Abstract
Click chemistry has found wide application in bioconjugation, enabling control over the site of modification in biomolecules. Demonstrations of this chemistry to construct chemically defined antibody-drug conjugates (ADCs) have increased in recent years, following studies that support benefits of homogeneity and site-specificity of drug placement on the antibody. In this chapter, a brief history of early applications of this chemistry in ADCs is presented. Examples of click chemistry that are utilized for ADC synthesis, including those currently undergoing clinical investigation, are enumerated. Protocols for two common methods based on carbonyl-aminooxy coupling and strain-promoted azide-alkyne cycloaddition are presented.
Keywords: Click chemistry, site-specific, antibody-drug conjugates (ADCs), aldehyde, oxime, strain-promoted azide-alkyne cycloaddition (SPAAC), bioconjugation
1. Introduction
The bioconjugation strategy to connect a drug molecule to an antibody continues to be an active area of research in the field of antibody-drug conjugates (ADCs) [1]. Among the four ADCs currently approved by the U.S. Food and Drug Administration (FDA), all of them (Mylotarg, Adcetris, Kadcyla, and Besponsa) are made using stochastic conjugation chemistries that target native lysine or cysteine residues of the antibody. Consequently, these ADCs have heterogeneous drug-to-antibody ratios (DARs) and conjugation sites. For instance, Mylotarg (gemtuzumab ozogamicin), the first FDA-approved ADC, is composed of a mixture of antibodies with a range of DARs, of which ~50% remains unconjugated and can compete with the active ADC for target cell uptake [2]. In addition, the ADCs with variable DARs can possess differential pharmacological properties. Indeed, corroborative studies over the past decade have reached a consensus that the DAR and the site of drug conjugation can have an impact on the pharmacokinetics and the therapeutic index of ADCs [3–5]. Therefore, methods and conjugation chemistries that enable the synthesis of homogeneous, site-specific ADCs are desired.
One seminal method for creating site-specific ADCs is the THIOMABs technology from Genentech [3]. In this approach, the antibody is engineered with cysteine residues at particular sites, to which cysteine-reactive (often maleimide-functionalized) drug linkers will solely attach if the interchain disulfides are left unreduced. A revolving concern regarding this approach is the stability of the conjugation, namely that the thiosuccinimidyl linkage formed from the reaction of cysteine with maleimide is reversible and can thus undergo premature cleavage upon exchange with circulating thiols in vivo. Continued research for stability improvement notwithstanding [4,6–8], interests in other conjugation chemistries forge another front working in parallel toward chemically defined and stable ADCs.
Concurrent development in bioorthogonal chemistries has provided a number of chemical strategies for ADC applications [9]. Often referred interchangeably to as click chemistries, these reactions involve the selective coupling of two unnatural functionalities that are orthogonal, or inert, to other biological functional groups. The product of these reactions should be stable in the biological milieu. The preconditions to be classified as bioorthogonal thus coincide with the attributes that are presently in demand for bioconjugation in ADC production.
While extended exploration of click chemistries for site-specific antibody-drug conjugation is relatively recent, examples of utility of such chemistry can be found even in early-generation ADCs, including Mylotarg [10]. Mylotarg contains a hydrazone bond, formed via one of the earliest bioorthogonal reactions – condensation of a carbonyl (aldehyde/ketone) with an α-effect amine. In this case, the carbonyl is an aromatic ketone installed in a lysine-targeted linker to the antibody and the α-effect amine is a hydrazide derivatized on the calicheamicin payload. It should be noted that the conjugation to the antibody still stems from the lysine-reactive component of the linker, hence the aforementioned heterogeneity of this ADC. The purpose of the hydrazone, which is acid-labile, is to render the ADC susceptible to drug cleavage upon cellular internationalization to the acidic lysosomal compartment. The same pH-sensitive linker is also present in the recently approved ADC, Besponsa (inotuzumab ozogamicin). Outside of serving as a cleavable linker, hydrazone ligation has also been applied for direct conjugation to antibodies that include predecessors of Mylotarg [10–12]. However, the conjugates lacked homogeneity, as the number of aldehydes introduced to the antibody through oxidation of the attached glycan was heterogeneous. Although site-specific ADCs using hydrazone ligation has subsequently been demonstrated [13], the hydrazone linkage has generally been deemed not sufficiently stable to avoid off-target cleavage at physiological condition [14].
Application of carbonyl-based chemistry with α-effect amine continues as the field shifts toward site-specific conjugation. Alkoxyamine is a common choice for the α-effect nucleophile. The condensation product, an oxime, is hydrolytically more stable than a hydrazone [15]. Some site-specific ADCs generated via oxime ligation are currently undergoing clinical development (e.g. ARX788 from Ambrx) [16] or preclinical assessment (e.g. LCB14–0110 from LegoChem Biosciences) [17].
Variations of the carbonyl-based chemistry have also been developed to improve from deficiencies of oxime ligation. Oxime formation suffers from slow kinetics and necessitates acidic condition as well as high concentration of reactants (or large excess of one) to reach appreciable conversion [18,19]. Hydrazino iso-Pictet-Spengler (HIPS) ligation, which pairs an aldehyde with an alkylhydrazine-functionalized indole, has been shown to perform optimally at near neutral pH (pH 6) [20]. The product contains a newly formed C-C bond and is reportedly more stable than the corresponding oxime. Utility of this chemistry for site-specific ADCs has been demonstrated [21] and one (Trph-222 from Triphase - licensed from Catalent) has recently entered clinical trial [22].
