Abstract
Objectives
How reconstruction affects function following total laryngectomy is unclear. This study seeks to determine whether reconstruction method is associated with differences in swallowing outcomes.
Methods
Retrospective review of reconstruction technique in patients undergoing TL was compared by pharyngeal transit time (PTT), patient‐reported dysphagia (EAT‐10), and diet‐tolerated (FOIS).
Results
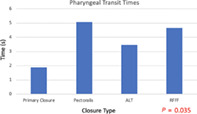
Ninety‐five patients met inclusion criteria, with 40 patients (42.1%) undergoing primary closure and 55 patients (57.9%) undergoing tissue transfer. There was no difference in EAT‐10 scores between the groups (P = .09). There was a significantly higher proportion of patients achieving oral diet (FOIS >3) with primary closure (P = .003). Patients undergoing PMC vs free flap had similar rates of g‐tube dependency. Primary closure had the shortest PTT (1.89 seconds) compared to free flap (3.47‐4.65 seconds) or PMC (5.1 seconds; P = .035).
Conclusions
When primary closure is achievable, these results suggest improved swallowing outcomes with better tolerance of oral diet and shorter pharyngeal transit times.
Level of evidence
IV
Keywords: dysphagia, head and neck cancer, microvascular reconstruction, total laryngectomy
The type of reconstruction chosen for the closure of total Laryngectomy defects affects swallowing outcomes. Primary closure is associated with shorter pharyngeal transit times and improved tolerance of oral diet.
1. INTRODUCTION
Cancer of the upper aerodigestive tract accounts for approximately 400 000 new cancer diagnoses each year, with approximately 14 400 cases attributable to laryngeal and hypopharyngeal cancer. 1 , 2 Following the publication of both the Veterans Affairs (VA) laryngeal cancer study in 1991, and the Radiation Therapy Oncology Group 91‐11 trial results in 2003, chemotherapy with radiation (CRT) became the primary treatment for patients with laryngeal cancer. 3 , 4 Long‐term results of this trial also demonstrated acceptable morbidity in patients who underwent salvage laryngectomy. Based on these results, total laryngectomy is now offered for surgical cure in advanced cases only, or for surgical salvage and for a nonfunctional larynx. 5 , 6
While the primary goal of therapy is cure, speech and swallowing function is of critical importance as well. Swallowing impairment is very common in this patient population before, during, and after treatment, leading to worse overall quality of life. 7 , 8 , 9 , 10 , 11 , 12 Dysphagia following total laryngectomy is attributed to stricture, diverticula, reduced transit in the neopharynx, and reflux. 8 , 13 Frequent complaints reported by patients include globus, regurgitation, prolonged mealtime, and food sticking. 8 , 14 , 15
Given current high success rates of free tissue transfer, it has become increasingly common in the reconstruction of pharyngeal defects, although many centers continue to utilize the pectoralis major flap. 16 , 17 , 18 The type of reconstruction for pharyngolaryngeal defects is dependent on tumor characteristics such as T‐stage, degree of pharyngeal mucosal involvement, history of prior radiation, and patients' comorbidities. 8 Although many studies have shown that the addition of a vascularized flap decreases complications such as fistula, and improves functional outcomes in the treatment of advanced laryngeal cancer, to date, only one compared different reconstructive options and examined post‐operative swallowing outcomes. However, this investigation did not use objective swallowing metrics in their analysis. 13 , 19 Therefore, the aim of this study was to determine whether or not closure type and flap choice is associated with differences in measurable post‐operative swallowing function following total laryngectomy.
