Abstract
Objective
Introduce novel methods and materials to limit microdroplet spread when performing transnasal aerosol generating procedures in the COVID‐19 era.
Methods
Prototypes of a negative pressure face shield (NPFS) were tested then used clinically to create a suction‐clearing negative pressure microenvironment with controlled access to the nose and mouth. Air pressure measurements within prototypes were followed by prospective evaluation of 30 consecutive patients treated with the device assessed through questionnaires and monitoring oximetry.
Results
The NPFS is a transparent acrylic barrier with two anterior instrumentation ports and a side port to which continuous suction is applied. It is positioned on a stand and employs a disposable antimicrobial wrap to secure an enclosure around the head. This assembly was successfully used to complete transnasal laryngoscopy in all 30 patients studied. Tolerance of the design was excellent, with postprocedure questionnaire identifying no shortness of breath (27/30), no claustrophobia (27/30), no pain (29/30), and no significant changes in pulse oximetry.
Conclusion
Diagnostic laryngoscopy was successfully performed in a negative pressure microenvironment created to limit dispersion of aerosols. Further application of the NPFS device is targeted for use with transnasal laryngeal laser and biopsy procedures to be followed by additional modification to enable intranasal and intraoral procedures in a similar protected environment.
Level of Evidence
Level 2b (Cohort Study).
Keywords: COVID‐19, diagnostic flexible, laryngoscopy, laryngoscopyaerosol generating procedure
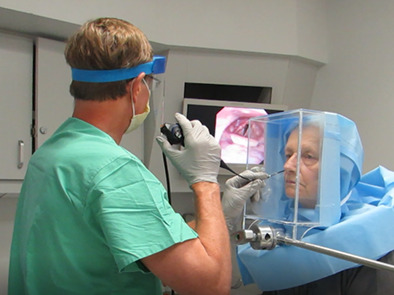
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic has radically altered the approach to medical care to impact the management of disorders of the upper aerodigestive tract. Risk of viral spread during the aerosol generating procedure (AGP) of transnasal laryngoscopy was addressed through a consensus development webinar involving 300 participants from the American laryngology community on March 24, 2020. 1 This consensus was presented early in the pandemic and recommended screening patients to radically limit use of this procedure. The recommendation at that time concluded that “flexible laryngoscopy should only be performed in critical cases” and “alternatives to laryngoscopy should be considered.” Additional recommendations were made for practitioner to use protective gear in a negative pressure or designated isolation room, and to attend to appropriate disinfection of equipment and the examination room.
Through evolving knowledge regarding protective measures, restrictions have been eased over the past months. Guidelines for safe return to practice to resume the practice of flexible laryngoscopy were disseminated online by the American Academy of Otolaryngology‐Head and Neck Surgery (AAO‐HNS) on May 8. 2 These guidelines identify that flexible nasal laryngoscopy “may potentially increase the likelihood of cough, gag, and sneeze, with possible subsequent aerosolization, and therefore appropriate precautions should be considered based on individual clinical circumstances.” The AAO‐HNS recommendations focused on use of personal protective equipment (PPE) and also discussed concern of the variability in COVID testing—emphasizing a high false negative rate.
Screening patients with nasopharyngeal or oropharyngeal swabs to test for SARS‐CoV‐2 infection with the reverse transcription polymerase chain reaction (RT‐PCR) assay has become common practice at some institutions before performing transnasal laryngoscopy but is still limited at others. A recent publication from the Johns Hopkins School for Public Health acknowledges a high false negative rate early in the course of infection and suggest that “care must be taken” when using these results as a basis for removing precautions. 3
In addition to use of PPE, other methods have been improvised to help separate the patient from the health care worker in addressing the concept that all patients should be managed as if they harbor transmissible virus. One approach has been to outfit the patient with a mask modified to permit passage of the flexible scope into the nose. 4 , 5
More elaborate barriers have been constructed to shield the health care workers during endotracheal intubation. 6 Similar barriers have not yet been reported to improve the safety of transnasal laryngoscopy.
We present development followed by implementation of a negative pressure face shield (NPFS) designed to establish a negative pressure microenvironment about the patient's face during transnasal and transoral procedures. The NPFS is a reusable acrylic apparatus implemented with the common clinical materials of wall suction, disposable tubing, tape, and draping material.
2. MATERIALS AND METHODS
The local institutional review board (IRB) directed and approved the clinical evaluation of the NPFS. All methods were in full accordance with the principles set out by the World Medical Association Declaration of Helsinki.
