Abstract
The use of complementary and alternative medicine at least once during or after cancer treatment has increased over the past years from an estimated 25% in the 1970s and 1980s to more than 32% in the 1990s and to 49% after 2000. The risk of herb‐drug interaction is therefore increasingly recognized as a public health problem. To the best of our knowledge, we report here the first case of interaction between ginger and anticancer drug, with serious consequences for the patient. There is an urgent need regarding complementary and alternative medicine: Both clinicians and patients should be aware of the potential interactions between herbs and prescribed drugs.
1.
Recent studies have confirmed that roughly half of the patients who are on conventional treatments for cancer also use complementary and alternative medicine (CAM).1, 2 Most of these health approaches fall into one of two subgroups: natural products or mind and body practices. Firkins et al concluded that there was a risk of interactions between a biological CAM method and conventional drugs in 54.9% of the patients using CAM.3 After vitamins, herbs were the second most frequently used substances in these cases. The risk of herb‐drug interaction is therefore increasingly recognized as a public health problem.4
Crizotinib is an orally available multiple tyrosine kinase inhibitor, currently approved for the first line treatment of anaplastic lymphoma kinase (ALK)‐positive non–small‐cell lung cancer. Crizotinib induces hepatic laboratory abnormalities in 30% of patients, typically grade 1 or 2, which mostly occur within two months of treatment introduction and is reversible on dose reduction or interruption.5 Ginger, roots of Zingiber officinale, is used for culinary and medicinal purpose, as it is thought to possess carminative, anti‐emetic, anti‐inflammatory, and antispasmodic properties.6 Ginger extract was recently tested in a double‐blind, placebo‐controlled trial to manage nausea and vomiting in cancer patients.7 No clinically relevant interaction involving ginger and anticancer drug has been described so far, despite the use of ginger for chemotherapy‐induced nausea and vomiting.
A 48‐year‐old woman was treated with crizotinib 250 mg twice a day since November 2016 after a first line of chemotherapy with cisplatin and pemetrexed, for a lung adenocarcinoma harbouring ALK rearrangement, with lung mass, pleural effusion, and mediastinal lymph nodes enlargement, diagnosed in March 2015. At the time of crizotinib initiation, acetylsalicylic acid 160 mg daily was also prescribed for a transient ischaemic attack in June 2015. While transaminases levels were normal until December 5, 2017 (aspartate aminotransferase, 33 UI/L; alanine aminotransferase, 41 UI/L), a severe hepatic cytolysis (alanine aminotransferase > 20 × ULN) was discovered in January 2018 (Figure 1). The abdominal ultrasound was subnormal with fatty liver and other laboratory tests (viral serologies, autoimmune, and parasitological tests) were negative. Liver biopsy showed histological lesions in favour of acute drug‐induced hepatitis and liver function gradually improved after discontinuation of crizotinib. Crizotinib plasma trough concentration was 384 μg/L 2 days after crizotinib discontinuation, against 205 μg/L in November 2017 (Figure 1). Finally, we found out that the patient consumed an increasing amount of a drink made from grated ginger, honey, lemon juice, and hot water since November 2017, up to more than 1 L/day, for nonmedical purposes.
Figure 1.
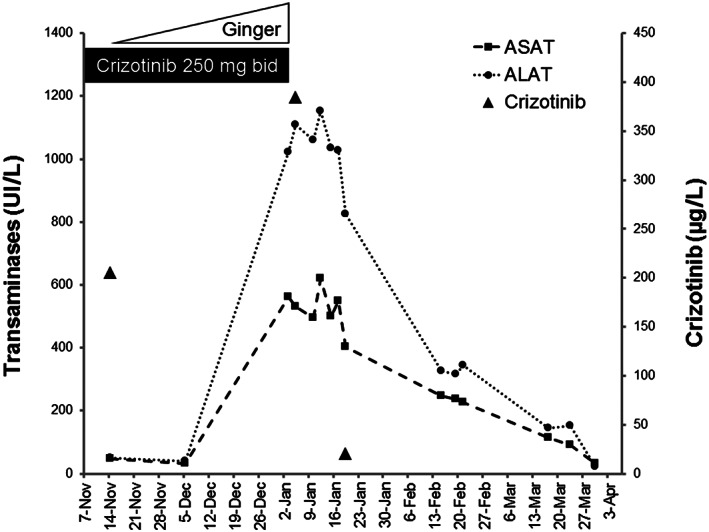
Evolution of serum transaminase levels and crizotinib concentration depending on the use of ginger and crizotinib. ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase
Crizotinib is mainly metabolized by CYP3A4, and its concomitant use with strong CYP3A4 inhibitors or inducers should be avoided. Coadministration of a single oral dose of crizotinib with ketoconazole, a strong CYP3A inhibitor, resulted in increases in crizotinib systemic exposure.8 In vitro studies also suggested that crizotinib is a substrate for P‐glycoprotein (P‐gp). Ginger significantly inhibits CYP3A4, CYP2C9, and P‐gp activities in vitro.9, 10 A previous study has shown that ginger increases blood concentrations of tacrolimus via CYP3A4 inhibition in rats.11 In humans, one study reported ginseng (Panax ginseng)‐mediated hepatotoxicity due to inhibition of CYP3A4 in a chronic leukaemia patient on imatinib, another tyrosine kinase inhibitor.12
Here, the tolerance of crizotinib was good before the introduction of ginger, and hepatic cytolysis disappeared after discontinuation of both crizotinib and ginger. The diagnostic evaluation did not reveal any cause of hepatotoxicity other than that due to raised crizotinib exposure. Indeed, concentration of crizotinib determined 2 days after crizotinib discontinuation was 1.8‐fold higher than previous level measured in November 2017 (Figure 1) and also higher than trough concentrations previously described at a dose of 250 mg twice a day (median trough concentration from 242 to 319 ng/mL).13 Thus, it is very likely that the reduced activity of CYP3A4 and possibly P‐gp in the presence of ginger led to the accumulation of crizotinib, which induced hepatotoxicity.
