Abstract
Introduction
Higher brain tocopherol levels have been associated with lower levels of Alzheimer's disease (AD) neuropathology; however, the underlying mechanisms are unclear.
Methods
We studied the relations of α‐ and γ‐tocopherol brain levels to microglia density in 113 deceased participants from the Memory and Aging Project. We used linear regression analyses to examine associations between tocopherol levels and microglia densities in a basic model adjusted for age, sex, education, apolipoprotein E (APOE)ε4 genotype (any ε4 allele vs. none) , and post‐mortem time interval, and a second model additionally adjusted for total amyloid load and neurofibrillary tangle severity.
Results
Higher α‐ and γ‐tocopherol levels were associated with lower total and activated microglia density in cortical but not in subcortical brain regions. The association between cortical α‐tocopherol and total microglia density remained statistically significant after adjusting for AD neuropathology.
Discussion
These results suggest that the relation between tocopherols and AD might be partly explained by the alleviating effects of tocopherols on microglia activation.
Keywords: amyloid, anti‐inflammatory, antioxidants, cerebral cortex, glial cells, inflammation, microglia, nutrition, pathology, tau, tocopherols, vitamin E
1. BACKGROUND
Vitamin E is considered a powerful antioxidant and has been often associated with reduced risk of cognitive decline and Alzheimer's disease (AD) dementia. 1 , 2 , 3 , 4 Although γ‐tocopherol is the major form of vitamin E in the U.S. diet, α‐ tocopherol is the most biologically active form of vitamin E and the main compound of vitamin E supplements. 5 In previous work, we examined the associations between brain α‐ and γ‐tocopherol levels and AD neuropathology. We showed that higher brain γ‐tocopherol levels were associated with lower levels of amyloid load and neurofibrillary tangle severity. In addition, we found that high brain α‐tocopherol levels in combination with low γ‐tocopherol levels were associated with higher amyloid load. 6 This work provided further support for the potential protective effects of vitamin E on AD, but has also highlighted the complexity of these relationships. Moreover, whereas there is expansive literature on the beneficial effects of dietary intake of vitamin E on cognition, clinical trials testing the effects of high‐dose α‐tocopherol supplementation have largely failed. 1 , 7 , 8 These apparent conflicting results have raised the need to unravel the potential underlying mechanisms by which tocopherols relate to AD.
Vitamin E is well‐known for its antioxidant properties, but might influence a variety of other neuroplasticity aspects that could explain their neuroprotective effects. For example, brain vitamin E has been related to neurogenesis, neuronal differentiation, synaptic functionality of the hippocampus, and cell signaling pathways. 9 Of interest, in vitro and animal models have shown that the effects of vitamin E on cell signaling pathways lower inflammatory responses that induce microglia activation. 10 , 11 Chronic microglia activation is likely to play a major role in AD as it relates to neuronal and synaptic loss and tau exacerbation. 12 We therefore hypothesized that if vitamin E can reduce microglia activation that this could be one of the biological mechanisms driving the inverse relation between tocopherols and accumulating AD damage.
In this study we examine the associations of brain α‐ and γ‐tocopherol levels with microglia activation and their relation with AD pathology among deceased and autopsied participants of a community cohort.
2. METHODS
2.1. Study sample
This study reports on 115 deceased and autopsied cases participants of the Rush Memory and Aging Project (MAP) who were analyzed for both brain tocopherols and neuropathology. The Rush MAP is an ongoing clinical‐neuropathological cohort study of persons living in or near Chicago in continuous care retirement communities and subsidized housing that began in 1997. 13 Clinical diagnosis of AD is made after review of annual clinical evaluations and neuropsychological test examinations by an expert neurologist who is blinded to neuropathologic findings. 14 Volunteers are enrolled without a known history of dementia, and all agreed to annual clinical evaluations and to brain autopsy at their death. Written informed consent was obtained from all study participants and the study was approved by the Rush University Medical Center Institutional Review Board.
HIGHLIGHTS
Higher brain α‐ and γ‐tocopherol levels are associated with lower activated microglia density in cortical but not in subcortical brain regions.
The association between higher brain α‐tocopherol and lower total microglia density remains intact even after adjustment for AD neuropathology.
Brain α‐tocopherol levels may create an anti‐inflammatory environment that reduces total microglia density.
Brain tocopherol levels might protect against AD neuropathology by reducing microglia density and activation.
RESEARCH IN CONTEXT
Systematic review: Literature review shows that higher vitamin E intake reduces the risks for cognitive decline and AD dementia. Moreover, higher levels of brain tocopherols have been related to lower levels of amyloid and neurofibrillary tangle severity. The mechanisms underlying this relation have however not been studied in human brain.
