Abstract
Introduction
Primary hyperparathyroidism (PHPT) is a condition in which one or more parathyroid glands secrete excess amounts of parathyroid hormone (PTH). In short, PHPT is characterized by hypercalcemia/hypercalciuria with concurrent elevated PTH levels. This condition is known to increase the risk of cardiovascular disease, osteoporosis, psychiatric disturbances, and renal complications. As of now, the disease typically runs a long course before being identified and treated. At present, surgery is the only viable treatment option for patients with this disease. Publications from other tertiary centers have identified a large‐scale underdiagnosis of PHPT. The aim of this study is to determine if similar trends exist at the University of Arkansas for Medical Sciences (UAMS). Moreover, this study was seen as a first step to developing a machine learning strategy to diagnose PHPT in large clinical data sets.
Methods
To evaluate for potential underdiagnosis of PHPT at UAMS, all patients from 2006 to 2018 with hypercalcemia and/or hypercalciuria (excluding those with known malignancies or other possible causes of excess serum calcium) were identified in electronic medical records. Then, it was evaluated whether these hypercalcemic/hypercalciuric patients received subsequent measurement of PTH levels necessary to confirm the diagnosis of HPT.
Results
At UAMS between 2006 and 2018, 28 831 patients were identified as having hypercalcemia and/or hypercalciuria. Of these patients, only 7984 ever had subsequent PTH levels tested. Therefore, 20 847 (72.3%) of these patients never had PTH labs drawn.
Conclusions
These findings may represent a significant patient population in which PHPT remains undiagnosed due to lack of follow‐up. PHPT is often a silent disease with an insidious onset. At the point of diagnosis, typically the treatment is surgical removal of the offending parathyroid gland(s) (parathyroidectomy). Identification of underdiagnosis is the first step for subsequent improvement in the diagnosis of PHPT. Detection of this disease in its earlier stages may open the door for medical and lifestyle interventions, thereby decreasing long‐term sequelae of the disease, such as osteoporosis, myocardial infarction, or stroke.
1. INTRODUCTION
The parathyroid glands are four small glands located in the neck, typically on the posterior aspect of the thyroid. Normally, one's size is comparable to that of a grain of rice, about 50 mg. These glands function in maintenance of appropriate calcium levels within the blood. The parathyroid glands produce parathyroid hormone (PTH), an 84‐amino acid polypeptide, which increases serum calcium through promoting osteoclast activity, decreasing the excretion of calcium in the urine, and enhancing absorption of calcium from the intestinal tract (Figure 1). 1 The output of PTH is regulated by calcium‐sensing receptors (CaSR) within the cell membranes of parathyroid glands. 2 Therefore, under normal conditions, there is an inverse relationship between blood calcium levels and PTH output.
FIGURE 1.
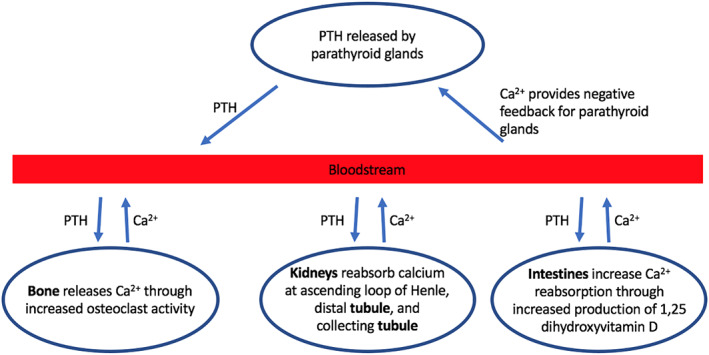
Normal physiology of calcium homeostasis via parathyroid hormone production
The disease primary hyperparathyroidism (PHPT) is characterized by one or more parathyroid glands producing excess amounts of PTH. These overactive parathyroid glands cease to respond to the intrinsic regulatory function by the CaSR, thereby causing an overabundance of calcium within the blood. In other words, PHPT is characterized by excess PTH levels with concurrent hypercalcemia. Patients with this disease often exhibit hypercalciuria as well. PHPT can arise due to several pathophysiological processes. Most commonly, PHPT occurs as a result of a parathyroid adenoma, which is a noncancerous adenomatous growth of normal cells within one parathyroid gland. Parathyroid hyperplasia, in which multiple parathyroid glands have become overgrown, accounts for a smaller proportion of PHPT cases. 3 Parathyroid cancer can also cause PHPT, but accounts for less than 1% of all cases. 4
