Abstract
Meristem fate is regulated by trehalose-6-phosphate phosphatases (TPPs), but their mechanism of action remains mysterious. Loss of the maize TPPs RAMOSA3 and TPP4 leads to reduced meristem determinacy and more inflorescence branching. However, analysis of an allelic series revealed no correlation between enzymatic activity and branching, and a catalytically inactive version of RA3 complements the ra3 mutant. Together with their nuclear localization, these findings suggest a moonlighting function for TPPs.
The body plan of plants is determined by meristems, self-renewing pools of pluripotent stem cells that produce new structures such as roots, leaves and floral organs. Meristem development is tightly controlled by multiple pathways1, and an important regulator of meristem fate is the phosphorylated disaccharide trehalose 6-phosphate (T6P). For example, loss-of-function mutations in TREHALOSE-6-PHOSPHATE SYNTHASE1 (TPS1), the major enzyme for T6P biosynthesis in Arabidopsis (Arabidopsis thaliana), are embryo-lethal, and when rescued during embryogenesis, impair the transition to reproductive development2. In maize (Zea mays), RAMOSA3 (RA3) encodes a TREHALOSE-6-PHOSPHATE PHOSPHATASE (TPP) that dephosphorylates T6P to trehalose3. ra3 mutants have reduced meristem determinacy, leading to increased tassel branching and ectopic branching in ears3. Despite its central role in controlling growth and development, knowledge on how T6P functions is limited. T6P levels correlate with sucrose levels, and are thought to be a signal that maintains appropriate sucrose levels for the tissue and developmental stage4. However, the molecular targets of T6P remain largely elusive. Sucrose non-fermenting1-related kinase 1 (SnRK1) functions in T6P-mediated growth control5, but the extent to which T6P signals through additional pathways is a subject of debate4,6. A better mechanistic understanding of T6P could increase crop yields, since expression of a rice TPP in developing maize ears enhances yields under drought7, and treatment with plant-permeable analogues of T6P increases grain yield and drought tolerance in wheat8.
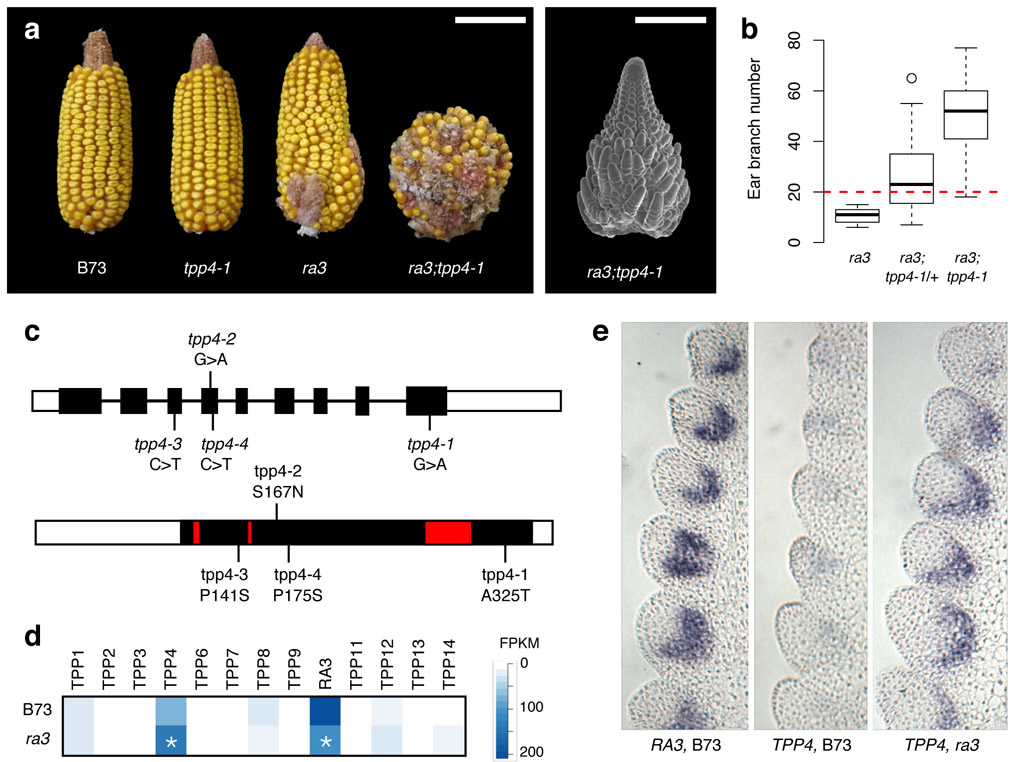
To better understand how TPPs regulate meristem determinacy, we isolated genetic modifiers by mutagenizing ra3 mutants using ethyl methanesulfonate (EMS) and screening for enhanced ear branching. In this screen, we identified four independent lines with mutations in the RA3 paralog TREHALOSE-6-PHOSPHATE PHOSPHATASE4 (TPP4, Zm00001d052227) that were associated with increased ear branching (Fig. 1a,b). All four alleles had missense mutations in residues that were conserved among plant TPPs (Fig. 1c, Supplementary Fig. 1). We confirmed that TPP4 was the causal gene by generating a null allele using CRISPR/Cas9, which enhanced ra3 to the same extent as our EMS alleles (Supplementary Fig. 2). tpp4 mutants had normal, unbranched ears in the presence of functional RA3, but in a ra3 mutant background, tpp4 semidominantly increased ear