Abstract
Objective
To assess the efficacy of saline nasal irrigation (S‐NI) and xylitol nasal irrigation (X‐NI) for chronic rhinosinusitis in participants with Gulf War illness (GWI).
Methods
This 26 week, 3‐arm (1:1:1) randomized controlled trial examined veterans meeting criteria for GWI with moderate‐to‐severe chronic rhinosinusitis and fatigue symptoms. All participants received standard of care for chronic rhinosinusitis (CRS); additionally, S‐NI or X‐NI participants added twice‐daily NI using 2% saline or 5% xylitol solutions. Outcomes included disease‐specific quality of life (primary; sino‐nasal outcome test [SNOT‐20]; 0‐100 points), overall quality of life (Short‐Form 36), and fatigue (Multidimensional Fatigue Index). Outcome assessors were blind to allocation group. Intention‐to‐treat analysis used repeated measures modeling; statistical significance was evaluated at the two‐sided α level of .05.
Results
Randomization (N = 40) produced three similar groups regarding sex (male, 80%), age (53.8 ± 7.8 years), duration (19.8 ± 7.7 years), and illness severity (48.5 ± 12.7 SNOT‐20 points). Age‐ and gender‐adjusted between‐group comparison showed that X‐NI participants, compared with control, reported improved SNOT‐20 scores at 8 weeks (13.5 points, 95% confidence interval [CI] −27.9 to 0.9) and at 26 weeks (15.4 points, 95% CI −30.1 to −0.6). S‐NI participants improved by 13.4 points (95% CI −28.8, 2.1) at 26 weeks compared with control.
The improvement in both NI groups approached minimal clinical important difference compared to control for the SNOT‐20 in the general population. Secondary outcomes were not different between groups. Satisfaction in both irrigation groups was high.
Conclusions
This randomized controlled trial suggests that NI with saline or xylitol improves chronic sinus symptoms among participants with GWI with improvement scores similar to those in the general population.
Level of Evidence
1b, individual randomized controlled trial.
Keywords: chronic rhinosinusitis, Gulf War illness, nasal irrigation, neti pot, saline, xylitol
1. INTRODUCTION
Up to 15% of the 700 000 veterans who returned from the Persian Gulf conflict of 1990‐1991 (Desert Shield/Desert Storm) are affected by Gulf War illness (GWI), 1 , 2 a disease affecting multiple organ systems. The condition can be disabling. Substantial research, 3 some of it controversial, 4 has failed to identify a specific cause; there is no cure. Treatment has focused on individualized supportive care. 5 The US Department of Defense (DoD) Gulf War Illness Research Program (GWIRP) has called for investigation of therapy to treat symptoms of GWI. 6
Especially common among GWI patients are chronic upper respiratory symptoms (with nasal congestion reported by 47% of patients) and fatigue (41%). 7 , 8 In the general population, these symptoms are primarily caused by infectious, irritant and allergic agents, and fatigue is often associated with chronic rhinosinusitis (CRS). 9 Pro‐inflammatory biomarkers in serum and nasal mucosa have been associated with fatigue 10 and chronic upper respiratory infection. 11 It is unclear whether the etiology of chronic upper respiratory symptoms in GWI, or therapy intended to treat such symptoms, is similar to that in the general population.
Nasal irrigation (NI) has been reported to effectively relieve symptoms of chronic upper respiratory conditions. 12 The procedure, which originated in the Ayurvedic medical tradition, involves rinsing the nasal cavity with therapeutic solution via the nostrils. While optimal solution type and procedural characteristics have not been objectively determined, two hypertonic solutions, saline (S‐NI) and xylitol (X‐NI), have been assessed. Each has been used to treat CRS in short‐ 13 , 14 and long‐term use. 15 S‐NI has been noted to be appropriate adjunctive therapy for symptomatic chronic rhinosinusitis 12 , 16 ; patient‐centered use involving personalizing some aspects of the irrigation protocol is associated with improved CRS symptom control and decreased side effects. 17 Xylitol is a naturally occurring five‐carbon sugar. Studies have reported that topical use of xylitol increases the effectiveness of natural killer cells in the nasal mucosa. 9 Xylitol may prevent dental caries, 18 treat acute otitis media, 19 and serve as effective therapy for CRS in postoperative care. 14 , 20 , 21 It has been assessed for chronic upper respiratory symptoms in limited trials. S‐NI and X‐NI have not been assessed in patients with GWI. We therefore conducted a three‐arm randomized controlled trial (RCT) to test the hypothesis that, compared with routine care alone, routine care plus either S‐NI or X‐NI improves sinus symptom‐related and fatigue‐related quality of life in patients with GWI.
2. METHODS
The study protocol has been reported. 22 This is a 26‐week RCT to assess the comparative effectiveness of three therapeutic approaches for management of CRS and fatigue in participants with GWI. All groups used routine care for CRS and fatigue. Groups 1 and 2 added S‐NI or X‐NI twice daily to their usual care regimen. Group 3 continued to use routine care only.