Testing of multiple prototypes led to development of the NPFS made of 0.2″ (5 mm) thick acrylic. This NPFS is a transparent 9″ × 10.5″ rectangular device with a depth of 3.5″ and an inferior stabilization flange used to engage a clamp on a stand for positioning in front of the patient (Figure 1). A heavily weighted camera stand employs a clamp engage the flange (Matthews Hollywood Century 40″ S Stand For Grip Arm Kit, Adorama, Inc, New York, New York) (Figure 2).
FIGURE 1.
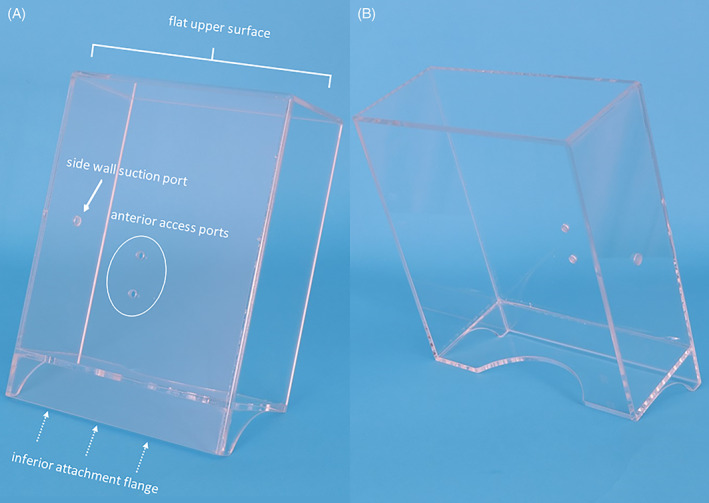
The acrylic negative pressure face shield oriented upright demonstrating inferior attachment flange (dotted arrows), flat upper surface, anterior access ports and suction port on the side
FIGURE 2.

A stable camera stand is employed to secure the negative pressure face shield in front of the patient's face
This reusable device was designed without any irregular surfaces to facilitate cleaning with antimicrobial wipes (either Virex or Sani‐Cloth) to disinfect it for reuse within following 3 minutes of air‐drying. The three openings in the NPFS were smoothed and sealed to ensure that these areas would not harbor unwanted particulate matter. These openings included two separate ¼‐inch access ports in the lower midline of the face shield and a 5/16‐in. suction port on the midlateral surface. This device was created in the University of Iowa Machine Shop with additional oversite from Infectious Disease, Hospital Epidemiology and Bioengineering Departments in the University of Iowa Hospital.
Prototype evaluation included initial testing with a standard wall suction (Vacutron Suction Regulators by Chemetron, Inc) with a maximum regulated suction of 320 ± 20 mmHg. Additional testing was done with a portable suction (Neptune 3 Waste Management System 120 VAC Rover, Stryker, Kalamazoo, Michigan) which includes a high efficiency particulate air (HEPA) Filter and is capable of generating continuous suction in a range from 50 to 520 mmHg.
All patient‐related experiments were performed in a standard examination room designated as having six air changes per hour (ACH) and employed standard wall suction as designated above. Sterile disposable suction tubing (0.6 mm × 3.7 mm, nonconductive suction tubing, 12 ft length; Cardinal Health, Waukegan, Illinois) was attached to the NPFS and used through the entire test.
Thirty‐one consecutive patients required transnasal laryngoscopy by the senior author (HTH) over a 24‐day period beginning May 5, 2020. Limited COVID testing at our facility during this period permitted viral assessment of the single patient with a tracheostomy. This patient was excluded from study, yielding 30 patients who were offered inclusion in the study with all consenting to participate.
A sterilization wrap (Halyard H100 sterilization wrap, O&M Halyard, Inc, Alpharetta, Georgia) was fashioned as a cylinder and secured to the NPFS with tape circumferentially (Figure 3). The wrap was adapted to drape over the patient's head as a hood to create a closed environment by drawing the lower aspect of the drape loosely around the patient with a 3 to 4 ft length of umbilical tie (white twill ½ inch, 36‐yard roll, Horn Textile, Inc, Titusville, Pennsylvania).
FIGURE 3.

