – lie immediately below the level of phenomena to be explained – e.g., recall effects and their relation to regional brain activation or focal lesions. Better still, being couched in function-neutral terms of operations and representations, these accounts are not limited to explaining phenomena that count as “memory”. Instead, they build bridges between memory, attention, perception, conceptual knowledge, decision-making, and more. Modern neuroscience tools, along with the new intermediate-level currency of operations and representations, are revising the textbook view of brain function by drawing parallels between phenomena that were previously consigned to separate chapters.
Highlights.
An intermediate level of biological scale is optimal for understanding human memory
Operations and representations are key components of brain-based memory theories
Modern memory research merges theory-driven and data-driven approaches
Acknowledgements
This work was funded by NSF award BCS-1554871 to R.A.C. and by NIMH award 1RF1MH114277 to R.A.C. and D.E.H.
Footnotes
conflicts of interest
I confirm that there are no conflicts of interest for either author in submitting this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bechtel W, The challenge of characterizing operations in the mechanisms underlying behavior. Journal of the Experimental Analysis of Behavior, 2005. 84(3): p. 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machamer P, Darden L, and Craver CF, Thinking about Mechanisms. Philosophy of Science, 2000. 67(1): p. 1–25. [Google Scholar]
- 3.Bechtel WD and Abrahamsen A, Explanation: A mechanist alternative. Studies in History and Philosophy of Biological and Biomedical Sciences, 2005. 36: p. 421–441. [DOI] [PubMed] [Google Scholar]
- 4.Marr D, Vision: A Computational Investigation into the Human Representation and Processing of Visual Information. 1982, San Francisco, CA: W. H. Freeman. [Google Scholar]
- 5.Churchland PS and Sejnowski TJ, Perspectives on cognitive neuroscience. Science, 1988. 242(4879): p. 741–45. [DOI] [PubMed] [Google Scholar]
- 6.Bechtel W, Mental Mechanisms: Philosophical Perspectives on Cognitive Neuroscience. 2008, New York: Taylor and Francis. [Google Scholar]
- 7.Cowell RA, Bussey TJ, and Saksida LM, Empiricists are from Venus, modelers are from Mars: Reconciling experimental and computational approaches in cognitive neuroscience. Neuroscience and Biobehavioral Reviews, 2012. 36(10): p. 2371–2379. [DOI] [PubMed] [Google Scholar]
- 8.Estes WK, Toward a Statistical Theory of Learning. Psychological Review, 1950. 57(2): p. 94–107. [Google Scholar]
- 9.Atkinson RC and Shiffrin R, Human memory: a proposed system and its control processes, in The psychology of learning and motivation: Advances in research and theory, Spence KW and Spence JT, Editors. 1968, Academic: New York: p. 89–195. [Google Scholar]
- 10.Raaijmakers JGW and Shiffrin RM, Search of Associative Memory. Psychological Review, 1981. 88(2): p. 93–134. [Google Scholar]
- 11.Gronlund SD and Shiffrin RM, Retrieval Strategies in Recall of Natural Categories and Categorized Lists. Journal of Experimental Psychology-Learning Memory and Cognition, 1986. 12(4): p. 550–561. [DOI] [PubMed] [Google Scholar]
- 12.Hintzman DL, Judgments of Frequency and Recognition Memory in a Multiple-Trace Memory Model. Psychological Review, 1988. 95(4): p. 528–551. [Google Scholar]
- 13.Murdock BB, A Theory for the Storage and Retrieval of Item and Associative Information. Psychological Review, 1982. 89(6): p. 609–626. [DOI] [PubMed] [Google Scholar]
- 14.Mensink GJ and Raaijmakers JGW, A Model for Interference and Forgetting. Psychological Review, 1988. 95(4): p. 434–455. [Google Scholar]
- 15.Howard MW and Kahana MJ, A distributed representation of temporal context. Journal of Mathematical Psychology, 2002. 46(3): p. 269–299. [Google Scholar]
- 16.Polyn SM, Norman KA, and Kahana MJ, A Context Maintenance and Retrieval Model of Organizational Processes in Free Recall. Psychological Review, 2009. 116(1): p. 129–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landauer TK and Dumais ST, A solution to Plato’s problem: The latent semantic analysis theory of acquisition, induction, and representation of knowledge. Psychological Review, 1997. 104(2): p. 211–240. [Google Scholar]
- 18.Scoville WB and Milner B, Loss of Recent Memory after Bilateral Hippocampal Lesions. Journal of Neurology Neurosurgery and Psychiatry, 1957. 20(1): p. 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milner B and Penfield W, The effect of hippocampal lesions on recent memory. Trans Am Neurol Assoc, 1955: p. 42–48. [PubMed] [Google Scholar]