In addition to HIPS, other aldehyde-based chemistries have also been reported for ADC synthesis. In a direct comparison of the rate of antibody-drug conjugation, the trapped-Knoevenagel ligation has a reported rate of 0.4 M−1s−1 at pH 7, compared to 0.03 M−1s−1 at pH 4.6 for oxime ligation [23]. Despite the improvement, the rate is still considered relatively slow. A substantially faster aldehyde-based reaction, with rate constants in the order of 103 M−1s−1 at neutral pH, involves an aromatic aldehyde with a boronic acid at the ortho position [24–27]. We have demonstrated that coupling this moiety with an α-amino-hydrazide as the nucleophile produces a unique zwitterionic boron-nitrogen heterocycle that is stable across a wide range of pH [28]. We have recently applied this chemistry to generate site-specific antibody conjugates [29]. Although the work presented thus far has used a fluorophore payload as proof-of-principle, the conjugate’s stability in human serum and its preservation of antibody function show promise for the utility of this developing chemistry for efficient ADC production.
Aside from carbonyl condensation chemistries, another class of click chemistry that is partaking in the development of site-specific ADCs is the azide-alkyne cycloaddition (AAC) reaction. Two major types that have been widely used for bioconjugation are the copper-catalyzed AAC (CuAAC) and the strain-promoted AAC (SPAAC). The former involves the coupling of an azide with a linear alkyne and the latter with a cyclooctyne. As the names suggest, CuAAC is catalyzed by copper while SPAAC relies on the ring strain on the cyclooctyne for its reactivity. Both reactions produce a 1,4-substituted triazole, though only the CuAAC product is regiospecific [30]. Site-specific ADCs conjugated via CuAAC and SPAAC have both been demonstrated [31–37] and some of them are currently under clinical evaluation (e.g. STRO-001 from Sutro Biopharma and ADCT-601 from ADC Therapeutics) [38]. For CuAAC, oxidation of certain amino acids on the antibody due to copper has been observed and is a factor to consider, as oxidized proteins may cause an immunogenic response [34].
Application of the inverse-electron-demand Diels Alder (IEDDA) reactions to construct site-specific ADCs has also been demonstrated. The IEDDA reactions involve the ligation of a strained alkene with a tetrazine and constitute some of the fastest bioorthogonal reactions to date [39]. In particular, in a recent study, an antibody equipped with a cyclopropene was site-specifically conjugated to a tetrazine-functionalized payload [40]. The conjugation was reportedly faster than most of the conjugations that made use of other bioorthogonal handles.
