Abstract
Turner syndrome (TS) is a genetic disorder affecting approximately 1:2000 live-born females. It results from partial or complete X monosomy and is associated with a range of clinical issues including a unique cognitive profile and increased risk for certain behavioral problems. Structural neuroimaging studies in adolescents, adults, and older children with TS have revealed altered neuroanatomy but are unable to identify when in development differences arise. In addition, older children and adults have often been exposed to years of growth hormone and/or exogenous estrogen therapy with potential implications for neurodevelopment. The study presented here is the first to test whether brain structure is altered in infants with TS. Twenty-six infants with TS received high-resolution structural MRI scans of the brain at 1 year of age and were compared to 47 typically developing female and 39 typically developing male infants. Results indicate that the typical neuroanatomical profile seen in older individuals with TS, characterized by decreased gray matter volumes in premotor, somatosensory, and parietal-occipital cortex, is already present at 1 year of age, suggesting a stable phenotype with origins in the prenatal or early postnatal period.
Keywords: infant, MRI, neuroimaging, Turner syndrome
Introduction
Females with Turner syndrome (TS), a well-defined genetic disorder resulting from the partial or complete loss of one of the sex chromosomes, represent a unique population for studying the effects of sex chromosomes and sex hormones on human brain development (Knickmeyer and Davenport 2011; Knickmeyer 2012). Affected individuals are haploinsufficient for genes that are normally expressed from both X chromosomes in females and also experience early loss of ovarian function, resulting in a postnatal developmental hormonal milieu that is estrogen- and androgen-deficient (Davenport et al. 2007). It is one of the most common human chromosomal abnormalities, occurring in approximately 1 in 2000 live female births (Jacobs et al. 1974; Nielsen and Wohlert 1991).
The most prominent physical features of the syndrome are short stature and pubertal delay, but individuals with TS also show a unique pattern of cognitive strengths and weaknesses. Girls and women with TS often exhibit specific deficits in visual-spatial functions (Murphy et al. 1994; Ross et al. 1996; Romans et al. 1998; Collaer et al. 2002; Rae et al. 2004; Hart et al. 2006), arithmetical abilities (Pennington et al. 1982; Rovet 1993; Temple and Carney 1996; Temple and Marriott 1998; Rae et al. 2004; Murphy et al. 2006), and executive functions (Murphy et al. 1994; Romans et al. 1998; Loesch et al. 2005; Green et al. 2015), with preserved or enhanced verbal ability (Temple and Carney 1996; Rae et al. 2004; Temple and Shephard 2012). Impairments in social skills and affective discrimination are common (Ross et al. 1997; Lawrence et al. 2003; Mazzola et al. 2006; Burnett et al. 2010; Hong et al. 2014; Lepage et al. 2014). Associated diagnoses may include attention deficit hyperactivity disorder (ADHD; present in approximately 25%; Russell et al. 2006; Green et al. 2015), specific learning disorders (dyscalculia present in approximately 75%; Mazzocco 2006), social communication and autism spectrum disorders (Hong et al. 2011), and developmental coordination disorder (Nijhuis-van der Sanden et al. 2003). As in other genetic syndromes, there is a high degree of individual variability in the somatic, cognitive, and psychosocial phenotypes that remains poorly understood.
Cognitive strengths and weaknesses presumably reflect changes in underlying neuroanatomy and function. Structural neuroimaging studies have consistently demonstrated a decrease in volume of parietal-occipital gray matter (GM; Brown et al. 2002, 2004; Cutter et al. 2006; Marzelli et al. 2011) and enlargement of the amygdala and orbitofrontal cortex (Good et al. 2003; Kesler et al. 2004; Cutter et al. 2006; Marzelli et al. 2011) in females with TS. Alterations in GM volume have also been reported in the insula, fusiform gyrus, posterior cingulate, inferior temporal gyrus, ventrolateral prefrontal cortex, anterior cingulate, hippocampus, basal ganglia, and around the superior temporal sulcus (Kesler et al. 2003; Cutter et al. 2006; Marzelli et al. 2011). These findings are of great interest, but previous studies have all been carried out in older children and adults; thus, they cannot address when in development these differences arise. Furthermore, older children and adults have often been exposed to many years of growth hormone or anabolic steroid therapy to enhance linear growth and exogenous estrogen therapy for feminization and health maintenance. Treatment may have produced some of the observed neural differences and it may have minimized or eliminated neural differences that were present before treatment began. For this reason, we have carried out the first structural neuroimaging study of infants with TS, prior to hormone therapy.
Materials and Methods
Subjects
A total of 26 females with TS (20 with complete X monosomy and 6 mosaic), 47 typically developing females, and 39 typically developing males are included in this study. All participants were approximately 1 year of age (see Table 1 for additional details). Participants with TS were recruited through the University of North Carolina (UNC) at Chapel Hill Pediatric Endocrinology and UNC Turner Syndrome Clinics, online advertisements with relevant support groups such as the Turner Syndrome Society, and mailings to health care providers both within and outside UNC, including genetic counselors, obstetricians, and pediatricians. Typically developing controls were drawn from an ongoing study of early brain development at UNC (Gilmore et al. 2007, 2012). Recruitment of controls occurred through community physicians, relevant clinics at UNC, including the general obstetrics clinics, and mass emails to the UNC community. Exclusion criteria for both groups included active substance or alcohol abuse or major medical illness in the mother during pregnancy (cancer and autoimmune disease); major psychiatric illness in the mother or the father (e.g., schizophrenia, schizoaffective disorder, bipolar disorder, and psychotic disorder not otherwise specified); extreme prematurity (birth prior to 32 weeks); and any major medical illness, learning delay, or congenital abnormality in the child not associated with a diagnosis of TS. Children were also excluded if health problems or metal implants precluded their participation in MRI. This study was approved by the Institutional Review Board of the UNC School of Medicine. Written informed consent was obtained from a parent or legal guardian prior to the study.
Table 1.