and tassel branch number (Fig. 1a,b, Supplementary Fig. 3), and branches extended up the ear rather than being restricted to the base as in ra3 single mutants (Fig. 1a). Therefore TPP4 is a fully redundant paralog of RA3, and accordingly its expression was lower than RA3 in developing ears9. However, TPP4 was strongly upregulated in ra3 mutants (Fig. 1d), suggesting it compensates for loss of RA3 in a responsive backup circuit10. In situ hybridization with TPP4-specific probes showed that this compensation occurred specifically in the expression domain of RA3, which subtends spikelet pair meristems3 (Fig. 1e). An additional TPP, TPP12, was also upregulated in developing ra3 mutant ears (Fig. 1d, Supplementary Fig. 4), but CRISPR/Cas9-induced tpp12 mutants did not enhance ra3 ear branching (Supplementary Fig. 4), indicating that RA3 and TPP4 are the major TPPs affecting meristem determinacy.
Figure 1. TPP4 acts as a redundant back-up for RA3.
a, Representative mature ears of B73 wild type (WT), tpp4-1, ra3 and ra3;tpp4-1, with enhanced branching compared to ra3. Phenotypes were consistent across all (≥ 5) growing seasons. Scale bar = 10 cm. A representative electron micrograph of a developing ra3;tpp4-1 ear on the right shows branching extending up the ear. Ears from ten plants were imaged, with similar results. Scale bar = 1 mm. b, Co-segregation of tpp4-1 with ear branch number in a ra3 mutant background. The red line indicates our arbitrary threshold for enhancement (20 ear branches). N = 11;40;13. Bottom and top of boxes = first and third quartile; middle line = median; whiskers = most extreme values within 1.5 x the interquartile distance below or above the first or third quartile; remaining outlier data points shown as individual dots. c, Overview of EMS-induced tpp4 alleles. Top, position of mutations in TPP4, with boxes indicating exons (CDS is in black) and lines indicating introns. Bottom, positions of amino acid substitutions in the TPP4 protein, with the solid black portion indicating the TPP domain and conserved phosphatase boxes in red. d, Heatmap of expression levels of all maize TPPs in developing ears in WT B73 and ra3 mutants. TPP5 is a pseudogene and was excluded. Asterisks indicate differential expression between B73 and ra3 ears (p < 0.05, exact negative binomial test in edgeR with Benjamini-Hochberg multiple testing correction; n = 3 biological replicates). TPP4 was significantly upregulated in ra3 (2.4-fold, p = 0.040), and RA3 was significantly downregulated (2-fold, p = 0.012). e, In situ hybridization using an antisense RA3 probe in a WT ear (left), showing expression subtending developing spikelet pair meristems; and using an antisense TPP4 probe in a WT (middle) or a ra3 mutant ear (right); TPP4 is expressed in the same domain as RA3, and is stronger in ra3 mutants. Representative images are shown; the experiment was performed twice with similar results, using ears from at least 3 independent plants per genotype per experiment. Scale bar = 100 μm.
