Abstract
Objectives
Chronic rhinosinusitis (CRS) is a multifactorial disease affecting up to 16% of the United States population and disproportionately affecting the cystic fibrosis (CF) patient population. Despite treating the underlying infection, the use of systemic antibiotics has shown little efficacy in alleviation of symptom burden. This review seeks to discuss recent research on novel antibiotic eluting stent therapy in vitro and within animal models as well as the factors that contribute to its efficacy.
Data Sources
PubMed literature review.
Review Methods
A review of all published literature related to antibiotic eluting sinus stents was conducted to integrate and summarize this innovative approach to chronic sinus infections.
Results
Placement of the ciprofloxacin sinus stent (CSS) and ciprofloxacin‐ivacaftor sinus stent (CISS) exhibited improvement in endoscopic and radiographic findings in rabbit CRS models. While the CSS showed an overall trend toward improvement in microscopic findings and a reduction in biofilm mass, there remained a significant quantity of planktonic bacteria due to antibiotic depletion from an initial burst release in the first 48 hours of stent placement. The CISS and ciprofloxacin‐azithromycin sinus stents (CASSs) exhibited controlled antibiotic release over the study period leading to greatly reduced planktonic bacterial load and biofilm mass. In vitro studies indicate that CASS may be just as efficacious at reducing biofilm mass.
Conclusion
Antibiotic eluting sinus stents show significant promise as a novel therapeutic strategy for CRS. The CISS may have particular promise for the CF patient population by addressing both the infectious and genetic components of disease. Animal studies demonstrate significant promise for translation into human studies. Human clinical trials are warranted to determine the efficacy of antibiotic sinus stents in human patients.
Level of Evidence
NA
Keywords: antibiotic stent, azithromycin, biofilm, chronic rhinosinusitis, ciprofloxacin, cystic fibrosis, ivacaftor, sinus stent, sinusitis, stent
1. INTRODUCTION
Chronic rhinosinusitis (CRS) is defined as a clinical syndrome of mucosal inflammation of the paranasal sinuses leading to a number of cardinal sinonasal symptoms including nasal congestion, nasal drainage, facial pain/pressure, and anosmia. 1 , 2 It is estimated to affect up to 16% of the population and cost the United States upward of $12.6 billion per year. 3 , 4 Medical therapies for CRS have included a broad array of tactics and mechanisms of delivery with varying degrees of success. Despite this variety in therapeutic attempts, outcomes for medical management alone for CRS remain poor with 28% requiring continued therapy after 2 years and 46% of patients requiring surgical management. 5
CRS is currently believed to be a multifactorial condition involving numerous endotypes and phenotypes with genetic, environmental, and infectious etiologies implicated in its development. 6 Two pathogenic factors of particular interest are cystic fibrosis (CF) and biofilm formation. Patients with CF have one of numerous mutations involving the cystic fibrosis transmembrane regulator (CFTR). These mutations lead to impaired mucociliary clearance causing chronic infection and often death secondary to pulmonary complications. Involvement of the sinuses in these patients is near universal with common organisms of infection being Pseudomonas aeruginosa and Staphylococcus aureus. In particular, chronic upper respiratory P. aeruginosa colonization with biofilm formation is believed to be a key pathogenic factor in the development of lower respiratory disease in patients with CF. 1 , 7 , 8 , 9 , 10 , 11 , 12 , 13 Furthermore, biofilm formation has been shown to play a major role in the development and severity of CRS and negatively affects surgical outcomes and symptom severity. 14 , 15 , 16 This review seeks to discuss the development and rationale for the use of antibiotic eluting sinus stents in CRS.
2. PATHOGENESIS AND CLINICAL IMPLICATIONS OF BIOFILMS IN CRS
The process of biofilm formation involves a complex development of colonization and bacterial metabolic differentiation. Development begins with bacterial adherence and formation of microcolonies. These colonies then produce a complex of polysaccharides, proteins, and nucleic acids which forms a protective matrix around the organisms. 17 , 18 As the biofilm develops, the bacteria begin to differentiate into planktonic and intrabiofilm subgroups. The biofilm subgroup functions to regulate expansion of the matrix, while free‐floating, planktonic organisms are released from the edges of the biofilm and form new microcolonies within the sinus cavity, thus perpetuating further biofilm formation. 17
These subgroups present a therapeutic challenge, as biofilm‐forming bacteria have been shown to have increased antibiotic resistance by up to 1000‐fold and protection against the host immune response. 17 , 19 The biofilm and planktonic organisms exhibit separate proteomic and genomic expression profiles which lead to separate resistance mechanisms within the subgroups. 20 , 21 , 22 For these reasons, patients with biofilm production have been consistently shown to have poor clinical outcomes compared to nonbiofilm controls. 14 , 16 , 23 , 24 , 25
On initial presentation, patients with biofilm production present with greater symptom burden and worse objective clinical indicators of disease. 16 , 25 While both biofilm and nonbiofilm controls tend to have symptomatic improvement after endoscopic sinus surgery (ESS), patients with biofilm tend to be less responsive with increased antibiotic utilization and higher rates of revision ESS. 14 , 16 , 23 , 24 , 25 This is particularly worrisome for at‐risk patient populations such as those with CF who have a greater disease burden at baseline.
Biofilms have been implicated as a major pathogenic factor in CF. Colonization of the upper respiratory tract with biofilm‐forming organisms, especially P. aeruginosa, is believed to be a precipitating factor in lower respiratory tract disease via direct seeding of organisms. Interestingly, surgical management of sinus disease in CF patients has been shown to lead to reduced hospitalization and symptom burden indicating that alleviating sinonasal disease burden has broader implications in the treatment of this patient population. 7 , 8 , 13 For these reasons, there is a growing need for novel therapy for sinus disease in at‐risk populations, such as those with CF, particularly directed at decreasing bacterial load and biofilm production.
3. A RATIONALE FOR TOPICAL THERAPIES
To overcome bacterial pathogenic factors such as biofilms, prolonged courses of antibiotics, such as fluoroquinolones, have traditionally been required, but systemic antibiotics have shown little efficacy in the treatment of CRS. 26 , 27 , 28 A major mechanism in the therapeutic inefficiency of antibiotics is multidiffusion barriers. Restrictive diffusion limits the type of antibiotic that can penetrate the biofilm with fluoroquinolones being one of the few antibiotics to consistently overcome this mechanism. 29 , 30 Retarded diffusion in biofilms reduces the concentration of the antibiotic directly proportional to the thickness of the biofilm. The simplest way to overcome this barrier is to provide sustained, high concentrations of the antibiotic of choice mandating high doses of systemic therapy for biofilm penetration at the sinonasal mucosa. 29 , 30 , 31 Unfortunately, recent safety concerns from the United States Food and Drug Administration (FDA) involving systemic fluoroquinolone therapy and an increased risk of tendinitis and tendon rupture have created a need for further investigation into nonsystemic therapeutic options for CRS sufferers. 26 , 32
In particular, topical therapies (eg, nasal rinses, sprays, and implants) are promising avenues of treatment with the capability to deliver high doses of drug to the site of interest with minimal systemic absorption. 17 Nasal rinses have shown efficacy and are the current first‐line recommendation for medical management of CRS by the American Association of Otolaryngology—Head and Neck Surgery. 28 , 33 , 34 In addition, rinses can be done alone or in combination with topical steroid use, which has been shown to improve symptom burden. 35 , 36 , 37 , 38 However, recent research has shown that patient adherence to saline irrigation and intranasal steroids are typically less than 25% and 50%, respectively. 39 Additionally, the duration of drug‐mucosa interaction of nasal sprays is typically less than a few hours secondary to ciliary clearance of the drug. 40 Low patient adherence combined with innately low mucosal‐drug contact time dictates the need for alternative topical therapies that allow for prolonged therapeutic intervention without concern for patient nonadherence.