Demographics, medical history, and cognitive performance of infants with TS and typically developing controls
| Variable | TS | Female control | Male control | P | |||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) range | N | Mean (SD) range | N | Mean (SD) range | ||
| Gestational age at birth (days) | 26 | 268 (11) 246–286 | 47 | 277 (9) 259–295 | 39 | 274 (11) 241–289 | 0.0061 |
| Birth weight (grams) | 26 | 2804 (421) 2155–3925 | 47 | 3380 (433) 2340–4414 | 39 | 3386 (475) 2375–4562 | <0.0001 |
| Age at MRI (days) | 26 | 389 (16) 359–419 | 47 | 383 (22) 339–439 | 39 | 382 (19) 343–422 | 0.2056 |
| Maternal age (years) | 25 | 30 (6) 22–43 | 47 | 31 (5) 21–41 | 39 | 31 (4) 21–42 | 0.5514 |
| Paternal age (years) | 24 | 33 (7) 24–51 | 47 | 33 (6) 22–51 | 37 | 33 (4) 23–40 | 0.8312 |
| Maternal education (years) | 24 | 15 (3) 6–20 | 47 | 16 (3) 9–23 | 39 | 16 (3) 10–22 | 0.2354 |
| Paternal education (years) | 23 | 14 (4) 3–23 | 46 | 16 (4) 8–22 | 39 | 16 (3) 9–22 | 0.0927 |
| Total household income (dollars) | 24 | 76 095 (69 420) 0–330 000 | 45 | 65 029 (41 482) 0–170 000 | 37 | 73 562 (47 868) 0–200 000 | 0.8229 |
| Mullen early learning composite | 24 | 100 (14) 72–120 | 47 | 120 (13) 84–144 | 39 | 122 (13) 78–150 | <0.0001 |
| Mullen gross motor* | 19 | 42 (8) 31–58 | 47 | 58 (13) 37–80 | 39 | 57 (15) 20–80 | <0.0001 |
| Mullen fine motor* | 24 | 54 (10) 22–66 | 47 | 64 (8) 44–77 | 39 | 66 (7) 49–80 | <0.0001 |
| Mullen visual reception* | 24 | 52 (8) 38–66 | 47 | 64 (11) 37–80 | 39 | 65 (9) 41–80 | <0.0001 |
| Mullen expressive language* | 24 | 48 (13) 20–71 | 47 | 61 (8) 42–78 | 39 | 60 (9) 33–80 | <0.0001 |
| Mullen receptive language* | 24 | 45 (8) 31–60 | 47 | 52 (8) 31–69 | 39 | 54 (8) 31–77 | <0.0001 |
| N | Percent | N | Percent | N | Percent | ||
| Maternal ethnicity | 0.0181 | ||||||
| White | 23 | 92 | 32 | 68 | 35 | 90 | |
| Black | 2 | 8 | 13 | 28 | 2 | 5 | |
| Asian | 0 | 0 | 2 | 4 | 2 | 5 | |
| Paternal ethnicity | 0.0567 | ||||||
| White | 23 | 92 | 32 | 68 | 32 | 86 | |
| Black | 2 | 8 | 13 | 28 | 3 | 8 | |
| Asian | 0 | 0 | 2 | 4 | 2 | 5 | |
| Smoking | 1.0000 | ||||||
| Yes | 1 | 4 | 3 | 6 | 2 | 5 | |
| No | 25 | 96 | 44 | 94 | 37 | 95 | |
| Scanner | 0.0160 | ||||||
| Trio | 13 | 50 | 11 | 23 | 7 | 18 | |
| Allegra | 13 | 50 | 36 | 77 | 32 | 82 | |
*T-scores; SD means standard deviation.
Image Acquisition
T1-weighted scans were acquired with either a Siemens Allegra head-only 3T scanner or a Siemens TIM Trio 3T scanner (Siemens Medical Supplies, Erlangen, Germany). Scan parameters for the Allegra were as follows: MP-RAGE time repetition (TR), 1900 ms; time echo (TE), 4.38 ms; flip angle, 7°; image resolution, 1 × 1 × 1 mm. Scan parameters for the Trio were as follows: MP-RAGE TR, 1820–1900 ms; TE, 3.74–3.75 ms; flip angle, 7°; image resolution, 1 × 1 × 1 mm.
Image Analysis
Brain tissue was classified as GM, white matter (WM), and cerebrospinal fluid (CSF) using an atlas-moderated iterative expectation maximization segmentation algorithm using the T1 images as previously described (Knickmeyer et al. 2008), following standard reference space alignment and inhomogeneity correction. In addition, GM was parcellated into 90 regions by nonlinear warping of a neonatal adaptation of the Automated Anatomical Labeling (AAL) atlas template as previously described (Shi et al. 2011; Gilmore et al. 2012). With these methods, we obtained measures of intracranial volume (ICV), total GM, total WM, total CSF, and 90 regional GM volumes.
Developmental Assessment
All children completed the Mullen Scales of Early Learning (Mullen 1995) to assess general cognitive and motor development. The Mullen consists of five scales (gross motor, visual reception, fine motor, expressive language, and receptive language) with their own age-group standardized normative T-scores and percentiles. The standardized T-scores of four scales (gross motor not included) are combined into an early learning composite similar to an IQ score. The Mullen has good standardization and reliability data, collected in two phases over an eight-year period in the 1980s, with median internal consistency scores ranging between 0.75 and 0.91, and test–retest correlations over 0.82 for 1–25 months.
Parent of Origin Analysis
To assign the parent of origin of the remaining X chromosome in individuals with nonmosaic TS, we extracted DNA from blood samples provided by the infants and their parents. Samples were available for 11 trios (infant, mother, and father) and 2 pairs (infant and mother). Samples were genotyped using the Illumina MEGA Array. We created two different algorithms for deriving the parent of origin in these families. The first required full trio data, and for each trio involved 1) identifying X chromosome variants with no missing genotypes and homozygous parental genotypes, one with allele A and one with allele B; 2) counting the total number of allele transmissions from mother and from father; 3) a two-sided binomial test of the proportion of mother/father transmissions under a null that 50% of transmissions will come from the mother. The second algorithm is useable on parent/child pairs. Here, for a particular parent/child pair we identified all variants where there is evidence of at least one nonreference allele in either the parent or child genotype. We then tested the null of a lack of nontransmission based on these genotypes, and tested this null using a one-sided binomial test versus an expected proportion of 0.01, which represents any nontransmissions in the data attributable to genotyping error. In cases where we rejected the null, there was considered to be sufficient evidence for the parent in the parent/child pair to not have contributed an X chromosome copy to the child. All classifications made with the parent/child algorithm were concordant with separate classifications made using the trio-based algorithm.
