Abstract
Introduction
Hyperprolactinaemia, a common adverse effect of antipsychotic drugs, is primarily linked to blockade of dopamine D2 receptors in the pituitary gland. Certain antipsychotic drugs, such as, for example risperidone and paliperidone, are more likely to induce hyperprolactinaemia compared to others. This effect is probably caused by a relatively high blood/brain concentration ratio, a consequence of being a substrate of P‐glycoprotein. Genetic variants of P‐glycoprotein with changed functional activity might influence the potential of risperidone and paliperidone to cause hyperprolactinaemia as the altered blood/brain concentration ratio would lead to a reduced therapeutic drug level within essential brain areas making dose adaptations necessary. This increases exposure of dopamine D2 receptors within the pituitary gland.
Aims
To investigate possible associations between MDR1/ABCB1 gene polymorphisms and antipsychotic drug‐induced hyperprolactinaemia in Russian patients with schizophrenia and to determine possible differences between risperidone/paliperidone and other antipsychotics.
Methods
In total, 446 patients with schizophrenia were included from 3 psychiatric hospitals in Siberia. Blood samples were obtained in a cross‐sectional study design for DNA extraction and prolactin measurement. Associations between hyperprolactinaemia and 8 MDR1/ABCB1 gene‐polymorphisms were assessed using logistic regression analysis accounting for covariates. The analysis was repeated in a patient subgroup using risperidone or paliperidone.
Results
We did not observe an association between any of the 8 single nucleotide polymorphisms and the prevalence of antipsychotic‐induced hyperprolactinaemia in the total patient population. However, in the risperidone/paliperidone subgroup, the single nucleotide polymorphism rs2032582 (G2677T) was found to be negatively associated with risperidone/paliperidone‐induced hyperprolactinaemia.
Conclusion
This study revealed a significant association between the ABCB1 gene polymorphism rs2032582 (G2677T) and risperidone/paliperidone‐induced hyperprolactinaemia.
Keywords: antipsychotics, hyperprolactinaemia, MDR1/ABCB1 gene, P‐glycoprotein, polymorphisms
What is already known about this subject
Hyperprolactinaemia is attributed to blockade of dopamine D2 receptors on the membranes of lactotroph cells within the pituitary gland
Certain antipsychotic drugs, e.g. risperidone and paliperidone, are more likely to induce hyperprolactinaemia compared to others.
The strong prolactin‐elevating effect of risperidone is probably caused by a relatively high blood/brain concentration ratio, a consequence of being a substrate of P‐glycoprotein.
What this study adds
Genetic variations in the MDR1/ABCB1 gene, such as rs2032582 (G2677T), might influence the occurrence of risperidone/paliperidone induced hyperprolactinaemia
1. INTRODUCTION
Prolactin is a polypeptide hormone that is excreted into the blood by lactotroph cells of the anterior pituitary gland. Increased excretion of this hormone causes hyperprolactinaemia (HPRL), a common side effect of both classical and atypical antipsychotic drugs. HPRL is characterized by both acute and chronic consequences such as sexual impairment, hypogonadism, galactorrhoea, amenorrhoea, reproductive dysfunctions, weight gain, osteoporosis, cardiovascular disease and depression. 1 , 2 , 3 , 4 , 5
Even though almost all antipsychotic drugs can cause HPRL, the highest rates of HPRL are commonly associated with the use of amisulpride, risperidone or paliperidone. 6 The occurrence of antipsychotic drug‐induced HPRL is attributed to blockade of the dopamine D2‐receptors on the membranes of lactotroph cells within the pituitary gland. A higher occupancy rate of the D2‐receptors seems to give a greater chance of developing HPRL. The threshold of D2‐receptor occupancy for the development of HPRL might differ among antipsychotic drugs, but a minimum of 72% D2‐receptor occupancy seems necessary. 6 , 7 , 8
Since pituitary D2‐receptor occupation seems important for developing HPRL, the penetration potency of an antipsychotic drug across the blood–brain barrier (BBB) might influence its occurrence. 6 The pituitary gland is anatomically not protected by the BBB. 9 The less an antipsychotic drug is capable to penetrate the BBB, the higher the concentration within the pituitary gland will be in order to reach a therapeutic drug level within essential brain areas. A higher drug concentration at the pituitary might result in a higher amount of D2‐receptor occupation and thus in an increased risk of HPRL. 6 , 10 The relatively high blood/brain concentration ratio of risperidone, partly caused by its high affinity for the P‐glycoprotein (P‐gp) efflux transporter 3 , 11 for example, may well be the origin of its common prolactin‐elevating effect.