Interpretation: This study, of over 100 human brain cases, showed that one potential biological mechanism underlying the relation between vitamin E and AD might be the anti‐inflammatory effects of brain tocopherols on microglia activation.
Future directions: This cross‐sectional study highlights the potential effect of tocopherols on microglia activation. Longitudinal animal or translocator protein positron emission tomography studies that measure microglia activation in vivo are, needed, however, to further determine the sites of action of brain tocopherols.
2.2. Brain neuropathology
Brain autopsies for the analyzed cases were performed, on average, 6.5 hours after death according to previously described methods. 13 Autopsied brains were processed and dissected into two hemispheres at the Rush Alzheimer's Disease Center laboratory, one fresh hemisphere was cut into 1 cm coronal slabs, stored in a −80°C freezer, and used for the tocopherol quantification as described below. The contralateral hemisphere was fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for at least 48 to 72 hours, cut into 1 cm coronal slabs, and used for brain neuropathology and microglia counts. Tissue blocks from multiple cortical regions of the brain were prepared for immunohistochemistry and quantification of amyloid load and Braak staging of neuronal neurofibrillary tangles (NFTs), as described previously. 6 Amyloid load was a composite measure of the percent area occupied by amyloid beta across eight cortical regions determined by averaging the values obtained by systematic computerized sampling in each region of interest. Braak NFT stage was determined using a modification of recommended criteria, resulting in a score on a scale of 0 (no NFT in any region) to 6 (frequent tangles in multiple regions). 15 Immunohistochemistry for microglia was performed using an Automated Leica Bond immunostainer (Leica Microsystems Inc., Bannockborn, IL) and antibodies to human HLA‐DP, HLA‐DQ, and HLA‐DR (clone CR3/43, 1:100, DakoCytomation, Carpentaria, CA) using Bond epitope retrieval and detection. 16 The Microbrightfield Stereology System outlined gray matter from the cortical and subcortical regions on each slide. The Stereo investigator 8.0 software program was used to place a 1000 µm by 750 µm sampling grid over the region, and sample 4.0% of the region with a 200 × 150 µm counting frame at 400× magnification at interval grid intersection points. Microglia have long fine processes; when these cells become activated, however, these processes thicken and contract and the cell body becomes more rounded. An investigator blinded to the clinical and pathologic data assigned separate tags to each microglia in the counting frames: stage I (thin ramified processes), stage II (plump cytoplasm and thicker processes), and stage III (appearance of macrophages) (Figure 1). 17 It is important to note that previous studies have shown that the classification system with using current MHCII+ marker used in this study correlates well with human genetic variants relating to microglial biology or AD, cognition, and AD pathology. 16 , 17 The total number of microglia per region was estimated by the stereology software based on the counts in each counting frame. Average microglia densities from two adjacent tissue blocks were used to obtain average densities of microglia in two cortical (inferior temporal and midfrontal) and two subcortical brain regions (posterior putamen and ventromedial caudate).
FIGURE 1.
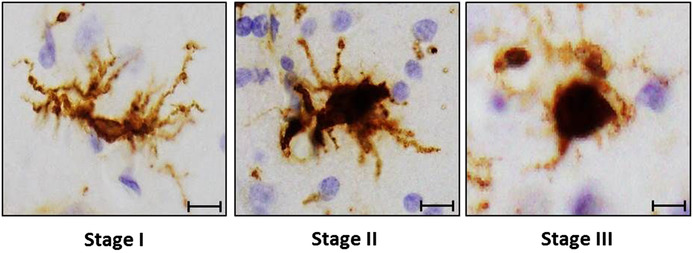
Representative photograph of microglial morphological states in the inferior temporal cortex. Scale bars are 10 µm
2.3. Brain tocopherol analyses
Frozen brain tissue from the same two cortical (inferior temporal and midfrontal) and two subcortical brain regions (posterior putamen and ventromedial caudate) were analyzed for tocopherol concentrations using high‐performance liquid chromatography (HPLC) coupled to electrochemical detection as described previously. 18 , 19 Extraction losses were corrected for recoveries of the internal standard, δ‐tocopherol. We eliminated from the analyses two cases with extreme values (α‐tocopherol >10,000 pmol/mg; γ‐tocopherol >900 pmol/mg).
2.4. Vitamin E intake
Vitamin E dietary and supplement intake was assessed using a semiquantitative food frequency questionnaire (FFQ) validated in a sample of older Chicago residents. 20 Daily intake of vitamin E was obtained by multiplying the vitamin E content of each food item (from the Harvard nutrient database) by reported frequency of intake, and summing over all food items. All nutrients were calorie adjusted by the regression‐residual method. Supplement use and dosage was calculated from vitamin E (α‐tocopherol) and multivitamin supplements. Dietary and supplement intake were averaged over all valid FFQs available for each participant.