Statistical Analysis
Statistical analyses were performed using SAS statistical software, version 9.4. For demographic data, two-sided Fisher’s exact tests were used to evaluate group differences in categorical variables. Two-sided nonparametric Kruskal–Wallis H tests were used for continuous variables. Subjects with missing data were excluded on a variable-by-variable basis. One-way analysis of covariance (ANCOVA) was used to test for differences in global and regional brain volumes between our three groups: females with TS (TS), typically developing females (control females), and typically developing males (control males). To achieve variance reduction, we included variables previously associated with imaging outcomes as covariates. For global brain volumes the following variables were included as covariates: birth weight, age at MRI, maternal education, paternal education, and scanner (Allegra or Trio). For regional GM volumes the following variables were included as covariates: ICV and scanner. For global brain volumes, the threshold for statistical significance was α = 0.05 uncorrected. For regional GM volumes, the threshold for statistical significance was α = 0.05 after performing an adjusted false discovery rate correction using the linear step-up method of Benjamini and Hochberg (1995) to control for multiple comparisons. If the overall test was significant post hoc tests (pairwise comparisons) were performed.
We also performed the following sensitivity analyses: 1) We repeated our primary analyses excluding individuals with nonmonosomic TS (N = 6). 2) We repeated our primary analyses excluding individuals with evidence of a developmental delay (early learning composite score on the Mullen scales of learning less than 70 at any point from birth to 4 years of age; N = 3; all with TS). 3) We repeated our primary analysis excluding individuals born prematurely (gestational age at birth less than 37 weeks; N = 9; 4 with TS, 5 control males). 4) We repeated our primary analyses including demographic and medical history variables that differed between the three groups as covariates.
Finally, we performed the following exploratory analyses: 1) In order to assess whether variation in brain volumes contributed to variation in cognitive and motor skills, we calculated Pearson correlations between the Mullen early learning composite score (and T-scores on the Mullen subscales) and regional brain volumes that showed significant differences in our primary analysis. Correlations were run within each group and in the combined sample. We also used one-way ANCOVAs to test for differences in Mullen scores between our three groups, using each regional brain volume as a covariate; 2) a t-test for equal least squares (LS) means from linear models was used to determine whether regional brain volumes that showed significant differences in our primary analysis differed between TS infants who received their remaining X chromosome from their mother (maternal X; n = 9) or their father (paternal X; n = 4), adjusting for ICV and scanner.
Results
Demographic and Medical History Variables
There were significant differences between the three groups in gestational age at birth, birth weight, and maternal ethnicity. Infants with TS were born earlier and weighed less at birth than both male and female controls. Female controls were less likely to have a white mother than male controls and females with TS. In addition, a greater proportion of infants with TS were scanned on the Trio scanner (and less on the Allegra) as compared to both male and female controls (Table 1).
Global Brain Volumes
There were no significant differences between the three groups in ICV, total GM, total WM, or total CSF in our primary analysis or our sensitivity analyses (see Table 2 and Supplementary Tables S1–S4).
Table 2.
Similar global brain volumes in infants with TS and typically developing controls
| Brain volume | LSMean (SE) TS | LSMean (SE) control female | LSMean (SE) control male | P-value ANCOVA |
|---|---|---|---|---|
| ICV | 892 235 (17 241) | 926 152 (12 064) | 940 315 (13 244) | 0.1135 |
| GM | 611 636 (11 032) | 636 502 (7719) | 640 016 (8474.47) | 0.1397 |
| WM | 213 653 (5977) | 220 509 (4178) | 228 199 (4587) | 0.1500 |
| CSF | 66 946 (1956) | 69 141 (1368) | 72 101 (1502) | 0.0980 |
SE means standard error.
Regional Brain Volumes
The following regions showed significant differences between groups on the three-way ANCOVA after adjustment for multiple comparisons: right calcarine cortex, left calcarine cortex, left lingual cortex, right lingual cortex, right precentral gyrus, left precentral gyrus, left frontal inferior operon, left frontal inferior trigonal, right parahippocampal cortex, left superior temporal gyrus, right middle temporal gyrus, left Heschl’s gyrus, and right supramarginal gyrus (see Table 3 and Fig. 1a). Post hoc t-tests indicated that TS females had smaller volumes than typical females in the right calcarine cortex, left calcarine cortex, left lingual cortex, right lingual cortex, right precentral gyrus, left precentral gyrus, left frontal inferior operon, left frontal inferior trigonal, and right middle temporal gyrus (see Table 3 and Fig. 1b). TS females had smaller volumes than typical males in right calcarine cortex, left calcarine cortex, left lingual cortex, right lingual cortex, and right precentral gyrus. TS females had larger volumes than typical males in right parahippocampal cortex, right superior temporal gyrus, and left Heschl’s gyrus (see Table 3 and Fig. 1c). Typical females had larger volumes than typical males in right parahippocampal cortex, right superior temporal gyrus left Heschl’s gyrus, left precentral gyrus, and the left frontal inferior trigonal (see Table 3 and Fig. 1d).
Table 3.
