Abstract
Intake despite negative consequences (compulsivity) contributes strongly to the harm of alcohol use disorder, making the underlying psychological and circuit mechanisms of great importance. To gain insight into possible underlying action strategies, we compared rat licking microstructure across compulsion-like and non-compulsive conditions. We previously showed that drinking under a moderate-challenge, quinine-alcohol model (Alc-ModQ) shows less variable responding in many measures, suggesting a more automatic strategy to overcome challenge. Here, we reanalyzed our original data, newly focusing on the behavioral profile of higher-challenge intake (100 mg/L quinine in alcohol, Alc-HighQ). Alc-HighQ greatly dropped consumption, yet retained aspects of greater automaticity and drive seen with Alc-ModQ, including earlier bout initiation and measures suggesting more stereotyped tongue control. In contrast, Alc-HighQ disordered bout generation and timing. Importantly, only fast-starting bouts persisted under Alc-HighQ, and while there were many fewer longer Alc-HighQ bouts, they still contributed >50% of consumption. Also, longer bouts under Alc-HighQ had an early, several-second period with greater chance of stopping, but afterwards showed similar persistence and recovery from slow licking as other drinking conditions. Together, our findings elucidate novel behavioral indicators of successful and unsuccessful epochs of Alc-HighQ, compulsion-like intake. We also relate findings to congruent human and animal work implicating anterior insula and medial prefrontal cortices as critical for compulsion-like alcohol responding, and where ventral frontal cortex has been more associated with overall action plan and tongue control (retained under Alc-HighQ), with medial cortex more related to proximal action timing (disrupted under Alc-HighQ except after faster bout initiation).
Keywords: automaticity, addiction, motivation, compulsion, insula
1. Introduction
Compulsion-like responding, especially alcohol intake in the face of negative consequences, is a major obstacle to treating addiction and a strong driver of excessive intake and its substantial harm [1–8]; consequence-resistant responding is also central to the DSM-V definition of Alcohol Use Disorder (AUD) [9]. Thus, improving human treatment requires better understanding of circuit and psychological mechanisms that drive aversion-resistant intake. The willingness of rodents to continue responding for reward even when paired with aversive consequences is considered to model some aspects of compulsion-like responding in human addiction [5–7] (see Discussion). Indeed, rats will continue drinking alcohol adulterated with 10 or 30 mg/L quinine [10–13] but drink very little water with the same quinine levels [11], indicating that alcohol-consumption under this level of quinine challenge is aversion-resistant [5]. In addition, human [14] and rodent [12,13,15] findings converge on the central importance of the anterior insula (AI) and medial prefrontal cortex (MPF) for driving compulsion-like responding for alcohol. This circuit is also likely critical for many other addictive behaviors [8,16–18] (cf [5,10]), including alcohol interoception and operant responding [19,20], in addition to promoting motivated responding more generally [17,18,21,22]. Other human findings also indicate that AI and MPF are important for action despite cost more generally, including overcoming task difficulty and risk of loss (see Supplemental Discussion).
In order to gain additional insight into psychological or action strategies that might be apparent during challenged alcohol drinking, we have examined the behavioral microstructure of licking, which is widely used to infer motivation (cf [10,23]). Interestingly, compulsion-like alcohol drinking under more moderate aversive challenge shows decreased variability in many response measures in rats [10], in agreement with the suggested importance of automaticity for habit and compulsion [5,6,24–26]. Accordingly, we proposed the Head Down and Push model [10], where decreased variability reflects a psychological strategy focused on stereotyped responding, which may minimize the need to attend to (and be impacted by) adverse consequences during action. However, high enough challenge can reduce compulsion-like alcohol drinking [5,10,11], perhaps reflecting inability to ignore cost which disrupts responding despite strong reward drive. To better understand behavioral strategies that could promote aversion-resistant alcohol intake under higher challenge (100mg/L quinine in alcohol, Alc-HighQ), we reanalyzed our previous data set [10], with a focus here on similarities and differences in licking patterns under Alc-HighQ and Alc-ModQ. In particular, we looked for behavioral indicators that could reflect better or worse ability to maintain consumption despite stronger challenge.
Here, we show that, even though Alc-HighQ strongly reduced alcohol consumption [10], Alc-HighQ responding retained several behavioral changes seen with Alc-ModQ, including less variable lick volume across sessions and earlier initiation of bouts within sessions. These suggested greater global commitment to drinking under aversive consequences, separate from challenge level. In contrast, Alc-HighQ exhibited shorter, more fragmented bouts and greater variability in lick speed, especially at the initiation of bouts. Longer, persisting bouts were of particular interest, since even though there were many fewer under Alc-HighQ, they contributed >50% of intake. Importantly, bout persistence under Alc-HighQ was associated with faster bout start, suggesting the possibility that the cognitive state at onset of licking was critical for allowing a bout to persist under Alc-HighQ. Together, these results suggest that, even when challenge substantially suppressed drinking, some aspects of responding still showed greater commitment to act. Also, while lick timing was disrupted during Alc-HighQ, faster-starting bouts were able to persist longer. Thus, while underlying mechanisms remain speculative, our findings define specific periods of interest within a bout (especially the onset) and across bout lengths, which will be of value for future studies examining challenge-related local brain activity. Further, since published findings indicate a cross-species importance of AI/MPF for compulsion-like alcohol responding [12,14,15], we also relate the present work to previous studies suggesting that ventral but not medial frontal cortex is associated with tongue control, while MPF inhibition is more associated with disrupted action organization and timing [27–39] (see Discussion).
2. Materials and methods
2.1. Drinking behaviors
All procedures followed Guide for Care and Use of Laboratory Animals provided by the NIH, with approval of the UCSF IACUC. Fourteen adult male Wistar rats (Harlan) arrived at P45–50, and were allowed ~2 wk to habituate to the vivarium. Rats then drank alcohol (20%, v/v) versus water under two-bottle choice for ~3 mo under intermittent access in the home cage. Drinking occurred in three overnight periods per week (usually starting late afternoon Monday, Wednesday and Friday) [10–13]. This allows development of compulsion-like responding for alcohol, defined as the willingness to keep responding even when alcohol is paired with a negative consequence [5,12,13], and which is considered to model some aspects of consequence-resistant drive for alcohol in humans with AUD [5–7]. Here we use quinine adulteration of alcohol, where quinine levels that are strongly rejected in water are tolerated when in alcohol [5,11–13]. Importantly, a similar cortico-accumbens circuit mediates alcohol responding in the face of both quinine and footshock [12], where footshock-resistance is considered a gold standard for compulsion-like responding in rodents, while quinine is technically much simpler [5]. Also, human compulsion-like responding for alcohol activates a congruent corticostriatal circuit (AI, MPF, striatum) [14] as implicated in rodents [12,13,15].
After ~ 3mo of intermittent access to alcohol, rats were switched to drink alcohol 20min/d, 5d/wk in the home cage. After ~1 mo, rats began drinking alcohol 20min/day in a Med Associates operant box with a lickometer system where the drinking lixit was somewhat recessed. All 20-min drinking sessions occurred at the end of the light cycle (2–3 hours before lights off), when animals previously had the onset of their 24 hr intermittent access sessions. Rats drank Alc-Only for ~4 weeks, except for 2–3 sessions in the second or third week with Alc-ModQ (10 mg/L quinine in alcohol) to habituate to the novelty of quinine. Experimental sessions then began. Each animal underwent 4 Alc-Only and 4 Alc-ModQ sessions on separate days, randomized across animals and sessions. We then tested 100 mg/L quinine in alcohol across 3 days subsequent to the Alc-Only and Alc-ModQ tests; this was done because, in the shorter term, exposure to very high quinine alcohol can disrupt subsequent Alc-Only intake in some animals (although, over the longer term, rats drink more robustly; not shown). Two animals didn’t drink during the third Alc-HighQ session, and thus we only have two Alc-HighQ sessions for these two rats. There were no significant differences in behavioral measures across the three Alc-HighQ sessions for rats that continued to drink (Fig.S1).