2.5. Statistical analyses
Microglia density scores were calculated for stage I/II/III (total count), stage II/III (activated microglia), and stage III (macrophages). Tocopherol levels and microglia densities were averaged across the two cortical (inferior temporal and midfrontal) and two subcortical regions (posterior putamen and ventromedial caudate). Paired t tests and Mann‐Whitney U tests were used to compare levels of tocopherols and microglia density in cortical and subcortical brain regions when appropriate. Next, all brain tocopherols levels and microglia densities (stages II/III and III) were log transformed to improve normality. In addition, all measures were standardized to z‐scores to enable comparison of effect sizes on a normalized scale. Spearman's correlations were used to assess correlations between cortical and subcortical tocopherol levels, cortical and subcortical microglia densities, and between dietary and total (dietary + supplement) intake of vitamin E and tocopherol brain levels. Linear regression models were used to examine the associations of brain tocopherols (continuous determinants) with microglia density (continuous outcome). The basic model was adjusted for age at death, sex, years of education, apolipoprotein E (APOE) ε4 genotype (any ε4 allele vs. none), and the time interval from death to autopsy (hours). In a second model we additionally adjusted for amyloid load and NFT severity. Subsequently, we repeated the basic model with additional adjustment for clinical AD. Finally, we performed a sensitivity analyses for the association of γ‐tocopherol levels with microglia density, excluding two outliers. All analyses were performed in R (3.4.2 [2017‐09‐28]). A probability level of P < .05 was considered statistically significant.
3. RESULTS
The study sample of 113 deceased MAP participants had a mean (SD) age of 88.5 (6.0) years at death, included more female than male participants (n = 68, 60%), and had a mean (SD) 14.9 (2.6) years of education. A total of 38 participants (34%) had a clinical diagnosis of AD at last evaluation prior to death and 30 participants (27%) had at least one APOE ε4 allele. The mean (SD) time to autopsy was 6.5 ± 3.2 hours (Table 1).
TABLE 1.
Descriptives of 113 memory and aging project participants
| Clinical characteristics | |
| Age at death, mean years ± SD | 88.5 ± 6.0 |
| Female, n(%) | 68 (60%) |
| Education, mean years ± SD | 14.9 ± 2.6 |
| APOEε4, n(%) with at least one allele | 30 (27%) |
| Clinical AD diagnosis, n(%) | 38 (34%) |
| Post‐mortem autopsy, mean hours ± SD | 6.5 ± 3.2 |
| α‐tocopherol, median pmol/mg (IQR) | |
| Cortical α–tocopherol | 232.2 (76.0, 356.4) |
| Subortical α–tocopherol | 158.7 (105.0, 286.6) |
| γ‐tocopherol, median pmol/mg (IQR) | |
| Cortical γ–tocopherol | 57.1 (42.9, 92.5) |
| Subcortical γ–tocopherol | 63.6 (48.4, 81.3) |
| Microglia stage I/II/III, median density (IQR) | |
| Cortical microglia stage I/II/III | 153.2 (111.2, 194.0) |
| Subcortical microglia stage I/II/III | 209.2 (175.8, 244.1) * |
| Microglia stage II/III, median density (IQR) | |
| Cortical microglia stage II/III | 3.9 (1.2, 11.7) |
| Subcortical microglia stage II/III | 4.3 (1.9, 9.5) |
| Microglia stage III, median density (IQR) | |
| Cortical microglia stage III | 0.8 (0.1, 2.9) |
| Subcortical microglia stage III | 1.1 (0.4, 2.4) |
Differences between cortical and subcortical tocopherol levels and cortical and subcortical microglia density were tested using t tests or Mann‐Whitney U tests when appropiate.
Microglia stages I to III represent stages of microglial activation; microglia density scores were calculated for total count (stage I/II/III), activated microglia (stage II/III), and macrophages (stage III).
AD, Alzheimer's disease; APOE, apolipoprotein E; IQR, interquartile range; SD, standard deviation.
P < .05 to cortical level.