2. MATERIALS AND METHODS
This study was performed under the approval of the university's institutional review board. The electronic medical records of patients >18 years of age with advanced laryngeal and hypopharyngeal cancer treated with total laryngectomy at a tertiary academic medical center from October 2007 to October 2018 were reviewed. All patients included in the study were evaluated for swallowing impairment with a video‐fluoroscopic swallow study (VFSS) post‐operatively, regardless of reconstruction or complaint of dysphagia. Swallow studies were interpreted by an experienced speech language pathologist and findings were reviewed by a laryngologist. All cases included could have been closed primarily, but at the surgeon discretion, a flap was chosen, most commonly due to history of radiation. All primary closures were done in a vertical fashion, and all flaps were inset as a cutaneous patch. All patients underwent a myotomy prior to pharyngeal closure. Exclusion criteria included missing clinical data, patients undergoing complete laryngopharyngectomy requiring a tubed closure, and patients with second primary cancer outside of the head and neck. Patient demographics as well as dysphagia metrics were collected. VFSS and swallowing metrics were collected every 3 months post‐operatively, but due to missing datapoints for some patients, the analysis only includes assessments completed at 1 year post‐operatively when all patients had a complete evaluation. Subjective evaluation of dysphagia was assessed by post‐operative Eating Assessment Tool‐10 scores (EAT‐10), a questionnaire with internal consistency, test‐retest reproducibility, criterion‐based validity, and normative data suggesting a score > 3 is abnormal. 20 Patients were divided into four groups based on reconstructive technique: (a) primary closure, (b) pectoralis major rotational flap (PMC), (c) radial forearm free flap (RFFF), and (d) anterolateral thigh free flap (ALT).
Statistical analysis was performed using SPSS statistics version 25 (IBM corporation, Armonk, NY). Subgroup analysis was performed via chi square testing to analyze the independent impact and association of each demographic factor on post‐operative swallowing impairment. Patients with EAT‐10 scores were compared using Kruskal‐Wallis analysis of variance. Any patient's lacking survey responses were omitted from the analysis. FOIS scores were analyzed by dividing groups into those feeding tube dependent (scores 1‐3) vs independent (4‐7) and compared with chi‐square analysis. Given the heterogeneity of the data, a Bonferroni correction was used where applicable. The threshold for significance was set at P < .05.
3. RESULTS
Ninety‐five patients met the inclusion criteria, with a mean age of 61 (SD 10.5), and 82.1% were male. Forty‐three patients (45.2%) underwent primary total laryngectomy, 38 (40%) underwent salvage laryngectomy, and 14 (14.7%) underwent functional laryngectomy. Primary closure was used in 40 patients (42.1%), most commonly in the primary laryngectomy group. Reconstruction with vascularized tissue transfer with either a regional or free flap was used in 55 cases (Table 1). One flap failure occurred in this cohort, with salvage performed by a PMC. This was not included as an additional reconstruction type during analysis.
TABLE 1.
Demographics
| N = 95 | Total | Primary TL | Salvage TL | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 78 (82.1%) | 45 | 33 | .325 |
| Female | 17 (17.9%) | 12 | 5 | |
| Closure | ||||
| Primary | 40 (42.1%) | 33 | 7 | |
| PMC | 19 (20.0%) | 10 | 9 | .0008 |
| ALT | 31 (32.6%) | 12 | 19 | |
| RFFF | 4 (4.2%) | 1 | 3 | |
| Tumor stage | ||||
| T1 | 4 (4.2%) | 0 | 4 | |
| T2 | 8 (8.4%) | 3 | 5 | .443 |
| T3 | 39 (41.1%) | 9 | 8 | |
| T4 | 43 (45.2%) | 26 | 18 | |
Reconstruction type did not differ between T‐stage ( P = .443; Table 1). There was no difference in EAT‐10 scores between the groups (P = .09; Figure 1). There was a significant difference in FOIS level, with a higher proportion of patients achieving oral diet (FOIS >3) with primary closure (P = .003; Bonferroni corrected P‐value .012; Figure 2). Patients undergoing PMC vs ALT free flap had similar rates of g‐tube dependency at 50% and 48%, respectively. Patients undergoing free flap reconstruction had significantly longer pharyngeal transit times (PTT) compared to primary closure, with ALT averaging 4.65 seconds, RFFF averaging 3.47 seconds, and primary closure averaging 1.89 seconds. Patients undergoing PMC had the longest PTT at 5.1 seconds (P = .035; Figure 3). Surprisingly, there was no correlation between EAT‐10, FOIS, or PTT, meaning some patients with fast PTT still had high EAT‐10 scores. Likewise, some patients with slower PTT had no g‐tube dependence, although there was a trend toward longer PTT and higher g‐tube dependence. Therefore, no correlations could be drawn among the outcome measures. Furthermore, among patients who underwent primary closure, in examining patients who underwent primary TL vs salvage TL, the EAT‐10, FOIS scores, and PTT were comparable (Table 2). Thirty patients underwent balloon dilation in the follow‐up period for continued dysphagia, 10 in the salvage TL group, and 20 in the primary TL group. There was no difference in the need for intervention between reconstruction techniques (P = .524), but there was a correlation between higher EAT‐10 scores and need for dilation. Furthermore, patients who underwent a series of three dilations spaced 3 months apart were more likely to achieve oral diet. Two patients required 14 dilations (primary closure) and 7 dilations (ALT) prior to achieving oral diet.