PHPT is known to increase the risk of cardiovascular disease, osteoporosis, psychiatric disturbances, and renal complications. 5 However, patients with PHPT most commonly exhibit mild, nonspecific symptoms, or are completely asymptomatic altogether. The diagnosis of PHPT is often made incidentally when blood draws are ordered for unrelated reasons. Nonspecific symptoms of the condition include joint aches, fatigue, appetite changes, and even psychiatric disturbances such as depression and impaired concentration. More severe and specific symptoms occur with significant disturbances in calcium homeostasis. These symptoms include impaired kidney function, nephrolithiasis, and osteoporosis. 3
Treatment/management of PHPT is largely dependent on the severity of symptoms. In asymptomatic individuals without significant chemical derangements, the condition may be managed with lifestyle modifications (eg, adequate hydration, physical activity, moderation of calcium intake, and consumption of vitamin D). For osteoporotic patients, further bone loss may be prevented by anti‐osteoporotic medications. However, the single definitive treatment for PHPT is parathyroidectomy, which is typically reserved for more symptomatic cases, with serum calcium at least 1 mg/dL greater than the reference normal cutoff. 6 , 7 Since surgical treatment remains the only treatment for severe/late‐stage PHPT, earlier detection of this condition may allow an opportunity for management of the disease through lifestyle changes and medical means. Some tertiary centers in the United States have published data suggesting large‐scale underdiagnosis of PHPT at their institutions. 8 , 9 , 10 This research manuscript discusses trends in the diagnosis of PHPT at the University of Arkansas for Medical Sciences (UAMS).
2. METHODS
With approval from the institutional review board (IRB #228381), a retrospective study of data from all UAMS patients from 2006 to 2018 was conducted via electronic health records (EHRs). Initially, patient health care data are captured in an Epic EHR. Patient demographic, medical history, and coverage details are then copied on a scheduled basis from the real‐time database to an operational reporting database residing on a Microsoft SQL server. The schedule of this extract, transform, and load (ETL) process is regular but varies by field. The UAMS Clinical Data Warehouse (CDW) contains a subset of data from the hospital EHR reporting system combined with legacy data from older discontinued software sources. The CDW data are also refreshed through an ETL process on a scheduled basis. Potential PHPT patients were gathered from the CDW. They were identified by the presence of hypercalcemia and/or hypercalciuria. Inclusion criteria were total calcium >10 mg/dL or ionized calcium >1.33 mmol/L, and/or diagnosis of hypercalciuria. Exclusion criteria included other conditions known to increase serum calcium: preexisting malignancy, vitamin D toxicity, hypermagnesemia, hyperphosphatemia, acute pancreatitis, active infection, herbicide exposure, familial hypocalciuric hypercalcemia, Bartter syndrome, milk‐alkali disease, or toxicity due to rifampin, aminoglycosides, bisphosphates, or proton pump inhibitors.
Finally, to evaluate the extent to which a diagnosis of PHPT, or lack thereof, was pursued, patients meeting these criteria were then screened by the binary question of whether or not they received subsequent testing of PTH levels. Reviewers inquired as to whether or not any identification of parathyroid gland abnormality was conducted via imaging, or if clinical courses were compared between patient cohorts. However, this study was conducted as a laboratory study focused on determining the prevalence of hypercalcemia/hypercalciuria at UAMS and the rate of appropriate follow‐up in these patients.
3. RESULTS
Of all UAMS patients from 2006 to 2018, 28 831 patients were identified to have hypercalcemia and/or hypercalciuria not explained by other medical conditions (as listed above). Of these patients, only 7984 subsequently received testing of PTH levels. Therefore, 20 847 patients (72%) with the diagnosis of hypercalcemia and/or hypercalciuria never had PTH levels drawn as part of their follow‐up (Figure 2). A comparison between the patients who received the PTH testing vs patients who did not is shown in Figures 3 and 4.