Tocopherol levels and microglia density did not differ between cortical and subcortical brain regions, except for total microglia density (stage I/II/III), which was lower in cortical than in subcortical regions (Table 1). Concentrations of α‐tocopherols but not γ‐tocopherols were moderately correlated between cortical and subcortical regions (r = 0.60, P < .001 for α‐tocopherol, and r = 0.14, P = .14 for γ‐tocopherol; Spearman correlation). The most predominant form of tocopherols in the brain was α‐tocopherol: α‐/γ‐tocopherol ratio was 70%/30% for cortical and 72%/28% for subcortical regions. Microglia density was modestly correlated between cortical and subcortical brain regions (r = 0.43, P < .001 for stage I/II/III, r = 0.55, P < .001 for stage II/III, r = 0.47, P < .001 for stage III; Spearman correlation). The predominant morphology of microglia was stage I, as the ratio of activated (stage II/III)/total count microglia was 4%/96% for cortical brain regions and 3%/97% for subcortical brain regions. Total intake (dietary + supplement) of vitamin E was marginally correlated with brain α‐tocopherol (Spearman r = 0.23, P = .02 cortex; r = 0.20, P = .04 subcortical regions) but not γ‐tocopherol brain levels (Spearman r = −0.10, P = .33 cortex; r = −0.14, P = .15 subcortical regions). Dietary intake alone was not correlated with brain α‐ or γ‐tocopherol levels (r[range] = −0.10,0.03).
In separate linear regression models, higher α‐ and γ‐tocopherol levels were associated with lower total (stage I/II/III) and activated (stage II/III and III) microglia density in the cortex (Table 2, Figures 2 and 3). When we adjusted additionally for amyloid load and NFT severity, effect estimates were smaller, most strongly for the associations with activated microglia (stage II/III and stage III). Only the association of higher α‐tocopherol levels with lower total microglia (stage I/II/III) density in the cortex remained statistically significant (B [SE] −0.20 [0.09], P = .03, Table 2). We found no associations between tocopherols and microglia density in the subcortical brain regions.
TABLE 2.
Association of brain levels of α‐ and γ‐tocopherol with microglia density by brain region
| Microglia stage I/II/III | Microglia stage II/III | Microglia stage III | ||||||
|---|---|---|---|---|---|---|---|---|
| Tocopherols | Brain region | Model | B (SE) | P | B (SE) | P | B (SE) | P |
| α‐Tocopherol | Cortical | Basic‐adjusted a | –0.25(0.09) | .01 | –0.20(0.10) | .04 | –0.19(0.09) | <.05 |
| +AD pathology b | –0.20(0.09) | .03 | –0.10(0.09) | .25 | ‐0.09(0.09) | .28 | ||
| α‐Tocopherol | Subcortical | Basic‐adjusted a | 0.11(0.10) | .26 | 0.12(0.09) | .20 | 0.04(0.09) | .69 |
| +AD pathology b | 0.11(0.10) | .28 | 0.12(0.09) | .20 | 0.04(0.09) | .69 | ||
| γ‐Tocopherol | Cortical | Basic‐adjusted a | –0.25(0.10) | .01 | –0.25(0.10) | .01 | –0.25(0.10) | .01 |
| +AD pathology b | –0.15(0.10) | .16 | –0.07(0.09) | .48 | ‐0.07(0.09) | .45 | ||
| γ‐Tocopherol | Subcortical | Basic‐adjusted a | –0.01(0.10) | .89 | 0.004(0.09) | .96 | –0.01(0.09) | .96 |
| +AD pathology b | –0.03(0.10) | .79 | 0.02(0.09) | .86 | 0.02(0.09) | .80 | ||
The association magnitudes (B) are reported in SD (±SE) change in microglia density per 1 SD increase in tocopherol concentration. Microglia stages I to III represent different stages of microglia activation, microglia density scores were calculated for total count (stage I/II/III), activated microglia (stage II/III), and macrophages (stage III).
SE, standard error.
Basic model adjusted for age at death (years), sex, education (years), APOE ε4 (any ε4 vs none) and post‐mortem autopsy time (hours.
Basic model +amyloid load and neurofibrillary tangle severity (Braak score).
FIGURE 2.
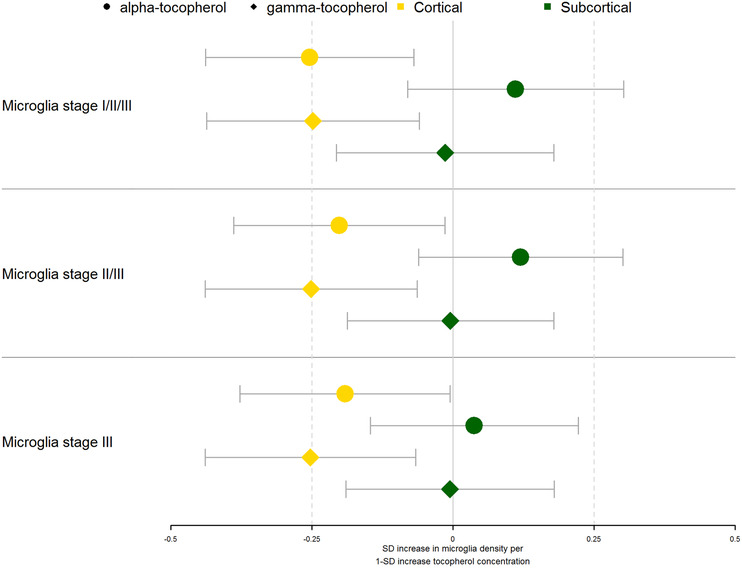
Associations of brain tocopherol levels with microglia density. The standardized effect estimates on microglia density of brain tocopherols adjusted for age, sex, education, post‐mortem time interval, and APOEε4 genotype (any ε4 allele vs. none) are shown. The estimates are shown for α‐tocopherol (circles) and γ‐tocopherol (diamonds) by region (cortical = yellow, subcortical = green). Point estimates are shown as boxes with whiskers denoting the 95% confidence interval of the effect estimates. SD, standard deviation
FIGURE 3.
