Abstract
This investigation examined whether the variation of cerebral structure is associated with genetic or environmental factors in children with autism spectrum disorder (ASD) compared with typically developing (TD) controls. T1-weighted magnetic resonance imaging scans were obtained from twin pairs (aged 6–15 years) in which at least one twin was diagnosed with ASD or both were TD. Good quality data were available from 30 ASD, 18 discordant, and 34 TD pairs (n = 164). Structural measures (volume, cortical thickness, and surface area) were generated with FreeSurfer, and ACE modeling was completed. Lobar structures were primarily genetically mediated in TD twins (a2 = 0.60–0.89), except thickness of the temporal (a2 = 0.33 [0.04, 0.63]) and occipital lobes (c2 = 0.61 [0.45, 0.77]). Lobar structures were also predominantly genetically mediated in twins with ASD (a2 = 0.70–1.00); however, thickness of the frontal (c2 = 0.81 [0.71, 0.92]), temporal (c2 = 0.77 [0.60, 0.93]), and parietal lobes (c2 = 0.87 [0.77, 0.97]), and frontal gray matter (GM) volume (c2 = 0.79 [0.63, 0.95]), were associated with environmental factors. Conversely, occipital thickness (a2 = 0.93 [0.75, 1.11]) did not exhibit the environmental contributions that were found in controls. Differences in GM volume were associated with social communication impairments for the frontal (r = 0.52 [0.18, 0.75]), temporal (r = 0.61 [0.30, 0.80]), and parietal lobes (r = 0.53 [0.19, 0.76]). To our knowledge, this is the first investigation to suggest that environmental factors influence GM to a larger extent in children with ASD, especially in the frontal lobe.
Keywords: cortical thickness, FreeSurfer, frontal lobe, heritability, social communication
Introduction
Autism spectrum disorder (ASD) is diagnosed in approximately 1 in 59 children in the United States of America (Baio et al. 2018) with variable presentation of the cognitive and behavioral social communication impairments (SCIs) and restricted, repetitive patterns of behavior and interests (RRB). Up to 20% of cases are associated with various rare genetic abnormalities, but the vast majority likely originate from interactions between common multifactorial genetic variation and environmental factors (Miles 2011). Individuals with this idiopathic form of autism may have a genetic vulnerability that interacts with environmental influences (Hallmayer et al. 2011) during critical developmental periods to alter the neuronal circuits that support the affected cognitive and behavioral domains.
Twin study designs have been applied in preliminary neuroimaging studies to assess the influence of genetic and environmental factors on brain development. Brain structure is primarily genetically mediated during typical development (Baare et al. 2001; Wallace et al. 2006; Panizzon et al. 2009; Winkler et al. 2010), and studies of twin pairs discordant for ASD indicate that volumetric alterations are present compared with both unaffected cotwins (Mitchell et al. 2009) and typically developing (TD) controls (Kates et al. 2004), see review (Mevel et al. 2015). Findings from these studies, and more recent examinations of a larger twin sample (Hegarty et al. 2018; Hegarty et al. 2019), suggest that cerebral development is also primarily genetically mediated in children with ASD. However, nongenetic factors also affect the structural variation of the brain, and even though certain brain regions are more heavily influenced by environmental factors during typical development (Wallace et al. 2006; Lenroot et al. 2007), children with ASD may be more sensitive to environmental influences compared with TD controls (Sajdel-Sulkowska et al. 2011). To date, it is not yet clear which brain regions and structural properties of the brain are most susceptible to environmental influences during development in children with autism.
The purpose of this investigation was to examine autism-related differences in the influence of genetic and environmental factors on the primary cerebral lobes. To our knowledge, the contributions from these factors on different brain structures have not yet been compared between twins with ASD and TD controls. Based on previous investigations (Baare et al. 2001; Kates et al. 2004; Wallace et al. 2006; Mitchell et al. 2009), we hypothesized that cerebral tissue volume, including gray matter (GM) and white matter (WM), would be primarily influenced by genetic factors in both TD twins as well as twins with ASD. Because some brain regions are more affected by adaptive, experience-based alterations during early brain development (Wallace et al. 2006; Lenroot et al. 2007), we also predicted that deviations from these primarily genetically driven models would most likely be found in the lobes containing the primary sensory processing regions (i.e., temporal and occipital). We expected that there would most likely be differential contributions from genetic and environmental factors on surface area and cortical thickness because adult studies have suggested that these factors are genetically and phenotypically independent (Panizzon et al. 2009; Winkler et al. 2010) and exhibit different developmental trajectories (Ecker et al. 2013; Wierenga et al. 2014). Although more recent research in infants (Jha et al. 2018) and children/adolescents (Schmitt et al. 2019) have also suggested that there may be considerable genetic overlap between surface area and cortical thickness earlier in development, significant differences in the impact of genetic and environmental influences on these structural brain measures are supported across twins studies. Following preliminary research of global structural measures of the brain (Hegarty et al. 2019), the current investigation examined regional lobar structures. We expected that surface area would be primarily genetically mediated but that cortical thickness would be primarily influenced by environmental factors in twins with ASD. We also expected that differences in environmental contributions to cortical thickness between twins with ASD and TD controls would be particularly robust in the frontal and temporal lobes because they contain some of the most frequently implicated brain regions (Carper et al. 2002; Ecker et al. 2012).
Materials and Methods
Participants
One-hundred and forty-nine twin pairs were initially enrolled in this study. Following formal screening procedures, 90 male or female same-sex twin pairs (180 individuals aged 6–15 years old) in which at least one twin was diagnosed with ASD or both were TD controls were invited to participate between 2010 and 2015. An initial power analysis, which was based on 80 probands and 40 unrelated controls allowing for 20% missing data (n = 96), indicated sufficient power to detect group-related differences within the expected effect size range (Sacco et al. 2015). Primary recruitment was from the California Autism Twin Study (Hallmayer et al. 2011) and Interactive Autism Network research databases and was supplemented with local/online advertisements. Twin pairs were excluded if either twin exhibited genetic or metabolic disorders, unstable medical conditions (e.g., seizures), a history of traumatic head injury or asphyxia at birth, or contraindication for magnetic resonance imaging (MRI). Control participants were also required to have no history of learning disabilities or severe psychiatric disorders and a full-scale IQ (FSIQ) > 70. Twin pair zygosity was confirmed from saliva samples based on nine short tandem repeat loci and the X/Y amelogenin compared between twins. Monozygotic (MZ) twin pairs exhibited concordance on all markers and dizygotic (DZ) twin pairs exhibited discordance on at least one marker (Hallmayer et al. 2011). The methodology of this investigation was approved by the Institutional Review Board. Written consent was obtained from parents/caregivers, and assent from participants in accordance with the Declaration of Helsinki.