To further investigate how TPPs affect inflorescence development, we measured T6P levels in developing ears of ra3 and ra3;tpp4-1 double mutants at the stage where RA3 and TPP4 expression peaks. Surprisingly, there was no difference in T6P levels compared to the B73 wild type (Fig. 2a). While it is possible that localized effects of loss of RA3 and TPP4 were masked by the analysis of entire ear primordia, we speculated that TPP4 enzymatic activity may not be important, consistent with the fact that our EMS-generated tpp4 alleles did not affect motifs important for phosphatase activity. We modelled TPP4 structure using structures of similar TPPs, and only the Ser167 residue mutated in tpp4-2 was close to the active site (Fig. 2b). We also predicted that mutating Ala325 to Thr in tpp4-1 would result in steric clash with the nearby highly conserved Phe302 residue and disrupt the catalytic pocket. However, residues mutated in tpp4-3 and tpp4-4 were far from the active site on exposed surface loops, where their effect on protein structure and activity was not obvious (Fig. 2b). To test our structural predictions, we asked if the proteins encoded by the different tpp4 alleles could complement the S. cerevisiae tpp mutant Δtps211. Expression of wild-type TPP4 rescued the Δtps2 growth defect, while a negative control, tpp4NYN, in which two catalytically important Asp residues were mutated to Asn, did not, suggesting enzymatic activity was required to complement the yeast mutant (Fig. 2c). We next tested our tpp4 alleles, and while tpp4-1 was unable to complement Δtps2, partial complementation was observed for tpp4-2, tpp4-3 and tpp4-4, suggesting that they maintain some level of activity, confirming our structural predictions (Fig. 2c). To validate these results and to measure enzymatic activity, we used purified MBP fusion proteins for in vitro assays. Similar to RA33, TPP4 activity was specific for T6P, and did not dephosphorylate other sugar phosphates (Supplementary Fig. 5). We quantified the degree of activity for different tpp4 alleles, and found that while tpp4-1 had no detectable activity, tpp4-2 had low activity, and tpp4-3 and tpp4-4 had significant activity, at ~25 and ~35% of wild-type levels, respectively (Fig. 2d).
Figure 2. TPP enzymatic activity does not correlate with ear branching phenotype.
a, Levels of T6P are unchanged in ra3 or ra3;tpp4-1 mutants (p = 0.80 for B73-ra3 comparison, p = 0.82 for B73-ra3;tpp4-1 comparison; two-sided Student’s t-test). Data points from three biological replicates are shown as dots; bars indicate the mean. b, Homology model of TPP4; the N-terminal extension is shown in grey and the TPP domain is in cyan; the N and C termini are labelled; T6P is modeled in the active site in yellow. Residues changed in the EMS mutants are shown in red, with all but Ser167 (tpp4-2) located on the protein surface. T6P is modeled in the active site in yellow. c, RA3, TPP4, and most tpp4 EMS alleles complemented the yeast tpp mutant Δtps2, whereas catalytically inactive tpp4NYN did not. EV = empty vector. Images from a representative experiment are shown; two independent experiments were performed with similar results. d, tpp4-3 and −4 mutant proteins have considerable TPP activity. Dots indicate data points from four independent biological replicates; bars show the mean. e, Relative increase in ear branch number in ra3;tpp4 heterozygous (blue) and ra3;tpp4 homozygous (red) mutants compared to segregating single ra3 mutants, plotted against TPP activity of the different alleles (from left to right, tpp4-1, tpp4-2, tpp4-3 and tpp4-4), showing no correlation between enzymatic activity and ear branching (Spearman’s ρ = −0.20, p = 0.47 for tpp4 heterozygotes; Spearman’s ρ = −0.08, p = 0.76 for homozygotes; p-values calculated using a two-sided Student’s t-test with asymptotic t approximation). Dots indicate data points from four independent biological replicates, with linear trend lines shown as dotted lines. f, RA3D110E mutant protein had no detectable TPP activity in vitro. g, ra3 mutants were complemented by catalytically inactive RA3D110E. Data are from segregating populations with siblings without the transgene in white and with the transgene in grey. Degree of complementation is similar for RA3D110E and WT RA3 constructs. Box plots defined as in Fig. 1b. P-values for each comparison were calculated using a two-sided Student’s t-test; N = 45;55; 39;53; 51;44; 43;43; 46;49.