4. POTENTIAL NONSTENT TOPICAL THERAPIES
When considering viable topical therapies, the clinician must take into account all of the factors previously mentioned: length of time the drug is delivered, the concentrations at which the drug is delivered over this length of time, the specific drug being delivered and its efficacy against biofilm resistance mechanisms, and patient adherence to the drug. Numerous topical therapies have been advanced in recent research that has attempted to meet these demands. 38 , 41 , 42
One method has been through placement of postoperative nasal packing material soaked in antibiotics or steroids. Recent studies have shown some success in short‐term use of nasal packing materials with antibiotic groups demonstrating improved endoscopic scoring compared to control. But when expanded to time periods greater than 90 days, significant differences are not observed between groups. 43 Due to the chronic nature of CRS, consistent, long‐term results are necessary to achieve appropriate improvement. Additionally, the nasal packing material has yet to show significant promise as a potential therapeutic intervention due to the lack of reliability in drug release. 40 Similar issues arise with mesh implants that are commonly used in orthopedic procedures to prevent postoperative infection. While drug is released over 4 weeks, a difficult to control burst release is identified within the first few days of implantation that contains up to 70% of the loaded drug. 44 There is some promise in multilayer meshes, but current research has not shown release of antipseudomonal drugs (especially ciprofloxacin) for time periods greater than 4 days. 45 , 46
A second potential method has been through the use of nanoparticle‐loaded hydrogels. Numerous polymeric hydrogels have been developed that are loaded with polyester, polylactic‐co‐glycolic acid (PLGA) nanoparticles that release over a variety of time frames. For long‐term drug release, the PLGA nanoparticles are modified into two populations with one immediate release population delivering drugs as the gel dissolves over the first month and the second population delaying release until after the first month and extending up to 90 days after gel placement. Unlike the nasal packing/mesh, these implants have been shown to release consistent, controlled levels of drug. 40 , 47 Finally, the gels have the benefit of prior FDA approval. 48 Despite these advantages, hydrogels have limited history of use in the nasal cavity. There remain concerns that expulsion or disintegration of hydrogels may be expedited by routine pre‐ and postoperative CRS treatment such as nasal sprays and irrigation. Due to the pitfalls seen in mesh, nasal packings, and hydrogels, other potential avenues of exploration are required to achieve prolonged, sinonasal topical therapy.
5. STEROID STENTS AS A TOPICAL THERAPY
A mometasone furoate‐releasing implant was introduced in 2011 and received FDA approval for use in the sinus cavity. 49 This implant is of particular interest due to its open‐lattice configuration that allows expansion in the nasal cavity. Its unique design is able to prevent the collapse of healing sinonasal tissues from middle turbinate lateralization and synechiae formation. The stents are made of a PLGA monofilament without any additives and incorporated with polyethylene glycol (PEG) to allow for coating of the sinus implant with mometasone. PEG is capable of holding water from the surrounding tissue and preventing noncovalent adsorption of proteins so that mometasone can be released in a sustained manner for 90 days. Additionally, Li et al reported that this sinus implant can supply mometasone steroid in the local sinus mucosa in a rabbit model without systemic absorption. 50
Steroid‐eluting sinus stents (SESSs) have shown impressive clinical success while also removing issues with patient adherence. The data show that placement of SESS after ESS reduces polyp development, lysis of adhesions, and future interventions. Additionally, placement of SESS is a more cost‐effective intervention than traditional management. 51 , 52 This clinical success in an FDA approved delivery system makes it a promising avenue for use with other drugs of interest. One major concern with current stent interventions, such as the SESS, is that they fail to address the pathogenic bacterial biofilm component of CRS. This is of particular concern, as prior studies have shown that stents serve as a reservoir for biofilm formation within the sinus tracts and at other mucosal surfaces. 53 , 54 An antibiotic eluting stent should theoretically provide prolonged release of the antibiotic of interest while also preventing biofilm formation on the stent and within the sinus cavity.
6. ANTIBIOTIC ELUTING SINUS STENTS IN ANIMAL MODELS
6.1. The ciprofloxacin sinus stent
Initial in vitro studies involving the ciprofloxacin sinus stent (CSS) showed an initial burst release phenomenon with 30% of ciprofloxacin released from the CSS within the first 48 hours with approximately 50% being released within 14 days. This excessive release of drug was concerning due to the potential for failure to treat in future preclinical studies, so stents in preclinical studies in rabbits were coated with higher drug content. 55 , 56
The capability of the CSS to reduce biofilm mass and improve clinical findings in a rabbit model of Pseudomonas sinusitis was determined utilizing endoscopic and radiographic tools. The CSS was shown to reduce a laboratory strain of biofilm‐forming PAO1 strain of P. aeruginosa bacterial load and improve endoscopic, microscopic, and radiographic findings; however, the stent only reduced bacterial biofilm mass to ~10% of control levels during the study period (Figure 1). Additionally, scanning electron microscopy showed presence of planktonic Pseudomonal organisms despite ciprofloxacin levels consistently measuring at 20x the minimum inhibitory concentration. 57 Given these findings, a simple increase in ciprofloxacin dosage on the stent was insufficient to show appropriate outcomes. It was believed that a sinus stent requires delivery of sustained, high doses of ciprofloxacin across several weeks to sufficiently eradicate Pseudomonal organisms and biofilms. This would be best accomplished through suppressing the initial burst release observed with the CSS stent.
FIGURE 1.