Significant differences in regional GM volumes between infants with TS and typically developing controls
| Brain region | LS means (SE) TS | LS means (SE) control female | LS means (SE) control male | P-value ANCOVA (adjusted FDR) | Percent difference TS versus control female | Percent difference TS versus control male | Percent difference control male versus control female |
|---|---|---|---|---|---|---|---|
| Typical males = typical females > TS females | |||||||
| Calcarine (right) | 6675 (185) | 7711 (144) | 7483 (162) | 0.0022 | −13%**** | −11%** | −3% |
| Calcarine (left) | 7280 (206) | 8188 (160) | 7890 (180) | 0.0363 | −11%*** | −8%* | −4% |
| Lingual (right) | 8233 (170) | 9193 (132) | 8901 (148) | 0.0022 | −10%**** | −8%** | −3% |
| Lingual (left) | 8123 (153) | 8987 (119) | 8872 (134) | 0.0022 | −10%**** | −8%*** | −1% |
| Precentral (right) | 8387 (184) | 9115 (143) | 8930 (160) | 0.0390 | −8%** | −6%* | −2% |
| Frontal inferior operon (left) | 2741 (72) | 3049 (56) | 2927 (62) | 0.0363 | −10%*** | −6%† | −4% |
| Supramarginal (right) | 6013 (101) | 6422 (79) | 6246 (88) | 0.0390 | −6%** | −4%† | −3% |
| Typical females > typical males = TS females | |||||||
| Precentral (left) | 8895 (159) | 9367 (124) | 8842 (139) | 0.0382 | −5%* | +1% | −6%** |
| Frontal inferior trigonal (left) | 5879 (123) | 6347 (96) | 6063 (108) | 0.0390 | −7%** | −3% | −5%* |
| Middle temporal (right) | 15 130 (194) | 15 629 (151) | 14 944 (170) | 0.0363 | −3%* | +1% | −4%** |
| Typical females = TS females > typical males | |||||||
| Parahippocampal (right) | 4535 (90) | 4367 (70) | 4167 (79) | 0.0390 | +4% | +9%** | −5%* |
| Heschl (left) | 1180 (37) | 1131 (29) | 1033 (33) | 0.0390 | +4% | +14%** | −9%* |
| Superior temporal (right) | 10 345 (138) | 10 420 (107) | 9962 (120) | 0.0390* | −1% | +4%* | −4%** |
****<0.0001, ***<0.001, **<0.01, *<0.05, †<0.1 (uncorrected P-value post hoc t-test); FDR means false discovery rate.
Figure 1.
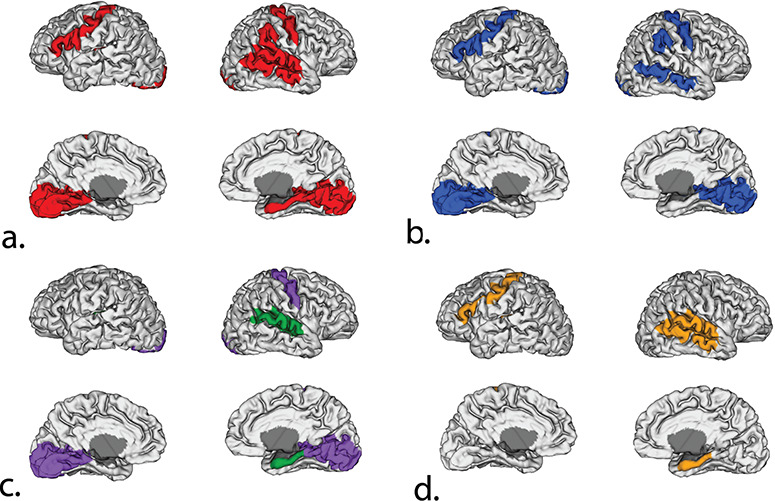
Significant differences in regional GM volumes between infants with TS and typically developing controls. (a) Results of three group ANCOVA on surface reconstruction; regions in red are significant after adjusted FDR correction. (b) Results of post hoc t-tests (TS females versus typical females); blue indicates TS females have significantly smaller volumes than typical females. (c) Results of post hoc t-tests (TS females versus typical males); purple indicates TS females have significantly smaller volumes than typical males; green indicates TS females have significantly larger volumes than typical males. (d) Results of post hoc t-tests (typical females versus typical males); orange indicates typical females have significantly larger volumes than typical males.
Results of our sensitivity analyses can be found in Supplementary Figs S1–S4. Reduced volumes of calcarine and lingual cortex in infants with TS, as compared to both typically developing females and typically developing males, were robust across all sensitivity analyses. A number of additional regions emerged as significant in sensitivity analysis 1 (excluding infants with nonmonosomic TS) including right anterior cingulate cortex (larger in infants with TS compared to typical females and males), left superior occipital gyrus and left postcentral gyrus (both smaller in infants with TS compared to typical females and males), left inferior temporal gyrus (smaller in infants with TS compared to typical females and smaller in typical males compared to typical females), right insular cortex, left posterior cingulate cortex, left hippocampus, left amygdala (all larger in infants with TS compared to typical males), and right Heschl’s gyrus (larger in infants with TS compared to typical males and larger in typical females compared to typical males).
Exploratory Analyses
In the combined sample, scores on the Mullen Scales of Early Learning showed significant correlations with right calcarine, right and left lingual, right precentral, and left frontal inferior trigonal cortex (Table 4). However, no significant correlations are seen within typical males and females (data not shown). Several nominally significant correlations were identified in TS infants (Table 4). Specifically, right lingual cortex volumes were positively correlated with Mullen composite scores and T-scores for receptive language. Right calcarine volumes were positively correlated with expressive language T-scores, and volumes of the left frontal inferior trigonal were positively correlated with gross motor T-scores. None of these relationships would survive correction for multiple comparisons. Group differences in Mullen scores are still significant when including individual brain volumes as covariates (data not shown). We did not observe any significant differences between infants with TS and a maternal X chromosome and those with TS and a paternal X chromosome for brain regions that emerged as significant in our primary analysis (Table 5). In general, individuals with a maternal X chromosome had smaller regional volumes than those with a paternal X chromosome.
Table 4.