We next used our tpp4 allelic series to ask if the branching phenotype correlated with enzymatic activity. All alleles were backcrossed to ra3 in B73 at least three times, and we phenotyped segregating populations grown in replicate in three different field locations. The degree of ear branching was normalized across locations using segregating controls to account for influence of environment on the phenotype. All tpp4 alleles enhanced ra3 semi-dominantly; however, despite the differences in enzyme activity, all alleles affected ear branching to the same degree, resulting in a two-fold increase in ra3;tpp4 heterozygotes (Spearman’s ρ = −0.20, p = 0.47), and a five-fold increase in ra3;tpp4 homozygotes (Spearman’s ρ = −0.08, p = 0.76; Fig. 2e, Supplementary Fig. 6). These results strongly suggested that TPP enzymatic activity is not important for control of branching.
To further test this hypothesis, we engineered a version of RA3 with a single amino acid change (RA3D110E) predicted to maintain overall protein structure while abolishing catalytic activity (Fig. 2f). Expression of this catalytically dead RA3 using its native promoter complemented the ra3 phenotype, leading to a ~50% reduction in ear branch number compared to control siblings without the transgene (Fig. 2g). Similar partial complementation was seen in families expressing a wild-type version of RA3 from the same promoter (Fig. 2g), suggesting the partial complementation was due to insufficient promoter elements in our constructs. Complementation of ra3 by a catalytically dead RA3 suggests that a non-enzymatic function is responsible for its role in meristem determinacy.
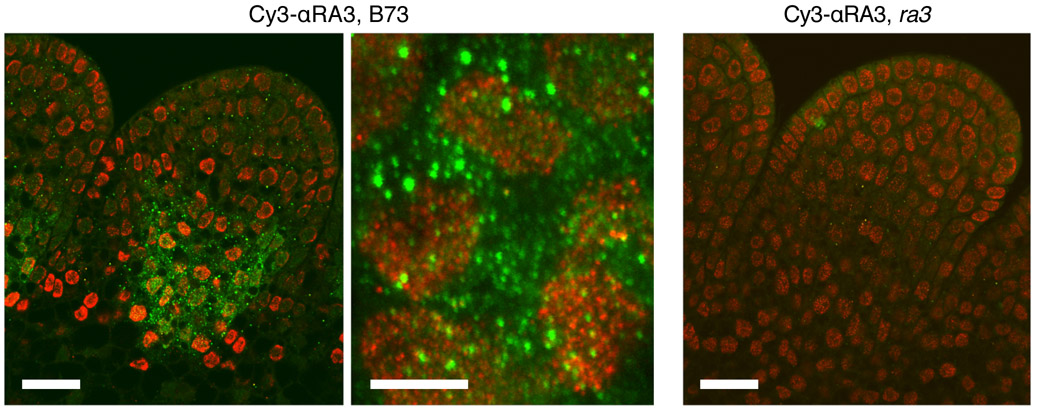
Regulatory functions were postulated for the TPP family in Arabidopsis, as its expansion is due to whole-genome duplications with a high degree of paralog retention, typical of regulatory proteins with stricter gene dosage requirements compared to enzymes12. A regulatory function was also proposed for rice TPP7, based on its low activity and lack of effect on T6P levels13. Some metabolic enzymes are known to have regulatory functions, a phenomenon known as moonlighting14, and two other sugar metabolic enzymes, HEXOKINASE1 (HXK1) and the fructose-1,6-bisphosphatase FRUCTOSE INSENSITIVE1, exhibit moonlighting activity in Arabidopsis15,16. As HXK1 regulates transcription in the nucleus15, we investigated the subcellular localization of RA3 and TPP4. Both proteins were predominantly localized in the nucleus when transiently expressed in tobacco (Supplementary Fig. 7), and we confirmed this by immunolocalization using RA3-specific antibodies in developing maize ears (Fig. 3). Interestingly, RA3 localized to speckles in cytoplasmic and nuclear compartments (Fig. 3), and these are typically associated with regulation of RNA processing and transcription17. As TPPs lack DNA- or RNA-binding domains, it is likely their hypothetical nuclear role requires interaction with other proteins, consistent with our observation that mutations on the surface of TPP4 impair its role in meristem determinacy. While the exact mechanism remains to be resolved, exploiting the dual roles of TPPs may open avenues for further crop improvement. TPP4 itself maps to QTL and near genome-wide association SNPs for tassel branch number18 and is in a region that shows strong evidence of selection during maize domestication and improvement19, making it an especially attractive target for improvement of maize productivity.
Figure 3. RA3 localizes to nuclear and cytoplasmic speckles.