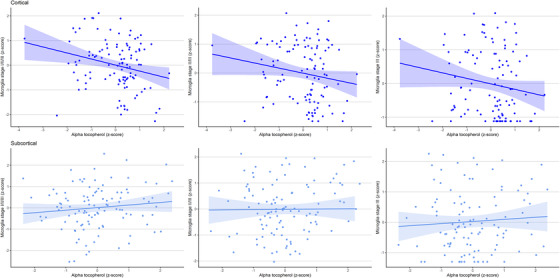
Scatterplots of the associations of α‐tocopherol levels with microglia density. The upper graphs in dark blue depict the associations in the cortical brain regions, whereas the lower graphs in light blue depict the associations in the subcortical brain regions. Microglia density and α‐tocopherol levels are shown as log‐transformed z‐scores
3.1. Clinical AD effects
To determine if the association between higher tocopherol levels and lower microglia density in the cortex was driven by a disease effect, we re‐analyzed the basic model additionally adjusting for clinical AD diagnosis. The associations between higher tocopherol levels and lower total and activated microglia density in the cortex as described for the basic model, remained, however, intact after statistical adjustment for a clinical AD diagnosis (Table S1).
3.2. Sensitivity analyses
In our sample, two cases had low levels of γ‐tocopherol (< 1 pmol/mg) and relatively high (activated) microglia density in the cortex. To determine the effect of these two cases on our findings for γ‐tocopherol we performed a sensitivity analysis. After exclusion of the two cases with low γ‐tocopherol levels the effect sizes of our associations between γ‐tocopherol and total and activated microglia density remained intact, but lost significance for stage II/III and total (stage I/II/III) microglia density (B(SE) −0.25 (0.14), P = .07 total microglia (stage I/II/III); B(SE) −0.27 (0.14), P = .05 stage II/III microglia; B(SE) −0.28 (0.14), P = .04 stage III microglia).
4. DISCUSSION
In this cross‐sectional study, higher α‐ and γ‐tocopherol levels were associated with lower activated microglia density in the cortical, but not in the subcortical, brain regions. The association between cortical α‐tocopherol levels and total microglia density remained statistically significant, even after additional adjustment for amyloid load and NFT severity. The cross‐sectional design of the analyses precludes conclusions on the directionality or causality of these observed associations. One possible explanation of these findings, however, is that microglia activation mediates the relation between tocopherols and AD neuropathology; that is, tocopherols might reduce microglia activation and thereby slow accumulating AD neuropathology. In addition, α‐tocopherol levels might protect the brain by creating an anti‐inflammatory environment independent of AD neuropathology. Below we discuss our findings and the potential mechanistic pathways that warrant further investigation.
The finding for higher tocopherol levels associated with lower total and activated microglia density confirms findings of previous in vitro and mice studies. In addition to its antioxidant function, vitamin E has been shown to reduce microglia activation and inflammatory responses. 10 , 11 , 21 , 22 For example, a study in AD mice showed that treatment with α‐tocopherol quinine suppressed microglia activation, decreased levels of amyloid β(Aβ) oligomers, and reduced cognitive decline. 10 Of interest, two other studies found that vitamin E supplementation increased microglia proliferation and activation, 23 , 24 but decreased levels of interleukin (IL)‐1β, indicating reduced inflammatory responses. 23 In contrast, a mice study found that, post‐stroke, vitamin E supplementation increased microglia activation and IL‐1β levels. 25 Together this could indicate that tocopherols are helpful to reduce chronic inflammation and keep an anti‐inflammatory state, but might exacerbate inflammation after acute brain injury. Longitudinal animal studies, comparing models of acute and chronic inflammation, will therefore help to better understand in which inflammatory setting tocopherols can be beneficial.