1.1. Cognitive and Behavioral Testing
ASD diagnosis was confirmed with the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994) and Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) (Lord et al. 2012). Discordance for autism was defined by one twin meeting diagnostic criteria on the ADI-R (Lord et al. 1994) and ADOS-2 (Lord et al. 2012) and the other twin not meeting criteria for ASD or exhibiting subthreshold autism-related impairments on either of these diagnostic measures. The social responsiveness scale (SRS) (Constantino and Gruber 2007) and short sensory profile (SSP) (Dunn 1999) were also obtained to compare autism-related symptoms between groups. The Stanford-Binet Intelligence Scales, Fifth Edition (Roid 2003), was used to assess general cognitive abilities, and handedness was evaluated with the Edinburgh Handedness Inventory (Oldfield 1971).
MRI Acquisition and Processing
Before attempting MRI acquisition, all participants were assessed on an MRI simulator to confirm their ability to successfully complete scanning procedures. Individuals that were not within acceptable motion thresholds (<2 mm in any direction) were provided additional training or excluded from the study. Alternatively, individuals with ASD who were unable to meet motion thresholds were offered the use of propofol under the supervision of an anesthesiologist (rate of 200–300 mcg/kg/min). Neuroimaging was conducted on two identical GE 3T MR750 scanners at the same institution using a standard eight-channel head coil. At least two T1-weighted IR SPGR echo pulse sequences were acquired from each participant (188 coronal slices, repetition time = 8.15 ms, echo time = 3.24 ms, inversion time = 600 ms, flip angle = 12°, slice thickness = 1.2 mm, field of view = 22 × 22 cm, in-plane resolution = 0.86 × 0.86, and acquisition matrix size = 256 × 192 mm, number of excitations = 1). This procedure was repeated as necessary until quality data were acquired. The highest quality image (e.g., no wrap around, drop out, or motion artefacts) was analyzed with FreeSurfer v 5.3 using the standard settings of the recon-all command with the 3T flag (Fischl 2012). Trained raters inspected all automated procedures and manually corrected segmentation errors. If significant motion artifacts were present or segmentation errors could not be corrected, the scans were excluded from further analysis. Structural measures were then evaluated with Qdec and smoothed using a standard 10-mm FWHM kernel. Lobar structural measures, excluding the cingulate, were then calculated by summing (volume, surface area) or averaging (thickness) the relevant gyral estimates from the Desikan–Killiany atlas (Desikan et al. 2006) across the left and right hemispheres, as described at https://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation and outlined in Supplemental Table 1. Lobar structures were the focus of this study to allow comparisons of regional structural brain measures to previous neuroimaging investigations of pediatric TD twins (Wallace et al. 2006).
As previously outlined (Hegarty et al. 2019), two sets of twins were scanned at both imaging sites, which were at the same institution. Within-subject comparisons indicated an approximately 6% difference in total brain volume between scanners. Affected scans (ASD and controls) were transformed using the FSL linear transformation package FLIRT with standard sinc interpolation to FSL standard orientation images prior to segmentation with FreeSurfer. The transformation matrix was designed to minimize site differences across all associated structural parameters. Importantly, twin pair analyses excluding the transformed data did not significantly differ from those reported for the full data set regarding total brain volume, surface area, or cortical thickness. Additionally, both twins from each pair were always scanned at the same location because our analyses were primarily based on variation within twin pairs.
Statistical Analysis
Intra-class correlations (ICC), adjusted for gender and diagnosis, were examined in 2017 and 2018 in all MZ and DZ twin pairs using STATA (StataCorp 2017) and the DeFries-Fulker model (DeFries and Fulker 1985) framework to evaluate whether our data met the general assumptions for twin modeling. ICCs were also generated separately for the ASD and control subgroups, adjusting for gender and excluding twin pairs discordant for autism, and compared with Fisher’s Z-transformation (Fisher 1915). Additional covariates of interest (e.g., age and socioeconomic status (SES)) were also evaluated. Importantly, ICC estimates did not significantly differ from those reported below and any marginal alterations were generally consistent across the MZ and DZ groups (i.e., twin modeling results were also consistent with those reported below). Twin pairs discordant for ASD were excluded from subgroup analyses to provide a more representative estimation of potential autism-related differences because they most likely differ to a greater degree regarding environmental factors compared with concordant twin pairs. The ACE model for broad sense heritability was then calculated based on Falconer’s formula (Falconer 1981). The ACE model estimates the variation in a trait of interest that is related to additive genetic (a2) and common/shared (c2) or unique (e2) environmental factors by comparing trait variability in MZ versus DZ twin pairs. Pearson’s correlations of within twin pair differences in lobar structures and symptom severity, as assessed with the SRS, were also examined to evaluate genetic and environmental influences on brain-behaviors relationships. False discovery rate (Benjamini and Hochberg 1995) correction was used to account for multiple comparisons.
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Participants
At the time of this analysis, MRI scans were available from 88 twin pairs (n = 176 twins), with good quality scans from 30 twin pairs in which both twins had ASD (15 MZ; 15 DZ), 18 twin pairs discordant for ASD (4 MZ; 14 DZ), and 34 TD control pairs (20 MZ; 14 DZ), n = 164 twins. Two twin pairs were excluded due to excessive motion artifacts, two twin pairs were excluded because one twin withdrew before completing imaging procedures, and two twin pairs were excluded due to inconclusive diagnostic results. Groups were generally well-matched, see Table 1, but SES was lower in twins with autism compared with control twins (M = −5.29, 95% CI [−10.51, −0.72]), which was predominantly due to DZ twin pairs (M = −10.06, 95% CI [−18.11, −2.01]). There were no other zygosity by diagnostic group differences and adjusting for SES did not significantly alter the twin modeling results. Twins from ASD pairs (M = −7.39, 95% CI [−14.26, −0.13]) and control twins (M = −4.28, 95% CI [−6.82, −1.74]) exhibited some within-group differences in total SRS scores comparing the DZ and MZ subgroups, which was likely related to the greater number of DZ twin pairs discordant for ASD. TD twins were also well below the threshold for any clinically relevant symptoms, regardless of zygosity. Interestingly, differences in cognitive functioning, FSIQ (M = −11.53, 95% CI [−21.32, −1.75]), and autism symptoms, SRS (M = 12.87, 95% CI [6.60, 19.13]), were observed in MZ twin pairs concordant for ASD, suggesting a relationship between nongenetic factors and symptom severity.