Our report highlights safety concerns arising from the use of CAM. Both clinicians and patients should be aware of the potential interactions between herbs and prescribed drugs.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,14 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.15, 16
COMPETING INTERESTS
There are no competing interests to declare.
ACKNOWLEDGEMENTS
We warmly thank the patient for her written consent.
Revol B, Gautier‐Veyret E, Arrivé C, et al. Pharmacokinetic herb‐drug interaction between ginger and crizotinib. Br J Clin Pharmacol. 2020;86:1892–1893. 10.1111/bcp.13862
REFERENCES
- 1. Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther. 2012;11(3):187‐203. [DOI] [PubMed] [Google Scholar]
- 2. Berretta M, Della Pepa C, Tralongo P, et al. Use of complementary and alternative medicine (CAM) in cancer patients: an Italian multicenter survey. Oncotarget. 2017;8:24401‐24414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Firkins R, Eisfeld H, Keinki C, et al. The use of complementary and alternative medicine by patients in routine care and the risk of interactions. J Cancer Res Clin Oncol. 2018;144(3):551‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Awortwe C, Makiwane M, Reuter H, Muller C, Louw J, Rosenkranz B. Critical evaluation of causality assessment of herb‐drug interactions in patients. Br J Clin Pharmacol. 2018;84(4):679‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schnell P, Safferman AZ, Bartlett CH, Tang Y, Wilner KD. Clinical presentation of hepatotoxicity‐associated crizotinib in ALK‐positive (ALK+) advanced non‐small cell lung cancer (NSCLC). J Clin Oncol. 2012;30:7598‐7598. [Google Scholar]
- 6. Bode AM, Dong Z. The Amazing and Mighty Ginger In: Benzie IFF, Wachtel‐Galor S, eds. Herb. Med. Biomol. Clin. Asp. 2nd ed. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. [PubMed] [Google Scholar]
- 7. Bossi P, Cortinovis D, Fatigoni S, et al. A randomized, double‐blind, placebo‐controlled, multicenter study of a ginger extract in the management of chemotherapy‐induced nausea and vomiting (CINV) in patients receiving high‐dose cisplatin. Ann Oncol. 2017;28:2547‐2551. [DOI] [PubMed] [Google Scholar]
- 8. Xu H, O'Gorman M, Tan W, Brega N, Bello A. The effects of ketoconazole and rifampin on the single‐dose pharmacokinetics of crizotinib in healthy subjects. Eur J Clin Pharmacol. 2015;71(12):1441‐1449. [DOI] [PubMed] [Google Scholar]
- 9. Kimura Y, Ito H, Hatano T. Effects of mace and nutmeg on human cytochrome P450 3A4 and 2C9 activity. Biol Pharm Bull. 2010;33(12):1977‐1982. [DOI] [PubMed] [Google Scholar]
- 10. Zhang W, Lim L‐Y. Effects of spice constituents on P‐glycoprotein‐mediated transport and CYP3A4‐mediated metabolism in vitro. Drug Metab Dispos Biol Fate Chem. 2008;36(7):1283‐1290. [DOI] [PubMed] [Google Scholar]
- 11. Egashira K, Sasaki H, Higuchi S, Ieiri I. Food‐drug interaction of tacrolimus with pomelo, ginger, and turmeric juice in rats. Drug Metab Pharmacokinet. 2012;27(2):242‐247. [DOI] [PubMed] [Google Scholar]
- 12. Bilgi N, Bell K, Ananthakrishnan AN, Atallah E. Imatinib and Panax ginseng: a potential interaction resulting in liver toxicity. Ann Pharmacother. 2010;44(5):926‐928. [DOI] [PubMed] [Google Scholar]
- 13. Hamilton G, Rath B, Burghuber O. Pharmacokinetics of crizotinib in NSCLC patients. Expert Opin Drug Metab Toxicol. 2015;11(5):835‐842. [DOI] [PubMed] [Google Scholar]
- 14. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alexander SP, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol. 2017;174(Suppl 1):S272‐S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexander SP, Kelly E, Marrion NV, et al. The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol. 2017;174(Suppl 1):S360‐S446. [DOI] [PMC free article] [PubMed] [Google Scholar]