The H100 “one step sterilization wrap” is unfolded and taped circumferentially around the back of the negative pressure face shield (NPFS) (black arrows) and then pulled forward to permit the patient to place their face in the device (white arrows)
The H100 sterilization wrap is a “a disposable infection control product” made of water‐repellant breathable “low lint spunbond meltblown spunbond (SMS) fabric” as is also used in production of face masks and lab jackets. 7
An electronic micromanometer (AirdataMultimeter ADM‐870C, Shortridge Instruments, Inc, Scottsdale, Arizona) was used to measure air pressure in the NPFS during use. Evaluation of the prototype involved assessment of the vacuum generated within the face shield. The probe was placed through one of the anterior ports with H100 sterilization wrap in place around the shield. Pressure readings were obtained both with the second anterior port opened and closed with tape (Figure 4).
FIGURE 4.
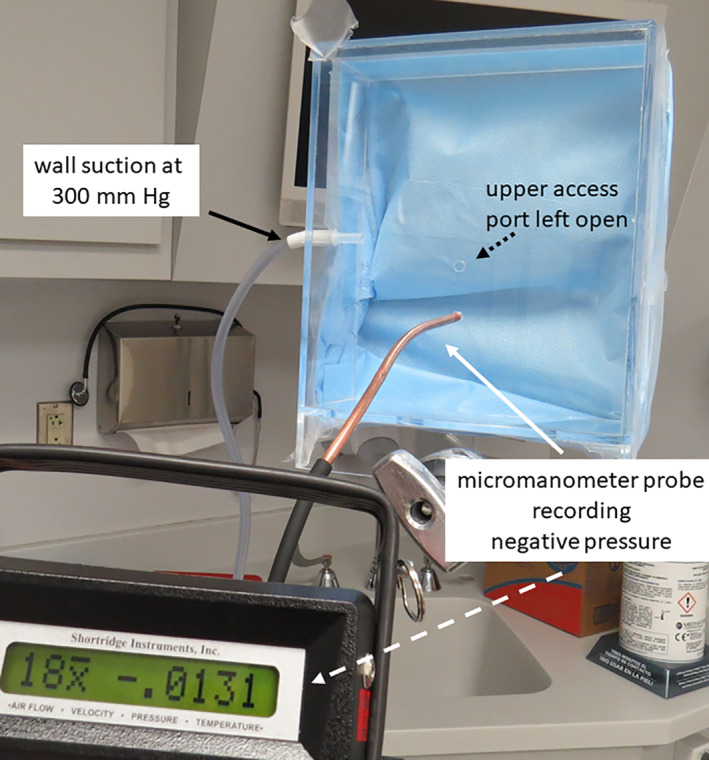
Testing negative pressure with full wall suction applied (here shown with second port opened). An average of 18 individual readings was −0.0131 in. of water
Initial testing in human volunteers included assessment of fluctuations in the negative pressure generated within the shield during inspiration and ventilation. Additional pressure testing was done employing a single vs double layer of the sterilization wrap as well as comparison of use of the wall suction on maximum vs portable suction with a HEPA filter.
The prepared NPFS with sterilization wrap applied is rotated into position with the patient leaning slightly forward and adjusting the exam chair to a level optimal for the exam. The most optimal of the two anterior access ports is chosen for use during the procedure (Figure 5A,B). The unused port is sealed with tape. A twill tape is drawn around the sterilization wrap and the patient to improve the seal and optimize negative pressure within the device (Figure 5C,D).
FIGURE 5.
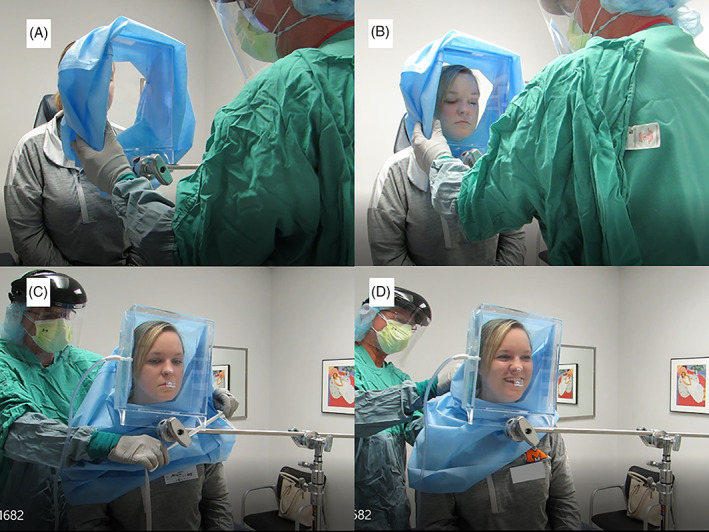
The negative pressure face shield with sterilization wrap is rotated in place and secured around the neck
Before performing transnasal laryngoscopy, all but one (who deferred anesthesia) of the 30 patients received 1 cc of a mixture of 4% lidocaine with 1% phenylephrine delivered to the nostril through an anterior access port by syringe spray employing the MADgic Laryngo‐tracheal mucosal atomization device (Teleflex Medical, Inc, Morrisville, North Carolina) (Figure 6).