Table 1 illustrates the various click chemistries that have been applied for the generation of homogeneous, site-specific ADCs. At present, two of the most common click chemistries used for antibody-drug conjugation are the oxime and the SPAAC ligations. Therefore, this method chapter will focus on these two chemistries, even as others may assume a broader role in the future.
Table 1.
Click chemistries for site-specific antibody-drug conjugation.
 |
To implement these chemistries for ADC assembly, the bioorthogonal reactive groups need to be introduced to the antibody and the payload. In general, for oxime and SPAAC ligations, the carbonyl or the azide, respectively, is installed on the antibody while the aminooxy or the cyclooctyne is placed on the drug-linker. Table 2 and 3 list select methods that have been developed to enable site-specific incorporation of the carbonyl/azide handle onto the antibody. A collection of previously synthesized drug-linkers that carry the complementary reactive group is also included. Readers are encouraged to refer to the cited references for detailed instructions on the derivatization process prior to conjugation. The method section will concentrate on the procedure to perform the conjugation using oxime and SPAAC chemistries.
Table 2.
Methods for site-specific incorporation of a carbonyl handle onto an antibody.
| Approach | Scheme a | Click Chemistry | Drug-Linker b | Reported DAR (Expected DAR) | Ref. |
|---|---|---|---|---|---|
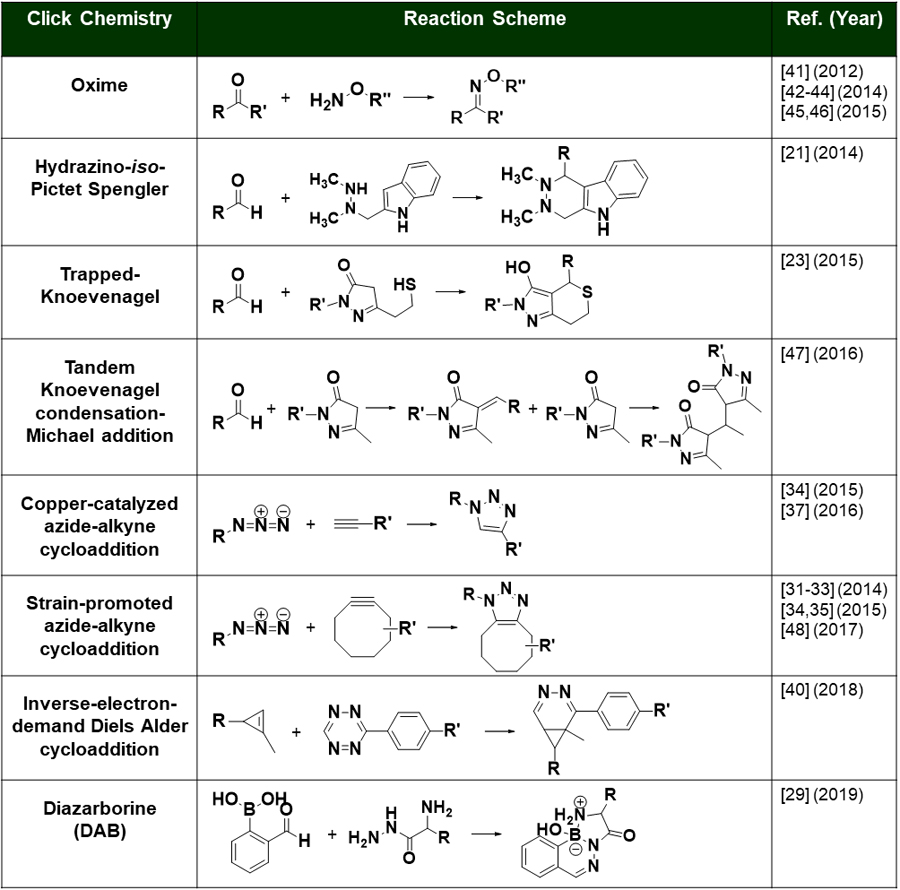
| Glycan Remodeling | 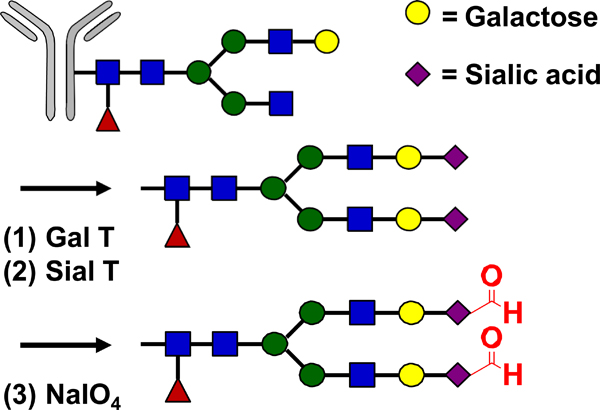 |
Oxime | Aminooxy-MMAE Aminooxy-Dol10 | 1.3–1.9 (4) | [43] |
| GlycanRemodeling |  |
Oxime | Aminooxy-AF | 4 (4) | [44] |
| Unnatural amino acid (UAA) mutagenesis |  |
Oxime | Aminooxy-AF Aminooxy-MMAD | >1.9 (2) | [41] [42] |
| N-terminal serine engineering | 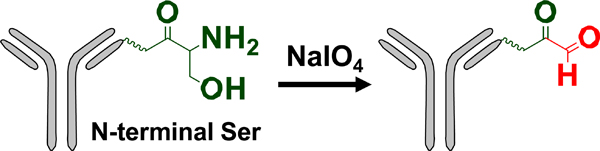 |
Oxime | Aminooxy-MMAE | 1.9 (2) | [45] |
| Enzymatic modification of peptide tag | 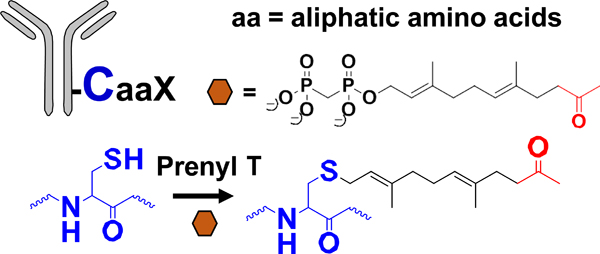 |
Oxime | Aminooxy-MMAF | 2 (2)c | [46,49] |
| Enzymatic modification of peptide tag | 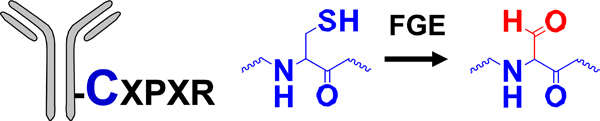 |
HIPS | HIPS-maytansine | 1.5 ->1.8 (2) | [21] |
| Knoevenagel | thioPz-maytansine | 2 (2)c | [23] | ||
| Pz-maytansine | 4 (4)c | [47] | |||
| Enzymatic modification of peptide tag | 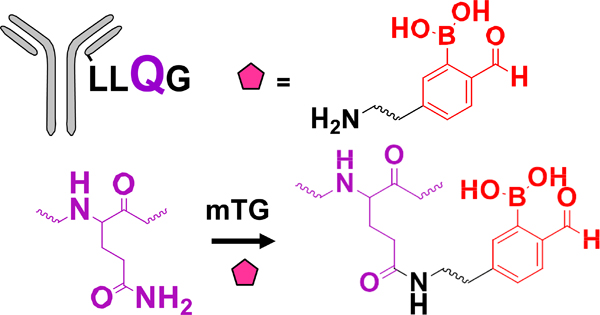 |
DAB | N/A | 1.6 ->1.9 (2) | [29] |
Gal T: β−1,4-galactosyltransferase; Sial T α−2,6-sialyltransferase; NaIO4: sodium periodate; β−1,4-T1-Y289L: Y289 mutant of β−1,4-galactosyltransferase; Prenyl T: prenyl transferase; FGE: formylglycine-generating enzyme; mTG: microbial transglutaminase.
MMAD/MMAE/MMAF: monomethyl auristatin D/E/F; Dol 10: dolastatin 10; AF: auristatin F; thioPz: thiopyrazolone; Pz: pyrazolone. Regarding the drug-linkers listed, only the functional group and the drug are specified. The linker connecting these two moieties may vary.
DAR not explicitly stated.
Table 3.
