Abstract
Objective
Endoscopic sinus surgery represents the gold standard for surgical treatment of chronic sinus diseases. Thereby, navigation systems can be of distinct use. In our study, we tested the recently developed KARL STORZ NAV1 SinusTracker navigation software that incorporates elements of augmented reality (AR) to provide a better preoperative planning and guidance during the surgical procedure.
Methods
One hundred patients with chronic sinus disease were operated on using either a conventional navigation software (n = 52, non‐AR, control group) or a navigation software incorporating AR elements (n = 48, AR, intervention group). Incidence of postoperative complications, duration of surgery, surgeon‐reported benefit from the navigation system and patient‐reported postoperative rehabilitation were assessed.
Results
The surgeons reported a higher benefit during surgery, used the navigation system for more surgical steps and spent longer time with preoperative image analysis when using the AR system as compared with the non‐AR system. No significant differences were seen in terms of postoperative complications, target registration error, operation time and postoperative rehabilitation.
Conclusion
The AR enhanced navigation software shows a high acceptance by sinus surgeons in different stages of surgical training and offers potential benefits during surgery without affecting the duration of the operation or the incidence of postoperative complications.
Level of evidence
1b.
Keywords: augmented reality, endoscopic sinus surgery, navigation systems, randomized controlled clinical trial
1. INTRODUCTION
Since more than 20 years functional endoscopic sinus surgery (FESS) represents the gold standard in surgical treatment of paranasal sinuses diseases. 1 , 2 Thereby, rigid 0° to 90° angled endoscopes with an approximately 25× magnification are used with their optical and technical properties being continuously improved. 2 Since the late 1980s, navigation systems are used for image guided sinus surgery. 3 These systems are based on preoperative CT or MRI scans and facilitate an intraoperative guidance using specific instruments, which are preoperatively adjusted to the imaging data. Image guidance facilitates anatomical orientation, which can be particularly helpful in cases of anatomical variants, revision surgery, skull base surgery and tumor diseases. 4 , 5 , 6 , 7 , 8 , 9 Mental workload of the surgeons can be reduced 10 and intraoperative complications can be avoided. 10 , 11 , 12 In addition, navigation systems can be a useful tool for young sinus surgeons at the beginning of their surgical training. 2 , 9 , 13 However, there are studies reporting no reduction of complications and a prolongation of operation time when using a navigation system. 14 , 15 , 16 , 17 , 18 , 19 Hence, the clinical benefit of navigation systems in FESS though being widely used in clinical practice still has to be substantiated.
Navigation systems show a continuous improvement in quality and accuracy as well as software properties. 20 While the illustration of an instrument's localization matched with the CT scan data represents the basic function of any navigation system in FESS, new systems enable additional functions, for example, integrated elements of augmented reality (AR), image fusions, acoustic warning signals and the usage of intraoperative CT. 21 , 22 , 23 , 24 , 25 The basic principle of all AR‐based navigation systems is an overlay of preoperative imaging on the conventional endoscopic view. 1 , 26 , 27 , 28 The development of new AR‐applications, a continuous improvement of the underlying software and a successful transfer of AR‐based navigation systems into clinic have the potential to become an important milestone in the modern treatment of sinus diseases.
Against this background, KARL STORZ SE & Co. KG developed the NAV1 SinusTracker navigation software integrating new AR elements that enable the surgeon to draw so called “surgical pathways” in the CT scan series. Intraoperatively, these pathways can be fused with the endoscopic image indicating the surgeon where to continue with preparation (Figure 1).
FIGURE 1.
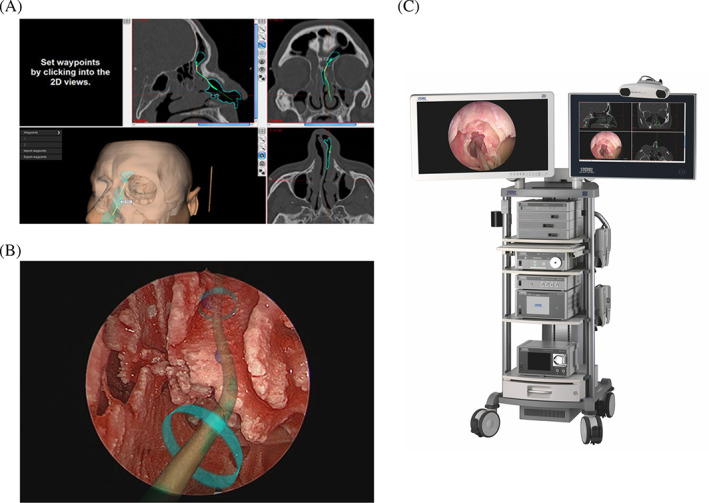
Surgical pathways as an element of augmented reality in the NAV1 SinusTracker software. A, Preoperative setting of the surgical pathway to the left frontal sinus. B, Intraoperative overlaying of the surgical pathway on the endoscopic image. C, Combined navigation cart with an optical and an electromagnetic navigation system
In our study, we investigated the potential benefit of these new tools incorporated in this AR navigation system (intervention group), which was applied during FESS procedures of 48 patients and compared with another 52 procedures using a non‐AR navigation system as a control (control group). The study was designed as a prospective, randomized, controlled monocentric clinical trial.