The study was approved by the University of Wisconsin‐Madison Institutional Review Board, the United States Army Human Research Protection Office, and the William S. Middleton Memorial Veterans Hospital Research and Development Committee. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice, as outlined by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines. Participants were recruited from the community and the William S. Middleton Memorial Veterans Hospital billing database between April 2013 and July 2015 and followed for 26 weeks. Inclusion criteria included: (a) deployment to the Persian Gulf for Operation Desert Shield or Operation Desert Storm during the first Gulf War (1990‐1991), (b) diagnosis of GWI based on a modified application of the “Kansas” GWI case definition 8 , 22 (personal communication with neuroepidemiologist and GWI content expert Lea Steele, 8‐26‐12), (c) a diagnosis of CRS 22 with a moderate‐to‐severe daily symptom impact score as defined by at least 3 points on a 0‐10 ordinal response scale, 15 and (d) moderate‐to‐severe fatigue severity as indicated by at least 3 points on a 0‐10 ordinal response scale. 22 Exclusion criteria included: (a) current use of NI, (b) any condition increasing risk of aspiration, (c) any condition preventing a potential participant from physically performing NI or attending study appointments, (d) nasal anatomical abnormalities requiring surgical evaluation, (e) unstable psychiatric illness or incompetency, and (f) self‐reported pregnancy.
2.1. Enrollment
Potential participants met with study personnel in a two‐part meeting to learn about the study. Education was provided via a slide presentation and a 30‐second film about NI. Interested persons provided written informed consent for screening procedures (including a sinus computed tomography [CT] scan, an endoscopic nasal exam to rule out sino‐nasal surgical disease, and a psychological evaluation), and signed Health Insurance Portability and Accountability Act (HIPAA) forms. Eligible participants were consented for the study and randomly allocated to one of three treatment groups using a computer‐generated randomization scheme in blocks of three: routine care, routine care plus S‐NI, or routine care plus X‐NI. Participants randomized to one of the two irrigation groups were taught how to do NI using a published approach. 13 All participants were contacted 1 to 3 days after enrollment to provide support for study procedures and trouble‐shooting for NI users. Outcome assessors, data entry personnel, and statistician were blind to allocation group. We did not attempt to blind active participants because xylitol (a sugar) and saline (salt) can be tasted during the procedure.
2.2. Treatment interventions
2.2.1. Interventions
All participants continued in their prior provider‐patient relationships. In addition, active participants delivered saline or xylitol solutions to their nasal cavity using a plastic version of the traditional NI cup or “neti pot” (SinuCleanse), a hand‐held vessel to gently irrigate the nasal cavity. Participants were instructed to irrigate the nasal cavity twice daily. Adherence was followed with self‐reported calendar entries. They received coaching and demonstrated NI proficiency prior to leaving the enrolment meeting consistent with prior studies. 13 To maximize safety, and consistent with US government guidelines, S‐NI and X‐NI participants were advised that saline and xylitol solution should be mixed with store‐bought distilled water and were provided funds for this purchase. 23
2.2.2. NI solutions
Each irrigant is administered as a 120 mL solution delivered using a protocol tested in prior clinical trials. 13 , 15 S‐NI solution was made using a pre‐packaged commercial product containing salt and sodium bicarbonate (SinuCleanse, 2% saline solution). X‐NI solution was made using a pre‐packaged product containing crystallized xylitol (Xivia provided by DuPont Nutrition & Biosciences and manufactured by Danisco Sweeteners Oy, Kotka, Finland, 5% xylitol solution). 14
2.3. Outcome measures
2.3.1. Primary outcome
We measured disease‐specific quality of life using the validated 20‐item sino‐nasal outcome test (SNOT‐20, 0‐100 points). 24 , 25 The SNOT‐20 is reliable and valid for patients with CRS, and is sensitive to clinical change. A change of a minimum of 16 points is considered clinically significant. 25
2.3.2. Secondary outcomes
We measured fatigue using the validated Multidimensional Fatigue Inventory (MFI; 0‐100 points), 26 which has good internal consistency, construct, and convergent validity. 16 Overall health‐related quality of life was assessed using the Medical Outcomes Survey Short Form‐36 (SF‐36; 0‐100 points), a validated questionnaire designed to assess health status, function, and overall health related quality of life. 27 Two items are specific to pain and were analyzed separately given that GWI patients often have substantial pain. 8 GWI and CRS both affect sleep and breathing parameters. Prior studies suggest that both may be improved with SNI in some patients. We have therefore administered 18 relevant sleep and breathing related questions from the Wisconsin Sleep Cohort 28 questionnaire and the Asthma Control Test, 29 respectively (0‐100 points).
2.3.3. Other measures
Demographics, tobacco use, duration of sinus and fatigue symptoms, and CT 9 and endoscopy 30 severity scores were collected at baseline but after enrolment to characterize the sample and to evaluate as covariates for statistical analysis. Adherence to NI and sinus‐related medication use was tracked using a monthly mail‐in calendar and analyzed as percentage of study days using NI or taking sinus medication, respectively.