Table 1.
Demographics and clinical characteristics
| Demographics | ASD (n = 30) | TD (n = 34) | ASD versus TD | ||||
|---|---|---|---|---|---|---|---|
| Concordant twin pairs | M | SD | M | SD | t or χ2 | P | |
| Age (years) | 10.90 | 2.80 | 9.59 | 2.69 | 1.91 | 0.06 | |
| Sex (male/female) | 24/6 | - | 24/10 | - | 0.75 | 0.39 | |
| Ethnicity (A/B/H/W/MO) | 2/1/3/19/5 | - | 2/0/1/27/4 | - | 3.27 | 0.51 | |
| SES (Hollingshead) | 50.22 | 11.86 | 55.52 | 8.60 | −2.03 | 0.05* | d |
| Clinical characteristics | ASD (n = 96) | TD (n = 68) | ASD versus TD | ||||
| Individuals | M | SD | M | SD | t or χ2 | P | |
| Handedness (right/left) | 85/11 | - | 62/6 | - | 0.30 | 0.59 | |
| Full scale IQ | 86.40 | 25.85 | 112.99 | 11.53 | −7.92 | <0.001† | c,d |
| Sensory Processing (SSP) | 149.33 | 25.54 | 175.10 | 14.85 | −7.05 | <0.001† | c,d |
| SRS | 67.15 | 17.47 | 43.63 | 5.49 | 10.64 | <0.001† | a,b,c,d |
| ADI-R diagnostic total | 33.99 | 18.91 | - | - | - | - | |
| ADOS-2 comp. score | 6.27 | 2.84 | - | - | - | - | |
The current samples are comprised of twin pairs in which both twins were concordant for ASD or were TD control twin pairs or all individuals, including those discordant for ASD. Mean (M) and standard deviations (SD) or counts within each category are presented. ADI-R diagnostic total is the sum of the social interaction, communication, and restricted/repetitive behavior subscales . ADOS-2 comp. score is the standardized comparison score across modules. Group comparisons (ASD vs. TD) were conducted with independent samples t tests (t) or chi-squared (χ2). A/B/H/W/MO = Asian/Black/Hispanic/White/multiple or other. Significant group comparison at *P < 0.05 or †false discovery rate (Benjamini and Hochberg 1995) corrected across the tests within each column is indicated.
a = MZ ASD vs. DZ ASD P ≤ 0.05,
b = MZ TD vs. DZ TD P ≤ 0.05,
c = MZ ASD vs. MZ TD P ≤ 0.05,
d = DZ ASD vs. DZ TD P ≤ 0.05
Of the 82 twin pairs included in this analysis, 37 pairs were assessed on one scanner and 45 pairs were assessed on the other scanner, see Supplementary Figure 1 for distributions of lobar volumes across sites. Comparison between scanners, controlling for transformation status, indicated minimal site-related effects that were limited to cortical thickness of the parietal lobe, F (1,161) = 5.71, P = 0.02. However, this difference was no longer evident when controlling for ASD severity as assessed by the SRS, P > 0.05, suggesting that it may be related to autism-related differences in brain morphology. There was also a similar observation regarding sedation status in twins with ASD, in which there was a marginal difference in parietal lobe thickness, t(94) = −2.27, P = 0.03, that was no longer evident when controlling for ASD severity, P > 0.05. Diagnostic group comparisons of lobar structures are included in the supplementary materials (Supplementary Tables 2 and 3). In general, autism-related differences were found within all of the lobar compartments comparing twins with ASD and TD controls, including cortical thickness of the parietal lobe, P < 0.001; however, these differences were only evident when controlling for total brain volume and age. Age-dependent differences would be expected for this patient group based on previous reports of developmental changes from early childhood to adolescence (Courchesne et al. 2011).
ICCs in MZ and DZ Twin Pairs
Examining all twin pairs regardless of diagnosis, we found the anticipated pattern of ICCs (Supplementary Table 4). They were positive, statistically significant, and higher in MZ compared with DZ twin pairs. The only exceptions were in DZ twins for temporal lobe surface area (ICC = 0.23, 95% CI [−0.03, 0.50]), and parietal lobe WM (ICC = 0.27, 95% CI [−0.07, 0.61]) and surface area (ICC = 0.22, 95% CI [−0.02, 0.46]). The zygosity subgroups also did not significantly differ in ICC estimates for thickness of the temporal (ICCMZ = 0.57, ICCDZ = 0.51; Z = 0.54, P = 0.59) and occipital lobes (ICCMZ = 0.74, ICCDZ = 0.61; Z = 1.55, P = 0.12).
Examining the ICCs from ASD and control twins separately (Fig. 1; Supplementary Table 5) revealed relevant differences between the diagnostic groups. All ICC estimates for MZ ASD and control twins were large magnitude (e.g., ~ 0.70–0.97) and statistically significant, P < 0.001 in all instances, with the exception of cortical thickness of the temporal lobe in control twins (ICC = 0.34, 95% CI [0.04, 0.64]). Comparing MZ twin pairs, ICCs were larger in MZ twins with ASD compared with MZ control twins for the thickness of the temporal (ICCASD = 0.79, ICCTD = 0.34; Z = 2.79, P = 0.01) and occipital lobes (ICCASD = 0.87, ICCTD = 0.67; Z = 2.09, P = 0.04) as well as parietal lobe total (ICCASD = 0.96, ICCTD = 0.86; Z = 2.82, P = 0.01), GM (ICCASD = 0.95, ICCTD = 0.83; Z = 2.54, P = 0.01) and WM volume (ICCASD = 0.97, ICCTD = 0.92; Z = 2.12, P = 0.03), and surface area (ICCASD = 0.95, ICCTD = 0.88; Z = 2.08, P = 0.04). However, these differences were generally low magnitude, especially regarding the parietal lobe, and did not survive correction for multiple comparisons. DZ twins with ASD also generally exhibited higher ICCs compared with control twins, including frontal lobe GM volume (ICCASD = 0.72, ICCTD = −0.02; Z = 3.32, P < 0.001), thickness (ICCASD = 0.78, ICCTD = 0.15; Z = 3.23, P = 0.001), and surface area (ICCASD = 0.62, ICCTD = 0.03; Z = 2.49, P = 0.01) as well as cortical thickness of the temporal (ICCASD = 0.75, ICCTD = 0.15; Z = 2.93, P = 0.003) and parietal lobes (ICCASD = 0.91, ICCTD = 0.08; Z = 5.25, P < 0.001). The ICC for occipital lobe WM volume was also lower in DZ twins with ASD compared with control twins (ICCASD = 0.01, ICCTD = 0.53; Z = −2.09, P = 0.04), but the only differences that survived correction for multiple comparisons were for frontal lobe GM volume and the thickness of the frontal, temporal, and parietal lobes.