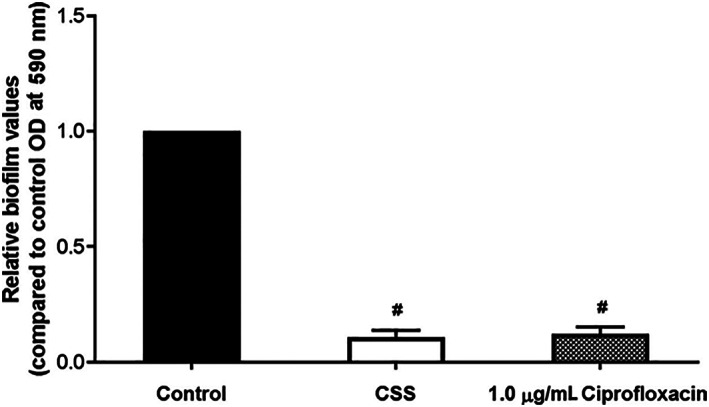
In vitro effects of the ciprofloxacin sinus stent on PAO1 biofilms. Utilizing crystal violet staining with optical density spectrometer analysis at 590 nm, CSS was shown to reduce biofilm mass to ~10% of the level of control. This is comparable to levels of biofilm found when grown in the presence of 1.0 μg/mL of ciprofloxacin. # = significance compared to control group. Adapted from Reference 57. CSS, ciprofloxacin sinus stent
6.2. The ciprofloxacin ivacaftor sinus stent
The initial burst release observed in the CSS is a well‐documented phenomenon among stent therapy including the SESS, which showed ~90% of drug release over the first 13 days. 50 , 58 , 59 Prior studies have shown that hydrophobic molecules loaded onto nanoparticles, as opposed to hydrophilic molecules such as ciprofloxacin, exhibit more sustained release over time. 58 It was thus hypothesized that developing a two layered stent with an inner, hydrophilic ciprofloxacin coating and an outer, hydrophobic drug would control the burst‐release phenomenon. Ivacaftor was selected to be incorporated into the external layer as it had the desired hydrophobic properties required while also integrating additional therapeutic benefits.
Ivacaftor is a CFTR modulator used for the treatment of CF with efficacy in patients with G551D CFTR mutations. Ivacaftor functions through potentiation of Cl− secretion of the airway epithelium allowing for partial correction of CFTR function and improved clinical outcomes. 9 , 12 , 60 , 61 , 62 , 63 , 64 , 65 , 66 In conjunction with CFTR potentiation, ivacaftor has recently been discovered to have weak antibacterial properties via a shared mechanism with the fluoroquinolone class of drugs through inhibition of bacterial DNA gyrase and topoisomerase IV. Use of ivacaftor in conjunction with antibiotics of an alternative mechanism of action has exhibited a synergistic effect on the antimicrobial properties of the drugs, including the ability to overcome drug resistance. However, ivacaftor's antimicrobial synergism had not been tested with antibiotics that have a similar mechanism of action. 67 , 68 To this end, further exploration of ivacaftor's interaction with ciprofloxacin was studied. Combination therapy was determined to have robust efficacy at elimination of planktonic organisms as well as reduction in biofilm mass (Figure 2). 69 These data confirmed that ivacaftor was an appropriate therapy to incorporate into the outer stent layer.
FIGURE 2.
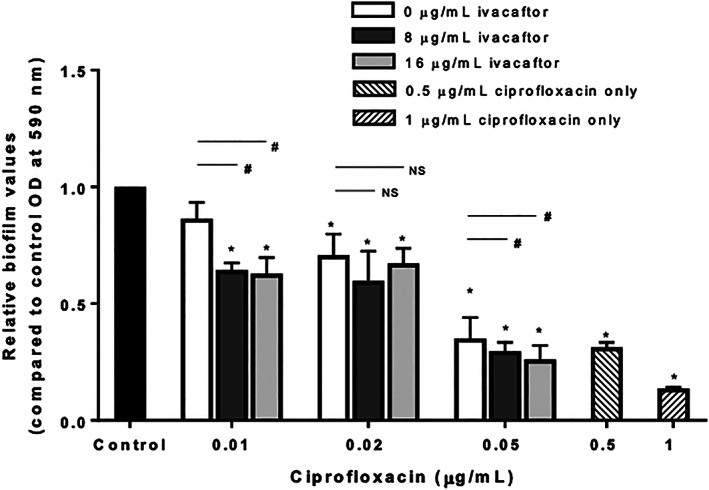
Effects of ciprofloxacin + ivacaftor on PAO1 biofilms. Utilizing crystal violet staining with optical density spectrometer analysis at 590 nm, a statistically significant overall reduction of biofilm mass was observed at 0.01 μg/mL and 0.05 mg/mL of ciprofloxacin with the addition of 8 and 16 μg/mL of ivacaftor. * = statistical significance compared to control. # = statistical significance within a ciprofloxacin group. Adapted from Reference 69. NS, not significant
The hydrophobic outer layer of ivacaftor exhibited promise in early studies as the ciprofloxacin ivacaftor sinus stent (CISS) displayed controlled release of ciprofloxacin and ivacaftor during in vitro analysis, with 50% of product released by 10 days and 80% by 21 days (Figures 3 and 4). 70 Further in vitro evaluation showed that the CISS was capable of not only reducing the total PAO1 bacterial load to <1% of control levels, but it also reduced biofilm formation and preformed biofilm mass as compared to negative control stents without loaded antibiotics. 70
FIGURE 3.
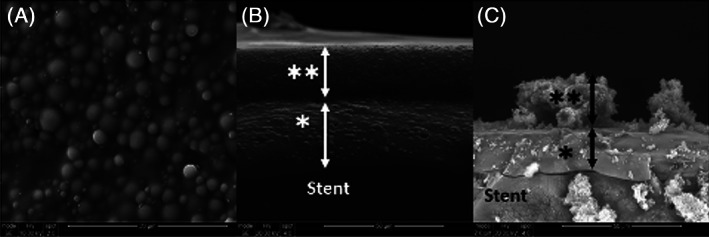
Scanning electron microscopy of the ciprofloxacin‐ivacaftor sinus stent. A, Aerial view of stent surface. B, Stent prior to use. C, Stent after 21 days of in vitro use. *: Inner (ciprofloxacin) layer of stent. **: Outer (ivacaftor) layer of stent. Arrows: Thickness of stent. Adapted from Reference 70
FIGURE 4.