FIGURE 2.
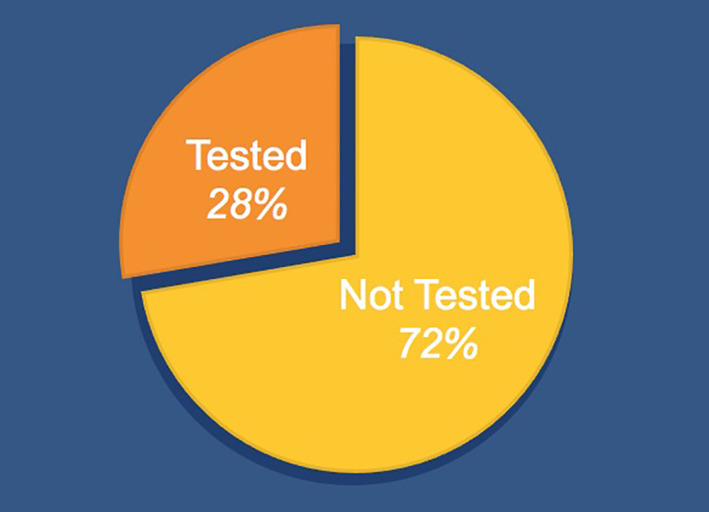
Proportions of University of Arkansas for Medical Sciences (UAMS) hypercalcemic/hypercalciuric patients 2006‐2018 (28 831 patients total) that were (28%) or were not (72%) tested for hyperparathyroidism (HPT)
FIGURE 3.
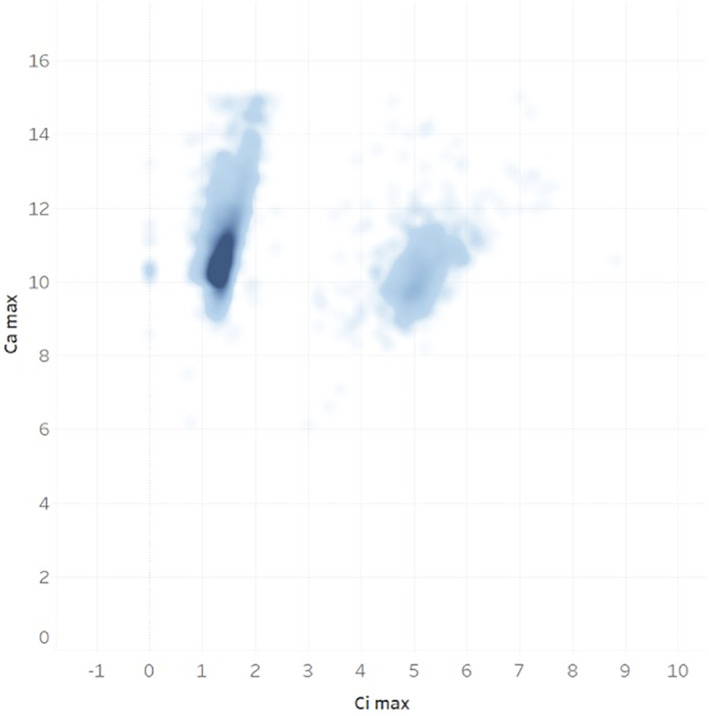
The distribution of maximum serum calcium (Ca max) and ionized calcium (Ci max) values (mg/dL) in hypercalcemic/hypercalciuric patients who received parathyroid hormone (PTH) test
FIGURE 4.