Figure 1.
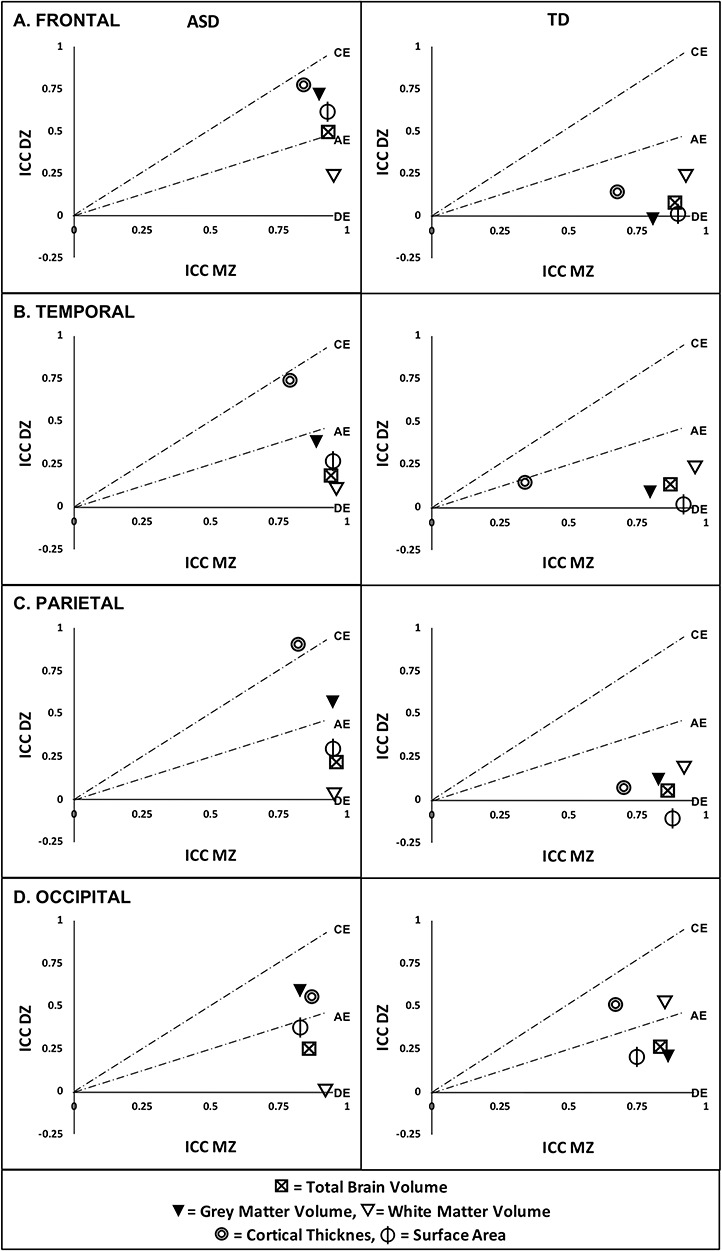
ICCs in ACE model space. ICCs from twin pairs in which both twins were diagnosed with ASD or were TD controls, adjusted for gender, were generated separately for MZ and DZ twin pairs and are displayed in relation to ACE model space [a = additive genetics; c = shared family environment; e = unique environment; d = genetic dominance] for the (A) frontal, (B) temporal, (C) parietal, and (D) occipital lobes. Under a primarily environmental CE model, ICCs in MZ and DZ twin pairs are equal or nearly equal, while under primarily genetic AE or DE models, the ICC in MZ twins is approximately twice, or more, that in DZ twin pairs.
ACE Modeling
ACE modeling provides an estimation of the magnitude for the relationship between genetic/environmental factors and variation in lobar structure (Table 2). Within control twin pairs, almost all structural measures that could be modeled were best fit with the AE model (a2 = 0.60–0.89), suggesting significant genetic contributions. Frontal lobe GM volume and parietal lobe surface area could not be modeled due to negative ICCs in the DZ control subgroup, but variation in these structures also appeared to be primarily influenced by genetic factors based on ICC comparisons. Temporal lobe thickness was also best fit with the AE model, but the majority of variation were attributed to the environmental factor, and the thickness of the occipital lobe was best fit with the CE model, suggesting primarily environmental influences.
Table 2.