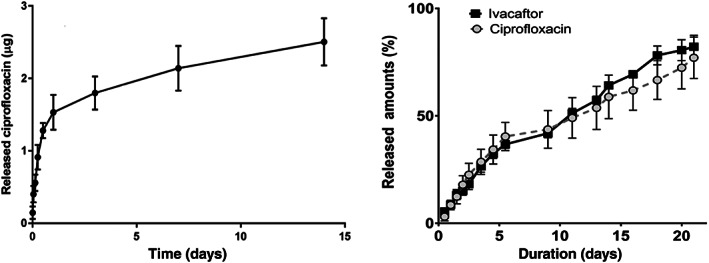
Comparison of the CSS (left) vs the CISS (right). The CSS exhibited an initial burst release phenomenon with 30% of ciprofloxacin released by day 2. Release rate slowed to <50% after day 7. The CISS showed sustained release of ciprofloxacin and ivacaftor over 21 days without an initial burst release. Adapted from References 55 and 70. CISS, ciprofloxacin‐ivacaftor sinus stent; CSS, ciprofloxacin sinus stent
The CISS was transitioned to preclinical studies with in vivo efficacy in a rabbit model determined through endoscopic examination, radiographic findings, bacterial load, and measurement of sinus potential difference. 57 , 71 , 72 , 73 Rabbit studies compared outcomes between organisms with stents placed without loaded antibiotics (sham, n = 5) and those with loaded antibiotics (CISS, n = 5). Endoscopic and radiographic improvement was robust with treated animals exhibiting a reduction in purulence on endoscopy and opacification using the Kerschner's rabbit sinus CT scoring system (Figure 5). Importantly, the CISS showed significant reduction in PAO1 bacterial load with 90% to 99% eradication of organisms while the sham group displayed a proliferation of bacteria. 74 An increased responsiveness to Cl− free ringers + forskolin was observed in the maxillary sinus in the CISS rabbit cohort indicating, at least in part, improved CFTR‐mediated Cl− transport. Additionally, submucosal thickness was reduced in treatment groups. 74 These data overall indicate normalization of epithelial function in the rabbit Pseudomonas sinusitis model with the CISS and supports translation of this modality to evaluate safety and efficacy in human clinical trials.
FIGURE 5.
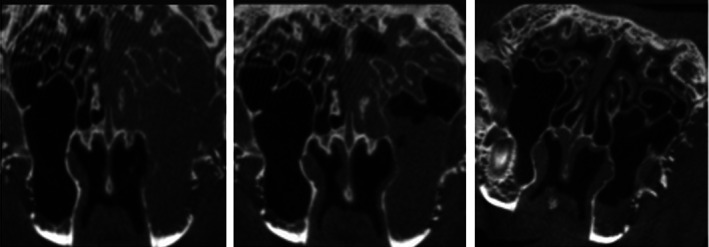
Axial micro‐CT findings of the CISS. Left: CT scan of the rabbit sinus with near complete opacification of the left maxillary sinus prior to stent placement. Middle: Continued opacification after 3 weeks of sham stent placement. Right: Radiograph showing reduced opacification after 3 weeks of CISS stent placement. Adapted from Reference 74. CISS, ciprofloxacin‐ivacaftor sinus stent; CT, computed tomographic
Although the inclusion of CFTR potentiators is an exciting prospect and showed appropriate efficacy in the preclinical models, its use in clinical practice is greatly limited by the FDA indications of the drug and limited patient reimbursement for use outside of individuals with CF and specific mutations. 9 , 75 For this reason, other hydrophobic drug options were sought to fit these needs.
6.3. The ciprofloxacin azithromycin sinus stent
Azithromycin is a commonly used antibiotic of the macrolide family that is indicated in the treatment of a number of lower and upper respiratory diseases, including sinusitis, otitis media, and strep throat. 76 Topical azithromycin has been routinely used in the ophthalmologic sphere with minimal adverse outcomes, but topical use in the treatment of airway disease remains an area of intrigue. 77 In addition to its antibacterial properties, azithromycin has significant anti‐inflammatory properties through immunomodulation of the neutrophilic response via blockade of NF‐kB and downstream interleukin (IL)‐8, IL‐6, and tumor necrosis factor alpha inflammatory activity. 78 , 79 , 80 The combined antibacterial and anti‐inflammatory mechanism of macrolides is believed to be part of the underlying mechanism in its ability to reduce polyp size, sinonasal outcome test scores, and IL‐8 levels in CRS, particularly in individuals with a noneosinophilic variant. 81 , 82 , 83 Additionally, topical azithromycin has recently been demonstrated to have in vitro ciliostimulatory effects on sinonasal epithelium. 84 The combined antibacterial, anti‐inflammatory, and ciliostimulatory effects of macrolides provided strong support for azithromycin as an ideal drug for use as the hydrophobic outer coating in antibiotic eluting stents.
Following development of the ciprofloxacin‐azithromycin sinus stent (CASS), in vitro releasing assays determined that ciprofloxacin release kinetics were comparable to the CISS with sustained release of the azithromycin as well (Figure 6). 85 There was also a significant reduction in the formation of PAO1 biofilms as well as the ability to eradicate preformed biofilms. After 3 days of exposure to the stent, the CASS displayed a greater than 50% reduction in biofilm mass measured with relative optical density, a robust reduction in percentage of living organisms (CASS 0.00% vs control 66.19%), and an overall loss of biofilm thickness measured utilizing confocal laser scanning microscopy analysis (CASS 14.7 μm vs control 44.68 μm). 85 These data support evaluation of efficacy in preclinical animal models in the future.
FIGURE 6.
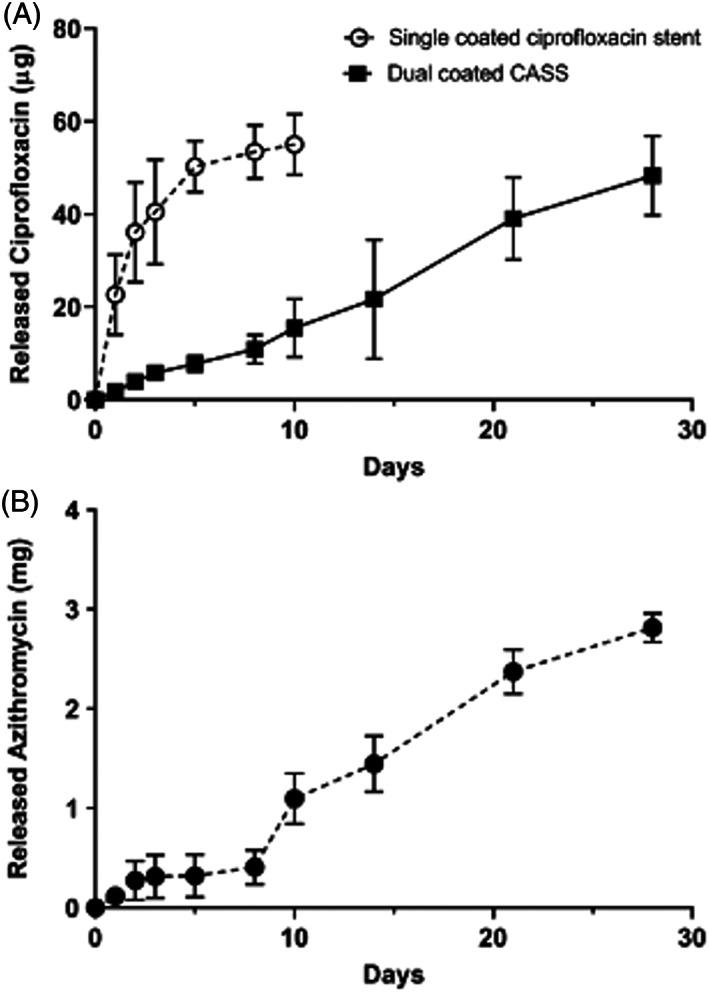
Release profiles of ciprofloxacin and azithromycin in the CASS vs CSS. A, Release profile of ciprofloxacin in the CASS vs CSS showing a sustained release over the 4‐week period in the CASS vs an initial burst release in the CSS. B, Release profile of azithromycin displaying sustained release over the 4‐week period in the CASS. Adapted from Reference 85. CASS, ciprofloxacin‐azithromycin sinus stent; CSS, ciprofloxacin sinus stent
7. DISCUSSION
Treatment of recalcitrant CRS continues to be challenging for otolaryngologists. There is a clinical need to design a topical drug delivery approach that delivers drug to the target tissue while eliminating adverse effects from systemic delivery. 17 The studies described in this review are the first to show efficacy of antibiotic eluting sinus stents in an animal model of Pseudomonas sinusitis.