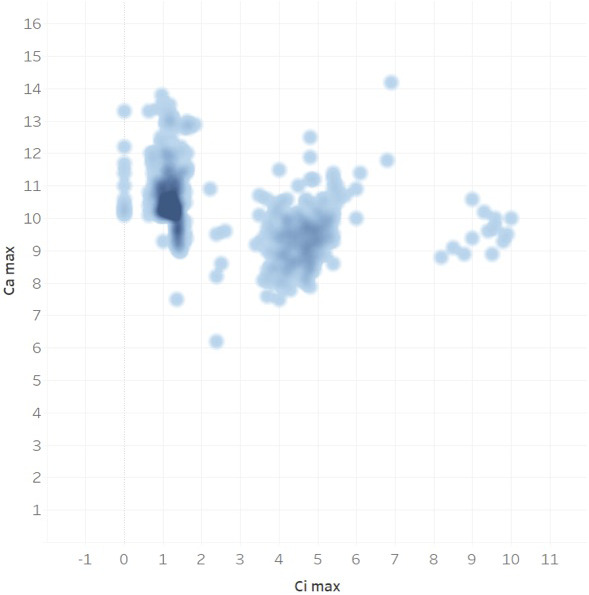
The distribution of maximum serum calcium (Ca max) and ionized calcium (Ci max) values (mg/dL) in hypercalcemic/hypercalciuric patients who did not receive parathyroid hormone (PTH) test
4. DISCUSSION
These data may indicate an extremely significant underdiagnosis of PHPT at UAMS. Literature review has shown that other US tertiary institutions have reported similar large‐scale lack of follow‐up for the potential diagnosis of PHPT. Delayed identification of this disease in patients worsens long‐term sequelae, which results in increased morbidity/mortality over time. In a study by Bandeira et al, a cohort of 25 patients with asymptomatic PHPT, 48% had osteoporosis in the lumbar spine. Furthermore, in patients with severe PHPT, the prevalence of osteoporosis was 100% in the lumbar spine, 86% in the femoral neck, and 86% in the 1/3 radius. 11 As one can infer, pathological fractures confer high rates of morbidity and mortality in elderly patients. In a study of nearly 100 000 Medicare patients with vertebral fracture by Lau et al, 7‐year risk of death was nearly doubled in an elderly person with vertebral fracture when compared to elderly counterparts without fracture, after adjustment for comorbidity. 12 Regarding hip fractures, a recent study by Edelmuth et al demonstrated a mortality rate of 11.9% during hospitalization for elderly patients admitted after surgery for hip fracture. 13
In addition to PHPT's catabolic effect on bone, disruption of calcium homeostasis in PHPT patients produces significant adverse cardiovascular effects. PHPT has been shown to contribute to hypertension, left ventricular hypertrophy, heart failure, and calcific disease. It is becoming well‐recognized that early diagnosis and treatment of PHPT can decrease cardiac sequelae of the disease. 14 Additionally, studies show an increased probability of cerebrovascular accidents in patients with PHPT vs those without the disease. 15 Rarely, stroke may even be the presenting symptom in a PHPT patient. 16 Although the mechanism has yet to be fully elucidated, PHPT has also been implicated as a cause of secondary depression. Most of these patients report resolution of their depressive symptoms following regulation of serum calcium levels. 17 From these data, one can infer that prompt diagnosis and early treatment of PHPT may decrease morbidity and mortality due to long‐term sequelae of the disease, as well as improving quality of life in these patients. Identification of underdiagnosis of PHPT at an institutional level opens the door for further characterization of this issue, as well as future improvement in the diagnosis rates of this disease.
5. CONCLUSIONS
This study seeks to increase physicians' consideration of PHPT on their diagnostic “radar,” especially given the fact that PTH quantification is not a particularly costly test. Improvement in diagnosis rates could be done at the level of primary care providers, hospitalists, and any other physicians that detect abnormally high serum/urine calcium and are in position to further screen the patient. Perhaps future collaboration with orthopedists regarding uniform PHPT screening after fracture would be of high yield. Similar recommendations may be made for urologists when a diagnosis of nephrolithiasis is made. In the future, notifications could be built into electronic medical records that would alert the physician if hypercalcemia/hypercalciuria is detected and the patient has never had PTH levels drawn. Given the long‐term sequelae of untreated PHPT, prompt diagnosis of this condition would undoubtedly improve morbidity and mortality in patients with this disease.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest or specific funding contributing to the publication of this article.