- 20.Tulving E, How Many Memory Systems Are There. American Psychologist, 1985. 40(4): p. 385–398. [Google Scholar]
- 21.Oakley DA, Brain mechanisms of mammalian memory. British Medical Bulletin, 1981. 37(2): p. 175–80. [DOI] [PubMed] [Google Scholar]
- 22.Squire LR and Zola-Morgan S, The Medial Temporal-Lobe Memory System. Science, 1991. 253(5026): p. 1380–1386. [DOI] [PubMed] [Google Scholar]
- 23.Squire LR and Knowlton B, Memory, hippocampus and brain systems, in The cognitive neurosciences, Gazzaniga MS, Editor. 1995, The MIT Press: Cambridge, MA: p. 825–837. [Google Scholar]
- 24.Tulving E and Schacter DL, Priming and Human Memory Systems. Science, 1990. 247(4940): p. 301–306. [DOI] [PubMed] [Google Scholar]
- 25.Nadel L, Multiple memory systems: what and why. J Cogn Neurosci, 1992. 4(3): p. 179–88. [DOI] [PubMed] [Google Scholar]
- 26.Bussey TJ, Multiple memory systems: Fact or fiction? Quarterly Journal of Experimental Psychology Section B-Comparative and Physiological Psychology, 2004. 57(1): p. 89–94. [Google Scholar]
- 27.Cowell RA, Barense MD, and Sadil PS, A Roadmap for Understanding Memory: Decomposing Cognitive Processes into Operations and Representations. eNeuro, 2019. 6(4).** Cowell et al. 2019: This review argues that cognitive processes – introspectively-identifiable “mental events” like recollection – are inadequate constructs for characterizing neural-cognitive mechanisms, because they conflate lower-level components of the mechanisms that we seek to understand. Recollection involves both an operation (pattern completion) and a neural representation (high-dimensional, associative content). To uncover the mechanisms of recollection, we must decompose it into its operations and representations.
- 28.Moscovitch M, Memory and Working-with-Memory: A Component Process Model Based on Modules and Central Systems. Journal of Cognitive Neuroscience, 1992. 4(3): p. 257–267. [DOI] [PubMed] [Google Scholar]
- 29.Blaxton TA, Investigating Dissociations among Memory Measures - Support for a Transfer-Appropriate Processing Framework. Journal of Experimental Psychology-Learning Memory and Cognition, 1989. 15(4): p. 657–668. [Google Scholar]
- 30.Roediger HL, Buckner RL, & McDermott KB (1999). Components of processing, in Memory: Systems, process or function?, Foster JK and Jelicic M, Editors. 1999, Oxford University Press: Oxford, UK: p. 31–65. [Google Scholar]
- 31.Yonelinas AP, Components of episodic memory: the contribution of recollection and familiarity. Philosophical Transactions of the Royal Society B-Biological Sciences, 2001. 356(1413): p. 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown MW and Aggleton JP, Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience, 2001. 2(1): p. 51–61. [DOI] [PubMed] [Google Scholar]
- 33.Vilberg KL and Rugg MD, Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia, 2007. 45(10): p. 2216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eichenbaum H, Yonelinas AP, and Ranganath C, The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 2007. 30: p. 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yonelinas AP, The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language, 2002. 46(3): p. 441–517. [Google Scholar]
- 36.Cowell RA, Bussey TJ, and Saksida LM, Components of recognition memory: dissociable cognitive processes or just differences in representational complexity? Hippocampus, 2010. 20(11): p. 1245–62. [DOI] [PubMed] [Google Scholar]
- 37.Ryle G, The Concept of Mind. 1949, London: Hutchinson. [Google Scholar]
- 38.McClelland JL, Mcnaughton BL, and O’Reilly RC, Why There Are Complementary Learning-Systems in the Hippocampus and Neocortex - Insights from the Successes and Failures of Connectionist Models of Learning and Memory. Psychological Review, 1995. 102(3): p. 419–457. [DOI] [PubMed] [Google Scholar]
- 39.Norman KA and O’Reilly RC, Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychological Review, 2003. 110(4): p. 611–646. [DOI] [PubMed] [Google Scholar]
- 40.Treves A and Rolls ET, Computational Analysis of the Role of the Hippocampus in Memory. Hippocampus, 1994. 4(3): p. 374–391. [DOI] [PubMed] [Google Scholar]
- 41.Marr D, Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci, 1971. 262(841): p. 23–81. [DOI] [PubMed] [Google Scholar]
- 42.O’Reilly RC and McClelland JL, Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus, 1994. 4(6): p. 661–82. [DOI] [PubMed] [Google Scholar]