In addition to the favorable pharmacokinetic profile and microscopic outcomes, the CASS and CISS are clinically promising due to the capability of two drug delivery, which may prove useful in the treatment of recalcitrant biofilm and/or multidrug resistant bacterial strains common to CRS infections. 68 , 86 The CISS is of particular interest to patients with CF (with class III gating or partial function mutations) who would directly benefit from both ivacaftor activity and reduced sinonasal disease burden.
Medical therapy remains first‐line for treatment of CRS with surgical treatment indicated in those that have failed prior medical intervention. 1 Current use of steroid releasing sinus stents has shown significant benefit when placed intraoperatively for those with medically refractory cases. 87 , 88 , 89 , 90 As the antibiotic stents progress to human trials, the authors believe these would best be utilized in a similar fashion with intraoperative placement with routine clinic postoperative follow‐up and monitoring of stent location. Alternatively, stents could be placed in the clinic in patients with previous sinus surgery and access to the maxillary and ethmoid cavities. Growing evidence indicates that CFTR dysfunction is not limited to patients with CF but includes a broad array of causes such as smoking and hypoxia. 13 , 65 , 91 , 92 , 93 , 94 , 95 , 96 Therefore, there are additional benefits to non‐CF patients as ivacaftor also potentiates wild‐type CFTR and will aid innate bacterial clearance of mucociliary function in a broad array of patients. 60 , 61 Unfortunately, until further trials are performed to assess ivacaftor therapy in these patient populations, it remains a niche to a specific patient cohort. For this reason, the CASS provides an alternative intervention to address multiple pathogenic factors present in CRS. Further research is needed to determine efficacy of the CASS in a preclinical models as well as assess whether the anti‐inflammatory effects of azithromycin are persistent when used in combination with ciprofloxacin.
PLGA is widely utilized in biomedical applications for tissue engineering scaffolds and drug delivery systems due to its biodegradability and nontoxic metabolites. 97 , 98 However, generation of acidic degradation products can decrease physiological pH and may cause an inflammatory response and fibrosis within the surrounding tissue. 99 Significant inflammation or fibrosis was not observed following placement of the antibiotic eluting stents in the in vivo animal studies, but several investigators have employed considerable efforts to reduce the inflammatory responses of PLGA by using various biological molecules and anti‐inflammatory drugs. 97 , 98 , 100 , 101 Therefore, adding an anti‐inflammatory agent to the PLGA material is a reasonable strategy until an ideal (nonimmunogenic, biocompatible, and biodegradable) biomaterial is available. While corticosteroids are one such anti‐inflammatory drug already incorporated into sinus stent delivery, some studies demonstrated that the drug downregulated endogenous vascular endothelial growth factors in a variety of cells leading to delayed healing and negatively impacting device performance. 97 , 101 , 102 Future investigations should focus on identifying biomaterials that do not cause tissue reaction and/or local effects independent of drug.
8. CONCLUSION
Drug‐eluting sinus stents represent a promising avenue of therapeutic development for recalcitrant CRS. Dual therapy stents overcome clinical challenges such as burst release, drug resistance, and biofilm mass. Future research should include transition to human clinical trials to assess safety and efficacy in human CRS patients.
CONFLICT OF INTEREST
B. A. W. is a consultant for Cook Medical, Smith and Nephew, and Baxter.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006‐04) and National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482‐05, CF Research Center Pilot Award) to B. A. W., and NIH/National Institutes of Allergy and Infectious disease (K08AI146220), John W. Kirklin Research and Education Foundation Fellowship Award, UAB Faculty Development Research Award, American Rhinologic Society New Investigator Award, and Cystic Fibrosis Foundation Research Development Pilot grant (ROWE15R0) to D.‐Y. C.
Thompson HM, Lim D‐J, Banks C, et al. Antibiotic eluting sinus stents. Laryngoscope Investigative Otolaryngology. 2020;5:598–607. 10.1002/lio2.423
Do‐Yeon Cho and Bradford A. Woodworth contributed equally to this work and serve as senior and corresponding coauthors.
Funding information American Rhinologic Society New Investigator Award; Cystic Fibrosis Foundation, Grant/Award Number: ROWE15R0; John W. Kirklin Research and Education Foundation Fellowship Award; National Heart, Lung, and Blood Institute, Grant/Award Number: 1 R01 HL133006‐04; National Institute of Allergy and Infectious Diseases, Grant/Award Number: K08AI146220; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: 5P30DK072482‐05; UAB Faculty Development Research Award
REFERENCES
- 1. Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(suppl 1):S22‐S209. [DOI] [PubMed] [Google Scholar]
- 2. Chaaban MR, Walsh EM, Woodworth BA. Epidemiology and differential diagnosis of nasal polyps. Am J Rhinol Allergy. 2013;27(6):473‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeConde AS, Soler ZM. Chronic rhinosinusitis: epidemiology and burden of disease. Am J Rhinol Allergy. 2016;30(2):134‐139. [DOI] [PubMed] [Google Scholar]
- 4. Blackwell DL, Collins JG, Coles R. Summary health statistics for U.S. adults: National Health Interview Survey, 1997. Vital Health Stat. 2002;10(205):1‐109. [PubMed] [Google Scholar]
- 5. Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims‐based study. Otolaryngol Head Neck Surg. 2011;144(3):440‐445. [DOI] [PubMed] [Google Scholar]
- 6. Bose S, Grammer LC, Peters AT. Infectious chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2016;4(4):584‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoiby N, Bjarnsholt T, Moser C, et al. Diagnosis of biofilm infections in cystic fibrosis patients. APMIS. 2017;125(4):339‐343. [DOI] [PubMed] [Google Scholar]
- 8. Gysin C, Alothman GA, Papsin BC. Sinonasal disease in cystic fibrosis: clinical characteristics, diagnosis, and management. Pediatr Pulmonol. 2000;30(6):481‐489. [DOI] [PubMed] [Google Scholar]