The P‐gp efflux transporter, encoded by the ATP binding cassette subfamily B member 1 (ABCB1) gene also known as multidrug resistance gene 1 (MDR1), is a transmembrane protein expressed at the BBB. It transports penetrated chemicals back from the brain into the bloodstream. 12 , 13 Several antipsychotic drugs are known to have more or less affinity for the P‐gp transporter. Quetiapine and risperidone for example are relatively good substrates of P‐gp. Clozapine and haloperidol on the other hand seem to have less affinity for the P‐gp transporter. 14
Genetic variations in the ABCB1 gene may cause differences in the expression level and/or the functionality of the P‐gp transporter. 15 , 16 More than 50 single nucleotide polymorphisms (SNPs) in the ABCB1 gene are currently known, of which at least 28 are at positions that can possibly influence the efficacy of the P‐gp transporter. 17 , 18
P‐gp transporter proteins with alternative functional activity by genetic variations might influence the potential of antipsychotic drugs to cause HPRL. When the functionality of the P‐pg transporter is reduced, the blood/brain ratio of substrate antipsychotic drugs will also be reduced. This results in a potentially higher penetration of antipsychotics into the brain at a normal dosage level, which increases the vulnerability for central nervous system side effects such as parkinsonism. Such central nervous system side effects can be expected to result in dosage adaptations, causing a lower drug concentration within the pituitary gland. This leads to a decrease in exposure and occupancy of the lactotroph dopamine D2 receptor by this antipsychotic drug. As a result, the chance of developing antipsychotic‐induced HPRL will also decrease.
To investigate the above theory, we studied the influence of 8 polymorphisms in the ABCB1 gene on the prevalence of antipsychotic‐induced HPRL in patients with schizophrenia and tried to determine possible differences between risperidone/paliperidone on the one hand and other antipsychotics on the other.
2. METHODS
2.1. Patients
For this study, 446 patients were included from psychiatric hospitals in Kemerovo, Tomsk and Chita oblasts in Siberia, Russian Federation. The study group and used methods are previously described by Ivanova et al. 19 , 20 Patients were included if they were diagnosed with schizophrenia according to ICD‐10 (F20), were using antipsychotic drugs for a sufficiently long period and were between 18 and 75 years old. Exclusion criteria were pregnancy, non‐Caucasian ethnicity, self‐reported or by observed physical appearance, organic brain disorders (Parkinson's disease, epilepsy), relevant thyroid or gynaecological disorders, or pharmacological withdrawal symptoms. Patients were treated for their schizophrenic disorder with conventional and/or atypical antipsychotic drugs in both oral and long‐acting formulations. Haloperidol was the most commonly used conventional antipsychotic drug. Other conventional antipsychotics included oral chlorpromazine, chlorprothixene, trifluoperazin and zuclopentixol, and long‐acting formulations of haloperidol, zuclopentixol and flupentixol. Atypical antipsychotics were used by 176 patients and included paliperidone, risperidone, amisulpride, clozapine, olanzapine, sertindole and quetiapine. A total of 79 patients used various combinations of classical and atypical drugs. All dosages are recalculated into chlorpromazine equivalents (CPZeq) to compare the antipsychotic exposure. 21
The study procedures were accepted by the Local Bioethics Committee of the Mental Health Research Institute (Tomsk). The study was recorded under protocol number N63/7.2014. Patients were included after providing written informed consent. All work was performed in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki 1975, revised in Fortaleza, Brazil, 2013) for experiments involving humans.
2.2. Sampling
Blood samples for DNA extraction and prolactin measurement were obtained after 8 hours of overnight fasting before intake of any food or medication in a cross‐sectional study design. For premenopausal women, samples were preferably collected in the follicular phase of the menstrual period. EDTA tubes were used for collecting the blood samples for DNA extraction. The tubes were stored at –20°C until DNA isolation. Tubes with CAT (clot activator) were used to collect serum for measuring prolactin concentrations (BD Vacutainer). The samples were centrifuged for 30 minutes at 200x g at 4°C to obtain serum.
2.3. Biochemical analysis
2.3.1. Prolactin concentration
The prolactin concentration was determined using an accuBind enzyme‐linked immunosorbent assay Microwells kit (Monobind Inc., USA). The sensitivity of the kit was 0.004 ng/well, which is equal to a prolactin concentration of 0.150 ng/mL. The upper limit for a normal prolactin concentration was fixed at ≤25 ng/mL for nonpregnant, non‐nursing women and at ≤20 ng/mL for men. These criteria for HPRL were confirmed in literature. 6 , 22
2.3.2. DNA analysis
For this study, 8 SNPs in the ABCB1 gene were selected based on a literature search for relevant polymorphic variants of the gene in patients with schizophrenia: rs1045642 (C3435T), rs2032582 (G2677T), rs4148739, rs28401781, rs2235040, rs9282564, rs2235015, and rs2032583. 15 , 23 , 24 , 25 , 26 , 27 , 28 , 29 SNPs were selected based on the relevance of the selected SNPs to associations with schizophrenia, drug‐induced side effects or treatment response. The minor allele frequency of selected SNPs should have been 0.05 (5%) or greater.
A standard phenol–chloroform micro method (as described by Ivanova et al.) 30 was used to isolate DNA from the leucocytes in the whole peripheral blood samples. Genotyping was performed with a MassARRAY system 4 (Agena Bioscience) in the Genome Analysis Facility, Department Genetics of the University Medical Center Groningen.
2.4. Statistics
Associations between the presence of HPRL in patients and the variant of the ABCB1 gene were assessed using logistic regression analysis accounting for covariates (sex, age, duration of the disease, smoking, leading symptoms [positive/negative] and drug dosage [CPZeq]). The analyses were carried out both in the total sample of patients and in a subgroup characterized by the use of risperidone/paliperidone, based on the strong affinity of risperidone and its major metabolite for the P‐gp transporter and their common prolactin‐elevating effect (n = 76). Sulpiride and amisulpride are also consistently reported to cause HPRL however, their sample size was insufficient for a proper subanalysis (n = 13). ABCB1 haplotypes were not analysed since the statistical power of such an analysis is limited caused by the low incidence rate of the recessive genotype/allele which we observed.