FIGURE 1.
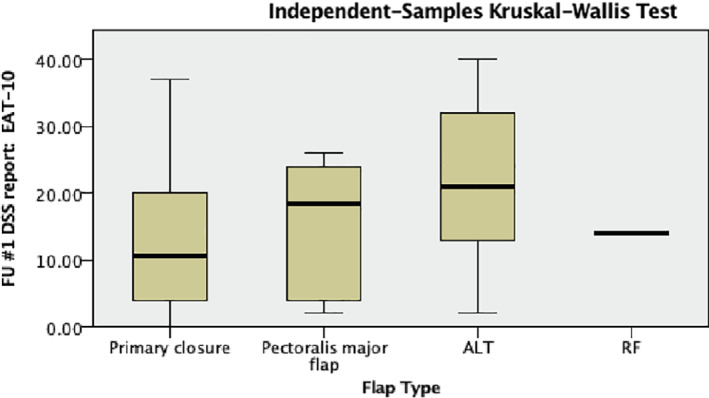
Kruskal‐Wallis ANOVA for flap type and EAT‐10 scores
FIGURE 2.
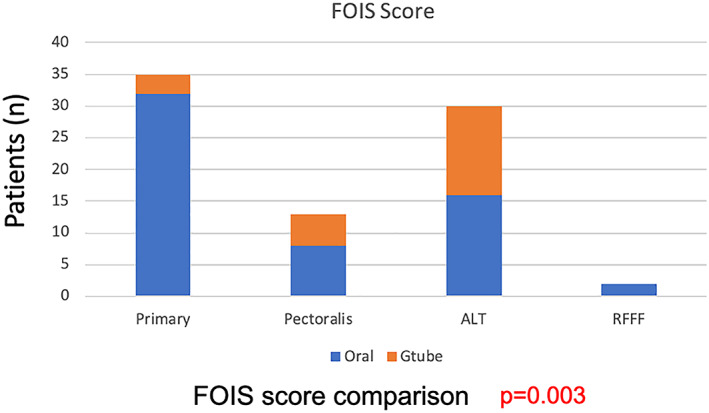
FOIS score comparison by closure
FIGURE 3.
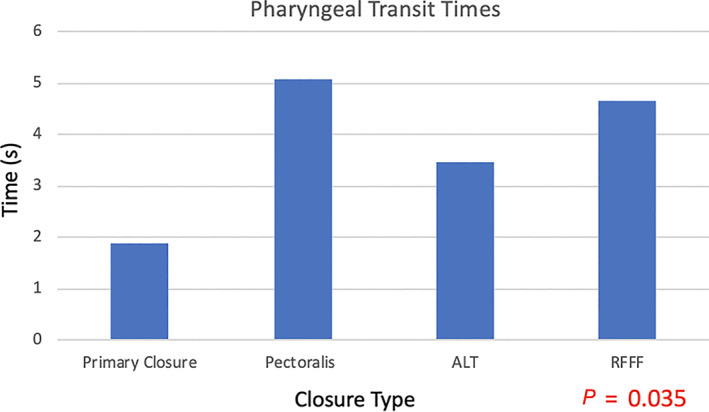
Pharyngeal transit times by closure technique
TABLE 2.