- 9. Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy. 2013;27(5):387‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karanth TK, Karanth V, Ward BK, Woodworth BA, Karanth L. Medical interventions for chronic rhinosinusitis in cystic fibrosis. Cochrane Database Syst Rev. 2019;10:CD012979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lowery AS, Gallant JN, Woodworth BA, et al. Chronic rhino‐sinusitis treatment in children with cystic fibrosis: a cross‐sectional survey of pediatric pulmonologists and otolaryngologists. Int J Pediatr Otorhinolaryngol. 2019;124:139‐142. [DOI] [PubMed] [Google Scholar]
- 12. Tipirneni KE, Woodworth BA. Medical and surgical advancements in the management of cystic fibrosis chronic rhinosinusitis. Curr Otorhinolaryngol Rep. 2017;5(1):24‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Curr Opin Pulm Med. 2014;20(6):623‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg. 2006;134(6):991‐996. [DOI] [PubMed] [Google Scholar]
- 15. Hochstim CJ, Masood R, Rice DH. Biofilm and persistent inflammation in endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2010;143(5):697‐698. [DOI] [PubMed] [Google Scholar]
- 16. Singhal D, Psaltis AJ, Foreman A, Wormald PJ. The impact of biofilms on outcomes after endoscopic sinus surgery. Am J Rhinol Allergy. 2010;24(3):169‐174. [DOI] [PubMed] [Google Scholar]
- 17. Fastenberg JH, Hsueh WD, Mustafa A, Akbar NA, Abuzeid WM. Biofilms in chronic rhinosinusitis: pathophysiology and therapeutic strategies. World J Otorhinolaryngol Head Neck Surg. 2016;2(4):219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rabin N, Zheng Y, Opoku‐Temeng C, Du Y, Bonsu E, Sintim HO. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med Chem. 2015;7(4):493‐512. [DOI] [PubMed] [Google Scholar]
- 19. Watters C, Fleming D, Bishop D, Rumbaugh KP. Host responses to biofilm. Prog Mol Biol Transl Sci. 2016;142:193‐239. [DOI] [PubMed] [Google Scholar]
- 20. Di Luca M, Navari E, Esin S, et al. Detection of biofilms in biopsies from chronic rhinosinusitis patients: in vitro biofilm forming ability and antimicrobial susceptibility testing in biofilm mode of growth of isolated bacteria. Adv Exp Med Biol. 2018;1057:1‐27. [DOI] [PubMed] [Google Scholar]
- 21. Macia MD, Rojo‐Molinero E, Oliver A. Antimicrobial susceptibility testing in biofilm‐growing bacteria. Clin Microbiol Infect. 2014;20(10):981‐990. [DOI] [PubMed] [Google Scholar]
- 22. Mulet X, Moya B, Juan C, et al. Antagonistic interactions of Pseudomonas aeruginosa antibiotic resistance mechanisms in planktonic but not biofilm growth. Antimicrob Agents Chemother. 2011;55(10):4560‐4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glowacki R, Tomaszewski KA, Strek P, et al. The influence of bacterial biofilm on the clinical outcome of chronic rhinosinusitis: a prospective, double‐blind, scanning electron microscopy study. Eur Arch Otorhinolaryngol. 2014;271(5):1015‐1021. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Z, Linkin DR, Finkelman BS, et al. Asthma and biofilm‐forming bacteria are independently associated with revision sinus surgeries for chronic rhinosinusitis. J Allergy Clin Immunol. 2011;128:221‐223. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Z, Adappa ND, Chiu AG, Doghramji LJ, Cohen NA, Palmer JN. Biofilm‐forming bacteria and quality of life improvement after sinus surgery. Int Forum Allergy Rhinol. 2015;5(7):643‐649. [DOI] [PubMed] [Google Scholar]
- 26. Kennedy JL, Borish L. Chronic rhinosinusitis and antibiotics: the good, the bad, and the ugly. Am J Rhinol Allergy. 2013;27(6):467‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Head K, Chong LY, Piromchai P, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1‐S39. [DOI] [PubMed] [Google Scholar]
- 29. Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45(4):999‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34‐39. [DOI] [PubMed] [Google Scholar]
- 31. Bayramov DF, Neff JA. Beyond conventional antibiotics ‐ new directions for combination products to combat biofilm. Adv Drug Deliv Rev. 2017;112:48‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open. 2015;5(11):e010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chong LY, Head K, Hopkins C, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Succar EF, Turner JH, Chandra RK. Nasal saline irrigation: a clinical update. Int Forum Allergy Rhinol. 2019;9(S1):S4‐S8. [DOI] [PubMed] [Google Scholar]
- 35. Huang ZZ, Chen XZ, Huang JC, et al. Budesonide nasal irrigation improved Lund‐Kennedy endoscopic score of chronic rhinosinusitis patients after endoscopic sinus surgery. Eur Arch Otorhinolaryngol. 2019;276(5):1397‐1403. [DOI] [PubMed] [Google Scholar]
- 36. Ghogomu N, Kern R. Chronic rhinosinusitis: the rationale for current treatments. Expert Rev Clin Immunol. 2017;13(3):259‐270. [DOI] [PubMed] [Google Scholar]
- 37. Chong LY, Head K, Hopkins C, Philpott C, Schilder AG, Burton MJ. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grayson JW, Harvey RJ. Topical corticosteroid irrigations in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9(S1):S9‐S15. [DOI] [PubMed] [Google Scholar]
- 39. Phillips KM, Hoehle LP, Caradonna DS, Gray ST, Sedaghat AR. Intranasal corticosteroids and saline: usage and adherence in chronic rhinosinusitis patients. Laryngoscope. 2020;130(4):852‐856. 10.1002/lary.28152. [DOI] [PubMed] [Google Scholar]
- 40. Parikh A, Anand U, Ugwu MC, Feridooni T, Massoud E, Agu RU. Drug‐eluting nasal implants: formulation, characterization, clinical applications and challenges. Pharmaceutics. 2014;6(2):249‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chaudhry AL, Chaaban MR, Ranganath NK, Woodworth BA. Topical triamcinolone acetonide/carboxymethylcellulose foam for acute exacerbations of chronic rhinosinusitis/nasal polyposis. Am J Rhinol Allergy. 2014;28(4):341‐344. [DOI] [PubMed] [Google Scholar]
- 42. Palmer JN, Jacobson KW, Messina JC, Kosik‐Gonzalez C, Djupesland PG, Mahmoud RA. EXHANCE‐12: 1‐year study of the exhalation delivery system with fluticasone (EDS‐FLU) in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(8):869‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grzeskowiak B, Wierzchowska M, Walorek R, Seredyka‐Burduk M, Wawrzyniak K, Burduk PK. Steroid vs. antibiotic impregnated absorbable nasal packing for wound healing after endoscopic sinus surgery: a randomized, double blind, placebo‐controlled study. Braz J Otorhinolaryngol. 2019;85(4):473‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gao P, Nie X, Zou M, Shi Y, Cheng G. Recent advances in materials for extended‐release antibiotic delivery system. J Antibiot. 2011;64(9):625‐634. [DOI] [PubMed] [Google Scholar]