The statistical program R with basic R functions and the SNPassoc package v.3.1.3 31 was used for all statistical analyses. P‐values <.05 were considered significant. Bonferroni correction was applied assuming 5 independent tests. The number of independent tests was estimated based on the correlation between the 8 SNPs.
The deviation from Hardy–Weinberg equilibrium was tested with χ2 test.
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.
3. RESULTS
3.1. Patients
A total of 446 patients from Siberia (225 female/221 male) participated in this study. 19 The general characteristics of the population are presented in Table 1. The median dosage of antipsychotic drugs was 425 CPZeq daily and the mean age of the patients was 41.5 years (SD ± 13.4). The mean duration of schizophrenia was 15 years. Of these, 191 patients were treated with conventional antipsychotics (42.8%), 176 with atypical antipsychotics (39.5%), and 79 with a combination of conventional and atypical antipsychotics (17.7%). Based on the established lower limit for the presence of HPRL at >25 ng/mL for females and >20 ng/mL for males, 227 of the 446 patients were classified as HPRL positive. 6 , 22 The mean prolactin concentration in the total population was 34.7 ng/mL. The mean prolactin concentration in the HPLR positive and negative group was 55.9 and 12.6 ng/mL, respectively. Female patients had a higher mean prolactin concentration compared to male patients.
TABLE 1.
Patient characteristics of the total population
| Trait | Total sample | Men | Women |
|---|---|---|---|
| n | 446 | 221 (49.6%) | 225 (50.4%) |
| Age (y) | |||
| Mean ± SD | 41.5 ± 13.4 | 37.8 ± 11.9 | 45.2 ± 13.9 |
| Median | 40 | 36 | 44 |
| Daily dose in CPZeq | |||
| Median | 425 | 500 | 372 |
| Duration of disease (y) | |||
| Mean ± SD | 15.4 ± 11.5 | 13.1 ± 10.0 | 17.6 ± 12.5 |
| Median | 13 | 11 | 15 |
| Type of therapy | |||
| Conventional antipsychotics | 191 (42.8%) | 91 (41.2%) | 100 (44.4%) |
| Atypical antipsychotics | 176 (39.5%) | 78 (35.3%) | 98 (43.6%) |
| Combination therapy | 79 (17.7%) | 52 (23.5%) | 27 (12.0%) |
| Smoking (+/−/?) | 259 (58.1%) /179 (40.1%) /8 (1.8%) | 173 (78.3%) /46 (20.8%) /2 (0.9%) | 86 (38.2%) /133 (59.1%) /6 (2.7%) |
| Hyperprolactinaemia (+/−) | 227 (50.9%) /219 (49.1%) | 98 (44.3%) /123 (55.7%) | 129 (56.9%) /96 (42.7%) |
| Prolactin (ng/mL) total | |||
| Mean ± SD | 34.7 ± 29.5 | 25.7 ± 22.5 | 43.5 ± 32.8 |
| Range | 1.5–140.6 | 1.5–122.1 | 2.1–140.6 |
| Prolactin (ng/mL; HPRL+) | |||
| Mean ± SD | 55.9 ± 27.5 | 43.5 ± 23.2 | 65.3 ± 26.9 |
| Range | 20.2–140.6 | 20.2–122.1 | 25.1–140.6 |
| Prolactin (ng/mL; HPLR–) | |||
| Mean ± SD | 12.6 ± 5.8 | 11.5 ± 5.0 | 14.1 ± 6.4 |
| Range | 1.5–24.9 | 1.5–20.0 | 2.1–24.9 |
CPZeq, chlorpromazine equivalents; HPRL, hyperprolactinaemia.
3.2. Patients using risperidone/paliperidone
Seventy‐six patients (45 female/31 male) were included in the risperidone/paliperidone subgroup. The baseline characteristics of this subgroup are presented in Table 2. The current median dosage of antipsychotic drugs was 300 CPZeq daily. The mean age of the patients was 38.1 years and patients suffered in general about 12.5 years of schizophrenia. Sixty‐six patients were treated with risperidone or paliperidone only (86.8%) and 10 with a combination of these with conventional antipsychotics (13.2%). Based on the established lower limit for the presence of HPRL at >25 ng/mL for females and >20 ng/mL for males, 59 of the patients were classified as HPRL positive. 6 , 22 The mean prolactin concentration in the risperidone/paliperidone population was 53.0 ng/mL. The mean prolactin concentration in the HPLR positive and negative group were 64.9 and 11.9 ng/mL, respectively. Female patients had a higher mean prolactin concentration in this group compared to male patients.
TABLE 2.