2. MATERIALS AND METHODS
2.1. Study concept
The aim of our study was to evaluate the potential benefit of the KARL STORZ NAV1 Sinus Tracker software (AR, intervention group) for the surgeon as well as for the patients when comparing it to the KARL STORZ NAV1 optical system as control (non‐AR, control group). Furthermore, we wanted to see if a different benefit could be seen for a surgeon at an early stage of surgical training compared with a more experienced surgeon. In total, 100 patients (≥18 years of age) were included in our study and distributed to four treatment arms (Figure 2A): one half of the patients was operated on using a conventional optical navigation system (non‐AR), and the other half was operated on using an electromagnetic navigation system with the AR system. In both groups, patients were again distributed to two subgroups, and either one senior physician or one resident as the surgeon performed their operations. The same surgeons (n = 2) operated with both navigation types. Both surgeons had no prior experience using the AR features apart from theoretical training by the company's representative. The surgical experience of the senior physician and the resident in paranasal sinus surgery comprised >3000 procedures for the senior physician and approximately 150 procedures for the resident, respectively. The distribution of the patients to the four treatment groups was performed using the stratified randomization technique considering age, sex and the extension of surgical intervention as covariates. The study was designed as single‐blinded study.
FIGURE 2.
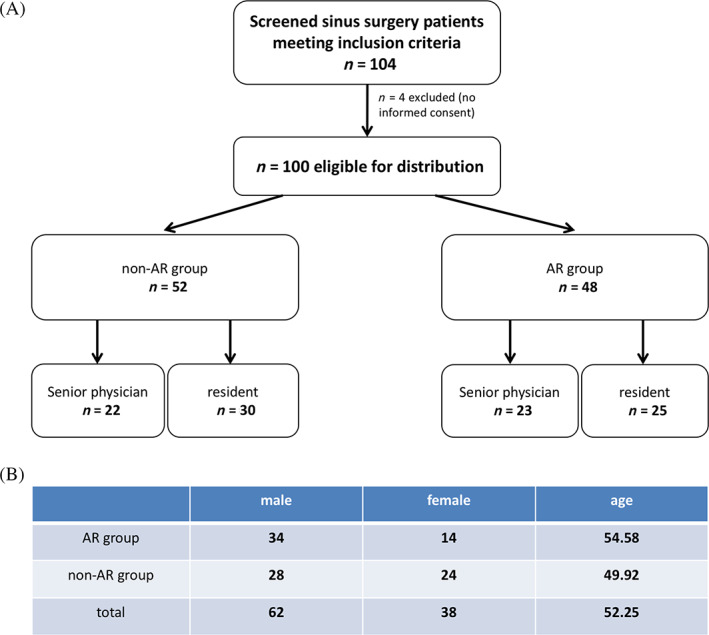
Study flowchart and distribution of age and sex in the different treatment groups. A, Study flow chart, the number of patients per study arm is indicated in the boxes. B, Distribution of age and sex in both study arms; for age, the mean value is indicated
2.2. Patient characteristics
Patients were recruited from September 2017 to December 2018 and treated at the Department of Otorhinolaryngology, Head and Neck Surgery (Saarland University Medical Centre, Homburg, Germany). Sample size calculation was based on a power analysis (Power (1 − ß) = 0.8; α = 0.05; Group A mean (μ A) = 6; Group B mean (μ B) = 8; SD (σ) = 3; sampling ratio (κ = n A/n B) = 1).
Inclusion criteria comprised an age ≥ 18 years, a medical indication for endoscopic sinus surgery due to chronic sinus disease excluding benign or malignant tumors as well as written informed consent. Exclusion criteria comprised an age < 18 years, a lacking medical indication for endoscopic sinus surgery, ongoing treatment with anticoagulants or thrombocyte aggregation inhibitors, inherited coagulation disorders as well as a permanent treatment with analgesic medication. The Saarland Medical Association ethics review committee approved this study (index‐number 168/17). Written informed consent was obtained from all patients. The study was prospectively registered at the German Clinical Trials Register (DRKS‐Nr. 00013508). Details of the distribution of age and sex among the four treatment groups are shown in Figure 2B. The extent of surgical intervention was balanced between the AR and non‐AR group is listed in Table 1.