- 45. Ghiorghita C‐A, Bucatariu F, Dragan ES. Influence of cross‐linking in loading/release applications of polyelectrolyte multilayer assemblies. A review. Mater Sci Eng C. 2019;105:110050. [DOI] [PubMed] [Google Scholar]
- 46. Gnanadhas DP, Ben Thomas M, Elango M, Raichur AM, Chakravortty D. Chitosan‐dextran sulphate nanocapsule drug delivery system as an effective therapeutic against intraphagosomal pathogen Salmonella. J Antimicrob Chemother. 2013;68(11):2576‐2586. [DOI] [PubMed] [Google Scholar]
- 47. Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. PLGA/PVA hydrogel composites for long‐term inflammation control following s.c. implantation. Int J Pharm. 2010;384(1‐2):78‐86. [DOI] [PubMed] [Google Scholar]
- 48. Patel GC, Dalwadi CA. Recent patents on stimuli responsive hydrogel drug delivery system. Recent Pat Drug Deliv Formul. 2013;7(3):206‐215. [DOI] [PubMed] [Google Scholar]
- 49. Kennedy DW. The PROPELtm steroid‐releasing bioabsorbable implant to improve outcomes of sinus surgery. Expert Rev Respir Med. 2012;6(5):493‐498. [DOI] [PubMed] [Google Scholar]
- 50. Li PM, Downie D, Hwang PH. Controlled steroid delivery via bioabsorbable stent: safety and performance in a rabbit model. Am J Rhinol Allergy. 2009;23(6):591‐596. [DOI] [PubMed] [Google Scholar]
- 51. Han JK, Marple BF, Smith TL, et al. Effect of steroid‐releasing sinus implants on postoperative medical and surgical interventions: an efficacy meta‐analysis. Int Forum Allergy Rhinol. 2012;2(4):271‐279. [DOI] [PubMed] [Google Scholar]
- 52. Han JK, Kern RC. Topical therapies for management of chronic rhinosinusitis: steroid implants. Int Forum Allergy Rhinol. 2019;9(S1):S22‐S26. [DOI] [PubMed] [Google Scholar]
- 53. Perloff JR, Palmer JN. Evidence of bacterial biofilms on frontal recess stents in patients with chronic rhinosinusitis. Am J Rhinol. 2004;18(6):377‐380. [PubMed] [Google Scholar]
- 54. Zumstein V, Betschart P, Albrich WC, et al. Biofilm formation on ureteral stents ‐ incidence, clinical impact, and prevention. Swiss Med Wkly. 2017;147:w14408. [DOI] [PubMed] [Google Scholar]
- 55. Cho DY, Hoffman K, Skinner D, et al. Tolerance and pharmacokinetics of a ciprofloxacin‐coated sinus stent in a preclinical model. Int Forum Allergy Rhinol. 2017;7(4):352‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haeseker M, Stolk L, Nieman F, et al. The ciprofloxacin target AUC: MIC ratio is not reached in hospitalized patients with the recommended dosing regimens. Br J Clin Pharmacol. 2013;75(1):180‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cho DY, Lim DJ, Mackey C, et al. Preclinical therapeutic efficacy of the ciprofloxacin‐eluting sinus stent for Pseudomonas aeruginosa sinusitis. Int Forum Allergy Rhinol. 2018;8(4):482‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rodrigues de Azevedo C, von Stosch M, Costa MS, et al. Modeling of the burst release from PLGA micro‐ and nanoparticles as function of physicochemical parameters and formulation characteristics. Int J Pharm. 2017;532(1):229‐240. [DOI] [PubMed] [Google Scholar]
- 59. Huang C, Cai XB, Guo LL, Qi XS, Gao Q, Wan XJ. Drug‐eluting fully covered self‐expanding metal stent for dissolution of bile duct stones in vitro. World J Gastroenterol. 2019;25(26):3370‐3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sermet‐Gaudelus I. Ivacaftor treatment in patients with cystic fibrosis and the G551D‐CFTR mutation. Eur Respir Rev. 2013;22(127):66‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rowe SM, Liu B, Hill A, et al. Optimizing nasal potential difference analysis for CFTR modulator development: assessment of ivacaftor in CF subjects with the G551D‐CFTR mutation. PLoS One. 2013;8(7):e66955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McCormick J, Cho DY, Lampkin B, et al. Ivacaftor improves rhinologic, psychologic, and sleep‐related quality of life in G551D cystic fibrosis patients. Int Forum Allergy Rhinol. 2019;9(3):292‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cho DY, Zhang S, Lazrak A, et al. Resveratrol and ivacaftor are additive G551D CFTR‐channel potentiators: therapeutic implications for cystic fibrosis sinus disease. Int Forum Allergy Rhinol. 2019;9(1):100‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cho DY, Lim DJ, Mackey C, et al. l‐methionine anti‐biofilm activity against Pseudomonas aeruginosa is enhanced by the cystic fibrosis transmembrane conductance regulator potentiator, ivacaftor. Int Forum Allergy Rhinol. 2018;8(5):577‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Woodworth BA. Resveratrol ameliorates abnormalities of fluid and electrolyte secretion in a hypoxia‐induced model of acquired CFTR deficiency. Laryngoscope. 2015;125(suppl 7):S1‐S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang S, Blount AC, McNicholas CM, et al. Resveratrol enhances airway surface liquid depth in sinonasal epithelium by increasing cystic fibrosis transmembrane conductance regulator open probability. PLoS One. 2013;8(11):e81589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reznikov LR, Abou Alaiwa MH, Dohrn CL, et al. Antibacterial properties of the CFTR potentiator ivacaftor. J Cyst Fibros. 2014;13(5):515‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schneider EK, Azad MA, Han ML, et al. An "unlikely" pair: the antimicrobial synergy of polymyxin B in combination with the cystic fibrosis transmembrane conductance regulator drugs KALYDECO and ORKAMBI. ACS Infect Dis. 2016;2(7):478‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cho DY, Lim DJ, Mackey C, et al. Ivacaftor, a cystic fibrosis transmembrane conductance regulator potentiator, enhances ciprofloxacin activity against Pseudomonas aeruginosa . Am J Rhinol Allergy. 2019;33(2):129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cho DY, Lim DJ, Mackey C, et al. In‐vitro evaluation of a ciprofloxacin‐ and ivacaftor‐coated sinus stent against Pseudomonas aeruginosa biofilms. Int Forum Allergy Rhinol. 2019;9(5):486‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leung HM, Birket SE, Hyun C, et al. Intranasal micro‐optical coherence tomography imaging for cystic fibrosis studies. Sci Transl Med. 2019;11(504):eaav3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cho DY, Skinner D, Mackey C, et al. Herbal dry extract BNO 1011 improves clinical and mucociliary parameters in a rabbit model of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9(6):629‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cho DY, Mackey C, Van Der Pol WJ, et al. Sinus microanatomy and microbiota in a rabbit model of rhinosinusitis. Front Cell Infect Microbiol. 2017;7:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lim D‐J, McCormick J, Skinner D, et al. Controlled delivery of ciprofloxacin and ivacaftor via sinus stent in a preclinical model of Pseudomonas sinusitis . Int Forum Allergy Rhinol. 2019;10:481‐488. 10.1002/alr.22514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Durmowicz AG, Lim R, Rogers H, Rosebraugh CJ, Chowdhury BA. The U.S. Food and Drug Administration's experience with ivacaftor in cystic fibrosis. Establishing efficacy using in vitro data in lieu of a clinical trial. Ann Am Thorac Soc. 2018;15(1):1‐2. [DOI] [PubMed] [Google Scholar]