FIGURE 6.
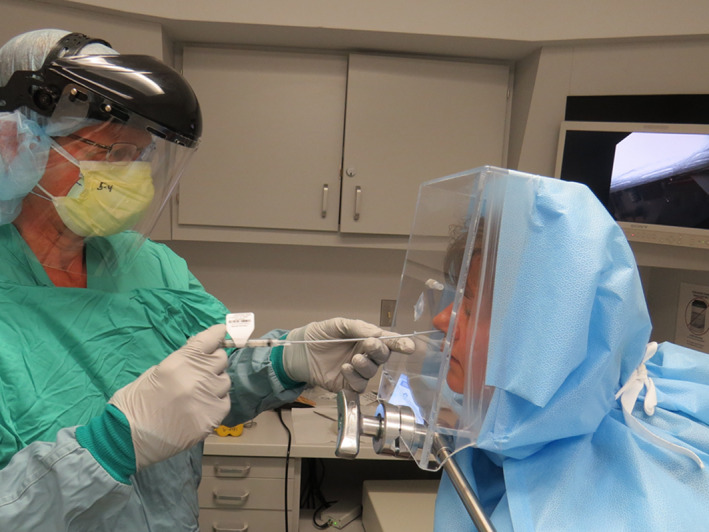
Instillation of nasal decongestant with anesthesia through anterior access port. Note the full protective equipment as depicted in examining this patient early in the study was modified with further experience to employ only a face shield, mask, and gloves without perceived need for gown or N95
The flexible transnasal laryngoscopies were performed with either a 2.6 mm Olympus ENF‐V3 Video or 3.9 mm ENF‐VH Olympus Rhinolaryngoscope during all patient encounters (Figure 7).
FIGURE 7.

The procedure is done with the flexible scope passed through the upper of two ports
If there is perceived need to further decrease the risk of aerosol spread, a tighter seal may be formed around the laryngoscope access port. Clear tape is placed over the access port, through which a narrow slit is created. The laryngoscope is then placed through the taped port, forming a tight barrier around the scope (Figure 8).
FIGURE 8.
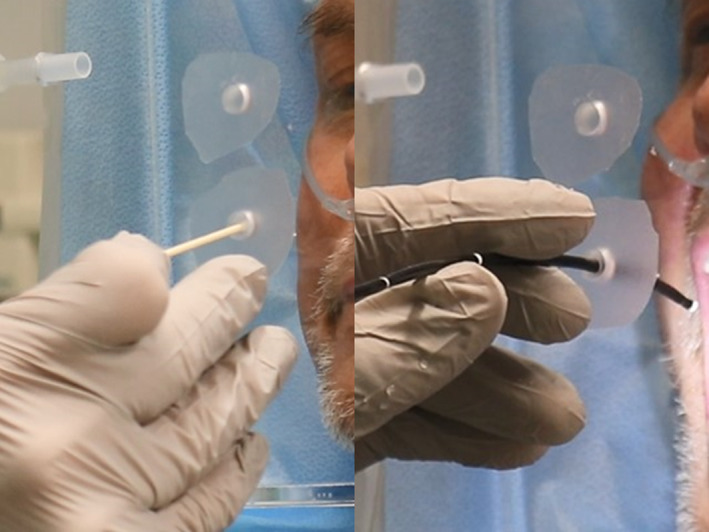
The end of a cotton tipped applicator is used to create a small opening providing a tight fit through which the flexible laryngoscope is placed. Note the procedure may be done with the patient on oxygen as this man required 24 hours/day at 3 L per minute
Early experience with the implementation of the NPFS provided sufficient confidence in the capacity to control aerosol generation that after the first three patients use of N95's and surgical gowns were discontinued (Figure 9).
FIGURE 9.

Successful experience in use of the negative pressure face shield permitted safely performing the flexible transnasal laryngoscopy without addition of gown or N95
Pulse oximetry (Mallinckrodt N‐20E Handheld Pulse Oximeter, Nellcor Puritan Bennett, Inc, Pleasanton, California) was performed on each patient prior to placement of the shield, during the procedure with the shield and drape in place and following completion of the procedure.