Methods for site-specific incorporation of an azide handle onto an antibody.
| Approach | Scheme a | Click Chemistry | Drug-Linker b | Reported DAR (Expected DAR) | Ref. |
|---|---|---|---|---|---|
| Glycan Remodeling | 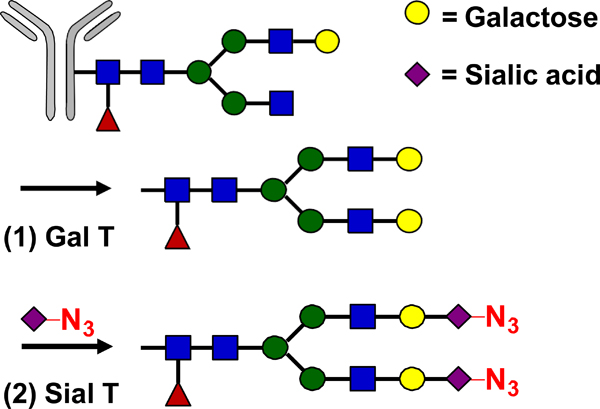 |
SPAAC | DIBO-Dox | 4.5 (4) | [31] |
| Glycan Remodeling | 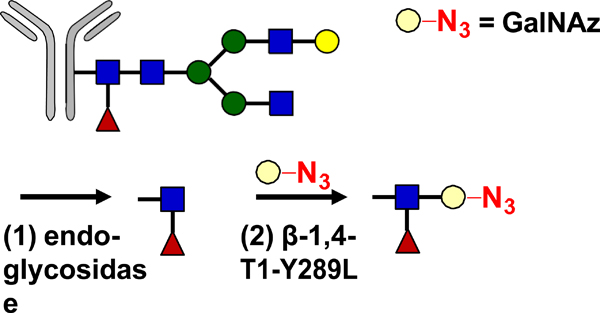 |
SPAAC | BCN-Dox BCN-MMAE BCN-MMAF BCN-maytansine BCN DUMSA | >1.9 (2) | [34] |
| CuAAC | Alkyne-PBD | >1.9 (2) 3.8 (4) | [37] | ||
| Unnatural amino acid (UAA) mutagenesis | 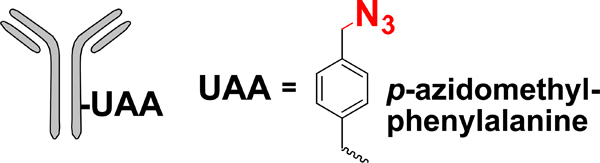 |
SPAAC | DBCO-MMAF | 1.2–1.9 (2) | [32] |
| UAA mutagenesis | 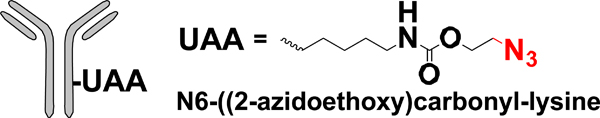 |
CuAAC SPAAC | Alkyne-PBD Alkyne-AF BCN-AF | 1.8 ->1.9 (2) | [35] |
| Enzymatic modification of peptide tag | 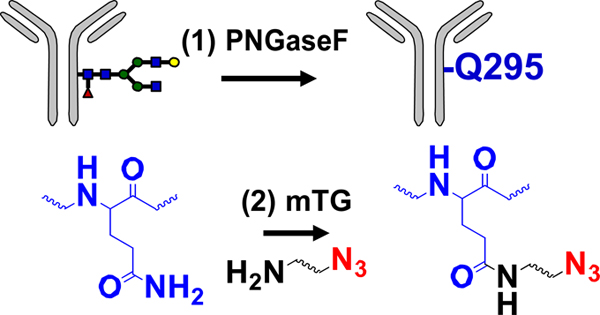 |
SPAAC | DBCO-MMAE | >1.9 (2) | [33] |
| Enzymatic modification of peptide tag | 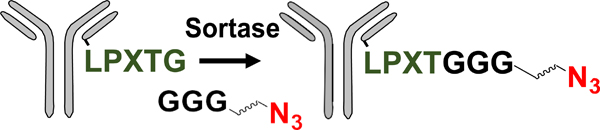 |
SPAAC | DBCO-MMAE | 3.3 (4) | [48] |
GalNAz: azido-modified N-acetyl-D-galactosamine; PNGase F: N-glycosidase F.
DIBO: dibenzocyclooctyne; BCN: bicyclo[6.1.0]nonyne; DUMSA: duocarmycin SA; PBD: pyrrolobenzodiazepine dimer; DBCO: dibenzoazacyclooctyne. Regarding the drug-linkers listed, only the functional group and the drug are specified. The linker connecting these two moieties may vary.
2. Materials
2.1. General
Dimethyl sulfoxide (DMSO) / dimethylformamide (DMF)/dimethylacetamide (DMA)
Desalting column
30 or 50 kDa MWCO ultracentrifugal filter
0.2 μm syringe filter
2.2. Oxime ligation
Acetate buffer, pH 4.5: 0.1 M sodium acetate, pH 4.5
PBS: 10 mM sodium phosphate, 150 mM NaCl, pH 7.4
Aldehyde/ketone functionalized antibody
Aminooxy-functionalized drug-linker
37 °C incubator / water bath
2.3. SPAAC ligation
PBS: 10 mM sodium phosphate, 150 mM NaCl, pH 7.4
Azide-functionalized antibody
Dibenzoazacyclooctyne (DBCO)-functionalized drug-linker
3. Method
3.1. Oxime ligation
Take the purified antibody functionalized with aldehyde/ketone, buffer exchange using a desalting column (e.g. PD-10 column containing Sephadex G-25 resin, GE Healthcare) equilibrated with acetate buffer, pH 4.5 (see Note 1).
Prepare a 26.7 mM stock solution of an aminooxy-functionalized drug-linker in DMSO (see Note 2).
To 10 mg of carbonyl-functionalized antibody in acetate buffer, pH 4.5, add 50 μL of 26.7 mM aminooxy-drug linker stock (see Note 3). The final volume of the sample is 1 mL. The following is the final composition of the sample in terms of concentration: 10 mg/mL (66.7 μM) antibody and 1.33 mM aminooxy-drug linker in acetate buffer, pH 4.5 containing 5% DMSO (see Note 4, 5, 6 & 7).
Allow the sample to incubate at 37 °C for 1–4 days (see Note 8).
Remove excess drug-linkers by subjecting the sample to a desalting column equilibrated with PBS (see Note 9).