Comparison of outcomes between primary and salvage TL
| Closure | EAT‐10 | P | FOIS >4 | PTT | ||||
|---|---|---|---|---|---|---|---|---|
| Primary TL | Salvage TL | Primary TL | Salvage TL | Primary TL | Salvage TL | P | ||
| Primary | 23 | 12.8 | .22 | 26 | 6 | 1.95 | 1.53 | .60 |
| PMC | 22 | 14.4 | .47 | 4 | 4 | 3.45 | 5.76 | .51 |
| ALT | 27.2 | 21 | .99 | 4 | 12 | 4.92 | 3.55 | .49 |
| RFFF | 14 | 23 | 1 | 1 | 6.76 | 2.53 | ||
4. DISCUSSION
The incidence of dysphagia following total laryngectomy has been reported in the range of 10%‐60%, with some studies reporting some level of persistent swallowing impairment in all patients post‐operatively. 11 To date, many studies have examined the success in free tissue transfer for limiting complications following total laryngectomy and have studied the functional outcomes associated with specific flaps. 9 , 12 , 21 Only one other study, however, has compared outcomes between different closure techniques, particularly with respect to post‐operative swallowing. 13
Among this cohort of total laryngectomy patients, we found no difference in self‐reported dysphagia between various reconstruction groups, as measured by EAT‐10 scores, regardless of whether or primary or salvage TL. This is consistent with Maclean et al who found no difference in the type of pharyngeal closure and patient reported dysphagia. 8 Similarly, Nguyen et al reported no difference in patient reported dysphagia between those undergoing PMC vs free flap reconstruction, although they did not utilize validated symptom indices such as the EAT‐10. 13 In their study of 126 patients undergoing salvage laryngectomy, their outcome measures were delay in oral intake, subjective complaint of dysphagia, diet consistency, need for feeding tube, and need for esophageal dilation due to pharyngoesophageal structure. They found a significantly lower number of patients tolerating oral diet in patients in the PMC group compared to the free flap group (38.7% vs 15.2%, P < .05). 13
In the present study, there was a similar rate of g‐tube dependency between the two types of reconstruction at 1 year post‐op, and this was consistent across primary and salvage TL patients.. In another study of 25 patients undergoing tubed RFFF reconstruction for total laryngopharyngectomy, only 1 patient remained g‐tube dependent at the end of their follow‐up period. 12 Yu et al reported on the success of the ALT for pharyngeal reconstruction. 21 Of 114 patients, 95% of the flaps were successful, 6% had strictures, and oral diet was achieved in 91%. 21 Many studies have corroborated these findings, although Parmar et al 22 are still proponents of the jejunal flap for circumferential defects given their high complication rate and low oral intake rate. It should be noted, though, that they had a very low number of patients (n = 6). Mahalingam et al 9 showed that 5/44 (11.4%) of ALT flap required long‐term enteral feeding in comparison with 18/461 (3.9%) of free jejunal flap patients (P < .05). They also found that pedicled flaps resulted in poorer outcomes than free flaps. The higher rates of g‐tube dependence in this study are unclear but it could be related to the significant proportion undergoing laryngectomy in the salvage setting, and shorter follow‐up time. Our findings indicate that closure choice is associated with ultimate diet tolerance as measured by FOIS. The highest proportion of patients achieving oral diet (FOIS >3) underwent primary closure. In a systematic review by Terlingen et al, 15 dysphagia in patients who underwent primary pharyngeal closure was related to impaired pharyngeal propulsion, outflow resistance, pharyngeal weakness, spasm, and nasopharyngeal reflux. A pseudodiverticulum was more frequently seen after vertical closure compared to T‐shaped closure. Furthermore, they found higher rates of stricture formation, as assessed with VFSS, with primary closure vs pedicled or microvascular free flap. 15 This could be due to a smaller overall lumen from primary closure as opposed to augmenting pharyngeal area with a flap, although the site of stricture was not specified. The improved swallowing outcomes with primary closure could be due to preserved pharyngeal musculature.