RA3 was detected by immunolocalization in nuclear and cytoplasmic speckles at the base of a spikelet pair meristem; RA3 in green and nuclei counterstained in red. Absence of signal in the ra3 mutant confirmed the specificity of the antibody. Representative images are shown; similar results were obtained in all five independent experiments. Scale bar = 25 μm in the outer panels, 5 μm in the middle panel.
Methods
Mapping
Pollen mutagenesis was performed as described20 on ra3-fea1 in B73 and ra3-NI in Mo17, and ~1500 M2 families were screened for enhanced branching. To generate F2 mapping populations, enhancer candidates were crossed to ra3-fea1 in the W23 or B73 background, and the resulting F1s were selfed. For each mutant, two pools were created using DNA from about 30 F2 individuals with either enhanced or non-enhanced ear branching, and single nucleotide polymorphism (SNP) genotyping was performed using MaizeSNP50 BeadChip microarrays (Illumina). After removal of low-quality SNPs (GC > 0.6) and non-polymorphic SNPs (allele frequency < 0.2 or > 0.8 in both pools), smoothed allele frequencies and their ratios were calculated using R 3.1.0 to find peaks associated with enhanced branching. Pooled genomic DNA from enhanced M2 individuals was used to make a library for next-generation sequencing using the NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs), and the sample was sequenced on a single lane of Illumina HiSeq2500 to 10x coverage. Reads were mapped to the maize B73 reference genome (AGPv3, https://www.maizegdb.org/assembly) using bwa-mem21, and SNPs called using the GATK unified genotyper22. High-confidence SNPs supported by at least three reads were selected if they were homozygous, and prioritized if they were G/C to A/T transitions and in exons within the mapping interval. To confirm the mutation and detect causal mutations in other alleles, TPP4 was amplified from genomic DNA of M2 populations of additional enhancer lines for Sanger sequencing.
Generation of CRISPR/Cas9-induced mutants
Guide RNAs (gRNAs) targeting TPP4 and TPP12 were designed using CRISPR-P23 (http://cbi.hzau.edu.cn/crispr/), and an array containing four gRNAs expressed from independent maize U6-derived promoters24 was synthesized, with gRNAs GCAGATTGCCAGCGCGTCCC and GTCGAGGTACCGCAGGGACA targeting TPP4 and gRNAs GTAGTGTTCAAGCAAAGCCC and GTGATCTTGCTGGGAGTAAA targeting TPP12. This array was cloned into the pMCG1005-Cas9 binary vector and transformed into the Hi-II background25. Mutations were identified by Sanger sequencing, and mutants were backcrossed at least three times to ra3-fea1(B73) before phenotyping. The inserted T-DNA containing Cas9 and the gRNA array was eliminated after the first backcross to ensure stability of mutations.
Generation of transgenic complementation lines
To generate pRA3::RA3D110E lines, the GAC codon coding for D110 was changed to GAG using site-directed mutagenesis of a RA3 genomic construct that spanned from 3,311 bp upstream of the ATG to the end of the 3’ UTR. To generate control pRA3::RA3-FLAG-HA lines, sequence encoding a FLAG tag followed by a triple HA tag was introduced just before the stop codon of RA3 in the same genomic construct. The resulting constructs were cloned into the binary vector pAM1006 and transformed into Hi-II. Transgenic lines were backcrossed at least five times to ra3-fea1(B73) before phenotyping. Individuals carrying transgenes were identified by scoring resistance to the selectable marker and/or by PCR.
Phenotyping
For ear and tassel phenotyping, plants were grown in field locations in Cold Spring Harbor, NY or
Lloyd Harbor, NY (June-September), or Valle De Banderas, Nayarit, Mexico (November-February). Ears and tassels were collected after anthesis to count branches. For scanning electron microscopy, developing ears were dissected around six weeks after planting, and mounted on stubs to image on a Hitachi S-3500N scanning electron microscope. All phenotyping experiments were performed on segregating populations, and each individual was genotyped.