Patient response to a 4‐question survey was performed at the conclusion of the procedure. Patients were asked to rate their tolerance of three factors: claustrophobia, shortness of breath, and pain. Responses were obtained on a 5‐point Likert scale, with ratings defined as (1) none, (2) slight, (3) moderate, (4) severe, and (5) intolerable. The fourth question was an open‐ended request for feedback on the experience, which was transcribed into the record and read‐back for patient approval.
All study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at the University of Iowa. 8 , 9 Redcap is a secure, web‐based software platform designed to support data capture for research studies, providing (a) an intuitive interface for validated data capture; (b) audit trails for tracking data manipulation and export procedures; (c) automated export procedures for seamless data downloads to common statistical packages; and (d) procedures for data integration and interoperability with external sources.
All statistical analysis was performed using Prism 8 (GraphPad Software, San Diego, California).
3. RESULTS
3.1. Prototype testing
Measurements at the anterior opening of the NPFS with a single layer of the grade H100 sterilization wrap identified a mean pressure of −0.013 in. of water, obtained from 18 samples at 10 second intervals. This finding was reproduced without change independent of whether the second access port was open or closed with tape.
Pressure measurements with the model in place were obtained at rest, during inspiration, and during expiration. Further measurements were obtained during sequential modifications to the size and shape of the shield as well as methods to occlude the region around the patient's face (combinations of towels, blankets, and positioning). Depending on the cycle of respiration, employing a single barrier wrap and wall suction at 300 mmHg, the pressure varied between −0.002 (expiration) to −0.020 (inspiration) inches of water. Consistently greater vacuum was achieved with a double layer of the barrier wrap and higher suction achievable (530 mmHg) with the Neptune portable suction system.
3.2. Patient evaluation
3.2.1. Duration of procedure
The duration of the procedure was measured from the initial placement of shield and barrier wrap around the patient to its removal and included the nasal spray, a purposeful delay to allow time for the topical application to work, and then performing the laryngoscopy. This duration ranged from 2 minutes and 10 seconds to 6 minutes and 5 seconds. The longest procedure included interaction with our speech pathologist involved to elicit additional vocalization maneuvers with the flexible laryngoscope in place.
3.2.2. Pulse oximetry
Intermittent pulse oximetry monitored the subjects' oxygenation status with frequent inquiries into the comfort of the process. A Friedman test of repeated measures, used due to the nonparametric nature of the data, was performed on the sample of 30 patients to determine if differences in SpO2 were due to the use of the NPFS. Results demonstrated that no statistically significant differences in mean SpO2 through the intervention (Fr = 0.17, P = .92). Specifically, no differences were noted in the SpO2 recorded preprocedure (98 ± 1.3%), intraprocedure (98 ± 1.2%), and postprocedure (98 ± 1.3%) by Dunn's multiple comparisons test (P > .99) (Figure 10). One patient with compromised pulmonary function at baseline was maintained on continuous oxygen treatment (3 L of oxygen per minute, administered by nasal cannula) throughout the process and maintained an SpO2 of 100% through the procedure.
FIGURE 10.

Pre‐, intra‐, and postprocedure pulse oximetry measurements identified saturation SpO2 consistently above 96%. NPFS, negative pressure face shield
3.3. Patient tolerance
Assessment of tolerance on a 5‐point Likert scale was assessed after the procedure. Ratings included (1) none, (2) slight, (3) moderate, (4) severe, and (5) intolerable.
Among the 30 patients, 27 reported no shortness of breath during the procedure, with the remainder only reporting “slight” shortness of breath. Twenty‐nine reported no pain in the process. The one patient reporting “slight” pain during the procedure attributed the discomfort to manipulation of the flexible laryngoscope and not the face shield. Twenty‐seven patients reported no claustrophobia, with the remainder reporting only “slight” claustrophobia. Among the comments from the three patients who reported “slight” shortness of breath was “shortness of breath wasn't a big deal—just a little nervous.”
Evaluation of scores in each category were significantly less than the compared, arbitrarily agreed‐upon, acceptable tolerance score of 2, as determined by the one sample sign test (P < .0001) (Figure 11).
FIGURE 11.