Not only was primary closure associated with oral diet success, it also correlated with faster pharyngeal transit times. This present study is the first to compare pharyngeal transit times (PTT) among different closure techniques. Patients undergoing free flap reconstruction had significantly longer PTT compared to primary closure, and patients undergoing PMC had the longest PTT. By comparison, Maclean et al studied intraluminal bolus pressures in 26 patients who underwent primary closure of the neopharynx. They found that patients who had mucosa‐alone pharyngeal closure had significantly greater pharyngeal diameter but no difference in intrabolus pressures. Patients closed with mucosa and muscle had superior swallowing, with near normal peak mid‐pharyngeal pressures. Furthermore, they did not find a difference in functional outcome between those who did and did not have a myotomy performed at the time of laryngectomy. As with prior studies, they found no difference in the type of pharyngeal closure and patient reported dysphagia. 8
Esophageal dilation has been shown to significantly improve dysphagia following TL. 23 Further studies have shown that intervention leading to improved dysphagia is correlated with improved quality of life. 24 Frequently, patients develop strictures, particularly at the anastomosis between the neopharynx and esophagus, leading to dysphagia requiring balloon dilation. 19 In the present study, approximately one‐third of patients underwent balloon dilation, but there was no difference in closure type between those who did and did not require dilation, or the frequency of dilation. Furthermore, need for dilation did not differ between the primary and salvage groups. In a study by Scharpf et al, nine patients (36%) who underwent RFFF reconstruction for hypopharyngeal defects required dilation. 12 A separate study by Mahalingam et al 9 found that 5 of 15 ALT, 3 or 11 gastro‐omental, and 87 of 810 free jejunum flaps developed strictures, a difference which was significant (P < .05). 9 Nguyen et al 13 found that PMC required more esophageal dilations related to stricture. They proposed that the free flaps may offer a higher freedom of movement than the PMC, and a higher degree of pharyngeal diameter. Esophageal dilation is clearly an important component of post‐operative follow‐up following total laryngectomy, but it does not appear to be related to the type of neopharyngeal closure.
The authors acknowledge several limitations to the present study including its retrospective design which lends to selection bias. Additionally, there were a small number of patients in the radial forearm free flap group making a direct comparison difficult. Finally, there was heterogeneity between the groups. We attempted to control for these factors by having strict inclusion criteria. Nevertheless, this is the first study comparing primary closure to free‐flap type and only the second study comparing outcomes between regional vs free flap reconstruction. Furthermore, this is the first study utilizing dynamic swallow studies to evaluate pharyngeal transit times between closure techniques, and the results suggest improved outcomes with primary closure.
5. CONCLUSION
Free flap reconstruction is a necessary part of total laryngectomy reconstruction, whether to augment mucosa for closure or to prevent complications. When achievable, preserving as much mucosa as possible, or closing primarily may result in improved pharyngeal transit times and oral tolerance.
CONFLICT OF INTEREST
The authors have no potential conflict of interest.
Harris BN, Hoshal SG, Evangelista L, Kuhn M. Reconstruction technique following total laryngectomy affects swallowing outcomes. Laryngoscope Investigative Otolaryngology. 2020;5:703–707. 10.1002/lio2.430
This abstract was presented as a scientific oral presentation at the COSM/AHNS meeting in Austin, TX, USA; May 1, 2019.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. Cancer. 2019;69:7‐34. [DOI] [PubMed] [Google Scholar]
- 2. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116:1‐13. [DOI] [PubMed] [Google Scholar]
- 3. The Department of Veterans Affairs Laryngeal Cancer Study Group . Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685‐1690. [DOI] [PubMed] [Google Scholar]
- 4. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091‐2098. [DOI] [PubMed] [Google Scholar]