Gene expression analysis
2-3 mm ears were dissected from 6-week old plants from a population segregating for ra3 and tpp4-1, and immediately frozen in liquid nitrogen. Tissue was ground using tungsten beads in a mixer mill, and total RNA was extracted using the DirectZol RNA Miniprep Kit (Zymo Research), according to the manufacturer’s instructions, including on-column digestion of genomic DNA with DNase I. cDNA synthesis were performed using the iScript cDNA Synthesis Kit (Bio-Rad). Quantitative real-time PCR was performed using Universal SybrGreen Master Mix (Bio-Rad) on the CFX96 Real-Time system (Bio-Rad). Primers GGTCCGTCCTGTTATTGATTG and ATTCCATCACCTCAGCTGGA were used for qRT-PCR detection of TPP12. UBIQUITIN1 (UBQ1, Zm00001d015327) was used for normalization (primers CCGCTTCAAGATGCAGATCTTTG and GAGACGGAGCACAAGGTGG).
RNA sequencing
Raw reads from previously described samples9 were trimmed and filtered using Trimmomatic 0.36, and paired reads were aligned against maize AGPv4.39 using HiSat2 2.1.026. Counts were generated using HTSeq 0.6.027 for all transcripts in the AGPv4.39 GTF, and differential expression was assessed using edgeR 3.22.028 in R 3.5.0 after filtering for genes with cpm > 1 in N samples, where N represents the number of biological replicates. Members of the TPP family were identified using Gramene (http://www.gramene.org), and the two members that were not previously described29 were named TPP13 (Zm00001d006375) and TPP14 (Zm00001d029371).
In situ hybridization
Developing ears (2-3 mm stage) were dissected from six-week old field-grown plants, followed by fixing, embedding and in situ hybridization as described30, with 16h substrate incubation. RA3 probes were as previously reported3, and for TPP4, two fragments were amplified for each from cDNA using primer pairs GCCTACATGAGCGACGTGAT/CCTCTTCCTCAGCACCTTGA and GTTGGGACGATCGAGAAAGT/GGCGTAGTAGAGCTCCGACA, and subcloned into pCR2.1. Clones carrying inserts in both orientations were identified and sequence-verified to generate antisense or sense probes by in vitro transcription with T7 polymerase (Roche).
In vitro TPP activity assays
RA3, TPP4 and TPP12 coding sequences were cloned into pMAL-c5x (New England Biolabs), and site-directed mutagenesis was performed on pMAL-c5x-TPP4 to introduce the various point mutations described in the Results section. All sequences were verified by Sanger sequencing before transformation into the Rosetta E. coli strain. Cultures were grown to an OD600 of 0.6 at 37°C, cooled to 16°C prior to addition of isopropyl β-D-1-thiogalactopyranoside to a final concentration of 0.5 mM, and grown for an additional 12-16 hours at 16°C. Culture harvesting and purification of MBP-TPPs were performed with the pMAL expression system (New England Biolabs) according to the manufacturer’s instructions. Protein purity and concentration were assessed using SDS-PAGE gels. For in vitro assays, equal amounts of purified TPPs were incubated for 30 minutes at 28°C in buffer containing 10 mM Tris-HCl pH 7.6, 0.2 mM EDTA, 5 mM MgCl2, 0.1 mg/mL BSA, and 0.5 mM T6P, S6P, G6P or F6P (Sigma-Aldrich). Phosphate release was measured using a colorimetric assay as OD600 using the Serine/Threonine Phosphatase Assay System (Promega). Activity was expressed as a percentage of the activity measured with purified recombinant TPP4.
Metabolite measurements
Developing ears (2-3 mm) were dissected from seven-week-old field-grown plants and immediately frozen in liquid nitrogen. T6P was extracted with chloroform-methanol and measured by high-performance anion-exchange chromatography coupled to tandem mass spectrometry6.
Protein structure homology model
The Phyre2 web portal for protein modelling and prediction31 was used to construct a homology model from the amino acid sequence of TPP4. The intensive search function of the web portal selected model templates 5GVX, 5DX9, 5DXF, 5HVO, 5LQD, and 3T5T, corresponding to Mycobacterium tuberculosis TPP32, the Cryptococcus neoformans TPP(D24N)-T6P complex33, the Candida albicans TPP N-terminal domain33, the Aspergillus fumigatus TPS in complex with UDP and validoxylamine A34, the Streptomyces venezuelae TPS35, and a structurally similar transferase from Streptomyces hygroscopus (unpublished), respectively. The TPP structures were necessary for modelling the phosphatase domain of TPP4, whereas the other structures including the TPS structures were incorporated for modelling the N-terminal domain of TPP4. From these templates, 96% of the residues were modeled at >90% confidence.