ACE model parameter estimates in concordant ASD and TD twin pairs
| A (additive genetics) | C (shared environment) | E (unique environment) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASD | CI | P | TD | CI | P | ASD | CI | P | TD | CI | P | ASD | CI | TD | CI | |
| Frontal lobe | ||||||||||||||||
| Total volume | 0.94 | [0.70, 1.18] | <0.001† | 0.77 | [0.55, 1.00] | <0.001† | x | x | x | x | x | x | 0.06 | [−0.18, 0.30] | 0.23 | [0.003, 0.45] |
| GM volume | x | x | x | - | - | - | 0.79 | [0.63, 0.95] | <0.001† | x | x | x | 0.21 | [0.05, 0.37] | - | - |
| WM volume | 0.86 | [0.58, 1.14] | <0.001† | 0.84 | [0.63, 1.05] | <0.001† | x | x | x | x | x | x | 0.14 | [−0.14, 0.42] | 0.16 | [−0.05, 0.37] |
| Thickness | x | x | x | 0.60 | [0.37, 0.83] | <0.001† | 0.81 | [0.71, 0.92] | <0.001† | x | x | x | 0.19 | [0.08, 0.29] | 0.40 | [0.17, 0.63] |
| Surface area | 1.00 | [0.78, 1.21] | <0.001† | 0.73 | [0.43, 1.03] | <0.001† | x | x | x | x | x | x | 0.01 | [−0.21, 0.22] | 0.27 | [−0.03, 0.57] |
| Temporal lobe | ||||||||||||||||
| Total volume | 0.81 | [0.46, 1.15] | <0.001† | 0.80 | [0.48, 1.12] | <0.001† | x | x | x | x | x | x | 0.19 | [−0.15, 0.54] | 0.20 | [−0.12, 0.52] |
| GM volume | 0.86 | [0.60, 1.12] | <0.001† | 0.74 | [0.41, 1.07] | <0.001† | x | x | x | x | x | x | 0.14 | [−0.12, 0.40] | 0.26 | [−0.07, 0.59] |
| WM volume | 0.80 | [0.44, 1.15] | <0.001† | 0.89 | [0.64, 1.13] | <0.001† | x | x | x | x | x | 0.21 | [−0.15, 0.56] | 0.11 | [−0.13, 0.36] | |
| Thickness | x | x | x | 0.33 | [0.04, 0.63] | 0.03† | 0.77 | [0.60, 0.93] | <0.001† | x | x | x | 0.24 | [0.07, 0.40] | 0.67 | [0.37, 0.96] |
| Surface area | 0.87 | [0.64, 1.11] | <0.001† | 0.79 | [0.50, 1.08] | <0.001† | x | x | x | x | x | x | 0.13 | [−0.11, 0.36] | 0.21 | [−0.08, 0.50] |
| Parietal lobe | ||||||||||||||||
| Total volume | 0.85 | [0.44, 1.25] | <0.001† | 0.75 | [0.50, 0.99] | <0.001† | x | x | x | x | x | x | 0.16 | [−0.25, 0.56] | 0.25 | [0.09, 0.50] |
| GM volume | 1.00 | [0.77, 1.25] | <0.001† | 0.76 | [0.54, 0.98] | <0.001† | x | x | x | x | x | x | 0.00 | [−0.25, 0.23] | 0.24 | [0.02, 0.46] |
| WM volume | 0.80 | [0.40, 1.19] | <0.001† | 0.88 | [0.59, 1.07] | <0.001† | x | x | x | x | x | x | 0.20 | [−0.19, 0.60] | 0.12 | [−0.07, 0.41] |
| Thickness | x | x | x | 0.63 | [0.43, 0.83] | <0.001† | 0.87 | [0.77, 0.97] | <0.001† | x | x | x | 0.13 | [0.03, 0.24] | 0.37 | [0.17, 0.57] |
| Surface area | 0.89 | [0.68, 1.10] | <0.001† | - | - | - | x | x | x | x | x | x | 0.11 | [−0.10, 0.32] | - | - |
| Occipital lobe | ||||||||||||||||
| Total volume | 0.76 | [0.45, 1.06] | <0.001† | 0.80 | [0.62, 0.97] | <0.001† | x | x | x | x | x | x | 0.24 | [−0.06, 0.55] | 0.21 | [0.03, 0.38] |
| GM volume | 0.93 | [0.70, 1.16] | <0.001† | 0.80 | [0.60, 1.00] | <0.001† | x | x | x | x | x | x | 0.07 | [−0.16, 0.30] | 0.20 | [0.00, 0.40] |
| WM volume | 0.70 | [0.34, 1.07] | <0.001† | 0.89 | [0.74, 1.05] | <0.001† | x | x | x | x | x | x | 0.30 | [−0.07, 0.67] | 0.11 | [−0.05, 0.26] |
| Thickness | 0.93 | [0.75, 1.11] | <0.001† | x | x | x | x | x | x | 0.61 | [0.45, 0.77] | <0.001† | 0.07 | [−0.11, 0.25] | 0.39 | [0.23, 0.55] |
| Surface area | 0.81 | [0.53, 1.10] | <0.001† | 0.69 | [0.45, 0.94] | <0.001† | x | x | x | x | x | x | 0.19 | [−0.10, 0.47] | 0.31 | [0.06, 0.56] |
The ACE model for broad sense heritability was calculated separately for twin pairs in which both twins were diagnosed with ASD or were TD controls based on Falconer’s formula (Falconer 1981) [a = additive genetics; c = shared family environment; e = unique environment] utilizing a bootstrapping method to calculate estimates across 1000 repetitions. Twin pairs discordant for ASD were excluded. When A or C was nonsignificant within the model (x), a constrained AE or CE model was utilized. When model assumptions were violated, model estimates were not generated, but the model parameters that would have been most appropriate based on ICC estimates are indicated (−). E estimates were based on the residuals and were not tested for significance. Significant model parameter at *P < 0.05 and †false discovery rate (Benjamini and Hochberg 1995) correction across the estimates within each column are indicated.
Within twin pairs with ASD, there were some significant deviations from the ACE models that were identified in control twins. Estimates for total and GM/WM volume as well as surface area of all of the lobar compartments were mostly best fit with AE models and also primarily genetically mediated (a2 = 0.70–1.00). However, frontal lobe GM volume and frontal, temporal, and parietal lobe thickness were best fit with the CE model in twins with ASD, suggesting significant environmental contributions. Conversely, occipital lobe thickness was best fit with the AE model and appeared to be primarily genetically mediated.
Relationships Between Lobar Structures and Autism-Related Symptoms
Correlations between twin pair differences in structural measures and SRS scores indicated some interesting brain-behavior relationships (Fig. 2). The primary associations that were identified were between differences in GM volume and the severity of SCIs. Across all DZ twins, regardless of diagnosis, frontal (r = 0.45, 95% CI [0.16, 0.67], P = 0.004), temporal (r = 0.50, 95% CI [0.22, 0.70], P = 0.001), and parietal lobe (r = 0.46, 95% CI [0.17, 0.67], P = 0.003) GM volume exhibited correlations that survived correction for multiple comparisons; whereas the occipital lobe did not (r = 0.27, 95% CI [−0.05, 0.54], P = 0.09). These relationships appeared to be primarily due to DZ twins with ASD who exhibited correlations that survived correction for multiple comparisons for the frontal (r = 0.52, 95% CI [0.18, 0.75], P = 0.01), temporal (r = 0.61, 95% CI [0.30, 0.80], P = 0.001), and parietal lobes (r = 0.53, 95% CI [0.19, 0.76], P = 0.004); whereas no significant correlations were found in DZ control twins, P > 0.05 in all instances. The lack of brain-behavior relationships in control twins is probably associated with the relatively low variability of autism-related symptoms, as measured by the SRS, in the current sample. Interestingly, these relationships were not found in MZ twins with or without ASD or with more general cognitive abilities as assessed with FSIQ from the SB-5, P > 0.05 in all instances.
Figure 2.