- 43.Cowell RA, Bussey TJ, and Saksida LM, Why does brain damage impair memory? A connectionist model of object recognition memory in perirhinal cortex. Journal of Neuroscience, 2006. 26(47): p. 12186–12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bussey TJ and Saksida LM, The organization of visual object representations: a connectionist model of effects of lesions in perirhinal cortex. European Journal of Neuroscience, 2002. 15(2): p. 355–364. [DOI] [PubMed] [Google Scholar]
- 45.Graham KS, Barense MD, and Lee ACH, Going beyond LTM in the MTL: A synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia, 2010. 48(4): p. 831–853. [DOI] [PubMed] [Google Scholar]
- 46.Diana RA, Yonelinas AP, and Ranganath C, Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences, 2007. 11(9): p. 379–386.* Ross et al. 2018: This study demonstrated that representational content, rather than mnemonic process, determines whether a brain region contributes to memory retrieval. It tested part-cued (i.e., pattern completion-like) retrieval of complex, associative memories (i.e., recollection) and simple, single-item memories (i.e., non-associative retrieval or “object recollection”). The hippocampus, traditionally assumed critical for recollection, was engaged in recall only for complex stimuli, and not for objects; object recall engaged lower-level regions, perirhinal and lateral occipital cortex.
- 47.Ross DA, et al. , Hippocampal Engagement during Recall Depends on Memory Content. Cereb Cortex, 2018. 28(8): p. 2685–2698. [DOI] [PubMed] [Google Scholar]
- 48.Barense MD, et al. , Intact Memory for Irrelevant Information Impairs Perception in Amnesia. Neuron, 2012. 75(1): p. 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee ACH, Scahill VL, and Graham KS, Activating the medial temporal lobe during oddity judgment for faces and scenes. Cerebral Cortex, 2008. 18(3): p. 683–696. [DOI] [PubMed] [Google Scholar]
- 50.Hannula DE, et al. , Medial temporal lobe contributions to cued retrieval of items and contexts. Neuropsychologia, 2013. 51(12): p. 2322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staresina BP, Cooper E, and Henson RN, Reversible information flow across the medial temporal lobe: the hippocampus links cortical modules during memory retrieval. J Neurosci, 2013. 33(35): p. 14184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohler S, et al. , Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: a comparison based on event-related fMRI. Hippocampus, 2005. 15(6): p. 763–74. [DOI] [PubMed] [Google Scholar]
- 53.Haxby JV, et al. , Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science, 2001. 293(5539): p. 2425–2430. [DOI] [PubMed] [Google Scholar]
- 54.Kriegeskorte N, Mur M, and Bandettini P, Representational similarity analysis - connecting the branches of systems neuroscience. Front Syst Neurosci, 2008. 2: p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki M, et al. , Neural basis of temporal context memory: a functional MRI study. Neuroimage, 2002. 17(4): p. 1790–6. [DOI] [PubMed] [Google Scholar]
- 56.Turk-Browne NB, Simon MG, and Sederberg PB, Scene representations in parahippocampal cortex depend on temporal context. J Neurosci, 2012. 32(21): p. 7202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang F and Diana RA, Neural correlates of temporal context retrieval for abstract scrambled phrases: Reducing narrative and familiarity-based strategies. Brain Res, 2017. 1655: p. 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dimsdale-Zucker HR, et al. , Hippocampal subfields integrate information about past temporal and cognitive contexts. BioRXiv, 2019. [Google Scholar]
- 59.Bransford JD and Johnson MK, Contextual Prerequisites for Understanding - Some Investigations of Comprehension and Recall. Journal of Verbal Learning and Verbal Behavior, 1972. 11(6): p. 717–726. [Google Scholar]
- 60.Bartlett FC, Remembering. 1932, Cambridge: Cambridge University Press. [Google Scholar]
- 61.McKenzie S, et al. , Hippocampal Representation of Related and Opposing Memories Develop within Distinct, Hierarchically Organized Neural Schemas. Neuron, 2014. 83(1): p. 202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zacks JM, et al. , Event perception: a mind-brain perspective. Psychol Bull, 2007. 133(2): p. 273–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DuBrow S and Davachi L, Temporal memory is shaped by encoding stability and intervening item reactivation. J Neurosci, 2014. 34(42): p. 13998–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ezzyat Y and Davachi L, Similarity breeds proximity: pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron, 2014. 81(5): p. 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brunec IK, Moscovitch M, and Barense MD, Boundaries Shape Cognitive Representations of Spaces and Events. Trends Cogn Sci, 2018. 22(7): p. 637–650. [DOI] [PubMed] [Google Scholar]