Correlations between regional brain volumes identified in the primary analysis and scores on the Mullen Scales of Early Learning (combined sample and TS only)
| Mullen composite | Gross motor | Fine motor | Visual reception | Expressive language | Receptive language | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain region | Combined | TS | Combined | TS | Combined | TS | Combined | TS | Combined | TS | Combined | TS |
| Calcarine (right) | 0.17† | 0.11 | 0.19† | −0.10 | 0.16 | −0.0004 | 0.13 | −0.12 | 0.25** | 0.45* | 0.04 | 0.03 |
| Calcarine (left) | 0.11 | −0.13 | 0.10 | −0.10 | 0.10 | −0.17 | 0.08 | −0.12 | 0.18 | 0.18 | −0.02 | −0.40† |
| Lingual (right) | 0.23*** | 0.46* | 0.09 | 0.27 | 0.27** | 0.38† | 0.29** | 0.18 | 0.28** | 0.31 | 0.23* | 0.62* |
| Lingual (left) | 0.33*** | 0.15 | 0.24* | 0.07 | 0.28** | 0.17 | 0.31** | 0.18 | 0.26** | −0.05 | 0.24* | 0.31 |
| Precentral (right) | 0.19* | 0.10 | 0.27** | 0.05 | 0.14 | 0.11 | 0.24* | −0.29 | 0.12 | 0.32 | 0.14 | 0.17 |
| Frontal inferior operon (left) | 0.12 | −0.06 | 0.15 | 0.09 | 0.18† | 0.07 | 0.09 | −0.0007 | 0.08 | −0.13 | 0.07 | 0.06 |
| Supramarginal (right) | −0.05 | −0.35† | 0.05 | −0.25 | −0.08 | −0.24 | 0.02 | −0.25 | −0.05 | −0.39† | −0.05 | −0.003 |
| Precentral (left) | 0.07 | 0.08 | 0.13 | 0.24 | 0.10 | 0.12 | 0.06 | 0.03 | 0.08 | 0.10 | 0.01 | 0.14 |
| Frontal inferior trigonal (left) | 0.21* | 0.30 | 0.27** | 0.48* | 0.15 | 0.34 | 0.20* | 0.40† | 0.19* | 0.05 | 0.12 | −0.004 |
| Middle temporal (right) | −0.03 | −0.35† | −0.003 | −0.12 | −0.02 | −0.27 | 0.06 | −0.20 | −0.07 | −0.30 | −0.06 | −0.17 |
| Parahippocampal (right) | −0.07 | −0.38† | −0.02 | 0.09 | −0.04 | −0.13 | −0.03 | −0.20 | −0.10 | −0.32 | −0.04 | −0.22 |
| Heschl (left) | 0.07 | 0.08 | −0.09 | −0.40† | 0.05 | −0.10 | 0.08 | −0.04 | 0.03 | 0.18 | −0.02 | −0.24 |
| Superior temporal (right) | 0.03 | −0.27 | 0.13 | 0.08 | 0.006 | −0.17 | 0.03 | −0.14 | 0.02 | −0.22 | 0.04 | −0.08 |
****<0.0001, ***<0.001, **<0.01, *<0.05, †<0.1 (uncorrected P-value); please note that no significant correlations were observed within typical males or typical females.
Table 5.
Parent of origin of the remaining X chromosome exerts minimal/no effect on regional brain volumes
| Brain region | LS means (SE) maternal X | LS means (SE) paternal X | LS mean difference (SE) | P-value test for equality of LS means | Percent difference maternal versus paternal X |
|---|---|---|---|---|---|
| Calcarine (right) | 6266 (241) | 6626 (361) | −360 (435) | 0.4297 | −6% |
| Calcarine (left) | 6966 (248) | 6584 (372) | 383 (448) | 0.4154 | 5% |
| Lingual (right) | 7705 (312) | 8204 (468) | −500 (565) | 0.3994 | −6% |
| Lingual (left) | 7493 (313) | 7810 (470) | −317 (567) | 0.5894 | −4% |
| Precentral (right) | 7798 (195) | 8112 (292) | −313 (352) | 0.3969 | −4% |
| Frontal inferior operon (left) | 2465 (78) | 2534 (117) | −68 (141) | 0.6393 | −2% |
| Supramarginal (right) | 6008 (183) | 5708 (274) | 300 (331) | 0.3875 | 4% |
| Precentral (left) | 8315 (224) | 8038 (336) | 277 (405) | 0.5106 | 3% |
| Frontal inferior trigonal (left) | 5687 (162) | 5796 (243) | −109 (293) | 0.7184 | −2% |
| Middle temporal (right) | 14 440 (280) | 14 703 (420) | −263 (506) | 0.6166 | −2% |
| Parahippocampal (right) | 4201 (150) | 4503 (225) | −302 (271) | 0.2937 | −7% |
| Heschl (left) | 1071 (62) | 1209 (93) | −137 (113) | 0.2520 | −12% |
| Superior temporal (right) | 9838 (94) | 10 013 (141) | −174 (170) | 0.3310 | −2% |
Discussion
We report results from the first quantitative neuroimaging study of infants with TS. Consistent with the literature in school age, adolescent, and adult individuals with TS (Brown et al. 2004; Kesler et al. 2004; Cutter et al. 2006; Marzelli et al. 2011; Green et al. 2014), we observed decreased GM volumes in premotor, somatosensory, and parietal-occipital cortex in comparison to typically developing females. When we restricted our analysis to individuals with monosomic TS, we also observed increased GM volumes in right insular cortex and left amygdala, similar to reports in older cohorts (Good et al. 2003; Kesler et al. 2004; Marzelli et al. 2011). Although post hoc comparisons for the latter two results were only significant when comparing TS females to typically developing males, examination of the least squares means (LSMean) suggests that TS females also have enlarged right insular cortex and left amygdala compared to typical females. Results suggest that many aspects of the neuroanatomical phenotype of TS are established in the prenatal and/or early postnatal period and remain relatively stable into adulthood. This is not entirely surprising given that the prenatal and early postnatal period is the foundational phase of human brain development, characterized by exuberant neurogenesis, neuronal migration, dendritic arborization, synaptogenesis, gyrification, myelinization, and waves of programmed cell death (Stiles and Jernigan 2010).
There were also some aspects of the neuroanatomical phenotype as described in older cohorts that were not present at this early age. In particular, we did not observe volume reductions in the cuneus or the superior parietal lobule, nor did we observe enlargement of the caudate or putamen. Our results suggest that volumetric reductions in primary visual cortex (centered around the calcarine sulcus) and secondary visual cortex (including the lingual gyrus) precede reductions in high-order visual-spatial processing areas. These findings may be relevant to the ongoing debate as to what foundational deficits might explain the broad range of visual-spatial and arithmetical difficulties exhibited by individuals with TS. Some researchers have hypothesized that the foundational deficit in girls with TS is altered development of spatiotemporal attention (Beaton et al. 2010). Others have suggested that impairments in executive function contribute to the emergence of cognitive difficulties in other domains (Lepage et al. 2011). The current study suggests that detailed assessments of the early stages of cortical visual processing in infants and toddlers with TS are warranted in order to understand how low-level computational processing or subtle differences in developmental timing might eventually produce the specific pattern of cognitive strengths and weaknesses observed in older individuals with TS.