TABLE 1.
FESS procedures in the intervention and control group
| Intervention group (AR) | Control group (non‐AR) | |
|---|---|---|
| Pansinus operation | 25 (52%) | 23 (44%) |
| Maxillary sinus + ethmoidectomy only | 9 (19%) | 12 (23%) |
| Maxillary sinus only | 3 (6%) | 4 (8%) |
| Frontal sinus only | 3 (6%) | 5 (10%) |
| Frontal sinus + ehtmoidectomy only | 8 (17%) | 8 (15%) |
| Total | 48 | 52 |
| One side | 7 (15%) | 9 (17%) |
| Both sides | 41 (85%) | 43 (83%) |
| Revision cases | 15 (31%) | 11 (21%) |
Abbreviation: FESS, functional endoscopic sinus surgery.
2.3. Navigation systems
In our study, two different navigation systems were compared and used in combination with a 2D endoscope, respectively: the NAV1 SinusTracker software with an electromagnetic navigation unit (AR, intervention group) and the NAV1 optical system with standard navigation software (non‐AR, control group). For both systems preoperative CT‐scans of the patients were used for registration. For all cases, a registration by setting four registration markers on the reconstructed patient surface was performed. Although both systems can illustrate the localization of special surgical devices placed in the intraoperative situs on a screen with the patient's CT images, the AR system has an additional key feature: the illustration of preoperatively defined “surgical pathways,” for example, to the frontal sinus, overlaying the endoscopic image and suggesting to the surgeon where to continue with the next surgical steps (AR element).
2.4. Postoperative treatment
All patients stayed in hospital for 5 days after surgery and were supplied with analgesic medication as requested. From the first postoperative day, patients were instructed how to syringe their nose with 0.9% sodium chloride solution and received a cleaning of their nasal cavity by a careful suctioning and removal of crusts. Depending on the surgeon's decision, patients received a nasal packing with either large tamponades (8 cm, removed on day 1) that were placed in the nasal cavity and/or small tamponades (3 cm, removed on day 4) that were placed in the middle nasal meatus or no nasal packing at all.
2.5. Questionnaires
The patients were asked to complete a questionnaire every day for the first five postoperative days starting at the first day after surgery. Herein, the patients had to answer two questions (Figure S1). First, pain level had to be valued on a numerical analogue scale (NAS) ranging from 1 (no pain) to 10 (strongest imaginable pain). Second, the impairment of general condition had to be assessed on a NAS ranging from 1 (no impairment) to 10 (strongest imaginable impairment of general condition). In the case of incomplete or lacking questionnaires, the respective patient was excluded from the study.
Additionally, the surgeons were asked to complete one questionnaire for each patient (Figure S2). First, they had to value the benefit during surgery supported by the navigation system ranging from 1 (no benefit) to 10 (highest imaginable benefit). Second, they were asked during how many surgical steps the navigation system was used (not at all [1 on NAS], 1‐2 steps [2 on NAS], 3‐5 steps [3 on NAS], more than 5 steps [4 on NAS]). Third, the surgeons had to state how much time they spent preoperatively with the analysis of the patient's CT images and the planning of the surgical procedure (not at all [1 on NAS], 1‐5 minutes [2 on NAS], 5‐15 minutes [3 on NAS], more than 15 minutes [4 on NAS]). Fourth, the surgeons were asked to state the accuracy of the navigation system (no TRE [1 on NAS], less than 1 mm [2 on NAS], 1‐3 mm [3 on NAS], more than 3 mm [4 on NAS]).
Furthermore, the treating physicians had to document the incidence of postoperative complications, for example, bleedings and postoperative infections, as well as the time of surgery for each patient. To register also late‐onset bleedings, all patients were called by phone 20 days after surgery and were asked for bleeding episodes.
The indicated benefit for the surgeons by using the navigation system was defined as primary outcome. Secondary outcomes comprised the number of surgical steps for which the navigation system was used, the time of preoperative planning of the surgical procedure using the CT imaging data, the accuracy of the navigation system (TRE), the pain level as well as the impairment of general condition indicated by the patients, the duration of the surgical procedure as well as the incidence of postoperative complications.
It has to be stated that neither the questionnaire for the surgeon nor the questionnaire for the patients were internally or externally validated before starting the study.
2.6. Statistical analysis
For statistical analysis, a Mann‐Whitney U‐test was used applying the commercially available software GraphPad Prism 7.0d (GraphPad Software, La Jolla, CA). P values <.05 were considered statistically significant.