Yeast complementation
RA3 and TPP4 coding sequences were cloned into pYX21212, and site-directed mutagenesis was performed to introduce the various point mutations described in the Results section. All sequences were verified by Sanger sequencing before transformation into the S. cerevisiae YSH448 Δtps2 strain. Clones were grown in liquid culture in SD-Ura medium to an OD600 of 1, spotted on SD-Ura plates with and without 1M NaCl, and grown at either 30°C or 39°C for two days. Expression of TPP-HA fusion proteins was assessed by Western blot using monoclonal anti-HA antibody from mouse (Sigma-Aldrich, clone HA-7).
Tobacco infiltration
RA3 and TPP4 were cloned into pK7FWG2 and pK7WGF236, respectively, and transformed into Agrobacterium tumefaciens strain GV3101. Tobacco infiltrations were performed as described37 with Agrobacterium at OD600 0.05, and a strain expressing p1938 was coinfiltrated with all constructs. After two days, leaves were stained with 1 μg/mL 4',6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific), and GFP and DAPI fluorescence were imaged using a Zeiss LSM 710 confocal microscope. For Western blot, total protein was prepared from tobacco leaves37, and GFP fusion proteins were detected using HRP-conjugated mouse anti-GFP antibody (Miltenyi Biotec).
Immunolocalization
Antibodies against RA3 were generated in rabbits using a peptide consisting of the first 80 residues of RA3 fused to a His-tag (produced in E. coli) as the antigen, and purified using the same peptide39. Immunolocalization was performed as described39 on 2-3 mm ears harvested from six-week old ra3 and B73 plants. RA3 was detected using the purified primary antibody and Cy3-coupled goat anti-rabbit IgG secondary antibody (ImmunoResearch Laboratories). Nuclei were counterstained with 3C5 mouse monoclonal antibody40,41 and Alexa Fluor 568-coupled anti-mouse secondary antibody (Invitrogen). Imaging was performed on a Zeiss LSM 710 confocal microscope.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Supplementary Material
Acknowledgments
We thank Prof. Dr. Patrick Van Dijck for sharing the pYX212 vector, Dr. Krishnamurthy Rao for discussion of RA3 protein structure, Tim Mulligan for plant care, and Sylvain Pouzet, Gavriela Carver, and all other Jackson lab summer students for their enthusiastic involvement in some of this work. This work was supported by funding from the National Science Foundation (IOS-1238202 and IOS-1755141), a collaborative agreement with Dupont Pioneer, the European Molecular Biology Organization (Long-Term Fellowship to H.C.), and the Vietnam National Foundation for Science and Technology Development (under grant number 106-NN.01-2014.48 to S.L.V.). The metabolite analysis was supported by the Max Planck Society (R.F and J.E.L.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Tanaka W, Pautler M, Jackson D & Hirano H-Y Grass meristems II: inflorescence architecture, flower development and meristem fate. Plant Cell Physiol. 54, 313–324 (2013). [DOI] [PubMed] [Google Scholar]
- 2.van Dijken AJH, Schluepmann H & Smeekens SCM Arabidopsis trehalose-6-phosphate synthase 1 is essential for normal vegetative growth and transition to flowering. Plant Physiol. 135, 969–977 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satoh-Nagasawa N, Nagasawa N, Malcomber S, Sakai H & Jackson D A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441, 227–230 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Figueroa CM & Lunn JE A Tale of Two Sugars: Trehalose 6-Phosphate and Sucrose. Plant Physiol. 172, 7–27 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes C et al. The trehalose 6-phosphate/SnRK1 signaling pathway primes growth recovery following relief of sink limitation. Plant Physiol. 162, 1720–1732 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueroa CM et al. Trehalose 6-phosphate coordinates organic and amino acid metabolism with carbon availability. Plant J. 85, 410–423 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Nuccio ML et al. Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat. Biotechnol 33, 862–869 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Griffiths CA et al. Chemical intervention in plant sugar signalling increases yield and resilience. Nature 540, 574–578 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Eveland AL et al. Regulatory modules controlling maize inflorescence architecture. Genome Res. 24, 431–443 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kafri R, Levy M & Pilpel Y The regulatory utilization of genetic redundancy through responsive backup circuits. Proc Natl Acad Sci USA 103, 11653–11658 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Virgilio C et al. Disruption of TPS2, the gene encoding the 100-kDa subunit of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae, causes accumulation of trehalose-6-phosphate and loss of trehalose-6-phosphate phosphatase activity. Eur. J. Biochem 212, 315–323 (1993). [DOI] [PubMed] [Google Scholar]