Patient characteristics of the patients in the risperidone/paliperidone subgroup
| Trait | Total sample | Men | Women |
|---|---|---|---|
| n | 76 | 31 (40.8%) | 45 (59.2%) |
| Age (y) | |||
| Mean ± SD | 38.1 ± 14.1 | 33.1 ± 11.5 | 41.6 ± 14.8 |
| Median | 34 | 31 | 40 |
| Daily dose in CPZeq | |||
| Median | 300 | 396 | 200 |
| Duration of disease (y) | |||
| Mean ± SD | 12.5 ± 12.3 | 9.6 ± 10.0 | 14.5 ± 13.4 |
| Median | 10.0 | 6.5 | 11.0 |
| Type of therapy | |||
| Atypical antipsychotics | 66 (86.8%) | 25 (80.6%) | 41 (91.1%) |
| Combination therapy | 10 (13.2%) | 6 (19.4%) | 4 (8.9%) |
| Smoking (+/−/?) | 31 (40.8%) /43 (56.6%) /2 (2.6%) | 21 (67.7%) /10 (32.3%) /0 | 10 (22.2%) /33 (73.3%) /2 (4.5%) |
| Hyperprolactinaemia (+/−) | 59 (77.6%)/17(22.4%) | 20 (64.5%)/11 (35.5%) | 39 (86.6%) /6 (13.3%) |
| Prolactin (ng/mL) total | |||
| Mean ± SD | 53.0 ± 34.4 | 35.8 ± 29.3 | 64.9 ± 32.9 |
| Range | 4.6–124.0 | 4.6–122.1 | 6.4–124.0 |
| Prolactin (ng/mL; HPRL+) | |||
| Mean ± SD | 64.9 ± 29.7 | 49.2 ± 28.5 | 72.9 ± 27.4 |
| Range | 20.2–124.0 | 20.2–122.1 | 28.1–124.0 |
| Prolactin (ng/mL; HPRL–) | |||
| Mean ± SD | 11.9 ± 4.8 | 11.6 ± 5.6 | 12.5 ± 3.5 |
| Range | 4.6–19.1 | 4.6–19.1 | 6.3–16.5 |
CPZeq, chlorpromazine equivalents; HPRL, hyperprolactinaemia.
3.3. Logistic regression model
The association between the SNPs and the occurrence of HPRL was assessed using logistic regression with adjustment for covariates (sex, age, smoking, duration of the disease, leading symptoms [positive/negative] and drug dosage [CPZeq]). The genotypes prevalence for all SNPs was in consistency with Hardy–Weinberg equilibrium. Sex and age had a significant relation with the occurrence of HPRL (P = .032 and P = .031 respectively).
A nominally significant association between 2 SNPs in the P‐gp gene (rs1045642 [C3435T] and rs4148739) and HPRL was established (Table 3). However, after Bonferroni correction for multiple testing, both associations were no longer significant (Figure 1).
TABLE 3.
Result logistic regression analysis between polymorphisms in the P‐glycoprotein transporter gene and hyperprolactinaemia in patients with schizophrenia
| SNP | OR | 95% CI | Raw P‐value |
|---|---|---|---|
| rs1045642 | 1.365 | 1.011–1.842 | .048 |
| rs2032582 | 0.813 | 0.606–1.091 | .173 |
| rs4148739 | 1.581 | 0.953–2.621 | .034 |
| rs28401781 | 1.517 | 0.931–2.472 | .084 |
| rs2235040 | 1.448 | 0.878–2.386 | .128 |
| rs9282564 | 0.850 | 0.550–1.314 | .554 |
| rs2235015 | 1.388 | 0.916–2.103 | .147 |
| rs2032583 | 1.603 | 0.980–2.622 | .052 |
CI, confidence interval; OR, odds ratio; SNP, single nucleotide polymorphism.
FIGURE 1.
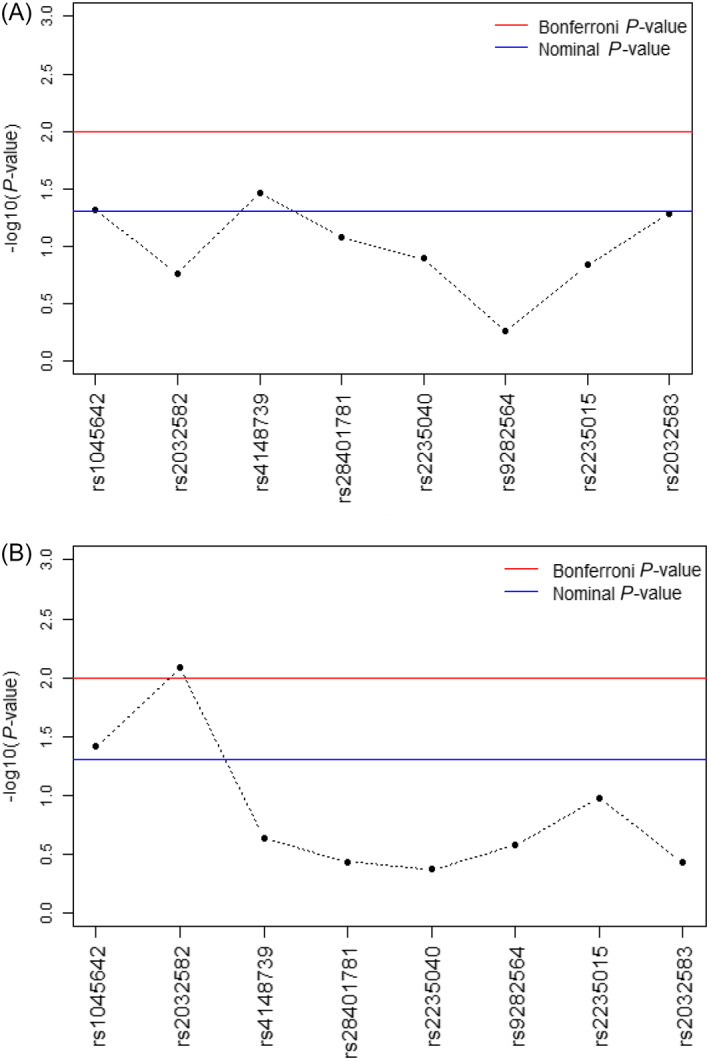
A, The –log10 P‐values of the association between polymorphisms in the P‐ glycoprotein receptor gene and hyperprolactinaemia in patients with schizophrenia. B, The –log10 P‐values of the association between polymorphisms in the P‐glycoprotein receptor gene and hyperprolactinaemia in patients with schizophrenia using risperidone or paliperidone
In the risperidone/paliperidone subgroup, the results of the logistic regression analysis showed also a significant association between 2 SNPs and the occurrence of HPRL (rs1045642 [C3435T] and rs2032582 [G2677T]; Table 4). After correction for multiple testing, the SNP rs2032582 (G2677T) was still significantly associated with the occurrence of HPRL (Figure 1).