An extra difficulty in evaluating the contrasting results of these studies is the possibility that microglia activation may have different effects on the brain depending on the stage of AD. That is, during acute inflammation and in early stages of AD, microglia activation might have beneficial effects. Microglia can restore homeostasis, remove debris, clear soluble Aβ, and build protective barriers around Aβ plaques. 12 , 26 As the disease progresses, however, continued microglia activation can have detrimental effects on neurons and synapses and can exacerbate tau pathology. 12 Altogether, whereas microglia proliferation and activation is not always a bad omen, continued microglia activation in AD is harmful, and alleviation of microglia activation by tocopherols is likely to be beneficial.
We found that high tocopherol levels were associated with reduced microglia activation in the cortical regions but,not in subcortical regions. Microglia activation is strongly related to AD pathology. 27 Cortical regions, such as the inferior temporal and medial temporal cortex measured in this study, are known to be affected by AD pathology relatively early in the disease process. 28 Moreover, a previous study found that activated microglia relate only to AD pathology in the cortical brain regions. 17 The regional differences we found could indicate that the association between tocopherols and microglia activation is in close relation with the level of AD neuropathology. The regional differences, might also be explained by tocopherol metabolism. Molecular determinants of tocopherol transport in the periphery, such as scavenger receptor class B type 1 and tocopherol transfer protein (TTP), are also found in the central nervous system, suggesting similar transport routes in the brain as in the periphery. 5 TTP has been detected in hippocampal cells of AD patients and in patients with other neurological oxidative stress‐related conditions, and not in controls, which has led to the hypothesis that TTP availability may be upregulated in oxidative stress settings and might help to get tocopherol to the sites where it is most needed. 29 , 30 However, in this study, we found no differences in tocopherol levels or activated microglia density between the cortical and subcortical regions. Future studies should include a larger sample, more brain regions, and a considerable number of subjects without AD pathology to test if level differences in tocopherol and/or microglia activation can be detected and to understand if regional differences are a consequence of physiological differences between brain regions or an effect of oxidative stress and AD pathological change.
To our knowledge this is the first study to report on associations of tocopherols with microglia activation in humans. Our sample, with 115 brain cases, is relatively large in comparison to other neuropathological studies and allowed us to adjust for the most important confounding factors. We performed a sensitivity analysis excluding two cases with very low levels of γ‐tocopherols. Reassuringly, the effect sizes for the associations between higher γ‐tocopherol levels and lower microglia density remained intact. The novelty of our data and the limited sample size of this study make it difficult, however, to further evaluate nonlinear relations. State of the art techniques were used to measure tocopherol levels and microglia density within two cortical (strongly affected by AD pathology) and two subcortical regions (relatively unaffected by AD pathology). Microglia were visualized using immunohistochemical markers of activation and sampled uniformly. There are, however, important limitations to consider. First, the relation between microglia morphology and function is complex. Microglia are, however, well known to change shape in response to brain injury, including neurodegeneration, which gives confidence that microglia morphology reflects changes in microglia function. 17 Moreover, the manual count of microglia causes some subjectivity. Manual count and automated methods have shown comparable inter‐operator variability. 31 Second, microglia morphology is complex with many intermediate phenotypes. 17 , 31 Single‐cell analysis, which can capture this heterogeneity, is, however, difficult to perform in large samples. 32 But combining morphologic count with data‐driven spatial statistics, as shown in a previous study, might be an interesting next step to get better insight in the heterogeneity of (activated) microglia. 31 Third, we find that infiltrating macrophages, if present in the brain tissue sample, are not separated from microglia, which may cause an error in our microglia counts, particularly when they are activated. However, recent single‐cell analysis studied performed on our brain samples have shown that these cells represent a very small minority of the cells (+/− 1%), and therefore we believe this unlikely to influence our results. 32 Furthermore, the α‐tocopherol, γ‐tocopherol levels are higher and the α‐/γ‐tocopherol ratio is lower in our study than reported by the Georgian Centenarian Study. 33 Currently, there are limited studies that measure tocopherols in human brain, so we can only speculate on the reasons for this difference. Possible explanations could be differences in population or measurement methods. The population of the present study was younger and included more males, in comparison to the Georgian Centenarian Study. 33 Moreover, although both studies used HPLC to measure the tocopherols, differences in internal standards or other (pre‐)analytical factors might explain the difference in findings. It would therefore be important in future research to measure tocopherols in more brain regions and to compare different analytical methods.