- 76. Bakheit AH, Al‐Hadiya BM, Abd‐Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1‐40. [DOI] [PubMed] [Google Scholar]
- 77. Kagkelaris KA, Makri OE, Georgakopoulos CD, Panayiotakopoulos GD. An eye for azithromycin: review of the literature. Ther Adv Ophthalmol. 2018;10:2515841418783622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Oakley GM, Harvey RJ, Lund VJ. The role of macrolides in chronic rhinosinusitis (CRSsNP and CRSwNP). Curr Allergy Asthma Rep. 2017;17(5):30. [DOI] [PubMed] [Google Scholar]
- 79. Suzuki H, Shimomura A, Ikeda K, Furukawa M, Oshima T, Takasaka T. Inhibitory effect of macrolides on interleukin‐8 secretion from cultured human nasal epithelial cells. Laryngoscope. 1997;107(12 pt 1):1661‐1666. [DOI] [PubMed] [Google Scholar]
- 80. Khan AA, Slifer TR, Araujo FG, Remington JS. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents. 1999;11(2):121‐132. [DOI] [PubMed] [Google Scholar]
- 81. Amali A, Saedi B, Rahavi‐Ezabadi S, Ghazavi H, Hassanpoor N. Long‐term postoperative azithromycin in patients with chronic rhinosinusitis: a randomized clinical trial. Am J Rhinol Allergy. 2015;29(6):421‐424. [DOI] [PubMed] [Google Scholar]
- 82. Wallwork B, Coman W, Mackay‐Sim A, Greiff L, Cervin A. A double‐blind, randomized, placebo‐controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope. 2006;116(2):189‐193. [DOI] [PubMed] [Google Scholar]
- 83. Yamada T, Fujieda S, Mori S, Yamamoto H, Saito H. Macrolide treatment decreased the size of nasal polyps and IL‐8 levels in nasal lavage. Am J Rhinol. 2000;14(3):143‐148. [DOI] [PubMed] [Google Scholar]
- 84. Workman AD, Carey RM, Kohanski MA, Adappa ND, Palmer JN, Cohen NA. Effects of ophthalmologic solutions on sinonasal ciliated epithelium. Int Forum Allergy Rhinol. 2017;7(8):801‐808. [DOI] [PubMed] [Google Scholar]
- 85. Lim D‐J, Skinner D, McLemore J, et al. In‐vitro evaluation of a ciprofloxacin and azithromycin sinus stent for Pseudomonas aeruginosa biofilms. Int Forum Allergy Rhinol. 2020;10(1):121‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gao W, Thamphiwatana S, Angsantikul P, Zhang L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(6):532‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bury S, Singh A. Evaluation of a steroid releasing sinus implant for the treatment of patients undergoing frontal sinus surgery for chronic rhinosinusitis. Expert Rev Med Devices. 2017;14(2):93‐101. [DOI] [PubMed] [Google Scholar]
- 88. Murr AH, Smith TL, Hwang PH, et al. Safety and efficacy of a novel bioabsorbable, steroid‐eluting sinus stent. Int Forum Allergy Rhinol. 2011;1(1):23‐32. [DOI] [PubMed] [Google Scholar]
- 89. Forwith KD, Chandra RK, Yun PT, Miller SK, Jampel HD. ADVANCE: a multisite trial of bioabsorbable steroid‐eluting sinus implants. Laryngoscope. 2011;121(11):2473‐2480. [DOI] [PubMed] [Google Scholar]
- 90. Marple BF, Smith TL, Han JK, et al. Advance II: a prospective, randomized study assessing safety and efficacy of bioabsorbable steroid‐releasing sinus implants. Otolaryngol Head Neck Surg. 2012;146(6):1004‐1011. [DOI] [PubMed] [Google Scholar]
- 91. Banks C, Freeman L, Cho DY, Woodworth BA. Acquired cystic fibrosis transmembrane conductance regulator dysfunction. World J Otorhinolaryngol Head Neck Surg. 2018;4(3):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tipirneni KE, Grayson JW, Zhang S, et al. Assessment of acquired mucociliary clearance defects using micro‐optical coherence tomography. Int Forum Allergy Rhinol. 2017;7(9):920‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cho DY, Skinner D, Zhang S, et al. Cystic fibrosis transmembrane conductance regulator activation by the solvent ethanol: implications for topical drug delivery. Int Forum Allergy Rhinol. 2016;6(2):178‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Illing EA, Cho DY, Zhang S, et al. Chlorogenic acid activates CFTR‐mediated Cl‐ secretion in mice and humans: therapeutic implications for chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2015;153(2):291‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Londino JD, Lazrak A, Noah JW, et al. Influenza virus M2 targets cystic fibrosis transmembrane conductance regulator for lysosomal degradation during viral infection. FASEB J. 2015;29(7):2712‐2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang S, Skinner D, Hicks SB, et al. Sinupret activates CFTR and TMEM16A‐dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS One. 2014;9(8):e104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lee Y, Kwon J, Khang G, Lee D. Reduction of inflammatory responses and enhancement of extracellular matrix formation by vanillin‐incorporated poly(lactic‐co‐glycolic acid) scaffolds. Tissue Eng Part A. 2012;18(19–20):1967‐1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yoon SJ, Kim SH, Ha HJ, et al. Reduction of inflammatory reaction of poly(d,l‐lactic‐co‐glycolic acid) using demineralized bone particles. Tissue Eng Part A. 2008;14(4):539‐547. [DOI] [PubMed] [Google Scholar]
- 99. Lih E, Park W, Park KW, et al. A bioinspired scaffold with anti‐inflammatory magnesium hydroxide and decellularized extracellular matrix for renal tissue regeneration. ACS Cent Sci. 2019;5(3):458‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Iwasaki Y, Sawada S, Ishihara K, Khang G, Lee HB. Reduction of surface‐induced inflammatory reaction on PLGA/MPC polymer blend. Biomaterials. 2002;23(18):3897‐3903. [DOI] [PubMed] [Google Scholar]
- 101. Thevenot PT, Nair AM, Shen J, Lotfi P, Ko CY, Tang L. The effect of incorporation of SDF‐1alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials. 2010;31(14):3997‐4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117(1):68‐79. [DOI] [PubMed] [Google Scholar]