Total alcohol intake was determined via a widely used method, by measuring the alcohol bottle weight before and after a drinking session, then subtracting 0.2 g which is the average amount of spillage determined from cages without rats. The weight was then converted to g alcohol and expressed relative to the animal’s body weight (giving g/kg).
The lickometer system we used combined an electrical detector of licks and a force lickometer system (Med Associates). Thus, tongue contact with the licking spout created a circuit and change in electrical signal indicating that a lick occurred, while concurrent signals from the force lickometer indicated a deflection in the lixit spout when a lick occurred. Force measures were deemed to be less quantitative and were not specifically analyzed further (not shown), but were useful for validating that lick detection across the two modes detected similar lick events. Also, we note that the licking measures we examined showed a broad distribution within each of the different drinking conditions, and thus any incorrect lick detection would similarly impact lick detection across conditions.
Our previous work [10] used this data set to examine average responding under a variety of licking measures during Alc-Only and Alc-ModQ alcohol drinking. Here, we reanalyzed the data set with a focus predominantly on behavioral microstructure patterns under Alc-HighQ, and how they compare to Alc-ModQ and Alc-Only. In particular, we were interested in behavioral measures that predicted successful maintenance of responding in the face of higher challenge. We also focus here more on the distribution of different response measures, with the overall goal to help develop a more unified model of behavioral strategies and potential brain regions that sustain responding under Alc-ModQ and Alc-HighQ compulsion-like alcohol drinking.
2.2. Analyses and statistics
Since the majority of data were non-normal, the Kruskal-Wallis (KW) test was used to compare across the three conditions, with Dunn’s multiple comparison posthoc. For normally distributed data, other tests including one-way and two-way ANOVA were used. Analyses were performed using GraphPad Prism, SPSS or R [10]. Bouts were 3+ licks occurring ≤1 sec apart [10]. For analyses in Fig. 6 we examined licking parameters for the 1st-12th ILI, and for licking beyond the 12th ILI. We chose the 12th ILI for two reasons: (1) in Fig. 5D, there is increased chance of stopping under Alc-HighQ until the 12th ILI, although the chance of stopping did not fully shift to Alc-Only levels until the ~16th ILI. (2) This was balanced with Fig. 4G, where licking speed in longer Alc-HighQ bouts slowed in the ~4th-10th ILI after bout onset, but recovered with somewhat faster licking by the 12th ILI.
Fig.6. Slower Intervals of Licking (SILs) in longer bouts: similar across drinking conditions.
(A) Example showing SILs across a bout. (B) No differences in the distribution of SIL length for longer bouts after the ~12th ILI (see Methods). In addition, the majority of SILs were only a single ILI in duration, indicating efficient recovery from slow licking that was similar during Alc-HighQ and other drinking conditions. (C) Small but significant shift in SIL duration in medium bouts, where Alc-HighQ and Alc-Only had longer SILs. (D) No differences in licking speed in SILs from longer bouts after the initial period of greater chance of stopping (up to ~12th ILI), suggesting that Alc-HighQ responding was similar to other drinking conditions during longer bouts after the first 2–3 seconds of licking. In contrast, (E) SILs were slower early in longer Alc-HighQ bouts, consistent with Fig.4F,G showing slower overall responding at bout onset under Alc-HighQ vs Alc-ModQ. (F) Similar abundance of SILs across longer bouts. For this analysis, SILs were assessed in terms of where they fell within a bout, with the first ILI being 0% and the last ILI being 100%. Combined with findings in Figs. 4,5, these results suggest that Alc-HighQ show behavioral evidence of similar regulation of licking as other drinking conditions during longer bouts after the early slowing/stopping period. *** p<0.005.
Fig.5. Greater chance of stopping early in Alc-HighQ bouts, although similar duration of longer bouts across drinking conditions.
(A-C) Bout length distributions for (A) Alc-Only, (B) Alc-ModQ, and (C) Alc-HighQ. Distributions are shown separately for bouts taken from the first (black line), middle (dotted line) and last (green line) tertile of bouts within a session, then aggregated across sessions of that drinking condition. For all drinking conditions, the bout length distributions were similar early and later in sessions. (D) Bout length distributions from (A-C) were used to calculate a hazard function, indicating the relative chance of stopping [P(stop)] after each ILI as bouts progressed; P(stop) was determined from the bout length distribution combined across the whole session. Higher-challenge showed increased chance of stopping across the first ~12 ILIs, in agreement with a several second slowing after Alc-HighQ bout onset (Fig.4G,H). (E) Even though Alc-HighQ drinking had significantly fewer longer bouts, longer bouts still contributed >50% of all intake during Alc-HighQ alcohol drinking. (F) No significant differences in length of longer bouts across drinking conditions (8–9 seconds on average), agreeing with the possibility that faster onset under Alc-HighQ (Fig.4) allowed bouts to persist for a similar duration under Alc-HighQ as seen with other, less challenging intake conditions.
Fig.4. Licking speed (calculated from time between licks) at onset of and across the bout.
(A) Cartoon showing how the licking speed (Time Between Licks) at bout start was calculated, specifically as the average of the first three ILIs in a bout (light grey bars). (B-D) Cumulative incidence of bout start speed for shorter (purple), medium (brown) and longer (black) bouts, for (B) Alc-Only, (C) Alc-ModQ, and (D) Alc-HighQ. A black-bounded yellow diamond is at the same position in (B-D), and is included to clarify that short and medium-length bouts started significantly slower than longer bouts under Alc-HighQ (D) and Alc-Only (B), while all Alc-ModQ bouts started faster (C) as previously reported [10]. (E-G) Inter-Lick Interval (in msec) across bouts, starting from bout initiation (the 1st ILI within the bout) and continuing across to the 10th, 20th and 30th ILIs in the bout; data are shown separately for shorter (purple), medium (brown) and longer (black) bouts, and for (E) Alc-Only, (F) Alc-ModQ, and (G) Alc-HighQ. (H) Greater slowing across bout initiation under Alc-HighQ; change in ILI (time between licks) across bout initiation was determined by subtracting the average speed of the 6th-9th ILIs (dark grey bars) from the average speed across the first three ILIs (light grey bars). * p<0.05, ** p<0.01; & p<0.05 between shorter Alc-ModQ bouts and shorter Alc-HighQ or Alc-Only bouts.
We calculated the hazard rate [h(x)] by first determining the empirical probability mass function (PMF) for the number of licks in a bout [f(x)], calculating its cumulative empirical (CMF) [F(x)], and then determining the hazard rate by setting [40–42]. This procedure has been previously used in the context of neuroscience research where there exists the need to calculate the instantaneous rate of an event occurring across time (such as reaction times following a “go” cue, see [40]).
3.3. Determining and interpreting measures of “tongue control”
The averaged lick volume for a given session was assessed as the intake volume divided by the number of licks. The lick-intake relation was calculated by measuring the perpendicular distance of each session’s values from the total-lick/total-intake regression line, using the equation , with (m,n) being the total intake and total lick values for each drinking session, respectively. The equation of the least-squares regression line (Ax+By+C=0) was determined separately for each condition. Data points farther from the regression line indicated more deviation in the lick-intake relationship. Data are show as mean±SEM.