The resultant ADC sample may be concentrated using a 30 or 50 kDa MWCO protein concentrator (e.g. Amicon Ultra centrifugal filter).
Filter the purified ADC with a 0.2 μm syringe filter.
The ADC sample can be stored at −20 °C to −80 °C until use.
3.2. SPAAC ligation
Take the purified antibody functionalized with azide, buffer exchange using a desalting column equilibrated with PBS (see Note 10).
Prepare a 26.7 mM stock solution of a DBCO-functionalized drug-linker in DMSO (see Note 2 & 11).
To 10 mg of azide-functionalized antibody in PBS, add 50 μL of 26.7 mM DBCO-drug linker stock (see Note 3). The final volume of the sample is 1 mL. The following is the final composition of the sample in terms of concentration: 10 mg/mL (66.7 μM) antibody and 1.33 mM DBCO-drug linker in PBS containing 5% DMSO (see Note 4, 5 & 6).
Allow the sample to incubate at room temperature for 2 hours (see Note 8).
Remove excess drug-linkers by subjecting the sample to a desalting column equilibrated with PBS (see Note 9).
The resultant ADC sample may be concentrated using a 30 or 50 kDa MWCO protein concentrator (e.g. Amicon Ultra centrifugal filter).
Filter the purified ADC with a 0.2 μm syringe filter.
The ADC sample can be stored at −20 °C to −80 °C until use.
4. Notes
Avoid amine-containing buffer, such as Tris or glycine.
Drug-linker may be dissolved in organic solvents other than DMSO, typically DMF or DMA.
The protocol here is for a scale of 10 mg antibody.
Here the antibody is assumed to have one reactive group on each light or heavy chain for a total of two reactive groups per antibody. The concentration of the drug-linker selected here (1.33 mM) corresponds to ten equivalents relative to the number of reactive group on the antibody.
The percentage of organic cosolvent is dependent on the solubility of the drug-linker. A less soluble drug-linker would require a higher percentage of organic cosolvent.
A catalyst may be added to speed up the reaction or to allow it to occur at neutral pH. Aniline or aniline derivatives are commonly used at 10 mM to 100 mM range as a nucleophilic catalyst [19,51,45,52,53]. We use 4-aminophenylalanine for catalysis, which is more biocompatible than aniline and accelerates the conjugation reaction rate at low temperature and neutral pH [54].
Analysis by LC-MS or hydrophobic interaction chromatography (HIC) is recommended to ensure that the conjugation is complete within the suggested time frame.
ADC can be further purified using HIC if the conjugation does not reach 100%. See chapter 20 for further instructions.
Avoid azide (such as sodium azide)-containing buffer. Buffers other than PBS with a buffer range near neutral pH may be used.
Other cyclooctyne-functionalized drug-linkers may be considered. Reported examples include dibenzocyclooctyne (DIBO) [31] and bicyclo[6.1.0]nonyne (BCN) [34,35]. BCN is reportedly able to conjugate with an azide-functionalized antibody more efficiently and is considered a less hydrophobic alternative to DBCO [34].
References
- 1.Beck A, Goetsch L, Dumontet C, Corvaia N (2017) Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov 16 (5):315–337. doi: 10.1038/nrd.2016.268 [DOI] [PubMed] [Google Scholar]
- 2.Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, Roy S, Sridhara R, Rahman A, Williams G, Pazdur R (2001) Approval Summary. Gemtuzumab Ozogamicin in Relapsed Acute Myeloid Leukemia 7 (6):1490–1496 [PubMed] [Google Scholar]
- 3.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, Lu Y, Meng YG, Ng C, Yang J, Lee CC, Duenas E, Gorrell J, Katta V, Kim A, McDorman K, Flagella K, Venook R, Ross S, Spencer SD, Lee Wong W, Lowman HB, Vandlen R, Sliwkowski MX, Scheller RH, Polakis P, Mallet W (2008) Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nature Biotechnology 26:925. doi: 10.1038/nbt.1480 [DOI] [PubMed] [Google Scholar]
- 4.Shen BQ, Xu K, Liu L, Raab H, Bhakta S, Kenrick M, Parsons-Reponte KL, Tien J, Yu SF, Mai E, Li D, Tibbitts J, Baudys J, Saad OM, Scales SJ, McDonald PJ, Hass PE, Eigenbrot C, Nguyen T, Solis WA, Fuji RN, Flagella KM, Patel D, Spencer SD, Khawli LA, Ebens A, Wong WL, Vandlen R, Kaur S, Sliwkowski MX, Scheller RH, Polakis P, Junutula JR (2012) Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol 30 (2):184–189. doi: 10.1038/nbt.2108 [DOI] [PubMed] [Google Scholar]