2.4. Analysis
2.4.1. Sample size calculation
We calculated the sample size based on detection of a significant overall F‐test in a 3‐arm trial, with a power of 80% and a type I error of 5% (α = .05). Based on our prior work, 13 we assumed that: (a) participants in the S‐NI and X‐NI treatment arms would report a 35% reduction in SNOT‐20 symptom scores compared with baseline, and (b) the routine care group will report minimal change. Therefore, an effective sample size of 20 subjects per study arm would provide 80% power (1‐beta = .81) to detect a significant overall F‐test of difference in treatment effects between the groups. Since we anticipated 20% loss to follow‐up or missing data, our targeted enrollment was 75 participants, or 25 participants per study arm.
Data were analyzed in an intention to treat manner using SAS 9.1 statistical software (SAS Institute Inc.; Cary, North Carolina). Descriptive statistics describe outcomes at each time point; mean ± SD was reported at baseline. Repeated measures analysis of variance compared treatment groups on follow‐up SNOT‐20, MFI, SF‐36, and Wisconsin Sleep Cohort questionnaire and the Asthma Control Test total and subscale scores controlling for age and gender. Statistical significance compared to baseline status and between treatment groups was assessed at each time point (group*time interaction) and comprehensively for the entire time frame (main treatment effect). Main treatment effects were evaluated by contrasting active treatment (S‐NI, X‐NI) to control. Medication use data were calculated for the full 26 weeks. The comparison to control for the active treatment arms was a chi‐square test. Statistical significance was evaluated at the two‐sided α level of .05. Effect size and 95% confidence intervals (CIs) around the effect size are reported.
3. RESULTS
In all, 629 persons were screened by phone; 48 met initial eligibility criteria; 40 persons were enrolled and randomized. No participants dropped out; therefore, 40 participants were included in the analysis (Figure 1). There were no significant baseline differences between groups (Table 1). Participants (53.8 ± 7.8 years, 80% men) reported sinus symptoms for 19.8 ± 7.7 years. Baseline scores on self‐reported (SNOT‐20, MFI, and SF‐36) and objectively assessed outcomes (sinus CT and endoscopy) suggest moderate‐to‐severe disease‐specific and overall quality of life.
FIGURE 1.
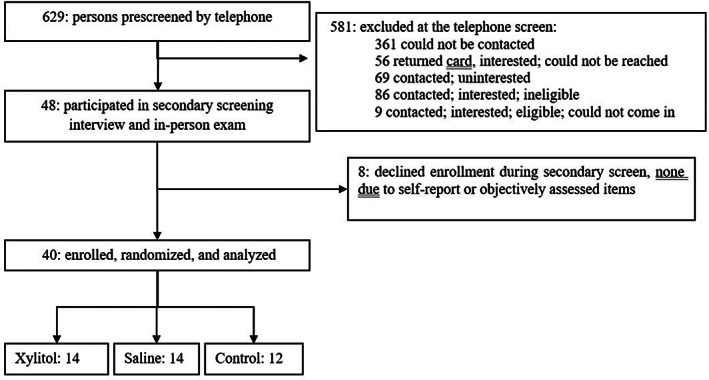
Consort flow diagram
TABLE 1.
Baseline participant characteristics
| Variables | Combined (n = 40) | Xylitol (n = 14) | Saline (n = 14) | Control (n = 12) | P value |
|---|---|---|---|---|---|
| Age in years, mean (SD) | 53.8 (7.8) | 53.7 (9.2) | 52.7 (6.7) | 55.0 (7.7) | .766 |
| Gender‐male, % (n) | 80.0 (32) | 71.4 (10) | 92.9 (13) | 75.0 (9) | .320 |
| Race/Ethnicity, % (n) | |||||
| Non‐Hispanic White | 77.5 (31) | 85.7 (12) | 57.1 (8) | 91.7 (11) | .072 |
| White Hispanic | 10.0 (4) | 7.1 (1) | 14.3 (2) | 8.3 (1) | |
| African American | 5.0 (2) | 7.1 (1) | 7.1 (1) | 0 | |
| Asian | 2.5 (1) | 0 | 7.1 (1) | 0 | |
| Pacific Islander/Hawaiian | 2.5 (1) | 0 | 7.1 (1) | 0 | |
| Multiple races | 2.5 (1) | 0 | 7.1 (1) | 0 | |
| Education, % (n) | |||||
| HS or less | 7.5 (3) | 7.1 (1) | 7.1 (1) | 8.3 (1) | .740 |
| Some college | 50.0 (20) | 57.1 (8) | 57.1 (8) | 33.3 (4) | |
| College graduate | 42.5 (17) | 35.7 (5) | 35.7 (5) | 58.3 (7) | |
| SNOT‐20 (SD) | 48.5 (12.7) | 50.8 (14.7) | 47.6 (13.7) | 47.2 (8.7) | .727 |
| SF‐36 (SD) | 57.3 (16.3) | 51.1 (15.8) | 59.0 (17.6) | 62.7 (14.1) | .177 |
| MFI (SD) | 60.4 (5.3) | 59.4 (5.4) | 60.5 (6.0) | 61.7 (4.7) | .578 |
| Sinusitis symptoms, years (SD) | 19.8 (7.7) | 20.5 (7.8) | 16.4 (9.9) | 22.4 (2.3) | .264 |
| Fatigue, years (SD) | 20.7 (6.6) | 19.3 (8.4) | 19.9 (6.0) | 23.8 (3.1) | .313 |
| CT | 3.9 (4.1) | 5.2 (4.4) | 3.7 (4.5) | 2.6 (3.1) | .274 |
| Endoscopy | 3.3 (1.9) | 3.1 (1.9) | 3.7 (1.8) | 3.1 (2.0) | .597 |
| Sleep | 42.9 (7.1) | 42.2 (5.6) | 44.7 (8.5) | 41.5 (7.0) | .473 |
| Wheeze | 21.5 (4.1) | 21.3 (4.4) | 21.6 (4.8) | 21.6 (3.3) | .975 |
| Tobacco use, % (n) | |||||
| Never | 40.0 (16) | 42.9 (6) | 42.9 (6) | 33.3 (4) | .323 |
| Current | 2.5 (1) | 0 | 0 | 1 (8.3) | |
| Past | 25.0 (8) | 35.7 (5) | 14.3 (2) | 8.3 (1) | |
Abbreviations: CT, computed tomography (0‐24 points); HS, high school; MFI, multidimensional fatigue index (0‐100 points); SF‐36, Short‐Form 36 (0‐100 points); SNOT‐20, sino‐nasal outcomes test‐20 (0‐100 points).