We also note that while some researchers posit that the cognitive profile observed in individuals with TS is indicative of right hemisphere dysfunction (also referred to as nonverbal learning disorder or NVLD; Hepworth and Rovet 2000), the majority of neuroanatomical differences we observed were bilateral. Recent literature reviews suggest there is a TS-specific social and cognitive profile which may overlap with other constructs, such as NVLD, but which cannot be reduced to it (Hong et al. 2009;Knickmeyer and Davenport 2011; Gravholt et al. 2017). Our findings support the hypothesis that this social and cognitive profile reflects a relatively uniform dysfunction of the left and right hemispheres (Ganou and Grouios 2008) and is not exclusively tied to WM impairment.
In general, our results suggest that early interventions could be important for improving cognitive and psychosocial outcomes for individuals with TS. Parallels might be drawn to children with autism where behavioral interventions applied in preschool have clinically significant influences on symptoms (Dawson 2008; Odom et al. 2012; Kasari et al. 2014), and emerging evidence indicates earlier interventions have even greater impact (Harris and Handleman 2000; Rogers et al. 2014; Green et al. 2017). Similarly, early life interventions for disadvantaged children, such as high-quality preschool programs, show greater return on investment than later interventions (Masten and Cicchetti 2010). Of course, this assumes that the neuroanatomical phenotypes we observed are linked in a meaningful way to behavioral function. In order to begin exploring this question, we tested whether scores on the Mullen Scales of Early Learning correlated with the 13 regional brain volumes showing significant group differences in our primary analysis. In evaluating these results, it is important to keep in mind that the Mullen scales assess general cognitive and motor ability; the procedure is not designed to target the specific constructs that are disrupted in older individuals with TS. In addition, although we observed significant differences between infants with TS and typical males and females, this did not necessarily reflect poor performance in the TS group. Instead, it appears to reflect exceptionally high performance in our control sample. For a more thorough analysis of early cognitive development in TS, readers are referred to another manuscript from our group (Pretzel et al., unpublished data).
Several significant correlations between key brain volumes and outcomes on the Mullen were observed in the combined sample, but this is likely a consequence of strong group differences between infants with TS and typical infants in both brain volume and Mullen scores. No significant correlations are seen within typical males and females. There are a few nominally significant correlations within infants with TS, although these would not survive correction for multiple comparisons. Larger studies are needed to confirm whether variation in regional brain volumes in infants with TS contributes to individual differences in cognitive, language, and motor development, and whether such findings vary with brain development over time. The current results suggest that variation in lingual and calcarine cortex, as well as the frontal inferior trigonal, may be relevant. Group differences in Mullen scores remain significant when including these individual brain volumes as covariates, which suggests that these particular brain volumes do not mediate the influence of TS diagnosis on Mullen scores.
One of the unique features of the current study is the inclusion of both a female and a male control group. Neuroanatomical differences in girls with TS may arise through a variety of mechanisms including haploinsufficiency of genes on the X chromosome, failure to express parentally imprinted genes, the uncovering of X chromosome mutations, gonadal steroid deficiency (Knickmeyer 2012), and changes in methylation of autosomal genes (Sharma et al. 2015). Comparing girls with TS to both male and female controls provides suggestive evidence regarding potential underlying mechanisms. A pattern of results where typical males = typical females ≠ TS females suggests the action of genes expressed from the inactive X chromosome in typical females that have homologues on the Y chromosome. These are primarily located in an area called the pseudoautosomal region (PAR; Carrel and Willard 2005). In the current study, we observed this pattern of results for calcarine and lingual cortex, the right precentral and supramarginal gyri, and the left frontal inferior operon. A pattern where typical males = TS females ≠ typical females suggests the effect of genes that escape X-inactivation but have no functionally equivalent Y homologues (most likely these would be outside the PAR). However, this result would also be compatible with an estrogen mediated effect or an imprinted X-linked gene. In the current study, we observed this pattern of results for the left precentral gyrus, left frontal inferior trigonal, and the right middle temporal gyrus. Analyses comparing infants with TS and a maternal X chromosome to infants with TS and a paternal X chromosome did not provide strong evidence for an imprinted X-linked gene, but these results must be considered with caution given the small sample size for this exploratory analysis. Finally, a pattern where typical females = TS females ≠ typical males suggests a testosterone-mediated effect but would also be compatible with social/experiential factors associated with sex. We observed this pattern for right parahippocampal cortex, right superior temporal gyrus, and left Heschl’s gyrus.
In conclusion, the current study represents an essential first step in constructing a developmental model of TS. Strengths of the study include the comprehensive analysis of brain structure, the inclusion of both male and female controls, and the careful consideration of mosaicism, developmental delay, and prematurity in our sensitivity analyses. Limitations include the moderate sample size. Although comparable to many studies carried out in older individuals with TS, our analyses may be underpowered to detect subtle differences in brain volume. Correlations between brain volumes and Mullen scores and results on parent of origin effects in particular must be considered as exploratory. Future studies ought to take a longitudinal approach, beginning prenatally or in early infancy, and incorporate neuroimaging, behavioral assessment, and evaluation of relevant clinical parameters such as endogenous hormone levels, growth failure and its treatment, cardiovascular malformations (and related surgeries), thyroid autoimmunity, and hearing issues. Ultimately, a better understanding of early development in girls with TS could lead to new interventions aimed at normalizing adverse ontogenetic pathways. It will also facilitate comparisons to other developmental conditions that may overlap with TS, including ADHD, dyscalculia, and autism, informing the question of whether interventions developed for non-TS populations will have similar, positive effects for girls with TS. Finally, a better understanding of the pathways leading to sexually dimorphic brain development will allow us to clarify how and why the sexes show differential vulnerability to certain psychiatric disorders and open up new possibilities for sex-tailored interventions and therapeutics.
Funding
National Institutes of Health, specifically the National Institute for Mental Health (K01MH083045 to R.C.K.; MH064065 and HD053000 to J.H.G.); Eunice Kennedy Shriver National Institute of Child Health and Human Development (5U54HD079124, Brain Imaging Core); National Center for Advancing Translational Sciences (UL1TR001111, pilot project to M.L.D.); Pfizer (WS1365413 and WS426679 to M.L.D.).
Supplementary Material
Notes
We would like to thank the staff of the University of North Carolina (UNC) MRI Research Center, the UNC BioSpecimen Processing Facility, the UNC Neuro Image Research and Analysis Laboratories, the Infant and Child Behavioral Assessment Laboratory at Frank Porter Graham Child Development Institute, the Carolina Institute for Developmental Disabilities, and the UNC Early Brain Development Program including Patricia Basta, Joseph Blocher, Wendy Neuheimer, Amy Dwy, Danielle Spiker, and Brittany Lindsay. We thank Shaili Jha for her assistance preparing the figures for this manuscript. We thank the staff at RUCDR Infinite Biologics for performing the genetic analysis. We also extend our gratitude to the children and families who participated in this study.