- 5.Strop P, Liu SH, Dorywalska M, Delaria K, Dushin RG, Tran TT, Ho WH, Farias S, Casas MG, Abdiche Y, Zhou D, Chandrasekaran R, Samain C, Loo C, Rossi A, Rickert M, Krimm S, Wong T, Chin SM, Yu J, Dilley J, Chaparro-Riggers J, Filzen GF, O’Donnell CJ, Wang F, Myers JS, Pons J, Shelton DL, Rajpal A (2013) Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem Biol 20 (2):161–167. doi: 10.1016/j.chembiol.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 6.Tumey LN, Charati M, He T, Sousa E, Ma D, Han X, Clark T, Casavant J, Loganzo F, Barletta F, Lucas J, Graziani EI (2014) Mild method for succinimide hydrolysis on ADCs: impact on ADC potency, stability, exposure, and efficacy. Bioconjug Chem 25 (10):1871–1880. doi: 10.1021/bc500357n [DOI] [PubMed] [Google Scholar]
- 7.Tumey LN, Li F, Rago B, Han X, Loganzo F, Musto S, Graziani EI, Puthenveetil S, Casavant J, Marquette K, Clark T, Bikker J, Bennett EM, Barletta F, Piche-Nicholas N, Tam A, O’Donnell CJ, Gerber HP, Tchistiakova L (2017) Site Selection: a Case Study in the Identification of Optimal Cysteine Engineered Antibody Drug Conjugates. AAPS J 19 (4):1123–1135. doi: 10.1208/s12248-017-0083-7 [DOI] [PubMed] [Google Scholar]
- 8.Vollmar BS, Wei B, Ohri R, Zhou J, He J, Yu SF, Leipold D, Cosino E, Yee S, Fourie-O’Donohue A, Li G, Phillips GL, Kozak KR, Kamath A, Xu K, Lee G, Lazar GA, Erickson HK (2017) Attachment Site Cysteine Thiol pKa Is a Key Driver for Site-Dependent Stability of THIOMAB Antibody-Drug Conjugates. Bioconjug Chem 28 (10):2538–2548. doi: 10.1021/acs.bioconjchem.7b00365 [DOI] [PubMed] [Google Scholar]
- 9.Agarwal P, Bertozzi CR (2015) Site-specific antibody-drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjug Chem 26 (2):176–192. doi: 10.1021/bc5004982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, Hallett W, Tsou H-R, Upeslacis J, Shochat D, Mountain A, Flowers DA, Bernstein I (2002) Gemtuzumab Ozogamicin, A Potent and Selective Anti-CD33 Antibody−Calicheamicin Conjugate for Treatment of Acute Myeloid Leukemia. Bioconjugate Chemistry 13 (1):47–58. doi: 10.1021/bc010021y [DOI] [PubMed] [Google Scholar]
- 11.Laguzza BC, Nichols CL, Briggs SL, Cullinan GJ, Johnson DA, Starling JJ, Baker AL, Bumol TF, Corvalan JRF (1989) New antitumor monoclonal antibody-vinca conjugates LY203725 and related compounds: design, preparation, and representative in vivo activity. Journal of Medicinal Chemistry 32 (3):548–555. doi: 10.1021/jm00123a007 [DOI] [PubMed] [Google Scholar]
- 12.Hinman LM, Hamann PR, Wallace R, Menendez AT, Durr FE, Upeslacis J (1993) Preparation and Characterization of Monoclonal Antibody Conjugates of the Calicheamicins: A Novel and Potent Family of Antitumor Antibiotics. Cancer Research 53 (14):3336–3342 [PubMed] [Google Scholar]
- 13.Zuberbühler K, Casi G, Bernardes GJL, Neri D (2012) Fucose-specific conjugation of hydrazide derivatives to a vascular-targeting monoclonal antibody in IgG format. Chemical Communications 48 (56):7100–7102. doi: 10.1039/c2cc32412a [DOI] [PubMed] [Google Scholar]
- 14.Senter PD (2009) Potent antibody drug conjugates for cancer therapy. Current Opinion in Chemical Biology 13 (3):235–244. doi: 10.1016/j.cbpa.2009.03.023 [DOI] [PubMed] [Google Scholar]
- 15.Kalia J, Raines RT (2008) Hydrolytic Stability of Hydrazones and Oximes. Angewandte Chemie International Edition 47 (39):7523–7526. doi:doi: 10.1002/anie.200802651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.https://clinicaltrials.gov/ct2/show/NCT03255070. Accessed 1 Feb 2019.
- 17.http://www.legochembio.com/m/eng/md/pipeline.asp. Accessed 1 Feb 2019.
- 18.Dirksen A, Dawson PE (2008) Rapid Oxime and Hydrazone Ligations with Aromatic Aldehydes for Biomolecular Labeling. Bioconjugate Chemistry 19 (12):2543–2548. doi: 10.1021/bc800310p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kölmel DK, Kool ET (2017) Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chemical Reviews 117 (15):10358–10376. doi: 10.1021/acs.chemrev.7b00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal P, Kudirka R, Albers AE, Barfield RM, de Hart GW, Drake PM, Jones LC, Rabuka D (2013) Hydrazino-Pictet-Spengler Ligation as a Biocompatible Method for the Generation of Stable Protein Conjugates. Bioconjugate Chemistry 24 (6):846–851. doi: 10.1021/bc400042a [DOI] [PubMed] [Google Scholar]
- 21.Drake PM, Albers AE, Baker J, Banas S, Barfield RM, Bhat AS, de Hart GW, Garofalo AW, Holder P, Jones LC, Kudirka R, McFarland J, Zmolek W, Rabuka D (2014) Aldehyde tag coupled with HIPS chemistry enables the production of ADCs conjugated site-specifically to different antibody regions with distinct in vivo efficacy and PK outcomes. Bioconjug Chem 25 (7):1331–1341. doi: 10.1021/bc500189z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Accessed 1 Feb 2019]. https://clinicaltrials.gov/ct2/show/NCT03682796.