Adherence to irrigation was high in both intervention groups; S‐NI participants reported irrigating one or more times on 84% ± 24% of days, and twice per day on 60.4% ± 36% of days; compared with X‐NI participants who reported irrigating one or more times on 95.5% ± 4% of days and twice per day on 78.4% ± 19.6% of days. There were no adherence differences between X‐NI and S‐NI groups across all outcomes, and at all follow‐up time points.
3.1. Sino‐nasal outcomes test‐20 (SNOT‐20)
Between‐group comparison showed that X‐NI participants, compared with routine care alone, reported improved SNOT‐20 scores at 8 weeks (13.5 points, 95% CI −27.9, 0.9; P = .07) and at 26 weeks (15.4 points, 95% CI −30.1, −0.6; P = .04). Improvement reported by S‐NI compared with Control participants was similar at 26 weeks (13.4 points, 95% CI −28.8, 2.1; P = .09). X‐NI participants reported significant improvement compared to their own baseline status at both 8 and 26 weeks, while S‐NI participants reported significant improvement compared to baseline only at 26 weeks (Table 2).
TABLE 2.
Change in outcome measures (SD) at 8 and 26 week follow‐up a
| Xylitol | Saline | Control | ||||
|---|---|---|---|---|---|---|
| 8 weeks | 26 weeks | 8 weeks | 26 weeks | 8 weeks | 26 weeks | |
| (n = 14) | (n = 14) | (n = 14) | (n = 12) | (n = 12) | (n = 11) | |
| SNOT‐20 b | 34.2 (18.6) | 32.3 (17.9) | 38.0 (20.2) | 32.2 (16.6) | 44.2 (8.6) | 44.1 (20.5) |
| Δ c/t baseline | −16.9 (4.9)** | −18.9 (4.9)** | −8.8 (5.0) | −16.9 (5.3)** | −3.4 (5.3) | −3.5 (5.6) |
| Δ c/t control | −13.5 (7.2) | −15.4 (7.4)* | −5.3 (7.3) | −13.4 (7.7) | ||
| (n = 14) | (n = 14) | (n = 14) | (n = 12) | (n = 12) | (n = 11) | |
| SF‐36 b | 52.0 (19.8) | 57.3 (21.5) | 63.6 (19.8) | 66.3 (19.2) | 61.9 (15.2) | 63.3 (20.6) |
| Δ c/t baseline | 1.1 (3.5) | 6.4 (3.5) | 3.9 (3.6) | 7.3 (3.6) a | −0.1 (3.8) | 2.0 (4.0) |
| Δ c/t control | 1.2 (5.2) | 4.4 (5.3) | 4.0 (5.2) | 5.3 (5.5) | ||
| (n = 12) | (n = 9) | (n = 14) | (n = 12) | (n = 11) | (n = 8) | |
| MFI‐20 b | 59.8 (7.1) | 61.1 (4.3) | 60.8 (7.0) | 59.3 (5.4) | 61.9 (5.6) | 73.5 (16.9) |
| Δ c/t baseline | 0.6 (2.4) | 1.9 (2.8) | 0.9 (2.3) | −1.4 (2.4) | 0.5 (2.5) | 10.4 (3.0) |
| Δ c/t control | 0.1 (3.5) | −8.5 (4.0) | 0.4 (3.4) | −11.7 (3.8) | ||
| (n = 14) | (n = 13) | (n = 14) | (n = 12) | (n = 12) | (n = 11) | |
| Sleep b | 38.7 (9.2) | 35.1 (9.8) | 41.5 (10.7) | 41.2 (8.8) | 42.8 (7.1) | 41.5 (6.9) |
| Δ c/t baseline | −3.2 (2.2) | −6.3 (2.3)** | −3.5 (2.2) | −4.4 (2.3) | 1.2 (2.4) | −0.0 (2.5) |
| Δ c/t control | −4.4 (3.2) | −6.3 (3.3) | −4.8 (3.2) | −4.4 (3.4) | ||
| (n = 13) | (n = 13) | (n = 14) | (n = 12) | (n = 12) | (n = 11) | |
| Wheezing b | 21.6 (4.1) | 22.9 (3.0) | 20.9 (5.9) | 20.8 (5.0) | 22.6 (2.7) | 21.3 (4.0) |
| Δ c/t baseline | 0.4 (1.0) | 1.8 (1.0) | −0.5 (1.0) | −0.4 (1.0) | 1.0 (1.0) | −0.2 (1.1) |
| Δ c/t control | −0.6 (1.4) | 1.9 (1.4) | −1.5 (1.4) | −0.2 (1.5) | ||
| Antibiotic % c | 7.4 (1.2)* | 0.2 (0.8) | 0.8 (2.3) | |||
| Other sinus medication % c | 19.5 (32.4)* | 8.2 (21.4)* | 53.3 (43.6) |
Abbreviations: Δ c/t, “change compared to”; MFI, multidimensional fatigue index; SF‐36, Short‐Form 36; SNOT‐20, sino‐nasal outcomes test‐20.