TABLE 4.
Result logistic regression analysis between polymorphisms in the P‐glycoprotein transporter gene and hyperprolactinaemia in patients with schizophrenia using risperidone or paliperidone
| SNP | OR | 95% CI | Raw P‐value |
|---|---|---|---|
| rs1045642 | 3.103 | 0.729–13.21 | .039 |
| rs2032582 | 0.165 | 0.035–0.790 | .008 |
| rs4148739 | 3.086 | 0.297–32.05 | .231 |
| rs28401781 | 2.567 | 0.255–25.89 | .369 |
| rs2235040 | 2.148 | 0.209–22.12 | .428 |
| rs9282564 | 0.270 | 0.052–1.407 | .267 |
| rs2235015 | 4.406 | 0.719–26.99 | .105 |
| rs2032583 | 2.567 | 0.255–25.89 | .369 |
CI, confidence interval; OR, odds ratio; SNP, single nucleotide polymorphism.
4. DISCUSSION
In this study, the association between 8 polymorphisms in the ABCB1 gene and antipsychotic drug‐induced HPRL was investigated in Russian patients with schizophrenia from Siberia. No association was found between any SNP and the prevalence of antipsychotic drug‐induced HPRL in the total group of patients with schizophrenia. However, the rs2032582 (G2677T) variant was found to be negatively associated with risperidone/paliperidone‐induced HPRL.
In this study, the prolactin level and occurrence of HPRL was determined in a large number of patients. Normal serum concentrations of prolactin can be affected by several factors such as sex, circadian variations, the menstrual cycle and menopause. Also, heavy meals, exercise, sexual intercourse, pregnancy, breastfeeding and epileptic seizures are known to increase the serum prolactin level. 6 , 23
In our study, patients with various physical and/or psychological disorders that can affect the serum prolactin level were excluded. We also corrected for sex differences by the use of different lower limits for HPRL for men and women. Also, all samples were obtained after 8 hours of overnight fasting, before intake of any food or medication, to reduce the influence of meals, exercise and circadian influences on the prolactin level.
The time between drug intake and sampling was pretty much random. The overnight fasting period, however, ensures that all samples were taken at least 8 hours after drug intake. The within‐day plasma level fluctuations of pituitary dopamine D2 occupancy are also assumed to be small when antipsychotics are taken chronically in a stable dosage. Lack of relationship between drug intake and prolactin levels was e.g. confirmed in schizophrenic patients using risperidone for at least 4 weeks by Yasui‐Furukori et al. 32
Despite the large number of patients and the accurate sampling procedure, our study has several limitations. For example, no distinction was made between pre‐ and postmenopausal woman and the phase of the menstrual cycle was not taken in account. For premenopausal women, samples were preferably collected in the follicular phase of the menstrual period. However, many women with schizophrenia do not have spontaneous periods caused by amenorrhoea, often a consequence of either psychosis and/or HPRL. Also, a fixed lower limit was used for the occurrence of HPRL. This lower limit was, however, accurately determined according to literature data. Also varying the lower limit during the statistical analysis did not to affect the outcome.
Furthermore, we did not measure serum concentrations of the antipsychotic drugs, so noncompliance could not be discovered. Patients were, however, titrated with the antipsychotic drug to optimal effect.
There is also no reason to assume that other factors that might influence the plasma level, such as noncompliance or CYP enzyme activity differences, are not randomly distributed between the different genotypes we studied, since the number of patients included in the study is sufficiently high.