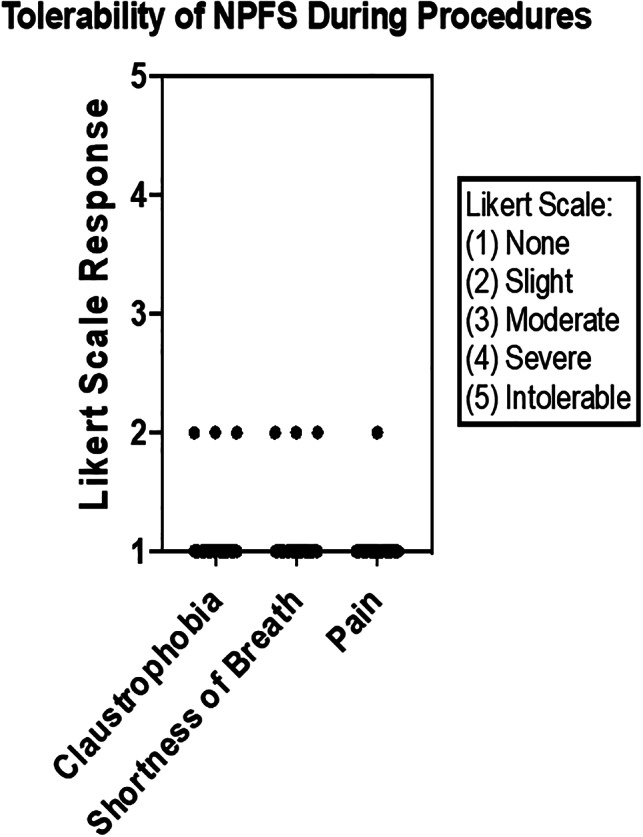
Postprocedure assessment by the patients identified no more than “slight” ratings for claustrophobia, shortness of breath and pain during the procedure. NPFS, negative pressure face shield
Additional qualitative assessments about the process were solicited from the patients following the procedure and included the following.
3.3.1. Selected quotations from patients
“I think this was a very good idea with COVID virus.”
“I thought it was absolutely ridiculous—because this is much ado about nothing a certain amount of people are going to croak anyway society is just a bunch of lemmings.”
“I think this is going to be a very good instrument to use in the future”; “anything you can do to prevent the spread of COVID 19 is very good.”
“Was worried I'd be claustrophobic but with the suction on and a slight breeze through the hood was calming and didn't feel claustrophobic.”
“I was more worried about being claustrophobic before the procedure than during” and “very professional and fast, in and out in a hurry.”
“Everything felt fine; didn't feel anxious at all.”
“The fact the walls of the box is clear makes claustrophobia unlikely.”
3.3.2. Summary of observations (wording provided by examiner and confirmed correct by the patient)
Airflow made it comfortable—better than wearing mask he came in with.
Did not like the spray (taste); caused sneeze, cough with face shield in place and [suction] activated.
Mentioned the scope in nose was irritating but process not painful.
3.4. Surgeon observations
Assembly of instrumentation and preparation of the face shield by assistants before the patient enters the room is facilitated by use of a checklist (see Supporting Information). It is helpful to optimize patient positioning prior to starting the procedure to minimize need for repositioning during the procedure. Positioning is initiated by adjusting the height of the exam chair, the angle of the stand, and the posture of the patient with the face shield in front of the patient before placing the antimicrobial wrap over the head. The full suction as applied to the NPFS is maintained through the end of the procedure to perform a vacuuming maneuver as the assembly is rotated from the patient's face with the blue draping material collapsing into the face shield under suction.
4. DISCUSSION
A high level of patient tolerance was identified in this effort to limit dispersion of bioaerosols created during transnasal laryngoscopy. As a feasibility study, these findings offer support for an approach that—as is true for many infection control strategies—is guided more by basic principles and common sense than evidence‐based medicine. 10
Other barriers to aerosolization have been developed to mitigate dispersion of aerosol during the COVID‐19 era and include the “aerosol box” or intubation box designed to provide protection to anesthesiologists during direct laryngoscopy. 11 , 12 , 13 Canelli et al reported trial of a transparent plastic cube placed over a mannequin's face with “crude simulation of a cough” found to be help contain the spread fluorescein droplets in a manner felt to provide “a modicum of additional protection” as “an adjunct to standard PPE.” 6 Other investigators identified shortcomings to this device including that it “introduces another contaminated device that must be properly handled during use and disinfected between use to prevent cross contamination.” 14 The NPFS addresses this concern by its simple design with smooth surfaces that permit rapid disinfection employing antimicrobial wipes followed by air‐drying in a 3‐minute period before reuse.