All estimates control for age and gender.
SNOT‐20, SF‐36, MFI; Sleep and Wheezing scales are 0‐100 points.
Antibiotic and Other Sinus Medication are percentage of study days on which participant took the medication.
P < .05.
P < .01.
3.2. Multidimensional fatigue inventory
There were no differences between groups on the MFI either compared with baseline status or between groups. Fewer participants completed the MFI survey and low sample size may have influenced results (Table 2).
3.3. Short‐Form 36
There were no differences between groups on the SF‐36, including on two items assessing pain scores. S‐NI participants reported improved overall quality of life compared to baseline status (7.3 points; 95% CI 0.0, 14.7), while change reported by X‐NI was similar but not significant (6.4 points, 95% CI −0.7, 13.4).
3.4. Sleep and wheezing
There were no significant changes in sleep or wheezing in the active groups compared to routine care alone. However, X‐NI participants reported improvement compared to baseline status in sleep.
3.5. Medication use
X‐NI participants used antibiotics on a significantly greater number of days (7.4%) in the 26‐week follow‐up than controls (0.8%); three participants in the X‐NI group used antibiotics on 25%‐37% of days. Anecdotally, each of the three had a history of significant antibiotic use. However, both X‐NI and S‐NI participants used other sinus medications on significantly fewer days (19.5% and 8.2%, respectively) than controls (53.3%) (Table 2).
There were no adverse events and only expected side effects (nasal stinging in both intervention groups). No baseline demographic variable predicted participant responsiveness to either X‐NI or S‐NI.
4. DISCUSSION
This is the first study of chronic nasal symptoms and fatigue in a population with GWI, and the first study to directly compare effects of X‐NI and S‐NI to control in a nonsurgical population. While there were no differences between NI groups, both X‐NI and S‐NI provided substantial improvement in several outcomes. Most robust is the finding that X‐NI participants reported significant improvements in SNOT‐20 scores compared to control at 26 weeks. Average change scores of both active groups fell just short of MCID criteria when compared with Control. However, participants using X‐NI at 8 and 26 weeks, and S‐NI participants at 26 weeks, reported improvement in SNOT‐20 scores in excess of the minimal clinical important difference (MCID) of 16 points compared with baseline status and likely experienced meaningful positive change. Secondary outcomes also improved in the active groups. NI‐X participants reported improved sleep scores compared with baseline status at 26 weeks. Fatigue was relatively unchanged across time for all groups. Participants in the X‐NI group used more antibiotics than control.
These data add to the literature about NI for chronic sinus symptoms. While the underlying mechanism for CRS in patients with GWI is not clear, CRS symptoms are not reported to be clinically different, or originate via different mechanisms, than CRS in the general populations. S‐NI and X‐NI have both been reported to improve symptoms of CRS. S‐NI has been assessed in four rigorous clinical trials testing either isotonic 31 or hypertonic saline. 13 , 16 , 31 Three of these reported significant improvement in validated outcome measures including the SNOT‐20 that meet or approach the MCID of the SNOT‐20. Three studies assessing xylitol and saline among postoperative ENT participants report improvements in SNOT‐20 and SNOT‐22 that favor X‐NI, also by values that approach or meet MCID criteria. 14 , 21 , 32 A trial is currently under way comparing X‐NI to S‐NI in a nonoperative cohort. 33
The main difference in self‐reported outcomes between the current trial and existing literature for both irrigation solutions is that in trials assessing S‐NI without X‐NI, participants have more often reported robust effect sizes at or near the MCID. 13 , 15 , 31 Two study design elements of X‐NI trials may have limited the reported effect size of S‐NI. All X‐NI trials used short NI intervention time periods of 30 days or less, compared with S‐NI only trials in which participants irrigated from 8 weeks to 12 months. S‐NI may require longer irrigation time to see an effect. In the current study, S‐NI participants at 8 weeks reported only half as much improvement as X‐NI participants, but caught up by 26 weeks. In addition, S‐NI trials more often used hypertonic saline solutions of approximately 2%, whereas X‐NI protocols have used isotonic (0.9%) saline. While evidence is limited regarding relative effect of saline concentration, hypertonicity appears to confer some improvement in symptoms compared with isotonic saline. 34
The mechanism of action of both saline and xylitol in CRS is not well understood and is likely multifaceted. Studies have reported several physiological effects at the mucosal and cellular levels that individually or in concert result in an improved function of the nasal mucosa and reduced symptoms. Physiologic effects of S‐NI include: (a) Direct cleansing: as it moves through the nasal cavity, saline thins and mechanically removes obstructive mucus and crusts; this mechanism is likely responsible for the immediate sense of improved breathing reported by many participants. 35 , 36 , 37 (b) Reduction or removal of inflammatory mediators associated with chronic sino‐nasal disease 38 , 39 ; S‐NI may acutely remove these mediators, reducing their inflammatory effects. (c) Improved mucociliary function as a result of increased ciliary beat frequency in the presence of hypertonic 40 or normal 41 saline.