In this study, we focused on the influence of 8 SNPs on the functionality and effectiveness of the P‐gp transporter present in the BBB. P‐gp transporters are, however, also present in various organs with a barrier or excretory function including the small intestine, lungs, liver and kidneys. 33 , 34 Therefore HPRL is not strictly related to the influence of a polymorphism of the ABCB1 gene on the functioning of the BBB as the blood/brain ratio is also affected by the functionality of P‐gp in various organs with a barrier or excretory function which might influence the antipsychotic blood concentration. Several studies attempted to find a relation between SNPs in the ABCB1 gene and pharmacokinetics of antipsychotic drugs but so far the results are inconsistent and sometimes conflicting. 35 , 36 , 37 , 38
The patients included in our study were using different types of antipsychotics. Since there are major differences between antipsychotics according to their affinity for the P‐gp transporter, the association between P‐gp gene polymorphisms and the occurrence of HPRL might also differ between antipsychotic drugs. To correct for this difference, an additive genetic model was tested, and the analysis was carried out both in the total sample of patients and in a subgroup characterized by the usage of risperidone/paliperidone (n = 76). In the risperidone/paliperidone subgroup, a negative association between the rs2032582 (G2677T) variant with risperidone/paliperidone‐induced HPRL was observed. In the total population, the association was also significant but did not survive correction for multiple testing.
The SNP rs2032582 (c.2677 T/A/G) polymorphism of the P‐gp gene is a nonsynonymous missense mutation in exon 21 that results in the substitution of alanine to threonine or serine. The c.2677 T/G polymorphism (p.Ala893Ser) is the most common SNP in human, while the 2677G/A (Ala893Thr) polymorphism is less often observed. 12 In our research, we analysed the (2677 T/G) variant.
The mechanism of the influence of the rs2032582 (G2677T) polymorphism on the concentration and function of the P‐pg transporter protein is unclear. Several studies reported no difference in P‐gp expression between carriers of various rs2032582 genotypes, 39 , 40 , 41 but a few report a significant reduction of the P‐gp gene expression and/or protein function in carriers of the variant allele. 42 , 43 The results of our study suggest that the rs2032582 polymorphism might alter the function of the P‐gp transporter within the BBB. However, it is also possible that the polymorphism is linked to another SNP that actually influences the efficacy of the P‐gp transporter.
There was no association found between the other tested polymorphisms of the P‐gp gene and the prevalence of antipsychotic‐induced HPRL in both the entire study population as in the risperidone/paliperidone subgroup. Unfortunately, from almost all SNPs in the P‐gp gene, it is unclear to what extent they actually influence the functionality and effectiveness of the P‐gp transporter. Also, most of the examined SNPs are located at intron regions and therefore might not directly influence the functionality of the P‐gp transporter.
In conclusion, no association was found between any of the 8 SNPs and antipsychotic drug‐induced HPRL. However, the rs2032582 (G2677T) variant appeared to be protective against HPRL in the subgroup of patients using risperidone or paliperidone. Further research to the influence of polymorphisms in the P‐gp gene on the functionality and efficacy of the P‐gp transporter and the related influence on the plasma levels of several antipsychotic drugs is eligible.
ACKNOWLEDGEMENT
The Russian Foundation for Basic Research partly funded this work, grant # 17‐29‐06035.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
L.M. Geers (LMG) and I.V. Pozhidaev (IVP) are shared first authors of this paper. LMG/IVP, D.J. Touw (DJT), A.J.M. Loonen (AJML) and S.A. Ivanova (SAI) instigated and designed the study. AJML, DJT and SAI coordinated and supervised the study. M.B. Freidin (MBF), designed and performed the statistical analysis and contributed to writing the paper. LMG and A.F. Schmidt (AFS) verified these statistical results. SAI wrote the study protocol and selected the SNPs. O.Yu. Fedorenko (OYF) and A.S. Boiko (ASB) monitored the study. A.V. Semke (AVS) collected clinical data. IVP and D.Z. Osmanova (DZO) isolated DNA, genotyped the samples and recorded all data in an Excel database. ASB measured prolactin levels. D. Cohen (DC) and N.A. Bokhan (NAB) supervised the clinical work. SAI, AJML, DJT, J.G.W. Kosterink (JGWK) and B. Wilffert (BW) supervised the technical work. LMG wrote the first draft of the manuscript and IVP thereafter adapted it. DJT, AJML, and SAI supervised the writing. All authors read the paper, when necessary commented on it and agree with its content. Their role justifies their (co)‐authorship to this paper.
Geers LM, Pozhidaev IV, Ivanova SA, et al. Association between 8 P‐glycoprotein (MDR1/ABCB1) gene polymorphisms and antipsychotic drug‐induced hyperprolactinaemia. Br J Clin Pharmacol. 2020;86:1827–1835. 10.1111/bcp.14288
Previous presentation: poster presented at the 30th ECNP congress, Paris 2–5 September 2017. The corresponding abstract is published in European Neuropsychopharmacology.
The authors confirm that there was no Principal Investigator for this paper. The chief physicians of the participating psychiatric hospitals had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Professor Svetlana A. Ivanova (ivanovaniipz@gmail.com) upon reasonable request and with permission of MHRI.