3. RESULTS
3.1. Correlation of surgical experience and the used navigation system with operation time
When comparing the operation time between operations performed with the non‐AR system and the AR system, no significant difference was seen neither for the resident nor for the senior physician (Figure 3A). Independent of the navigation system that was used, operations performed by the resident lasted significantly longer than operations performed by the senior physician (resident: mean 108.9 minutes, 95% CI 97.68‐120.2 minutes; senior physician: mean 70.62 minutes, 95% CI 55.68‐85.57 minutes; P < .0001; Figure 3B). Importantly, all four groups were matched for extension of surgical intervention as shown in Table 1 thereby trying to minimize a potential bias by differences in surgical procedures regarding the primary and secondary endpoints of the study.
FIGURE 3.
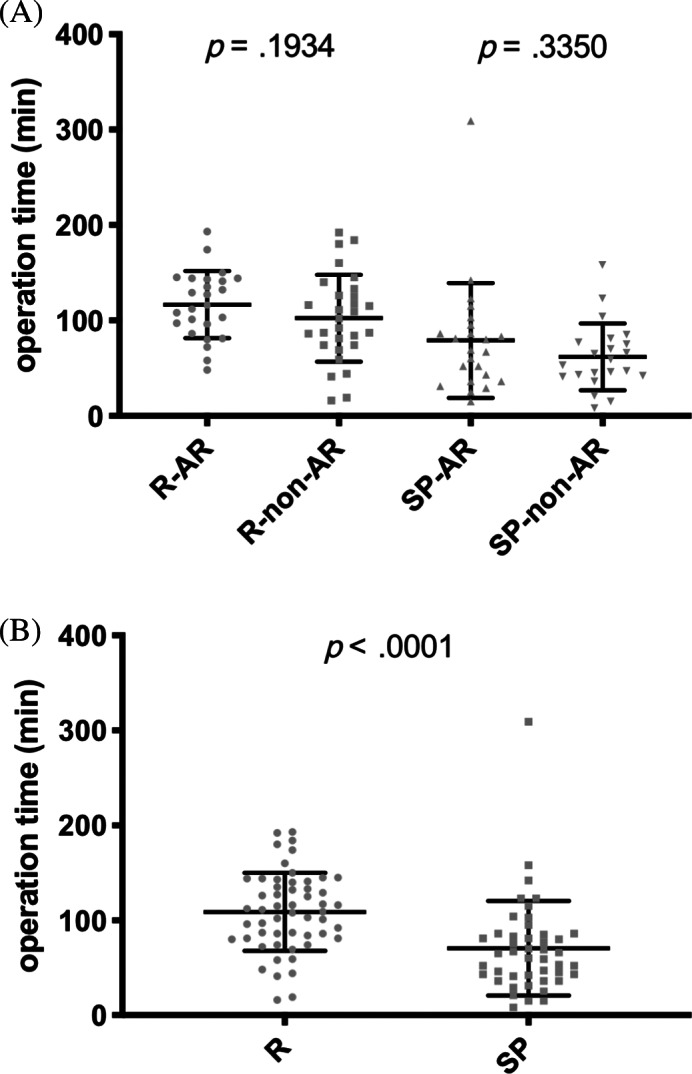
Comparison of operation time between the intervention and control group. A, Operation time (min) for all four groups of the study. B, Comparison between operations performed by the resident and the senior physician independent of the navigation system that was used. R‐AR: operation performed by the resident with navigation system including augmented reality elements (intervention group); R‐non‐AR: operation performed by the resident with navigation system not including augmented reality elements (control group); SP‐AR: operation performed by the senior physician with navigation system including augmented reality elements (intervention group); SP‐non‐AR: operation performed by the senior physician with navigation system not including augmented reality elements (control group)
3.2. Evaluation of the navigation systems by the surgeons
To evaluate the benefit provided by both navigation systems, the surgeons had to complete one questionnaire after finishing the operation as described in materials and methods. As shown in Figure 4, when using the AR system, both the resident as well as the senior physician stated a significantly higher benefit during surgery (Figure 4A), used the new system during the operation with a higher frequency (Figure 4B) and spent a significantly longer time period with preoperative image analysis and image‐based planning of the surgical procedure (Figure 4C) as compared with the non‐AR system. No significant difference between both navigation systems was seen in terms of the indicated target registration error (Figure 4D). When comparing all operations performed by the resident with all operations performed by the senior physician independent of the navigation system that was used, we found that the resident valued the benefit of using a navigation system in general as significantly lower (P = .0001) and indicated a significantly higher TRE (P = .0013). No significant difference between the resident and the senior physician was seen in terms of the frequency of using the navigation system during an operation and the time of preoperative image analysis and image‐based operation planning (data not shown). Both surgeons reported a high accuracy level of the AR images and AR based target pathways with no relevant changes in accuracy during operation.
FIGURE 4.