X‐NI may also have such direct cleansing effects; in addition, it is reported to increase the ability of the airway surface layer (ASL) of the nasal mucosa to kill respiratory pathogens. Agents in the ASL including lysozyme, lactoferrin, and β defensins form part of the local pulmonary host defense system. Their antibacterial activity is salt‐sensitive 42 , 43 and is more potent at lower salt concentrations. 44 , 45 , 46 When added to the mucosal surface of airway epithelia, xylitol lowers salt concentration of the ASL, potentially enhancing antimicrobial properties. A randomized, double‐blind, crossover study of 21 healthy volunteers assessed the number of colonized coagulase‐negative Staphylococcus in the nasal cavity in the presence of 5% xylitol compared to saline. Nasal swab cultures showed that NI‐X significantly reduced the number of coagulase‐negative Staphylococcus aureus on the nasal surface compared to saline. 42
4.1. Limitations
This study has several limitations, including small sample size. Recruitment of this narrowly defined cohort was challenging and we did not meet planned enrolment. This likely lead to the study being underpowered to detect statistical differences between S‐NI and control should they exist. However, the change scores of both X‐NI and S‐NI on the SNOT‐20 were large enough to detect between‐group and within‐group differences. We were not able to determine the type and duration of antibiotic use. The study's sample size limits our ability to detect uncommon adverse events. We did not directly assess pain, a common component of GWI. Blinding of participants was not possible, potentially introducing bias. Strengths include pragmatic assessment using validated, patient‐oriented outcomes.
5. CONCLUSIONS
In patients with chronic rhinosinusitis in the context of GWI, X‐NI, or S‐NI along with standard of care is associated with decreased sino‐nasal symptoms and improved disease‐specific quality of life compared with standard of care alone. Participants reported high adherence to the protocol; there were no adverse events or unanticipated side effects. While there were no differences between active groups, it is unclear whether a given patient would experience both forms of NI in the same way. Therefore, clinicians may reasonably conclude that each is a potentially effective modality in this patient population.
ACKNOWLEDGMENTS
Individual coauthors, materials, and participant‐related research services are funded in part by support provided by DuPont Nutrition & Biosciences (xylitol sachets) and Med‐Systems (salt sachets and nasal irrigation (neti) pots; participant‐related research services were supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Rabago D, Kille T, Mundt M, Obasi C. Results of a RCT assessing saline and xylitol nasal irrigation for CRS and fatigue in Gulf War illness. Laryngoscope Investigative Otolaryngology. 2020;5:613–620. 10.1002/lio2.425
Abstracts presented of in‐progress work in two national conference settings:
Obasi C, Hayer S, Kille T, Molander R, Hauffe I, Kansariwala I, Fehrenbach D, Comp L, Van Leuven L, Rabago D. Nasal irrigation for chronic rhinosinusitis and fatigue in patients with gulf war illness: Preliminary data from a 3‐arm randomized controlled trial (Abstract presented at the Integrative Medicine for the Underserved; Tufts University; Boston, MA; August June 8, 2015 and published in Global Adv Health Med, 2015;4(6):65‐72.
Interim data related to the current paper have been presented in a conference setting:
Rabago D, Obasi C, Hayer S, Kille T, Amaza I, Mundt M, Molander R, Kansariwala I. Saline and Xylitol Nasal Irrigation for Chronic Rhinosinusitis and Fatigue in Patients With Gulf War Illness: Results from A Randomized Controlled Pilot Study; North American Primary Care Research Group (NAPCRG) 45th Annual Conference; Montreal Quebec Canada; November 19, 2017.
Methodological information related to the current paper has been published in a methods paper:
Hayer SD, Rabago D, Amaza IP, Kille T, Coe C, Zgierska A, Zakletskaia L, Mundt M, Krahn D, Obasi C, Molander R. Effectiveness of saline and xylitol nasal irrigation for chronic rhinosinusitis and fatigue in patients with gulf war illness: protocol for a randomized controlled trial. Contemporary Clinical Trials, 2015;41:219‐226; PMID 25625809.