Underlying mechanisms that explain the relation between vitamin E and AD pathology are of interest for the design of future dietary intervention trials. If replication studies can confirm that higher tocopherol levels relate to lower activated microglia density, this would further contribute to the need for attention for dietary interventions trials that optimize vitamin E levels. Ideally, new vitamin E intervention studies would evaluate treatment effect of vitamin E on microglia activation. Measurement of microglia activation in vivo is still difficult, but as positron emission tomography (PET) tracers are quickly improving this might be feasible in the near future. 34 , 35 First, however, our model in which vitamin E reduces microglia activation, should be validated with longitudinal animal studies or PET studies. It is notable that several of our findings support a model in which nutritional intake is responsible for the brain tocopherols instead of disease effects. First, we found that total (dietary + supplement) intake of vitamin E was moderately correlated with brain α‐tocopherol. A similar moderate association has been reported previously between total intake and serum α‐tocopherol levels and between serum and brain α‐ and γ‐tocopherol levels. 33 , 36 These associations support the concept that dietary intake is likely to influence brain tocopherol levels. Second, if these findings were a consequence of global disease effects we would have expected to find similar effects in cortical and subcortical brain areas. Finally, when we correct our models for a clinical diagnosis of AD, our associations of higher tocopherol levels with lower microglia activation in the cortex remained intact.
To conclude, higher tocopherol levels are associated with lower levels of activated microglia density in cortical but not in subcortical regions. Strong inverse associations between α‐tocopherol levels and total microglia density remained intact, even after controlling for AD pathology. Together this implies that reduction of microglia activation is one of the potential mechanisms by which tocopherols protect against AD neuropathology.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
This study was funded by the National Institute on Aging (R01AG031553, R01AG054476 and R01AG17917). F.d.L is appointed to the NUDAD project, which is funded by NWO‐FCB (project number 057‐14‐004). F.d.L. was supported by the Alzheimer Nederland Fellowship Grant No. WE. 15‐2018‐03.
de Leeuw FA, Schneider JA, Agrawal S, Leurgans SE, Morris MC. Brain tocopherol levels are associated with lower activated microglia density in elderly human cortex. Alzheimer's Dement. 2020;6:e12021 10.1002/trc2.12021
REFERENCES
- 1. Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59(7):1125‐1132. [DOI] [PubMed] [Google Scholar]
- 2. Morris MC, Evans DA, Tangney CC, et al. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr. 2005;81(2):508‐514. [DOI] [PubMed] [Google Scholar]
- 3. Mangialasche F, Solomon A, Kåreholt I, et al. Serum levels of vitamin E forms and risk of cognitive impairment in a Finnish cohort of older adults. Exp Gerontol. 2013;48(12):1428‐1435. [DOI] [PubMed] [Google Scholar]
- 4. Joseph JA, Shukitt‐Hale B, Denisova NA, et al. Long‐term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age‐related neuronal signal‐transduction and cognitive behavioral deficits. J Neurosci. 1998;. 1(18):8047‐8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee P, Ulatowski LN. Vitamin E: mechanism of transport and regulation in the CNS. Iubmb Life. 2019;71(4):424‐429. [DOI] [PubMed] [Google Scholar]
- 6. Morris MC, Schneider JA, Li H, et al. Brain tocopherols related to Alzheimer's disease neuropathology in humans. Alzheimers Dement. 2015;11(1):32‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farina N, Isaac MG, Clark AR, Rusted J, Tabet N. Vitamin E for Alzheimer's dementia and mild cognitive impairment. Cochrane Database Syst Rev. 2017;. 4:CD002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li FJ, Shen L, Ji HF. Dietary intakes of vitamin E, vitamin C, and beta‐carotene and risk of Alzheimer's disease: a meta‐analysis. J Alzheimers Dis. 2012;31(2):253‐258. [DOI] [PubMed] [Google Scholar]
- 9. Ambrogini P, Betti M, Galati C, et al. alpha‐Tocopherol and Hippocampal Neural Plasticity in Physiological and Pathological Conditions. Int J Mol Sci. 2016;17(12):2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang SW, Yang SG, Liu W, et al. Alpha‐tocopherol quinine ameliorates spatial memory deficits by reducing beta‐amyloid oligomers, neuroinflammation and oxidative stress in transgenic mice with Alzheimer's disease. Behav Brain Res. 2016;. 296:109‐117. [DOI] [PubMed] [Google Scholar]
- 11. Li Y, Liu L, Barger SW, Mrak RE, Griffin WS. Vitamin E suppression of microglial activation is neuroprotective. J Neurosci Res. 2001;66(2):163‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer's disease. J Cell Biol. 2018;217(2):459‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and Findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical Diagnosis of Alzheimer's Disease: report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939‐944. [DOI] [PubMed] [Google Scholar]