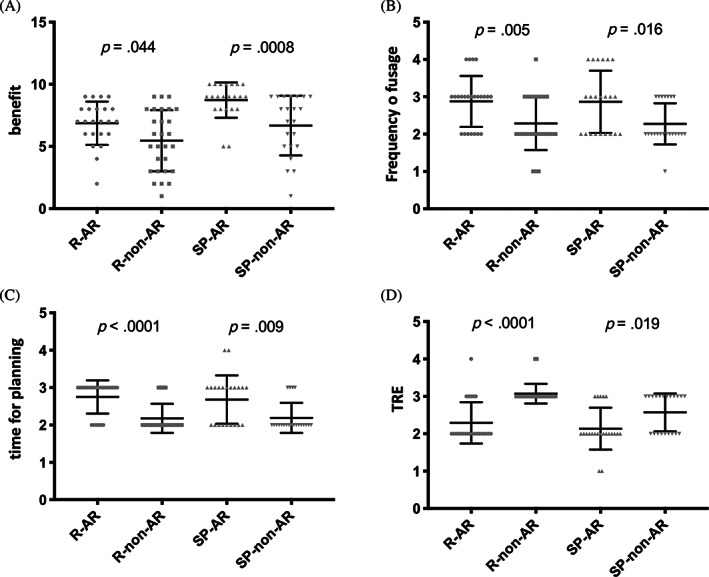
Evaluation of the two tested navigation systems by the surgeons. A, Benefit during surgery supported by the navigation system ranging from 1 (no benefit) to 10 (best imaginable benefit). B, Number of surgical steps during which the navigation system was used ranging from 1 (not at all) to 4 (more than 5 steps). C, Amount of time the surgeon spent preoperatively with the analysis of the patient's CT imaging and the planning of the surgical procedure based on these images ranging from 1 (not at all) to 4 (more than 15 minutes). D, Accuracy of the navigation system by assessing the target registration error (TRE) ranging from 1 (no TRE) to 4 (TRE > 3 mm). In panels A to D, medians and inter‐quartile ranges are indicated. R‐AR: operation performed by the resident with navigation system including augmented reality elements (intervention group); R‐non‐AR: operation performed by the resident with navigation system not including augmented reality elements (control group); SP‐AR: operation performed by the senior physician with navigation system including augmented reality elements (intervention group); SP‐non‐AR: operation performed by the senior physician with navigation system not including augmented reality elements (control group)
3.3. Incidence of postoperative complications
In total, only minor postoperative trouble was observed among all participants including slight bleedings not requiring surgical revision (5 patients in the intervention group, 4 patients in the control group) and oral antibiotic treatment due to supposed local wound infection (2 patients in the treatment and 3 patients in the control group).
3.4. Effect of the applied navigation system on postoperative rehabilitation
For all patients, we found a stepwise decrease of pain intensity (Figure 5A) as well as a stepwise improvement of their general condition after the operation with no significant difference between the AR and the non‐AR group (Figure 5B). There were also no significant differences in terms of the median pain levels as well as the median impairment of general condition on days 1 to 5 after surgery when comparing the patients operated by the resident with the patients operated by the senior physician (data not shown).
FIGURE 5.
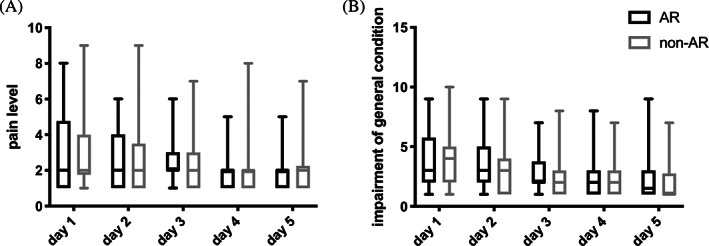
Postoperative rehabilitation of the patients. A, Pain level indicated by the patients on a numerical analogue scale ranging from 1 (no pain) to 10 (strongest imaginable pain) for the first 5 days after surgery. B, Impairment of general condition indicated by the patients on a numerical analogue scale ranging from 1 (no impairment) to 10 (strongest imaginable impairment). Values for patients of the treatment group (NAV1 SinusTracker software) are shown in black and values for patients of the control group (NAV1 optical system) are shown in grey using box and whisker blots. Each box represents the range from the first quartile to the third quartile. The median is indicated by a line. The whiskers outside the boxes represent the ranges from the minimum to the maximum value of each group. AR: navigation system with augmented reality elements (intervention group); non‐AR: navigation system without augmented reality elements (control group)
4. DISCUSSION
In our study, we demonstrated that the AR navigation system shows a high acceptance by sinus surgeons in different stages of surgical training and can offer benefits during surgery when compared with the non‐AR system without affecting the duration of the operation or the incidence of postoperative complications. Both surgeons indicated a significantly higher benefit during surgery, used the navigation system during significantly more surgical steps and spent significantly longer time with preoperative image analysis and image‐based planning of the surgical procedure when using the AR system as compared with the non‐AR system. No differences were seen between both groups in terms of postoperative complications, target registration error, operation time and postoperative pain as well as the patients' general condition.