Additional concerns about enclosures restricting access to the patient 15 during intubation were addressed by the original investigators who reiterated in their reply that “operators should be ready to abandon use of the box should airway management prove difficult.” 16 The NPFS is deployed in a manner that permits it to be immediately rotated away from the patient. However, the excellent patient tolerance identified in the course of our study makes this process less of a concern.
The limited access to the nose and oral cavity through use of the NPFS was not an issue for diagnostic imaging but is problematic if other manipulations such as nasal packing or cautery are needed. Modifications to the NPFS, including the strength of suction, the positioning of the suction, as well as the size, shape and covering of the aperture are under evaluation to address these issues.
Management options other than placing a barrier around the patient have been proposed to limit viral spread. These options include pretreatment testing of patients for COVID‐19. Greater access to COVID‐19 testing has permitted us to apply this option to patients who are not candidates for use of the NPFS—including those with tracheostomies and those requiring transoral procedures. Current accessibility to testing remains limited and is still unavailable for use prior to routine laryngoscopy at our institution (as of June 5, 2020). Shortcomings to testing all patients include the need for patients to make a separate trip for testing before the laryngoscopy to allow time for processing. Additionally, concerns about reliability of testing have been raised and have identified false negative results to occur.
Other strategies to decrease viral spread include use of topical preparations to reduce the viral load in the upper aerodigestive tract. Parhar et al advocate application of a dilute povidone‐iodine solution to the nasal and oral cavities prior to instrumentation of the upper airway as safe and potentially helpful in decreasing the viral load. 17
Studies addressing influenza have identified that both infectivity and the severity of an infection are dependent on the viral load to which a patient is exposed. 18 , 19 While this dose‐dependence has not been specifically identified for SARS‐CoV‐2, eliminating or minimizing the inoculum of exposure is a reasonable goal for any aerosolized virus that has the potential for infection. Although there is currently no single strategy available to completely eradicate the risk of coronavirus transmission, methods to decrease dissemination such as the NPFS are likely to be beneficial.
As identified by Rameau et al “Flexible laryngoscopy in the age of COVID‐19 requires adaptation.” 1 The NPFS provides an adaptation designed to decrease dissemination of virus and has been successfully used clinically to examine the larynx. It is under active study for use in transnasal surgical procedures including laser treatments and biopsies.
Shortcomings to this study of the NPFS include the lack of a comparison of its efficacy in diminishing aerosol distribution when compared to other methods. Sophisticated techniques to identify bioaerosol dissemination are available and have been used by a member of the research team to assess generation of bioaerosols with toilet flushing in the hospital setting. 20 Evaluation is planned to employ these techniques to quantify and compare the degree to which the bioaerosols dispersion is mitigated with the NPFS.
5. CONCLUSION
A new device is presented with accompanying methods and materials designed to limit exposure to aerosols and to enhance the safety of transnasal AGPs. We have incorporated the NPFS into routine clinical practice due to its ease of use, rapid disinfection, and high level of patient tolerance. The NPFS has the potential to extend its application beyond diagnostics to include procedures at even higher risk for generating contagious aerosols, including transnasal laser and biopsy procedures.
CONFLICT OF INTEREST
Henry T. Hoffman: (a) COOK Medical: Research consultant and patent. (b) UpToDate: author. (c) IoataMotion: Research consultant with patent application. Coauthors Miller, Walsh, Stegall, and Diekema have no conflict of interest.
Supporting information
Data S1 Checklist for transnasal laryngoscopy with NPFS.
Hoffman HT, Miller RM, Walsh JE, Stegall HR, Diekema DJ. Negative pressure face shield for flexible laryngoscopy in the COVID‐19 era. Laryngoscope Investigative Otolaryngology. 2020;5:718–726. 10.1002/lio2.437
Funding information Institute for Clinical and Translational Science (NIH and CTSA), Grant/Award Number: UL1TR002537; University of Iowa Department of Otolaryngology
BIBLIOGRAPHY
- 1. Rameau A, Young VN, Amin MR, Sulica L. Flexible Laryngoscopy and COVID‐19. Otolaryngol Head Neck Surg. 2020;162(6):813‐815. 10.1177/0194599820921395. [DOI] [PubMed] [Google Scholar]
- 2.AAO‐HNS COVID‐19 RESOURCES Guidance for return to practice for Otolaryngology‐Head and Neck Surgery. https://www.entnet.org/content/guidance-return-practice-otolaryngology-head-and-neck-surgery. Accessed June 21, 2020.