- 12.Vandesteene L et al. Expansive Evolution of the TREHALOSE-6-PHOSPHATE PHOSPHATASE Gene Family in Arabidopsis. Plant Physiol. 160, 884–896 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kretzschmar T et al. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nat Plants 1, 15124 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Copley SD Moonlighting is mainstream: paradigm adjustment required. Bioessays 34, 578–588 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Cho Y-H, Yoo SD & Sheen J Regulatory Functions of Nuclear Hexokinase1 Complex in Glucose Signaling. Cell 127, 579–589 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Cho Y-H & Yoo S-D Signaling role of fructose mediated by FINS1/FBP in Arabidopsis thaliana. PLoS Genet. 7, e1001263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spector DL & Lamond AI Nuclear speckles. Cold Spring Harb Perspect Biol 3, a000646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown PJ et al. Distinct genetic architectures for male and female inflorescence traits of maize. PLoS Genet. 7, e1002383 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hufford MB et al. Comparative population genomics of maize domestication and improvement. Nat. Genet 44, 808–811 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeling M & Walbot V The Maize Handbook. (Springer-Verlag, 1994). [Google Scholar]
- 21.Li H Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. (2013). [Google Scholar]
- 22.McKenna A et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei Y et al. CRISPR-P: A Web Tool for Synthetic Single-Guide RNA Design of CRISPR-System in Plants. Mol Plant 7, 1494–1496 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, Regan M, Furukawa H & Jackson D Role of heterotrimeric Ga proteins in maize development and enhancement of agronomic traits. PLoS Genet. 14, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Char SN et al. An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant Biotechnol. J 15, 257–268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim D, Langmead B & Salzberg SL HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anders S, Pyl PT & Huber W HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson MD, McCarthy DJ & Smyth GK edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou M-L et al. Trehalose Metabolism-Related Genes in Maize. J Plant Growth Regul 33, 256–271 (2014). [Google Scholar]
- 30.Jackson DP in Molecular Plant Pathology: A Practical Approach (eds. Bowles DJ, Gurr SJ & McPherson M) (1991). [Google Scholar]
- 31.Kelley LA, Mezulis S, Yates CM, Wass MN & Sternberg MJE The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10, 845–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shan S, Min H, Liu T, Jiang D & Rao Z Structural insight into dephosphorylation by trehalose 6-phosphate phosphatase (OtsB2) from Mycobacterium tuberculosis. FASEB J. 30, 3989–3996 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Miao Y et al. Structures of trehalose-6-phosphate phosphatase from pathogenic fungi reveal the mechanisms of substrate recognition and catalysis. Proc. Natl. Acad. Sci. U.S.A 113, 7148–7153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao Y et al. Structural and In Vivo Studies on Trehalose-6-Phosphate Synthase from Pathogenic Fungi Provide Insights into Its Catalytic Mechanism, Biological Necessity, and Potential for Novel Antifungal Drug Design. mBio 8, e00643–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asenci0n Diez MD et al. The Production and Utilization of GDP-glucose in the Biosynthesis of Trehalose 6-Phosphate by Streptomyces venezuelae. J. Biol. Chem 292, 945–954 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karimi M, Inze D & Depicker A GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7, 193–195 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Xu F, Copeland C & Li X Protein Immunoprecipitation Using Nicotiana benthamiana Transient Expression System. Bio-protocol 5, (2015). [Google Scholar]
- 38.Voinnet O, Rivas S, Mestre P & Baulcombe D An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 33, 949–956 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Smith LG, Greene B, Veit B & Hake S A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development 116, 21–30 (1992). [DOI] [PubMed] [Google Scholar]
- 40.Turner BM & Franchi L Identification of protein antigens associated with the nuclear matrix and with clusters of interchromatin granules in both interphase and mitotic cells. Journal of Cell Science 87 ( Pt 2), 269–282 (1987). [DOI] [PubMed] [Google Scholar]
- 41.Fang Y, Hearn S & Spector DL Tissue-specific expression and dynamic organization of SR splicing factors in Arabidopsis. Mol. Biol. Cell 15, 2664–2673 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.