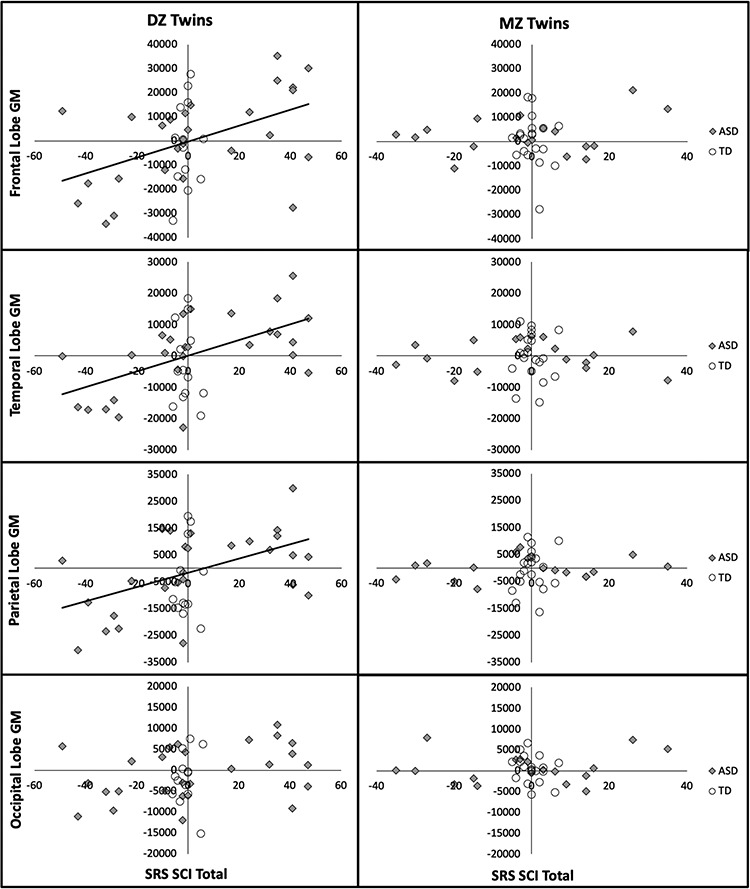
Relationships between intra-twin pair differences in GM volume and SCI. Within twin pair differences in lobar GM volume (mm3) and SCIs, as assessed with the SRS, are displayed for DZ and MZ twin pairs in which at least one twin was diagnosed with ASD (squares) or both were TD controls (circles). Pearson’s correlations that survived false discovery rate (Benjamini and Hochberg 1995) correction for multiple comparisons across all lobar structures (solid line) are displayed for ASD twin pairs. There were no significant correlations in TD twins or MZ twins with ASD.
Discussion
Structural variation in the primary lobar compartments of the brain was predominantly genetically mediated in TD control twins, except for cortical thickness of the temporal and occipital lobes. These findings support that while brain structure is primarily influenced by genetic factors during typical development, environmental factors also modify the development of certain brain structures, such as the lobes that contain the primary sensory processing regions (Wallace et al. 2006; Lenroot et al. 2007). As expected, the majority of structural variation in the lobar compartments was also predominantly genetically mediated in twins with ASD. However, cortical thickness of the frontal, temporal, and parietal lobes, as well as GM volume of the frontal lobe, were primarily associated with environmental factors. Additionally, occipital lobe thickness did not exhibit the expected environmental influences compared with TD controls. These findings support that brain development is considerably genetically mediated during typical development but also suggest that environmental factors may impact GM to a larger extent in children with ASD, especially in the frontal lobe which may be particularly vulnerable. Twin pair differences in GM volume were also associated with differences in the severity of autism-related symptoms in DZ but not MZ twins, indicating that genetic differences may have influenced these brain-behavior relationships. Conversely, these correlations could have been related to environmental differences within twin pairs discordant for ASD. Cumulatively, these observations suggest that genetic susceptibility for ASD may increase vulnerability to environmental insults and the interaction between these factors could alter regional brain structure to impact cognitive and behavioral development.
Our findings are consistent with what has been previously reported in TD twins (Peper et al. 2007), which supports our approach and choice of control group. Variation in brain structure is primarily associated with genetic factors during typical brain development (66–97%) (Bartley et al. 1997; Pennington et al. 2000; Baare et al. 2001; Wright et al. 2002; Wallace et al. 2006), including cortical GM (65–82%) and WM (73–93%) volume (Baare et al. 2001; Wallace et al. 2006; Yoon et al. 2011; Blokland et al. 2012), surface area (71–89%), and cortical thickness (60–81%) (Panizzon et al. 2009; Winkler et al. 2010). In addition to these primarily genetically mediated models, there are also some brain structures that are more sensitive to environmental factors. For instance, temporal lobe volume is influenced by environmental factors to a larger extent during typical brain development (Geschwind et al. 2002; Wallace et al. 2006). This may also be the case for the occipital and parietal lobes (Geschwind et al. 2002), but reports regarding these structures are less consistent. Differences across studies could be associated with the variable influences of genetic and environmental factors during diverse developmental periods (Wallace et al. 2006) or other factors (e.g., epigenetics) that cannot be easily segregated with this approach. Nonetheless, the relatively high heritability of brain structure during typical development is well-supported across investigations.
Dynamic changes in the effects of genetic and environmental factors on variation in brain structure have also been reported depending on the region and developmental period (Giedd et al. 2007; Lenroot and Giedd 2008; Jha et al. 2018; Schmitt et al. 2019). Most twin studies of adults indicate that brain structure is primarily genetically mediated, but investigations of pediatric samples suggest that environmental factors may affect some brain regions to a larger extent during childhood, with increasing influence of genetic factors over time (Wallace et al. 2006; Jha et al. 2018; Schmitt et al. 2019). For instance, environmental factors have a variable but significant impact on the development of cortical thickness across most of the brain in neonates (Jha et al. 2018). The magnitude of these influences may increase with age during childhood in regions in the temporal and occipital lobes that are associated with sensory processing, but decrease with age in higher order brain regions, such as the frontal lobe regions involved with executive control and language (Lenroot et al. 2007). Conversely, it appears that surface area is predominantly genetically mediated throughout the lifespan (Panizzon et al. 2009; Winkler et al. 2010; Jha et al. 2018; Schmitt et al. 2019). Consistent with these observations, environmental factors had the greatest influence on cortical thickness of the temporal and occipital lobes in the current TD twin sample, and surface area was primarily influenced by genetic factors across all of the lobes. In general, evolutionarily older regions may undergo greater early genetic control on some brain structures, which could be modulated by increased environmental influences over time; whereas more recently evolved regions may be susceptible to early environmental exposures with increasing genetic control later in life (Lenroot and Giedd 2008). Cortical thickness may also be particularly impacted by environmental influences during early neonatal development (Jha et al. 2018). Based on this model, the neurobiological alterations that are often observed in individuals with idiopathic forms of ASD may be associated with the interaction between genetic susceptibility for the disorder and environmental exposures altering the typical shift between genetic control and adaptive, experience-based development.