We previously found that Alc-ModQ drinking exhibits less variable responding in many licking measures, including some that attempt to address lick efficiency related to tongue shape. In this regard, classic studies indicate that ventral lateral prefrontal cortex (PFC) but not medial PFC lesions impair tongue protrusion [34–37], and disruption of AI (part of ventral lateral PFC) also reduces tongue protrusion [43]. In agreement, inactivating lateral orbitofrontal [38] but not medial PFC [27] alters lick volume, a simple measure often assessed as the average volume per lick across a session. Thus, these studies converge on the importance of ventral lateral PFC for regulating tongue shape and licking efficiency (which we call “tongue control”), and, importantly, validate the use of lick volume measures to assess this aspect of tongue regulation. Notably, this is in contrast to assessment of lick timing, the rate and pattern of licks across time, which is conceptually distinct from the tongue shape during each lick. In our present results, we assessed two different aspects of lick volume, the average volume per lick and the lick-intake relation (related but not identical measures, Fig. 2E), with a particular focus on their variability across drinking sessions. As described further in the Discussion, these tongue control measures are less variable across Alc-ModQ session compared with Alc-Only (which lacks the explicit aversive challenge) [10]; we speculate this reflects adoption of a more stereotyped response strategy to allow continued drinking with less need to attend to and guide ongoing licking (which would also increase awareness of the aversion and increase chance of stopping) [10]. Importantly, our present work showed that Alc-HighQ drinking also exhibited more regular, less variable lick volume, similar to Alc-ModQ, suggesting that some drivers of licking are similar under high and moderate challenge, even though Alc-HighQ significantly reduces intake. This is taken as part of the evidence suggesting that AI/ventral-lateral PFC makes a similar contribution to motivation to drink under both Alc-HighQ and Alc-ModQ (compared with Alc-only).
Fig.2. Tongue control was significantly less variable during both Alc-ModQ and Alc-HighQ drinking.
(A) Less variable lick volume in both compulsion-like intake conditions versus Alc-Only, where lick volume was assessed as the total ml intake / total licks for each session. (B) Distribution of lick-intake relations; this measure is the distance of each session’s lick-intake value from the lick-intake regression line in a given drinking condition; outlines reflect lick-intake distributions for Alc-Only and Alc-ModQ conditions which were previously shown in [10]. (C) Cumulative distribution of lick-intake relation across conditions, showing that lick-intake relation values were lower in both challenged conditions relative to Alc-Only, indicating less variability around the lick-intake regression line. (D) Lower lick-intake relation, relative to Alc-Only, under both challenge conditions even when considering only sessions with ≥0.3 g/kg intake. (E) Lick volume and lick-intake relation were not related in a simple way, and thus may assess somewhat different measures (see also Fig.S9). (F) Lick volume was not related to basal intake level (p>0.1 in all intake conditions). # p<0.05 difference in variance, * p<0.05, ** p<0.01, *** p<0.005.
3. Results
3.1. Overall procedure
Many studies have inferred underlying motivation and drives from lick microstructure (cf [10,23]). Here, we compared response patterns during higher-challenge compulsion-like intake (Alc-HighQ; alcohol plus 100 mg/L quinine; red symbols) with moderate-challenge compulsion-like drinking (Alc-ModQ; alcohol plus 10 mg/L quinine; blue symbols) and Alc-Only (black symbols), in order to better understand the behavioral profile of different aspects of aversion-resistant intake under Alc-HighQ. Each of 14 animals underwent 4 Alc-Only, 4 Alc-ModQ, and 3 Alc-HighQ sessions (see Methods). Bouts were 3+ licks occurring ≤1sec apart (Fig. 1A), as widely used (see [10]). Data for sessions and bouts were analyzed separate from animal identity, as done before [10,44], giving 56 sessions each for Alc-Only and Alc-ModQ and 40 sessions for Alc-HighQ (as two rats didn’t drink on the third Alc-HighQ session). In particular, this allowed us to study the distribution and variability of responding, as well as shifts in average responding.
Fig.1. Bouts started significantly sooner during both Alc-ModQ and Alc-HighQ drinking.
14 animals underwent 4 Alc-Only, 4 Alc-ModQ, and 3 Alc-HighQ sessions; data were primarily analyzed separate of animal identity, as in [10,44], and thus our results show 56 (14×4) sessions for Alc-Only and Alc-ModQ, and 40 sessions (14×3, with two rats not drinking in the third session) for Alc-HighQ. See Methods for experimental timing and other details. (A) Licks grouped into a bout (≥3 licks with ≤1 sec between licks, as used before, see [10]). (B) Alc-ModQ (blue, 10 mg/L quinine in alcohol) did not reduce total intake relative to Alc-Only (black), demonstrating aversion-resistant consumption, while Alc-HighQ (red, 100 mg/L quinine in alcohol) significantly and strongly reduced overall drinking levels. (C) Cumulative distribution showing that the first lick of the session (session start time) did not differ across intake conditions. (D) Cumulative distribution of bout start time was shifted to the left under both challenged drinking conditions, indicating that more bouts occurred earlier, with a similar change under Alc-HighQ and Alc-ModQ relative to Alc-Only; entire cumulative distribution in Fig.S2C. Cumul: cumulative. *** p<0.005.
3.2. Earlier start time and more regular tongue control under both Alc-HighQ and Alc-ModQ drinking
Alc-HighQ reduced intake by ~50% (Fig. 1B; [10]), and thus we predicted a priori that Alc-HighQ drinking might be delayed, reflecting increased time to overcome the expected higher aversion. The time of the first lick in the session was not different across drinking conditions (Figs. 1C,S2A; KW=0.119, p=0.942), likely because rats didn’t know which alcohol solution was present until the first lick. However, once rats determined that drinking involved challenging consequences, bout initiation actually occurred significantly earlier during both Alc-HighQ and Alc-ModQ sessions, relative to Alc-Only (Figs. 1D,S2B–C; Kruskal-Wallis test [KW]; KW=23.47, p<0.0001), with no difference between Alc-HighQ and Alc-ModQ (p>0.9 post-hoc). Thus, rats adopted a strategy of licking sooner under both challenge conditions.
We then examined whether other measures exhibited similar changes in the two challenged-drinking conditions relative to Alc-Only. Interestingly, several aspects of licking that assess tongue control (rationale detailed in Methods) [27,34–38,43] showed a comparable shift under Alc-ModQ and Alc-HighQ intake, with less variability in both challenge conditions versus Alc-Only. For example, both challenged drinking conditions showed less variable lick volume across sessions (Fig. 2A; Levene’s test F(2,149)=4.236, p=0.0163; p>0.5 post-hoc between challenge conditions, F-test). However, there were no differences in average lick volume (KW=1.206, p=0.5473), indicating reduced response variability under both Alc-HighQ and Alc-ModQ rather than altered response level.