Funding information NIH National Center for Advancing Translational Sciences, Grant/Award Number: UL1TR000427; U.S. Department of Defense, Grant/Award Number: W81XWH‐11‐1‐0390
BIBLIOGRAPHY
- 1. CDC . Current estimates from the national health interview survey, 1994. US Department of Health and Human Services: Vital Health Statistics; 1995; PHS 96‐1521.
- 2. Conboy L, St John M, Schnyer R. The effectiveness of acupuncture in the treatment of Gulf War illness. Contemp Clin Trials. 2012;33(3):557‐562. [DOI] [PubMed] [Google Scholar]
- 3. Binns J. Research Advisory Committee on Gulf War Veterans' Illnesses Gulf War Illness and the Health of Gulf War Veterans: Scientific Findings and Recommendations. Washington, DC: US Government Printing Office; 2008. [Google Scholar]
- 4. Miller J, Coffman M. Gulf War: What Kind of Care Are Veterans Receiving 20 Years Later? Hearing Before the Subcommittee on Oversight and Investigations of the Committee on Veterans' Affairs. Committee on Veterans' Affairs, ed. https://veterans.house.gov. Vol Serial Number 113‐0. Washington, DC: US Government Printing Office; 2013:1‐66. [Google Scholar]
- 5. Steele L, Sastre A, Gerkovich MM, Cook MR. Complex factors in the etiology of Gulf War illness: wartime exposures and risk factors in veteran subgroups. Environ Health Perspect. 2012;120:112‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aubert B, Barate R, Bona M, et al. Measurement of the branching fraction and photon energy moments of B–>Xs gamma and A(CP)(B –> X[s+d gamma]). Phys Rev Lett. 2006;97(17):171803. [DOI] [PubMed] [Google Scholar]
- 7. Fukada K, Nisenbaum R, Stewart G, Thompson WW. Chronic multisystem illness affecting air force verterans of the Gulf War. JAMA. 1998;280:981‐988. [DOI] [PubMed] [Google Scholar]
- 8. Steele L. Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place and time of military service. Am J of Epidemiol. 2000;152(10):992‐1002. [DOI] [PubMed] [Google Scholar]
- 9. Bhattacharyya N. Clinical and symptom criteria for the accurate diagnosis of chronic rhinosinusitis. Laryngoscope. 2006;116(S110):1‐22. [DOI] [PubMed] [Google Scholar]
- 10. Broderick G, Fuite J, Kreitz A, Vernon S, Klimas N, Fletcher M. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav Immun. epublished ahead of print. 2010;24(7):1209‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reichelmann H, Deutschle T, Rozasi A, Keck T, Polzehl D, Burner H. Nasal biomarker profiles in acute and chronic rhinosinusitis. Clin Exp Allergy. 2005;35:1186‐1191. [DOI] [PubMed] [Google Scholar]
- 12. Harvey R, Hannan S, Badia L, Scadding G. Nasal saline for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;3:CD006394. [DOI] [PubMed] [Google Scholar]
- 13. Rabago D, Zgierska A, Mundt MP, Barrett B, Bobula J, Maberry R. Efficacy of daily hypertonic saline nasal irrigation among patients with sinusitis: a randomized controlled trial. J FAM Practice. 2002;51(12):1049‐1055. [PubMed] [Google Scholar]
- 14. Weissman JD, Fernandez F, Hwang PH. Xylitol nasal irrigation in the management of chronic rhinosinusitis: a pilot study. Laryngoscope. 2011;122:468‐472. [DOI] [PubMed] [Google Scholar]
- 15. Rabago D, Pasic T, Zgierska A, Barrett B, Mundt M, Maberry R. The efficacy of hypertonic saline nasal irrigation for chronic sinonasal symptoms. Otolaryngol Head Neck Surg. 2005;133:3‐8. [DOI] [PubMed] [Google Scholar]
- 16. Little PSB, Mullee M, Thomas T, et al. Effectiveness of steam inhalation and nasal irrigation for chronic or recurrent sinus symptoms in primary care: a pragmatic randomized controlled trial. CMAJ. 2016;188:940‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rabago D, Barrett B, Marchand L, Maberry R, Mundt M. Qualitative aspects of nasal irrigation use by patients with chronic sinus disease in a multimethod study. Ann Fam Med. 2006;4(4):295‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maguire A, Rugg‐Gunn AJ. Xylitol and carries prevention‐is it magic bullet? Br Dent J. 2003;194:429‐436. [DOI] [PubMed] [Google Scholar]
- 19. Uhari M, Kontiokari T, Niemelä M. A novel use of xylitol sugar in preventing acute otitis media. Pediatrics. 1998;102(4):879‐974. [DOI] [PubMed] [Google Scholar]
- 20. Kim DH, Kim Y, Lim G, Cho JH, Park YJ, Kim SW. Effect of postoperative xylitol nasal irrigation on patients with sinonasal diseases. Otolaryngol Head Neck Surg. 2018;160:550‐555. 10.1177/0194599818802815. [DOI] [PubMed] [Google Scholar]
- 21. Lin L, Tang X, Wei J, Fei D, Sun G. Xylitol nasal irrigation in the treatment of chronic rhinosinusitis. Am J Otolaryngol. 2017;38:383‐389. [DOI] [PubMed] [Google Scholar]
- 22. Hayer SD, Rabago DP, Amaza IP, et al. Effectiveness of nasal irrigation for chronic rhinosinusitis and fatigue in patients with Gulf War illness: protocol for a randomized controlled trial. Cont Clin Trials. 2015;41:219‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Is rinsing your sinuses with neti pots safe? US Food and Drug Administration , https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm316375.htm. 2017. Accessed May 17, 2019.