- 23.Kudirka R, Barfield Robyn M, McFarland J, Albers Aaron E, de Hart Gregory W, Drake Penelope M, Holder Patrick G, Banas S, Jones Lesley C, Garofalo Albert W, Rabuka D (2015) Generating Site-Specifically Modified Proteins via a Versatile and Stable Nucleophilic Carbon Ligation. Chemistry & Biology 22 (2):293–298. doi: 10.1016/j.chembiol.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 24.Dilek O, Lei Z, Mukherjee K, Bane S (2015) Rapid formation of a stable boron-nitrogen heterocycle in dilute, neutral aqueous solution for bioorthogonal coupling reactions. Chem Commun (Camb) 51 (95):16992–16995. doi: 10.1039/c5cc07453c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt P, Stress C, Gillingham D (2015) Boronic acids facilitate rapid oxime condensations at neutral pH. Chem Sci 6 (6):3329–3333. doi: 10.1039/c5sc00921a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stress CJ, Schmidt PJ, Gillingham DG (2016) Comparison of boron-assisted oxime and hydrazone formations leads to the discovery of a fluorogenic variant. Organic & Biomolecular Chemistry 14 (24):5529–5533. doi: 10.1039/c6ob00168h [DOI] [PubMed] [Google Scholar]
- 27.Gillingham D (2016) The role of boronic acids in accelerating condensation reactions of alpha-effect amines with carbonyls. Org Biomol Chem 14 (32):7606–7609. doi: 10.1039/c6ob01193d [DOI] [PubMed] [Google Scholar]
- 28.Gu H, Chio TI, Lei Z, Staples RJ, Hirschi JS, Bane S (2017) Formation of hydrazones and stabilized boron-nitrogen heterocycles in aqueous solution from carbohydrazides and ortho-formylphenylboronic acids. Org Biomol Chem 15 (36):7543–7548. doi: 10.1039/c7ob01708a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chio TI, Gu H, Mukherjee K, Tumey LN, Bane S (2019) Bioconjugation via the rapid bioorthogonal formation of zwitterionic boron-nitrogen heterocycles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickens CJ, Johnson SN, Pressnall MM, Leon MA, Berkland CJ (2018) Practical Considerations, Challenges, and Limitations of Bioconjugation via Azide–Alkyne Cycloaddition. Bioconjugate Chemistry 29 (3):686–701. doi: 10.1021/acs.bioconjchem.7b00633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Fang T, Boons G-J (2014) Preparation of Well-Defined Antibody–Drug Conjugates through Glycan Remodeling and Strain-Promoted Azide–Alkyne Cycloadditions. Angewandte Chemie 126 (28):7307–7310. doi:doi: 10.1002/ange.201402606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmerman ES, Heibeck TH, Gill A, Li X, Murray CJ, Madlansacay MR, Tran C, Uter NT, Yin G, Rivers PJ, Yam AY, Wang WD, Steiner AR, Bajad SU, Penta K, Yang W, Hallam TJ, Thanos CD, Sato AK (2014) Production of Site-Specific Antibody–Drug Conjugates Using Optimized Non-Natural Amino Acids in a Cell-Free Expression System. Bioconjugate Chemistry 25 (2):351–361. doi: 10.1021/bc400490z [DOI] [PubMed] [Google Scholar]
- 33.Dennler P, Chiotellis A, Fischer E, Bregeon D, Belmant C, Gauthier L, Lhospice F, Romagne F, Schibli R (2014) Transglutaminase-based chemo-enzymatic conjugation approach yields homogeneous antibody-drug conjugates. Bioconjug Chem 25 (3):569–578. doi: 10.1021/bc400574z [DOI] [PubMed] [Google Scholar]
- 34.van Geel R, Wijdeven MA, Heesbeen R, Verkade JMM, Wasiel AA, van Berkel SS, van Delft FL (2015) Chemoenzymatic Conjugation of Toxic Payloads to the Globally Conserved N-Glycan of Native mAbs Provides Homogeneous and Highly Efficacious Antibody–Drug Conjugates. Bioconjugate Chemistry 26 (11):2233–2242. doi: 10.1021/acs.bioconjchem.5b00224 [DOI] [PubMed] [Google Scholar]
- 35.VanBrunt MP, Shanebeck K, Caldwell Z, Johnson J, Thompson P, Martin T, Dong H, Li G, Xu H, D’Hooge F, Masterson L, Bariola P, Tiberghien A, Ezeadi E, Williams DG, Hartley JA, Howard PW, Grabstein KH, Bowen MA, Marelli M (2015) Genetically Encoded Azide Containing Amino Acid in Mammalian Cells Enables Site-Specific Antibody–Drug Conjugates Using Click Cycloaddition Chemistry. Bioconjugate Chemistry 26 (11):2249–2260. doi: 10.1021/acs.bioconjchem.5b00359 [DOI] [PubMed] [Google Scholar]
- 36.Tang F, Yang Y, Tang Y, Tang S, Yang L, Sun B, Jiang B, Dong J, Liu H, Huang M, Geng M-Y, Huang W (2016) One-pot N-glycosylation remodeling of IgG with non-natural sialylglycopeptides enables glycosite-specific and dual-payload antibody–drug conjugates. Organic & Biomolecular Chemistry 14 (40):9501–9518. doi: 10.1039/c6ob01751g [DOI] [PubMed] [Google Scholar]
- 37.Thompson P, Ezeadi E, Hutchinson I, Fleming R, Bezabeh B, Lin J, Mao S, Chen C, Masterson L, Zhong H, Toader D, Howard P, Wu H, Gao C, Dimasi N (2016) Straightforward Glycoengineering Approach to Site-Specific Antibody–Pyrrolobenzodiazepine Conjugates. ACS Medicinal Chemistry Letters 7 (11):1005–1008. doi: 10.1021/acsmedchemlett.6b00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.https://clinicaltrials.gov/ct2/show/NCT03424603. Accessed 1 Feb 2019 https://clinicaltrials.gov/ct2/show/NCT03700294. Accessed 1 Feb 2019.