References
- Beaton EA, Stoddard J, Lai S, Lackey J, Shi JR, Ross JL, Simon TJ. 2010. Atypical functional brain activation during a multiple object tracking task in girls with Turner syndrome: neurocorrelates of reduced spatiotemporal resolution. Am J Intellect Dev Disabil. 115:140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 57:289–300. [Google Scholar]
- Brown WE, Kesler SR, Eliez S, Warsofsky IS, Haberecht M, Reiss AL. 2004. A volumetric study of parietal lobe subregions in Turner syndrome. Dev Med Child Neurol. 46:607–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WE, Kesler SR, Eliez S, Warsofsy IS, Haberecht M, Patwardhan A, Ross JL, Neely EK, Zeng SM, Yankowitz J et al. 2002. Brain development in Turner syndrome: a magnetic resonance imaging study. Psychiatry Res. 116:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AC, Reutens DC, Wood AG. 2010. Social cognition in Turner’s syndrome. J Clin Neurosci. 17:283–286. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF. 2005. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 434:400–404. [DOI] [PubMed] [Google Scholar]
- Collaer ML, Geffner ME, Kaufman FR, Buckingham B, Hines M. 2002. Cognitive and behavioral characteristics of Turner syndrome: exploring a role for ovarian hormones in female sexual differentiation. Horm Behav. 41:139–155. [DOI] [PubMed] [Google Scholar]
- Cutter WJ, Daly EM, Robertson DMW, Chitnis XA, Amelsvoort TAMJ, Simmons A, Ng VWK, Williams BS, Shaw P, Conway GS et al. 2006. Influence of X chromosome and hormones on human brain development: a magnetic resonance imaging and proton magnetic resonance spectroscopy study of Turner syndrome. Biol Psychiatry. 59:273–283. [DOI] [PubMed] [Google Scholar]
- Davenport ML, Hooper SR, Zeger M. 2007. Turner syndrome in childhood In: Mazzocco MM, Ross JL, editors. Neurogenetic Developmental Disorders: Manifestation and Identification in Childhood. Cambridge, MA: MIT Press, pp. 3–46. [Google Scholar]
- Dawson G. 2008. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 20:775–803. [DOI] [PubMed] [Google Scholar]
- Ganou M, Grouios G. 2008. Cerebral laterality in Turner syndrome: a critical review of the literature. Child Neuropsychol. 14:135–147. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA et al. 2007. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 27:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, Zhu H, Hamer RM, Styner M, Shen D. 2012. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex. 22:2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Lawrence K, Thomas NS, Price CJ, Ashburner J, Friston KJ, Frackowiak RSJ, Oreland L, Skuse DH. 2003. Dosage-sensitive X-linked locus influences the development of amygdala and orbitofrontal cortex, and fear recognition in humans. Brain. 126:2431–2446. [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, Lin AE, Mauras N, Quigley CA, Rubin K et al. 2017. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 177:G1–G70. [DOI] [PubMed] [Google Scholar]
- Green J, Pickles A, Pasco G, Bedford R, Wan MW, Elsabbagh M, Slonims V, Gliga T, Jones EJ, Cheung CH et al. 2017. Randomised trial of a parent-mediated intervention for infants at high risk for autism: longitudinal outcomes to age 3 years. J Child Psychol Psychiatry. 58:1330–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Bade Shrestha S, Chromik LC, Rutledge K, Pennington BF, Hong DS, Reiss AL. 2015. Elucidating X chromosome influences on attention deficit hyperactivity disorder and executive function. J Psychiatr Res. 68:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Chromik LC, Mazaika PK, Fierro K, Raman MM, Lazzeroni LC, Hong DS, Reiss AL. 2014. Aberrant parietal cortex developmental trajectories in girls with Turner syndrome and related visual-spatial cognitive development: a preliminary study. Am J Med Genet B Neuropsychiatr Genet. 165B:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SL, Handleman JS. 2000. Age and IQ at intake as predictors of placement for young children with autism: a four- to six-year follow-up. J Autism Dev Disord. 30:137–142. [DOI] [PubMed] [Google Scholar]
- Hart SJ, Davenport ML, Hooper SR, Belger A. 2006. Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain. 129:1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth SL, Rovet JF. 2000. Visual integration difficulties in a 9-year-old girl with Turner syndrome: parallel verbal disabilities? Child Neuropsychol. 6:262–273. [DOI] [PubMed] [Google Scholar]
- Hong D, Kent JS, Kesler S. 2009. Cognitive profile of Turner syndrome. Dev Disabil Res Rev. 15:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DS, Bray S, Haas BW, Hoeft F, Reiss AL. 2014. Aberrant neurocognitive processing of fear in young girls with Turner syndrome. Soc Cogn Affect Neurosci. 9:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DS, Dunkin B, Reiss AL. 2011. Psychosocial functioning and social cognitive processing in girls with Turner syndrome. J Dev Behav Pediatr. 32:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs PA, Melville M, Ratcliff S, Keay AJ, Syme J. 1974. Cytogenetic survey of 11,680 newborn infants. Ann Hum Genet. 37:359–376. [DOI] [PubMed] [Google Scholar]
- Kasari C, Shire S, Factor R, McCracken C. 2014. Psychosocial treatments for individuals with autism spectrum disorder across the lifespan: new developments and underlying mechanisms. Curr Psychiatry Rep. 16:512. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Blasey CM, Brown WE, Yankowitz J, Zeng SM, Bender BG, Reiss AL. 2003. Effects of X-monosomy and X-linked imprinting on superior temporal gyrus morphology in Turner syndrome. Biol Psychiatry. 54:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Garrett A, Bender B, Yankowitz J, Zeng SM, Reiss AL. 2004. Amygdala and hippocampal volumes in Turner syndrome: a high-resolution MRI study of X-monosomy. Neuropsychologia. 42:1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer R, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. 2008. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 28:12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC. 2012. Turner syndrome: advances in understanding altered cognition, brain structure and function. Curr Opin Neurol. 25:144–149. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Davenport M. 2011. Turner syndrome and sexual differentiation of the brain: implications for understanding male-biased neurodevelopmental disorders. J Neurodev Disord. 3:293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence K, Kuntsi J, Coleman M, Campbell R, Skuse D. 2003. Face and emotion recognition deficits in Turner syndrome: a possible role for X-linked genes in amygdala development. Neuropsychology. 17:39–49. [PubMed] [Google Scholar]