Beyond our study, only a few studies investigated the benefit of AR elements in navigation based endoscopic sinus surgery so far. Li et al. developed a system capable of fusing endoscopic images to three‐dimensional virtual images and compared this display mode with conventional navigation systems. 25 The use of this new navigation mode shortened the duration of surgery and reduced the mental workload of the surgeons. Citardi et al tested a newly developed “hybrid navigation system” (Scopis Hybrid Navigation) in a cadaver study. 29 Thereby, a pre‐dissection planning was performed including a modeling of the frontal sinus outflow pathway on CT images. The optic nerve and the internal carotid artery were labeled as anti‐targets. Intraoperatively, both the pathway to the frontal sinus as well as the anti‐targets were superimposed onto the endoscopic image. The authors concluded that this AR system has the potential to reduce surgical complications and morbidity. Leonard et al. developed a video‐based navigation system enabling a surgeon to asynchronously register a sequence of endoscopic images to a CT scan. 30 Intraoperatively, this system then allows an overly of anatomical structures, visible, or occluded, on top of video images. While the authors focused on the stability of this system reporting a position error of 1.09 mm or less, no data are available on the potential clinical use. These data are consistent with our study where both, the resident as well as the senior physician, reported a significantly higher benefit provided by the AR navigation system, which strengthens the previously published data on cadaver dissections indicating a usefulness of AR in endoscopic sinus surgery. In contrast, Yeh and Wickens could show that AR can aid in target detection for expected targets, that is, targets that were preoperatively marked as critical structures, but draw attention away from the presence of unexpected targets implicating a potentially higher risk of intraoperative complications. 31 However, we saw no higher rate of intra‐ and postoperative complications in our study when using the AR system.
In terms of postoperative rehabilitation, Riley et al. reported a maximum of patient‐reported pain on the third day after endoscopic sinus surgery with a rapid decreasing afterwards, 32 which is comparable with our results and goes along with our clinical experience in general.
Considering the accuracy of the navigation systems, the surgeon‐reported TRE did not significantly differ between the intervention and the control group. For the majority of cases, both surgeons indicated a TRE of more than 1 mm, which is consistent with previous studies using comparable navigation software. 1 , 33 , 34 , 35 , 36 , 37
From a critical point of view, it must be stated that despite a careful planning and randomization process the primary endpoint of our study is still based on subjective evaluation of the navigation system and an RCT is anyhow unable to remove this bias. However, no other study design would be able to address this bias either, as blinding of the surgeon is not possible. Furthermore, several factors may have a relevant influence on postoperative pain and general condition and could not be considered in our study. Though a careful matching of all four study groups for the extension of surgical intervention (see Table 1) differences in surgical procedures between the groups were only minimized but not fully eliminated. Furthermore, the fact that an electromagnetic system was used in the intervention group and an optical system in the control group cannot be excluded as a potential bias regarding the subjective benefit stated by the surgeons and the number of surgical steps during which the navigation system was used. Though significant differences were seen between the AR and non‐AR group in terms of preoperative time spent with the navigation system and the number of surgical steps when navigation was used, there are numerous potential factors that influence these secondary endpoints so that differences that were seen between the groups cannot exclusively be related to the additional AR technology.
It is hard to speculate on why the resident valued the benefit of intraoperative navigation in general lower than the senior physician. One possible explanation is that due to the limited experience in working with navigation systems, the TRE was in fact higher when registration was performed by the resident as compared with the senior physician as it is also indicated by the results of the surgeons' questionnaires and therefore, the navigation system showed a lower accuracy when used by the resident.
5. CONCLUSION
Taken together, the results of our study demonstrated that the incorporation of AR elements in navigation software provides potential benefits during endoscopic sinus surgery as compared to non‐AR navigation systems without affecting operation time and complication rates. Both, the experienced surgeon as well as the surgeon in training highly appreciated AR enhanced navigation and recommend an application of this technology especially for revision cases and challenging anatomy. Future studies will have to show if this benefit for the surgeons can also result in significantly better surgical outcome, which due to the limited number of patients and the short follow‐up period was not properly addressed in our study.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Figure S1 Questionnaire for the surgeon.
Figure S2 Questionnaire for the patients.
ACKNOWLEDGMENTS
We thank the whole team of the Saarland University Medical Center ENT Department for their worthful support in conducting our study. We also express our deep gratitude to our patients who participated in this study.