Quilao RJ, Greer M, Stack BC Jr. Investigating the potential underdiagnosis of primary hyperparathyroidism at the University of Arkansas for Medical Sciences. Laryngoscope Investigative Otolaryngology. 2020;5:773–777. 10.1002/lio2.415
Presented at UAMS College of Medicine 3‐Minute Thesis Competition, 1st Place
BIBLIOGRAPHY
- 1. Khan M, Sharma S. Physiology, parathyroid hormone (PTH) StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK499940/. [PubMed] [Google Scholar]
- 2. Chen R, Goodman W. Role of the calcium‐sensing receptor in parathyroid gland physiology. Am J Physiol Renal Physiol. 2004;286:F1005‐F1011. [DOI] [PubMed] [Google Scholar]
- 3. Spiegel AM. Pathophysiology of primary hyperparathyroidism. J Bone Miner Res. 1991;6(suppl 2):S15‐S17. [DOI] [PubMed] [Google Scholar]
- 4. Ruda J, Hollenbeak CS, Stack BC Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995‐2003. Otolaryngol Head Neck Surg. 2005;132(3):359‐372. [DOI] [PubMed] [Google Scholar]
- 5. MacKenzie‐Feder J, Sirrs S, Anderson D, Sharif J, Khan A. Primary hyperparathyroidism: an overview. Int J Endocrinol. 2011;2011:1‐8. 10.1155/2011/251410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pokhrel B, Levine SN. Primary hyperparathyroidism StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK441895/. [PubMed] [Google Scholar]
- 7. Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3561‐3569. 10.1210/jc.2014-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen H, Balentine CJ, Xie R, Kirklin JK. Failure to diagnose hyperparathyroidism in 10,432 patients with hypercalcemia: opportunities for system‐level intervention to increase surgical referrals and cure. Ann Surg. 2017;266(4):632‐640. 10.1097/SLA.0000000000002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Press DM, Siperstein AE, Berber E, et al. The prevalence of underdiagnosed and unrecognized primary hyperparathyroidism: a population‐based analysis from the electronic medical record. Surgery. 2013;154(6):1232‐1237. 10.1016/j.surg.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 10. Alore EA, Suliburk JW, Ramsey DJ, et al. Diagnosis and management of primary hyperparathyroidism across the veterans affairs health care system. JAMA Intern Med. 2019;179(9):1220‐1227. 10.1001/jamainternmed.2019.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bandeira F, Griz LH, Bandeira C, et al. Prevalence of cortical osteoporosis in mild and severe primary hyperparathyroidism and its relationship with bone markers and vitamin D status. J Clin Densitom. 2009;12:195‐199. [DOI] [PubMed] [Google Scholar]
- 12. Lau E, Ong K, Kurtz S, Schmier J, Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am. 2008;90:1479‐1486. [DOI] [PubMed] [Google Scholar]
- 13. Edelmuth SVCL, Sorio GN, Sprovieri FAA, Gali JC, Peron SF. Comorbidities, clinical intercurrences, and factors associated with mortality in elderly patients admitted for a hip fracture. Rev Bras Ortop. 2018;53(5):543‐551. 10.1016/j.rboe.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown SJ, Ruppe MD, Tabatabai LS. The parathyroid gland and heart disease. Methodist Debakey Cardiovasc J. 2017;13(2):49‐54. 10.14797/mdcj-13-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boström H, Alveryd A. Stroke in hyperparathyroidism. Acta Med Scand. 1972;192(4):299‐308. [DOI] [PubMed] [Google Scholar]
- 16. Mitre N, Mack K, Babovic‐Vuksanovic D, Thompson G, Kumar S. Ischemic stroke as the presenting symptom of primary hyperparathyroidism due to multiple endocrine neoplasia type 1. J Pediatr. 2008;153:582‐585. [DOI] [PubMed] [Google Scholar]
- 17. Hurst K. Primary hyperparathyroidism as a secondary cause of depression. J Am Board Fam Med. 2010;23(5):677‐680. 10.3122/jabfm.2010.05.090199. [DOI] [PubMed] [Google Scholar]