- Lepage J, Dunkin B, Hong D, Reiss AL. 2011. Contribution of executive functions to visuospatial difficulties in prepubertal girls with Turner syndrome. Dev Neuropsychol. 36:988–1002. [DOI] [PubMed] [Google Scholar]
- Lepage JF, Lortie M, Deal CL, Theoret H. 2014. Empathy, autistic traits, and motor resonance in adults with Turner syndrome. Soc Neurosci. 9:601–609. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Kelso W, Huggins RM, Slater H, Warne G, Bergman PB, Rodda C, Mitchell RJ, Prior M. 2005. Effect of Turner’s syndrome and X-linked imprinting on cognitive status: analysis based on pedigree data. Brain Dev. 27:494–503. [DOI] [PubMed] [Google Scholar]
- Marzelli MJ, Hoeft F, Hong DS, Reiss AL. 2011. Neuroanatomical spatial patterns in Turner syndrome. Neuroimage. 55:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. 2010. Developmental cascades. Dev Psychopathol. 22:491–495. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM. 2006. The cognitive phenotype of Turner syndrome: specific learning disabilities. Int Congr Ser. 1298:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola F, Seigal A, MacAskill A, Corden B, Lawrence K, Skuse DH. 2006. Eye tracking and fear recognition deficits in Turner syndrome. Soc Neurosci. 1:259–269. [DOI] [PubMed] [Google Scholar]
- Mullen EM. 1995. Mullens Scales of Early Development. Circle Pines, MN: American Guidance Service Inc. [Google Scholar]
- Murphy DG, Allen G, Haxby JV, Largay KA, Daly E, White BJ, Powell CM, Schapiro MB. 1994. The effects of sex steroids, and the X chromosome, on female brain function: a study of the neuropsychology of adult Turner syndrome. Neuropsychologia. 32:1309–1323. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Mazzocco MM, Gerner G, Henry AE. 2006. Mathematics learning disability in girls with Turner syndrome or fragile X syndrome. Brain Cogn. 61:195–210. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Wohlert M. 1991. Chromosome abnormalities found among 34910 Newborn children—results from a 13-year incidence study in Arhus, Denmark. Hum Genet. 87:81–83. [DOI] [PubMed] [Google Scholar]
- Nijhuis-van der Sanden MW, Eling PA, Otten BJ. 2003. A review of neuropsychological and motor studies in Turner syndrome. Neurosci Biobehav Rev. 27:329–338. [DOI] [PubMed] [Google Scholar]
- Odom S, Hume K, Boyd B, Stabel A. 2012. Moving beyond the intensive behavior treatment versus eclectic dichotomy: evidence-based and individualized programs for learners with ASD. Behav Modif. 36:270–297. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Bender B, Puck M, Salbenblatt J, Robinson A. 1982. Learning disabilities in children with sex chromosome anomalies. Child Dev. 53:1182–1192. [PubMed] [Google Scholar]
- Pretzel R, Waitt A, Reinhartsen D, DeRamus M, Knickmeyer RC, Davenport ML, Submitted HSR. Cognitive and behavioral development in infants and toddlers with Turner syndrome.
- Rae C, Joy P, Harasty J, Kemp A, Kuan S, Christodoulou J, Cowell CT, Coltheart M. 2004. Enlarged temporal lobes in Turner syndrome: an X-chromosome effect? Cereb Cortex. 14:156–164. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, Ozonoff S. 2014. Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants. J Autism Dev Disord. 44:2981–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romans SM, Stefanatos G, Roeltgen DP, Kushner H, Ross JL. 1998. Transition to young adulthood in Ullrich–Turner syndrome: neurodevelopmental changes. Am J Med Genet. 79:140–147. [PubMed] [Google Scholar]
- Ross JL, Feuillan P, Kushner H, Roeltgen D, Cutler GB Jr. 1997. Absence of growth hormone effects on cognitive function in girls with Turner syndrome. J Clin Endocrinol Metab. 82:1814–1817. [DOI] [PubMed] [Google Scholar]
- Ross JL, Kushner H, Roeltgen DP. 1996. Developmental changes in motor function in girls with Turner syndrome. Pediatr Neurol. 15:317–322. [DOI] [PubMed] [Google Scholar]
- Rovet JF. 1993. The psychoeducational characteristics of children with Turner syndrome. J Learn Disabil. 26:333–341. [DOI] [PubMed] [Google Scholar]
- Russell HF, Wallis D, Mazzocco MM, Moshang T, Zackai E, Zinn AR, Ross JL, Muenke M. 2006. Increased prevalence of ADHD in Turner syndrome with no evidence of imprinting effects. J Pediatr Psychol. 31:945–955. [DOI] [PubMed] [Google Scholar]
- Sharma A, Jamil MA, Nuesgen N, Schreiner F, Priebe L, Hoffmann P, Herns S, Nothen MM, Frohlich H, Oldenburg J et al. 2015. DNA methylation signature in peripheral blood reveals distinct characteristics of human X chromosome numerical aberrations. Clin Epigenetics. 7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, Shen D. 2011. Infant brain atlases from neonates to 1- and 2-year olds. PLoS One. 14:e18746. doi: 10.1371/journal.pone.0018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. 2010. The basics of brain development. Neuropsychol Rev. 20:327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple CM, Carney R. 1996. Reading skills in children with Turner’s syndrome: an analysis of hyperlexia. Cortex. 32:335–345. [DOI] [PubMed] [Google Scholar]
- Temple CM, Marriott AJ. 1998. Arithmetical ability and disability in Turner’s syndrome: a cognitive neuropsychological analysis. Dev Neuropsychol. 14:47–67. [Google Scholar]
- Temple CM, Shephard EE. 2012. Exceptional lexical skills but executive language deficits in school starters and young adults with Turners syndrome: implications for X chromosome effects on brain function. Brain Lang. 120:345–359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