Linxweiler M, Pillong L, Kopanja D, et al. Augmented reality‐enhanced navigation in endoscopic sinus surgery: A prospective, randomized, controlled clinical trial. Laryngoscope Investigative Otolaryngology. 2020;5:621–629. 10.1002/lio2.436
REFERENCES
- 1. Citardi MJ, Batra PS. Intraoperative surgical navigation for endoscopic sinus surgery: rationale and indications. Curr Opin Otolaryngol Head Neck Surg. 2007;15:23‐27. [DOI] [PubMed] [Google Scholar]
- 2. Weber RK, Hosemann W. Comprehensive review on endonasal endoscopic sinus surgery. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2015. 10.3205/cto000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wise SK, DelGaudio JM. Computer‐aided surgery of the paranasal sinuses and skull base. Expert Rev Med Devices. 2005;2:395‐408. [DOI] [PubMed] [Google Scholar]
- 4. Caversaccio M, Zheng G, Nolte LP. Computer‐aided surgery of the paranasal sinuses and the anterior skull base. Eur Arch Otorhinolaryngol. 2008;56:376‐378. [DOI] [PubMed] [Google Scholar]
- 5. Franz L, Isola M, Bagatto D, Tuniz F, Robiony M. A novel approach to skull‐base and orbital osteotomies through virtual planning and navigation. Laryngoscope. 2018;129:823‐831. 10.1002/lary.27479. [DOI] [PubMed] [Google Scholar]
- 6. Hepworth EJ, Bucknor M, Patel A, Vaughan WC. Nationwide survey on the use of image‐guided functional endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2006;135:68‐73. [DOI] [PubMed] [Google Scholar]
- 7. Oakley GM, Barham HP, Harvey RJ. Utility of image‐guidance in frontal sinus surgery. Otolaryngol Clin North Am. 2016;49:975‐988. [DOI] [PubMed] [Google Scholar]
- 8. Reiss‐Zimmermann M, Schulz T, Kahn T, Hofer M. Imaging of the sinuses for functional sinus surgery using navigational guidance. Laryngorhinootologie. 2012;91:160‐166. [DOI] [PubMed] [Google Scholar]
- 9. Zavattero E, Viterbo S, Gerbino G, Ramieri G. Navigation‐aided endoscopic sinus surgery. J Craniofac Surg. 2015;26:326‐327. [DOI] [PubMed] [Google Scholar]
- 10. Dalgorf DM, Sacks R, Wormald PJ, et al. Image‐guided surgery influences perioperative morbidity from endoscopic sinus surgery: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2013;149:17‐29. [DOI] [PubMed] [Google Scholar]
- 11. Fried MP, Moharir VM, Shin J, Taylor‐Becker M, Morrison P, Kennedy DW. Comparison of endoscopic sinus surgery with and without image guidance. Am J Rhinol. 2002;16:193‐197. [PubMed] [Google Scholar]
- 12. Javer AR, Genoway KA. Patient quality of life improvements with and without computer assistance in sinus surgery: outcomes study. J Otolaryngol. 2006;35:373‐379. [DOI] [PubMed] [Google Scholar]
- 13. Theodoraki MN, Ledderose GJ, Becker S, et al. Mental distress and effort to engage an image‐guided navigation system in the surgical training of endoscopic sinus surgery: a prospective, randomized clinical trial. Eur Arch Otorhinolaryngol. 2015;272:905‐913. [DOI] [PubMed] [Google Scholar]
- 14. Al‐Swiahb JN, Al Dousary SH. Computer‐aided endoscopic sinus surgery: a retrospective comparative study. Ann Saudi Med. 2010;30:149‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Metson R, Cosenza M, Gliklich RE, Montgomery WW. The role of image‐guidance systems for head and neck surgery. Arch Otolaryngol Head Neck Surg. 1999;125:1100‐1104. [DOI] [PubMed] [Google Scholar]
- 16. Mueller SA, Caversaccio M. Outcome of computer‐assisted surgery in patients with chronic rhinosinusitis. J Laryngol Otol. 2010;124:500‐504. [DOI] [PubMed] [Google Scholar]
- 17. Ramakrishnan VR, Orlandi RR, Citardi MJ, Smith TL, Fried MP, Kingdom TT. The use of image‐guided surgery in endoscopic sinus surgery: an ecidence‐based review with recommendations. Int Forum Allergy Rhinol. 2013;3:236‐241. [DOI] [PubMed] [Google Scholar]
- 18. Tabaee A, Hsu AK, Shrime MG, et al. Quality of life and complications following image‐guided endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2006;135:76‐80. [DOI] [PubMed] [Google Scholar]