In the current investigation, twins with ASD exhibited significant environmental influences on cortical thickness of the frontal, temporal, and parietal lobes, as well as frontal lobe GM volume, but did not exhibit the anticipated effects on the occipital lobe. Considering the aforementioned early environmental influences on cortical thickness across much of the brain (Jha et al. 2018), which may reduce in magnitude throughout the lifespan (Panizzon et al. 2009; Winkler et al. 2010; Schmitt et al. 2019), our findings suggest that differences in cortical thickness in individuals with ASD (Hardan et al. 2006) may be associated with vulnerability to environmental factors for a longer period during development than is found in TD controls. Although it is not yet clear which specific developmental factors or insults are driving these effects (e.g., in utero exposure to drugs or stress, perinatal complications, postnatal toxins or pollution, etc.), recent studies of infants at high risk for ASD reported that alterations in cortical thickness are not yet present during early infancy (Hazlett et al. 2017), suggesting that they may occur after the first 2 years of postnatal development. Our findings also suggest that higher order frontal regions may be susceptible to environmental factors for a longer period in children and adolescents with ASD and that lower order sensory regions of the occipital and temporal lobes may not experience the same adaptive neuroplasticity that is found during typical brain development. This is particularly relevant considering the relatively high rates of intellectual disability and sensory processing abnormalities in this patient group.
An altered shift between genetic versus environmental modulation, or vice versa, on the development of different brain regions might be related with the presence of autism-related symptoms and could underlie some of the heterogeneity across diagnosed individuals. Based on the increased environmental influences that were found in twins with ASD, it appeared as if this shift may be due to the increased environmental susceptibility of cortical thickness. However, we also found a relationship between GM differences and core symptoms that may have been associated with genetic factors. This is based on the significant brain-behavior relationships within DZ twins with autism, who differ in ~ 50% of their genetic makeup, but not in MZ twins, who share the same genetic background. Considering that GM volume is composed of two independent components (Panizzon et al. 2009; Winkler et al. 2010), cortical thickness and surface area, genetic-modulation of the latter may account for some variation in these brain-behavior relationships. This is especially relevant because autism-related differences in surface area appear very early in postnatal development (Hazlett et al. 2017). However, we also cannot rule out that postnatal environmental differences within twin pairs discordant for ASD could have contributed because our twin pair approach assumes that shared environmental effects are the same for MZ and DZ twins, which is not the case for twins discordant for a disorder that affects the manner in which they experience and react to the environment. Additionally, these relationships did not extend to measures of general cognitive abilities, such as IQ, suggesting that they may be more specific to autism-related differences in cognitive and behavioral processing, which is supported by a recent twin study of social cognition in which the relationship between social cognition abilities and autism severity appeared to be influenced by environmental factors (Isaksson et al. 2019). Importantly, the most obvious indicator of environmental effects is the existence of differences in symptomatology in MZ twins who are concordant for ASD, such as the within pair variation in IQ and SCIs that were observed. Thus, genetic factors might increase vulnerability for the development of ASD and alter GM development via pathways that affect cortical surface area expansion (e.g., 16p11.2 deletions or duplications, which are associated with increased susceptibility for ASD [Weiss et al. 2008], may alter cortical surface area in a dose-dependent manner [Qureshi et al. 2014]); whereas environmental influences could play a role in the expression and severity of the different symptom domains via pathways that affect cortical thickness. Clearly, further research will be necessary to elucidate these complex brain-behavior-environment relationships.
There are several limitations that should be considered regarding our research. Although our sample is rather large for an MRI study, it might be underpowered to apply twin modeling techniques to examine some regional structural brain measures across smaller units of segmentation. The basic ACE model that was utilized assumes that there are no gene by environment interactions, which may overestimate the magnitude of some genetic factors, and is unable to estimate the influence of nonadditive genetic effects. The use of two separate scanners may have also introduced some additional variability, but data transformation was applied and site-effects did not significantly alter the reported results. Finally, our preliminary findings indicate the magnitude that genetic and environmental factors may influence brain structure and resulting symptomatology but not the specific mechanisms that are driving these effects, which warrants further investigation.
Summary
Brain structure is primarily genetically mediated during typical development, but there are also considerable environmental influences, such as factors that alter cortical thickness in higher order cognitive regions during early development and lower order processing regions during continued adaptive development later in life (Lenroot et al. 2007). In conjunction with these reports, our findings suggest that individuals with ASD may not experience the same shift in genetic versus environmental control of brain development. As such, differences in genetic susceptibility and environmental exposures during different developmental periods may affect the presence of the disorder and mediate some heterogeneity in SCI, RRB, and other associated cognitive and behavioral symptoms across individuals. Future investigations should assess the specific mechanisms that may be altered during different developmental periods and examine their effects on different symptom domains by phenotyping and genotyping a larger sample of younger twin pairs at high risk for or already exhibiting autism-related impairments, and their siblings, and follow them longitudinally.
Supplementary Material
Funding
This work is supported by an award from the National Institute of Mental Health at the National Institutes of Health (grant R01MH083972 to A.Y.H.), and J.P.H was supported by the Bass Society Pediatric Fellowship Program at Stanford University.
Notes
We would like to thank Sean Berquist, Serena Tamura, and Phoebe Cates for their data acquisition support and the many behavioral technicians that conducted the cognitive/behavioral testing. We would like to especially thank the participants and their families, many of whom traveled great distances. The authors have no competing or conflicts of interest to disclose.