- 24. Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(3 [suppl]):S1‐S32. [DOI] [PubMed] [Google Scholar]
- 25. Piccirillo JF, Merritt MG Jr, Richards ML. Psychometric and clinimetric validity of the 20‐item sino‐nasal outcome test (SNOT‐20). Otolaryngol Head Neck Surg. 2002;126(1):41‐47. [DOI] [PubMed] [Google Scholar]
- 26. Smets EM, Garssen B, Bonke B, De Haes JC. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315‐325. [DOI] [PubMed] [Google Scholar]
- 27. Ware J, Sherborne C. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;30:473‐483. [PubMed] [Google Scholar]
- 28. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep‐disordered breathing among middle‐aged adults. New England J Med. 1993;328(17):1230‐1235. [DOI] [PubMed] [Google Scholar]
- 29. Schatz M, Sorkness CA, Li JT. Asthma control test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549‐556. [DOI] [PubMed] [Google Scholar]
- 30. Bhattacharyya N, Lee L. Evaluating the diagnosis of chronic rhinosinusitis based on clinical guidelines and endoscopy. Otolaryngol Head Neck Surg. 2010;143:147‐151. [DOI] [PubMed] [Google Scholar]
- 31. Pynnonen MA, Mukerji SS, Kim HM, Adams ME, Terrell JE. Nasal saline for chronic sinonasal symptoms: a randomized controlled trial. Arch Otolaryngol Head Neck Surg. 2007;133:1115‐1120. [DOI] [PubMed] [Google Scholar]
- 32. Kim DH, Kim YK, Lim IG, et al. Effect of post operative xylitol irrigation on patients with sinonasal diseases. Otolaryngol Head Neck Surg. 2019;160:550‐555. [DOI] [PubMed] [Google Scholar]
- 33. Durairaj L. Xylitol versus saline in chronic sinusitis. Clinical Trialsgov 2014. https://clinicaltrials.gov/ct2/show/NCT00924404
- 34. Kanjanawasee D, Seresirikachorn W, Chitsuthipakorn W, Snidvongs K. Hypertonic saline versus isotonic saline nasal irrigation: systematic review and meta‐analysis. Am J Rhinol Allergy. 2018;32:269‐279. [DOI] [PubMed] [Google Scholar]
- 35. Ozsoylu S. Nose drops and the common cold. Eur J Pediatr. 1985;144:294.4054173 [Google Scholar]
- 36. Karadag A. Nasal saline for acute sinusitis. Pediatrics. 2002;109:165. [DOI] [PubMed] [Google Scholar]
- 37. Kurtaran H, Karadag A, Catal F, Avci Z. A reappraisal of nasal saline solution use in chronic sinusitis. Chest. 2003;124:2036‐2037. [DOI] [PubMed] [Google Scholar]
- 38. Georgitis JW. Nasal hyperthermia and simple irrigation for perennial rhinitis. Changes in inflammatory mediators. Chest. 1994;106(5):1487‐1492. [DOI] [PubMed] [Google Scholar]
- 39. Ponikau JU, Sherris DA, Kephart DM, Kern EB, Congdon DJ, et al. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005;116(2):362‐369. [DOI] [PubMed] [Google Scholar]
- 40. Talbot AR, Herr TM, Parsons DS. Mucocilliary clearance and buffered hypertonic saline solution. Laryngoscope. 1997;107:500‐503. [DOI] [PubMed] [Google Scholar]
- 41. Boek WM, Graamans K, Natzijl H, van Rijk PP, Huizing EH. Nasal mucociliary transport: new evidence for a key role of ciliary beat frequency. Laryngoscope. 2002;112:570‐573. [DOI] [PubMed] [Google Scholar]
- 42. Zabner J, Seiler P, Launspach JL, et al. The osmolyte xylitol reduces the salt concentration of airway surface liquid and may enhance bacterial killing. PNAS. 2000;97:11614‐11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lehrer RI, Ganz T. Antimicrobial peptides in mammalian and insect host defense. Curr Opin Immunol. 1999;11:23‐27. [DOI] [PubMed] [Google Scholar]
- 44. Travis SM, Singh PK, Welsh MJ. Antimicrobial peptides and proteins in the innate defense of the airway surface. Curr Opin Immunol. 2001;13:89‐95. [DOI] [PubMed] [Google Scholar]
- 45. Huttner KM, Bevins CL. Antimicrobial peptides as mediators of epithelial host defense. Pediatr Res. 1999;45:785‐794. [DOI] [PubMed] [Google Scholar]
- 46. Bals R, Weiner DJ, Wilson JM. The innate immune system in cystic fibrosis lung disease. J Clin Invest. 1999;103:303‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]