- 5. Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the radiation therapy oncology group trial 91‐11. Arch Otolaryngol Head Neck Surg. 2003;129:44‐49. [DOI] [PubMed] [Google Scholar]
- 6. Harris BN, Bhuskute AA, Rao S, Farwell DG, Bewley AF. Primary surgery for advanced‐stage laryngeal cancer: a stage and subsite‐specific survival analysis. Head Neck. 2016;28:1380‐1386. [DOI] [PubMed] [Google Scholar]
- 7. De Oliveira R, dos Santos R, de Carvalho S, et al. Prospective evaluation of quality of life in patients with head and neck cancer. Oral Med Oral Surg Oral Pathol Oral Radiol. 2017;123:350‐357. [DOI] [PubMed] [Google Scholar]
- 8. Maclean J, Szczesniak M, Cotton S, Cook I, Perry A. Impact of laryngectomy and surgical closure technique on swallow biomechanics and dysphagia severity. Otolaryngol Head Neck Surg. 2011;144:21‐28. [DOI] [PubMed] [Google Scholar]
- 9. Mahalingam S, Srinivasan R, Spielmann P. Quality‐of‐life and functional outcomes following pharyngolaryngectomy: a systematic review of literature. Clin Otolaryngol. 2015;41:25‐43. [DOI] [PubMed] [Google Scholar]
- 10. Thrasyvoulou G, Vlastarakos PV, Thrasyvoulou M, Sismanis A. Horizontal (vs. vertical) closure of the neo‐pharynx is associated with superior postoperative swallowing after total laryngectomy. ENT J. 2018;97:E31‐E35. [DOI] [PubMed] [Google Scholar]
- 11. Ward EC, Bishop B, Frisby J, Stevens M. Swallowing outcomes following laryngectomy and pharyngolaryngectomy. Arch Otolaryngol Head Neck Surg. 2002;128:181‐186. [DOI] [PubMed] [Google Scholar]
- 12. Scharpf J, Esclamado RM. Reconstruction with radial forearm flaps after ablative surgery for hypopharyngeal cancer. Head Neck. 2003;25:261‐266. [DOI] [PubMed] [Google Scholar]
- 13. Nguyen S, Thuot F. Functional outcomes of fasciocutaneous free flap and pectoralis major flap for salvage total laryngectomy. Head Neck. 2017;39:1797‐1805. [DOI] [PubMed] [Google Scholar]
- 14. Coffey MM, Tolley NHoward D, Drinnan M, Hickson M. An investigation of the post‐laryngectomy swallow using videofluoroscopy and fiberoptic endoscopic evaluation of swallowing (FEES). Dysphagia. 2018;33:369‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Terlingen LT, Pilz W, Kuijer M, Kremer B, Baijens LW. Diagnosis and treatment of oropharyngeal dysphagia after total laryngectomy with or without pharyngoesophageal reconstruction: systematic review. Head Neck. 2018;40:2733‐2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aladimi MT, Han B, Li C, Helal H, Gao Z, Li L. Factors to consider when deciding on the type of free‐flap reconstruction of head and neck soft tissue defects. ORL J Otorhinolaryngol Relat Spec. 2017;79(4):230‐238. [DOI] [PubMed] [Google Scholar]
- 17. Wang K, Tsai C, Chang C, et al. Comparison of flap outcomes between single‐and multiple‐perforator‐based free anterolateral thigh flap in head and neck reconstruction. Microsurgery. 2019;39(2):150‐155. [DOI] [PubMed] [Google Scholar]
- 18. Schusterman MA, Miller MJ, Reece GP, Kroll SS, Marchi M, Goepfert H. A single center's experience with 308 free flaps for repair of head and neck cancer defects. Plast Reconstr Surg. 1994;93(3):472‐478. [PubMed] [Google Scholar]
- 19. Ho MW, Houghton L, Gillmartin E, et al. Outcomes following pharyngolaryngectomy reconstruction with the anterolateral thigh (ALT) free flap. Br J Oral Maxillofac Surg. 2012;50:19‐24. [DOI] [PubMed] [Google Scholar]
- 20. Belafsky P, Mouadeb D, Rees C, et al. Validity and reliability of the eating assessment tool (EAT‐10). Ann Otol Rhinol Laryngol. 2008. Dec;117(12):919‐924. [DOI] [PubMed] [Google Scholar]
- 21. Yu P, Hanasono MM, Skoracki RJ, et al. Pharyngoesophageal reconstruction with the anterolateral thigh flap after total laryngopharyngectomy. Cancer. 2010;116:1718‐1724. [DOI] [PubMed] [Google Scholar]
- 22. Parmar S, Al Asaadi Z, Martin T, Jennings C, Pracy P. The anterolateral fasciocutaneous thigh flap for circumferential pharyngeal defects‐ can it really replace the jejunum? Br J Oral Maxillofac Surg. 2014;52:247‐250. [DOI] [PubMed] [Google Scholar]
- 23. Wu PI, Szczesniak M, Maclean J, et al. Endoscopic dilatation improves long‐term dysphagia following head and neck cancer therapies: a randomized control trial. Dis Esophagus. 2018;0:1‐9. [DOI] [PubMed] [Google Scholar]
- 24. Jones E, Speyer R, Kertscher B, Denman D, Swan K, Cordier R. Health‐related quality of life and oropharyngeal dysphagia: a systematic review. Dysphagia. 2018;33(2):141‐172. [DOI] [PubMed] [Google Scholar]