- 19. Tschopp KP, Thomaser EG. Outcome of functional endonasal sinus surgery with and without CT‐navigation. Rhinology. 2008;46:116‐120. [PubMed] [Google Scholar]
- 20. Citardi MJ, Yao W, Luong A. Next‐generation surgical navigation systems in sinus and skull base surgery. Otolaryngol Clin North Am. 2017;50:617‐632. [DOI] [PubMed] [Google Scholar]
- 21. Batra PS, Kanowitz SJ, Citardi MJ. Clinical utility of intraoperative volume computed tomography scanner for endoscopic sinonasal and skull base procedures. Am J Rhinol. 2008;22:511‐515. [DOI] [PubMed] [Google Scholar]
- 22. Dixon BJ, Daly MJ, Chan H, Vescan A, Witterick IJ, Irish JC. Augmented real‐time navigation with critical structure proximity alerts for endoscopic skull base surgery. Laryngoscope. 2014;124:853‐859. [DOI] [PubMed] [Google Scholar]
- 23. Jackman AH, Palmer JN, Chiu AG, Kennedy DW. Use of intraoperative CT scanning in endoscopic sinus surgery: a preliminary report. Am J Rhinol. 2008;22:170‐174. [DOI] [PubMed] [Google Scholar]
- 24. Leong JL, Batra PS, Citardi MJ. CT‐MRT image fusion for the management of skull base lesions. Otolaryngol Head Neck Surg. 2006;134:868‐876. [DOI] [PubMed] [Google Scholar]
- 25. Li L, Yang J, Chu Y, et al. A novel augmented reality navigation system for endoscopic sinus and skull base surgery: a feasibility study. PLoS One. 2016;11:e0146996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Besharati Tabrizi L, Mahvash M. Augmented reality‐guided neurosurgery: accuracy and intraoperative application of an image projection technique. J Neurosurg. 2015;123:206‐211. [DOI] [PubMed] [Google Scholar]
- 27. Suzuki N, Hattori A, Limura J, et al. Development of AR surgical navigation systems for multiple surgical regions. Stud Health Technol Inform. 2014;196:404‐408. [PubMed] [Google Scholar]
- 28. Winne C, Khan M, Stopp F, Jank E, Keeve E. Overlay visualization in endoscopic ENT surgery. Int J Comput Assist Radiol Surg. 2011;6:401‐406. [DOI] [PubMed] [Google Scholar]
- 29. Citardi MJ, Agbetoba A, Bigcas JL, Luong A. Augmented reality for endoscopic sinus surgery with surgical navigation: a cadaver study. Int Forum Allergy Rhinol. 2016;6:523‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leonard S, Sinha A, Reiter A, et al. Evaluation and stability analysis of video‐based navigation system for functional endoscopic sinus surgery on in vivo clinical data. IEEE Trans Med Imaging. 2018;37:2185‐2195. [DOI] [PubMed] [Google Scholar]
- 31. Yeh M, Wickens CD. Display signaling in augmented reality: effects of cue reliability and image realism on attention allocation and trust calibration. Hum Factors. 2001;43:355‐365. [DOI] [PubMed] [Google Scholar]
- 32. Riley CA, Kim M, Sclafani AP, et al. Opioid analgesic use and patient‐reported pain outcomes after rhinologic surgery. Int Forum Allergy Rhinol. 2018;9:339‐344. 10.1002/alr.22260. [DOI] [PubMed] [Google Scholar]
- 33. Chang CM, Jaw FS, Lo WC, Fang KM, Cheng PW. Three‐dimensional analysis of the accuracy of optic and electromagnetic navigation systems using surface registration in live endoscopic sinus surgery. Rhinology. 2016;54:88‐94. [DOI] [PubMed] [Google Scholar]
- 34. Govindaraj S, Adappa ND, Kennedy DW. Endoscopic sinus surgery: evolution and technical innovations. J Laryngol Otol. 2012;124:242‐250. [DOI] [PubMed] [Google Scholar]
- 35. Grauvogel TD, Becker C, Hassepass F, Arndt S, Laszig R, Maier W. Comparison of 3D C‐arm‐based registration to conventional pair‐point registration regarding navigation accuracy in ENT surgery. Otolaryngol Head Neck Surg. 2015;152:266‐271. [DOI] [PubMed] [Google Scholar]
- 36. Labadie RF, Davis BM, Fitzpatrick JM. Image‐guided surgery: what is the accuracy? Curr Opin Otolaryngol Head Neck Surg. 2005;13:27‐31. [DOI] [PubMed] [Google Scholar]
- 37. Soteriou E, Grauvogel J, Laszig R, Grauvogel TD. Prospects and limitations of different registration modalities in electromagnetic ENT navigation. Eur Arch Otorhinolaryngol. 2016;273:3979‐3986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Questionnaire for the surgeon.
Figure S2 Questionnaire for the patients.