To better understand response variability, we then examined the relationship between total licks and total intake across sessions. In particular, we determined the distance of each session’s value from the lick-intake regression line for each condition (see Methods); this measure is related to lick volume and examines overall lick regularity [10]. We previously showed that this lick-intake relation is significantly reduced during Alc-ModQ versus Alc-Only (blue versus black outlines in Fig. 2B show range of data; see [10]), suggesting that tongue control during Alc-ModQ licking is more stereotyped and less variable than during Alc-Only. Here, we demonstrate that the lick-intake relation under Alc-HighQ was also significantly lower than Alc-Only (Fig. 2B,C; KW=30.03, p<0.0001), and Alc-HighQ lick-intake values were lower than Alc-ModQ (p<0.05 post-hoc), again suggesting more stereotyped tongue shape during licking under both Alc-HighQ and Alc-ModQ. However, reduced consumption during Alc-HighQ (Fig. 1B) could produce less dynamic range, and thus we examined the lick-intake relation for sessions with ≥0.3 g/kg intake. Alc-HighQ and Alc-ModQ drinking sessions still deviated less from their lick-intake regression lines compared with Alc-Only (Fig. 2D; KW=14.74, p=0.0006), with no difference between the challenge conditions (p>0.5 pos-hoc). Together, these findings support the suggestion that tongue control was more regular and less variable during both Alc-HighQ and Alc-ModQ consumption relative to Alc-Only. In addition, the intake-lick relation was not simply related to lick volume (Fig. 2E), suggesting that the two measures can indicate separable aspects of licking. Also, lick volume was not related to basal intake level (Fig. 2F), suggesting that less variable licking may allow drinking despite negative consequences to occur at all, but does not relate directly to consumption levels, similar to intake defense [45] (see Discussion). Together, these results suggest that Alc-HighQ intake showed important aspects of more regular tongue shape during licking that are seen under Alc-ModQ [10], suggesting use of a similar, more stereotyped action strategy to cope with challenge, separate from the actual challenge level.
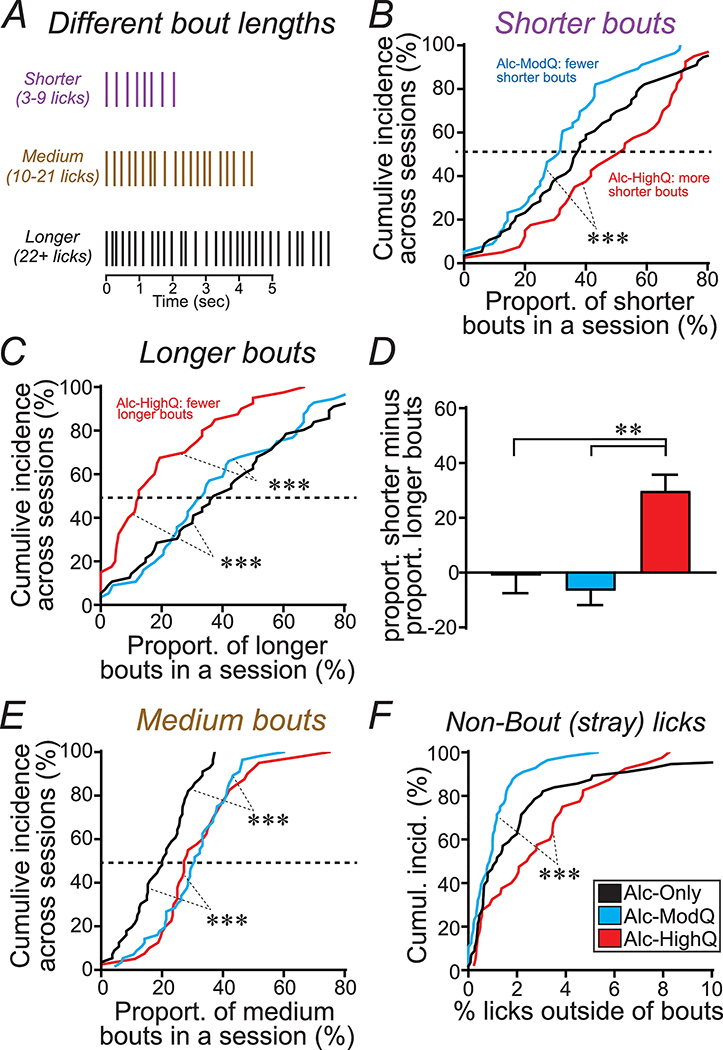
3.3. Bout disorganization under Alc-HighQ drinking
Even though Alc-HighQ retained several indicators of higher drive to act, it still significantly reduced drinking levels, and we next considered the possibility that decreased ability to maintain responding could be reflected in shortening of bouts. When examining bout length, we and others find that the distribution of bout lengths is not normal, and instead approximates an exponential distribution, with decreased bout abundance as bouts get longer (cf [10]) (see Fig. 5A–C). Because of this non-linearity, we first divided bout lengths into three groups (Fig. 3A), shorter (3–9 licks, purple), medium (10–21 licks, brown) and longer (22+ licks, black), since there are about equal numbers of the three bout length groups during Alc-Only and Alc-ModQ drinking [10]. We measured bout duration in terms of number of licks, which was highly correlated to bout duration calculated in seconds (Fig.S3). Relative to other drinking conditions, Alc-HighQ intake was shifted towards shorter and away from longer bouts (Fig. 3B–D). First, we determined the proportion of shorter, medium and longer bouts in each session, and we then examined the cumulative distribution of the proportion of each different bout length, and separately for each three drinking conditions. Alc-HighQ sessions had significantly more shorter bouts (cumulative distribution shifted to the right; Fig. 3B; KW=13.94, p=0.0009) and substantially fewer longer bouts (cumulative distribution strongly shifted to the left; Fig. 3C; KW=20.49, p<0.0001), while both challenge conditions had more medium bouts (Fig. 3E; KW=25.96, p<0.0001). We also subtracted the proportion of longer bouts from the proportion of shorter bouts in each session. This again showed that Alc-HighQ shifted drinking towards shorter and away from longer bouts (Fig. 3D; one-way ANOVA F(2,149)=9.383, p=0.0001). There were also more licks outside of bouts (“stray licks”) during Alc-HighQ versus Alc-ModQ intake (Fig. 3F; KW=16.475, p=0.0003 across three intake conditions). These results strongly suggest that Alc-HighQ increased bout fragmentation.
Fig.3. Alc-HighQ intake had more shorter bouts and many fewer longer bouts.
(A) Cartoon showing different bout length groups. (B-C) Cumulative incidence, across sessions, of the relative proportion of (B) shorter bouts and (C) longer bouts in a given session. Lines to the left indicate that sessions had fewer of that bout length group, while lines to the right indicate that sessions had more of that bout length group. In particular, Alc-HighQ bouts had (B) more shorter and (C) many fewer longer bouts. (D) We calculated the proportion of longer minus proportion of shorter bouts within each session, showing an increase in relative proportion of shorter bouts; these findings agree with a bout fragmentation during Alc-HighQ. (E) Cumulative incidence of the relative proportion of medium bouts (KW=25.96, p<0.0001). (F) Alc-HighQ intake had significantly more licks outside of bouts (stray licks, licks >1 sec apart) than Alc-ModQ. Proport: proportion. *** p<0.005
3.4. Only faster-starting Alc-HighQ bouts lasted longer
Several licking measures showed a similar shift during the two challenge conditions when compared with Alc-Only (Figs. 1, 2), but Alc-HighQ clearly disrupted intake (Fig. 1B) and bout length (Fig. 3). Thus, we examined other lick measures considered to reflect motivation. In particular, licking speed has been used as a measure of motivation (cf [10,23]), e.g. where Alc-ModQ drinking is faster and less variable than Alc-Only, especially at bout onset [10]. However, higher cost under Alc-HighQ may impact motivation more strongly than under Alc-ModQ, which could slow licking. Thus, we first examined licking speed at the start of bouts, since Alc-ModQ bouts overall start faster than Alc-Only bouts [10]. Specifically, we assessed the average time between adjacent licks across the first 4 licks (i.e., across the first 3 inter-lick intervals, ILIs) for each bout (grey boxes in Fig. 4A) [10]. Shorter Alc-HighQ bouts started significantly slower than shorter Alc-ModQ bouts, indicated by a shift to the right in the cumulative distribution of initial licking speed (Figs. 4C–D,S4A; two-way ANOVA: Substance, F(2,3329)=16.398, p<0.0001; Bout length, F(2,3329)=16.417, p<0.0001; Substance-Bout length Interaction: F(4,3329)=2.605, p=0.0341; one way ANOVA for post-hocs F(8,3329)=9.881, p<0.001). Importantly, during Alc-HighQ drinking, longer bouts started significantly faster than shorter bouts (Fig. 4D, p<0.05 post-hoc), similar to Alc-Only (Fig. 4B). Thus, the initial licking speed across Alc-HighQ bouts was overall more variable than Alc-ModQ, with longer bouts starting significantly faster than shorter bouts (Fig. 4C–D). Further, overall bout start speed and whole bout speed were faster during Alc-ModQ versus other conditions (Fig.S4B,C). Thus, quicker bout start and overall licking under Alc-ModQ [10] were disrupted by Alc-HighQ, although faster bout onset was associated with greater persistence of licking under Alc-HighQ; thus, we speculate that commitment to licking began before bout onset (detailed in Discussion).