References
- Baare WF, Hulshoff Pol HE, Boomsma DI, Posthuma D, Geus EJ, Schnack HG, Haren NE, Oel CJ, Kahn RS. 2001. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 11:816–824. [DOI] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T et al. 2018. Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 67:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley AJ, Jones DW, Weinberger DR. 1997. Genetic variability of human brain size and cortical gyral patterns. Brain. 120(Pt 2):257–269. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 57:289–300. [Google Scholar]
- Blokland GAM, Zubicaray GI, McMahon KL, Wright MJ. 2012. Genetic and environmental influences on neuroimaging phenotypes: a meta-analytical perspective on twin imaging studies. Twin Res Hum Genet. 15:351–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. 2002. Cerebral lobes in autism: early hyperplasia and abnormal age effects. NeuroImage. 16:1038–1051. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. 2007. Social responsiveness scale (SRS). Los Angeles (CA): Western Psychological Services. [Google Scholar]
- Courchesne E, Campbell K, Solso S. 2011. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 1380:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFries JC, Fulker DW. 1985. Multiple regression analysis of twin data. Behav Genet. 15:467–473. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- Dunn W. 1999. Short sensory profile. San Antonio (TX): Psychological Corporation. [Google Scholar]
- Ecker C, Ginestet C, Feng Y et al. 2013. Brain surface anatomy in adults with autism: the relationship between surface area, cortical thickness, and autistic symptoms. JAMA Psychiat. 70:59–70. [DOI] [PubMed] [Google Scholar]
- Ecker C, Suckling J, Deoni SC et al. 2012. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch Gen Psychiatry. 69:195–209. [DOI] [PubMed] [Google Scholar]
- Falconer DS. 1981. Introduction to quantitative genetics. London: Longman. [Google Scholar]
- Fischl B. 2012. FreeSurfer. NeuroImage. 62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. 1915. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika. 10:507–521. [Google Scholar]
- Geschwind DH, Miller BL, DeCarli C, Carmelli D. 2002. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci. 99:3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Schmitt JE, Neale MC. 2007. Structural brain magnetic resonance imaging of pediatric twins. Hum Brain Mapp. 28:474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K et al. 2011. Genetic heritability and shared environmental factors among twin pairs with autism. JAMA Psychiat. 68:1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ. 2006. An MRI study of increased cortical thickness in autism. Am J Psychiatry. 163:1290–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KN et al. 2017. Early brain development in infants at high risk for autism spectrum disorder. Nature. 542:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty JP II, Gu M, Spielman DM, Cleveland SC, Hallmayer JF, Lazzeroni LC, Raman MM, Frazier TW, Phillips JM, Reiss AL et al. 2018. A proton MR spectroscopy study of the thalamus in twins with autism spectrum disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 81:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty JP II, Pegoraro LFL, Lazzeroni LC, Raman MM, Hallmayer JF, Monterrey JC, Cleveland SC, Wolke ON, Phillips JM, Reiss AL et al. Forthcoming 2019. Genetic and environmental influences on structural brain measures in twins with autism spectrum disorder. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson J, Westeinde A, Cauvet É, Kuja-Halkola R, Lundin K, Neufeld J, Willfors C, Bölte S. 2019. Social cognition in autism and other neurodevelopmental disorders: a co-twin control study. J Autism Dev Disord. 49:2838–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha SC, Xia K, Schmitt JE, Ahn M, Girault JB, Murphy VA, Li G, Wang L, Shen D, Zou F et al. 2018. Genetic influences on neonatal cortical thickness and surface area. Hum Brain Mapp. 39:4998–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D, Landa R, Reiss AL, Pearlson GD. 2004. Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. Am J Psychiatr. 161:539–546. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. 2008. The changing impact of genes and environment on brain development during childhood and adolescence: initial findings from a neuroimaging study of pediatric twins. Dev Psychopathol. 20:1161–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN. 2007. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp. 30:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. 2012. Autism diagnostic observation schedule–2nd edition (ADOS-2). Torrance (CA): Western Psychological Corporation. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. 1994. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24:659–685. [DOI] [PubMed] [Google Scholar]
- Mevel K, Fransson P, Bölte S. 2015. Multimodal brain imaging in autism spectrum disorder and the promise of twin research. Autism. 19:527–541. [DOI] [PubMed] [Google Scholar]
- Miles JH. 2011. Autism spectrum disorders—a genetics review. Genet Med. 13:278. [DOI] [PubMed] [Google Scholar]
- Mitchell SR, Reiss AL, Tatusko DH, Ikuta I, Kazmerski DB, Botti J-AC, Burnette CP, Kates WR. 2009. Neuroanatomic alterations and social and communication deficits in monozygotic twins discordant for autism disorder. Am J Psychiatr. 166(8):917–925. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9:97–113. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE et al. 2009. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Filipek PA, Lefly D, Chhabildas N. 2000. A twin MRI study of size variations in the human brain. J Cogn Neurosci. 12:223–232. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. 2007. Genetic influences on human brain structure: a review of brain imaging studies in twins. Hum Brain Mapp. 28:464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AY, Mueller S, Snyder AZ, Mukherjee P, Berman JI, Roberts TPL, Nagarajan SS, Spiro JE, Chung WK, Sherr EH et al. 2014. Opposing brain differences in 16p11.2 deletion and duplication carriers. J Neurosci. 34:11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid GH. 2003. Stanford-Binet intelligence scales. Itasca (IL): Riverside Publishing. [Google Scholar]
- Sacco R, Gabriele S, Persico AM. 2015. Head circumference and brain size in autism spectrum disorder: a systematic review and meta-analysis. Psychiatry Res Neuroimaging. 234:239–251. [DOI] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, Xu M, McGinnis W, Koibuchi N. 2011. Brain region-specific changes in oxidative stress and Neurotrophin levels in autism spectrum disorders (ASD). Cerebellum. 10:43–48. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Neale MC, Clasen LS, Liu S, Seidlitz J, Pritikin JN, Chu A, Wallace GL, Lee NR, Giedd JN et al. 2019. A comprehensive quantitative genetic analysis of cerebral surface area in youth. J Neurosci. 39:3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp 2017. Stata statistical software. College Station (TX): StateCorp LLC. [Google Scholar]
- Wallace GL, Schmitt JE, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Molloy EA, Clasen LS, Kendler KS, Neale MC et al. 2006. A pediatric twin study of brain morphometry. J Child Psychol Psychiatry. 47:987–993. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira MAR, Green T et al. 2008. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 358:667–675. [DOI] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S. 2014. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 87:120–126. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. 2010. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 53:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IC, Sham P, Murray RM, Weinberger DR, Bullmore ET. 2002. Genetic contributions to regional variability in human brain structure: methods and preliminary results. NeuroImage. 17:256–271. [DOI] [PubMed] [Google Scholar]
- Yoon U, Perusse D, Lee J-M, Evans AC. 2011. Genetic and environmental influences on structural variability of the brain in pediatric twin: deformation based morphometry. Neurosci Lett. 493:8–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