- 15. Braak H, Braak E. Neuropatholocial stageing of Alzheimer‐related changes. Neuropathologica. 1991(4):239‐259. [DOI] [PubMed] [Google Scholar]
- 16. Bradshaw EM, Chibnik LB, Keenan BT, et al. CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nat Neurosci. 2013(7):848‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Felsky D, Roostaei T, Nho K, et al. Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat Commun. 2019;. 10(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hensley K, Barnes LL, Christov A, et al. Analysis of postmortem ventricular cerebrospinal fluid from patients with and without dementia indicates association of vitamin E with neuritic plaques and specific measures of cognitive performance. J Alzheimers Dis. 2011;24(4):767‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williamson KS, Gabbita SP, Mou S, et al. The nitration product 5‐nitro‐gamma‐tocopherol is increased in the Alzheimer brain. Nitric Oxide. 2002;6(2):221‐227. [DOI] [PubMed] [Google Scholar]
- 20. Morris MC. Validity and Reproducibility of a Food Frequency Questionnaire by Cognition in an Older Biracial Sample. Am J Epidemiol. 2003;158(12):1213‐1217. [DOI] [PubMed] [Google Scholar]
- 21. Egger T, Schuligoi R, Wintersperger A, Amann R, Malle E, Sattler W . Vitamin E (alpha‐tocopherol) attenuates cyclo‐oxygenase 2 transcription and synthesis in immortalized murine BV‐2 microglia. Biochem J. 2003;. 370:459‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heppner FL, Roth K, Nitsch R, Hailer NP. Vitamin E induces ramification and downregulation of adhesion molecules in cultured microglial cells. Glia. 1998;22(2):180‐188. [PubMed] [Google Scholar]
- 23. Flanary BE, Streit WJ. Alpha‐tocopherol (vitamin E) induces rapid, nonsustained proliferation in cultured rat microglia. Glia. 2006;53(6):669‐674. [DOI] [PubMed] [Google Scholar]
- 24. Bialowas‐McGoey LA, Lesicka A, Whitaker‐Azmitia PM. Vitamin E increases S100B‐mediated microglial activation in an S100B‐overexpressing mouse model of pathological aging. Glia. 2008;56(16):1780‐1790. [DOI] [PubMed] [Google Scholar]
- 25. Khanna S, Heigel M, Weist J, et al. Excessive alpha‐tocopherol exacerbates microglial activation and brain injury caused by acute ischemic stroke. FASEB J. 2015;29(3):828‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hopperton KE, Mohammad D, Trépanier MO, Giuliano V, Bazinet RP. Markers of microglia in post‐mortem brain samples from patients with Alzheimer's disease: a systematic review. Mol Psychiatry. 2018;23(2):177‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brettschneider J, Del Tredici K, Lee VMY, Trojanowski JQ. Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci. 2015;16(2):109‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Copp RP, Wisniewski T, Hentati F, Larnaout A, Ben Hamida M, Kayden HJ. Localization of alpha‐tocopherol transfer protein in the brains of patients with ataxia with vitamin E deficiency and other oxidative stress related neurodegenerative disorders. Brain Res. 1999;822(1‐2):80‐87. [DOI] [PubMed] [Google Scholar]
- 30. Ulatowski L, Dreussi C, Noy V, Barnholtz‐Sloan J, Klein E, Manor D. Expression of the alpha‐tocopherol transfer protein gene is regulated by oxidative stress and common single‐nucleotide polymorphisms. Free Radic Biol Med. 2012;53(12):2318‐2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davis BM, Salinas‐Navarro M, Cordeiro MF, Moons L, De Groef L. Characterizing microglia activation: a spatial statistics approach to maximize information extraction. Sci Rep. 2017;. 7(1):1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Olah M, Menon V, Habib N, et al. A single cell‐based atlas of human microglial states reveals associations with neurological disorders and histopathological features of the aging brain. Preprint at https://www.biorxiv.org/content/10.1101/343780v1, 2018.
- 33. Tanprasertsuk J, Mohn ES, Matthan NR, et al. Serum Carotenoids, Tocopherols, Total n‐3 Polyunsaturated Fatty Acids, and n‐6/n‐3 Polyunsaturated Fatty Acid Ratio Reflect Brain Concentrations in a Cohort of Centenarians. J Gerontol A Biol Sci Med Sci. 2019;74(3):306‐314. [DOI] [PubMed] [Google Scholar]
- 34. Kreisl WC, Henter ID, Innis RB. Imaging Translocator Protein as a Biomarker of Neuroinflammation in Dementia. Adv Pharmacol. 2018;82:163‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horti AG, Naik R, Foss CA, et al. PET imaging of microglia by targeting macrophage colony‐stimulating factor 1 receptor (CSF1R). Proc Natl Acad Sci U S A. 2019;116(5):1686‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Talegawkar SA, Johnson EJ, Carithers T, Taylor HA, Bogle ML, Tucker KL. Total alpha‐tocopherol intakes are associated with serum alpha‐tocopherol concentrations in African American adults. J Nutr. 2007;137(10):2297‐2303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.