Alc-HighQ bouts which started faster lasted longer (Fig. 4D), suggesting that initial licking speed could be a useful metric to predict bout persistence under higher challenge. We then examined the time between licks farther across the bout, allowing us to examine how licking speed might change across bouts as they progressed after their onset. In Fig. 4E–G, “1st” on the x-axis indicates the first ILI in the bout, “10th” the tenth ILI in the bout, etc. Lick timing was relatively well controlled during Alc-ModQ intake, for longer and shorter bouts (Fig. 4F), as we previously described [10]. In contrast, longer Alc-HighQ bouts started faster, but soon slowed relative to other conditions (Fig. 4G,H; examining [average duration of 6–9th ILIs, black bars] minus [average ILI duration across the bout start, grey bars]; KW=11.53, p=0.0031), with a similar trend for medium bouts (Fig.S4D). This might suggest that, even though faster speed at bout onset predicted the persistence of bouts under higher challenge, there were still significant challenges when Alc-HighQ intake was initiated. To further explore this possibility, we used hazard rate to calculate the probability of stopping [P(stop)] as bouts progressed; hazard rate can provide an instantaneous, unbiased estimator of a measure across time (here, the chance of bout termination as the bout progresses) [40–42] (see Methods). This hazard rate was calculated from the bout length distribution (see Methods): bout length distributions showed an approximately exponential drop in abundance as bout duration increased (Fig. 5A–C), which is widely observed (cf [10]). Also, bout length distributions were similar early and late in the session for each drinking condition (addressed below); P(stop) was determined from bouts pooled across the entire session. There was a clear increase in chance of termination for Alc-HighQ bouts until about the 12th lick (Fig. 5D) (see Methods). In contrast, the hazard rate of starting to lick showed smaller differences (Fig.S5). Thus, our results suggest that there was greater slowing (Fig. 4G,H) and greater possibility of bout termination (Fig. 5D) during the first seconds of Alc-HighQ bouts, and that only Alc-HighQ bouts that started faster were able to overcome such obstacles and persist much longer.
3.5. Similar longer bouts under Alc-HighQ and other drinking conditions
Faster bout initiation in longer Alc-HighQ bouts perhaps suggests a benefit to persistent licking (e.g. where the ability to have longer bouts might make any extra focus worth the effort). Thus, we examined the properties of longer bouts across drinking conditions. Indeed, even though Alc-HighQ intake had fewer longer bouts (Figs. 3C,D), these longer bouts still contributed >50% of intake (Fig. 5E). In addition, there were no significant differences in duration of longer bouts across drinking conditions (Fig. 5F; KW=5.553, p=0.063), suggesting similar persistence of longer bouts when successfully initiated. Also, longer bouts continued for ~8–9 seconds on average, which was much longer than the initial few seconds of Alc-HighQ drinking which showed greater slowing and chance of stopping; this perhaps suggests that Alc-HighQ had limited impact once beyond this initial period. In order to address this possibility, we defined periods of slower licking during longer bouts, which we called Slow Intervals of Licking (SILs), where each SIL contains one or more ILIs >250 msec in a row (Fig. 6A). A priori, we predicted that slowness during persistent bouts might increase risk of termination under challenge, with greater chance of stopping with higher challenge (i.e. where slowed licking would require attention to reestablish rapid licking, but would also increase awareness and impact of aversiveness). In strong contrast, our results suggest that these SIL events were very similar across the drinking conditions. First, recovery from slow licking periods in longer bouts seemed efficient in all conditions, with ~80% of SILs only a single ILI long, and with no differences in SIL duration across drinking conditions in longer bouts past the 12th ILI (i.e, after the initial Alc-HighQ slowing/stopping period) (Fig. 6B; KW=0.8754, p=0.6455) or across the whole bout (Fig.S6); medium bouts did show small differences in SIL duration across conditions (Fig. 6C; KW=16.92, p=0.0002). In addition, SILs during longer bouts were similarly slow across intake conditions (Fig. 6D; KW=4.402, p=0.1107, assessed after the 12th ILI); we note that Alc-HighQ SILs were slower early in longer bouts (Fig. 6E; KW=17.41, p=0.0002), consistent with overall slowed licking speed at bout onset during Alc-HighQ consumption (Fig. 4). Finally, SILs were similarly distributed across the bout in each drinking condition (Fig. 6F), again suggesting similar regulation of slow licking across drinking conditions during the longer licking periods. Thus, Alc-HighQ responding later in longer bouts was behaviorally similar to other drinking conditions, in particular where intake persisted with seemingly limited impact of the higher challenge, suggesting a benefit to longer bouts.
3.6. Evidence for sustained action strategy under challenged alcohol drinking
In addition to more moment-to-moment regulation of intake under challenge (implicated by results in Figs. 3–5), aversion-resistant intake has been suggested to represent use of a single, session-long response strategy [10]. Several lines of evidence here support this. First, one might predict longer bouts early in the session (cartoon in Fig. 7A), when motivation is ostensibly higher, as widely observed (cf [10,23]). Instead, as shown in Fig. 5A–C, the bout length distribution within each condition was similar across early, middle and later bouts (first, second, and third tertile of bouts) within a session. Second, SILs were similarly distributed across longer bouts (Fig. 6F), suggesting consistent regulation of recovery from slowed licking that was similar across drinking conditions. Third, Alc-ModQ intake showed consistent licking speed across the session for both whole bout speed (Fig. 7B, S7A) and bout start speed (Fig. 7C,S7B). Alc-HighQ results were more variable, but still showed no significant differences across time (see Fig. Legend for statistical analyses). Together, these findings support the idea that challenge-related shifts in bout duration and speed were present across the session, in accordance with use of a consistent, sustained response strategy across each drinking session (as we described in [10]).
Fig.7. Evidence that alcohol drinking involved a session-long intake strategy.