REFERENCES
- 1. Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin Endocrinol (Oxf). 2011;74(2):141‐147. [DOI] [PubMed] [Google Scholar]
- 2. Serri O, Chik CL, Ur E, Ezzat S. Diagnosis and management of hyperprolactinemia. CMAJ. 2003;169(6):575‐581. [PMC free article] [PubMed] [Google Scholar]
- 3. Ejsing TB, Pedersen AD, Linnet K. P‐glycoprotein interaction with risperidone and 9‐OH‐risperidone studied in vitro, in knock‐out mice and in drug‐drug interaction experiments. Hum Psychopharmacol. 2005;20(7):493‐500. [DOI] [PubMed] [Google Scholar]
- 4. Haddad PM, Wieck A. Antipsychotic‐induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291‐2314. [DOI] [PubMed] [Google Scholar]
- 5. Moons T, de Roo M, Claes S, Dom G. Relationship between P‐glycoprotein and second‐generation antipsychotics. Pharmacogenomics. 2011;12(8):1193‐1211. [DOI] [PubMed] [Google Scholar]
- 6. Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double‐blind PET study of first‐episode schizophrenia. Am J Psychiatry. 2000;157(4):514‐520. [DOI] [PubMed] [Google Scholar]
- 8. Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: a new hypothesis. Am J Psychiatry. 2001;158(3):360‐369. [DOI] [PubMed] [Google Scholar]
- 9. Bushe CJ, Bradley A, Pendlebury J. A review of hyperprolactinaemia and severe mental illness: are there implications for clinical biochemistry? Ann Clin Biochem. 2010;47(Pt 4):292‐300. [DOI] [PubMed] [Google Scholar]
- 10. Kapur S, Langlois X, Vinken P, Megens AA, De Coster R, Andrews JS. The differential effects of atypical antipsychotics on prolactin elevation are explained by their differential blood‐brain disposition: a pharmacological analysis in rats. J Pharmacol Exp Ther. 2002;302(3):1129‐1134. [DOI] [PubMed] [Google Scholar]
- 11. Doran A, Obach RS, Smith BJ, et al. The impact of P‐glycoprotein on the disposition of drugs targeted for indications of the central nervous system: evaluation using the MDR1A/1B knockout mouse model. Drug Metab Dispos. 2005;33:165‐174. [DOI] [PubMed] [Google Scholar]
- 12. Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P‐glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75(1):13‐33. [DOI] [PubMed] [Google Scholar]
- 13. Macdonald N, Gledhill A. Potential impact of ABCB1 (p‐glycoprotein) polymorphisms on avermectin toxicity in humans. Arch Toxicol. 2007;81(8):553‐563. [DOI] [PubMed] [Google Scholar]
- 14. Boulton DW, DeVane CL, Liston HL, Markowitz JS. In vitro P‐glycoprotein affinity for atypical and conventional antipsychotics. Life Sci. 2002;71(2):163‐169. [DOI] [PubMed] [Google Scholar]
- 15. Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug‐resistance gene: multiple sequence variations and correlation of one allele with P‐glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S a. 2000;97(7):3473‐3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pauli‐Magnus C, Kroetz DL. Functional implications of genetic polymorphisms in the multidrug resistance gene MDR1 (ABCB1). Pharm Res. 2004;21(6):904‐913. [DOI] [PubMed] [Google Scholar]
- 17. Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta. 2009;1794:860‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolking S, Schaeffeler E, Lerche H, Schwab M, Nies AT. Impact of genetic polymorphisms of ABCB1 (MDR1, P‐glycoprotein) on drug disposition and potential clinical implications: update of the literature. Clin Pharmacokinet. 2015;54(7):709‐735. [DOI] [PubMed] [Google Scholar]
- 19. Ivanova SA, Osmanova DZ, Freidin MB, et al. Identification of 5‐hydroxytryptamine receptor gene polymorphisms modulating hyperprolactinaemia in antipsychotic drug‐treated patients with schizophrenia. World J Biol Psychiatry. 2017;18(3):239‐246. [DOI] [PubMed] [Google Scholar]
- 20. Ivanova SA, Osmanova DZ, Boiko AS, et al. Prolactin gene polymorphism (−1149 G/T) is associated with hyperprolactinemia in patients with schizophrenia treated with antipsychotics. Schizophr Res. 2017;182:110‐114. [DOI] [PubMed] [Google Scholar]
- 21. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose‐years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly DL, Wehring HJ, Earl AK, et al. Treating symptomatic hyperprolactinemia in women with schizophrenia: presentation of the ongoing DAAMSEL clinical trial (dopamine partial agonist, aripiprazole, for the Management of Symptomatic ELevated prolactin). BMC Psychiatry. 2013;13(1):214,244X‐13‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yasui‐Furukori N, Tsuchimine S, Saito M, Nakagami T, Sato Y, Kaneko S. Association between major multidrug resistance 1 (MDR1) gene polymorphisms and plasma concentration of prolactin during risperidone treatment in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(6):1230‐1234. [DOI] [PubMed] [Google Scholar]