- 3. Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in False‐Negative Rate of Reverse Transcriptase Polymerase Chain Reaction‐Based SARS‐CoV‐2 Tests by Time Since Exposure. Ann Intern Med. 2020;M20‐1495 10.7326/M20-1495. [Online ahead of print, 2020 May 13]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffman HT, ed. Iowa Head and Neck Protocols “Trans‐nasal laryngoscopy in COVID‐19 era—method for additional protection with disposable mask”. https://medicine.uiowa.edu/iowaprotocols/transnasal-laryngoscopy-covid-19-era-method-additional-protection-disposable-mask. Accessed May 3, 2020.
- 5. Narwani V, Kohli N, Lerner MZ. Application of a Modified Endoscopy Face Mask for Flexible Laryngoscopy During the COVID‐19 Pandemic. Otolaryngol Head Neck Surg. 2020;163(1):107‐109. 10.1177/0194599820928977. [DOI] [PubMed] [Google Scholar]
- 6. Canelli R, Connor CW, Gonzalez M, Nozari A, Ortega R. Barrier enclosure during endotracheal intubation. N Engl J Med. 2020;382:1957‐1958. 10.1056/NEJMc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.510(k) Summary for the Kimberly‐Clark Corporation Kimguard Sterilization Wrap. https://www.accessdata.fda.gov/cdrh_docs/pdf8/K082554.pdf. Accessed February 5, 2020.
- 8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software partners. J Biomed Inform. 2019;95:103208 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Masse V, Edmond MB, Diekema DJ. Infection prevention strategies for procedures performed outside operating rooms: a conceptual integrated model. Am J Infect Control. 2018;46:94‐96. [DOI] [PubMed] [Google Scholar]
- 11. Workman AD, Jafari A, Welling DB, et al. Airborne Aerosol Generation During Endonasal Procedures in the Era of COVID‐19: Risks and Recommendations. Otolaryngol Head Neck Surg. 2020;194599820931805 10.1177/0194599820931805. [Online ahead of print, 2020 May 26]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Workman AD, Welling DB, Carter BS, et al. Endonasal instrumentation and aerosolization risk in the era of COVID‐19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. 2020;10(7):798‐805. 10.1002/alr.22577. [DOI] [PubMed] [Google Scholar]
- 13. Malik JS, Jenner C, Ward PA. Application of the aerosol box in protecting healthcare workers during the COVID‐19 pandemic. Anaesthesia. 2020;75:974‐975. 10.1111/anae.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenblatt WH, Sherman JD. More on barrier enclosure during endotracheal intubation. N Engl J Med. 2020;382(21):e69. [DOI] [PubMed] [Google Scholar]
- 15. Kovatsis PG, Matava CT, Peyton JM. More on barrier enclosure during endotracheal intubation. N Engl J Med 2020;382(21):e69. 10.1056/NEJMc2012960. [DOI] [PubMed]
- 16. Ortega R, Nozari A, Canelli R. More on barrier enclosure during endotracheal intubation. Reply. N Engl J Med. 2020;382(21):e69 10.1056/NEJMc2012960. [DOI] [PubMed] [Google Scholar]
- 17. Parhar HS, Tasche K, Brody RM, et al. Topical preparations to reduce SARS‐CoV‐2 aerosolization in head and neck mucosal surgery. Authorea. 2020;42(6):1268‐1272. 10.22541/au.158636956.63067038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han A, Czajkowski LM, Donaldson A, et al. A dose‐finding study of a wild‐type influenza a(H3N2) virus in a healthy volunteer human challenge model. Clin Infect Dis. 2019;69(12):2082‐2090. 10.1093/cid/ciz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paulo AC, Correia‐Neves M, Domingos T, Murta AG, Pedrosa J. Influenza infectious dose may explain the high mortality of the second and third wave of 1918‐1919 influenza pandemic. PLoS One. 2010;5(7):e11655 10.1371/journal.pone.0011655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knowlton SD, Boles CL, Perencevich EN, Diekema DJ, Nonnenmann MW, CDC Epicenters Program . Bioaerosol concentrations generated from toilet flushing in a hospital‐based patient care setting. Antimicrob Resist Infect Control. 2018;7:16 10.1186/s13756-018-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Checklist for transnasal laryngoscopy with NPFS.