(A) Cartoon depicting the hypothesis from classic studies which predicts that there would be more longer bouts earlier in a session, when motivation is ostensibly higher (cf [10,23]). Instead, the bout length distribution was similar early and later in the session (Fig.5A–C). (B,C) Alc-ModQ showed similar whole bout speed (B) and bout start speed (C) across the session (speed determined from the time between licks). For this assessment, we divided the bouts from each session into ten groups, and licking measures were calculated for the first tenth, second tenth, etc of bouts within a session. Kruskal-Wallis was determined across session for each measure and in each drinking condition separately. (B) Whole bout speed did not vary across Alc-ModQ sessions (KW=9.882, p=0.3601) or Alc-HighQ (KW=15.79, p=0.0714). (C) Starting bout speed did not significantly vary across Alc-ModQ sessions (KW=14.47, p=0.148) or Alc-HighQ (KW=16.27, p=0.0615). However, Alc-Only bouts did show significant differences across the session in both whole bout speed (B, KW=19.83, p=0.019) and start speed (C, KW=20.48, p=0.0152), and the similar variation under Alc-HighQ suggests there may have been differences in licking speed across Alc-HighQ sessions that we were underpowered to detect.
3.7. Individual variability across rats
We note that our data were primarily analyzed separate of animal identity, as in [10,44], in order to focus on the overall response variability during compulsion-like alcohol drinking under moderate or higher challenge when compared with Alc-Only. Averaging results across multiple sessions from a given animal would remove some information about response variability. However, Fig.S8 shows, for each individual rat, the mean/SEM values across the three drinking conditions for a number of experimental variables. Some rats are “outliers” relative to other rats for some measures, but, in general, there was no overall pattern in terms of specific rats showing a constellation of differences across licking measures. In addition, to further understand individual variability, we examined results in Fig. 2E to determine whether subgroups of rats contributed to results with higher lick-intake/higher lick-volume or with higher lick-intake/lower lick-volume (described in detail in Fig.S9,Table.S1); a minority rats did have a predominance of sessions within these two “outlier” groups. However, there was no overall relation between lick-intake and licking speed (Fig.S9). Thus, while there were some individual differences across rats, further work with much larger sample sizes (like the n>600 for compulsion-like alcohol responding in [46]) will likely be required to understand how individual differences in particular response parameters could contribute to the overall strong impact of Alc-HighQ on licking measures.
4. Discussion
Compulsion-like alcohol consumption is a strong contributor to the harm of human AUD, making it critically important to understand the psychological and circuit mechanisms that promote intake despite negative consequences. Here, we used behavioral microstructure of licking to examine behavioral patterns during Alc-HighQ drinking; in particular, we attempted to distinguish licking changes that might be observed during both Alc-ModQ and Alc-HighQ intake (relative to Alc-Only), and changes that differentiate Alc-HighQ consumption attempts that were less or more successful at overcoming aversion. Interestingly, even though Alc-HighQ significantly reduced intake, it retained several behavioral changes seen with Alc-ModQ, including less variable lick volume and lick-intake relation, and earlier initiation of bouts, compared with Alc-Only. In contrast, while Alc-ModQ alcohol drinking shows less variable timing of licks [10], Alc-HighQ intake here exhibited greater variability in lick timing and more fragmented bouts relative to Alc-ModQ. Also, even though Alc-HighQ significantly reduced the number of longer bouts, these persisting bouts contributed >50% of consumption. Thus, it was interesting that longer Alc-HighQ bouts started significantly faster than shorter bouts. Also, once Alc-HighQ licking passed an ~2 second period of slowed licking and greater probability of stopping, longer bouts were similar in length and had similar, rapid recovery from slower licking intervals across drinking conditions. Hence, faster bout onset was associated with bout persistence under Alc-HighQ, and longer Alc-HighQ bouts were behaviorally similar to other drinking conditions after the first few seconds. Thus, even when challenge was high enough to suppress drinking, some aspects of responding (less variable tongue control, earlier bout start time) still showed greater commitment to act, and while lick timing and total intake were disrupted by Alc-HighQ, faster-starting bouts persisted longer (and similar to other drinking conditions). Our results are in agreement with automaticity being a central mechanism for habit/compulsion expression [5,6,24,25], and, importantly, our findings define specific periods of interest within a bout (especially the onset) and across bouts of different lengths which will be of value for future studies examining challenge-related brain activity as well as psychological mechanisms that promote compulsive-like responding.
Several licking measures examined showed a similar change under Alc-HighQ and Alc-ModQ when compared with Alc-Only (earlier bout onset, more stereotyped tongue control). In contrast, Alc-HighQ responding showed disrupted bout organization and timing, except for faster-starting Alc-HighQ bouts which persisted longer. Thus, it is interesting that particular brain regions implicated in compulsion-like addiction, anterior insula (AI) and medial prefrontal cortex (MPF) (see below), may regulate different aspects of behavioral organization during licking. In particular, increased response variability under Alc-HighQ was similar to changes observed after MPF inhibition [27,28], which often produces less orderly action (including greater timing variability) in rodent [27–31] and human [32,33]. In contrast, tongue control is not impaired by MPF inhibition [27,34,35] but is disrupted by ventral-frontal cortical inhibition [34–37,43], which has little impact on MPF-related action timing ([32,33,38]; AI tested specifically in [29,39]). In this light, a number of human studies also suggest that AI is related more to overall action plans, while MPF contributions are more proximal to specific costs and actions (see Supplemental Discussion). These extensive previous studies, taken together with our present findings, lead us to propose a model where AI/ventral-frontal cortex contributes to more overall response measures (such as earlier bout start) and tongue control, which are maintained under Alc-HighQ, while MPF regulates bout organization and timing which are largely disrupted under Alc-HighQ. While speculative at present, we believe this model helps define specific predictions for future studies examining how AI and MPF activity may differentially reflect specific epochs during challenged licking, work that we are initiating in our own lab.
Our interest in AI and MPF as regulators of specific aspects of licking is also motivated by a larger literature that implicates AI and MPF in regulation of compulsion-like, aversion-resistant action, as well as addiction [8,16–20], and motivation [17,18,21,22] more generally. Both AI and MPF inputs to ventral striatum are important for compulsion-like alcohol drinking in rats [12], with AI adaptations also critical in mice [15]. Interestingly, AI-MPF circuitry are also implicated in compulsion-like responding for alcohol in heavy human drinkers [14], suggesting a strong cross-species concurrence [5]. Further, non-canonical NMDA receptors are also implicated for conflict-related alcohol drives but not alcohol-only intake in both rodents and humans [12,13,47], although extant studies are limited. In addition, in rodents, AI connections to ventral striatum regulate compulsion for palatable food [48], and AI regulates cocaine compulsion [16]. In humans, AI also is implicated in compulsive drive during obsessive-compulsive disorder [49]. Thus, the likely central importance of AI and MPF for the expression of aversion-resistant responding for alcohol bolsters the specific interest in AI versus MPF as more specific regulators of specific aspects of licking for alcohol under challenge (overall commitment to act and tongue control versus organization of action timing).
One central question for future work is the nature of the consequence-resistance during compulsion-like responding. A considerable literature suggests that AI-MPF are important for discounting costs (see Supplemental Discussion), which would facilitate aversion-resistant action. Alternately, these structures could promote effort to acquire goals, which secondarily permits overcoming associated negative consequences. These processes may occur in concert, and will require extensive future studies of neural encoding in putative reward and cost related regions to help disentangle underlying mechanisms. Another challenge is that human addiction often involves discounting of negative consequences that occur in the future, rather than the immediate bad outcomes in rodent models. Assessing future discounting is highly challenging in rodents, but one possible (although speculative) relevant point is that intake-related costs are much more immediate for treatment-seeking individuals (discussed in [47,50,51]), and we have previously argued that our rodent investigations are relevant primarily for treatment-seekers who are in conflict about their intake. Thus, there are a number of important conceptual and psychological questions that require further investigation when seeking to understand mechanisms of compulsion-like responding.