- 24. de Klerk OL, Willemsen AT, Bosker FJ, et al. Regional increase in P‐glycoprotein function in the blood‐brain barrier of patients with chronic schizophrenia: a PET study with [(11)C]verapamil as a probe for P‐glycoprotein function. Psychiatry Res. 2010;183(2):151‐156. [DOI] [PubMed] [Google Scholar]
- 25. Jovanovic N, Bozina N, Lovric M, Medved V, Jakovljevic M, Peles AM. The role of CYP2D6 and ABCB1 pharmacogenetics in drug‐naive patients with first‐episode schizophrenia treated with risperidone. Eur J Clin Pharmacol. 2010;66(11):1109‐1117. [DOI] [PubMed] [Google Scholar]
- 26. Nikisch G, Baumann P, Oneda B, et al. Cytochrome P450 and ABCB1 genetics: association with quetiapine and norquetiapine plasma and cerebrospinal fluid concentrations and with clinical response in patients suffering from schizophrenia. A pilot study. J Psychopharmacol. 2011;25(7):896‐907. [DOI] [PubMed] [Google Scholar]
- 27. Mas S, Gasso P, Alvarez S, Parellada E, Bernardo M, Lafuente A. Intuitive pharmacogenetics: spontaneous risperidone dosage is related to CYP2D6, CYP3A5 and ABCB1 genotypes. Pharmacogenomics J. 2012;12(3):255‐259. [DOI] [PubMed] [Google Scholar]
- 28. Vijayan NN, Mathew A, Balan S, et al. Antipsychotic drug dosage and therapeutic response in schizophrenia is influenced by ABCB1 genotypes: a study from a south Indian perspective. Pharmacogenomics. 2012;13:1119‐1127. [DOI] [PubMed] [Google Scholar]
- 29. Llerena A, Berecz R, Penas‐Lledo E, Suveges A, Farinas H. Pharmacogenetics of clinical response to risperidone. Pharmacogenomics. 2013;14(2):177‐194. [DOI] [PubMed] [Google Scholar]
- 30. Ivanova SA, Loonen AJ, Pechlivanoglou P, et al. NMDA receptor genotypes associated with the vulnerability to develop dyskinesia. Transl Psychiatry. 2012;2:e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. González JR, Armengol L, Solé X, et al. SNPassoc: an R package to perform whole genome association studies. Bioinformatics. 2007;23:644‐655. [DOI] [PubMed] [Google Scholar]
- 32. Yasui‐Furukori N, Furukori H, Sugawara N, et al. Prolactin fluctuation over the course of a day during treatments with three atypical antipsychotics in schizophrenic patients. Hum Psychopharmacol. 2010;25(3):236‐242. [DOI] [PubMed] [Google Scholar]
- 33. Linnet K, Ejsing TB. A review on the impact of P‐glycoprotein on the penetration of drugs into the brain. Focus on psychotropic drugs. Eur Neuropsychopharmacol. 2008;18:157‐169. [DOI] [PubMed] [Google Scholar]
- 34. Van Strater AC, Bogers JP. Interaction of St John's wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121‐124. [DOI] [PubMed] [Google Scholar]
- 35. Saiz‐Rodriguez M, Belmonte C, Roman M, et al. Effect of ABCB1 C3435T polymorphism on pharmacokinetics of antipsychotics and antidepressants. Basic Clin Pharmacol Toxicol. 2018;123(4):474‐485. [DOI] [PubMed] [Google Scholar]
- 36. Kim KA, Joo HJ, Lee HM, Park JY. Influence of ABCB1 and CYP3A5 genetic polymorphisms on the pharmacokinetics of quetiapine in healthy volunteers. Pharmacogenet Genomics. 2014;24(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 37. Belmonte C, Ochoa D, Roman M, et al. Influence of CYP2D6, CYP3A4, CYP3A5 and ABCB1 polymorphisms on pharmacokinetics and safety of aripiprazole in healthy volunteers. Basic Clin Pharmacol Toxicol. 2018;122(6):596‐605. [DOI] [PubMed] [Google Scholar]
- 38. Gonzalez‐Vacarezza N, Dorado P, Penas‐Lledo EM, Farinas H, Estevez‐Carrizo FE, Llerena A. MDR‐1 genotypes and quetiapine pharmacokinetics in healthy volunteers. Drug Metabol Drug Interact. 2013;28(3):163‐166. [DOI] [PubMed] [Google Scholar]
- 39. Hitzl M, Schaeffeler E, Hocher B, et al. Variable expression of P‐glycoprotein in the human placenta and its association with mutations of the multidrug resistance 1 gene (MDR1, ABCB1). Pharmacogenetics. 2004;14(5):309‐318. [DOI] [PubMed] [Google Scholar]
- 40. Siegmund W, Ludwig K, Giessmann T, et al. The effects of the human MDR1 genotype on the expression of duodenal P‐glycoprotein and disposition of the probe drug talinolol. Clin Pharmacol Ther. 2002;72(5):572‐583. [DOI] [PubMed] [Google Scholar]
- 41. Owen A, Goldring C, Morgan P, Chadwick D, Park BK, Pirmohamed M. Relationship between the C3435T and G2677T(a) polymorphisms in the ABCB1 gene and P‐glycoprotein expression in human liver. Br J Clin Pharmacol. 2005;59(3):365‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hemauer SJ, Nanovskaya TN, Abdel‐Rahman SZ, Patrikeeva SL, Hankins GD, Ahmed MS. Modulation of human placental P‐glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem Pharmacol. 2010;79(6):921‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanabe M, Ieiri I, Nagata N, et al. Expression of P‐glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)‐1 gene. J Pharmacol Exp Ther. 2001;297(3):1137‐1143. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Professor Svetlana A. Ivanova (ivanovaniipz@gmail.com) upon reasonable request and with permission of MHRI.