- 66.Kim H, Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage, 2010. 50(4): p. 1648–57. [DOI] [PubMed] [Google Scholar]
- 67.Shimamura AP, Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci, 2011. 11(3): p. 277–91. [DOI] [PubMed] [Google Scholar]
- 68.Thakral PP, Wang TH, and Rugg MD, Decoding the content of recollection within the core recollection network and beyond. Cortex, 2017. 91: p. 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raichle ME, The brain’s default mode network. Annu Rev Neurosci, 2015. 38: p. 433–47. [DOI] [PubMed] [Google Scholar]
- 70.Kaplan R, et al. , Hippocampal Sharp-Wave Ripples Influence Selective Activation of the Default Mode Network. Curr Biol, 2016. 26(5): p. 686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sreekumar V, et al. , The experience of vivid autobiographical reminiscence is supported by subjective content representations in the precuneus. Sci Rep, 2018. 8(1): p. 14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weidemann CT, et al. , Neural activity reveals interactions between episodic and semantic memory systems during retrieval. J Exp Psychol Gen, 2019. 148(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang H, Fell J, and Axmacher N, Electrophysiological mechanisms of human memory consolidation. Nat Commun, 2018. 9(1): p. 4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacDonald CJ, et al. , Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron, 2011. 71(4): p. 737–49.* Mau et al. 2018: This study reported ensembles of “time cells” in hippocampus that simultaneously carry information about temporal sequence order on timescales of seconds, minutes and days, providing compelling empirical evidence for mechanisms proposed in a computational model of hippocampal function [ref 75].
- 75.Mau W, et al. , The Same Hippocampal CA1 Population Simultaneously Codes Temporal Information over Multiple Timescales. Curr Biol, 2018. 28(10): p. 1499–1508 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Howard MW, et al. , A unified mathematical framework for coding time, space, and sequences in the hippocampal region. J Neurosci, 2014. 34(13): p. 4692–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Howard MW, et al. , A distributed representation of internal time. Psychol Rev, 2015. 122(1): p. 24–53. [DOI] [PubMed] [Google Scholar]
- 78.Eichenbaum H, On the Integration of Space, Time, and Memory. Neuron, 2017. 95(5): p. 1007–1018.** Aly and Ranganath 2018: This guest editorial introduces a special issue discussing new perspectives on hippocampal function, in which the various articles outline a diversity of functional roles for the hippocampus, including perception, working memory, attention and implicit memory. Together, the arguments laid out by these authors usher in a new philosophy on how to approach brain function.
- 79.Aly M and Ranganath C, New perspectives on the hippocampus and memory. Neurosci Lett, 2018. 680: p. 1–3. [DOI] [PubMed] [Google Scholar]
- 80.Cordova NI, Turk-Browne NB, and Aly M, Focusing on what matters: Modulation of the human hippocampus by relational attention. Hippocampus, 2019. 29(11): p. 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hasson U, Chen J, and Honey CJ, Hierarchical process memory: memory as an integral component of information processing. Trends Cogn Sci, 2015. 19(6): p. 304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bussey TJ and Saksida LM, Memory, perception, and the ventral visual-perirhinalhippocampal stream: Thinking outside of the boxes. Hippocampus, 2007. 17(9): p. 898–908. [DOI] [PubMed] [Google Scholar]
- 83.Bartko SJ, et al. , Perceptual functions of perirhinal cortex in rats: Zero-delay object recognition and simultaneous oddity discriminations. Journal of Neuroscience, 2007. 27(10): p. 2548–2559.* Mack, Love and Preston, 2018: This theoretical synthesis suggests that the hippocampus transforms episodic experiences into conceptual knowledge by deploying many different cognitive operations including attentional biasing, pattern completion-based retrieval, memory integration, pattern separation and the generation of prediction error.
- 84.Mack ML, Love BC, and Preston AR, Building concepts one episode at a time: The hippocampus and concept formation. Neurosci Lett, 2018. 680: p. 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aly M and Turk-Browne NB, Flexible weighting of diverse inputs makes hippocampal function malleable. Neuroscience Letters, 2018. 680: p. 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]