In addition to changes in response timing, our results provide evidence that compulsion-like responding may involve adoption of a singular, session-long strategy [10]. One advantage of using a predetermined action plan is the ability to act more stereotypically or automatically, decreasing cognitive load and the need to attend to aversive challenge. Here, bout length distributions were similar early and late in sessions. This is unlike previous studies with sucrose, which show larger bouts early in the session when motivation is ostensibly higher (cf [10,23]). In addition, bout speed was not different across the session during Alc-ModQ and Alc-HighQ. Other studies described in Supplemental Discussion also support the likely central importance of AI-MPF for protracted action strategies, including those somewhat detached from moment-to-moment events. We speculate that the use of a single, session-long strategy during compulsion-like responding is similar to intake defense, where optimal intake is sacrificed to allow consumption without moment-to-moment attention to responding, reward, and/or cost [45].
We note the more variable and overall slower licking (Fig. 6) during Alc-HighQ was different from Alc-ModQ, but similar to Alc-Only. This might suggest that some aspects of drive to drink were similar under Alc-HighQ and Alc-Only. However, Alc-HighQ was very different from Alc-Only, with significantly reduced intake and disrupted bout organization. Thus, we consider it more likely that Alc-HighQ licking speed reflects a (partial) loss of the greater response regularity during Alc-ModQ. In this view, the more variable licking during Alc-HighQ versus Alc-ModQ reflects inability to maintain stereotyped responding against higher challenge, rather than the presence of a similar motivational state as Alc-Only. This remains speculative, but helps define possible state differences across drinking conditions for future studies.
Finally, all the present studies were performed in male rats, and studies in female rats might observe somewhat different patterns. Females often drink more alcohol than males under Alc-Only conditions (e.g. [52]), and can exhibit either no differences in quinine-resistant alcohol drinking [52] or greater quinine-resistance [53,54] relative to males, perhaps depending on the species and drinking model. At present, the mechanisms mediating greater intake in females remain somewhat unclear, but we speculate that greater motivation for alcohol in females could be reflected in greater number of longer Alc-HighQ bouts and shifts to faster licking, especially at bout onset; these could magnify the putative motivation-related behavioral patterns reported here.
5. Summary
We propose that our results help define a number of specific periods during licking which will be of interest for future studies examining neuronal activity in particular brain regions (including AI and MPF) and particular aspects of compulsion-like drive for alcohol. Bout onset is of particular importance, since faster licking at bout initiation (which we speculate reflects strong drive to drink) seemed essential for Alc-HighQ bouts to persist beyond an early period with higher risk of stopping. In addition, once beyond this early period, responding in longer Alc-HighQ bouts continued essentially the same behaviorally across conditions. Thus, our study provides a number of specific, novel, testable predictions, including particular behavioral changes within defined periods of responding which might indicate level of motivation and drive to overcome challenge. However, considerable work will be needed to robustly address these possibilities, requiring not only multiple, randomized drinking sessions of each challenge condition in the same animal (since longer bouts are also lowest in abundance), but also important internal controls for more basic positive (e.g. sucrose) and negative (e.g. quinine-only) consummants in each animal. Nonetheless, our findings provide a critical roadmap for such ongoing and future work. Our findings are also likely relevant to anxiety, OCD and related conditions where AI and MPF circuitry is strongly implicated [17,18].
Supplementary Material
Highlights.
Compulsive intake contributes strongly to harm of alcohol use disorder & addiction.
High challenge reduced intake, but not better tongue control or earlier bout start.
Higher challenge overall disordered bout generation and timing.
Higher-challenge bouts that started faster lasted much longer, with >50% of intake.
Model: Insula drive for all challenge, and mPFC for bout timing, disrupted or not.
Acknowledgements
The authors declare no conflicts of interest. Supported by AA024109 (FWH).
Footnotes
Raw data are available at (site with raw data presently being put together).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sinha R, Modeling stress and drug craving in the laboratory: implications for addiction treatment development, Addict Biol 14 (2009) 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton RF, Obsessive-compulsive aspects of craving: development of the Obsessive Compulsive Drinking Scale, Addiction 95 Suppl 2 (2000) S211–7. [DOI] [PubMed] [Google Scholar]
- 3.Larimer ME, Palmer RS, Marlatt GA, Relapse prevention. An overview of Marlatt’s cognitive-behavioral model, Alcohol Res Health 23 (1999) 151–60. [PMC free article] [PubMed] [Google Scholar]
- 4.CDC, Excessive Drinking Costs U.S. $223.5 Billion. 2014, Center for Disease Control: Atlanta, GA. [Google Scholar]
- 5.Hopf FW Lesscher HM, Rodent models for compulsive alcohol intake, Alcohol 48 (2014) 253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koob GF Volkow ND, Neurocircuitry of addiction, Neuropsychopharm 35 (2010) 217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein DH Kowalczyk WJ, Compulsive Seekers: Our take. Two Clinicians’ Perspective on a New Animal Model of Addiction, Neuropsychopharmacology (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naqvi NH, Gaznick N, Tranel D, Bechara A, The insula: a critical neural substrate for craving and drug seeking under conflict and risk, Annals of the New York Academy of Sciences 1316 (2014) 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diagnostic and Statistical Manual of Mental Disorders (DSM–5), (2013). [DOI] [PubMed]
- 10.Darevsky D, Gill TM, Vitale KR, Hu B, Wegner SA, Hopf FW, Drinking despite adversity: behavioral evidence for a head down and push strategy of conflict-resistant alcohol drinking in rats, Addict Biol 24 (2018) 426–37. [DOI] [PubMed] [Google Scholar]
- 11.Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A, Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration, Alcohol Clin Exp Res 34 (2010) 1565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW, Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake, Nat Neurosci 16 (2013) 1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO, Hopf FW, D-Serine and D-Cycloserine Reduce Compulsive Alcohol Intake in Rats, Neuropsychopharm 40 (2015) 2357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grodin EN, Sussman L, Sundby K, Brennan GM, Diazgranados N, Heilig M, Momenan R, Neural Correlates of Compulsive Alcohol Seeking in Heavy Drinkers, Biol Psych Cogn Neuro Neuroimag 2 (2018) 1022–31. [DOI] [PubMed] [Google Scholar]
- 15.Chen NM Lasek AM, Perineuronal Nets in the Insula Regulate Aversion-Resistant Alcohol Drinking, Addict Biol Epub ahead of print (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belin-Rauscent A, Daniel ML, Puaud M, Jupp B, Sawiak S, Howett D, McKenzie C, Caprioli D, Besson M, Robbins TW, Everitt BJ, Dalley JW, Belin D, From impulses to maladaptive actions: the insula is a neurobiological gate for the development of compulsive behavior, Mol Psychiatry 21 (2016) 491–9. [DOI] [PubMed] [Google Scholar]