- 39.Oliveira BL, Guo Z, Bernardes GJL (2017) Inverse electron demand Diels–Alder reactions in chemical biology. Chemical Society Reviews 46 (16):4895–4950. doi: 10.1039/c7cs00184c [DOI] [PubMed] [Google Scholar]
- 40.Oller-Salvia B, Kym G, Chin JW (2018) Rapid and Efficient Generation of Stable Antibody-Drug Conjugates via an Encoded Cyclopropene and an Inverse-Electron-Demand Diels-Alder Reaction. Angew Chem Int Ed Engl 57 (11):2831–2834. doi: 10.1002/anie.201712370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Halder R, Forsyth JS, Santidrian AF, Stafin K, Lu Y, Tran H, Seller AJ, Biroc SL, Szydlik A, Pinkstaff JK, Tian F, Sinha SC, Felding-Habermann B, Smider VV, Schultz PG (2012) Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci U S A 109 (40):16101–16106. doi: 10.1073/pnas.1211023109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian F, Lu Y, Manibusan A, Sellers A, Tran H, Sun Y, Phuong T, Barnett R, Hehli B, Song F, DeGuzman MJ, Ensari S, Pinkstaff JK, Sullivan LM, Biroc SL, Cho H, Schultz PG, DiJoseph J, Dougher M, Ma D, Dushin R, Leal M, Tchistiakova L, Feyfant E, Gerber HP, Sapra P (2014) A general approach to site-specific antibody drug conjugates. Proc Natl Acad Sci U S A 111 (5):1766–1771. doi: 10.1073/pnas.1321237111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q, Stefano JE, Manning C, Kyazike J, Chen B, Gianolio DA, Park A, Busch M, Bird J, Zheng X, Simonds-Mannes H, Kim J, Gregory RC, Miller RJ, Brondyk WH, Dhal PK, Pan CQ (2014) Site-Specific Antibody–Drug Conjugation through Glycoengineering. Bioconjugate Chemistry 25 (3):510–520. doi: 10.1021/bc400505q [DOI] [PubMed] [Google Scholar]
- 44.Ramakrishnan B, Li J, Wang Y, Feng Y, Prabakaran P, Colantonio S, Dyba MA, Qasba PK, Dimitrov DS (2014) Site-specific antibody-drug conjugation through an engineered glycotransferase and a chemically reactive sugar AU - Zhu, Zhongyu. mAbs 6 (5):1190–1200. doi: 10.4161/mabs.29889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson P, Bezabeh B, Fleming R, Pruitt M, Mao S, Strout P, Chen C, Cho S, Zhong H, Wu H, Gao C, Dimasi N (2015) Hydrolytically Stable Site-Specific Conjugation at the N-Terminus of an Engineered Antibody. Bioconjugate Chemistry 26 (10):2085–2096. doi: 10.1021/acs.bioconjchem.5b00355 [DOI] [PubMed] [Google Scholar]
- 46.Lee J-j, Choi H-J, Yun M, Kang Y, Jung J-E, Ryu Y, Kim TY, Cha Y-j, Cho H-S, Min J-J, Chung C-W, Kim H-S (2015) Enzymatic Prenylation and Oxime Ligation for the Synthesis of Stable and Homogeneous Protein–Drug Conjugates for Targeted Therapy. Angewandte Chemie International Edition 54 (41):12020–12024. doi:doi: 10.1002/anie.201505964 [DOI] [PubMed] [Google Scholar]
- 47.Kudirka RA, Barfield RM, McFarland JM, Drake PM, Carlson A, Banas S, Zmolek W, Garofalo AW, Rabuka D (2016) Site-Specific Tandem Knoevenagel Condensation-Michael Addition To Generate Antibody-Drug Conjugates. ACS Med Chem Lett 7 (11):994–998. doi: 10.1021/acsmedchemlett.6b00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y, Jin S, Zhao W, Liu W, Ding D, Zhou J, Chen S (2017) A Versatile Chemo-Enzymatic Conjugation Approach Yields Homogeneous and Highly Potent Antibody-Drug Conjugates. International Journal of Molecular Sciences 18 (11):2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.https://www.imapac.com/wp-content/uploads/2015/06/12.00pm-Yong-Zu-Kim-can-share.pdf. Accessed 1 Feb 2019.
- 50.Saito F, Noda H, Bode JW (2015) Critical Evaluation and Rate Constants of Chemoselective Ligation Reactions for Stoichiometric Conjugations in Water. ACS Chemical Biology 10 (4):1026–1033. doi: 10.1021/cb5006728 [DOI] [PubMed] [Google Scholar]
- 51.Dirksen A, Hackeng TM, Dawson PE (2006) Nucleophilic Catalysis of Oxime Ligation. Angewandte Chemie International Edition 45 (45):7581–7584. doi:doi: 10.1002/anie.200602877 [DOI] [PubMed] [Google Scholar]
- 52.Kumar A, Kinneer K, Masterson L, Ezeadi E, Howard P, Wu H, Gao C, Dimasi N (2018) Synthesis of a heterotrifunctional linker for the site-specific preparation of antibody-drug conjugates with two distinct warheads. Bioorganic & Medicinal Chemistry Letters 28 (23):3617–3621. doi: 10.1016/j.bmcl.2018.10.043 [DOI] [PubMed] [Google Scholar]
- 53.Kumar A, Kinneer K, Masterson L, Ezeadi E, Howard P, Wu H, Gao C, Dimasi N (2018) Characterization and in vitro data of antibody drug conjugates (ADCs) derived from heterotrifunctional linker designed for the site-specific preparation of dual ADCs. Data in Brief 21:2208–2220. doi: 10.1016/j.dib.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanden AR, Mukherjee K, Dilek O, Loew M, Bane SL (2011) 4-Aminophenylalanine as a Biocompatible Nucleophilic Catalyst for Hydrazone Ligations at Low Temperature and Neutral pH. Bioconjugate Chemistry 22 (10):1954–1961. doi: 10.1021/bc2001566 [DOI] [PMC free article] [PubMed] [Google Scholar]