- 17.Namkung H, Kim SH, Sawa A, The Insula: An Underestimated Brain Area in Clinical Neuroscience, Psychiatry, and Neurology: (Trends in Neuroscience 40, 200–207, 2017), Trends Neurosci 41 (2018) 551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O, Structure and Function of the Human Insula, J Clin Neurophysiol 34 (2017) 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaramillo AA, Agan VE, Makhijani VH, Pedroza S, McElligott ZA, Besheer J, Functional role for suppression of the insular-striatal circuit in modulating interoceptive effects of alcohol, Addict Biol 23 (2017) 1020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaramillo AA, Van Voorhies K, Randall PA, Besheer J, Silencing the insular-striatal circuit decreases alcohol self-administration and increases sensitivity to alcohol, Behav Brain Res 348 (2018) 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolling N, Scholl J, Chekroud A, Trier HA, Rushworth MFS, Prospection, Perseverance, and Insight in Sequential Behavior, Neuron 99 (2018) 1069–1082 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterzer P Kleinschmidt A, Anterior insula activations in perceptual paradigms: often observed but barely understood, Brain Struct Funct 214 (2010) 611–22. [DOI] [PubMed] [Google Scholar]
- 23.Lardeux S, Kim JJ, Nicola SM, Intermittent access to sweet high-fat liquid induces increased palatability and motivation to consume in a rat model of binge consumption, Physiol Behav 114–115 (2013) 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ersche KD, Lim TV, Ward LHE, Robbins TW, Stochl J, Creature of Habit: A self-report measure of habitual routines and automatic tendencies in everyday life, Pers Individ Dif 116 (2017) 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voon V, Derbyshire K, Ruck C, Irvine MA, Worbe Y, Enander J, Schreiber LR, Gillan C, Fineberg NA, Sahakian BJ, Robbins TW, Harrison NA, Wood J, Daw ND, Dayan P, Grant JE, Bullmore ET, Disorders of compulsivity: a common bias towards learning habits, Mol Psychiatry 20 (2015) 345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ersche KD, Ward LHE, Lim TV, Lumsden RJ, Sawiak SJ, Robbins TW, Stochl J, Impulsivity and compulsivity are differentially associated with automaticity and routine on the Creature of Habit Scale, Pers Individ Dif 150 (2019) 109493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parent MA, Amarante LM, Liu B, Weikum D, Laubach M, The medial prefrontal cortex is crucial for the maintenance of persistent licking and the expression of incentive contrast, Front Integr Neurosci 9 (2015a) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parent MA, Amarante LM, Swanson K, Laubach M, Cholinergic and ghrelinergic receptors and KCNQ channels in the medial PFC regulate the expression of palatability, Front Behav Neurosci 9 (2015b) 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horst NK Laubach M, The role of rat dorsomedial prefrontal cortex in spatial working memory, Neuroscience 164 (2009) 444–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krigolson OE, Heinekey H, Kent CM, Handy TC, Cognitive load impacts error evaluation within medial-frontal cortex, Brain Res 1430 (2012) 62–7. [DOI] [PubMed] [Google Scholar]
- 31.Narayanan NS, Horst NK, Laubach M, Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus, Neuroscience 139 (2006) 865–76. [DOI] [PubMed] [Google Scholar]
- 32.Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Borowiec A, Katz DI, Multiple frontal systems controlling response speed, Neuropsychologia 43 (2005) 396–417. [DOI] [PubMed] [Google Scholar]
- 33.Stuss DT, Murphy KJ, Binns MA, Alexander MP, Staying on the job: the frontal lobes control individual performance variability, Brain 126 (2003) 2363–80. [DOI] [PubMed] [Google Scholar]
- 34.Whishaw IQ Kolb B, “Stick out your tongue”: tongue protrusion in neocortex and hypothalamic damaged rats, Physiol Behav 30 (1983) 471–80. [DOI] [PubMed] [Google Scholar]
- 35.Whishaw IQ Tompkins GJ, An optic-fiber photocell detector for measuring tongue protrusion in the rat: evaluation of recovery from localized cortical lesions, Physiol Behav 43 (1988) 397–401. [DOI] [PubMed] [Google Scholar]
- 36.Brimley CC Mogenson GJ, Oral motor deficits following lesions of the central nervous system in the rat, Am J Physiol 237 (1979) R126–31. [DOI] [PubMed] [Google Scholar]
- 37.Castro AJ, The effects of cortical ablations on tongue usage in the rat, Brain Res 45 (1972) 251–3. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez R, Carmena JM, Nicolelis MA, Simon SA, Orbitofrontal ensemble activity monitors licking and distinguishes among natural rewards, J Neurophysiol 95 (2006) 119–33. [DOI] [PubMed] [Google Scholar]
- 39.Smith NJ, Horst NK, Liu B, Caetano MS, Laubach M, Reversible Inactivation of Rat Premotor Cortex Impairs Temporal Preparation, but not Inhibitory Control, During Simple Reaction-Time Performance, Front Integr Neurosci 4 (2010) 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen P Shadlen MN, A representation of the hazard rate of elapsed time in macaque area LIP, Nat Neurosci 8 (2005) 234–41. [DOI] [PubMed] [Google Scholar]
- 41.Wilson RC, Nassar MR, Gold JI, Bayesian online learning of the hazard rate in change-point problems, Neural Comput 22 (2010) 2452–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sozou PD, On hyperbolic discounting and uncertain hazard rats, Proc R Soc Lond B 265 (1998) 2015–20. [Google Scholar]
- 43.Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, Bird CW, Davies S, Savage DD, Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior, Behav Brain Res 269 (2014) 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffin WC 3rd, Randall PK, Middaugh LD, Intravenous cocaine self-administration: individual differences in male and female C57BL/6J mice, Pharmacol Biochem Behav 87 (2007) 267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaplan JM, Baird JP, Grill HJ, Dissociation of licking and volume intake controls in rats ingesting glucose and maltodextrin, Behav Neurosci 115 (2001) 188–95. [DOI] [PubMed] [Google Scholar]
- 46.Augier E, Barbier E, Dulman RS, Licheri V, Augier G, Domi E, Barchiesi R, Farris S, Natt D, Mayfield RD, Adermark L, Heilig M, A molecular mechanism for choosing alcohol over an alternative reward, Science 360 (2018) 1321–1326. [DOI] [PubMed] [Google Scholar]
- 47.Hopf FW, Do specific NMDA receptor subunits act as gateways for addictive behaviors?, Genes Brain Behav 16 (2017) 118–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spierling S, de Guglielmo G, Kirson D, Kreisler A, Roberto M, George O, Zorrilla EP, Insula to ventral striatal projections mediate compulsive eating produced by intermittent access to palatable food, Neuropsychopharmacology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luigjes J, Figee M, Tobler PN, van den Brink W, de Kwaasteniet B, van Wingen G, Denys D, Doubt in the Insula: Risk Processing in Obsessive-Compulsive Disorder, Front Hum Neurosci 10 (2016) 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hogarth L, Addiction is driven by excessive goal-directed drug choice under negative affect: translational critique of habit and compulsion theory, Neuropsychopharmacology 45 (2020) 720–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hopf FW, Recent perspectives on orexin/hypocretin promotion of addiction-related behaviors, Neuropharmacology 168 (2020) 108013. [DOI] [PubMed] [Google Scholar]
- 52.Sneddon EA, White RD, Radke AK, Sex Differences in Binge-Like and Aversion-Resistant Alcohol Drinking in C57BL/6J Mice, Alcohol Clin Exp Res 43 (2019) 243–249. [DOI] [PubMed] [Google Scholar]
- 53.Radke AK, Held IT, Sneddon EA, Riddle CA, Quinn JJ, Additive influences of acute early life stress and sex on vulnerability for aversion-resistant alcohol drinking, Addict Biol (2019) e12829. [DOI] [PubMed] [Google Scholar]
- 54.Fulenwider HD, Nennig SE, Price ME, Hafeez H, Schank JR, Sex Differences in Aversion-Resistant Ethanol Intake in Mice, Alcohol Alcohol 54 (2019) 345–352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.